Abstract
This study investigates the viability of a native Scenedesmus sp. strain for use in a 50 L bubble column photobioreactor designed to reduce greenhouse gas emissions under simulated spring, extreme summer, and winter conditions. The experiments were conducted by placing the reactor in a controlled climatic chamber, which allowed us to regulate the temperature, light intensity, and day–night cycles throughout the entire experiment. The results showed that under simulated spring conditions (a maximum temperature of 22 °C), the algal culture grew continuously for 61 days. Under extreme summer conditions (a maximum temperature of 39 °C), an initial drop in cell density was followed by recovery and continued growth over 75 days, although biomass production was 35% lower. Under winter conditions (a maximum temperature of 10 °C), the culture failed, indicating the need to prevent temperatures below 10 °C. In terms of biomass production, the culture densities achieved were 1.04 g L−1 and 0.68 g L−1 in the spring and summer trials, respectively. The Scenedesmus sp. strain demonstrated high carbon capture efficiency, tolerance to extreme heat, and sustained growth without the need for fresh medium or pH adjustments for over 60 days during spring and extreme summer conditions, confirming its potential for outdoor applications.
1. Introduction
The accelerated increase in CO2 emissions in recent years is largely driven by anthropogenic activities such as industrial processes, electricity generation, and land-use changes. Many techniques related to CO2 capture and its storage in geological wells have been investigated [1,2,3,4,5]. To mitigate greenhouse gas emissions, carbon sequestration through microalgae has gained attention as a promising strategy for achieving carbon neutrality due to its high carbon capture efficiency, ability to produce valuable by-products, and environmental sustainability [6,7].
Recent research has also highlighted the potential of microalgae in the removal of nitrogen compounds from wastewater by leveraging their superior photosynthetic efficiency [8,9]. This application aligns with the principles of the circular economy, where waste is repurposed to generate valuable by-products, thereby contributing to sustainable development. In addition to their potential in carbon sequestration, microalgae are recognized as a significant source of natural bioactive compounds, including carotenoids, chlorophyll, long-chain polyunsaturated fatty acids, phycobiliproteins, and enzymes, all of which have substantial industrial applications [10].
Carbon capture by microalgae typically takes place in a bubble column photobioreactor, where a gas mixture containing CO2 is introduced into the algal suspension in the form of bubbles [7]. Photobioreactors (PBRs) play a crucial role in optimizing the cultivation of microalgae by regulating key environmental factors such as CO2 supply, light intensity, pH, temperature, and nutrient concentrations [11]. Scaling up production requires careful control of gas flow rates, temperature, and carbon availability in order to maximize biomass yield and ensure commercial viability [12].
Numerous studies have demonstrated the potential of Scenedesmus sp. for wastewater treatment, highlighting its effectiveness in nutrient removal and biomass production [9,13,14]. The global environment is currently undergoing significant climatic changes due to human activity. In this context, the use of indigenous microalgal strains may enhance the feasibility of outdoor cultivation by reducing the need for strict temperature regulation.
Given the potential of microalgae for CO2 capture, this study developed a photobioreactor for CO2 sequestration. A native strain of Scenedesmus sp., isolated from southeastern Spain, was cultivated under three simulated climatic conditions: spring, extreme summer, and winter. The ultimate objective is to design a microalgae-based photobioreactor capable of being integrated into an urban lighting system, enabling ambient CO2 capture under diverse environmental conditions with minimal maintenance. Accordingly, several parameters were measured to evaluate algal growth and CO2 sequestration, including biomass accumulation and total carbon and nitrogen content throughout the experiment.
2. Materials and Methods
2.1. Microalgal Strain and Cultivation
The Scenedesmus sp. strain used in this study was provided by the Algal Biology and Ecology Research Group at the University of Murcia, having been isolated from different habitats of southeastern Spain. The algae were grown in a standard Gold Medium (FWS, Aqualgae S.L., A Coruña, Spain) [15] without pH correction. The composition of Gold Medium FWS, once prepared, contains 515 mg/L of sodium nitrate (NaNO3) and potassium dihydrogen phosphate (KH2PO4), 150 mg/L of magnesium sulfate (MgSO4·7H2O), 50 mg/L of calcium chloride (CaCl2·2H2O), and trace elements and vitamins at a concentration of 8 mg/L. These chemicals were supplied by Sigma-Aldrich-Fluka Chemical Co. (Darmstadt, Germany) and was of the highest purity available.
2.2. Photobioreactor and Working Conditions
A 50 L bubble column photobioreactor (PBR), equipped with an air bubble supply, was used for the cultivation of the microalgae (design details are protected under industrial secrecy). The optimal photoperiod for this Scenedesmus sp. strain is 16 h per day, as determined in our laboratory. The system in which the bioreactor is installed is designed not only to illuminate the streets but also to provide supplemental light to the reactor when sunlight is not available in order to ensure a total of 16 h of illumination per day.
The photobioreactor contained 43 L of algal culture. Environmental conditions were simulated by placing the bioreactor inside a bioclimatic chamber (FITOCLIMA S600 PLH, ARALAB, Sintra, Portugal), which allowed for precise control of temperature and light to replicate different seasonal scenarios: spring (13 h of light, 14–22 °C), extreme summer (15 h of light, 19–39 °C), and winter (10 h of light, 0–10 °C). As mentioned above, illumination inside the chamber (500 µmol m−2 s−1) was supplemented by the photobioreactor’s own integrated lighting system during the dark periods of natural light to maintain a consistent 16 h photoperiod under all experimental conditions. For this purpose, supplemental illumination was set at 3, 1, and 6 h for the spring, summer, and winter trials, respectively (Figure 1).
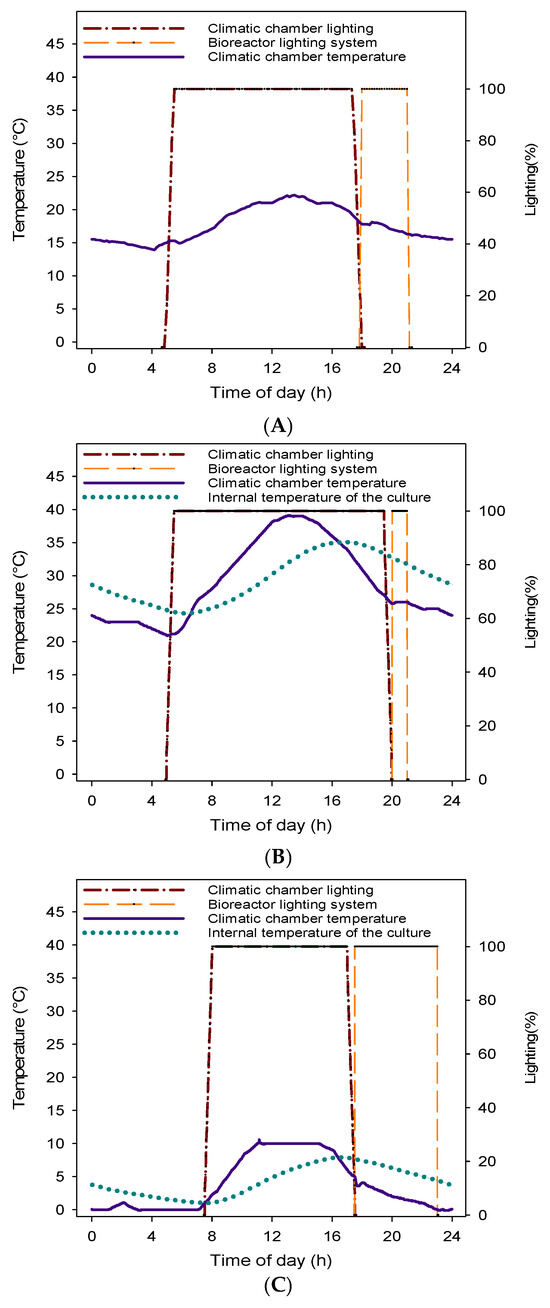
Figure 1.
Climatic lighting and temperature conditions established in the climatic chamber for the simulated spring (A), summer (B), and winter (C) trials, along with internal temperature recordings of the culture for the summer (B) and winter (C) trials.
The photoperiod and temperature settings were based on hourly average data for air temperature (°C) and solar radiation (µmol photons m−2 s−1) corresponding to typical spring, summer, and winter days in Murcia. These data were obtained from the SIAM (Agricultural Information System of Murcia-IMIDA) database. A weighted hourly average was calculated for each parameter, which served as the basis for simulating the seasonal light and temperature conditions.
The bioclimatic chamber, located indoors at ambient laboratory temperature, ensured a stable and controlled environment for the experiments. Ambient air was used as the inlet gas for the bioreactor, pumped directly without any additional CO2 enrichment. A temperature sensor continuously recorded the internal temperature of the algal culture, enabling real-time monitoring and comparison with the programmed environmental settings, particularly during the extreme summer and winter trials.
2.3. Analytical Methodology
Samples were collected on day 0 and every 7 days for analysis. Each sampling involved withdrawing 80 mL using a 100 mL syringe connected to the outlet hose and transferring the sample into a sterile 250 mL+ container. All analyses were performed on the same day as sampling, except for the carbon and nitrogen content of the culture medium, which were stored for subsequent analysis by the Scientific and Technical Research Area (ACTI) of the University of Murcia.
Biomass production was evaluated weekly by measuring dry weight after filtration and drying the filter at 105 °C, following APHA protocols [16]. Additionally, the optical densities at 680 nm and 750 nm were monitored using a UV-VIS spectrophotometer (T80 PG Instruments Ltd., Leicestershire, UK). Cell growth and density were monitored through cell counts under a microscope using a Neubauer chamber, as described by [17]. Total and organic carbon contents as well as nitrogen content were measured weekly using ISO method 20236 and ISO method 13878. Electrical conductivity, pH, and oxidation–reduction potential (ORP) were measured weekly using a portable multiparameter probe (sensION+ MM150, Hach, Loveland, CO, USA). These analyses provided comprehensive data on the culture’s growth and physiological state under varying environmental conditions.
2.4. Statistical Analysis
Statistical analyses were performed using PASW Statistics 28 for Windows (SPSS Inc., Chicago, IL, USA) to calculate descriptive statistics (mean and standard deviation). Additionally, bilateral correlations between parameters were assessed using Pearson’s correlation with a 95% confidence interval. Data will be made available upon request.
3. Results and Discussion
The main objective of this study is to evaluate the ability of the microalga Scenedesmus sp. to capture CO2 in a photobioreactor designed for long-term outdoor use under varying environmental conditions. The photobioreactor is also equipped with its own lighting system powered by solar panels and batteries, which, in addition to illuminating streets and public spaces, provide light to the bioreactor during dark periods, enabling continued CO2 capture without relying on natural light. While commercial photobioreactor systems for CO2 capture and air treatment do exist, most are not specifically designed for outdoor integration in urban environments, nor do they assess long-term viability under highly variable environmental conditions. The gap this study addresses is the real-world operational resilience of a native Scenedesmus sp. strain cultivated in a semi-autonomous PBR under simulated seasonal temperature regimes (including extreme summer and winter conditions). Furthermore, this work provides a foundational model for a dual-purpose photobioreactor system capable of both microalgae cultivation and integration into urban lighting infrastructure.
Figure 2 shows the growth of the microalga, measured by the optical density of the suspension at 680 and 750 nm using spectrophotometry, as well as by cell density, under different environmental conditions—namely, spring, extreme summer, and winter. The specific conditions for each scenario are described in the Materials and Methods Section and were simulated in a climatic chamber. As observed, the growth trends measured by both methods are consistent. Under spring conditions, continuous and more pronounced growth was observed, followed by a decline in microalgal concentration after approximately 55 days, mainly due to nutrient depletion caused by the discontinuous feeding strategy.
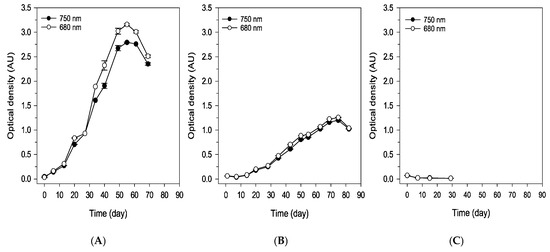
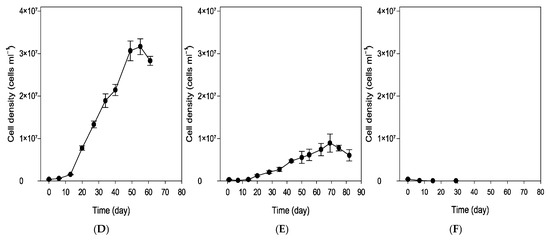
Figure 2.
Monitoring of cell growth through optical densities determined by absorbance at 680 and 750 nm (Absorbance Units) and cell density (cells mL−1) in the simulated spring (A,D), summer (B,E), and winter (C,F) trials. Values are the mean standard deviation of at least 2 replicates.
Under extreme summer conditions, the growth rate was lower; however, microalgal growth continued for nearly 75 days. Although nutrient concentrations in the medium were not measured, the delayed growth observed after the initial inoculum loss due to high temperatures suggests that substrate depletion likely occurred at a later stage. This may explain the extended growth period observed in the summer trial, which lasted up to 75 days (longer than the 61-day growth observed under spring conditions), although the exact timing of nutrient exhaustion cannot be confirmed.
Under winter conditions, no algal growth was observed; as shown in Figure 2A, a discontinuous feeding strategy was chosen for the cultivation process, as the system is intended to be as simple and autonomous as possible, requiring minimal maintenance. Based on the results obtained, maintenance operations could be limited to every 80 days under spring conditions and every 90 days under extreme summer conditions.
Figure 3 presents the biomass productivity expressed in grams of dry weight for the simulated spring (A) and summer (B) trials. Biomass productivity followed the same trend as the optical and cell densities, reaching a maximum around day 55 for spring and day 75 for summer conditions, with lower productivity observed in the winter condition.
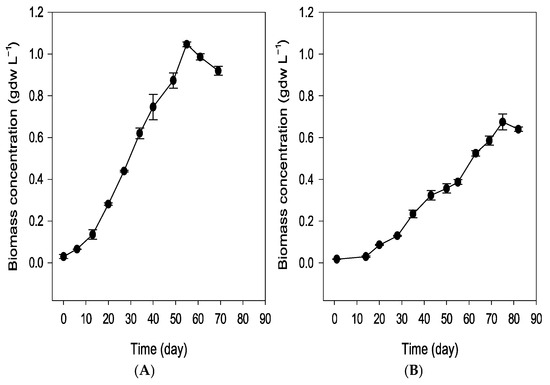
Figure 3.
Biomass productivity expressed in grams of dry weight in the simulated spring (A) and summer (B) trials. The winter trial was not analyzed due to the culture’s non-survival. Values are the mean standard deviation of at least 2 replicates.
The spring conditions were the most favorable for the cultivation of Scenedesmus sp. during the spring trial; the culture initially entered a lag phase, characterized by slow growth during the first two weeks, as indicated by the optical density, cell density, and dry biomass measurements (Figure 2 and Figure 3). Following this acclimation period, the culture entered an exponential growth phase, reaching its peak biomass on day 55. A stationary phase was anticipated afterward; however, a decline in biomass occurred by day 61, alongside signs of degradation within the bioreactor. Brown deposits accumulated on the bioreactor walls (Figure 4C), particularly in areas exposed to light, suggesting photooxidative damage [18]. By day 69, the biomass had significantly decreased, leading to the conclusion of the trial (Figure 2A,D and Figure 3A).
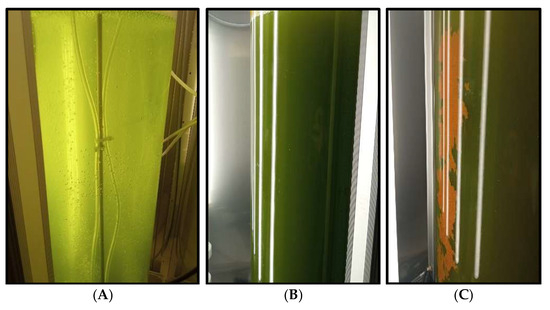
Figure 4.
Images of the microalgae culture in the photobioreactor taken on the day of culture initiation (A), on days 55 (B) and 61 of cultivation, and (C) during the simulated spring trial.
During the summer trial, a marked reduction in culture color intensity was clearly visible to the naked eye by day 2, accompanied by substantial sedimentation of the inoculum at the bottom of the reactor. Although the culture recovered by day 7, the summer trial did not reach the biomass levels achieved in spring, exhibiting slower growth throughout the experiment (Figure 2B,E and Figure 3B). The final biomass concentration in summer was 35% lower than in spring, likely due to the initial loss of inoculum caused by exposure to elevated temperatures. Similar brown deposits, as seen during the spring trial, appeared on the reactor walls exposed to light at the end of the trial.
In contrast, during the winter trial, all parameters indicated the culture’s non-viability (Figure 2C,F), with visible evidence in the reactor, where a significant portion of the inoculum settled at the bottom after day 2 of the trial. The results indicate the inviability of Scenedesmus sp. under winter conditions. In this case, the incorporation of a heating system into the reactor is proposed to enable its operation under typical winter environmental conditions. The solar panels and batteries integrated into the reactor could facilitate the sustainable integration of the heating system within the photobioreactor.
Elemental analysis confirmed the capture of CO2 by the culture during the viable trials, as shown by an increase in total carbon (TC) in the culture medium, which correlated with biomass production (Figure 5). In the spring trial, liquid-phase analysis revealed that up to 91% of the total carbon increase was attributable to organic carbon.
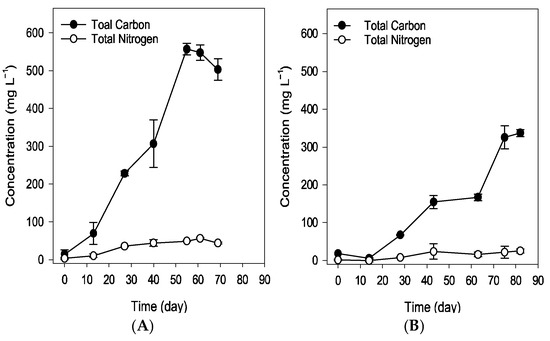
Figure 5.
The macroelemental content of total carbon and total nitrogen in dry biomass samples during the simulated spring (A) and summer (B) trials. The winter trial was not analyzed due to the culture’s non-survival. Values are the mean standard deviation of at least 2 replicates.
A strong correlation was observed between cell density, measured through optical microscopy, and optical density at 760 nm and 680 nm, as well as dry biomass weight and carbon content from elemental analysis (Figure 2, Figure 3 and Figure 5). Statistically significant correlations (p < 0.01) were found across all parameters, with Pearson’s correlation coefficients exceeding 0.93, highlighting a robust relationship between these variables.
On the other hand, the physicochemical analysis showed that the culture’s initial pH was approximately 7 (Table 1). In both the spring and summer trials, pH rapidly increased to around 10 and remained stable, without negatively affecting culture viability, as indicated by continued growth and biomass production (Figure 2 and Figure 3, respectively). Additionally, reductions in oxidation–reduction potential (ORP) and electrical conductivity (EC) were observed in the viable cultures, while in the non-viable winter trial, no significant changes in pH, ORP, or EC were detected (Table 1). Several studies have documented the rise in pH due to microalgal photosynthetic activity [19,20]. Although optimal microalgal growth typically occurs within a pH range of 7 to 9 [13], this native Scenedesmus sp. strain exhibited growth under study conditions without pH adjustment, reaching values above 10.

Table 1.
Analysis of pH, electrical conductivity (EC), and oxidation–reduction potential (ORP) of samples of the microalgae culture inside the photobioreactor throughout the trials.
The reduction in oxidation–reduction potential (ORP) commonly observed during microalgal cultivation can be attributed to several interrelated biological and physicochemical processes [21]. The decrease in ORP during microalgal cultivation results from a combination of organic matter accumulation, oxygen consumption, microbial activity, and pH-driven chemical equilibria—all of which create a more reduced environment in the culture medium.
Regarding the internal temperature of the culture in the photobioreactor (PBR), the results showed that the internal temperature of the tank exhibited some buffering relative to external environmental conditions (Figure 1). In the summer trial, the internal temperature was 4 °C lower than the external maximum, while in the winter trial, it was 2 °C lower than the external maximum and 1 °C higher than the external minimum.
The tolerance of Scenedesmus to high temperatures is well-documented. Studies have shown that species such as S. obliquus and S. almeriensis are mesophilic microalgae, with optimal growth temperatures ranging from 31 to 32 °C and maximum growth temperatures of 34–36 °C [13,22,23]. Additionally, Sánchez et al. [13] reported that these species can tolerate temperatures as high as 45 °C. The findings from this study are consistent with these reports, demonstrating that the native Scenedesmus sp. strain can survive high-temperature exposure, although an initial population decline was observed following inoculation [13,22,23]. Other microalgae, such as Phaeodactylum tricornutum, Dinobryon divergens, and Porphyridium cruentum, show growth rates approaching zero at 30 °C and below [24]. Conversely, previous studies indicate that while Scenedesmus sp. showed diminished growth at 10 °C, it was nonetheless able to grow under these conditions, effectively treating wastewater and accumulating valuable compounds [13,14,25]. However, in our study, at lower temperatures, the native strain evaluated did not recover from the initial population decrease. We should consider that we use more extreme conditions in our experiments since temperature range from 1 °C to 9 °C. Scenedesmus sp. can be considered thermostable over a relatively wide temperature range compared to other microalgae.
In terms of biomass production, the culture densities achieved were 1.04 g L−1 and 0.68 g L−1 in the spring and summer trials, respectively. These values were comparable to those reported in other studies of microalgae cultivated in bubbling photobioreactor trials without renewal of the growth medium [6,7].
These results underscore the high biomass productivity potential of Scenedesmus sp. under favorable environmental conditions, particularly during spring and to a lesser degree at very high temperatures. However, low temperatures pose significant challenges to the viability and biomass production of the culture. As previously mentioned, the system of solar panels and batteries can be utilized to integrate a heating system into the photobioreactor, allowing it to operate effectively under cold winter conditions.
4. Conclusions
In conclusion, this native strain of Scenedesmus sp. demonstrated compatibility with the pilot photobioreactor design, as well as efficiency in the transformation of environmental CO2 into organic carbon, comparable to other algae cultivation studies in bubble column photobioreactors. Moreover, this strain exhibited a high tolerance to elevated temperatures, sustaining cultivation for over 60 days without the addition of fresh medium or pH regulation, with the potential to extend cultivation to approximately 90 days under temperate and high-temperature conditions. However, the culture was not viable under cold temperatures. In this case, a sustainable heating system could be incorporated into the photobioreactor. In terms of biomass production, the culture densities achieved were 1.04 g L−1 and 0.68 g L−1 in the spring and summer trials, respectively. The system composed of Scenedesmus sp. and the custom-designed photobioreactor may enable the use of microalgae for CO2 capture through a semi-autonomous, low-maintenance setup under spring and extreme summer conditions. In the case of cold winters, the system allows for the sustainable integration of a heating mechanism, thanks to the inclusion of solar panels and batteries.
Author Contributions
Conceptualization, F.J.H.-F., A.P.d.l.R., J.Q.M., J.D.S. and Y.G.; methodology, Y.G., A.H.-F., A.S.-Z. and E.I.-L.; formal analysis, F.J.H.-F., A.P.d.l.R. and J.Q.M.; software, Y.G., A.S.-Z., A.H.-F. and E.I.-L.; investigation, Y.G., J.D.S., F.J.H.-F., A.P.d.l.R., J.Q.M., A.S.-Z., E.I.-L. and A.H.-F.; data curation, Y.G., A.S.-Z., E.I.-L. and A.H.-F.; writing—review and editing, F.J.H.-F., A.P.d.l.R., J.Q.M. and Y.G.; supervision, J.Q.M., F.J.H.-F., J.D.S. and A.P.d.l.R.; project administration, J.Q.M., F.J.H.-F., J.D.S. and A.P.d.l.R.; funding acquisition, J.Q.M., F.J.H.-F., J.D.S. and A.P.d.l.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors wish to acknowledge the financial support of the Spanish Company “Ecología y Nuevas Tecnologías, More Than Light (NTE)”; the Ministry of Science, Innovation, and Universities (MCIN/AEI/10.13039/501100011033 and Union European Next Generation EU/PRTR, Ref. TED2021-129220B-I00); and the Seneca Foundation Science and Technology Agency of the Region of Murcia Ref. 22017/PI/22. Adrián Hernández Fernández has a grant (21817/FPI/22) from the Seneca Foundation Science and Technology Agency of the Region of Murcia. Eduardo Iniesta López has a grant (22345/FPI/23) from the Seneca Foundation Science and Technology Agency of the Region of Murcia.
Data Availability Statement
Data supporting the reported results can be provided upon reasonable request to the corresponding author where appropriate.
Acknowledgments
The authors also wish to acknowledge the Scientific and Technical Research Area (ACTI) of University of Murcia for performing the elemental analyses of this study.
Conflicts of Interest
José David Sánchez is CEO of the company Ecología y Nuevas Tecnologías, More Than Light (NTE). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct capture of CO2 from ambient air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Thiedemann, T.M.; Wark, M. A compact review of current technologies for carbon capture as well as storing and utilizing the captured CO2. Processes 2025, 13, 283. [Google Scholar] [CrossRef]
- Bondarenko, A.V.; Islamov, S.R.; Ignatyev, K.V.; Mardashov, D.V. Laboratory studies of polymer compositions for well-kill under increased fracturing. Perm J. Pet. Min. Eng. 2020, 20, 37–48. [Google Scholar] [CrossRef]
- Belousov, A.; Lushpeev, V.; Sokolov, A.; Sultanbekov, R.; Tyan, Y.; Ovchinnikov, E.; Shvets, A.; Bushuev, V.; Islamov, S. Experimental research of the possibility of applying the Hartmann–Sprenger effect to regulate the pressure of natural gas in non-stationary conditions. Processes 2025, 13, 1189. [Google Scholar] [CrossRef]
- Cailly, B.; Le Thiez, P.; Egermann, P.; Audibert, A.; Vidal-Gilbert, S.; Longaygue, X. Geological storage of CO2: A state-of-the-art of injection processes and technologies. Oil Gas Sci. Technol.—Rev. IFP 2005, 60, 517–525. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Castillo-Vacas, E.I.; Castañeda-Hernández, L.; Gradiz-Menjivar, A.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Sosa-Hernández, J.E.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. CO2 biocapture by Scenedesmus sp. grown in industrial wastewater. Sci. Total Environ. 2021, 790, 148222. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, W.; Li, J.; Zhang, X.; Liu, L.; Li, H. Effects of bubble cutting dynamic behaviors on microalgal growth in bubble column photobioreactor with a novel aeration device. Front. Bioeng. Biotechnol. 2023, 11, 1225187. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef]
- Hernández-Fernández, A.; Iniesta-López, E.; Hernández Baños, A.I.; Garrido, Y.; Sánchez Zurano, A.; Hernández-Fernández, F.J.; De los Ríos, A.P. Optimization of recovery of nutrients from pig manure slurry through combined microbial fuel cell and microalgae treatment. Processes 2024, 12, 1989. [Google Scholar] [CrossRef]
- Yan, N.; Fan, C.; Chen, Y.; Hu, Z. The potential for microalgae as bioreactors to produce pharmaceuticals. Int. J. Mol. Sci. 2016, 17, 962. [Google Scholar] [CrossRef] [PubMed]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef]
- Reddy, S.D.M.; Deepika, N.; Dropathi, M.R.; Vishwanutha, S.; Daaman, J.D.; Reddy, C.N.; Yadavalli, R. Chapter 4—Factors affecting microalgal biomass productivity in photobioreactors. In Current Developments in Biotechnology and Bioengineering; Sirohi, R., Pandey, A., Sim, S., Chang, J.-S., Lee, D.-J., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 59–88. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández-Sevilla, J.M.; Acién, F.G.; Cerón, M.C.; Pérez-Parra, J.; Molina-Grima, E. Biomass and lutein productivity of Scenedesmus almeriensis: Influence of irradiance, dilution rate and temperature. Appl. Microbiol. Biotechnol. 2008, 79, 719–729. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.-y.; Zhang, Y.-p. Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour. Technol. 2011, 102, 3098–3102. [Google Scholar] [CrossRef]
- Aqualgae. Culture Media for Microalgae. Available online: https://aqualgae.com/portfolio/culture-media-for-microalgae/ (accessed on 21 July 2025).
- APHA American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 20th ed; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]
- Zhang, M.; Gu, L.; Zheng, P.; Chen, Z.; Dou, X.; Qin, Q.; Cai, X. Improvement of cell counting method for Neubauer counting chamber. J. Clin. Lab. Anal. 2020, 34, e23024. [Google Scholar] [CrossRef]
- Molina Grima, E.; Fernández, F.G.A.; García Camacho, F.; Chisti, Y. Photobioreactors: Light regime, mass transfer, and scale-up. J. Biotech. 1999, 70, 231–247. [Google Scholar] [CrossRef]
- Goldman, J.C.; Brewer, P.G. Effect of nitrogen source and growth rate on phytoplankton-mediated changes in alkalinity. Limnol. Oceanogr. 1980, 25, 352–357. [Google Scholar] [CrossRef]
- Hinga, K.R. Effects of pH on coastal marine phytoplankton. Mar. Ecol. Prog. Ser. 2002, 238, 281–300. [Google Scholar] [CrossRef]
- Foladori, P.; Petrini, S.; Andreottola, G. Evolution of real municipal wastewater treatment in photobioreactors and microalgae-bacteria consortia using real-time parameters. Chem. Eng. J. 2018, 345, 507–516. [Google Scholar] [CrossRef]
- Soede, C.J.; Hegewald, E. Scenedesmus. In Micro-Algal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 59–84. [Google Scholar]
- Martínez, M.E.; Jiménez, J.M.; El Yousfi, F. Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresour. Technol. 1999, 67, 233–240. [Google Scholar] [CrossRef]
- Ras, M.; Steyer, J.-P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 153–164. [Google Scholar] [CrossRef]
- Ma, C.; Wen, H.; Xing, D.; Pei, X.; Zhu, J.; Ren, N.; Liu, B. Molasses wastewater treatment and lipid production at low temperature conditions by a microalgal mutant Scenedesmus sp. Z-4. Biotechnol. Biofuels Bioprod. 2017, 10, 111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).