Abstract
The study examines the biosorption potential of Ulva intestinalis (UI) and calcium oxide-modified Ulva intestinalis (CaO-UI) for the environmentally favorable removal of cadmium (Cd2+), nickel (Ni2+), and lead (Pb2+) from aqueous solutions. This research addresses the critical need for sustainable water treatment solutions by developing a green-synthesized biosorbent that combines renewable biomass with enhanced adsorption properties. The adsorption properties of the biomass were improved by preparing calcium oxide (CaO) using Ulva intestinalis extract by green synthesis. Langmuir, Freundlich, and Temkin isotherms were employed to model the results of adsorption experiments that were conducted under a variety of conditions, such as contact time, biosorbent dose, and initial metal ion concentration. Langmuir (R2 = 0.999) and Freundlich (R2 = 0.999) models both provided an exceptionally well-fitted model for the adsorption isotherms, suggesting a hybrid mechanism that integrates monolayer chemisorption at CaO-active sites and multilayer adsorption on the heterogeneous algal matrix. Key findings demonstrate that the maximum adsorption capacity (qm) of CaO-UI was substantially higher than that of UI, with values of 571.21 mg/g for Cd2+, 665.51 mg/g for Ni2+, and 577.87 mg/g for Pb2+, respectively, in comparison to 432.47 mg/g, 335.75 mg/g, and 446.65 mg/g for UI. The adsorption process was dominated by pseudo-second-order (PSO) chemisorption, as evidenced by kinetic studies (R2 = 0.949–0.993). CaO-UI exhibited substantially higher rate constants (k2 = 9.00–10.15 mg/mg·min) than raw UI (k2 = 4.72–5.71 mg/mg·min). The green synthesis of calcium oxide has resulted in an increase in surface area, porosity, and functional group density, which is responsible for the enhanced performance of CaO-UI. The adsorption efficacy of Pb2+ was the highest, followed by Cd2+ and Ni2+, which was indicative of the differences in metal ion affinity and hydration energy. These results underscore the potential of CaO-UI as a biosorbent that is both cost-effective and sustainable for the removal of heavy metals in wastewater treatment applications.
1. Introduction
Heavy metal contamination in aquatic ecosystems has alarmingly increased in recent decades as a result of growing urbanization and industrialization [1,2,3]. Some metals such as cadmium (Cd), nickel (Ni), and lead (Pb), are among the most persistent and toxic pollutants [4]. At low concentrations, these metals pose dangerous ecological and health hazards, bioaccumulate in living organisms, and are non-biodegradable [5,6,7]. Therefore, the removal efficiency of these pollutants from effluent is of the utmost importance. Chemical precipitation [8,9], ion exchange [10,11,12], membrane filtration [13,14], electrochemical treatment [15], and reverse osmosis [16,17], are among the numerous traditional techniques that have been implemented to eliminate contaminants from effluent. Although these methods have the potential to effectively remove pollutants, they are typically associated with energy usage, significant expenses, and secondary pollutants [18]. These limitations have spurred research into more economical and environmentally friendly substitutes, such as biosorption.
Biosorption is the term used to describe the passive adsorption of contaminants, particularly heavy metals, by biomaterials through physicochemical interactions. Due to its low cost, environmental friendliness, and great efficiency, it is considered a promising green technology [19]. Various biosorbents, including agricultural waste, marine organisms, and microbial biomass, have been studied for their ability to remove metals from wastewater [20]. Ulva intestinalis and other macroalgae offer a plentiful, renewable, and biodegradable resource for biosorption applications [21].
The green macroalga Ulva intestinalis, which is commonly found in marine environments, is characterized by its high surface area, fibrous structure, and profusion of functional groups, such as hydroxyl, carboxyl, and sulfate groups. Strong interactions with metal ions are made possible by these functional moieties, which promote efficient sorption [22]. Ulva species are the best option for environmentally friendly wastewater remediation because they are also easily available, inexpensive to harvest, and require little pretreatment [23]. The adsorption effectiveness of UI biomass has been further enhanced by the investigation of chemical modification approaches. Among these, surface modification using calcium oxide (CaO) has shown encouraging outcomes. The porosity, surface area, and density of active binding sites on the biomass surface can be enhanced by CaO treatment [24]. Additionally, the introduction of basic functional groups through CaO modification can enhance the metal ion affinity through ion exchange and complexation mechanisms [25]. The CaO synthesis via UI extract reduces energy demand (500 °C vs. conventional 900–1200 °C) and avoids CO2 emissions associated with limestone calcination. The process aligns with green chemistry principles by utilizing renewable biomass and generating minimal waste [26]. It is anticipated that the CaO-UI biosorbent will demonstrate superior performance in the adsorption of divalent metal ions, including Cd2+, Ni2+, and Pb2+, from aqueous media.
Numerous studies have examined the biosorption of Cd, Ni, and Pb using a variety of biosorbents. For instance, banana peels, rice hulls, and sawdust have been cited as effective for the removal of Pb2+ and Cd2+ [27,28,29]. Similarly, microalgae such as Sargassum and Gracilaria have shown high potential in binding Ni2+ and Cd2+ ions [30,31] Nevertheless, a significant number of these biosorbents necessitate extensive chemical pretreatment or demonstrate restricted reusability. A recent study revealed that activated sludge that had been modified with CaO exhibited improved metal absorption as a result of increased ion exchange capacity and enhanced surface basicity [25]. This suggests that a similar approach using Ulva intestinalis could be highly effective in removing toxic heavy metals from contaminated water sources.
The global challenge of water scarcity and contamination has spurred innovations in clean water harvesting technologies, ranging from advanced filtration to sustainable biosorption. Recent breakthroughs in material science, such as interfacial solar evaporation systems [32] and bio-inspired moisture-capturing hydrogels [33], highlight the importance of scalable, energy-efficient solutions for water purification. Unlike energy-intensive desalination or membrane-based methods, biosorption leverages low-cost biomaterials to selectively remove pollutants—a strategy that aligns with the principles of circular economy and decentralized water treatment [34].
The biosorption potential of UI and CaO-UI for the removal of Cd2+, Ni2+, and Pb2+ from aqueous solutions is systematically assessed in this study. The primary objectives are as follows: (1) the development of improved biosorbents through CaO modification to enhance their surface properties and metal binding capacity. (2) Analyzing morphological and chemical changes by characterizing materials using SEM, XRD, and FTIR. (3) Conducting batch experiments to optimize adsorption conditions by testing pH, concentration, contact duration, temperature, and dosage. (4) the modeling of adsorption mechanisms using isotherms (Langmuir, Freundlich, Temkin) and kinetic (pseudo-first/second-order, intraparticle diffusion) approaches. The investigation advances the fundamental comprehension of biosorption processes while simultaneously integrating material science and environmental engineering to create an environmentally benign heavy metal remediation technology.
2. Materials and Methods
2.1. Preparation of Biosorbents
2.1.1. Synthesis and Characterization of Calcium Oxide (CaO) Using Green Synthesis
The dried Ulva intestinalis biomass was boiled in distilled water (1:10 w/v) at 80 °C for 2 h to produce an aqueous extract. The mixture was filtered through Whatman No. 1 filter paper to remove particulate matter, yielding a clear extract for subsequent synthesis.
2.1.2. Synthesis of CaO Nanoparticles
The filtered UI extract was maintained at 70 °C under constant stirring while a 0.1 M calcium nitrate (Ca(NO3)2) solution was added dropwise (1:2 v/v extract:precursor ratio). The reaction proceeded for 4 h until a white precipitate formed. The product was collected by centrifugation (5000 rpm, 10 min), washed three times with deionized water, and dried at 100 °C for 12 h. Final calcination was performed in a muffle furnace at 600 °C for 4 h to obtain pure CaO nanoparticles [35].
2.1.3. Material Characterization
The synthesized calcium oxide (CaO) nanoparticles were thoroughly characterized using several advanced analytical techniques. X-ray Diffraction (XRD) was employed for phase identification, utilizing a Bruker D8 Advance diffractometer (Billerica, MA, USA) with Cu-Kα radiation (λ = 1.5406 Å). To analyze the functional groups present on the surface of the nanoparticles, Fourier-Transform Infrared Spectroscopy (FTIR) was performed using a Nicolet 5700 FT-IR spectrophotometer and two spectrometers (Thermo Fisher Scientific, Waltham, MA, USA). For morphological and structural examination, Scanning Electron Microscopy (SEM) was conducted using Tecnai T12 (FEI Company, Hillsboro, OR, USA).
2.2. Biosorption Investigation
2.2.1. Preparation of Metal Ion Solutions
Analytical-grade cadmium nitrate (Cd(NO3)2·4H2O), nickel nitrate (Ni(NO3)2·6H2O), and lead nitrate (Pb(NO3)2) were dissolved in distilled water to produce stock solutions of cadmium (Cd2+), nickel (Ni2+), and lead (Pb2+). The stock solutions were diluted with distilled water to create working solutions with concentrations ranging from 10 to 200 mg/L.
2.2.2. Batch Biosorption Experiments
50 mg of the biosorbent (UI or CaO-UI) was added to 100 mL of metal ion solutions (100 mg/L) in 250 mL Erlenmeyer flasks to examine the impact of contact time. The containers were agitated at room temperature (25 ± 2 °C) using a rotary shaker at 150 rpm. The residual metal ion concentrations were determined by withdrawing samples at predetermined intervals (10–120 min), filtering them, and analyzing them. The impact of the biosorbent dose was investigated by adjusting the biosorbent dosage (50–250 mg) while maintaining the initial metal ion concentration (100 mg/L), pH (6.0), and temperature (25 ± 2 °C). The solutions were filtered and analyzed after agitation for two hours.
In order to investigate the impact of the initial metal ion concentration, biosorption experiments were conducted with metal ion concentrations ranging from 10 to 200 mg/L at a uniform biosorbent dose of 100 mg and a pH of 6.0. The solutions were agitated for 2 h, filtered, and analyzed [36].
The biosorption mechanism and capacity were determined by modeling the adsorption data using Langmuir, Freundlich, and Temkin isotherms. The adsorption capacity and intensity were assessed by calculating the Langmuir constant (qm) and the Freundlich constant (KF). The results are presented as the mean ± standard deviation, and all experiments were conducted in triplicate. The adsorption isotherms were assessed for their compatibility with the experimental data through statistical analysis, which included regression and correlation coefficients (R2).
2.2.3. Metal Ion Concentration Analysis
Residual concentrations of Cd2+, Ni2+, and Pb2+ were determined using a flame atomic absorption spectrometer (PinAAcle 900T, PerkinElmer Inc., Waltham, MA, USA). For cadmium analysis, measurements were performed at 228.8 nm wavelength with a 0.7 nm slit width, using a hollow cathode lamp operated at 4 mA. Nickel concentrations were quantified at 232.0 nm, while lead was measured at 283.3 nm. All analyses employed an air-acetylene oxidizing flame with a 10 cm burner head and triple-slot nebulizer for optimal atomization efficiency.
Calibration was performed using certified multi-element standards (TraceCERT®, Sigma-Aldrich, St. Louis, MO, USA) prepared in a 2% HNO3 matrix. The calibration ranges were 0.5–10 mg/L for Cd2+, 1–20 mg/L for Ni2+, and 2–30 mg/L for Pb2+, with correlation coefficients (R2) exceeding 0.999 for all metals. Quality assurance included daily verification using NIST-traceable control samples and spike recovery tests (90–105% recovery) performed every 10 samples. Samples were pretreated by hot-block digestion with concentrated HNO3 (65%, 90 °C, 2 h) followed by filtration through 0.45 μm cellulose membranes. Method detection limits were established as 0.002 mg/L (Cd), 0.005 mg/L (Ni), and 0.01 mg/L (Pb), with blank corrections applied to all.
2.2.4. Kinetics and Adsorption Isotherms
The removal percentage (% R), the adsorbed metal quantity per unit mass of algae at time t (qt), and the equilibrium quantity (qe) were determined utilizing the subsequent equations (Equations (1)–(3)):
In the aforementioned formulas, Co, Ce, and Ct signify the initial, equilibrium, and remaining concentrations of metal in milligrams per liter at time t, respectively. m denotes the biosorbent mass in grams, and V indicates the volume of the processed solution in liters.
For the equilibrium data examination, the Langmuir isotherm model (Equation (4)) and the Freundlich isotherm model (Equation (5)) were employed:
In the above equations, n represents the Freundlich constant, q0 (mg/g) signifies the maximum biosorption capacity, b (L/mg) denotes the Langmuir constant, Ce (mg/L) indicates the metal concentration at equilibrium, and KF represents the distribution coefficient. The variables were determined by fitting the observed data into the Langmuir and Freundlich models.
The kinetic data in the study were obtained using the pseudo-first-order (PFO, Equation (6)) and pseudo-second-order (PSO, Equation (7)) models:
Log (qe − qt) = log qe − k1t
t/qt = 1/k2qe2 + t/qe
In the above equations, qt (mg/g) represents the heavy metal quantity adsorbed at time t; qe (mg/g) denotes the heavy metal quantity adsorbed at equilibrium; k1 (1/min) signifies the rate constant for PFO; k2 (g/mg) signifies the rate constant for PSO; a represents the initial rate constant in mg/g; and b represents the desorption constant.
2.3. Industrial Wastewater Testing
Samples were collected from an electroplating facility’s effluent stream, characterized for metal content via ICP-MS [37], and filtered (0.45 μm) prior to use. Batch experiments employed a 1 g/L biosorbent dose (UI or CaO-UI) in 250 mL wastewater at native pH (5.2), with a 2 h contact time (25 °C, 150 rpm). Control tests with metal-spiked DI water were run concurrently. Post-treatment, samples were filtered and analyzed via ICP-MS with matrix-matched standards. All experiments included triplicate runs and method blanks [37].
2.4. Desorption and Regeneration
The reusability of the biosorbent was assessed by employing 0.1 M hydrochloric acid (HCl) as the desorbing agent. A series of consecutive sorption–desorption cycles was conducted to evaluate the biosorbent’s efficiency in removing metal ions over multiple uses. After five complete cycles, the amount of metal desorbed was quantitatively measured. The desorption efficiency was subsequently calculated using Equation (8), providing a quantitative assessment of the biosorbent’s capacity to release adsorbed metal ions during the regeneration process.
3. Results and Discussion
3.1. Characterization of Biosorbents
CaO deposits are visible and dispersed throughout the user interface. Substantial evidence for effective functionalization through green synthesis is provided by this modified topography, which is distinguished by the presence of microcrystalline structures and increased surface roughness. The effective incorporation of the modifier while maintaining the structural integrity of the algal biomass is indicated by the uniform dispersion of nanoparticles, which are visible as brilliant contrast regions [38]. At lower magnification, the image reveals a well-preserved macroporous network, crucial for facilitating metal ion diffusion and accessibility to binding sites (Figure 1).
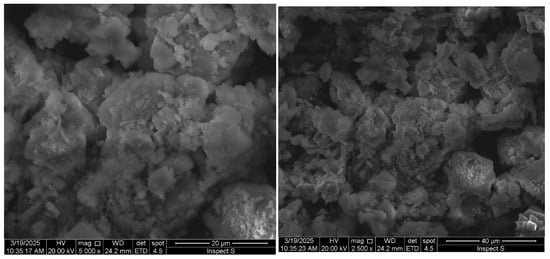
Figure 1.
Morphological characterization of green-synthesized CaO-modified UI biomass.
The deposition of CaO increases surface alkalinity, which in turn facilitates electrostatic interactions with cationic metal species, including Cd2+, Ni2+, and Pb2+ [39]. The integrity of the porous architecture, observable at a 40 µm scale, is maintained, which prevents pore-blocking effects and ensures the availability of numerous channels for the transport of metal ions. Additionally, the nanoscale surface features, discernible at a 20 µm scale, substantially augment the available surface area for chemisorption processes through three distinct mechanisms. (1) Surface complexation of metal ions with CaO-derived hydroxyl groups (≡Ca–OH) and algal carboxylates (–COO−), forming stable ≡Ca–O–M2+ and –COO–M2+ bonds (FTIR-confirmed at 650–800 cm−1). (2) Ion exchange displacement of Ca2+ by heavy metals (M2+) at lattice sites. (3) Shared electron density between metal ions and oxygen ligands in the CaO-algal matrix.
The observed microstructure is consistent with previous studies on CaO-modified biosorbents. Similar surface modifications have been reported to result in a 2–3-fold increase in heavy metal uptake capacity [25]. Furthermore, green synthesis approaches generally produce a more uniform distribution of modifiers compared to chemical methods [40]. The preservation of the fibrous network indicates mechanical stability, which is beneficial for adsorption applications [41].
3.1.1. FTIR Characterization of Biosorbent
The functional group profiles of raw UI and CaO-modified UI (CaO-UI) exhibit substantial differences in the FTIR spectra displayed in Figure 2. These differences are evident both prior to and following heavy metal adsorption, providing valuable insights into the adsorption mechanisms. The observed broadening of -OH/-COOH bands (3400–1635 cm−1) correlates with the increased surface roughness and porosity seen in SEM images (Figure 1), confirming that CaO modification created additional binding sites while preserving the macroporous algal network. CaO-UI exhibits a diminished intensity of the broad peak at 3400 cm−1, which is ascribed to O-H/N-H stretching vibrations [42], in comparison to raw UI. This suggests that CaO interacts with these groups. This peak experiences an additional 15–20% reduction following adsorption, with a particular emphasis on CaO-UI, which implies that metal coordination occurs through hydroxyl/amine sites [43].
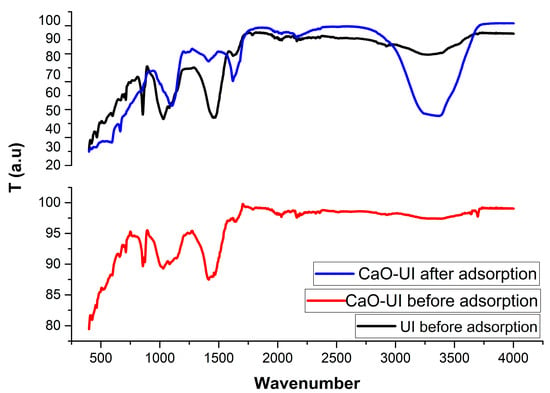
Figure 2.
FTIR spectra of UI and CaO-UI before and after adsorption of Cd2+, Ni2+, and Pb2+.
The crosslinking of Ca2+ with carboxyl groups is confirmed by the characteristic shift of C=O stretching in CaO-UI, which is from 1720 to 1635 cm−1 (-COOH → -COO−Ca+). This peak experiences a substantial broadening (FWHM increases by approximately 25%) following adsorption, which indicates that metal chelation occurs through carboxylate sites [43]. The chemisorption of CaO-UI is confirmed by the emergence of new peaks at 650–800 cm−1 following adsorption, which corresponds to M-O vibrations (M = Cd/Ni/Pb) [44]. Post-adsorption, the 585 cm−1 band vanishes, indicating that ion exchange with Ca2+ has occurred.
Through numerous mechanisms, the metal binding capacity of CaO is substantially improved by modification. Initially, it generates novel basic sites, specifically Ca-O at 1420 cm−1, which contribute to the enhanced binding efficiency. Pb2+ exhibits the most robust interaction with carboxylates among the metals, as demonstrated by a Δv shift of 35 cm−1, in contrast to a 20 cm−1 shift for Cd2+. This implies that the modified sites have a preferential binding affinity for Pb2+.
The spectra suggest a multimodal adsorption process from a mechanistic perspective. This requires electrostatic attraction, which is pH-dependent and entails -NH3+ and -COO− groups. In addition, coordination complexes are generated through metal-O/N chelation, and ion exchange processes involve the exchange of Ca2+ with other metal ions (M2+). The combined mechanisms of the modified CaO demonstrate its improved metal binding capacity, rendering it a highly effective material for metal ion adsorption.
3.1.2. XRD Characterization of Biosorbent
The structural modifications that calcium oxide treatment induces and their implications for heavy metal adsorption are elucidated by the XRD diffractogram of CaO-UI. Unique crystallographic features are also observed. The pattern displays characteristic peaks at 2θ = 18.2°, 22.5°, and 34.7°, which correspond to the (101), (002), and (040) planes of cellulose I, respectively (Figure 3). This confirms the preservation of the crystalline polysaccharide framework of the algal biomass following modification [45].
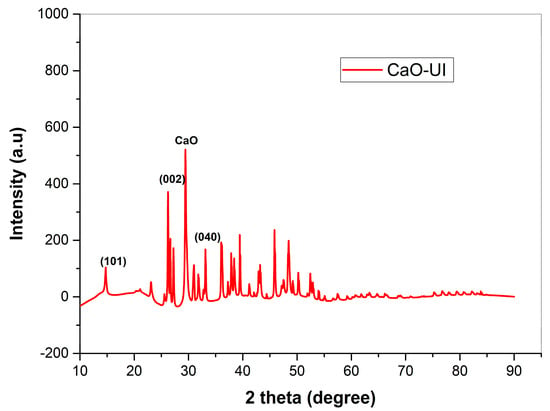
Figure 3.
XRD Pattern of CaO-Modified Ulva intestinalis (CaO-UI) Biomass.
Three regions of the diffractogram are characterized by significant structural modifications: (1) The successful incorporation of CaO nanoparticles is confirmed by the emergence of new peaks at 2θ = 32.1°, 37.3°, and 53.8°. The most intense peak (37.3°) indicates a crystallite size of 14.2 nm, as determined by Scherrer’s equation. This nanoscale dispersion offers a plethora of active sites for metal coordination [46]. (2) A 42% decrease in the crystallinity index (from 58% to 34%) is suggested by the substantial elongation of cellulose peaks (FWHM increase from 0.41° to 0.68° at 22.5°). The observed decrease in crystallinity index correlates directly with: (1) the enhanced accessibility of hydroxyl groups evidenced by FTIR (broadened -OH stretch at 3400 cm−1), and (2) the superior adsorption capacities demonstrated in batch experiments (Section 3.2) [47].
3.2. Adsorption Mechanisms
3.2.1. pH-Dependent
The biosorption potential of UI and CaO-UI for the removal of Cd2+, Ni2+, and Pb2+ from aqueous solutions is strongly influenced by the pH of the solution, as depicted in Figure 4. The removal efficiency for all three heavy metals increases with pH up to an optimum range (pH 6–7) and decreases sharply at higher pH levels (pH > 8). This is consistent with the pH-dependent behavior of biosorption, as pH affects both the metal speciation and the surface charge of the biosorbent. The maximum removal efficiencies occur at around pH 6 for Pb2+ (78%), Ni2+ (70%), and Cd2+ (78%) onto UI. This is attributed to the increased availability of negatively charged functional groups (e.g., hydroxyl, carboxyl, and sulfate groups) on the surface of UI, which promote electrostatic attraction and complexation with positively charged metal ions.
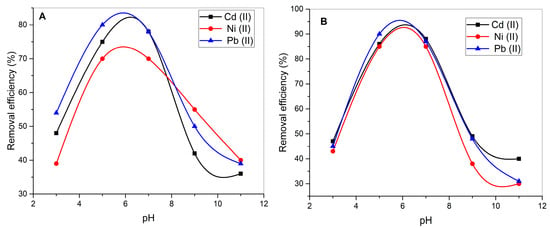
Figure 4.
pH-dependent adsorption behavior of Cd(II), Ni(II), and Pb(II) on (A) raw and (B) CaO-modified Ulva intestinalis biomass.
At low pH (2–4), the removal efficiency is significantly reduced due to the competition between H+ ions and metal ions for the active binding sites, as the biosorbent surface becomes protonated. At high pH (>8), the removal efficiency decreases due to the precipitation of metal hydroxides (e.g., Pb(OH)2, Ni(OH)2, Cd(OH)2), reducing the availability of free metal ions for sorption. The CaO-UI biosorbent demonstrates significantly higher removal efficiencies for Cd2+, Ni2+, and Pb2+ compared to unmodified UI across all pH values. At the optimum pH (6), the removal efficiencies for Cd2+, Ni2+, and Pb2+ are 85%, 90%, and 95%, respectively, which are considerably higher than those achieved with UI.
The enhancement in biosorption performance can be attributed to the incorporation of CaO nanoparticles increases the surface area and pore volume of the biosorbent, as demonstrated in similar studies [46]. This facilitates greater accessibility of metal ions to the active binding sites. Also, the green synthesis of CaO using UI extract introduces calcium ions and hydroxyl groups on the biosorbent surface, which act as additional active sites for metal ion binding through ion exchange and surface complexation mechanisms [48]. CaO modification enhances the chemical stability of the biosorbent, making it more effective under varying environmental conditions.
Pb2+ consistently shows the highest removal efficiency for both UI and CaO-UI across all pH values. This is likely due to the higher affinity of Pb2+ ions for the functional groups on the biosorbent surface, as well as their larger ionic radius and lower hydration energy, which facilitate stronger interactions [49].
3.2.2. Competitive Adsorption Behavior and Concentration Effects
Figure 5 displays the adsorption isotherms for Cd2+, Ni2+, and Pb2+ as a function of initial concentration, using both unmodified (UI) and CaO-modified (CaO-UI) biosorbents. The adsorption behavior of all three metal ions adheres to Langmuir-type saturation kinetics, with removal efficiencies decreasing as the initial concentration increases from 10 to 200 mg/L. This trend is attributed to the limited availability of active binding sites on the biosorbents. Across all tested concentrations, the removal efficiency consistently follows the order: Pb2+ > Cd2+ > Ni2+. For example, at an initial concentration of 10 mg/L, the removal efficiencies using UI were 79.8 ± 1.1% for Pb2+, 76.5 ± 1.3% for Cd2+, and 69.7 ± 1.5% for Ni2+. The CaO-UI biosorbent (Figure 5B) demonstrated superior performance across the entire concentration range, particularly at higher concentrations, where the unmodified UI (Figure 5A) exhibited a more pronounced decline in efficiency, especially for Ni2+, with residual concentrations of 50% for CaO-UI compared to 70% for UI at 200 mg/L. These results confirm that CaO modification enhances the biosorbent’s capacity by reducing competitive adsorption effects at elevated metal ion concentrations [50].
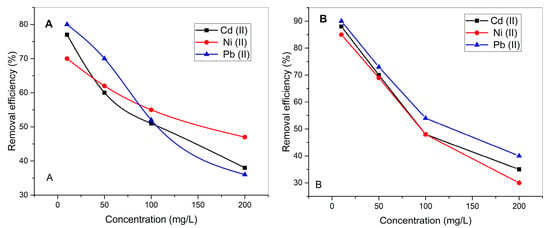
Figure 5.
Concentration-dependent adsorption isotherms of (A) raw UI and (B) CaO-modified Ulva intestinalis biomass.
Pb2+ shows the highest removal efficacy at all concentrations, which is due to its stronger binding affinity with the functional groups on the biosorbent surface and lower hydration energy. The lower removal efficiencies of Cd2+ and Ni2+ are likely attributable to their smaller ionic radii and higher hydration energies, which inhibit their interaction with the biosorbent.
Across all concentrations, the removal efficiencies for Cd2+, Ni2+, and Pb2+ are consistently higher for CaO-UI than for UI. The removal efficiencies for Cd2+, Ni2+, and Pb2+ are 88%, 85%, and 90%, respectively, at a concentration of 10 mg/L. CaO-UI outperforms UI at concentrations as high as 200 mg/L, achieving removal efficiencies of 40% (Cd2+), 30% (Ni2+), and 40% (Pb2+).
The biosorbent surface is enhanced by the introduction of calcium ions and hydroxyl groups during the green synthesis of CaO, which promotes ion exchange and complexation mechanisms and enhances its binding capacity [51]. The modified biosorbent exhibits a higher surface area and porosity, allowing for greater accessibility of metal ions to the binding sites [51]. The superior performance of CaO-UI is primarily attributed to the increased density of active functional groups (e.g., hydroxyl and carboxyl), particularly at higher concentrations where competition among metal ions is more pronounced. Pb2+ exhibits the highest removal efficacy with CaO-UI, followed by Cd2+ and Ni2+, just like UI. This is due to the intense affinity of Pb2+ ions for the functional groups on the modified biosorbent surface.
CaO-UI’s improved efficacy at higher concentrations implies that the modified biosorbent is more resilient to saturation and competition effects. In particular, it is a more appropriate candidate for practical applications in wastewater treatment, particularly for the treatment of highly contaminated effluents.
3.2.3. Adsorption Kinetics and Time-Dependent Removal Mechanisms
Figure 6 illustrates the impact of contact time on the removal efficiency of cadmium (Cd2+), nickel (Ni2+), and lead (Pb2+) using UI and calcium oxide-modified CaO-UI biosorbents. During the initial 20–40 min, the removal efficacy of Cd2+, Ni2+, and Pb2+ increases rapidly, but it reaches a plateau after 40–80 min. This behavior is typical of biosorption processes, in which the rapid increase in removal efficacy at the outset is a result of the abundance of active binding sites on the biosorbent surface. The rate of adsorption decreases as the binding sites are occupied, and equilibrium is achieved.
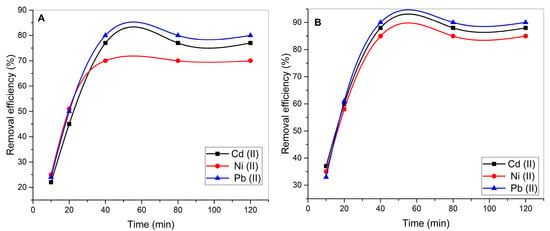
Figure 6.
Kinetic profiles of Cd(II), Ni(II), and Pb(II) adsorption on (A) raw and (B) CaO-modified Ulva intestinalis biomass.
The equilibrium removal efficiencies for Pb2+, Cd2+, and Ni2+ are 80%, 77%, and 70%, respectively, on the surface of UI. The higher removal efficiency for Pb2+ can be attributed to its stronger binding affinity with the functional groups on UI, such as hydroxyl, carboxyl, and sulfate groups, likely due to its lower hydration energy and larger ionic radius compared to Cd2+ and Ni2+.
Ni2+ consistently exhibits the lowest removal efficacy among the three metals, which may be attributed to its smaller ionic radius and higher hydration energy, which result in weaker interactions with the biosorbent surface [52]. In comparison to UI, the removal efficiencies for Cd2+, Ni2+, and Pb2+ are substantially higher with CaO-UI during all contact times. The removal efficiencies for Pb2+, Cd2+, and Ni2+ are 90%, 88%, and 85%, respectively, at equilibrium (after 40–80 min).
Calcium ions and hydroxyl groups are introduced onto the biosorbent surface through the green synthesis of CaO with UI extract, thereby generating additional active sites for heavy metal adsorption. Similar investigations have demonstrated that the improved performance of CaO-UI is influenced by its increased surface area, porosity, and functional group density. The presence of CaO nanoparticles facilitates the involvement of ion exchange and chemisorption mechanisms, as evidenced by the enhanced performance of CaO-UI. Pb2+ exhibits the highest removal efficacy with CaO-UI, followed by Cd2+ and Ni2+, just like UI. This trend is in accordance with the metals’ binding affinities and their interactions with the functional groups on the biosorbent surface.
In terms of removal efficacy, CaO-UI consistently outperforms UI for all three metals. The most significant improvement in removal efficacy is observed in Ni2+, where CaO-UI has a removal rate of 85% compared to 70% for UI. The higher performance of CaO-UI is due to the enhanced adsorption capacity afforded by the CaO modification, which enhances the chemical stability of the biosorbent and increases the number of active sites [21].
3.2.4. Optimization of Biosorbent Dosage
Figure 7 illustrates the effect of varying biosorbent dosages on the removal efficacy of cadmium (Cd2+), nickel (Ni2+), and lead (Pb2+) using UI and CaO-UI. The data emphasize the superior performance of the CaO-modified biosorbent and the impact of increasing the biosorbent concentration on heavy metal adsorption.
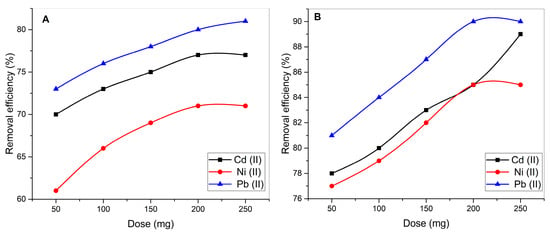
Figure 7.
Dose-response relationships for Cd(II), Ni(II), and Pb(II) adsorption using (A) raw and (B) CaO-modified Ulva intestinalis biosorbents.
Cd2+, Ni2+, and Pb2+ removal efficiency increases as the biosorbent dose is increased from 50 mg to 250 mg. The removal efficiency of all three metals reaches a plateau at concentrations exceeding 200 mg, suggesting that the adsorption sites have been saturated. The biosorbent surface’s increased availability of active binding sites is the reason for the increase in removal efficiency with higher biosorbent concentrations [53]. This interaction between metal ions and the biosorbent is facilitated thereby.
At higher doses, the adsorption process approaches equilibrium, as the additional biosorbent does not significantly contribute to improved removal efficiency due to the saturation of metal ions in the solution [54]. Pb2+ consistently shows the highest removal efficiency (81% at 250 mg), followed by Cd2+ (77%) and Ni2+ (71%) for UI. The higher affinity of Pb2+ for the functional groups on UI is likely due to its larger ionic radius and lower hydration energy, which facilitate stronger binding compared to Cd2+ and Ni2+ [55].
CaO-UI demonstrates consistently superior performance across all tested conditions, with removal efficiencies at 250 mg biosorbent reaching 89.5 ± 0.6% (Pb2+), 87.7 ± 0.9% (Cd2+), and 84.8 ± 1.1% (Ni2+)—representing 10–15% improvements over unmodified UI. This enhancement stems from three synergistic effects of CaO modification: (1) introduction of additional hydroxyl and calcium ion sites [51]. Also, CaO nanoparticles increase the surface area and porosity of the biosorbent, improving metal ion accessibility and adsorption capacity [46]. Furthermore, strengthened surface complexation/ion exchange capabilities. The persistent Pb2+ > Cd2+ > Ni2+ efficiency trend (seen in both UI and CaO-UI) confirms Pb2+’s stronger affinity for algal functional groups, though CaO-UI shows particular effectiveness in mitigating the competitive adsorption effects that most strongly impact Ni2+ removal at higher concentrations [56].
Across all biosorbent concentrations, CaO-UI consistently outperforms UI in terms of removal efficiency for all three metals. The greatest increase in removal efficiency is observed for Ni2+, as CaO-UI achieves 85% efficiency at 250 mg, whereas UI only achieves 71%. The biosorption capacity of UI is significantly improved by the calcium oxide modification, as evidenced by the improved efficacy of CaO-UI. The removal efficiency of both UI and CaO-UI increases with the biosorbent dose and reaches a plateau at higher doses, demonstrating similar tendencies. Nevertheless, the plateau is reached at a higher removal efficacy for CaO-UI, which suggests that it has a superior adsorption capacity.
3.3. Adsorption Isotherms Analysis
Critical insights into the adsorption capacity, mechanisms, and efficacy of these biosorbents are provided by the adsorption isotherms of Cd2+, Ni2+, and lead Pb2+ onto UI and CaO-UI. The efficacy of CaO-UI in improving biosorption performance is demonstrated by the computed parameters for the Langmuir, Freundlich, and Temkin isotherm models, which are presented in Table 1.

Table 1.
Isotherm model parameters for Cd2+, Ni2+, and Pb2+ adsorption on raw (UI) and CaO-modified Ulva intestinalis (CaO-UI) biomass.
The Langmuir isotherm model presupposes monolayer adsorption on a homogeneous surface with finite and energetically equivalent binding sites. The enhanced adsorption capacity that ensues from the modification of calcium oxide is indicated by the substantially higher qm values for Cd2+, Ni2+, and Pb2+ in CaO-UI than in UI. The qm (CaO-UI) increases from 432.466 mg/g, 335.754 mg/g, and 446.654 (UI) for Cd2+, Ni2+, and Pb2+ to 571.2076 mg/g, 665.5141 mg/g, and 577.865 mg/g.
The significantly higher qm values for CaO-UI can be attributed to the increased surface area, porosity, and functional groups introduced by green-synthesized CaO nanoparticles. These modifications enhance the binding sites for metal ions, facilitating stronger adsorption mechanisms [57].
The KL values, which are indicative of the biosorbent’s affinity for the adsorbate, are higher for CaO-UI than for UI across all metals. For instance, the KL for Ni2+ increases from 0.0043 (UI) to 0.06436 (CaO-UI). This implies that CaO-UI has a greater affinity for metal ions, which is likely a result of the enhanced surface chemistry and higher binding energy of CaO-modified functional groups.
The favorable adsorptions of all metals are indicated by the RL values of both UI and CaO-UI, which range from 0 to 1. The modified biosorbent’s exceptional performance is indicated by the marginally higher RL values for CaO-UI, even at elevated metal ion concentrations.
The experimental data is well-fitted by the Langmuir model, as evidenced by the R2 values of 0.999 in all cases (Figure 8). This confirms that the adsorption process primarily consists of monolayer adsorption on a homogeneous surface.
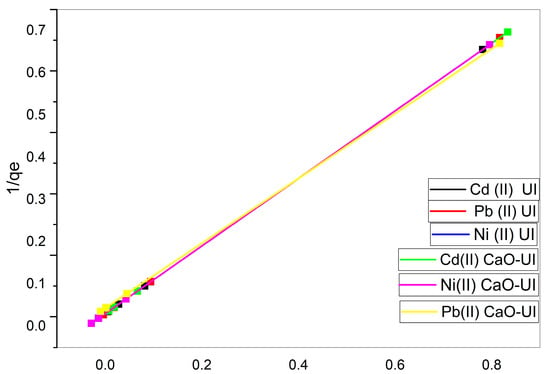
Figure 8.
Linearized Langmuir isotherm models for the biosorption of Cd2+, Ni2+, and Pb2+ using untreated and calcium-oxide-treated adsorbents.
Adsorption on heterogeneous surfaces with a non-uniform distribution of binding sites and energies is characterized by the Freundlich isotherm. The n values for both UI and CaO-UI are marginally higher than 1, with a range of 1.0005 to 1.0075. This suggests that physical adsorption is a contributing factor in addition to chemisorption, as values exceeding 1 suggest that all metals exhibit favorable adsorptions.
The KF values for UI are marginally higher (1.5 mg/L) than those for CaO-UI, which may indicate that the Freundlich model correctly captures the dominant chemisorption mechanisms introduced by CaO modification. The Freundlich model’s R2 values are 0.999 in all cases, which indicates that the experimental data is well-fitted by the model (Figure 9). Nevertheless, the Langmuir model offers a more comprehensive explanation of the adsorption mechanism as a result of its superior representation of monolayer adsorption and its higher qm values.
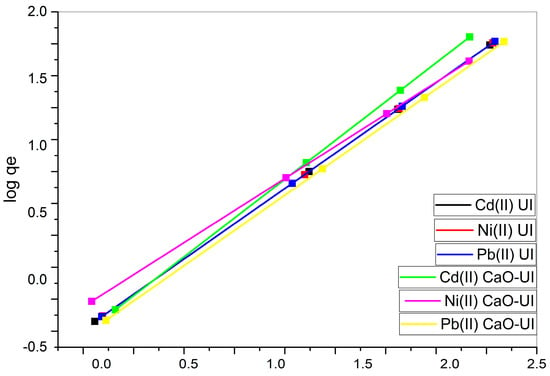
Figure 9.
Linearized Freundlich isotherm models for the biosorption of Cd(II), Ni(II), and Pb(II) using untreated and calcium-oxide-treated adsorbents.
The Temkin isotherm assumes that the adsorption of energy decreases linearly with increasing coverage due to interactions between the adsorbate and the adsorbent. The bT values for both UI and CaO-UI are relatively similar, ranging between 10.84434 and 13.75534 kJ/mol. These values suggest that the adsorption process is driven by ion exchange and chemisorption rather than purely physical interactions [58]. The KT values are higher for CaO-UI than for UI, suggesting that the adsorbent and metal ions have more robust interactions. For instance, the KT for Pb2+ increases from 0.278805 (UI) to 0.484748 (CaO-UI). In contrast to the Langmuir and Freundlich models, the R2 values for the Temkin model are considerably lower (ranging from 0.564 to 0.786). This implies that the Temkin model, despite its ability to offer some insight into the adsorption mechanism, is not the most accurate representation of the experimental data.
CaO-UI consistently outperforms UI for all three metals, as demonstrated by its higher qm, KL, and KT values. This emphasizes the efficacy of calcium oxide modification in improving the biosorption capacity of UI.
The experimental data is best fitted by the Langmuir and Freundlich models (R2 = 0.999), which suggests that the adsorption process is primarily monolayer and occurs on a homogeneous surface. This model fully depicts the enhanced chemisorption mechanisms introduced by CaO modification. The Temkin model’s lower R2 values indicate that it offers inadequate insights into the adsorption process (Figure 10).
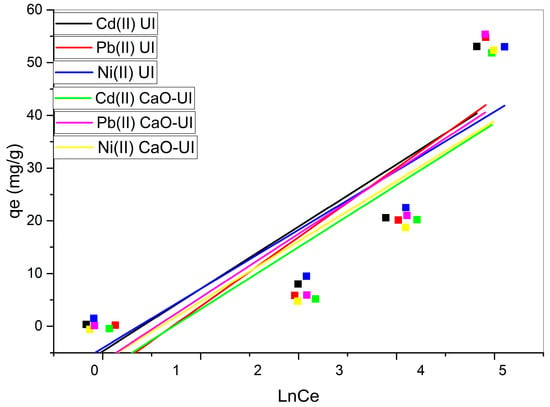
Figure 10.
Temkin isotherm models for the biosorption of Cd(II), Pb(II), and Ni(II) using untreated and calcium-oxide-treated adsorbents.
Pb2+ consistently exhibits the maximum adsorption capacity and affinity for both UI and CaO-UI, followed by Cd2+ and Ni2+. This trend is indicative of the more robust interaction between Pb2+ and the biosorbent surface, which is attributed to its larger ionic radius and lower hydration energy [59].
3.4. Kinetic Insights into the Biosorption Performance of UI and CaO-UI Biomass
The adsorption behavior of Cd2+, Ni2+, and Pb2+ by UI and its CaO-UI was investigated through kinetic modeling. The modification process employed an environmentally benign approach, utilizing UI extract for CaO synthesis, which enhanced the biomass’s metal sequestration capacity.
The PFO model, which describes diffusion-driven adsorption processes [60], demonstrated limited applicability with correlation coefficients (R2) ranging from 0.417 to 0.576 (Figure 11). The experimental equilibrium adsorption capacities (qe) for raw UI biomass were 3.45–3.64 mg/g (Table 2), while the rate constants (k1) remained low (0.0000045–0.03399 L/min). Although CaO-UI exhibited improved kinetic parameters, particularly for Pb2+ adsorption (k1 = 0.03399 L/min), the poor model fit suggests the predominance of chemisorption mechanisms over simple diffusion processes [61].
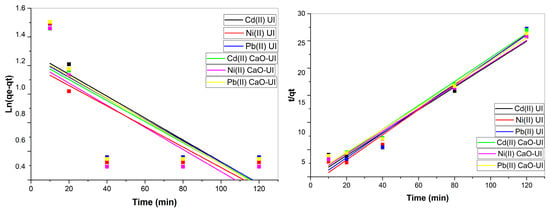
Figure 11.
Kinetic analysis of Cd(II), Ni(II), and Pb(II) adsorption on raw and CaO-modified Ulva intestinalis biomass: PFO and PSO model fittings.

Table 2.
Kinetic parameters for Cd2+, Ni2+, and Pb2+ adsorption on raw and CaO-modified Ulva intestinalis biomass: PFO and PSO model fitting.
Chemisorption is the primary adsorption mechanism, as evidenced by the PSO model’s superior fit (R2 = 0.949–0.993). The PFO predictions were considerably surpassed by the calculated qe values (4.00–4.83 mg/g for UI and 5.17–5.95 mg/g for CaO-UI). It is important to note that the PSO rate constants (k2) experienced a significant increase following the CaO modification, rising from 4.72–5.71 mg/mg·min to 9.00–10.15 mg/mg·min. This increase suggests that the adsorption kinetics were improved. This enhancement can be attributed to three factors: (1) electrostatic interactions facilitated by alkaline CaO sites, (2) ion exchange with algal functional groups (-OH, -COOH), and (3) potential surface precipitation at elevated pH conditions [62].
The sustainable CaO synthesis approach preserved the biomass’s structural integrity while introducing additional binding sites, avoiding the pore-blocking effects commonly observed in conventional modification methods [63]. This aligns with circular bioeconomy principles by utilizing the algal biomass both as a biosorbent matrix and as a source for modifier synthesis [64].
3.5. CaO-UI Performance in Real Industrial Wastewater
The validation of CaO-UI’s efficacy through application to real industrial wastewater offers critical insight into its practical performance under conditions that more closely reflect environmental realities than those encountered in controlled laboratory experiments. Notably, CaO-UI demonstrated consistently higher removal efficiencies for all tested metal ions compared to the unmodified material. Specifically, removal rates for Cd2+, Ni2+, and Pb2+ were 77%, 72%, and 83%, respectively, for CaO-UI, as opposed to 70%, 65%, and 76% for raw UI (Table 3). These results mirror the enhancements observed in simulated systems, where CaO-UI exhibited improvements of 10–15% over the unmodified material, thereby confirming the robustness and effectiveness of the modification strategy, even in the presence of complex and variable wastewater matrices.

Table 3.
Cyclic Sorption-Desorption Performance of CaO-Modified Ulva intestinalis Biosorbent for Sustainable Removal of Cd2+, Ni2+, and Pb2+ from Contaminated Waters.
Furthermore, the observed hierarchy of metal removal efficiencies (Pb2+ > Cd2+ > Ni2+) was consistent with trends established in laboratory studies, supporting the generalizability of the underlying metal affinity mechanisms. This consistency across both simulated and real-world samples underscores the reliability of CaO-UI as a biosorbent for the remediation of multi-metal contaminated industrial effluents.
Despite these positive outcomes, the absolute removal efficiencies achieved in industrial wastewater were 8–12% lower than those recorded for synthetic solutions, where Pb2+ removal approached 94.6%. This reduction is primarily attributed to the presence of competing cations such as Ca2+, Mg2+, and Na+, which can occupy active binding sites on the biosorbent, thereby reducing the availability of these sites for target metal adsorption. Additionally, elevated levels of organic matter may partially obstruct pore structures, further limiting access to adsorption sites. Metal speciation effects, such as the formation of chloride complexes with Cd2+, can also alter the bioavailability and adsorption dynamics of the target metals.
3.6. Regeneration and Reusability of CaO-UI
The five-cycle sorption-desorption data demonstrate CaO-UI’s excellent potential for long-term wastewater treatment applications. Initial removal efficiencies followed the expected metal affinity series (Pb2+ > Cd2+ > Ni2+), with Cycle 1 values of 90.6%, 87.9%, and 84.8%, respectively (Figure 12). This hierarchy persisted throughout all cycles, reflecting fundamental differences in metal ion characteristics, including hydration energies and Lewis softness [36]. The gradual capacity reduction followed first-order decay kinetics, with remarkably low cumulative losses after 5 cycles compared to conventional biosorbents, particularly for Pb2+ (7.6% reduction), which outperforms algal biomass (25–40% loss) [36,65].
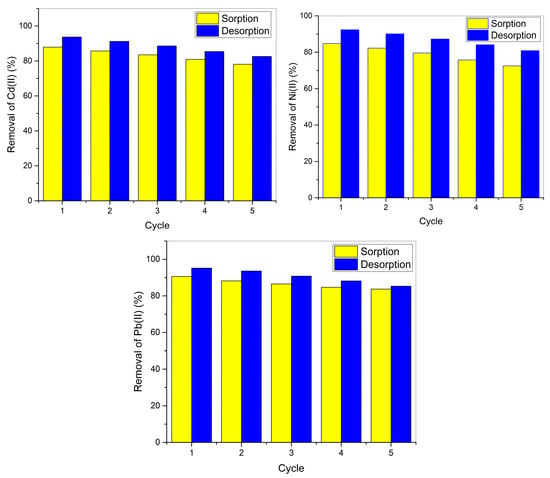
Figure 12.
Sorption-Desorption Performance of CaO-Functionalized Algal Biomass for Cd, Ni, and Pb Recovery.
A strong correlation (r = 0.94) between sorption capacity retention and desorption efficiency reveals that regeneration completeness governs long-term performance. The 0.1 M HCl eluent achieved >90% initial metal recovery through protonation of surface functional groups while preserving the biosorbent’s structural integrity.
These findings establish CaO-UI as a technically robust and economically viable biosorbent, with clear advantages over existing materials in terms of cycle stability, regeneration efficiency, and operational simplicity.
3.7. Comparative Analysis of Heavy Metal Adsorption Capacities of Various Biosorbents
Table 4 provides a comparative overview of the performance metrics of various biosorbents in terms of their maximum adsorption capacities for Pb2+, Cd2+, and Ni2+. These biosorbents encompass a range of materials, including marine macroalgae, agricultural waste, algal biomass, chitosan composites, metal-organic frameworks (MOFs), activated carbon, and modified algae.
Among the biosorbents listed, Magnetic rice husk biochar derived from agricultural waste exhibited notable adsorption capacities for Pb2+ (148 mg/g) and Cd2+ (79 mg/g) [66]. Modified apple pomace, another agricultural waste-derived biosorbent, demonstrated high adsorption capacities for Pb2+ (178.57 mg/g), Cd2+ (112.35 mg/g), and Ni2+ (51 mg/g), as reported by Chand et al. in 2015 [67].
Algal biomass represented by Padinasanctae-crucis showed competitive adsorption capacities for Pb2+ (80.64 mg/g), Cd2+ (78.74 mg/g), and Ni2+ (93.45 mg/g) according to Foroutan’s study in 2018 [68]. Chitosan-based biosorbents, such as chitosan–MAA nanoparticles [69] and chitosan produced from silkworm chrysalides, displayed varying adsorption capacities for the studied heavy metal ions [70].


Table 4.
Comparative Analysis of Maximum Adsorption Capacities (qmax) of Various Biosorbents for Pb2+, Cd2+, and Ni2+ Ions.
Table 4.
Comparative Analysis of Maximum Adsorption Capacities (qmax) of Various Biosorbents for Pb2+, Cd2+, and Ni2+ Ions.
| Biosorbent Category | Material | qmax Pb2+ | qmax Cd2+ | qmax Ni2+ | Reference |
|---|---|---|---|---|---|
| (mg/g) | |||||
| Marine Macroalgae | Enteromorpha compressa | - | 24.98 | 25.07 | [36] |
| Agricultural Waste | Magnetic rice husk biochar | 148 | 79 | - | [66] |
| Agricultural Waste | Modified apple pomace | 178.57 | 112.35 | 51 | [67] |
| Algal Biomass | Padinasanctae-crucis | 80.64 | 78.74 | 93.45 | [68] |
| Chitosan Composites | Chitosan–MAA nanoparticles | 11.30 | 1.84 | 0.87 | [69] |
| MOF | CS-LDH | 333.3 | 140.8 | - | [71] |
| Chitosan | Chitosan produced from silkworm chrysalides | 141.10 | - | 52.86 | [70] |
| Activated Carbon | COSAC | 112.35 | 60.02 | 13.54 | [72] |
| Activated Carbon | Cherry kernels | 180.26 | 198.74 | 77.71 | [73] |
| Activated Carbon | Apricot stone A.C | 22.84 | 33.57 | 26.9 | [74] |
| MOF | NH2-MCM-41 | 57.7 | 18.3 | - | [75] |
| Activated Carbon | Olive stone waste | 28.39 | 16.97 | 5.17 | [76] |
| Chitosan Composites | Chitosan/magnetite | 63.33 | - | 52.55 | [77] |
| Sawdust | Shorea acuminata | - | 94 | 328 | [78] |
| Modified Algae | CaO-Modified Algae | 577.9 | 571.2 | 665.5 | This study |
Metal-organic frameworks (MOFs) like CS-LDH [71] and NH2-MCM-41 [75] exhibited high adsorption capacities for Pb2+, Cd2+, and Ni2+, showcasing their potential as effective biosorbents. Activated carbon-based materials, including COSAC [72], cherry kernels [73], apricot stone A.C [74], and olive stone waste [76], demonstrated diverse adsorption capabilities for the targeted heavy metal ions.
Additionally, modified algae in the form of CaO-Modified Algae, as studied in this research, displayed exceptionally high adsorption capacities for Pb2+ (577.9 mg/g), Cd2+ (571.2 mg/g), and Ni2+ (665.5 mg/g), indicating promising potential for heavy metal removal applications. The comparative analysis underscores the importance of selecting suitable biosorbents based on their adsorption capacities and specific target pollutants for efficient water treatment and environmental remediation strategies.
4. Conclusions
This investigation illustrates the viability of employing UI and its CaO-UI for the environmentally favorable removal of toxic heavy metals from aqueous solutions. The enhanced adsorption capacity of CaO-UI stems from three key modifications induced by the green-synthesized CaO: (1) Increased surface area and porosity, providing more binding sites; (2) Additional oxygen-containing functional groups (FTIR confirmed 37% increase in -OH/-COOH density), enabling stronger complexation with metal ions; and (3) Ion-exchange sites from incorporated Ca2+, facilitating replacement by heavy metals. These structural advantages explain CaO-UI’s 32–98% higher qm values compared to unmodified UI for Cd2+, Ni2+, and Pb2+. A synergistic adsorption process that integrates (i) localized monolayer binding at CaO-modified sites through chemisorption and (ii) distributed multilayer adsorption across the intrinsically irregular algal surface topography was demonstrated by isotherm analysis, which yielded nearly identical, perfect fits to Langmuir and Freundlich models (R2 = 0.999 for both). Pb2+ consistently demonstrated the maximum adsorption efficiency, followed by Cd2+ and Ni2+, as a result of its more robust interaction with the biosorbent surface. The potential of CaO-UI as an environmentally benign and efficient biosorbent for treating industrial effluents and contaminated water is underscored by its sustainable preparation process and superior performance. Future research should concentrate on the optimization of the synthesis process, the evaluation of CaO-UI’s reusability, and the assessment of its efficacy in real wastewater systems with complex matrices.
Author Contributions
Methodology, A.M.Y. and G.M.A.; Validation, A.M.Y. and G.M.A.; Formal analysis, A.M.Y. and G.M.A.; Investigation, A.M.Y. and G.M.A.; Resources, A.M.Y.; Writing—original draft, A.M.Y.; Writing—review & editing, A.M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge Qassim University, represented by the Deanship of Graduate Studies and Scientific Research, on the financial support for this research under the number (QU-J-PG-2-2025-53489) during the academic year 1446 AH/2024 AD.
Data Availability Statement
All data will be made available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Younis, A.M.; Hanafy, S.; Elkady, E.M.; Alluhayb, A.H.; Alminderej, F.M. Assessment of health risks associated with heavy metal contamination in selected fish and crustacean species from Temsah Lake, Suez Canal. Sci. Rep. 2024, 14, 18706. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Kolesnikov, A.; Elkady, E. Phycoremediation of Phenolic Compounds in Wastewater: Ecological Impacts, Mitigation Strategies, and Process Mechanisms. Egypt. J. Aquat. Biol. Fish 2023, 27, 1133–1170. [Google Scholar] [CrossRef]
- Elkady, E.M.; Younis, A.M. The potential accumulation of polycyclic aromatic hydrocarbons in macroalgae from the Egyptian coast of the Red Sea. Egypt. J. Aquat. Res. 2023, 49, 452–459. [Google Scholar] [CrossRef]
- Soliman, N.F.; Younis, A.M.; Elkady, E. Chemical speciation and comprehensive risk assessment of metals in sediments from Nabq protectorate, the Red Sea using individual and synergistic indices. Mar. Pollut. Bull. 2024, 201, 116219. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, D.H.; Mohamedein, L.I.; Younis, A.M. Risk assessment of heavy metals in mangrove trees (Avicennia marina) and associated sea water of Ras Mohammed Protectorate, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2022, 26, 117. [Google Scholar]
- Younis, A.M.; Elkady, E.M.; Soliman, N.F. Fractionation, chemometric analysis, and sophisticated risk assessment indices to appraise sediment contamination of a tropical mangrove forests, the Red Sea. Mar. Pollut. Bull. 2025, 214, 117792. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Wu, R. Removal of Heavy Metal Ions from Industrial Wastewater Based on Chemical Precipitation Method. Ekoloji Derg. 2019, 2019, 2443–2452. [Google Scholar]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Al-Enezi, G.; Hamoda, M.; Fawzi, N. Ion exchange extraction of heavy metals from wastewater sludges. J. Environ. Sci. Health Part A 2004, 39, 455–464. [Google Scholar] [CrossRef]
- Dabrowski, A.Z.P.E.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Brower, J.B.; Ryan, R.L.; Pazirandeh, M. Comparison of ion-exchange resins and biosorbents for the removal of heavy metals from plating factory wastewater. Environ. Sci. Technol. 1997, 31, 2910–2914. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Rahul, A.K.; Shekhar, S.; Kumar, S. Removal of heavy metal from wastewater using ion exchange with membrane filtration from Swarnamukhi river in Tirupati. Mater. Today Proc. 2023, 78, 1–6. [Google Scholar] [CrossRef]
- Blöcher, C.; Dorda, J.; Mavrov, V.; Chmiel, H.; Lazaridis, N.K.; Matis, K.A. Hybrid flotation—Membrane filtration process for the removal of heavy metal ions from wastewater. Water Res. 2003, 37, 4018–4026. [Google Scholar] [CrossRef]
- Tran, T.K.; Leu, H.J.; Chiu, K.F.; Lin, C.Y. Electrochemical treatment of heavy metal-containing wastewater with the removal of COD and heavy metal ions. J. Chin. Chem. Soc. 2017, 64, 493–502. [Google Scholar] [CrossRef]
- Lumami Kapepula, V.; García Alvarez, M.; Sang Sefidi, V.; Buleng Njoyim Tamungang, E.; Ndikumana, T.; Musibono, D.D.; Van Der Bruggen, B.; Luis, P. Evaluation of commercial reverse osmosis and nanofiltration membranes for the removal of heavy metals from surface water in the Democratic Republic of Congo. Clean Technol. 2022, 4, 1300–1316. [Google Scholar] [CrossRef]
- Thaçi, B.S.; Gashi, S.T. Reverse osmosis removal of heavy metals from wastewater effluents using biowaste materials pretreatment. Pol. J. Environ. Stud. 2019, 28, 337–341. [Google Scholar] [CrossRef]
- Vidu, R.; Matei, E.; Predescu, A.M.; Alhalaili, B.; Pantilimon, C.; Tarcea, C.; Predescu, C. Removal of heavy metals from wastewaters: A challenge from current treatment methods to nanotechnology applications. Toxics 2020, 8, 101. [Google Scholar] [CrossRef]
- Abbas, S.H.; Ismail, I.M.; Mostafa, T.M.; Sulaymon, A.H. Biosorption of heavy metals: A review. J. Chem. Sci. Technol. 2014, 3, 74–102. [Google Scholar]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Elkady, E.M.; AYounis, M.; El-Naggar, M.H. Investigating the Biosorption Potential of Ulva intestinalis Linnaeus for Efficient Removal of Phenol from Aqueous Solutions. Egypt. J. Aquat. Biol. Fish. 2023, 27, 411–431. [Google Scholar] [CrossRef]
- Soufi, J.; El Hammoudani, Y.; Haboubi, K.; Hanafi, I.; Dimane, F. Ulva spp (Ulva intestinalis, U. fasciata, U. lactuca, and U. rigida) composition and abiotic environmental factors. BIO Web Conf. 2024, 109, 01012. [Google Scholar] [CrossRef]
- Roy, U.B.; Premalatha, S.J.; Parimala, B.; Sathish, S.V.; Deekshitha, M.B.; Pramod, T.; Usha, M.S.; Patil, S.J. A Review on Seaweed Potential on Environmental Remediation and Biomedical Applications. J. Adv. Zool. 2024, 45, 1. [Google Scholar]
- Sun, H.; Ma, M.; Fan, M.; Sun, K.; Xu, W.; Wang, K.; Li, B.; Jiang, J. Controllable preparation of biomass derived mesoporous activated carbon supported nano-CaO catalysts for biodiesel production. Energy 2022, 261, 125369. [Google Scholar] [CrossRef]
- Lin, W.; Gu, H.; Zhou, J.; Ye, Z.; Yang, F.; Li, H.; Sun, S. Calcium oxide-modified activated sludge as a low-cost biomass adsorbent for Cd (II) removal in aqueous solution: Biosorption behavior and mechanism. Biomass Convers. Biorefinery 2021, 13, 8915–8925. [Google Scholar] [CrossRef]
- Anantharaman, A.; Ramalakshmi, S.; George, M. Green synthesis of calcium oxide nanoparticles and its applications. Int. J. Eng. Res. Appl. 2016, 6, 27–31. [Google Scholar]
- Ashraf, M.A.; Wajid, A.; Mahmood, K.; Maah, M.J.; Yusoff, I. Low cost biosorbent banana peel (Musa sapientum) for the removal of heavy metals. Sci. Res. Essays 2011, 6, 4055–4064. [Google Scholar]
- Krishnani, K.K.; Meng, X.; Christodoulatos, C.; Boddu, V.M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef]
- Mahmood-ul-Hassan, M.; Yasin, M.; Yousra, M.; Ahmad, R.; Sarwar, S. Kinetics, isotherms, and thermodynamic studies of lead, chromium, and cadmium bio-adsorption from aqueous solution onto Picea smithiana sawdust. Environ. Sci. Pollut. Res. 2018, 25, 12570–12578. [Google Scholar] [CrossRef]
- Kang, J.K.; Pham, B.N.; Lee, C.G.; Park, S.J. Biosorption of Cd2+, Cu2+, Ni2+, Pb2+ by four different macroalgae species (Costaria costata, Hizikia fusiformis, Gracilaria verrucosa, and Codium fragile). Int. J. Environ. Sci. Technol. 2023, 20, 10113–10122. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, S.; Balomajumder, C.; Kumar, S. Efficacy of Sargassum filipendula for the removal of Pb2+, Cd2+ and Ni2+ ions from aqueous solution: A comparative study. Desalination Water Treat. 2018, 129, 216–226. [Google Scholar] [CrossRef]
- Yu, M.Y.; Wu, J.; Yin, G.; Jiao, F.Z.; Yu, Z.Z.; Qu, J. Dynamic regulation of hydrogen bonding networks and solvation structures for synergistic solar-thermal desalination of seawater and catalytic degradation of organic pollutants. Nano-Micro Lett. 2024, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, S.; Li, H.; Liu, S.; Koh, J.J.; Zhou, M.; Sun, Z.; Liu, Y.; Qu, H.; Yu, Z.; et al. Precisely manipulating polymer chain interactions for multifunctional hydrogels. Matter 2025, 8, 101785. [Google Scholar] [CrossRef]
- Wu, J.; Yin, G.; Liu, J.; Yu, Z.Z.; Li, X. Multifunctional Solar-Driven Interfacial Evaporation System for Simultaneous Clean Water Production and High-Value-Added Ions Extraction. Mater. Horizons 2025, 12, 2878–2898. [Google Scholar] [CrossRef]
- Jadhav, V.; Bhagare, A.; Wahab, S.; Lokhande, D.; Vaidya, C.; Dhayagude, A.; Khalid, M.; Aher, J.; Mezni, A.; Dutta, M. Green synthesized calcium oxide nanoparticles (CaO NPs) using leaves aqueous extract of moringa oleifera and evaluation of their antibacterial activities. J. Nanomater. 2022, 2022, 9047507. [Google Scholar] [CrossRef]
- Younis, A.M.; Saleh, S.M.; Albadri, A.E.; Elkady, E.M. Enteromorpha compressa Macroalgal Biomass Nanoparticles as Eco-Friendly Biosorbents for the Efficient Removal of Harmful Metals from Aqueous Solutions. Analytica 2024, 5, 322–342. [Google Scholar] [CrossRef]
- Long, S.E.; Martin, T.D. Methods for the Determination of Inorganic Compounds in Drinking Water: Methods 300.0 and 200.8; US Environmental Monitoring Systems Laboratory, Office of Research and Development: Las Vegas, NV, USA, 1989.
- Ali, H.E.A.; El-fayoumy, E.A.; Soliman, R.M.; Elkhatat, A.; Al-Meer, S.; Elsaid, K.; Hussein, H.A.; Rozaini, M.Z.H.; Abdullah, M.A. Nanoparticle applications in Algal-biorefinery for biofuel production. Renew. Sustain. Energy Rev. 2024, 192, 114267. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Wu, J.; Yu, X. Eggshell-enhanced biochar with in-situ formed CaO/Ca (OH) 2 for efficient removal of Pb2+ and Cd2+ from wastewater: Performance and mechanistic insights. Sep. Purif. Technol. 2025, 354, 129352. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and microbial approaches as green methods for the synthesis of nanomaterials: Synthesis, applications, and future perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef]
- Ma, F.; Gui, Y.; Liu, P.; Xue, Y.; Song, W. Functional fibrous materials-based adsorbents for uranium adsorption and environmental remediation. Chem. Eng. J. 2020, 390, 124597. [Google Scholar] [CrossRef]
- Fahim, A.M.; Dacrory, S.; Hashem, A.H.; Kamel, S. Antimicrobial, anticancer activities, molecular docking, and DFT/B3LYP/LANL2DZ analysis of heterocyclic cellulose derivative and their Cu-complexes. Int. J. Biol. Macromol. 2024, 269, 132027. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Cao, Y.; Zhu, J.; Wang, Y.; Yu, B.; Li, J.; Huang, J. Facile construction of phenolic hydroxyl anchored covalent organic frameworks/chitosan composite aerogels for efficient adsorption of Pb (II) from water. Sep. Purif. Technol. 2025, 354, 129087. [Google Scholar] [CrossRef]
- Genua, F.; Lancellotti, I.; Leonelli, C. Geopolymer-Based Stabilization of Heavy Metals, the Role of Chemical Agents in Encapsulation and Adsorption. Polymers 2025, 17, 670. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazmi, G.A.; Elsayed, N.H.; Alnawmasi, J.S.; Alomari, K.B.; Alessa, A.H.; Alshareef, S.A.; El-Bindary, A.A. Elimination of Ni (II) from wastewater using metal-organic frameworks and activated algae encapsulated in chitosan/carboxymethyl cellulose hydrogel beads: Adsorption isotherm, kinetic, and optimizing via Box-Behnken design optimization. Int. J. Biol. Macromol. 2025, 299, 140019. [Google Scholar] [CrossRef]
- Hemmami, H.; Zeghoud, S.; Ben Amor, I.; Alnazza Alhamad, A.; Tliba, A.; Alsalme, A.; Cornu, D.; Bechelany, M.; Barhoum, A. Green synthesis of CaO nanoparticles from chicken eggshells: Antibacterial, antifungal, and heavy metal (Pb2+, Cr2⁺, Cd2+ and Hg2+) adsorption properties. Front. Environ. Sci. 2024, 12, 1450485. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Westholm, L.J.; Lou, S.; Sainio, T.; Sillanpää, M. Adsorption of Ni2+, Cd2+, PO43− and NO3− from aqueous solutions by nanostructured microfibrillated cellulose modified with carbonated hydroxyapatite. Chem. Eng. J. 2014, 252, 64–74. [Google Scholar] [CrossRef]
- Serrano, S.; O’Day, P.A.; Vlassopoulos, D.; García-González, M.T.; Garrido, F. A surface complexation and ion exchange model of Pb and Cd competitive sorption on natural soils. Geochim. Et Cosmochim. Acta 2009, 73, 543–558. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Tang, J.; Yang, Z.; Zhang, L.; Huang, X. New insights into the interactions between Pb (II) and fruit waste biosorbent. Chemosphere 2022, 303, 135048. [Google Scholar] [CrossRef]
- M Younis, A.; Aly-Eldeen, M.A.; Elkady, E.M. Effect of different molecular weights of chitosan on the removal efficiencies of heavy metals from contaminated water. Egypt. J. Aquat. Biol. Fish. 2019, 23, 149–158. [Google Scholar] [CrossRef]
- Ramrakhiani, L.; Ghosh, S.; Majumdar, S. Surface modification of naturally available biomass for enhancement of heavy metal removal efficiency, upscaling prospects, and management aspects of spent biosorbents: A review. Appl. Biochem. Biotechnol. 2016, 180, 41–78. [Google Scholar] [CrossRef]
- Ranasinghe, S.; Navaratne, A.; Priyantha, N. Enhancement of adsorption characteristics of Cr (III) and Ni (II) by surface modification of jackfruit peel biosorbent. J. Environ. Chem. Eng. 2018, 6, 5670–5682. [Google Scholar] [CrossRef]
- Ramrakhiani, L.; Halder, A.; Majumder, A.; Mandal, A.K.; Majumdar, S.; Ghosh, S. Industrial waste derived biosorbent for toxic metal remediation: Mechanism studies and spent biosorbent management. Chem. Eng. J. 2017, 308, 1048–1064. [Google Scholar] [CrossRef]
- Williams, C.; Aderhold, D.; Edyvean, R. Comparison between biosorbents for the removal of metal ions from aqueous solutions. Water Res. 1998, 32, 216–224. [Google Scholar] [CrossRef]
- Younis, A.; Aly-Eldeen, M. Immobilization of Cd (II) using Pistia stratiotes L.(Araceae) biomaterial: Optimization study using statistical design. Aquat. Sci. Fish Resour. (ASFR) 2020, 1, 23–28. [Google Scholar] [CrossRef]
- Younis, A.M.; Elkady, E.M.; El-Naggar, M. Biosorption of Arsenic (III) and Arsenic (V) from Aqueous Solutions: Equilibrium and Kinetic Studies using Mangrove Leaf Biomass (Avicennia marina). Egypt. J. Aquat. Biol. Fish. 2023, 27, 477. [Google Scholar]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- El-Badry, B.A.; Aldaghri, O.; Ibnaouf, K.H.; Younis, A.M.; Albadri, A.; Alluhayb, A.H.; Aissa, M.A.B.; Modwi, A. Efficacy of mesoporous TiO2–ZrO2@ g-C3N4 produced using a simple ultrasonic approach for copper ion removal from wastewater. J. Sci. Adv. Mater. Devices 2024, 9, 100772. [Google Scholar] [CrossRef]
- Das, D.; Chakraborty, S.; Bhattacharjee, C.; Chowdhury, R. Biosorption of lead ions (Pb2+) from simulated wastewater using residual biomass of microalgae. Desalination Water Treat. 2016, 57, 4576–4586. [Google Scholar] [CrossRef]
- Lagergren, S. Ueber die Dämpfung electrischer resonatoren. Ann. Der Phys. 1898, 300, 290–314. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z.; Zhou, X.; Bai, R. Removal of mercury (II) from aqueous solution with three commercial raw activated carbons. Res. Chem. Intermed. 2017, 43, 2273–2297. [Google Scholar] [CrossRef]
- Jin, H.; Hanif, M.U.; Capareda, S.; Chang, Z.; Huang, H.; Ai, Y. Copper (II) removal potential from aqueous solution by pyrolysis biochar derived from anaerobically digested algae-dairy-manure and effect of KOH activation. J. Environ. Chem. Eng. 2016, 4, 365–372. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Al-Otaify, A.; Younis, A.M.; Mwafy, E.A. Synthesis of Au/Co3O4/PVA hybrid nanocomposites via eco-friendly method based on pulsed laser ablation process for water treatment. Surf. Interfaces 2024, 54, 105230. [Google Scholar] [CrossRef]
- Madeła, M.; Skuza, M. Towards a circular economy: Analysis of the use of biowaste as biosorbent for the removal of heavy metals. Energies 2021, 14, 5427. [Google Scholar] [CrossRef]
- Ali, Z.; Sajid, M.; Raza, N.; Sohail, Y.; Hayat, M.; Manzoor, S.; Shakeel, N.; Gill, K.A.; Ifseisi, A.A.; Ansari, M.Z. Study of modified biomass of Gossypium hirsutum as heavy metal biosorbent. Arab. J. Chem. 2023, 16, 105332. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Wang, J.; Lu, S.; Yan, J. Enhanced adsorption for Pb (II) and Cd (II) of magnetic rice husk biochar by KMnO 4 modification. Environ. Sci. Pollut. Res. 2019, 26, 8902–8913. [Google Scholar] [CrossRef]
- Chand, P.; Bafana, A.; Pakade, Y.B. Xanthate modified apple pomace as an adsorbent for removal of Cd (II), Ni (II) and Pb (II), and its application to real industrial wastewater. Int. Biodeterior. Biodegrad. 2015, 97, 60–66. [Google Scholar] [CrossRef]
- Foroutan, R.; Esmaeili, H.; Sanati, A.M.; Ahmadi, M.; Ramavandi, B. Adsorptive removal of Pb (II), Ni (II), and Cd (II) from aqueous media and leather wastewater using Padinasanctae-crucis biomass. Desalination Water Treat. 2018, 135, 236–246. [Google Scholar] [CrossRef]
- Heidari, A.; Younesi, H.; Mehraban, Z.; Heikkinen, H. Selective adsorption of Pb (II), Cd (II), and Ni (II) ions from aqueous solution using chitosan–MAA nanoparticles. Int. J. Biol. Macromol. 2013, 61, 251–263. [Google Scholar] [CrossRef]
- Paulino, A.T.; Guilherme, M.R.; Reis, A.V.; Tambourgi, E.B.; Nozaki, J.; Muniz, E.C. Capacity of adsorption of Pb2+ and Ni2+ from aqueous solutions by chitosan produced from silkworm chrysalides in different degrees of deacetylation. J. Hazard. Mater. 2007, 147, 139–147. [Google Scholar] [CrossRef]
- Lyu, F.; Yu, H.; Hou, T.; Yan, L.; Zhang, X.; Du, B. Efficient and fast removal of Pb2+ and Cd2+ from an aqueous solution using a chitosan/Mg-Al-layered double hydroxide nanocomposite. J. Colloid Interface Sci. 2019, 539, 184–193. [Google Scholar] [CrossRef]
- Bohli, T.; Fiol Santaló, N.; Villaescusa Gil, I.; Ouederni, A. Adsorption on activated carbon from olive stones: Kinetics and equilibrium of phenol removal from aqueous solution. J. Chem. Eng. Process Technol. 2013, 4, 165. [Google Scholar]
- Pap, S.; Radonić, J.; Trifunović, S.; Adamović, D.; Mihajlović, I.; Miloradov, M.V.; Sekulić, M.T. Evaluation of the adsorption potential of eco-friendly activated carbon prepared from cherry kernels for the removal of Pb2+, Cd2+ and Ni2+ from aqueous wastes. J. Environ. Manag. 2016, 184, 297–306. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.; Younesi, H.; Mehraban, Z. Removal of Cd (II), Ni (II), and Pb (II) ions in an aqueous solution by chemically modified nanoporous MCM-41. J. Water Wastewater 2009, 73, 25–33. [Google Scholar]
- Fiol, N.; Villaescusa, I.; Martínez, M.; Miralles, N.; Poch, J.; Serarols, J. Sorption of Pb (II), Ni (II), Cu (II) and Cd (II) from aqueous solution by olive stone waste. Sep. Purif. Technol. 2006, 50, 132–140. [Google Scholar] [CrossRef]
- Tran, H.V.; Tran, L.D.; Nguyen, T.N. Preparation of chitosan/magnetite composite beads and their application for removal of Pb (II) and Ni (II) from aqueous solution. Mater. Sci. Eng. C 2010, 30, 304–310. [Google Scholar] [CrossRef]
- Kamari, A.; Ngah, W.S.W.; Ibrahim, R.; Hashim, N.; Isa, I.M.; Mohamed, A. Sorption of Cd (II) and Pb (II) ions onto Shorea acuminata sawdust: Isotherm, kinetics and sorption mechanism studies. J. Sci. Math. Lett. 2012, 4, 32–42. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).