Abstract
In this study, particles of ground grape seeds were utilized to adsorb phosphate ions from a prepared solution, aiming to reduce phosphate concentration. Through a series of adsorption experiments, the effects of the adsorbent concentration, initial phosphate ion concentration, temperature, and pH on the phosphate ion uptake were studied. The removal efficiency of the phosphate ion decreased from 77 to 61% as a 25 to 45 °C increment in temperature was observed, which indicated the exothermicity in the adsorption process. The phosphate ion movement onto the adsorbent surface that exhibited the highest uptake value favored a neutral reaction environment with a pH value of seven. The experimental results, when compared using different adsorption isotherms, showed that the best fit was exhibited by the Jovanovic isotherm, which was further confirmed owing to its high 0.974 R2 value. Intraparticle diffusion and pseudo second order models describe the kinetics of phosphate adsorption onto grape seeds, with reaction constants of 8.8 × 10−3 (mg/g min) and 0.412 (mg/g·min0.5), respectively. The adsorption was physiosorptive, spontaneous, exothermic, and favorable. Furthermore, the negative entropy with a value of −0.0887 kJ/mol·K revealed reduced randomness in the adsorption process system.
1. Introduction
Phosphate ion () can be considered one of the most essential elements of living cells. It is a component of bone, DNA, RNA, cell membrane, energy production, and transportation mechanisms, in addition to being a constituent of the chemical structure of some enzymes [1]. Along with nitrogen and potassium, phosphate ions are a vital nutrient to achieve successful crop growth and production. It is usually added to the soil as an inorganic fertilizer or organic as manure. It is absorbed by plants and transported throughout the plant via the xylem and phloem, where it contributes to several physiological processes like energy metabolism [2]. Human and animal waste, in addition to the wastes of dead animals and plants, are biodegraded, releasing phosphate ions back into the soil to complete the cycle [3].
It is widely understood that small amounts of phosphate ions are used as fertilizer and become a part of the human food chain. A large portion of the phosphate ions remains in the soil [4]. This portion that remains in the soil usually moves into surface water and, consequently, deposits in different locations or accumulates in rivers or large water bodies. This fact usually causes nutrient pollution or what is known as water eutrophication [5]. Eutrophication usually originates from excess-nutrient driven processes, such as the soluble reactive phosphate ion (SRP) algae and aquatic plant growth [6]. This drives a drastic fluctuation in dissolved oxygen between the day and the night, which leads to mortality of marine fauna, including fish. Consequently, this can cause serious damage to biodiversity and food chains in aquatic ecosystems. Water runoff and soil erosion, triggered by rainfall or snowmelt, combined with water coming from municipal wastewater treatment facilities, are the main reasons behind eutrophication of water bodies [7].
Reducing nutrient overload and its negative environmental consequences in the form of water eutrophication is an important objective that has received significant attention from municipalities and scientists worldwide. This objective is achievable through phosphate ion reduction and recovery, together with other nutrients that are found in different wastewater effluents. Phosphorus is present in aquatic environments in multiple forms, the most prominent of which are dissolved reactive phosphorus (SRP), organic phosphorus, and phosphorus bound to minerals, particularly iron, calcium, and aluminum oxides. SRP is the most bioavailable form in the water column and is used directly by microorganisms, making it the primary cause of eutrophication. In sediments, it is often bound to minerals, but reducing conditions, such as hypoxia in the lower layers, can result in the release of phosphate back into the water column, causing internal loading and persistent contamination. Therefore, understanding these forms and their environmental behavior is essential for developing effective remediation strategies [1,6]. The removal can be accomplished by using biological or physicochemical processes or a combination of them. This depends on the source and the effluent’s physical characteristics. Physiochemical methods, biological treatment, and/or combinations of both, are some of the techniques that are well known for removal of phosphate ions from wastewater effluents [8]. Among these techniques, chemical precipitation is one of the most common, where iron, aluminum, or calcium salts are used to form insoluble phosphate compounds that are removed by sedimentation. Enhanced biological phosphorus removal (EBPR) is another effective technique, relying on microorganisms capable of storing phosphate within their cells under alternating aerobic and anaerobic conditions. Additionally, constructed wetlands are used as a low-cost and environmentally friendly solution, where aquatic plants and substrates (such as gravel and sand) contribute to the absorption and sequestration of phosphate through adsorption or sedimentation. These methods are key technologies for phosphate management in various ecosystems and aquatic systems, and their effectiveness has been tested in numerous practical applications [6]. So far, precipitation or biological processes have been more reliably applied to the removal of phosphate ions, with majority relying on the production of hydroxyapatite or struvite. A shortcoming and complication of these methods is the need for a high phosphate concentration or presence of ammoniacal nitrogen. Thus, adsorption has been favored due to its ability to work in waters contaminated with low phosphate levels [9]. For instance, the use of calcium salt can form hydroxyapatite, achieving removal efficiencies of 75–85% [10]. Table 1 summarizes some of the common adsorbents along with their advantages and disadvantages.

Table 1.
Advantages and disadvantages of various adsorbents for phosphate ion removal.
The physicochemical processes have many advantages but suffer from many drawbacks and limitations. These limitations include the formation of solids, the need to control the effluent pH, and the inclusion of chemicals before any final discharge [27] Cost and energy utilized in removal techniques represent a significant challenge, especially for small wastewater plants and if a low-level concentration is to be attained. Nevertheless, it is many times less for agricultural practice than that for municipal effluent [28]. Thus, the adsorption process using cheap and available adsorbents can be applied as an attractive and inexpensive alternative treatment approach for phosphate ion removal [29]. Phosphate ion removal from a polluted waterbody was conducted with adsorbents of different kinds [30]. Many researchers have applied many cost-effective and efficacious adsorbents for phosphate ion removal, such as activated carbon [31], calcite [32], coal [33], iron [34], chitosan [35], fruit juice residue and rice husk [36], ZnO nanoparticles [37], and modified bentonite [38]. An excellent candidate for adsorption is grape seeds, which contain organic acids that act as metal chelating agents and form stable complexes, which augment phosphate adsorption from the environment. Globally, approximately 1.4 × 106 tons of grape seeds are generated as waste from the food industry, which results from processing 46.5 million tons of grapes for various products, including wine and grape juice, assuming a seed panicle ratio of 3% by weight [39]. This constitutes anywhere between 2 and 5% of the grapes’ mass, which makes the amount of grape seed being discarded much more significant on the upper bound [40]. In the wine industry alone, other estimates have shown that 20–25% by weight of grapes used is discarded as waste [41]. These statistics indicate that grape seed valorization in the form of adsorbents for phosphate is important, as it will not only decrease phosphate pollution but also make use of large amount of agricultural waste, i.e., grape seeds, which would otherwise go to waste [42]. Grape seeds contain a high amount of polyphenolic compounds, which gives them an advantage in binding well with phosphate ions [43]. Polyphenols are known for forming complexes with various pollutants, which permits pollutant removal [44]. It has been reported that grape seeds account for 60 to 70% of grape polyphenol content [45]. The carboxyl and hydroxyl functional groups in the grape seeds also augment their ability to absorb ions through electrostatic forces and hydrogen bonding, which was illustrated in a study that investigated nitrate ion removal from water using grape seeds [46]. Another advantage of grape seeds is the ease in modifying their physiochemical properties, which can enhance their adsorptive effects [47]. Another interesting study investigated the benefit of surface area of grape seeds and processing them into an activated carbon form, which exhibited improved adsorptive action due to the porous structures, allowing for larger volumes of adsorbate to be adsorbed [48]. In line with these excellent properties and large quantities of grape seeds being wasted in modern production chains, it can be deduced that their valorization is a step toward waste reduction and a more circular and sustainable economy.
In this research, phosphate ion removal from an aqueous solution was studied via adsorption onto ground grape seed particles. Different operational factors like adsorbent concentration, contact time, and temperature were considered. Additionally, initial phosphate ion concentration and solution pH influence the process; hence, these were investigated. Isotherms of adsorption together with kinetics and thermodynamics were also studied.
2. Materials and Methods
2.1. Preparation of Adsorbents and Characterization
Black-grape seeds were studied for adsorbing phosphate ions from synthetic wastewater. To investigate their adsorption capacity, the seeds were squeezed from grape fruit that was locally grown in Jordan; the seeds then were washed (distilled water), after which were left to dry naturally in the sun for a few days. This was followed by mechanical grounding and sieving to different particle sizes, the largest of which was about 0.71 mm.
The adsorbent was characterized further with Fourier transform infrared spectroscopic (FTIR) analysis (on at a wavelength range of 4000–500 cm−1 using the PerkinElmer machine, Waltham, MA, USA), using the attenuated total reflectance (ATR-FTIR) technique. The analysis was performed under ambient air conditions without the use of KBr pellets or a vacuum. This analysis was performed to understand the bond intensity and identify the functional groups present on the surface of the grape seeds.
Scanning electron microscopy (SEM) was performed with a Tescan VEGA XMU machine (Tescan orsay Holding, Brno, Czech Republic) for the evaluation of grape-seed surface morphology. X-ray diffraction (XRD) analysis was performed using a Bruker D8 Advance scintillation point and 1-D detector (Bruker, Billerica, MA, USA).
2.2. Phosphate Stock Solution
A standard 1000 ppm solution of potassium dihydrogen phosphate (KH2PO4) was prepared and then diluted to 100 ppm. The pH of the standard solution was adjusted to 6.8 using hydrochloric acid and sodium hydroxide solutions, each at a concentration of 0.1 N.
Temperature effect was investigated to determine the optimum adsorption temperature that results in the highest phosphate ion uptake; different temperatures (25, 35, and 45 °C) were applied at different phosphate initial concentration levels (100, 60, and 40 ppm), and 20 ppm adsorbent concentration, while keeping a constant solution pH = 6.8 during the experiment. The optimum pH value to achieve the highest phosphate ion removal was studied over a range of 3 to 11 at a fixed temperature value of 25 °C, and using 20 ppm initial adsorbent concentration and 100 ppm phosphate ion concentration. The mixtures were placed in a shaker at 100 rpm for 4 h to ensure equilibrium was reached. Adsorbent concentration effects were studied by varying its value (5, 10, 15, and 20 ppm) whilst the initial phosphate ion concentration was kept fixed. Samples were agitated by applying a rotary shaker at 100 rpm and given sufficient time to achieve equilibrium. The temperature and pH value were both maintained constant at 25 °C and 6.8.
Adsorption kinetics to determine the reaction order was investigated utilizing three initial phosphate levels (100 ppm, 60 ppm, and 40 ppm) at fixed pH = 6.8, temperature = 25 °C, and adsorbent concentration = 20 ppm. The adsorption or removal percentage was recorded at different time intervals of up to 250 min; sampling was conducted using five-minute increments.
2.3. Phosphate Concentration Measurement
Phosphate concentrations in the solution before and after adsorption were determined using a colorimetric method based on the reaction of phosphate with vanadium molybdenum phosphoric acid, a reliable and validated method for environmental and chemical measurements, according to the APHA Guide to Standard Analytical Procedures for phosphate ions with a reagent containing vanadium and molybdate in an acidic medium [49]. Measurements were performed using a Hach DR/4000 visible-light spectrophotometer (Loveland, CO, USA), where the absorbance was read at the appropriate wavelength (470 nm). A calibration curve was prepared using standard phosphate solutions (KH2PO4) of known concentrations to determine the relationship between absorbance intensity and concentration. This curve was used to calculate the residual concentrations in the samples after adsorption. To ensure reliability and statistical accuracy, each experiment was repeated three times, and the arithmetic mean of the three readings was used to present the results and analyze the adsorption efficiency.
2.4. Theory
Phosphate ion uptake per adsorbent mass, qt (mg/g) is calculated using Equation (1):
where C is the phosphate ion concentration at time t and C0 is the initial phosphate ion concentration in ppm. Vs (L) represents sample volume and m is the sorbent weight expressed in grams. The equilibrium phosphate ion uptake qe (mg/g) is determined according to the Equation (2):
where Ce represents the equilibrium concentration (ppm) of phosphate ions in the solution.
2.5. Kinetic Study
The adsorption reaction rate law was determined by plotting the results obtained using the integrated linear forms for different proposed reaction orders. The correlation coefficient (R2) was applied to validate the model that provided the best fit. A good model should produce a straight line and very high correlation coefficient. The following mathematical formulas for reaction rates were studied [50]:
- 1.
- Pseudo first order:
- 2.
- Pseudo second order:
- 3.
- Intraparticle diffusion model, given by Equation (5):
2.6. Isotherms of Adsorption
The experimental results, usually concentration versus time at constant pH value and temperature, obtained from the phosphate ion adsorption process by the particles of grape seeds, are characterized and modeled by well-known mathematical models called isotherms. Some of the isotherms, such as Henry’s model, given in Equation (7), is a simple one-parameter model, which relates the quantity of adsorbate uptake per adsorbent unit mass to the concentration of adsorbate at equilibrium. The model is not recommended for use at high temperature and pressure when using gases or high concentration, as in the case of aqueous solutions.
where HHE is Henry’s adsorption constant [51].
The Freundlich isotherm, given in Equation (8), is a very common model and widely used for experimental fitting of adsorption data. It is one of the simplest nonlinear empirical isotherms, initially formulated to relate pressure to the amounts of adsorbed gas [50]. The isotherm is based on the assumptions that surfaces can be heterogeneous, or sites have variable heat of adsorption, and multilayer layer adsorption is possible. It has been widely used by researchers to correlate the concentration of adsorbate in solutions with the concentration of solute on the surface of adsorbents under equilibrium.
where KF is a constant that represents an adsorption capacity estimate, followed by n, which measures the intensity of adsorption. Both KF and n vary with the nature and characteristics of the adsorbate and adsorbent. Values of n and KF are good indicators for equilibrium uptake and are visualized by graphing the Freundlich isotherm in linearized form, which is described by Equation (9):
The Langmuir isotherm assumes that the adjutant adsorbed molecules experience no interaction, there exists only one adsorption layer, and the adsorption energy is constant. The linearized form of Langmuir isotherm can be illustrated through Equation (10):
where b and qm are Langmuir adsorption isotherm constants. To account for adsorbent–adsorbate interaction, Temkin suggested that the enthalpy of adsorption decreases with coverage, and on that based his isotherm, given by Equation (11):
where T is the temperature (K), AT and bT are Temkin’s isotherm constants, and R is the gas constant (J/mol·K). Using the assumptions made by Langmuir and adding the possibility of having mechanical interaction between the adsorbent and adsorbate, Jovanovic formulated his isotherm; the linear form is given by Equation (12):
where KJ is Jovanovic constant and qmax is the maximum phosphate ion uptake. The experimental results obtained were fit to some of the isotherms described above. The extent of applicability of each model to fit the experimental results was validated via calculating the correlation coefficient (R2); when the R2 value is higher, the representation the model provides for the experimental data is superior.
2.7. Statistical Analysis
The effect of various operational factors on phosphate concentration and removal efficiency during the adsorption process was analyzed by calculating the standard error to ensure the accuracy of the results and assess the variability among measured values.
All experiments were conducted in two independent replicates, and the average phosphate concentration () was calculated using a statistical equation based on the number of replicates (N) and individual concentration values (Ci):
The standard error of the mean () was calculated using the well-known relationship based on the standard deviation (σ), which is calculated using the variance among experimental values.
Phosphate concentrations were reported as mean ± standard error (). This formula was used to represent error bars to demonstrate the confidence level in the results.
3. Results
3.1. Adsorbent Characterization
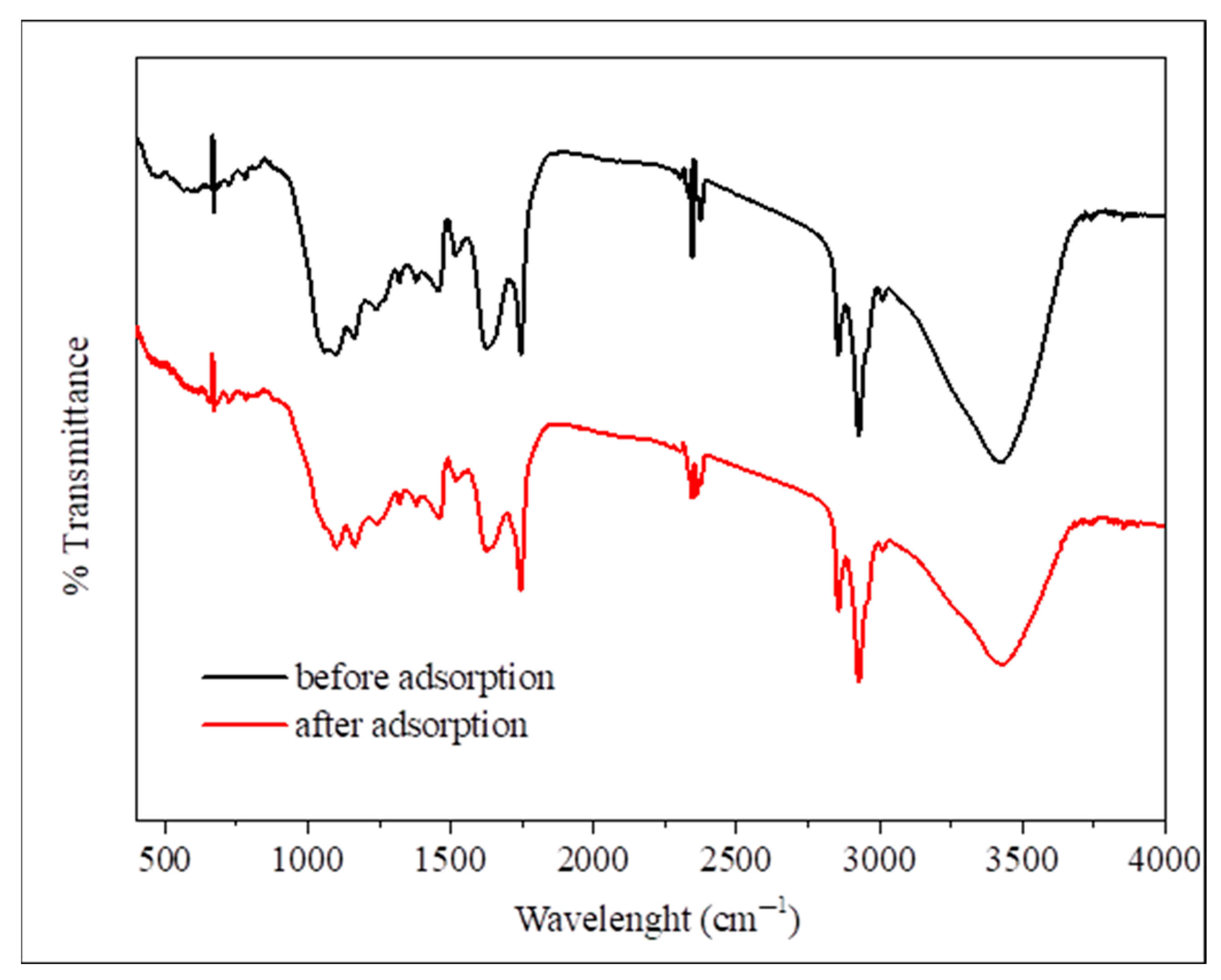
FTIR spectroscopy is a tool for functional group identification and molecular structure classification in grape seeds, wherein it has been used for chemical composition classification of their extracts, which indicated the presence of flavonoids and phenolic acids [52]. The FTIR spectra shown in Figure 1 collate functional groups that are present on the grape seed surface and their interaction with phosphate ions during adsorption. Before adsorption, the spectrum shows several characteristic bands, such as a broad band at 3200–3500 cm−1, which represents O-H stretching.
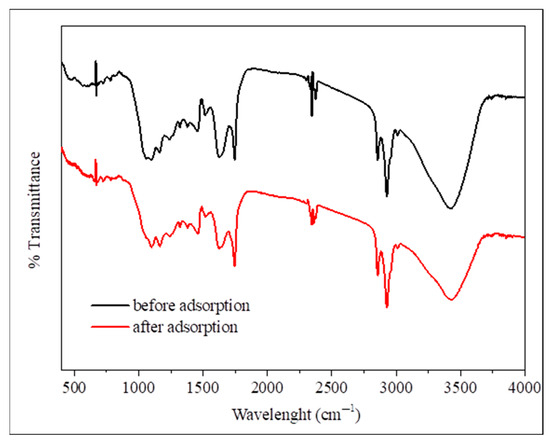
Figure 1.
FTIR analysis before adsorption (black line) and after phosphate adsorption (red line).
More distinct peaks at 2920–2850 cm−1 are observed, which are attributed to asymmetric and symmetric stretching vibrations of CH2 or CH3 groups. Probably, they are related to the hydrocarbon chains of the lipids or lignins, followed by another band at around 1744 cm−1, which originates from C=O bond stretching within the ester groups. After adsorption, the spectra showed no visually noticeable changes in most bands, which may indicate that surface interaction with phosphate was limited or that the changes were within the spectral resolution limits of the instrument. In the fingerprint region (1000–800 cm−1), some minor changes in peak intensity or position were observed. However, this region is inherently complex due to the overlap of many bands, making it difficult to provide precise assignments or definitive conclusions. Therefore, a general description of this region is sufficient, in line with what is reported in the scientific literature.
These results indicate the presence of negative functional groups (such as –OH and –COOH) on the surface of grape seeds, which potentially contribute to the adsorption mechanism through electrostatic interactions or hydrogen bonding to H2PO4− or HPO42− ions, especially at moderate pH, without direct spectroscopic evidence for the formation of clear surface complexes with phosphate [32]. After phosphate adsorption, the O-H stretching band exhibits decreased intensity along with modifications in the C-O stretching region and altered peak patterns in the fingerprint region. These spectral changes are evidence for phosphate ions interacting with the surface functional groups on the grape seeds. Similar functional group assignments have been reported in grape waste studies, where bands at 3439 and 3423 cm−1 were attributed to O-H stretching vibrations, and peaks at 2925 and 2854 cm−1 were assigned to aliphatic C-H stretching [53]. Previous research has also shown that grape-based adsorbents exhibiting altered intensities in the O-H, C=C, and C=O bands post-adsorption indicate successful interaction with metallic adsorbates [54]. Furthermore, FTIR results have also reported peaks in the fingerprint region between 1500 and 800 cm−1, which contain much information on the presence of various alcohols, organic acids, and sugars. However, it is difficult to analyze due to complexity [53]. Peaks at 1020, 1235, and 1318 cm−1 were also assigned to polysaccharides, lignins, and pectins collectively, in no specific order, likely due to P–O bond vibrations, which is evidence for phosphate binding to the adsorbent [55]. These FTIR results purport that grape seeds are rich in polyphenols and polyunsaturated fatty acids, which also deems them as a great nutraceutical source.
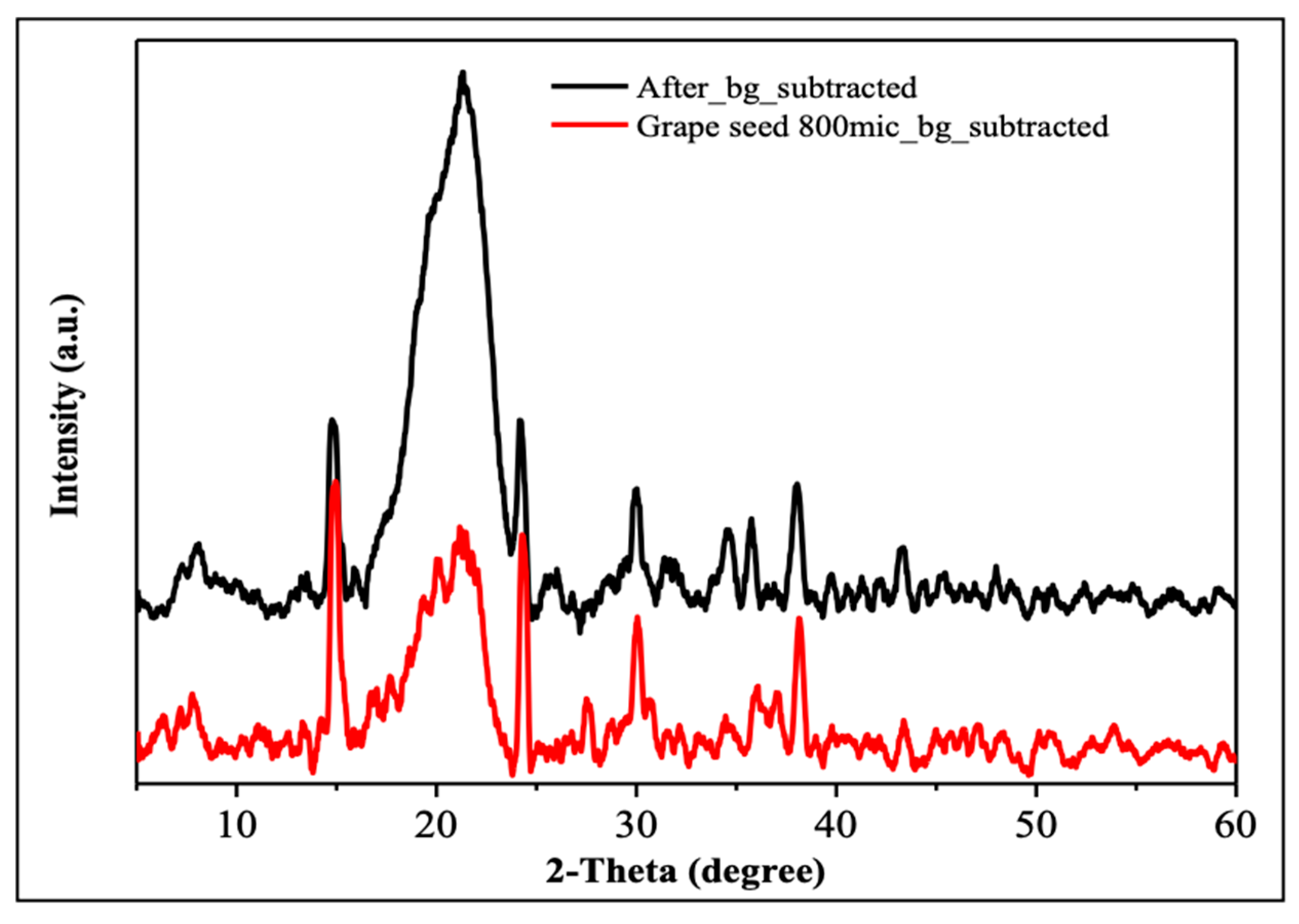
The XRD analysis allows for the examination of the crystalline structure of grape seeds and their degree of crystallinity [52]. Figure 2 compares the background-subtracted XRD patterns for grape seed particles (red line) and for the material after further processing/treatment (black line). Both diffractograms show largely broad reflections in the 2θ range of roughly 15–30°, which is characteristic of largely amorphous lignocellulosic materials. The pronounced peak near 2θ ≈ 22° is more intense in the black-line (which is after adsorption) sample and is attributed to the planes of semicrystalline cellulose. The presence of only a few minor and low-intensity peaks elsewhere in the patterns indicate that no highly crystalline mineral phases were formed in significant amounts. These results corroborate that ground grape seeds are predominantly amorphous with a small fraction of cellulose-like domains responsible for the diffuse peaks. Thus, the bulk structure of the grape seeds remains largely intact before and after phosphate adsorption, which implies that the mechanism of adsorption involves the interaction with organic functional groups on the grape seed surface rather than formation of new crystalline compounds.
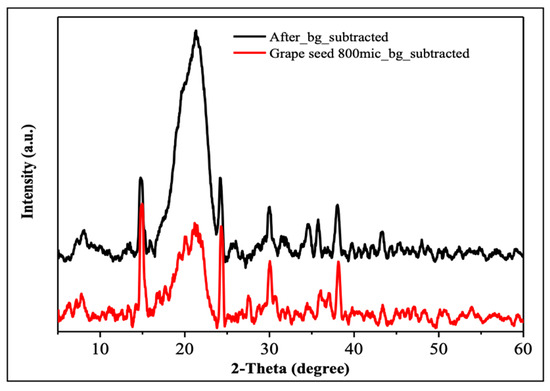
Figure 2.
XRD results before adsorption (red) and after adsorption (black).
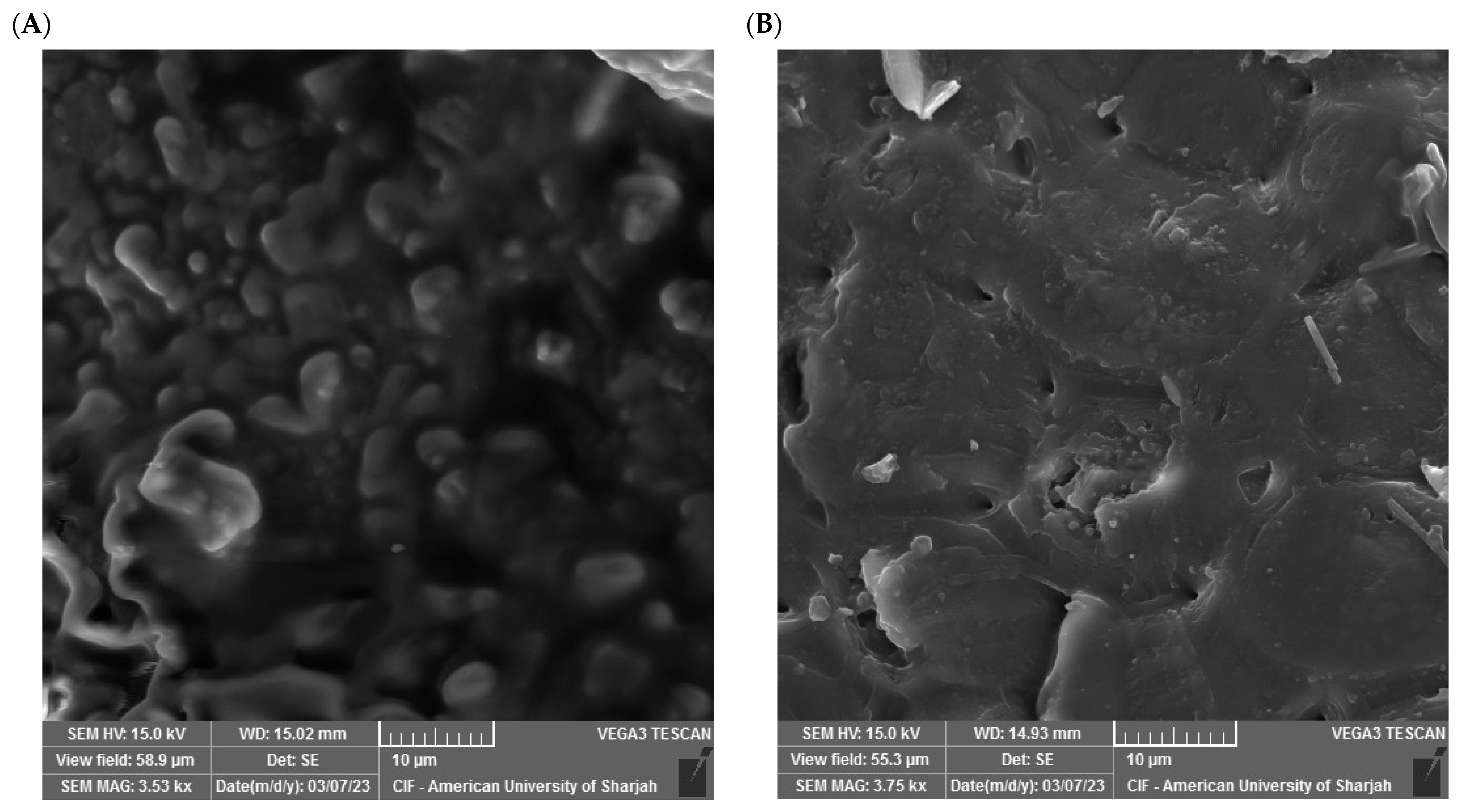
SEM allows for visualizing the surface area and porosity of the grape seeds, which permits gauging their capability in adsorbing metals and organic pollutants. Various studies have shown that different grape seeds can have different surface features due to differences in variety of the grape and processing conditions [56]. The SEM micrographs in Figure 3 visualize the grape seeds’ surface morphology before adsorption (Figure 3A) and after phosphate adsorption (Figure 3B).
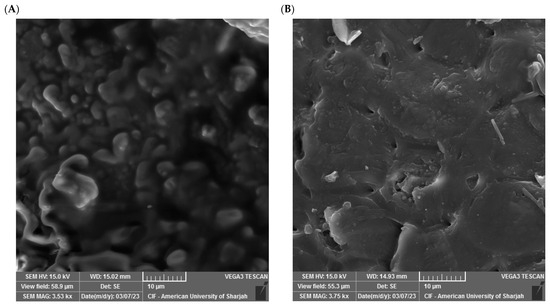
Figure 3.
SEM image before adsorption (A) and after adsorption (B).
The observed surface porosity was high before adsorption. It also exhibits heterogeneous features with irregular shapes and multiple cavities, representing increased surface area that allows for interphase interaction. After phosphate uptake, the surface appeared smoother with some deposits, which is a sign indicating successful phosphate ion adsorption. Similar SEM studies have attributed the heterogeneity of these features to the fact that the material consists of different fruit parts [57]. These results are consistent with the literature on lignocellulosic materials with amorphous structures that exhibit broad diffraction patterns in the 2θ range between 15° and 30° due to the presence of components such as lignin and hemicellulose, which do not exhibit clear crystalline regularity [58]. The peak observed at 2θ ≈ 22° is often associated with the presence of partial regions of semi-crystalline cellulose, and this peak has been observed in similar studies to be more pronounced after treatment, indicating a slight structural rearrangement without actual crystallization [59]. The absence of sharp or new peaks after adsorption confirms that the mechanism remains superficial and related to interactions with functional groups, rather than the formation of new crystalline phases [58,60]. Thus, the XRD analysis results, despite their formal simplicity, play a supporting role in confirming that the overall structure of grape seeds does not undergo radical crystallographic changes during the adsorption process.
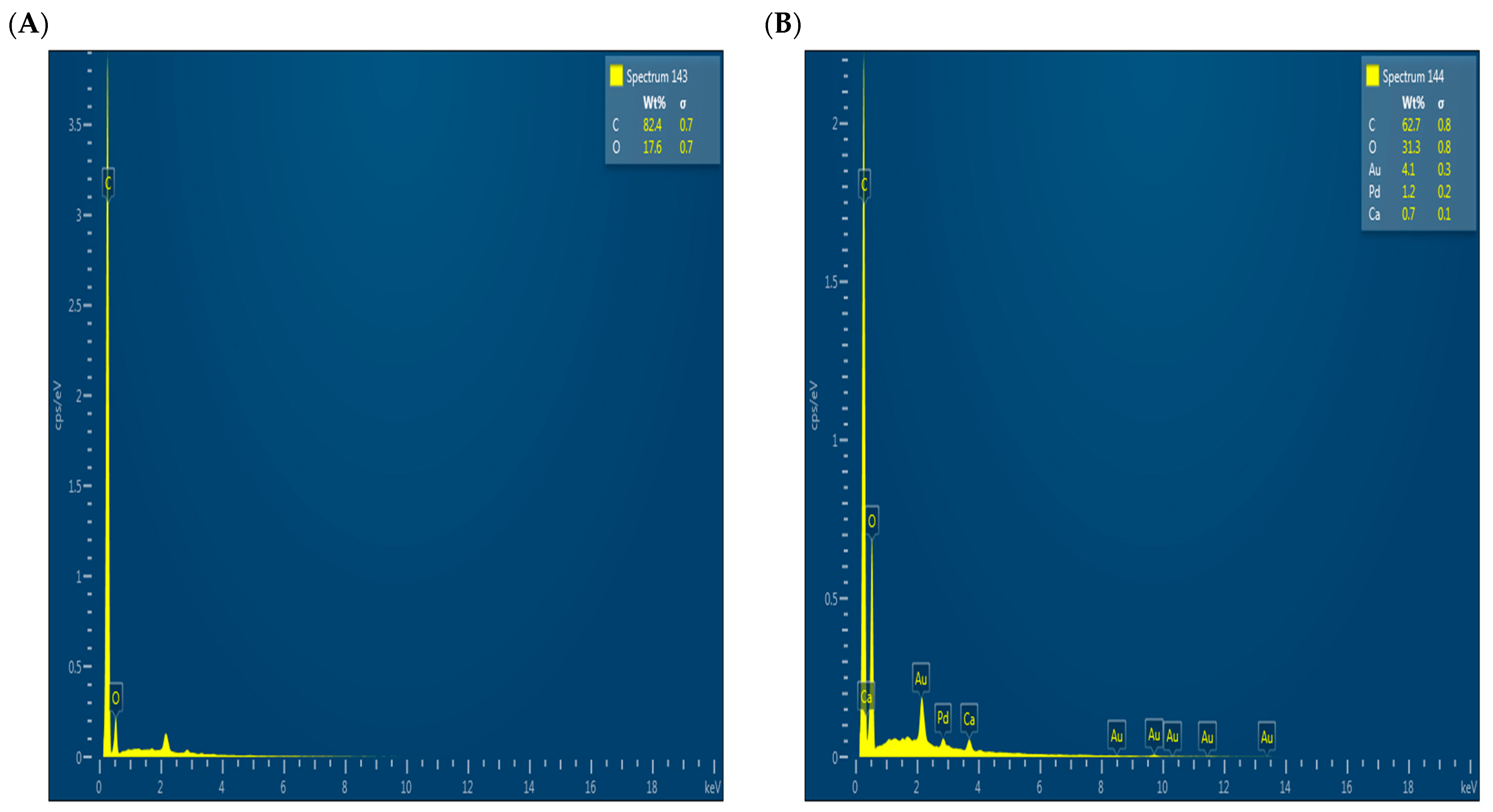
To complement the scanning electron microscopy (SEM) results, EDX analysis was performed, as shown in Figure 4, to characterize the surface elemental composition before (Figure 4A) and after (Figure 4B) adsorption. However, it should be noted that the sample after adsorption was gold-coated to improve conductivity during SEM analysis, which may explain the appearance of elements such as Au and Pd, but should not be considered evidence for their presence because of adsorption. Therefore, the elemental ratios cannot be directly compared between the two cases. The absence of phosphorus after adsorption may be attributed to its low concentration or to its adsorption depth below the surface, outside the detection range of EDX.
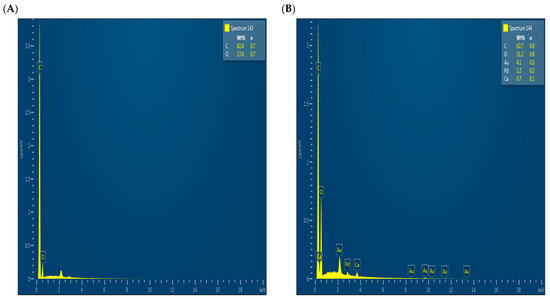
Figure 4.
EDX results before adsorption (A) and after adsorption (B).
The regeneration capacity of an adsorbent is a vital factor affecting its practical application in removing phosphate from water. The ease and efficiency of grape seed regeneration depend largely on the dominant adsorption mechanism, whether physical or chemical. In the case of physical adsorption, which relies on weak forces such as hydrogen interactions and van der Waals forces, the adsorbent can be effectively recovered by washing with aqueous solutions or changing pH and temperature conditions, allowing phosphate to be removed without significant damage to the surface structure of the material [61]. Chemical adsorption, however, involves the formation of strong bonds between grape seed functional groups and phosphate ions, which may require the use of strong chemical solutions or thermal processes to break these bonds, potentially limiting the number of reuse cycles and affecting the material’s stability [62]. FTIR spectroscopy results indicate that the interactions between the grape seed surface and phosphate were predominantly physical, with no signs of strong chemical bond formation observed. Accordingly, the adsorbent can be regenerated using relatively simple methods, such as pH adjustment or washing with aqueous solutions, without significantly affecting the surface properties. Thus, grape seeds provide a sustainable and effective option, while reducing the costs and environmental impacts associated with single-use sorbent waste, supporting their use in environmental water treatment systems [63].
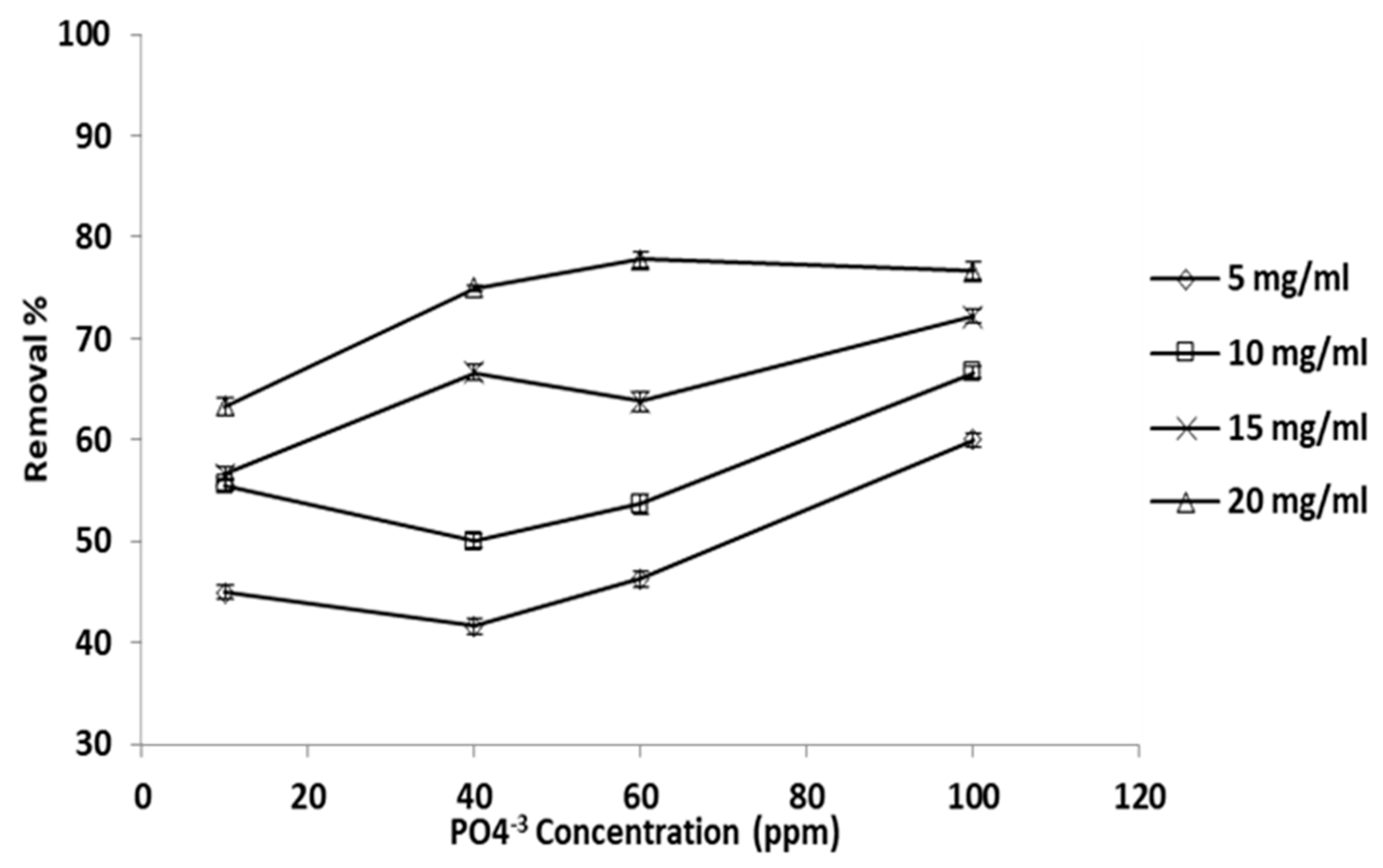
3.2. Adsorbent Concentration Effects
Percentage of phosphate ion removed versus phosphate ion initial concentration, at constant adsorbent concentration, is given in Figure 5. In this set of experiments, four phosphate ion initial concentration levels were employed (100, 60, 40, and 10 ppm); the temperature and pH were maintained constant at 25 °C and 6.08. It is very evident that the removal percentage increased with increasing initial concentration, and that the trend was almost linear and clearly demonstrated at all initial phosphate ion concentration levels.
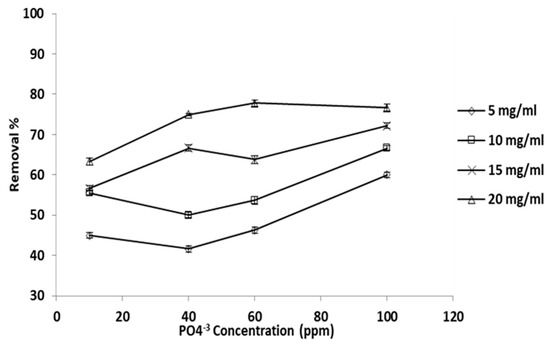
Figure 5.
Adsorbent does concentration effect the percentage removal (T: 25 °C, pH: 6.8).
The ultimate removal percentage was 78% when the adsorbent dose and initial phosphate ion concentration was 20 and 100 ppm, respectively. At 20 mg/mL adsorbent concentration, the removal was almost the same for all initial concentration levels except that at 10 ppm, which exhibited a lower removal percentage. This result is due either to higher binding site competition among phosphate ions available on the grape seed particle surface or to the rise in the grape seed particles’ quantity surrounding the phosphate ion ions [64].
3.3. Effect of Time
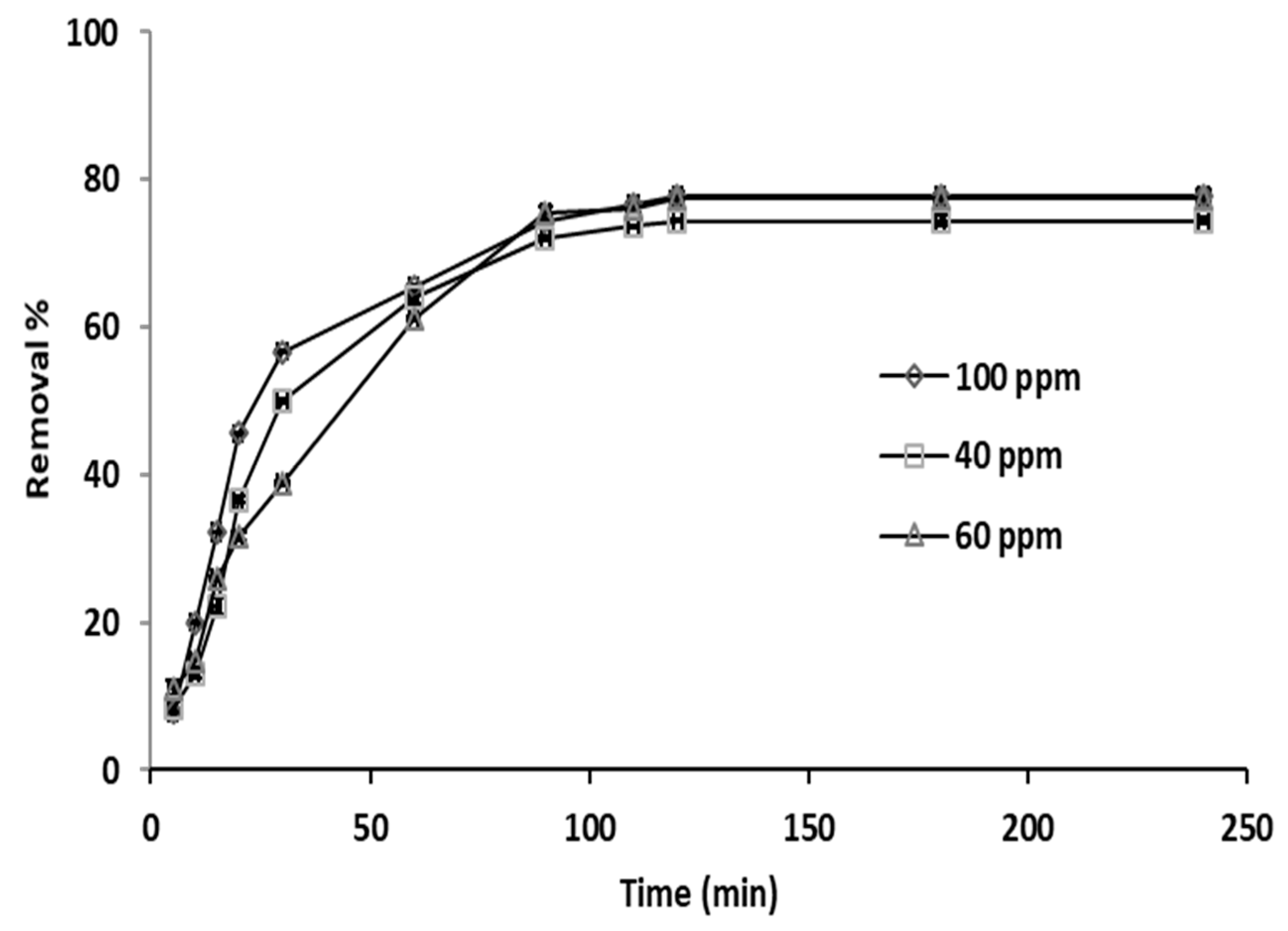
Figure 6 depicts the percent removal versus contact time for three initial phosphate ion concentrations (40, 60, and 100 ppm).
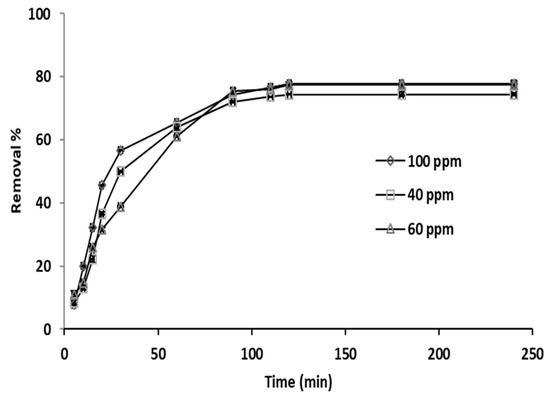
Figure 6.
Adsorption kinetics of phosphate ions on grape seeds at different initial phosphate ion concentrations (dose: 20 mg/mL, T: 25 °C, pH: 6.8).
The pH, temperature, and adsorbent concentration remained constant at 6.8, 25 °C, and 20 ppm, respectively. Adsorption was increasing at relatively high rate for almost 60 min and was higher for the phosphate ion concentration of 100 ppm than the other two concentration levels (40 and 60 ppm), and then continued to increase but at a lower rate for the next 30 min, as clearly shown by the slope of the trend lines, and then remained constant, as the curves reached a plateau for the rest of the experiment that lasted for more than 4 h. The plateau shape of the curves is an indication of reaching a state of equilibrium by the adsorption reaction. Therefore, equilibrium was nearly attained after 90 min. The enhancement in the percentage removal with rise in the initial phosphate ion concentration is a result of the phosphate ion concentration gradient increase between the grape seeds surface and the bulk. Consequently, the phosphate ion–adsorbent interaction was increased; hence, greater removal percentage was observed [64,65].
Furthermore, the rapid adsorption at the start can be explained as follows: at the beginning, all vacant sites available on the adsorbent surface were unoccupied and the adsorbate concentration gradient was high, which results in increasing the mass transfer driving force. Subsequently, the amount of phosphate ion removal experienced a considerable reduction with contact time. However, as the adsorption process continued, accumulation of phosphate ion on the grape seeds resulted in the development of a negatively charged surface, consequently slowing the rate of adsorption, as observed. This is in compliance with the findings reported previously by Abdelhay et al. [66].
3.4. Effect of pH
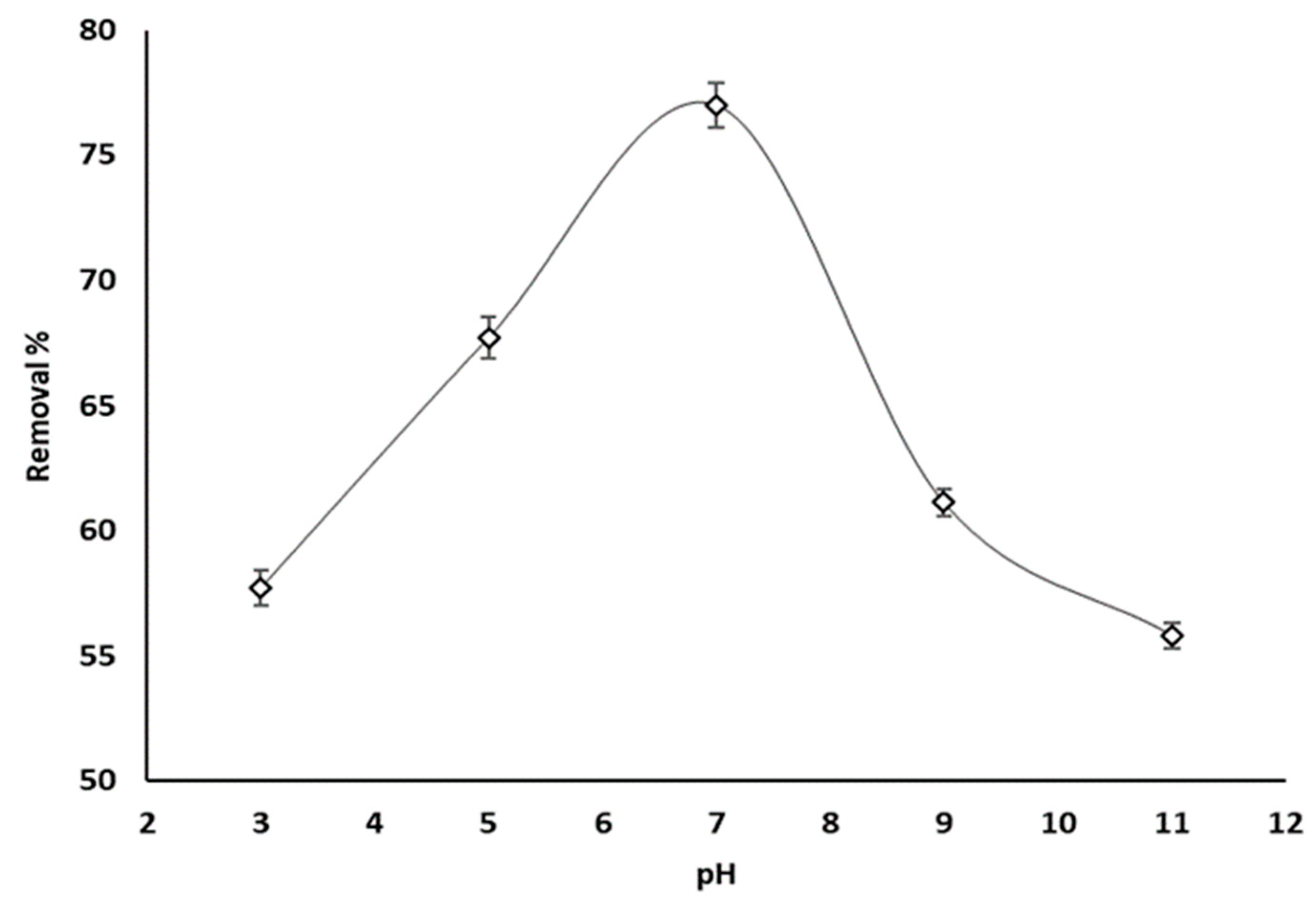
Variations in phosphate ion uptake with pH are given in Figure 7. In this experiment the temperature was held at 25 °C, the adsorbent concentration and initial phosphate concentration were 20 ppm and 100 ppm, and the pH was varied between 3 and 11.
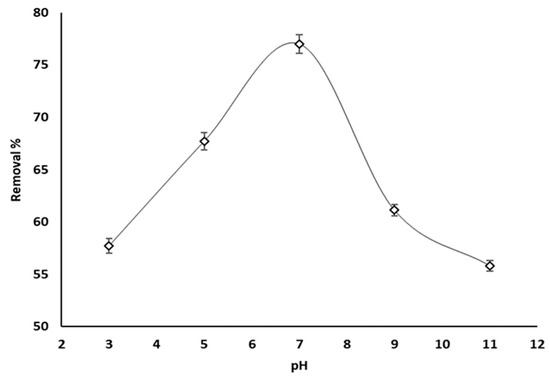
Figure 7.
Phosphate ion adsorption by grape seeds as a function of pH (dose: 20 mg/mL, T: 25 °C, C0: 100 ppm).
Based on Figure 3, an increase in phosphate ion removal between 57.7% and 75.5% was recorded as the pH rose from 3 to 7, and then dropped to 55.8% as the pH increased to 11. Therefore, an optimum phosphate ion uptake was exhibited when the solution is neutral. Phosphate ions have a pKa value of 7.2 [67]. A basic environment results in the generation of a negatively charged surface of adsorbents and H2PO4− as the predominant species of phosphate [68]. Therefore, the phosphate removal percentage decreased as a result of the electrostatic repulsive forces H2PO4− and the deprotonated adsorbents surface. However, decreasing the pH decreases the influence of electrostatic repulsion by the positively charged adsorbent surface, and the electrostatic attraction progressively controls the adsorption process between the adsorbent surface and phosphate ions.
3.5. Effect of Temperature
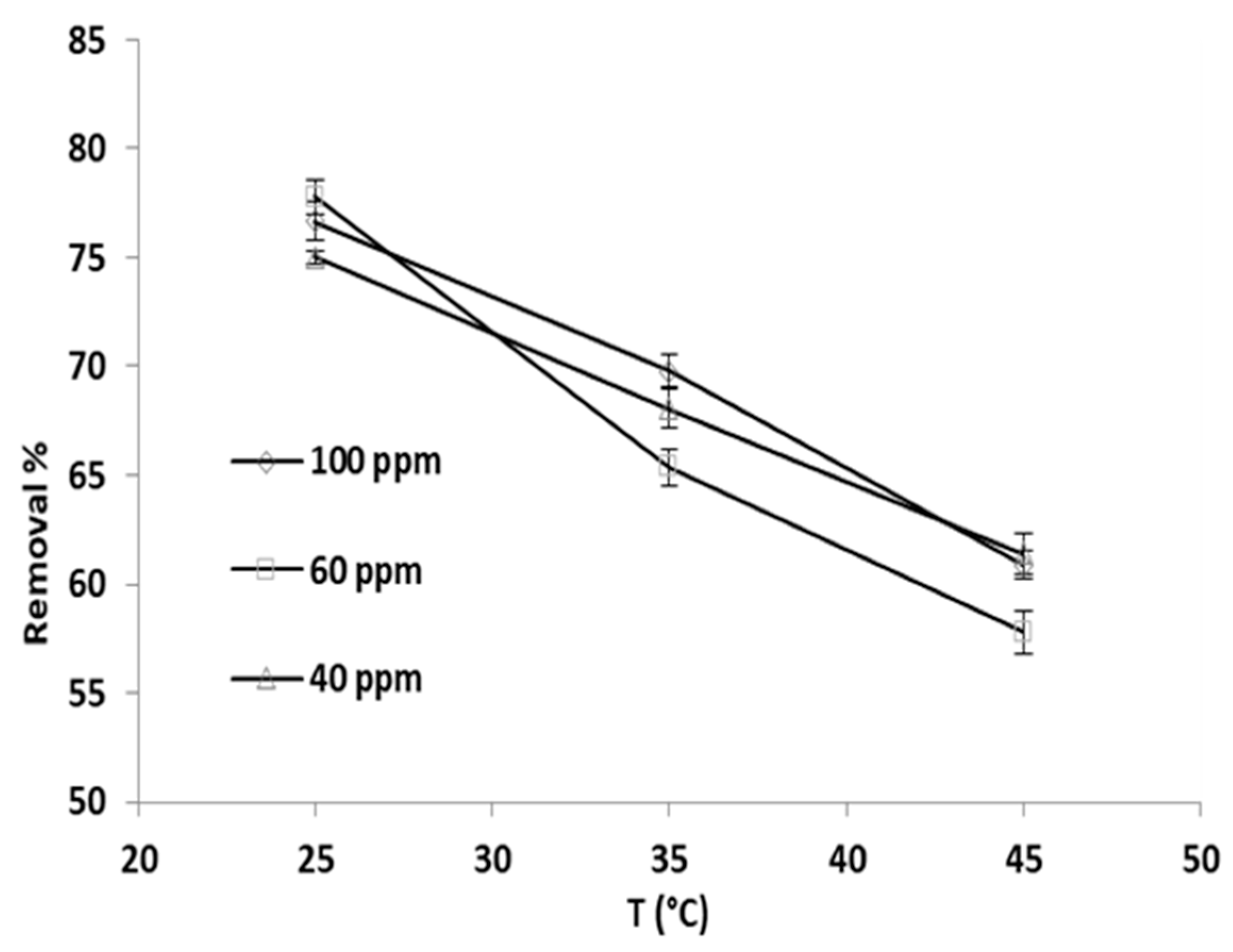
Temperature influence on the percentage removal of phosphate ions was explored via applying three temperatures, specifically 25 °C, 35 °C, and 45 °C, and the initial phosphate ion concentrations were set to 40 ppm, 60 ppm, and 100 ppm; the findings are depicted in Figure 8.
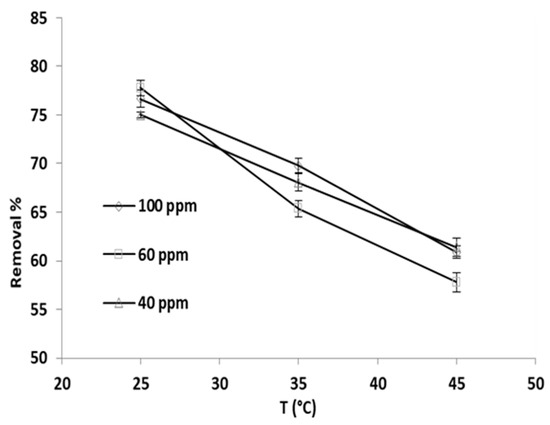
Figure 8.
Adsorption temperature effect on the phosphate ion removal percentage by grape seeds at varying phosphate ion initial concentrations (dose: 20 mg/mL, pH:6.8, T: 25 °C).
Evidently, the percent removal decreased between 18 and 25% as the temperature increased from 25 to 45 °C. The drop in percent removal was almost the same for the three initial phosphate ion concentrations, which implies that the adsorption could be an exothermic process.
3.6. Adsorption Process Kinetics
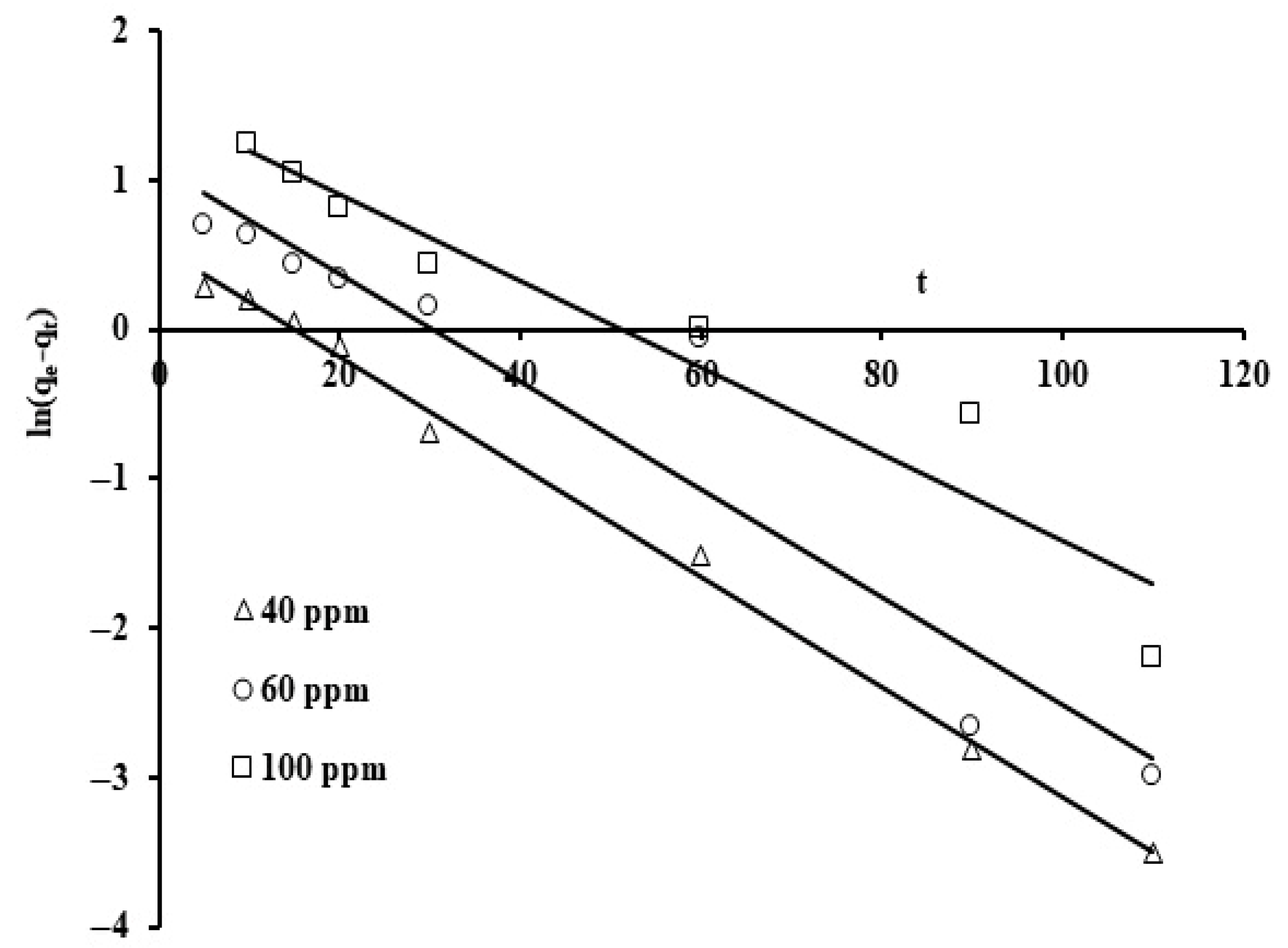
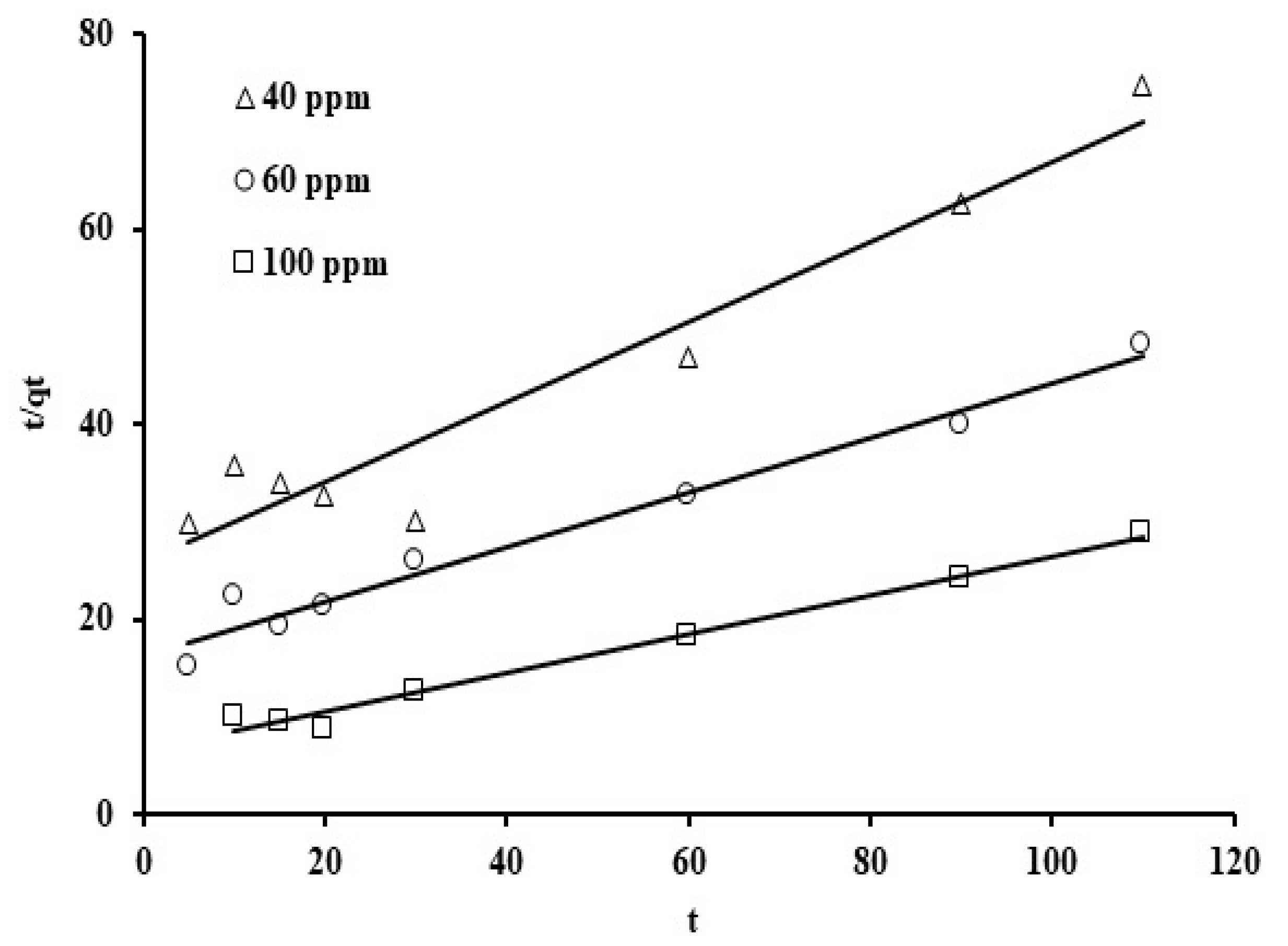
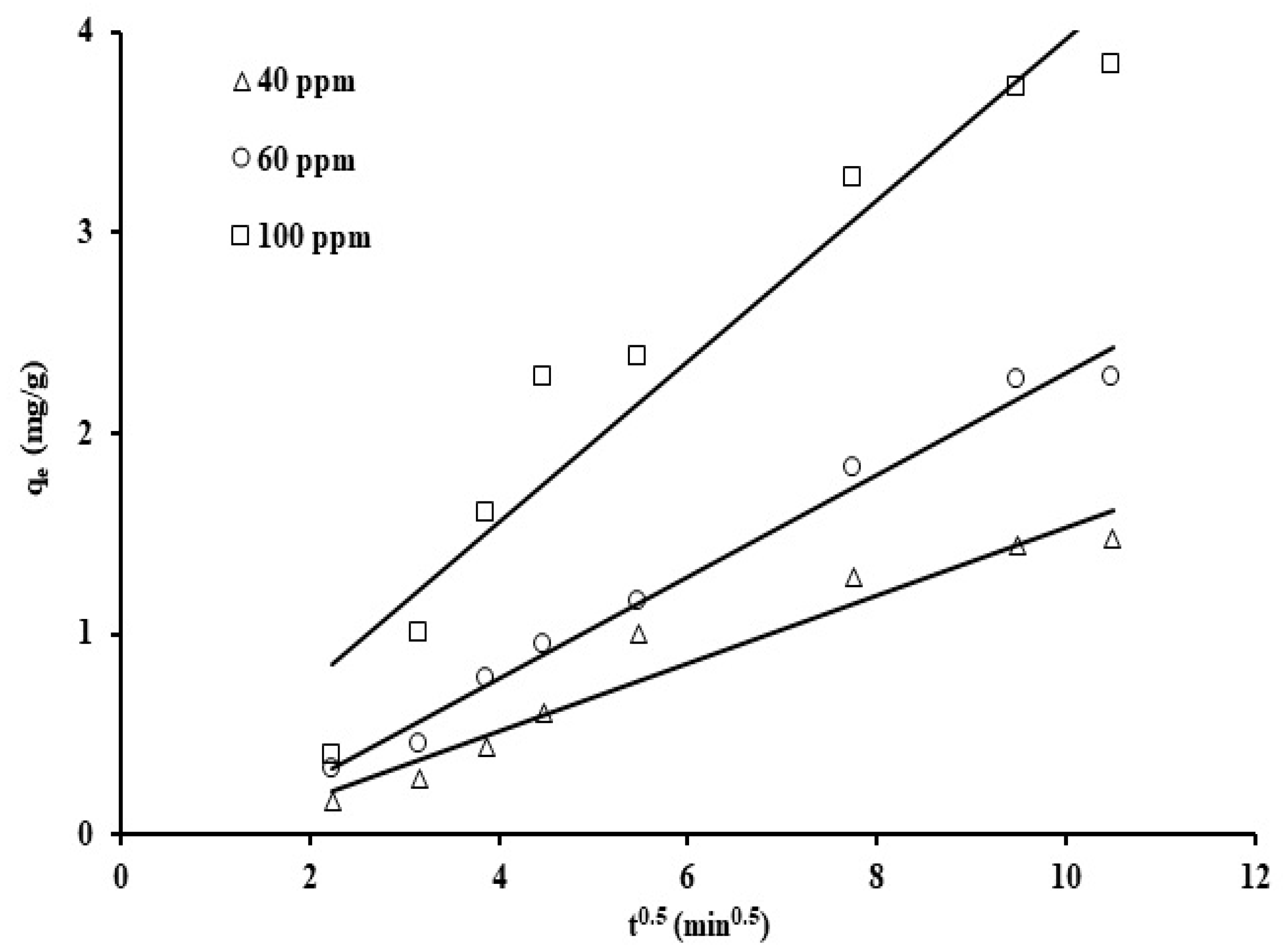
The results were plotted utilizing the linear form for various reaction rate orders displayed in Equations (3)–(5) (Figure 9, Figure 10 and Figure 11). The rate constant values, the standard deviation, and the correlation coefficients for the three kinetic models are displayed in Table 2.
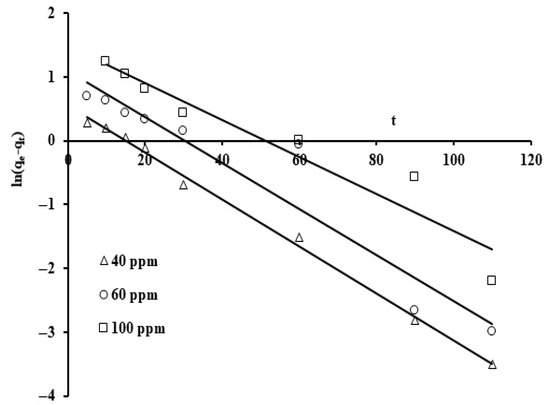
Figure 9.
Pseudo first order adsorption graph on grape seeds at varying phosphate ion initial concentrations (dose: 20 mg/mL, pH: 6.8, T: 25 °C).
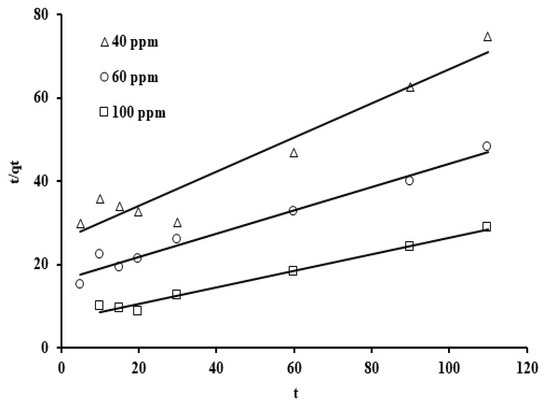
Figure 10.
Pseudo second order adsorption graph depicting trends in grape seed adsorption at varying initial phosphate ion concentration (dose: 20 mg/mL, pH: 6.8, T: 25 °C).
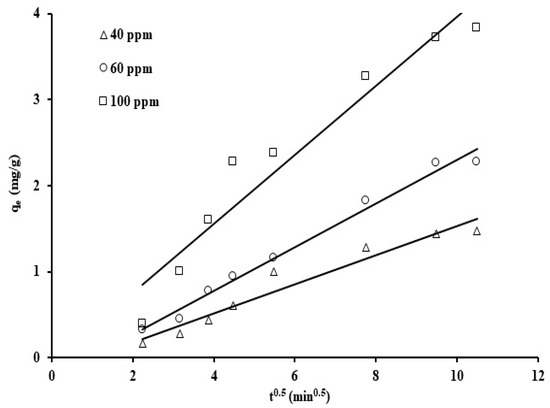
Figure 11.
Intraparticle diffusion model adsorption graph for grape seeds at various initial phosphate ion concentrations (dose: 20 mg/mL, pH: 6.8, T: 25 °C).

Table 2.
Kinetic parameters for the adsorption from aqueous solution by grape seeds at varying initial concentrations.
The linear form of the pseudo first order kinetic model was applied to the results as exhibited in Figure 9. The y-intercept and gradient of ln(qe − qt) against t provided the rate constants K1.
Note that the corresponding reaction rate constants were not strongly affected as the initial phosphate concentrations were increased. The average value for the reaction constant (k1) is 0.033 min−1 and the correlation coefficient (R2) ranges from 0.91 to 0.99. Thus, the correlation coefficient (R2) and standard deviation values indicate that this model represents a good fit. When the values of the standard deviation are compared, the pseudo second order rate law provides an excellent fit for the adsorption results. This suggests that external adsorption predominates over micropore adsorption [69]. Figure 6 presents the plots for the three initial phosphate concentration levels (40, 60, and 100 ppm) using the second order rate formula in linearized form, t/q against t.
The reaction rate constants and initial adsorption rate (h) were determined from the values of the y-intercept and the gradient value. As can be seen, straight lines are clearly exhibited, with correlation coefficients (R2) between 0.93 and 0.99 and reaction constants in the range of 6.25, 4.9, and 6.48 × 10−2 (mg/g min) for the three initial phosphate concentration levels (40 ppm, 60 ppm, and 100 ppm). Furthermore, based on the initial adsorption rate (h) values, the phosphate uptake was noted to be kinetically the fastest at the phosphate concentration of 100 ppm. Observed improvement in the phosphate adsorption rate, upon increasing the initial phosphate concentration, may be due to the rise in the mass transfer driving force as the concentration gradient was increased [70]. The results of this study showed that the sorbent derived from grape seed waste achieved an adsorption capacity of 3.83 mg/g (Figure 11), demonstrating excellent performance compared to some other low-cost biomaterials reported in the literature. For example, raw okra recorded an adsorption capacity of approximately 2.45 mg/g, while coconut tar showed an adsorption efficiency of approximately 4.35 mg/g, as reported in the reference study [71,72]. This good performance of grape seed is attributed to the abundance of active functional groups on its surface, such as carboxyl and hydroxyl, which effectively contribute to electrostatic interactions and bond formation with phosphate ions. In addition to its efficiency, this material is characterized by its high availability, low cost, and environmental friendliness, making it a promising and practical option within the category of alternative adsorbents for wastewater treatment.
The intraparticle diffusion model reaction constant was determined by obtaining the slope of ln(qt) against t0.5 plots for varying phosphate ion concentrations, as displayed in Figure 11.
The intraparticle diffusion values, Kp, for different phosphate ion concentrations (40, 60, and 100 ppm) are 0.169, 0.255, and 0.401 mg/g min0.5. The increase in Kp values with the phosphate initial concentration may be assigned to the rise in the concentration gradient, which in return improved the phosphate ion diffusion within the solid and reduced the diffusion in the boundary layer. Based on the correlation coefficients values (0.93–0.99) and standard deviation values shown in Table 2, the results indicate that the intraparticle diffusion model is applicable and show that the adsorption rate is strongly affected by the adsorbate migration rate to the surface of the adsorbent.
3.7. Adsorption Isotherms
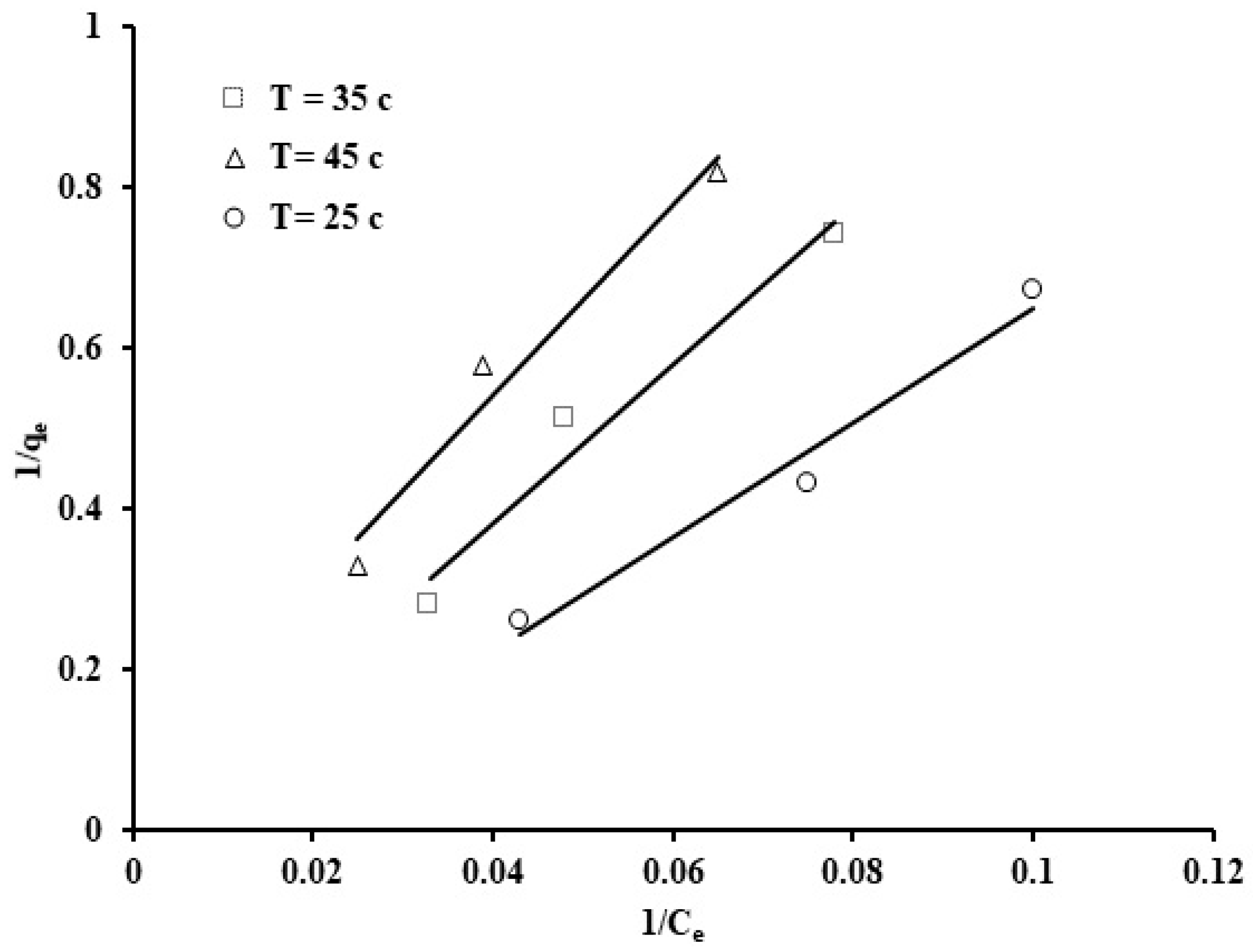
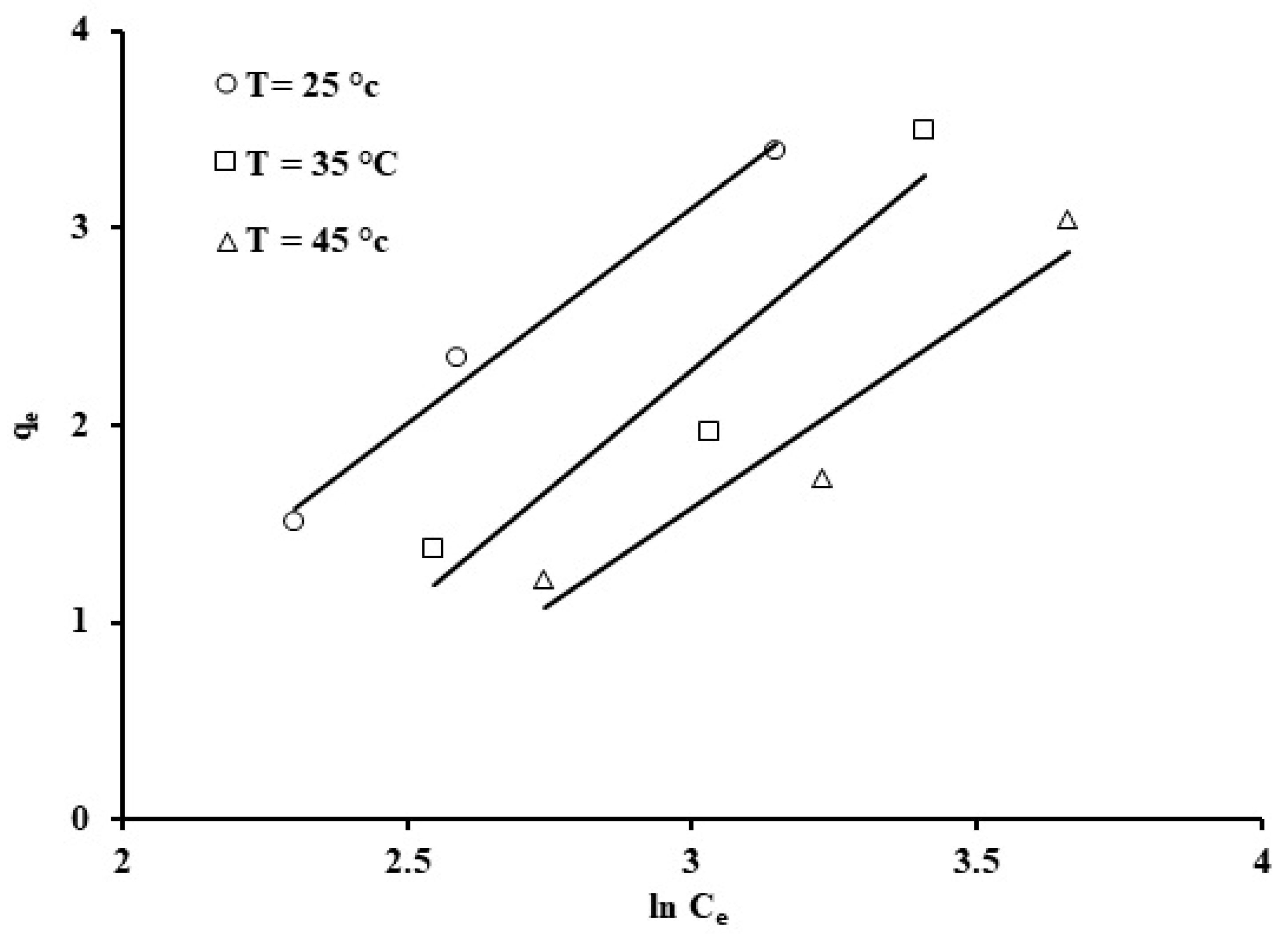
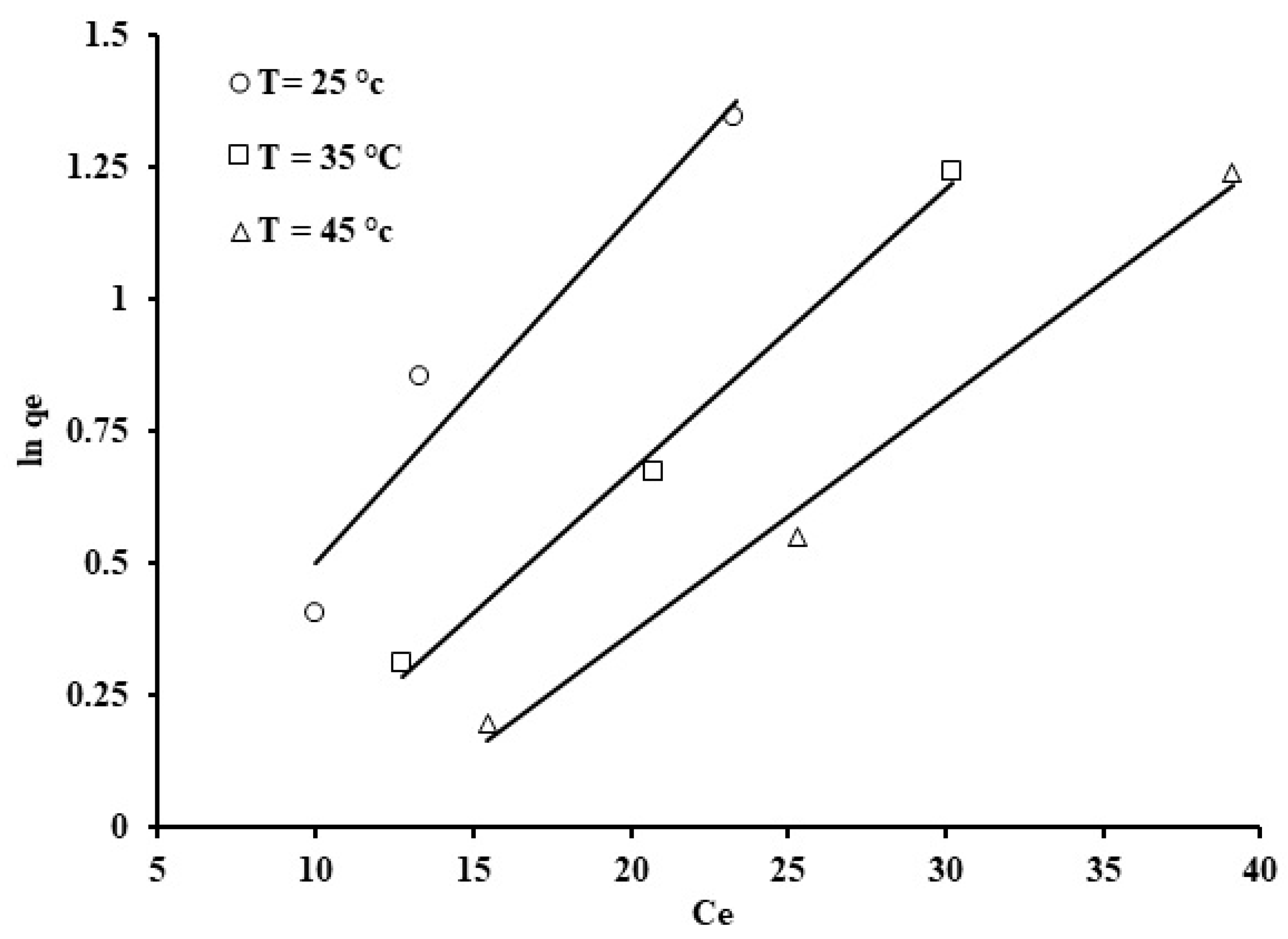
Freundlich, Langmuir, Temkin, and Jovanovic adsorption isotherms were applied to the results, as depicted in Figure 12, Figure 13, Figure 14 and Figure 15, respectively.
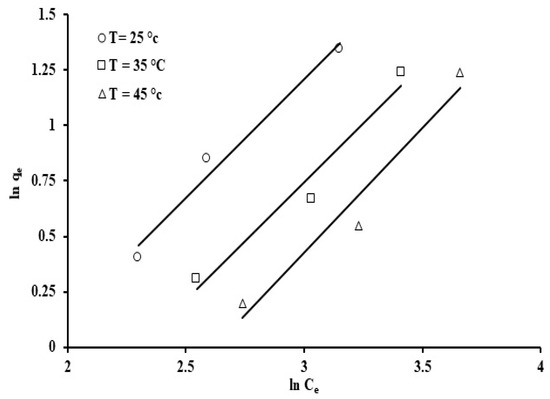
Figure 12.
Freundlich isotherm representing phosphate ion uptake by grape seeds (dose: 20 mg/mL, pH: 6.8, C0: 100 ppm).
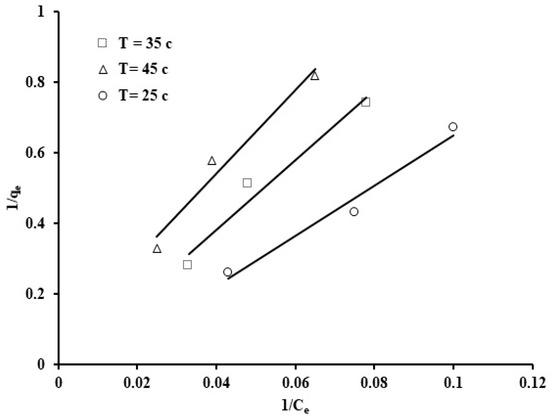
Figure 13.
Langmuir isotherm for the phosphate ion uptake by grape seeds (pH: 6.8, C0: 100 ppm, dose: 20 mg/mL).
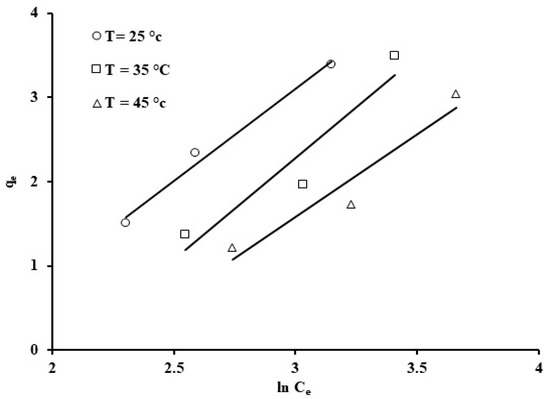
Figure 14.
Temkin isotherm for the phosphate ion uptake by grape seeds (dose: 20 mg/mL, pH: 6.8, C0: 100 ppm).
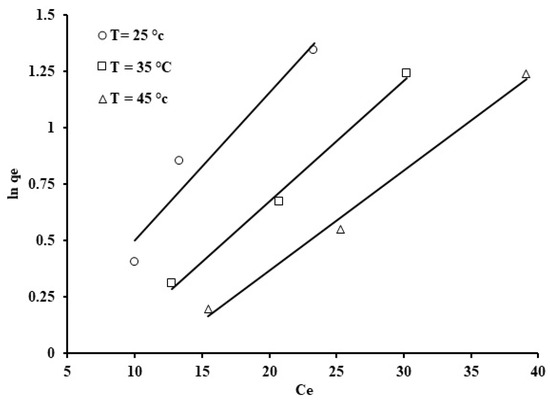
Figure 15.
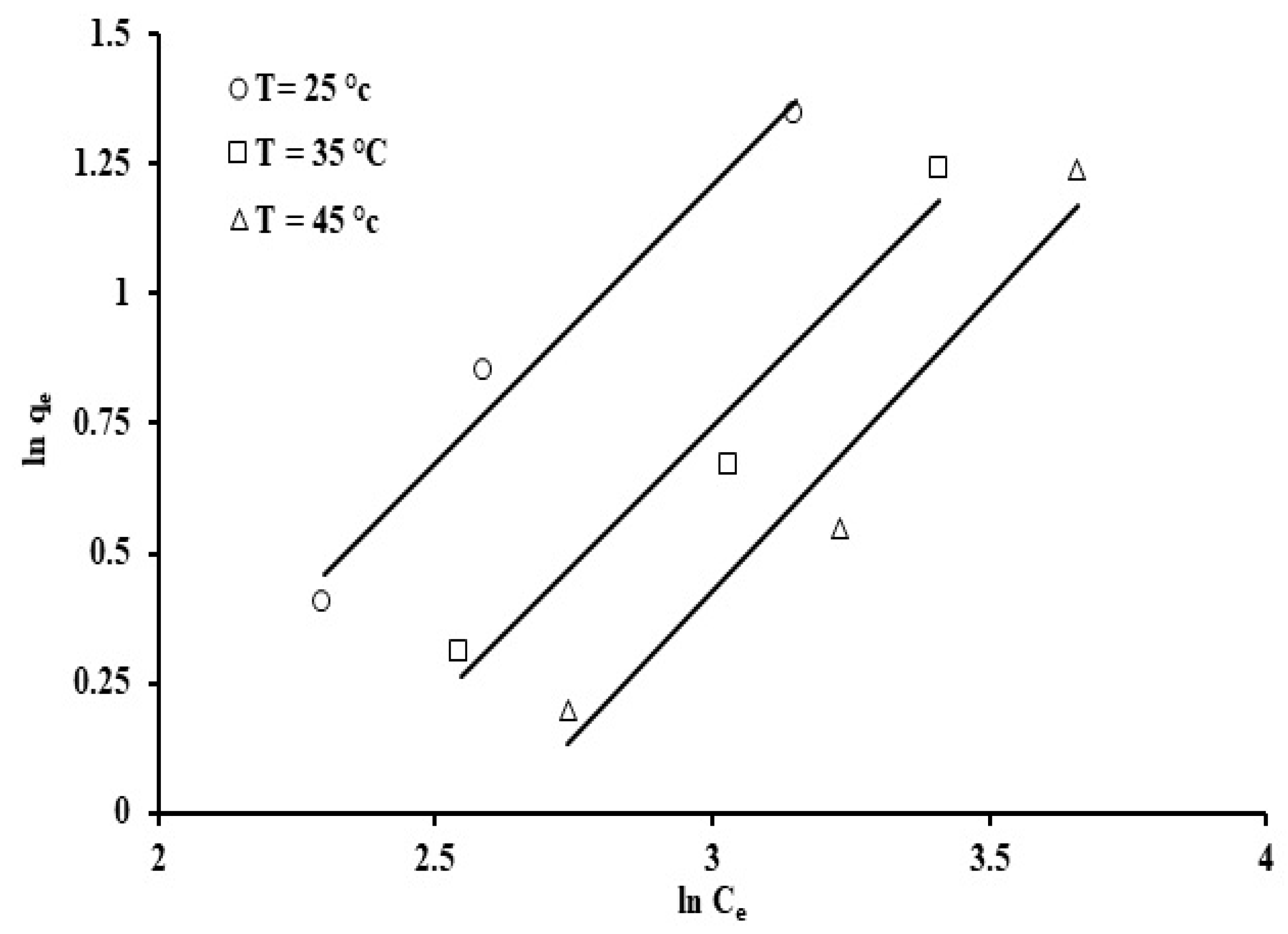
Jovanovic isotherm for the phosphate ion uptake by grape seeds (pH: 6.8, C0: 100 ppm, dose: 20 mg/mL).
The experiment was conducted at three temperatures, 25, 35, and 45 °C, the pH was retained constant at 7, and the initial concentration of phosphate ions and adsorbent concentration were 100 ppm and 20 ppm, respectively. The isotherm parameter values and their correlation coefficients are presented in Table 3.

Table 3.
Isotherm parameters for the phosphate ion adsorption by grape seeds from aqueous solution at different initial concentrations.
The correlation coefficient values were applied to validate these models. Table 3 reveals that the highest correlation coefficient values were, in decreasing order, Jovanovic > Langmuir > Freundlich > Temkin models.
Amongst the models that were studied, the Jovanovic model provided the best fit for the adsorption results when compared to the other models (R2 = 0.974). A plot of ln qe against Ce allows estimation of the Jovanovic constants, qmax and KJ, which are the y-intercept and the gradient, respectively (Figure 15). The correlation coefficients (R2 = 0.94–0.99) indicate that the Jovanovic isotherm provides the best fit for the phosphate ion adsorption onto grape seeds. The qmax values, listed in Table 3, suggest that a higher temperature (45 °C) caused a decrease in phosphate ion uptake. The latter results agree with the point that phosphate ion adsorption on the grape seeds is an exothermic process, as mentioned earlier.
The linearized form of the Temkin isotherm was applied to test its adsorption data representation, as shown in Figure 14. Temkin constants b and kT, which represent the adsorption enthalpy (kJ/mol) and constant related to the binding energy (L/g), were estimated based on the gradient and intercept values from the plot of qe against ln (Ce); the Temkin constants are displayed in Table 3. Based on Figure 10, the following values were calculated: kT = 1.5 L/g, b = 1.17 kJ/mol. The heat of adsorption in the Temkin model, b, is positive for phosphate ion adsorption onto grape seeds, which indicates exothermicity in the adsorptive process [73].
The Freundlich model, as shown in Figure 12, indicates a positive fit for the adsorption results, which exhibit variation in ln qe against ln Ce. Evidently, the data produced straight lines with an average correlation coefficient of 0.968. The value of the constant “n”, which is less than unity, indicates the process is chemisorption in nature [74]. The Freundlich constant, kF, decreased with increasing adsorption temperature (Table 3). The results obtained are in good agreement with this, and also indicate that the adsorption rate was higher at a lower temperature rather than at higher temperature.
The results from the Langmuir isotherm in linear form are shown in Table 3. The plot of 1/qe against 1/Ce, exhibited in Figure 9, was utilized to estimate values of the Langmuir coefficients KL and b. It can be observed that the Langmuir coefficients have negative values, which suggests that the experimental results do not follow the Langmuir model despite the high correlation coefficient value (0.974).
3.8. Adsorption Thermodynamics
The adsorption thermodynamic parameters, entropy, enthalpy, and Gibbs free energy, are correlated by Equation (15).
where ∆H represents adsorption heat in kJ/mol, T and R represent temperature (K) and the universal gas constant in kJ/mol·K. Equation (16) represents the adsorption equilibrium constant (b).
where Ce,l and Ce,s are concentrations at equilibrium in bulk and on the solid surface, respectively. The change in Gibbs free energy and entropy during the process is calculated from Equations (17) and (18), respectively, as follows:
The thermodynamic parameter values at various temperatures were determined and recorded in Table 4.

Table 4.
Calculated thermodynamic parameters for equilibrium adsorption of phenol onto grape seeds.
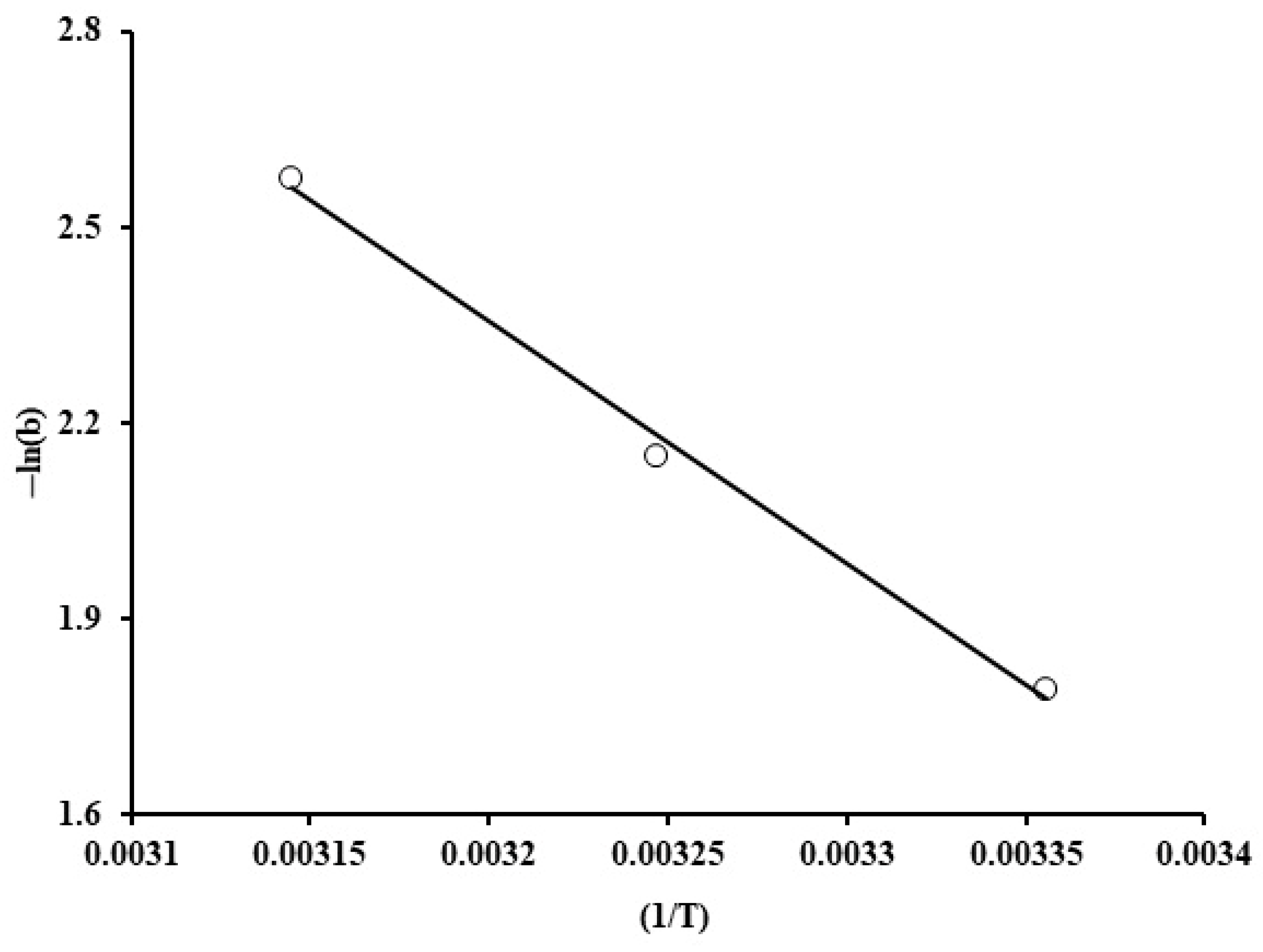
Figure 16 exhibits 1/T against ln (b) variations. The heat of adsorption was estimated from the slope, which was equal to −30.9 kJ/mol. For physical adsorption, ΔH is typically within the range of −20 and −40 kJ/mol, whereas chemisorption is characterized by high enthalpy of adsorption, which is about −400 to −80 kJ/mol. Therefore, the enthalpy change value indicates that phosphate adsorption by the grape seed particles involves physical adsorption with an exothermic nature, as revealed by the negative value. This result was also validated by the data shown on Figure 5; specifically, at high initial phosphate ion concentration (100 ppm), a decline in the uptake was experienced upon increasing the temperature.
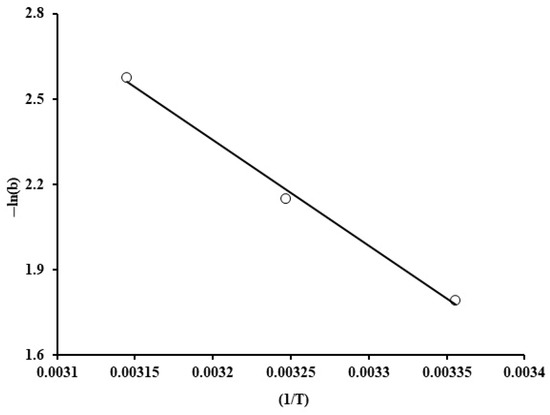
Figure 16.
Reciprocal of temperature (1/T) against ln(b) for phosphate adsorption by grape seeds (dose: 20 mg/mL, pH: 6.8, C0: 100 ppm).
Evidently, the randomness was decreased at the adsorbent–solution surface during the adsorption process, could take place as indicated by the negative value of entropy (∆S = −88.7 J/mol K) at 298 K. Furthermore, the Gibbs free energy has negative values at 298 K, as exhibited in Table 4, which is an indication of spontaneity in the adsorptive process.
4. Conclusions
Conclusively, ground black grape seeds can be utilized as an alternative inexpensive adsorbent, to efficiently reduce the concentration of phosphate ion via adsorption. Grape seed morphological studies show a highly porous structure, which is very suitable for adsorption and demonstrated positive results for phosphate adsorption onto the seeds. Greater initial phosphate ion concentration resulted in slightly increasing initial removal percentage rate and the total removal percentage. The removal percentage was mostly enhanced with incrementally larger adsorbent dosage but decreased with a rise in temperature. The removal was more efficient in a neutral solution and when close to room temperature (25 °C). Four isotherm models were investigated, and equilibrium data were best defined by a Jovanovic isotherm, which revealed fairly high correlation coefficient values. Kinetic analysis of the adsorption process obeyed a pseudo second order model, and the thermodynamic properties imply favorable adsorption with exothermicity and reduction in randomness through the process. Experimental results from the current studies will be helpful in designing and operating a full-scale wastewater treatment unit. Grape seeds can be regenerated and recycled to save on expenses and prevent environmental complications associated with the suggested disposal method. Next, it will be exciting to carry out an economic assessment to clarify the preparation cost of the adsorbent from the grape waste byproduct.
5. Recommendations
Optimization of Adsorption Conditions: Future studies can focus on optimizing the adsorption conditions, such as temperature, pH, contact time, and adsorbent dosage, to enhance phosphate removal efficiency using grape seed adsorbents.
Regeneration and Reusability of Grape Seed Adsorbent: Investigating the regeneration and reusability of grape seed adsorbents can help assess their economic feasibility and sustainability in large-scale wastewater treatment applications.
Application in Actual Wastewater Treatment: Conducting experiments using actual wastewater samples from agricultural and industrial sources would provide insight into the performance of grape seed adsorbents in real-world conditions, including the effects of competing ions.
Modification of Grape Seeds for Enhanced Adsorption: Exploring chemical or physical modifications of grape seeds, such as activation with metal oxides or carbonization, could enhance their adsorption capacity and selectivity for phosphate ions.
Author Contributions
Conceptualization, A.A.-B., K.A.b.A. and K.B.-M.; methodology, A.A.-B. and M.T.; formal analysis, A.A.A.-T., M.H. and A.A.-B.; investigation, A.A.-B.; resources, Z.A.-Q. and M.H.; data curation, A.A.-B., M.T., K.A.b.A., K.B.-M. and Z.A.-Q.; writing—original draft preparation, E.G., M.T., A.A.-B. and A.A.A.-T.; supervision, K.A.b.A., M.T., A.A.-B. and M.H.; project administration, A.A.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The authors declare that the data are available in this article. Should any other raw data be needed, reasonable requests should be directed to the corresponding author. All relevant data are included in the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yu, D.; Yan, L.; Shi, J.; Liu, Y.; Zhang, A.; Wang, Y.; Zhang, Y.; Xie, T. Phosphorus Removal and Recovery During Microalgae-Based Wastewater Treatment: A Mini-Review. Int. J. Environ. Res. 2024, 18, 34. [Google Scholar] [CrossRef]
- Rani, I.D.; Dermiyati, D.; Suharjo, R.; Niswati, A.; Pangaribuan, D.H. Soil Organisms Activities in Red Onion Cultivation with Application of Plant Extract Suspension and Compost. J. Trop. Soils 2022, 27, 89. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Su, Y.; Staehelin, C.; Xu, C. T-DNA Insertion Mutagenesis in Penicillium Brocae Results in Identification of an Enolase Gene Mutant Impaired in Secretion of Organic Acids and Phosphate Solubilization. Microbiology 2023, 169, 001325. [Google Scholar] [CrossRef]
- Pu, J.; Wang, S.; Ni, Z.; Wu, Y.; Liu, X.; Wu, T.; Wu, H. Implications of Phosphorus Partitioning at the Suspended Particle-Water Interface for Lake Eutrophication in China’s Largest Freshwater Lake, Poyang Lake. Chemosphere 2021, 263, 128334. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Hou, S.; Wang, J.; Zhu, H.; Shutes, B.; Yan, B. Biochar-Amended Constructed Wetlands for Eutrophication Control and Microcystin (MC-LR) Removal. Chemosphere 2022, 295, 133830. [Google Scholar] [CrossRef]
- Bowes, M.J.; Jarvie, H.P.; Halliday, S.J.; Skeffington, R.A.; Wade, A.J.; Loewenthal, M.; Gozzard, E.; Newman, J.R.; Palmer-Felgate, E.J. Characterising Phosphorus and Nitrate Inputs to a Rural River Using High-Frequency Concentration-Flow Relationships. Sci. Total Environ. 2015, 511, 608–620. [Google Scholar] [CrossRef]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A Review of Phosphorus Removal Technologies and Their Applicability to Small-Scale Domestic Wastewater Treatment Systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.M.A.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Performance and Prospects of Different Adsorbents for Phosphorus Uptake and Recovery from Water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Myllymäki, P.; Pesonen, J.; Romar, H.; Hu, T.; Tynjälä, P.; Lassi, U. The Use of Calcined Paper Mill Sludge as a Chemical Precipitant in the Simultaneous Removal of Ammonium and Phosphate-Paper Mill Waste Recycling and Reuse. Desalination Water Treat. 2020, 194, 459–467. [Google Scholar] [CrossRef]
- Fang, L.; Shi, Q.; Nguyen, J.; Wu, B.; Wang, Z.; Lo, I.M.C. Removal Mechanisms of Phosphate by Lanthanum Hydroxide Nanorods: Investigations Using EXAFS, ATR-FTIR, DFT, and Surface Complexation Modeling Approaches. Environ. Sci. Technol. 2017, 51, 12377–12384. [Google Scholar] [CrossRef]
- Rajendran, S.; Sai Bharadwaj, A.V.S.L.; Barmavatu, P.; Palani, G.; Trilaksanna, H.; Kannan, K.; Meenakshisundaram, N. A Review on Lanthanum-Based Materials for Phosphate Removal. ChemEngineering 2024, 8, 23. [Google Scholar] [CrossRef]
- Zhu, Y.; Yue, X.; Xie, F. Adsorptive Removal of Phosphate by a Fe–Mn–La Tri-Metal Composite Sorbent: Adsorption Capacity, Influence Factors, and Mechanism. Adsorpt. Sci. Technol. 2020, 38, 254–270. [Google Scholar] [CrossRef]
- Yu, Y.; Paul Chen, J. Key Factors for Optimum Performance in Phosphate Removal from Contaminated Water by a Fe–Mg–La Tri-Metal Composite Sorbent. J. Colloid Interface Sci. 2015, 445, 303–311. [Google Scholar] [CrossRef]
- Oktor, K.; Yuzer, N.Y.; Hasirci, G.; Hilmioglu, N. Optimization of Removal of Phosphate from Water by Adsorption Using Biopolymer Chitosan Beads. Water Air Soil Pollut. 2023, 234, 271. [Google Scholar] [CrossRef]
- Pokhrel, M.R.; Poudel, B.R.; Aryal, R.L.; Paudyal, H.; Ghimire, K.N. Removal and Recovery of Phosphate from Water and Wastewater Using Metal-Loaded Agricultural Waste-Based Adsorbents: A Review. J. Inst. Sci. Technol. 2019, 24, 77–89. [Google Scholar] [CrossRef]
- Zhu, X.F.; Liu, H.Y.; Shi, P.H.; Wu, J.F.; Guo, Y.F. Removal of Phosphate from Aqueous Solution by Using Red Mud. AMR 2011, 291–294, 1804–1807. [Google Scholar] [CrossRef]
- Yue, Q.; Zhao, Y.; Li, Q.; Li, W.; Gao, B.; Han, S.; Qi, Y.; Yu, H. Research on the Characteristics of Red Mud Granular Adsorbents (RMGA) for Phosphate Removal. J. Hazard. Mater. 2010, 176, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.L.; Xia, W.T.; An, J.; Yang, W.Q.; Yin, J.G. Removal of Phosphate Anions from Aqueous Solutions Using Dolomite as Adsorbent. AMR 2013, 864–867, 1454–1457. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing Phosphate Adsorption by Mg/Al Layered Double Hydroxide Functionalized Biochar with Different Mg/Al Ratios. Sci. Total. Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef]
- Sisay, G.B.; Bezabeh, B.; Getachew, E. Copper Oxide Nanobiochar from Spent Coffee Grounds for Phosphate Removal and Its Application as an Antibacterial Activity. Res. Sq. 2023; in review. [Google Scholar] [CrossRef]
- Chansuvarn, W. Removal of Phosphate from Wastewater Using Carbonized Filter Cake. AMM 2018, 879, 125–130. [Google Scholar] [CrossRef]
- Nyakairu, G.W.; Ntale, M.; Usman, M.O. Adsorption of Phosphate by Synthesized Silver/Calcium Oxide-Activated Carbon Nanocomposite. Adv. Environ. Eng. Res. 2023, 04, 1–20. [Google Scholar] [CrossRef]
- Siwek, H.; Bartkowiak, A.; Włodarczyk, M.; Sobecka, K. Removal of Phosphate from Aqueous Solution Using Alginate/Iron (III) Chloride Capsules: A Laboratory Study. Water Air Soil Pollut. 2016, 227, 427. [Google Scholar] [CrossRef]
- Atnafu, T.; Leta, S. Plasticized Magnetic Starch-Based Fe3O4 Clay Polymer Nanocomposites for Phosphate Adsorption from Aqueous Solution. Heliyon 2021, 7, e07973. [Google Scholar] [CrossRef]
- Civan Çavuşoğlu, F.; Özçelik, G.; Bayazit, Ş.S. Comparative Investigation of Phosphate Adsorption Efficiencies of MOF-76 (Ce) and Metal Oxides Derived from MOF-76 (Ce). Langmuir 2024, 40, 4255–4266. [Google Scholar] [CrossRef]
- Tóth, A.J.; Fózer, D.; Mizsey, P.; Varbanov, P.S.; Klemeš, J.J. Physicochemical Methods for Process Wastewater Treatment: Powerful Tools for Circular Economy in the Chemical Industry. Rev. Chem. Eng. 2023, 39, 1123–1151. [Google Scholar] [CrossRef]
- Oleszkiewicz, J.; Kruk, D.; Devlin, T.; Yuan, Q.; Lashkarizadeh, M. Options for Improved Nutrient Removal and Recovery from Municipal Wastewater in the Canadian Context; Canadian Water Network: Waterloo, ON, Canada, 2015. [Google Scholar]
- Kim, M.-J.; Lee, J.-H.; Lee, C.-G.; Park, S.-J. Thermal Treatment of Attapulgite for Phosphate Removal: A Cheap and Natural Adsorbent with High Adsorption Capacity. Desalination Water Treat. 2018, 114, 175–184. [Google Scholar] [CrossRef]
- Hasan, M.N.; Altaf, M.M.; Khan, N.A.; Khan, A.H.; Khan, A.A.; Ahmed, S.; Kumar, P.S.; Naushad, M.; Rajapaksha, A.U.; Iqbal, J.; et al. Recent Technologies for Nutrient Removal and Recovery from Wastewaters: A Review. Chemosphere 2021, 277, 130328. [Google Scholar] [CrossRef]
- Isiuku, B.; Oze, N. Adsorption performance of acid-activated carbon derived from Gmelin arborea in a fixed bed column. J. Appl. Sci. Environ. Manag. 2019, 44, 1070–1079. [Google Scholar]
- Li, Z.; Sun, X.; Huang, L.; Liu, D.; Yu, L.; Wu, H.; Wei, D. Phosphate Adsorption and Precipitation on Calcite under Calco-Carbonic Equilibrium Condition. Chemosphere 2017, 183, 419–428. [Google Scholar] [CrossRef]
- Khan, S.; Ishaq, M.; Ahmad, I.; Hussain, S.; Ullah, H. Evaluation of Coal as Adsorbent for Phosphate Removal. Arab. J. Geosci. 2013, 6, 1113–1117. [Google Scholar] [CrossRef]
- Nagoya, S.; Nakamichi, S.; Kawase, Y. Mechanisms of Phosphate Removal from Aqueous Solution by Zero-Valent Iron: A Novel Kinetic Model for Electrostatic Adsorption, Surface Complexation and Precipitation of Phosphate Under Oxic Conditions. Sep. Purif. Technol. 2019, 218, 120–129. [Google Scholar] [CrossRef]
- Aswin Kumar, I.; Viswanathan, N. Development and Reuse of Amine-Grafted Chitosan Hybrid Beads in the Retention of Nitrate and Phosphate. J. Chem. Eng. Data 2018, 63, 147–158. [Google Scholar] [CrossRef]
- Yadav, D.; Kapur, M.; Kumar, P.; Mondal, M.K. Adsorptive Removal of Phosphate from Aqueous Solution Using Rice Husk and Fruit Juice Residue. Process. Saf. Environ. Prot. 2015, 94, 402–409. [Google Scholar] [CrossRef]
- Muhmood, A.; Cui, S.; Wang, J.; Wang, D.; Pugliese, L.; Wu, S. Eco-Nano Solutions for Rapid Phosphorus Recovery: Closing the Loop for Sustainable Agriculture. Sci. Total. Environ. 2025, 964, 178477. [Google Scholar] [CrossRef]
- Xavier, G.T.M.; Nunes, R.S.; Urzedo, A.L.; Tng, K.H.; Le-Clech, P.; Araújo, G.C.L.; Mandelli, D.; Fadini, P.S.; Carvalho, W.A. Removal of Phosphorus by Modified Bentonite: Polyvinylidene Fluoride Membrane—Study of Adsorption Performance and Mechanism. Environ. Sci. Pollut. Res. 2024, 31, 53718–53728. [Google Scholar] [CrossRef]
- Odabaşioğlu, M.İ.; Gürsöz, S. Effects of Drought-Tolerant Grapevine Rootstocks on the Mineral Contents and Fatty Acid Compositions of Grape Seeds. J. Berry Res. 2022, 12, 383–400. [Google Scholar] [CrossRef]
- Ergović-Ravančić, M.; Obradović, V.; Mesić, J.; Svitlica, B.; Marčetić, H.; Prtenjača, K.; Škrabal, S. The Influence of Grape Seed Drying Temperature on the Quality of Grape Seed Oil. J. Process. Energy Agric. 2020, 24, 22–25. [Google Scholar] [CrossRef]
- Escudero, L.B.; Vanni, G.; Duarte, F.A.; Segger, T.; Dotto, G.L. Biosorption of Silver from Aqueous Solutions Using Wine Industry Wastes. Chem. Eng. Commun. 2018, 205, 325–337. [Google Scholar] [CrossRef]
- Zanini, M.; Silvestre, W.P.; Baldasso, C.; Tessaro, I.C. Valorization of Wastes Generated in Organic Grape Processing. Braz. Arch. Biol. Technol. 2024, 67, e24230183. [Google Scholar] [CrossRef]
- Alvarez-Gonzales, R.; Bejar-Aramburú, D.G.; Cama-Ortiz, F.; Gonzales-Condori, E.G.; Gonzales-Condori, J.; Gutiérrez-Delgado, J.A.; Zúñiga Torres, J.C. Valorization of Grape Seed Waste for Use in the Production of Antioxidant Soaps and as an Adsorbent for a Textile Dye. In Proceedings of the 3rd LACCEI International Multiconference on Entrepreneurship, Innovation and Regional Development (LEIRD 2023): “Igniting the Spark of Innovation: Emerging Trends, Disruptive Technologies, and Innovative Models for Business Success”, Virtual, 4–6 December 2023; Latin American and Caribbean Consortium of Engineering Institutions: Boca Raton, FA, USA, 2023. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, H. Phytochemical Constituents, Health Benefits, and Industrial Applications of Grape Seeds: A Mini-Review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef]
- Fidan, M.; Erez, M.E.; Ć°Nal, B.; Pinar, S.M.; Altintaş, S. Antioxidant Capacity and Phylogenetic Analysis of Twenty Native Grape Cultivars in Siirt Province, Turkey. Cell. Mol. Biol. 2018, 64, 14–18. [Google Scholar] [CrossRef]
- Stjepanović, M.; Velić, N.; Habuda-Stanić, M. Modified Grape Seeds: A Promising Alternative for Nitrate Removal from Water. Materials 2021, 14, 4791. [Google Scholar] [CrossRef]
- Mohammed, A.J.; Ibrahim, M.H.; Zulkifli, S.Z.; Salman, J.M. Synthesis and Characterization of a Nano-Adsorbent Derivative Derived from Grape Seeds for Cadmium Ion Removal in an Aqueous Solution. Water 2021, 13, 2896. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Castello, D.; Fiori, L. Granular Activated Carbon from Grape Seeds Hydrothermal Char. Appl. Sci. 2018, 8, 331. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standards Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]
- Al Bsoul, A.; Hailat, M.; Abdelhay, A.; Tawalbeh, M.; Al-Othman, A.; Al-kharabsheh, I.N.; Al-Taani, A.A. Efficient Removal of Phenol Compounds from Water Environment Using Ziziphus Leaves Adsorbent. Sci. Total. Environ. 2021, 761, 143229. [Google Scholar] [CrossRef]
- Petropoulos, J.H.; Havredaki, V.I. On the fundamental concepts underlying Henry-law adsorption and adsorbed gas transport in porous solids. J. Chem. Soc. Faraday Trans. 1986, 82, 2531–2545. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.; Romani, A.; Lampe, A.; Ferrari Nicoli, S.; Gabrielli, P.; et al. Grape Seeds: Chromatographic Profile of Fatty Acids and Phenolic Compounds and Qualitative Analysis by FTIR-ATR Spectroscopy. Foods 2019, 9, 10. [Google Scholar] [CrossRef]
- Da Silva, R.N.F.; De Azevedo Mello, P.; Penteado Holkem, A.; Silva, L.F.O.; Oliveira, M.L.S.; Nawaz, A.; Manoharadas, S.; Dotto, G.L. Recovery of Ce and La from Phosphogypsum Leachate by Adsorption Using Grape Wastes. Environ. Sci. Pollut. Res. 2023, 30, 118366–118376. [Google Scholar] [CrossRef]
- Zúñiga-Muro, N.M.; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E.; Duran-Valle, C.J.; Ghalla, H.; Sellaoui, L. Recovery of Grape Waste for the Preparation of Adsorbents for Water Treatment: Mercury Removal. J. Environ. Chem. Eng. 2020, 8, 103738. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Rooney, A.; Hernández-Hierro, J.M.; Byrne, H.J.; Heredia, F.J. Study of Phenolic Extractability in Grape Seeds by Means of ATR-FTIR and Raman Spectroscopy. Food Chem. 2017, 232, 602–609. [Google Scholar] [CrossRef]
- Banjanin, T.; Özcan, M.M.; Al Juhaimi, F.; Ranković-Vasić, Z.; Uslu, N.; Mohamed, I.A.; Ghafoor, K.; Babiker, E.E.; Osman, M.A.; Gassem, M.A.; et al. Effect of Varieties on Bioactive Compounds, Fatty Acids, and Mineral Contents in Different Grape Seed and Oils from Bosnia and Herzegovina. J. Food Process. Preserv. 2019, 43. [Google Scholar] [CrossRef]
- Antunes, M.; Esteves, V.I.; Guégan, R.; Crespo, J.S.; Fernandes, A.N.; Giovanela, M. Removal of Diclofenac Sodium from Aqueous Solution by Isabel Grape Bagasse. Chem. Eng. J. 2012, 192, 114–121. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of Nanocellulose Fibrils from Lignocellulosic Fibres: A Novel Approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Forte, M.M.C.; Santana, R.M.C. Thermal Decomposition of Wood: Influence of Wood Components and Cellulose Crystallite Size. Bioresour. Technol. 2012, 109, 148–153. [Google Scholar] [CrossRef]
- Babel, S. Low-Cost Adsorbents for Heavy Metals Uptake from Contaminated Water: A Review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Al Bsoul, A. The Use of Eucalyptus Leaves as Adsorbent for Copper Ion Removal. Desalination Water Treat. 2014, 52, 7838–7844. [Google Scholar] [CrossRef]
- Bousba, S.; Meniai, A.H. Removal of Phenol from Water by Adsorption onto Sewage Sludge Based Adsorbent. Chem. Eng. Trans. 2014, 40, 235–240. [Google Scholar] [CrossRef]
- Abdelhay, A.; Al Bsoul, A.; Al-Othman, A.; Al-Ananzeh, N.M.; Jum’h, I.; Al-Taani, A.A. Kinetic and Thermodynamic Study of Phosphate Removal from Water by Adsorption onto (Arundo Donax) Reeds. Adsorpt. Sci. Technol. 2018, 36, 46–61. [Google Scholar] [CrossRef]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Selective Adsorption of Phosphate from Seawater and Wastewater by Amorphous Zirconium Hydroxide. J. Colloid Interface Sci. 2006, 297, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, P.; Li, L.; Huang, Y.; Pu, Y.; Hou, X.; Song, L. Identification and Antioxidant Activity of Flavonoids Extracted from Xinjiang Jujube (Ziziphus jujube Mill.) Leaves with Ultra-High Pressure Extraction Technology. Molecules 2019, 24, 122. [Google Scholar] [CrossRef]
- Iacomi, P.; Alabarse, F.; Appleyard, R.; Lemaire, T.; Thessieu, C.; Wang, S.; Serre, C.; Maurin, G.; Yot, P.G. Structural Insight of MOFs under Combined Mechanical and Adsorption Stimuli. Angew. Chem. Int. Ed. 2022, 61, e202201924. [Google Scholar] [CrossRef]
- Liu, Y.; Villalba, G.; Ayres, R.U.; Schroder, H. Global Phosphorus Flows and Environmental Impacts from a Consumption Perspective. J. Ind. Ecol. 2008, 12, 229–247. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhou, J.L.; Wang, J.; Liang, H.; Li, G. Phosphorus Elimination from Aqueous Solution Using ‘Zirconium Loaded Okara’ as a Biosorbent. Bioresour. Technol. 2014, 170, 30–37. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Haridas, A. Removal of Phosphate from Aqueous Solutions and Sewage Using Natural and Surface Modified Coir Pith. J. Hazard. Mater. 2008, 152, 527–535. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Isotherms Studies of Equilibrium Sorption of Zn 2+ Unto Phosphoric Acid Modified Rice Husk. IOSRJAC 2012, 3, 38–45. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Naushad, M.; Ali, R. Kinetic, Equilibrium Isotherm and Thermodynamic Studies of Cr(VI) Adsorption onto Low-Cost Adsorbent Developed from Peanut Shell Activated with Phosphoric Acid. Environ. Sci. Pollut. Res. 2013, 20, 3351–3365. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).