Abstract
This work focuses on the effectiveness of removing ammonium from real municipal wastewater using synthetic faujasite (FAU-type) and β (BEA-type) zeolites and a commercial β (BEA-type) sample. The results demonstrated that synthetic samples presented enhanced performance on ammonium removal in comparison with commercial zeolite due to higher Al content and larger specific surface area, promoting better accessibility to active adsorption sites of the adsorbents. Synthetic FAU-type and BEA-type zeolites achieved a maximum adsorption capacity of 28.87 and 12.62 mg·g−1, respectively, outperforming commercial BEA-type zeolite (6.50 mg·g−1). Adsorption assays, associated with kinetic studies and adsorption isotherms, were better fitted using the pseudo-second order model and the Langmuir model, respectively, suggesting that chemisorption, involving ion exchange, and monolayer formation at the zeolite surface, was the main mechanism involved in the NH4+ adsorption process. After ammonium adsorption, the NH4+-loaded zeolite samples were used to stimulate the growth of tomato seedlings; the results revealed a change in the biomass production for seedlings grown in vitro, especially when the BEA_C_NH4 sample was employed, leading to a 15% increase in the fresh mass in comparison with the control sample. In contrast, the excess of ammonium adsorbed over the BEA_S_NH4 and FAU_NH4 samples probably caused a toxic effect on seedling growth. The elemental analysis results supported the hypothesis that the presence of NH4+-loaded zeolite into the culture medium was important for the release of nitrogen. The obtained results show then that the investigated zeolites are promising both as efficient adsorbents to mitigate the environmental impact of ammonium-contaminated water bodies and as nitrogen-rich fertilizers.
1. Introduction
Water is an indispensable resource for life, present in essential physiological processes, in addition to functioning as a habitat and ecological niche for countless animal and plant species. Nitrogen from sewage effluents, when discharged into water bodies, becomes a nutrient for aquatic plants, causing eutrophication of rivers [1,2,3,4]. In the form of ammoniacal nitrogen or ammoniacal-N (NH4+ and NH3), it is easily oxidized by nitrifying organisms that consume dissolved oxygen, making aerobic aquatic life unviable [5,6].
At pH values near 7.5, NH4+ ions are dominant (99%), with only 1% of NH3, as estimated by speciation analysis [7,8]. Raising the pH tends to transform NH4+ ions into NH3. Ammoniacal-N is a parameter included in Brazilian federal legislation concerning wastewater, for which CONAMA Resolution 430/2011 has established a maximum ammoniacal-N concentration of 20 mg·L−1 [9].
Biological treatment is the most cost-effective and successful NH4+ remediation approach by nitrification-denitrification, which consists of converting ammonium to nitrate using aerobic bacteria, followed by the transformation of nitrate into nitrogen gas under anoxic conditions [7,10,11]. However, some drawbacks, including high costs, secondary pollution, and labor intensity, are involved in the process. Therefore, low-cost and easy-to-apply technologies offering an environmentally friendly and effective alternative are required. Among these, the use of zeolites becomes attractive, since these materials are non-toxic, insoluble in water, present a high removal rate, and have a simple operation capacity [3,12,13,14].
Zeolites are excellent adsorbents since the system of channels and cavities present in these solids, associated with the high specific surface area, allows the access and diffusion of several ions and molecules into the internal adsorption sites [15,16,17]. Additionally, the high insertion capacity of structural aluminum is responsible for the generation of cation exchange sites [15,18]. As a consequence, zeolites provide adsorption sites with exchangeable cations for ammonium ions, and porosity for ammonia; therefore, ion exchange and molecular adsorption are the main mechanisms expected for this system, respectively [13,19,20].
In this sense, synthetic zeolites with distinct topologies of BEA-type and FAU-type stand out, since the elevated surface areas associated with accessible pores and high Al contents found for these zeolites are fundamental properties for adsorption processes [21,22]. Moreover, FAU-type zeolite is still more interesting, since its synthesis is conducted without the presence of structural directing agents (SDAs) that are expensive and comprise environmentally harmful organic molecules [22,23]. Consequently, an additional step for SDA removal by calcination at relatively high temperatures (>500 °C) is avoided, which would require considerable energy expenditure and cause a significant amount of polluting volatile organic compounds to be released into the atmosphere [22].
Zeolite is a non-toxic porous material with soil-amended capacity and a slow rate of ammonium release, while nitrogen is an essential nutrient for plant growth [24,25]. The use of NH4+-loaded zeolite residue as a substrate is promising to enhance seedling growth [25]. Few studies have explored the use of zeolite containing ammonium ions removed from wastewater for seedling growth. The recycling of zeolite-based waste and its use as a source of NH4+ can contribute to reducing production costs and environmental impacts. This work innovates by integrating NH4+-loaded zeolites into seedling growth, closing the waste-to-resource cycle.
Thus, the present study explores the use of synthetic BEA-type and FAU-type zeolites for the removal of ammonium ions from real effluents; the performances of these samples were compared with that corresponding to a commercial BEA-type zeolite. The ability of the NH4+-loaded zeolites to stimulate plant growth was investigated on tomato seedlings, promoting a complete reuse cycle of the adsorbed material based on sustainability and eco-friendly processes. The main hypothesis to be explored here is that NH4+-loaded zeolites recovered after the treatment of wastewater can be used as potential slow-release fertilizers. In order to investigate this issue, the synthetic zeolites were prepared, characterized, and then used as adsorbents for the removal of ammonium ions from wastewater. After that, the ability of the NH4+-loaded zeolites to stimulate plant growth was investigated on tomato seedlings, promoting a complete reuse cycle of the adsorbed material based on sustainability and eco-friendly processes.
2. Materials and Methods
2.1. Synthesis of Zeolites
The synthesis of β-zeolite (or BEA-type zeolite, labeled as BEA_S) took place by mixing 0.20 g of NaOH and 0.90 g of sodium aluminate (NaAlO2) in 32.00 g of tetraethylammoniumhydroxide aqueous solution (TEAOH 20% wt). Afterwards, 33.00 g of tetraethylorthosilicate (TEOS) were added. The mixture was mechanically stirred for 4 h before its transference to a stainless-steel autoclave lined with Teflon, and then it was hydrothermally treated at 140 °C for 48 h. The obtained solid product was washed, filtered, and dried overnight in an oven at 60 °C before calcination at 550 °C at 2 °C·min−1 in air for 6 h. A commercial β-zeolite (from Zeolyst International, labeled as BEA_C) was tested on the ammonium adsorption experiments, without any prior treatment, for comparison purposes.
The green synthesis of faujasite (or FAU-type zeolite, labeled as FAU) took place by preparing two systems: In system I, 3.00 g of NaOH, 1.50 g of NaAlO2, and 12.30 g of sodium metasilicate were added in 26.0 mL of H2O. The suspension remained mechanically stirred for 24 h. System II was obtained by adding 0.14 g of NaOH, 9.40 g of NaAlO2, and 76.60 g of sodium metasilicate in 160.0 mL of H2O. System II was kept under mechanical stirring for 30 min, and then 12.00 g of system I was added to system II, with the mixture being stirred for 24 h. The resulting suspension was hydrothermally treated in a stainless-steel autoclave lined with Teflon at 100 °C for 5 h. The FAU sample was washed, filtered, and dried overnight in an oven at 60 °C.
2.2. Characterization
The prepared samples were structurally characterized by X-ray diffraction (XRD) in a Rigaku Miniflex powder diffractometer with CuKα radiation, 40 kV voltage, 40 mA current, and with the diffraction angle (2θ) scanned from 5 to 50° at a scanning rate of 2° min−1. The XRD patterns of all analyzed samples were fitted using the MAUD (Materials Analysis Using Diffraction, Version 2.9999) program (freeware) in order to determine the average crystallite sizes. Semi-quantitative elemental analysis was conducted by X-ray fluorescence (XRF) using a Bruker S8/Tiger instrument.
N2 physisorption experiments were performed at 77 K using a Quantachrome Autosorb-1 instrument to investigate the porous structures of the adsorbents. The isotherms were recorded in the relative pressure (P/P0) range from 10−5 to 1, and the BET specific surface area (SBET) values for each sample were determined by the Brunauer, Emmett, and Teller method. The non-local density functional theory (NLDFT) approach was used to obtain the values of micropore, mesopore, and total pore volumes (Vmicro, Vmeso, and Vtotal, respectively), assuming the occurrence of cylindrical pores.
The local environment of the Al and Si atoms was investigated by solid state 27Al and 29Si nuclear magnetic resonance (NMR) spectroscopy. Experiments were conducted using a Varian/Agilent VNMR 400 MHz spectrometer operating at a magnetic field of 9.4 T. The 27Al NMR experiments were recorded at 104.16 MHz, with single pulse excitation (SPE) and magic angle spinning (MAS), with accumulation of 200 transients, pulse duration of 1.0 μs, recycle delay of 1.0 s, and MAS rate of 14 kHz. An aqueous solution of Al(NO3)3 was used as the primary shift reference. The 29Si SPE/MAS NMR spectra were recorded at 79.41 MHZ, with accumulation of 400 transients, acquisition time of 0.032 s, pulse duration of 4 μs, recycle delay of 20 s and MAS rate of 10 kHz; the spectra were externally referenced to tetramethylsilane (TMS), using the 29Si NMR signal of kaolinite (at −91.2 ppm) as the secondary reference.
Raman spectra of powder synthetic zeolites were performed by an Anton Paar Strabe 20 FT-Raman instrument with a laser at 785 nm and a power of 400 mW. A secondary filter was used to remove the Rayleigh line.
Scanning electron microscopy (SEM) images were obtained in a MEV-FEG Tescan Mira 2 microscope, with 20 kV voltage, equipped with an energy dispersive X-ray spectroscopy (EDS) accessory.
2.3. Adsorption Experiments
To perform the adsorption tests, real municipal wastewater samples were collected from a sewage treatment plant in Volta Redonda (SAAE-VR) (Supplementary Material Figure S1A–C), Rio de Janeiro, Brazil. The collected samples were subjected to analysis of ammonium levels on the same day of collection, using the 919 IC Autosampler plus ion chromatograph. The parameters of the wastewater sample are presented in Table S1.
The pH of wastewater is an important parameter in the adsorption process, since it affects the physical–chemical properties of the solution, influencing the chemical balance between the ammonium and ammonia species. The pH of the collected effluent was 7.55, and according to the speciation diagram obtained using Medusa-Hydra v.1.5.8 software (Figure S2), no pH adjustment took place because practically all ammonia was in the form of NH4+-N. The effect of adsorbent dosage was investigated from 50 to 200 mg, and aliquots were withdrawn in triplicate for all adsorption tests in 120 mL of wastewater solution.
The removal efficiency R (%) and the amount of adsorbed ammonium qa (mg·g−1) were obtained according to Equations (1) and (2), respectively:
In these expressions, C0 and Ce (mg·L−1) are the initial and equilibrium ammonium concentrations in wastewater, respectively, V is the solution volume (L), and m is the adsorbent dosage (g).
The non-linear kinetics data were evaluated according to the pseudo-first order (PFO) and pseudo-second order (PSO) models (Equations (3) and (4)) in the time interval between 0 and 120 min. The adsorption isotherms studies in their non-linear forms were assessed by Langmuir and Freundlich models (Equations (5) and (6)) [22]. For these experiments, solutions with different concentrations were obtained from dilutions of the wastewater sample.
where qt and qe (mg·g−1) are the amounts of ammonium uptake at a specific time t (min) and at equilibrium, respectively; K1 (min−1) and K2 (g·mg−1·min−1) are the PFO and PSO rate constants of adsorption; qmax is the maximum adsorption capacity (mg·g−1); KL (L·mg−1) is the Langmuir equilibrium constant; KF (mg·g−1) is the Freundlich constant (related to the adsorption capacity of the adsorbent); and 1/n is the empirical constant associated with surface heterogeneity in the Freundlich model.
Samples after ammonium ion adsorption will be labeled as FAU_NH4, BEA_S_NH4 and BEA_C_NH4, and will be investigated in plant cultivation experiments.
2.4. Plant Cultivation in a Medium Enriched with NH4+-Loaded Zeolites
Tomato seedlings were grown in a cell-culture plate in agar/water culture medium using a ratio of 1.5% (w/v). The culture medium was individually enriched with 50 mg of FAU_NH4, BEA_S_NH4, and BEA_C_NH4 samples. Fifty seeds of Solanum lycopersicum (Cherry Tomato, Isla®) were used on each cell culture, in triplicate for each type of zeolite and with three control replicates (only the culture medium, without zeolites), totaling 12 experimental units. The seeds were purchased from local stores and used only after disinfection using 0.5% (v/v) of sodium hypochlorite for 15 min, followed by triple washing with distilled water. The experimental units remained for 14 days in a growth chamber under controlled conditions (12 h photoperiod (light/dark), average temperature of 27 °C, and relative humidity of 60%). At the end of the cultivation period, the seedlings were weighed to obtain fresh mass and then dried in a forced air circulation oven at 60 °C. The dry material was crushed and subjected to elemental analysis to determine the nitrogen content using a CHNS Elemental Analyzer. All the steps involved in this work, starting from the synthesis of zeolites up to seedling growth, are presented in Scheme 1.
Scheme 1.
Steps involved in the present work.
2.5. Statistical Analysis
The experiments were carried out in a completely randomized design (CRD) with four replications. Data normality (Shapiro–Wilk) and homogeneity (Bartlett’s test) were verified before ANOVA and Tukey’s post hoc test (p < 0.05), using the ExpDes.pt package [26].
3. Results and Discussion
3.1. Characterization of Zeolites
3.1.1. X-Ray Diffraction and X-Ray Fluorescence (XRF) Analysis
The XRD pattern of the FAU and BEA_S samples (Figure 1) suggests that the synthesized solids are characteristic of single pure phases of both FAU-type and BEA-type zeolites, respectively [27,28,29,30]. The XRD pattern of the FAU sample reveals a set of broad diffraction peaks, pointing to the presence of a nanostructured phase; this result is consistent with the solid-state 29Si NMR results, as discussed below. The same observations also apply to the BEA_S zeolite. An average crystallite size of 8.3 nm was determined for the FAU sample, which is considerably smaller than the values found for BEA_S (14.5 nm) and BEA_C (16.4 nm) solids, respectively (Table 1). It is noteworthy that the crystallite size can affect the textural properties of solids and influence the performance of materials in adsorption tests [22].
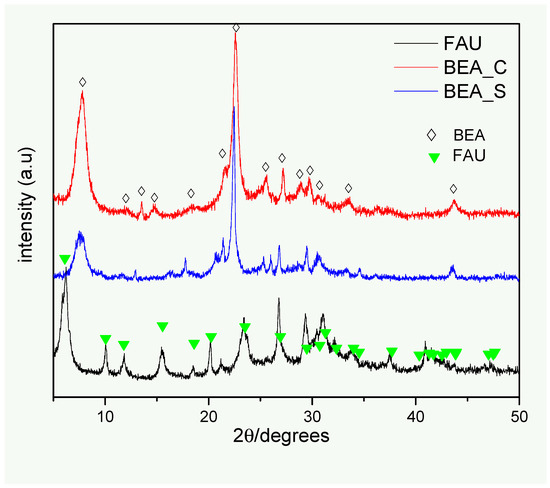
Figure 1.
XRD patterns of the zeolites investigated in this work. The symbols indicate the angles and relative intensities of the diffraction peaks expected for each identified phase (corresponding to the faujasite and β zeolite structures).

Table 1.
Si/Al molar ratios in the synthesis gel (nominal values) and in the final products (from XRF and EDS).
Chemical analysis results obtained by XRF and EDS (Table 1) agree well with each other, and reveal lower Si/Al molar ratios for all produced samples in comparison with the nominal values that would be expected considering the composition of the synthesis gel; this indicates that an expressive amount of Si-containing species remains solubilized after hydrothermal treatment due to the high alkalinity of the reaction medium [21]. The high amount of Al in the structure of the materials compared to the starting composition can be a key parameter for ammonium uptake, since a larger number of negative charges responsible for ion exchange are expected. On the other hand, the commercial sample (BEA_C) presented a relatively low Si/Al molar ratio.
3.1.2. Textural Analysis
The N2 physisorption isotherms of the samples (Figure 2) are typical of microporous/mesoporous materials, with profiles displaying a combination of types I and IV isotherms [22,31]. The quick increase in the N2 uptake at low relative pressures indicates the adsorption in micropores, whereas the presence of mesopores (associated with the formation of particle aggregates) can be recognized by the observation of hysteresis loops (H4-type hysteresis) in the adsorption/desorption branches and the absence of saturation of the adsorbed gas volume at high relative pressures.
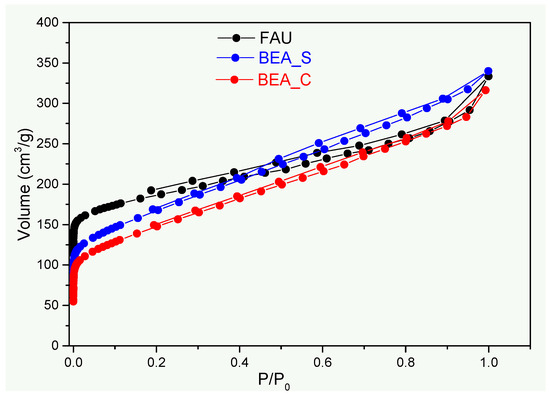
Figure 2.
N2 adsorption/desorption isotherms for the studied adsorbents.
The specific surface area (SBET) values found for the samples follow the order: FAU > BEA_S > BEA_C. Furthermore, the higher total pore volume of the synthetic samples compared to the commercial sample (Table 2) suggests that the produced samples can provide improved adsorption performance of ammonium ions. Considering that the pore sizes of faujasite (7.4 Å) and β-zeolite (5.5Å × 5.5 Å) [32,33] are larger than the hydration radius of the NH4+ ion (3.31 Å) [34], no hindrance to accessing the active adsorption sites of the zeolites is to be expected.

Table 2.
Pore volumes and specific surface area obtained from the analysis of the N2 adsorption/desorption isotherms of the studied adsorbents.
3.1.3. Short-Range Binding and Sample Morphology
The solid-state NMR spectra shown in Figure 3 reveal remarkable differences in the analyzed samples regarding the local chemical environments of the 29Si and 27Al probe nuclei. The 29Si NMR spectrum of the FAU sample contains peaks at −80 ppm (corresponding to Si(4Al) units, i.e., a Si atom with four Al atoms in the second coordination sphere, forming Si-O-Al bridges, −85 ppm (Si(3Al) units), −90 ppm (Si(2Al) units, and −95 ppm (Si(1Al) units), which are typical of FAU-type zeolites with high Al content (in agreement with the XRF results) [22,32,35]. In contrast, the 29Si NMR spectra of the BEA_S and BEA_C samples reveal typical profiles of zeolites with low aluminum content, showing the most intense signal at −106 ppm due to Si(0Al) units [36].
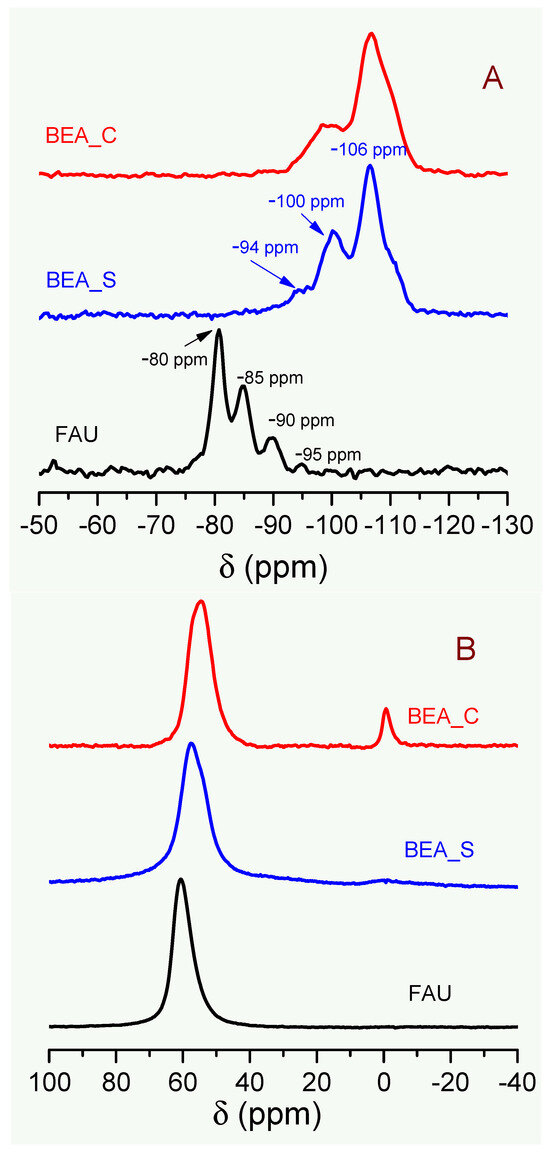
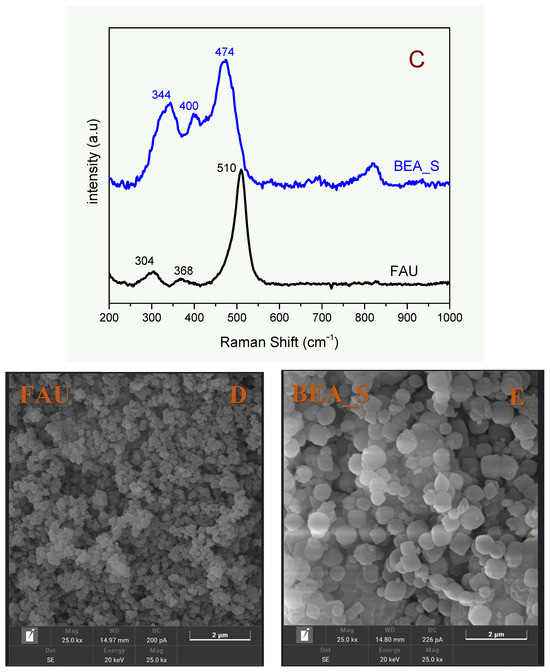
Figure 3.
Solid state 29Si (A) and 27Al (B) NMR spectra of the adsorbents and (C–E) Raman spectra and micrographs (SEM) of the synthetic zeolites FAU and BEA_S, respectively.
Furthermore, the well-defined peaks found in the 29Si NMR spectrum of the FAU sample evidence a high degree of structural order around the Si atoms; this finding is consistent with the occurrence of a nanocrystalline arrangement, rather than the presence of amorphous matter. In fact, whereas XRD gives information regarding long-range structural ordering, solid-state NMR spectroscopy provides a short-range perspective for the chemical and structural features around the probe nuclei in the zeolite framework. Thus, both the broad diffraction peaks in the XRD pattern and the well-defined peaks in the 29Si NMR spectrum of the FAU sample are consistent with the presence of nanocrystals in the zeolite [22,32,35].
As shown in Figure 3B, the 27Al NMR spectra obtained for all samples reveal an intense peak at around 55–60 ppm, indicating the presence of aluminum atoms in tetrahedral coordination within the zeolite framework, responsible for ion exchange sites [37,38]. On the other hand, the small signal at around 0 ppm, found for the BEA_C sample, indicates the presence of extra-framework Al species in octahedral coordination [38,39], which are absent in the synthetic samples.
Figure 3C shows the Raman spectra of the synthetic samples (FAU and BEA_S), respectively. The bands related to the bonding modes of the T-O-T atoms due to six- and four-membered rings (6MR and 4MR) typical of crystalline FAU-type zeolite framework are found at 304, 368, and 510 cm−1 [32]. On the other hand, the bands related to crystalline BEA zeolite framework are shown at 334 cm−1 (6MR), 404 cm−1 (5MR), and 474 cm−1 (4MR) [40]. These results demonstrate that the synthesized structures consist of highly ordered solids and the absence of amorphous matter, as suggested by 29Si-NMR spectroscopy. These results demonstrate that the synthesized materials exhibit short-range ordered structures, in agreement with the solid-state 29Si NMR and XRD results.
The SEM images obtained for the synthetic samples (FAU and BEA_S) are shown in Figure 3D,E. All images are composed of particles (corresponding to aggregates of nanocrystals) with irregular sizes and shapes. It is worth noting that the particles present in the BEA_S sample are larger than those of the FAU zeolite, in agreement with the crystallite sizes obtained from XRD.
Thus, based on the characterization results, it should be reasonable to expect improved performance on ammonium removal for FAU than BEA_S adsorbents, which present high Al content, smaller average crystallite and particle sizes, and large specific surface area, leading to an elevated density of accessible active sites. Following the same reasoning, no remarkable performance on NH4+ uptake is to be expected for BEA_C.
3.2. Adsorption Tests
3.2.1. Effect of Adsorbent Dosage, Adsorption Isotherms, and Kinetic Studies
The first set of experiments is concerned with the effect of adsorbent dosage on ammonium removal (Figure 4A). The studies were carried out only with the FAU zeolite, which was the sample with the best overall performance in removing ammonium from wastewater (Figure 4B,C). It can be seen from Figure 4A that the removal efficiency (%) strongly increases with the rise in zeolite mass; however, the adsorbed amount (mg·g−1) increases up to 0.15 g and then decreases. Particle aggregation, interference among binding sites, and reduced mixing at elevated zeolite loads may cause a decrease in the availability of active sites for adsorption and, consequently, reduce the amount of adsorbed ammonium ions [32].
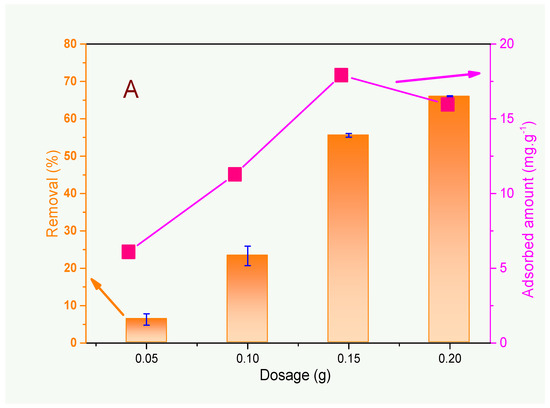
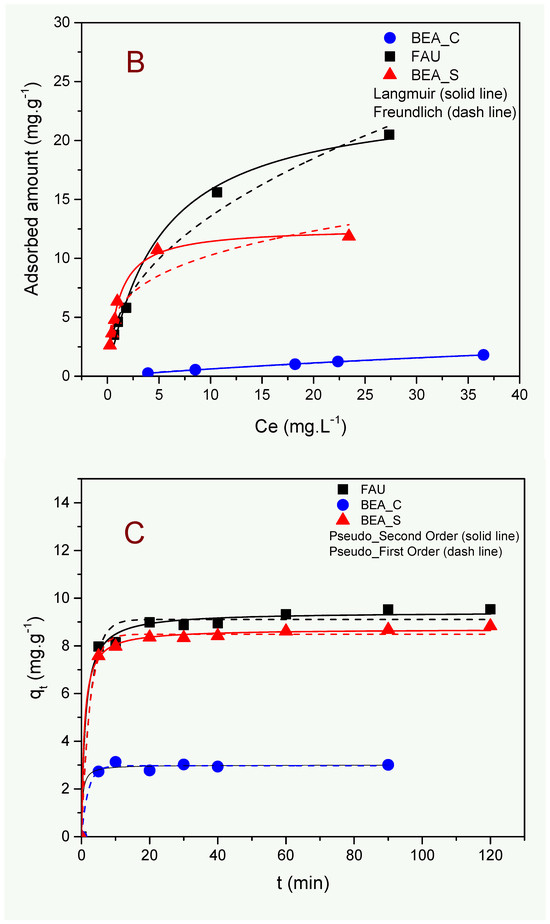
Figure 4.
Effect of adsorbent dosage (A), adsorption isotherms (B), and kinetics studies (C) on ammonium uptake from wastewater using the zeolite samples described in this work. Conditions: V = 120 mL, pH = 7.55, time = 60 min (for the experiments in Figure 4A,B), m = 200 mg, and [NH4+] ≈ 40 mg·L−1 (for the experiments in Figure 4A,C).
As the proposal here is to reduce the ammonium ion levels to the limits permitted by Brazilian legislation (20 mg·L−1) [9], the relevant parameter to be considered is the removal efficiency (%), and thus, the adsorbent dosage of 200 mg was chosen to be used in subsequent studies.
To understand the interactions involving the adsorbent and adsorbate, the adsorption isotherms were analyzed using the non-linear Langmuir and Freundlich models, as shown in Figure 4B; the corresponding fitting parameters are given in Table 3. The downward concave shape observed for all isotherms suggests a strong affinity between the surface of zeolites and ammonium ions [32]. Moreover, the rise in adsorption amount as the initial ammonium concentration increases is a consequence of the variations in the diffusion rates of the NH4+ ions towards the zeolite adsorption sites under more diluted solutions [35]. At higher concentrations, the plateau formation indicates the attainment of the maximum adsorption capacity of the adsorbents.

Table 3.
Langmuir and Freundlich parameters obtained by fitting the ammonium ions equilibrium adsorption isotherms recorded using the FAU, BEA_S, and BEA_C adsorbents.
According to Table 3, the coefficients of determination (R2) in all cases were higher for the Langmuir model than the Freundlich model. This suggests that the adsorption of ammonium ions took place by a monolayer formation over the zeolite surface [31]. The order of maximum adsorption capacity (qmax) was FAU > BEA_S > BEA_C, which can be attributed to the higher Al content (lower Si/Al molar ratio) responsible for the generation of negative charges. Moreover, the high specific surface area and the small average crystallite and particle sizes (notably for the FAU sample) are also expected to play a significant role in this trend. The Freundlich parameter 1/n was less than 1 in all cases, evidencing a favorable adsorption process for ammonium ion capture by the investigated zeolites.
From the results of the maximum adsorption capacity (qmax), it is observed that the synthetic zeolites presented superior performance in relation to the commercial sample, revealing that the design of adsorbents with specific chemical and textural properties represents significant improvements on the adsorptive properties of the materials.
Considering the kinetic studies (Figure 4C), a fast adsorption equilibrium was achieved at around 20 min in all cases, which can be attributed to a large availability of adsorption sites located on the surfaces of zeolites. As stated earlier, no hindrance to accessing the active adsorption sites of the adsorbents is expected due to the larger pores of zeolites in comparison with the size of the NH4+ ions. Fast adsorptive kinetics and high removal rates are very attractive for large-scale industrial applications.
According to Table 4, the better fit using the PSO model in comparison with the PFO model (i.e., higher R2) suggests that chemisorption, probably via ion exchange, is the rate-limiting step controlling the adsorption process [31].

Table 4.
Kinetic parameters for the adsorption of ammonium ions onto FAU, BEA_S, and BEA_C adsorbents.
3.2.2. Comparison with Literature Results and Proposed Adsorption Mechanism
Table 5 shows a remarkably high adsorption capacity for the samples studied in this work in comparison with other zeolite adsorbents reported in the literature, highlighting the performance of the FAU zeolite (qmax = 23.87 mg·g−1), produced by the green method. This demonstrates the potential application of this material on an industrial scale, especially considering its relatively low production cost; moreover, it is worth noting the excellent performance of the FAU sample on ammonium ions uptake even when using a chemically complex matrix such as wastewater from the sewage treatment plant, where several organic and inorganic species can coexist, making this material highly promising.

Table 5.
Comparative literature data on the maximum adsorption capacity (qmax) for ammonium capture by zeolite adsorbents.
Regarding the mechanism proposal in Figure 5, the parameters that affect adsorption are surface area, pore size, OH group density, and the Si/Al ratio (the latter is responsible for the generation of negative charges). Since the surface area of zeolites is relatively high (>500 m2g−1) and their pore sizes are larger than the ammonium ion size, no hindrance for these ions to access the zeolite adsorption sites is expected.
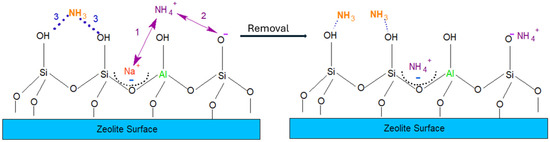
Figure 5.
Proposal mechanism for ammoniacal-N uptake from wastewater.
On the other hand, throughout this work, it was assumed that practically all the ammoniacal-N found in the effluent is in the form of ammonium ions. This makes perfect sense considering that the removal efficiency increased with decreasing Si/Al molar ratio. However, it is possible that some residual nitrogen (in the NH3 form) can be found and will be considered for the mechanism proposal (Figure 5) of ammoniacal-N removal from wastewater.
The presence of NH4+ ions causes an ion exchange with Na+ ions (route 1). Thus, high amounts of Al atoms tetrahedrally coordinated in the zeolite structure result in expressive cation exchange capacities. Furthermore, deprotonated silanol groups (Si-O-) can promote electrostatic interaction (route 2), but are not expected to occur to a large extent since the medium is not alkaline enough to deprotonate the silanol groups significantly. In contrast, the presence of protonated silanol groups (Si-OH) is expected to have a high affinity with NH3 groups through hydrogen bond formation (route 3), but as discussed earlier, it is unlikely that there is a considerable amount of NH3 in the solution. Thus, the cation exchange played the main role for sequestration of ammonium-N by zeolites in agreement with kinetics studies.
3.3. Plant Cultivation in a Medium Enriched with NH4+-Loaded Zeolites
The addition of the NH4+-loaded zeolites into the culture medium resulted in modification in the biomass production of seedlings grown in vitro (Figure 6 and Figure 7), with emphasis on the increase in fresh mass when the cultivation was carried out in the presence of the BEA_C_NH4 sample compared to the control, both when the biomass was weighed (Figure 6) and visually analyzed (Figure 7A,C).
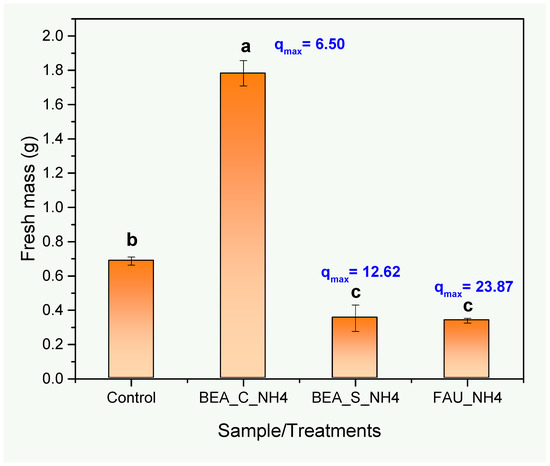
Figure 6.
Fresh mass (g) of seedlings (Solanum lycopersicum) grown in vitro, subjected to four treatments: Control (without addition of zeolites) and three treatments involving the addition of the NH4+-loaded zeolites. Means (n = 3) followed by distinct lowercase letters indicate a significant difference between the treatments (Tukey test, p < 0.05).
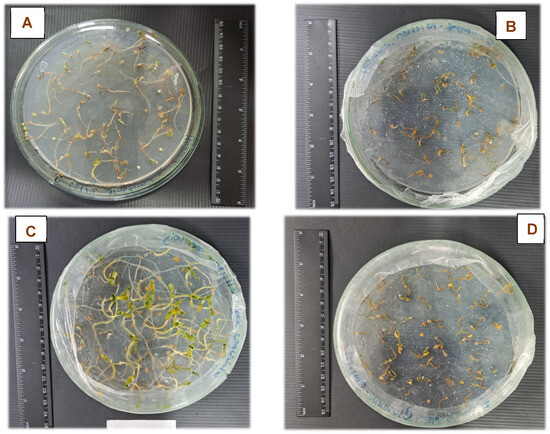
Figure 7.
Images of cell-culture plates after 14 days of seedling cultivation (Solanum lycopersicum), subjected to four treatments: (A) Control (without zeolite addition), (B) FAU_NH4 addition, (C) BEA_C_NH4 addition, and (D) BEA_S_NH4 addition.
The synthetic zeolites (FAU_NH4 and BEA_S_NH4) exhibited superior adsorption capacities (23.87 and 12.62 mg·g−1, respectively) compared to the commercial zeolite (6.50 mg·g−1). It is believed that the excess availability of NH4+ causes ammonium toxicity, which can result in metabolic imbalance, significant reduction of fresh biomass, leaf chlorosis, accumulation of reactive oxygen species, and inhibition of root growth [47,48,49]. Regarding the FAU_NH4 and BEA_S_NH4 zeolites, a reduction in biomass production was detected when compared to the control (Figure 6), possibly indicating a toxic effect also observed visually (Figure 7A,B,D). It is worth noting that the order of fresh mass of cultivated seedlings followed the inverse order of the maximum ammonium adsorption capacity obtained using the Langmuir model (Table 3). Thus, it is assumed that the FAU_NH4 and BEA_S_NH4 zeolites presented an excessively high amount of adsorbed ammonium ions when these zeolites were added to the culture medium.
In this sense, it is necessary to consider that ammonium toxicity leads to a significant reduction in fresh mass in different plant species, reflecting a direct effect of the excessive accumulation of ammonium in the tissues, which may result in metabolic and physiological disorders [50,51,52]. As future steps, studies with different dosages of NH4+-loaded zeolites will be used to optimize seedling growth processes and to determine the limits between toxicity and controlled release of NH4+ at adequate doses. The results presented demonstrate remarkable differences in the efficiency of different types of zeolites (FAU and BEA) in the adsorption of ammoniacal nitrogen and its subsequent availability for seedling growth.
When the nitrogen contents of the grown tomato seedlings’ tissues are analyzed (Table 6), it is observed that the insertion of zeolites into the cultivation medium influenced the nitrogen absorption, suggesting that the specific physicochemical properties of the used zeolites play a significant role in this process. The commercial zeolite BEA_C_NH4 apparently exhibited the best agronomic performance, promoting superior biomass growth (15% higher than control), while FAU_NH4 and BEA_S_NH4 caused toxicity due to excess ammonium, as evidenced by reduced biomass despite greater nitrogen accumulation in the tissues. The results suggest that the effectiveness of zeolitic fertilizers critically depends on the applied dosage. Although synthetic zeolites demonstrate higher adsorption capacity, this characteristic can be counterproductive when it results in excessive NH4+ release, causing toxicity [49]. Future studies should focus on optimizing the dosage for each type of zeolite. These results suggest important structural and functional differences between the types of zeolites evaluated, with direct implications for the subsequent release of nitrogen.

Table 6.
Nitrogen contents in tomato seedling tissues (Solanum lycopersicum) subjected to different treatments.
The desorption of ammonium from zeolites is critical for its application as a slow-release fertilizer. A recent study revealed that different zeolites have varying efficiency in the recovery of NH4+ from aqueous solutions, under the influence of several factors such as pH, temperature, and the presence of other ions in solution [53].
The controlled release mechanism minimizes nutrient loss and allows greater control of nitrogen availability to plants. The effectiveness of zeolites as slow-release fertilizers is related to their ability to retain and gradually release ammonium ions as plants need. Our results indicate that FAU-type zeolite has the potential to be used in a similar application, as the FAU_NH4 treatment resulted in an increase both in the percentage of nitrogen (4.81% vs. 4.38% in control) and in the absolute nitrogen content in the seedlings (3.05 vs. 2.03 mg in control).
All these variables reveal that studies involving zeolites containing adsorbed ammonium from wastewater are highly promising, and more detailed future investigations related to the development of different plants and with optimized experimental conditions will be carried out. The present study clearly demonstrates that it is possible to perform a complete cycle from pollutant removal with zeolite, especially regarding ammonium ions (which, in high concentrations, are harmful to humans and the environment if inappropriately disposed of), and recycling the zeolite-based waste for practical applications, promoting sustainable and eco-friendly processes in accordance with green methods.
4. Conclusions
The present work investigated the use of synthetic FAU-type and BEA-type zeolites and a commercial sample of BEA-type zeolite for ammonium ion uptake from wastewater collected in a sewage treatment plant. The synthetic samples showed superior performance in removing ammonium ions compared to the commercial sample due to (i) lower Si/Al molar ratio, (ii) larger specific surface area, and (iii) smaller average crystallite and particle sizes, which facilitates access and diffusion of ammonium ions into adsorption sites. Furthermore, the best performance found for the FAU sample becomes even more attractive, since this sample is produced without the presence of structural directing agents, eliminating the final calcination step, which makes the synthesis process environmentally and energetically interesting.
The insertion of the NH4+-loaded zeolites into the culture medium resulted in a change in the biomass production of seedlings grown in vitro, especially for the BEA_C_NH4 sample, where the fresh mass increased in relation to the control. On the other hand, the use of both the BEA_S_NH4 and FAU_NH4 adsorbents led to a toxic effect on seedlings’ growth, which can be attributed to the excess of ammonium adsorbed in these samples. Future studies with these or other zeolites should optimize the adsorbent dosage to avoid NH4+ toxicity and test the scalability of the process in continuous-flow systems. Thus, zeolite adsorbents offer a dual solution: wastewater treatment and nutrient recycling for agriculture, aligning with circular economic principles.
Finally, the elemental analysis results support the hypothesis that the addition of zeolite into the culture medium acted to reduce losses by leaching and gradually releasing the nutrients, which allows the recovered zeolites to be considered environmentally friendly and promising slow-release fertilizers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13082426/s1, Figure S1A–C: Images of the wastewater collection sites at the sewage treatment plant; Table S1: Physicochemical properties of the wastewater sample obtained from the sewage treatment plant considered in this work. Figure S2: Ammonium speciation (from Medusa-Hydra v.1.5.8 software) diagram at varying pH values for a concentration of 40 mg·L−1.
Author Contributions
M.S.C.A.: Investigation; M.A.d.S.: investigation; G.d.S.C.: investigation; F.S.d.S.: formal analysis, review and editing; D.F.C.: investigation; D.N.F.: investigation; formal analysis; A.M.d.S.: funding acquisition; formal analysis; writing—review and editing; project administration; J.C.C.F.: funding acquisition; formal analysis; writing—review and editing; M.K.P.: conceptualization; funding acquisition; formal analysis; writing—original draft; writing—review and editing; project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by FAPERJ (grant Nº E-26/210.138/2022 and No E-26/010.001842/2015). JCCF acknowledges the support from the Brazilian agencies FAPES (grant 892/2023) and CNPq (grant 310528/2022-4).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors also acknowledge the Volta Redonda Sewage Treatment Plant (SAAE-VR), Rio de Janeiro, Brazil, for providing the wastewater samples.
Conflicts of Interest
The authors declare there are no conflicts of interest.
Abbreviations
| BEA-type | β-zeolite |
| EDS | energy dispersive X-ray spectroscopy |
| Fau-type | faujasite zeolite |
| PFO | pseudo-first order |
| PSO | pseudo-second order |
| SBET | specific surface area |
| SEM | Scanning electron microscopy |
| SDA | structure direct agent |
| NMR | nuclear magnetic resonance |
| Vmeso | mesopore volume |
| Vmicro | micropore volume |
| Vtotal | total pore volume |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
References
- Adam, M.R.; Othman, M.H.D.; Hubadillah, S.K.; Abd Aziz, M.H.; Jamalludin, M.R. Application of Natural Zeolite Clinoptilolite for the Removal of Ammonia in Wastewater. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Zhu, F.; Ren, G.; Cao, G.; Song, L. Application of Modified Zeolite for Ammonium Removal from Drinking Water. Desalination 2011, 271, 295–300. [Google Scholar] [CrossRef]
- Yadav, V.; Kumar, L.; Saini, N.; Yadav, M.; Singh, N.; Murugasen, V.; Varathan, E. Effective Removal of Ammonia from Water Using Pre-Treated Clinoptilolite Zeolite-A Detailed Study. Water Air Soil. Pollut. 2023, 234, 435. [Google Scholar] [CrossRef]
- Pereyra, N.; Kamran, U.; Aguilar-Mamani, W.; Akhtar, F. Conversion of Glass Waste into Zeolite A Adsorbent for Efficient Ammonium Ion Adsorption from Aqueous Solution: Kinetic and Isotherm Studies. Processes 2025, 13, 678. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Wang, J.; Du, R.; Xue, X.; Peng, Y. Pilot-Scale Partial Nitrification and Anaerobic Ammonium Oxidation System for Nitrogen Removal from Municipal Wastewater. Commun. Eng. 2025, 4, 36. [Google Scholar] [CrossRef]
- Eljamal, O.; Eljamal, R.; Maamoun, I.; Khalil, A.M.E.; Shubair, T.; Falyouna, O.; Sugihara, Y. Efficient Treatment of Ammonia-Nitrogen Contaminated Waters by Nano Zero-Valent Iron/Zeolite Composite. Chemosphere 2022, 287, 131990. [Google Scholar] [CrossRef]
- Hoang-Minh, T.; Hai, N.T.; Hieu, D.T.; Hoai, T.T.; Van Dong, B.; Dung, L.V.; Ha, N.T.H. Removal of Ammonium from Water by a KOH-Treated Bentonite Biochar Composite. Colloid. Polym. Sci. 2025, 303, 81–94. [Google Scholar] [CrossRef]
- Li, L.; Lollar, B.S.; Li, H.; Wortmann, U.G.; Lacrampe-Couloume, G. Ammonium Stability and Nitrogen Isotope Fractionations for –NH3(Aq)–NH3(Gas) Systems at 20–70 °C and PH of 2–13: Applications to Habitability and Nitrogen Cycling in Low-Temperature Hydrothermal Systems. Geochim. Cosmochim. Acta 2012, 84, 280–296. [Google Scholar] [CrossRef]
- Zanol, M.B.; Lima, J.P.P.; Assemany, P.; Aguiar, A. Assessment of Characteristics and Treatment Processes of Wastewater from Slaughterhouses in the State of Minas Gerais, Brazil. J. Environ. Manag. 2024, 358, 120862. [Google Scholar] [CrossRef] [PubMed]
- Taher, T.; Melati, E.K.A.; Febrina, M.; Maulana, S.; Asagabaldan, M.A.; Rianjanu, A.; Lesbani, A.; Mukti, R.R. Effect of Desilication on Indonesian Natural Zeolite for the Enhancement of Ammonium Ion Removal from Aqueous Solutions. Silicon 2024, 16, 1309–1319. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, M.; Zeng, H.; Min, R.; Wang, J.; Zhang, G. Application of Physicochemical Techniques to the Removal of Ammonia Nitrogen from Water: A Systematic Review. Environ. Geochem. Health 2024, 46, 344. [Google Scholar] [CrossRef]
- Phetrungnapha, A.; Wiengnak, N.; Maikrang, K. A Low-Cost Water Hyacinth-Based Adsorbent for Free Fatty Acids Removal from Waste Cooking Oil: Kinetic, Isotherm, and Thermodynamic Studies. Braz. J. Chem. Eng. 2024, 42, 727–740. [Google Scholar] [CrossRef]
- Feng, L.; Qiu, T.; Yan, H.; Liu, C.; Chen, Y.; Zhou, X.; Qiu, S. Removal of Ammonia Nitrogen from Aqueous Media with Low-Cost Adsorbents: A Review. Water Air Soil. Pollut. 2023, 234, 280. [Google Scholar] [CrossRef]
- Kurniawan, T.; Bahri, S.; Diyanah, A.; Milenia, N.D.; Nuryoto, N.; Faungnawakij, K.; Thongratkaew, S.; Roil Bilad, M.; Huda, N. Improving Ammonium Sorption of Bayah Natural Zeolites by Hydrothermal Method. Processes 2020, 8, 1569. [Google Scholar] [CrossRef]
- de Magalhães, L.F.; da Silva, G.R.; Peres, A.E.C. Zeolite Application in Wastewater Treatment. Adsorpt. Sci. Technol. 2022, 2022, 4544104. [Google Scholar] [CrossRef]
- Arenhardt, W.D.; Ketzer, F.; Wancura, J.H.C.; Seraglio, J.; Carasek, F.L.; Zin, G.; Calisto, J.F.F.; Rodrigues, C.A.; de Meneses, A.C.; Oliveira, J.V.; et al. Fe3+ and Mn2+ Removal from Water Solutions by Clinoptilolite Zeolites as a Potential Treatment for Groundwater Wells. Processes 2025, 13, 1060. [Google Scholar] [CrossRef]
- Mohammadi, P.; Mirghaffari, N.; Razavi, Z.; Soleimani, M. Synthesis and Characterization of Zeolite X from Granite Stone Sludge. Next Mater. 2025, 6, 100466. [Google Scholar] [CrossRef]
- Das, D.; Sengupta, S. Alkaline Hydrothermal Treatment of Chabazite to Enhance Its Ammonium Removal and Recovery Capabilities through Recrystallization. Processes 2023, 12, 85. [Google Scholar] [CrossRef]
- Yadav, V.; Rani, M.; Kumar, L.; Singh, N.; Ezhilselvi, V. Effect of Surface Modification of Natural Zeolite on Ammonium Ion Removal from Water Using Batch Study: An Overview. Water Air Soil. Pollut. 2022, 233, 465. [Google Scholar] [CrossRef]
- Ellersdorfer, M.; Pesendorfer, S.; Stocker, K. Nitrogen Recovery from Swine Manure Using a Zeolite-Based Process. Processes 2020, 8, 1515. [Google Scholar] [CrossRef]
- Pratti, L.M.; Reis, G.M.; dos Santos, F.S.; Gonçalves, G.R.; Freitas, J.C.C.; de Pietre, M.K. Effects of Textural and Chemical Properties of β-Zeolites on Their Performance as Adsorbents for Heavy Metals Removal. Environ. Earth Sci. 2019, 78, 553. [Google Scholar] [CrossRef]
- Teixeira, R.S.; Schmidt, D.V.C.; dos Santos, F.S.; Cipriano, D.F.; Faria, D.N.; Freitas, J.C.C.; Pietre, M.K. Nanostructured Faujasites with Different Structural and Textural Properties for Adsorption of Cobalt and Nickel. Braz. J. Chem. Eng. 2023, 41, 1271–1283. [Google Scholar] [CrossRef]
- Chaves, T.F.; Pastore, H.O.; Cardoso, D. A Simple Synthesis Procedure to Prepare Nanosized Faujasite Crystals. Microporous Mesoporous Mater. 2012, 161, 67–75. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Campisi, T.; Abbondanzi, F.; Faccini, B.; Di Giuseppe, D.; Malferrari, D.; Coltorti, M.; Laurora, A.; Passaglia, E. Ammonium-Charged Zeolitite Effects on Crop Growth and Nutrient Leaching: Greenhouse Experiments on Maize (Zea mays). Catena 2016, 140, 66–76. [Google Scholar] [CrossRef]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: An R Package for ANOVA and Experimental Designs. Appl. Math. 2014, 05, 2952–2958. [Google Scholar] [CrossRef]
- Wu, R.; Lv, P.; He, X.; Bai, Y.; Wang, J.; Wei, J.; Su, W.; Song, X.; Yu, G. Tandem Catalytic Upgrading of Cow Manure Pyrolysis Vapors by Beta Zeolite and Nitrogen-Doped Activated Carbon Dual Catalysts Prepared from Coal Gasification Fine Slag to Produce Light Aromatic Hydrocarbons. J. Environ. Chem. Eng. 2025, 13, 115508. [Google Scholar] [CrossRef]
- Reinoso, D.; Adrover, M.; Pedernera, M. Green Synthesis of Nanocrystalline Faujasite Zeolite. Ultrason. Sonochem 2018, 42, 303–309. [Google Scholar] [CrossRef]
- Bacariza, C.; Karam, L.; El Hassan, N.; Lopes, J.M.; Henriques, C. Carbon Dioxide Reforming of Methane over Nickel-Supported Zeolites: A Screening Study. Processes 2022, 10, 1331. [Google Scholar] [CrossRef]
- Sobuś, N.; Czekaj, I. Catalytic Transformation of Biomass-Derived Glucose by One-Pot Method into Levulinic Acid over Na-BEA Zeolite. Processes 2022, 10, 223. [Google Scholar] [CrossRef]
- Moraes, C.S.; Carneiro, P.A.; Faria, D.N.; Cipriano, D.F.; Freitas, J.C.C.; Amorim, R.G.; da Silva, R.S.; Pietre, M.K. High Efficiency of Myclobutanil Adsorption by CTAB-Zeolite Structures: Experimental Evidence Meets Theoretical Investigation. Silicon 2024, 16, 3737–3753. [Google Scholar] [CrossRef]
- Profeta, D.O.; da Silva, M.A.; Faria, D.N.; Cipriano, D.F.; Freitas, J.C.C.; dos Santos, F.S.; Lima, T.M.; Vasconcelos, S.C.; Pietre, M.K. Zeolite/Calcium Carbonate Composite for a Synergistic Adsorption of Cadmium in Aqueous Solution. Next Mater. 2025, 6, 100493. [Google Scholar] [CrossRef]
- Belviso, C.; Guerra, G.; Abdolrahimi, M.; Peddis, D.; Maraschi, F.; Cavalcante, F.; Ferretti, M.; Martucci, A.; Sturini, M. Efficiency in Ofloxacin Antibiotic Water Remediation by Magnetic Zeolites Formed Combining Pure Sources and Wastes. Processes 2021, 9, 2137. [Google Scholar] [CrossRef]
- Wang, Y.; Kuchena, S.F. Recent Progress in Aqueous Ammonium-Ion Batteries. ACS Omega 2022, 7, 33732–33748. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.B.; Schmidt, D.V.C.; dos Santos, F.S.; Cipriano, D.F.; Gonçalves, G.R.; Freitas, J.C.C.; de Pietre, M.K. Nanostructured Faujasite Zeolite as Metal Ion Adsorbent: Kinetics, Equilibrium Adsorption and Metal Recovery Studies. Water Sci. Technol. 2021, 83, 358–371. [Google Scholar] [CrossRef]
- Brouwer, D.H.; Brouwer, C.C.; Mesa, S.; Semelhago, C.A.; Steckley, E.E.; Sun, M.P.Y.; Mikolajewski, J.G.; Baerlocher, C. Solid-State 29Si NMR Spectra of Pure Silica Zeolites for the International Zeolite Association Database of Zeolite Structures. Microporous Mesoporous Mater. 2020, 297, 110000. [Google Scholar] [CrossRef]
- Mansuri, B.; Lodhi, A.; Parmar, H.; Dalai, A.; Maheria, K. Synthesis, Characterization and Catalytic Assessment of Novel Hierarchical Zeolite Hβ for the Synthesis of Biologically Active Amido Alkyl Naphthols. Res. Chem. Intermed. 2025, 51, 1187–1212. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.S.; Nguyen, D.D. Ultrasonic-Assisted Dealumination of Y Zeolite for Improving VOCs Adsorption and Moisture Resistance. Water Air Soil. Pollut. 2025, 236, 533. [Google Scholar] [CrossRef]
- Toyama, N.; Nagashima, T.; Wada, K.; Deguchi, K.; Ohki, S.; Mogami, Y.; Tansho, M.; Goto, A.; Furukawa, S. Synthesis of Mesoporous SiO2–Al2O3 Hollow Spheres Using Ultrasonic Irradiation and Their Activity for Hydrolysis of Ammonia Borane. Next Mater. 2025, 8, 100777. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, Y.; Deng, F.; Feng, Z.; Meng, X.; Xiao, F.-S. Evolution of D6R Units in the Interzeolite Transformation from FAU, MFI or *BEA into AEI: Transfer or Reassembly? Inorg. Chem. Front. 2020, 7, 2204–2211. [Google Scholar] [CrossRef]
- Widiastuti, N.; Wu, H.; Ang, H.M.; Zhang, D. Removal of Ammonium from Greywater Using Natural Zeolite. Desalination 2011, 277, 15–23. [Google Scholar] [CrossRef]
- Lei, L.; Li, X.; Zhang, X. Ammonium Removal from Aqueous Solutions Using Microwave-Treated Natural Chinese Zeolite. Sep. Purif. Technol. 2008, 58, 359–366. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Xu, D.; Han, L.; Niu, D.; Tian, B.; Zhang, J.; Zhang, L.; Wu, W. Removal of Ammonium from Aqueous Solutions Using Zeolite Synthesized from Fly Ash by a Fusion Method. Desalination 2011, 271, 111–121. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.; Yu, Z.; Shen, J.; Li, J.; Zhang, M.; Ding, T.; Xu, L.; Lee, S.S. Ammonium Removal Using a Calcined Natural Zeolite Modified with Sodium Nitrate. J. Hazard. Mater. 2020, 393, 122481. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Belviso, C.; Cavalcante, F.; Lettino, A.; Vagliasindi, F.G.A.; Roccaro, P. Removal of Ammonium from Wastewater by Zeolite Synthetized from Volcanic Ash: Batch and Column Tests. J. Environ. Chem. Eng. 2022, 10, 107539. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamada, H.; Tanaka, J.; Komatsu, Y.; Moriyoshi, Y. Ammonium Ion Exchange of Synthetic Zeolites: The Effect of Their Open-Window Sizes, Pore Structures, and Cation Exchange Capacities. Sep. Sci. Technol. 2005, 39, 2091–2104. [Google Scholar] [CrossRef]
- Qin, C.; Yi, K.; Wu, P. Ammonium Affects Cell Viability to Inhibit Root Growth in Arabidopsis. J. Zhejiang Univ. Sci. B 2011, 12, 477–484. [Google Scholar] [CrossRef]
- Xie, L.-B.; Sun, L.-N.; Zhang, Z.-W.; Chen, Y.-E.; Yuan, M.; Yuan, S. Phenotype Assessment and Putative Mechanisms of Ammonium Toxicity to Plants. Int. J. Mol. Sci. 2025, 26, 2606. [Google Scholar] [CrossRef] [PubMed]
- Shilpha, J.; Song, J.; Jeong, B.R. Ammonium Phytotoxicity and Tolerance: An Insight into Ammonium Nutrition to Improve Crop Productivity. Agronomy 2023, 13, 1487. [Google Scholar] [CrossRef]
- Souri, M.K.; Römheld, V. Split Daily Applications of Ammonium Can Not Ameliorate Ammonium Toxicity in Tomato Plants. Hortic. Environ. Biotechnol. 2009, 50, 384–391. [Google Scholar]
- Silva, G.; Prado, R.; Ferreira, R. Absorption of Nutrients, Growth and Nutritional Disorders Resulting from Ammonium Toxicity in Rice and Spinach Plants. Emir. J. Food Agric. 2016, 28, 882. [Google Scholar] [CrossRef]
- Roosta, H.R.; Schjoerring, J.K. Effects of Ammonium Toxicity on Nitrogen Metabolism and Elemental Profile of Cucumber Plants. J. Plant Nutr. 2007, 30, 1933–1951. [Google Scholar] [CrossRef]
- Muscarella, S.M.; Badalucco, L.; Cano, B.; Laudicina, V.A.; Mannina, G. Ammonium Adsorption, Desorption and Recovery by Acid and Alkaline Treated Zeolite. Bioresour. Technol. 2021, 341, 125812. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).