Abstract
In this work, zeolite LTA (Linde Type A) was synthesised from natural clay as a novel adsorbent for copper and lead ions removal from water effluents. The applied process allowed the reuse of kaolin, as natural clay, for the production of zeolite LTA through a stepwise process, which involved the formation of metakaolin. The results of characterisation showed the formation of crystalline cubic crystals of zeolite with a mean dimension of 2–3 microns, indicating the successful nucleation and development of the LTA zeolite phase. Batch adsorption studies were carried out to study the removal ability of zeolite LTA by testing Cu2+ and Pb2+ ions. Effects of contact time, pH, and adsorbent dosage were investigated. At pH > 5, the removal efficiency for both metals exceeded 95%. As the zeolite dosage increases from 2 to 10 g/L, the removal effectiveness for both metals markedly enhances (>95% at 10 g/L for lead ions and >90% at 10 g/L for copper ions). The adsorbent showed a higher adsorption capacity in removing lead compared to copper (Qm = 81.5 mg/g for Pb2+ and 67.5 mg/g for Cu2+). The adsorption process was well described by the pseudo-second-order kinetic model, while the Langmuir isotherm adequately depicted the equilibrium behavior. Notably, the kinetics revealed distinct contributions from chemisorption and physisorption, with the AOAS model effectively quantifying their respective roles in metal ion removal. The findings revealed that prepared zeolite LTA acts as an efficient adsorbent to remove heavy metals.
1. Introduction
The developing world is recently facing the scarcity of pure and clean water due to an immense increase in population and industrialization [1]. The contamination of wastewater with heavy metals, resulting from rapid industrialisation and intensive human activities such as mineral processing, petrochemical operations, metal plating, mining, smelting, and battery manufacturing, has become a major global concern due to its significant environmental and health risks [2,3]. The concentration of heavy metals in soil, groundwater, and various water bodies is exceeding the acceptable limits in many countries as a result of unplanned urbanisation, industrialisation, population growth, and poor management policies, leading to a hidden threat to the human food chain [4]. Heavy metals like lead and copper, commonly found in industrial effluents, are non-biodegradable and tend to accumulate in the environment, leading to serious health issues in humans, including stunted growth, neurological impairments, organ damage, and cancer, as well as causing severe harm to ecosystems [5,6]. If these toxic heavy metals in the aquatic environment are transferred and amplified by aquatic animals, they may pose severe health risks to humans [7]. To address these challenges, a variety of techniques have been developed for the removal of heavy metal ions from wastewater, including chemical precipitation, ion exchange, chemical coagulation, membrane processes, and adsorption [3,8]. However, many treatment methods have some drawbacks, such as high energy requirements, complex operation, and limited conditions [9,10,11]. Among these, adsorption stands out as one of the simplest and most effective methods [12], owing to its low initial cost, straightforward design, and ease of operation, especially when using adsorbents with a high affinity for target metals [13]. Zeolites, in particular, are highly promising adsorbents due to their unique properties such as ion exchange capacity, strong affinity for heavy metal cations, and proven chemical and mechanical stability, making them excellent candidates for the treatment of metal-contaminated wastewater [14,15,16].
Copper (Cu) and lead (Pb) are among the most problematic heavy metals found in industrial and municipal wastewater due to their persistence, toxicity, and cumulative effects in biological systems [17]. Chronic exposure to Cu2+ can trigger hepatic and renal dysfunctions, gastrointestinal distress, and neurological damage [18], while Pb2+ is even more hazardous, causing severe neurotoxicity, hematological disorders, developmental delays in children, and irreversible organ damage [19]. Both metals resist natural degradation, accumulate in aquatic environments, and readily enter the food chain, amplifying risks for humans and ecosystems. While various adsorbents have been tested for their ability to capture heavy metals, most, including activated carbon, natural clays, and biosorbents, show significant limitations. These include a lack of selectivity for specific ions, rapid saturation, poor regeneration performance, and inefficiency in the presence of competing pollutants or fluctuating pH conditions [20,21,22]. Ashraff Aziz Marhoon et al. synthesized high-purity single-phase zeolite X from treated coal fly ash using a fusion-hydrothermal method, achieving efficient removal of nickel and vanadium ions from aqueous solutions with high adsorption capacity and excellent crystallinity [20]. Prepilková et al. investigated the adsorption efficiency of cadmium and manganese using natural sorbents, including bentonite, zeolite, and stabilized digested dewatered sludge, highlighting the superior metal removal capacity of the sludge in neutral mine drainage conditions, a less studied environment compared to acid mine drainage [23]. In contrast, synthetic zeolites such as LTA stand out for their well-defined, uniform microporous framework and high cation exchange capacity, which confer strong selectivity for heavy metal ions like Cu2+ and Pb2+.
Zeolites are crystalline hydrated aluminosilicates characterized by distinct three-dimensional microporous frameworks, synthesized via processes including hydrothermal treatment (preferred for its simplicity), alkali fusion, microwave irradiation, and ultrasonic procedures. In recent years, sustainable synthesis methods utilizing natural resources such as kaolin (calcined at 600–900 °C and activated with alkali), smectite clays (hydrothermal conversion), and diatomite (alkali fusion with Na2CO3) have facilitated the environmentally friendly production of zeolites, including types A, X, and Na-P1, achieving cost reductions of up to 40% while valorizing mineral waste [24,25,26,27]. These naturally sourced zeolites are gaining significance in environmental applications, such as heavy metal adsorption (up to 200 mg/g for Pb2+), wastewater treatment (98% elimination of NH4+ in 15 min), CO2 capture (4.2 mmol/g), and soil remediation [28,29]. Their porous architecture and elevated cation exchange capacity (2.8 meq/g for Na-P1) render them advantageous in low-carbon cements and solar energy storage systems, establishing natural zeolites as essential elements in the circular economy, with the global market anticipated to attain USD 2.8 billion by 2030. The characteristics and properties of zeolites have attracted attention for their efficiency in adsorbing heavy metals [30,31]. Their adsorption properties are unique since the ion exchange property allows cation exchange with other ions like heavy metals [32].
Zeolite Na-A (LTA) is a highly adaptable and extensively utilized synthetic aluminosilicate characterized by the formula Na12[(AlO2)12(SiO2)12].27H2O. It possesses a distinctive three-dimensional structure composed of interlinked double sodalite cages, forming extensive alpha cavities and a network of microporous channels. This structure features three categories of exchangeable cation sites: located on the six-membered rings of the sodalite cages, adjacent to the centers of the eight-membered rings encircling the alpha cavities, and on the four-membered rings, which accommodate 8, 3, and 12 cations per cavity, respectively. These attributes confer upon Na-A exceptional molecular sieving capabilities and significant hydrophilicity, rendering it advantageous in catalysis and separation operations. Na-A is commercially synthesized hydrothermally from aluminosilicate hydrogels employing silica and aluminum sources; however, there is growing interest in sustainable synthesis methods that utilize abundant and inexpensive raw materials, such as kaolin clay, slags, fly ash, and mining byproducts [33]. Kaolin is significant because its Si/Al ratio closely resembles that of zeolite A, facilitating the synthesis of highly crystalline Na-A by calcination and alkaline activation. By modifying synthesis parameters—such as the transformation temperature of kaolin to reactive metakaolin, the molar ratios of SiO2/Al2O3, Na2O/SiO2, and H2O/Na2O, along with agitation and crystallisation conditions—the structural and chemical properties of the zeolite can be precisely optimized for targeted industrial and environmental applications, including wastewater treatment, gas separation, and CO2 capture [34]. This study focuses on the synthesis of LTA zeolite from kaolin for the efficient removal of lead (Pb2+) and copper (Cu2+) ions from water effluents. The effects of various process parameters on the adsorption performance of the synthesized adsorbent were systematically investigated, aiming to develop a highly effective and durable material for mitigating heavy metal contamination in wastewater.
2. Materials and Methods
2.1. Materials
Sodium hydroxide (NaOH) and kaolin (designated as SZWMK1) were utilized for the production of zeolite A. The kaolin, provided by the ALGERIAN BENTONITES COMPANY EPE/SPA (Maghnia-Tlemcen, northwestern Algeria), is described in Table 1 with its chemical composition. The Loss on Ignition (LOI) represents the percentage of weight lost by the kaolin sample when heated to a high temperature; this loss corresponds mainly to the removal of moisture, organic matter, and other volatile substances during the heating process. The chemical composition of the kaolin is expressed in molar percentages, as detailed in Table S1 (see the Supplementary Data). Before synthesis, the kaolin was carefully dried at 110 °C for 24 h in a Pyrex beaker to remove residual moisture. High-purity sodium hydroxide (99.99%) was obtained from Sigma-Aldrich. Copper(II) nitrate trihydrate (Cu(NO3)2·3H2O, 99.9%, Sigma-Aldrich) and lead(II) nitrate (Pb(NO3)2, 99.95%, Sigma-Aldrich) were used as metal ion sources for adsorption studies. All chemicals utilized were of exceptional purity. Deionised water (Milli-Q Millipore, 18.20 MΩ·cm) was consistently employed throughout the synthesis process.

Table 1.
Chemical Composition of Kaolin.
2.2. Experimental Methodology
An amalgamated fusion/hydrothermal methodology, as described in previous studies [14], was employed to produce zeolite A from kaolin. Based on the results of the XRF analysis, the SiO2/Al2O3 molar ratio of the raw kaolin was determined to be 2.362 (Table 1). NaOH was used by considering the NaOH/kaolin ratio of 0.9. Fused kaolin was created by combining 10 g of kaolin with 9 g of NaOH, grinding the mixture in a mortar for 15 min, and then fusing it for two hours at 550 °C. The combined product was ground up, dissolved in 100 mL of deionised water, then stirred for 5 h, and left to stir at room temperature for five hours. The mixture was put in a Teflon-coated stainless-steel autoclave, and the heating temperature and crystallization time were adjusted to 90 °C and 20 h, respectively. After the heated product was filtered through a suction device, it was repeatedly cleaned with distilled water to eliminate any remaining NaOH, and it was left to dry overnight at 80 °C in an oven. Figure 1 illustrates the synthesis process steps of LTA zeolite.
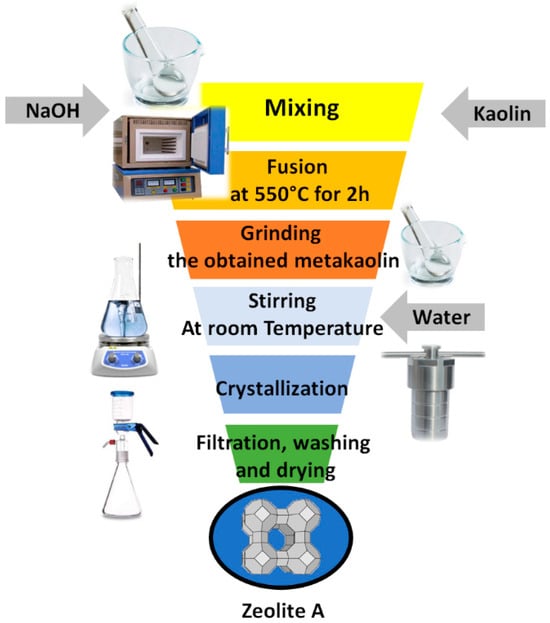
Figure 1.
Process flow chart for the preparation of LTA zeolite from kaolin.
2.3. Batch Adsorption Studies
The preliminary adsorption performance of Cu2+ (C0 = 80 mg/L) and Pb2+ (C0 = 80 mg/L) using LTA zeolite was evaluated through batch experiments by separately testing the heavy metal ion solutions. To systematically investigate the effects of zeolite dosage (2–10 g/L), pH (1–11), and contact time (0–250 min) on the adsorption performance, experiments were conducted using 100 mL of solution in 250 mL conical flasks. These flasks were agitated at 150 rpm in shaking incubators (Zhicheng ZWYR-2102C, China). This approach enabled the controlled assessment of how each factor influenced the zeolite’s ability to adsorb Cu2+ and Pb2+ from aqueous solutions. Solutions were prepared from a stock solution, and their pH was adjusted with HNO3 or NaOH. All experiments were performed at 25 °C, and solution separation from the adsorbent was achieved by filtration through 0.45 μm membrane filters. Heavy metal ion concentrations were then determined using atomic absorption spectrometry (AAS, PerkinElmer PinAAcle 900T, USA). The adsorption capacity (Qt) was evaluated through Equation (1), while the removal efficiency (R%) was calculated by using Equation (2):
where C0 represents the initial concentration, Ct is the concentration at time t, V is the volume of the solution, and m is the mass of the adsorbent [12]. To increase the accuracy of the data, each experiment was repeated 3 times.
3. Results and Discussion
3.1. Transformation Pathway from Kaolin to Zeolite LTA
The transformation of kaolin into LTA zeolite typically follows a multi-step process, which can be described and refined as follows (Figure 2). Initially, kaolin was combined with NaOH in a 10:9 mass ratio and subjected to calcination at 550 °C, yielding metakaolin by dihydroxylation and structural reconfiguration, wherein aluminium transitions to tetrahedral/pentahedral coordination. In the second phase, hydrothermal synthesis is conducted by including water at a 1:10 ratio to the precursor mixture, followed by heating at 90 °C. This improved alkaline activation enhances the crystallization of pure LTA zeolite, reducing amorphous byproducts in contrast to traditional techniques. Meticulous regulation of these ratios improves the reactivity of metakaolin and the purity of the end product. The resultant LTA zeolites, characterised by their cubic architecture and elevated surface area, exhibit significant potential for heavy metal adsorption and other advanced applications.

Figure 2.
The transformation process: from kaolin to metakaolin to LTA zeolite.
The transformation of kaolin into LTA zeolite entails a stepwise procedure of thermal activation followed by alkaline hydrothermal synthesis, each facilitating critical structural and phase changes. This technique facilitates the synthesis of highly reactive and functional materials for sophisticated industrial applications. The thermal Activation Pathway involves:
- Key process: Dehydroxylation (550–650 °C).
- Structural change: Al coordination shifts from octahedral to tetrahedral/pentahedral.
- Industrial significance: Enhanced pozzolanic activity for cement/concrete.
Followed by the aluminosilicate rearrangement (Metakaolin as a Precursor for Zeolite LTA Synthesis) based on:
- Critical step: Alkaline activation (NaOH solutions or fusion at 550 °C).
- Phase evolution: Amorphous → crystalline LTA frameworks via hydrothermal treatment.
- Morphological features: Cubic crystals (2 µm avg. size) with intergrown twins.
Hierarchical Transformation Process
The synthesis of zeolite LTA from kaolin involves a series of controlled thermal and chemical treatments that facilitate the transformation of the raw clay mineral into a highly crystalline zeolite structure. Each major stage in this process is characterized by distinct reaction conditions and leads to specific phase developments, as outlined below in Table 2:

Table 2.
Process conditions and phase development in the synthesis of zeolite LTA from kaolin.
3.2. XRD Analysis
The X-ray diffraction patterns presented in Figure 3 compare the crystalline structures of kaolin, metakaolin, and LTA zeolite. From Figure 3, the kaolin sample exhibits sharp and well-defined peaks characteristic of its highly ordered layered silicate structure. The result, suggesting the existence of several phases, such as illite (K2Al4Si8O24) and amorphous phases of quartz (SiO2), in addition to kaolin (Al2Si2O5(OH)4) [35,36]. Upon calcination, the transformation to metakaolin was evidenced by the broadening and reduction in intensity of the diffraction peaks, indicating a loss of long-range order and the formation of an amorphous aluminosilicate phase [37,38]. Following hydrothermal synthesis, the XRD pattern of the LTA zeolite displays new, intense peaks distinct from those of kaolin and metakaolin, confirming the successful crystallisation of the LTA zeolite framework. The XRD pattern of LTA zeolite exhibits characteristic peaks at specific angles (2θ°) corresponding to its distinct crystal planes. The main peaks generally appear around 2θ° of 7.2°, 10.2°, 12.4°, 16.2°, 21.6°, 24°, and 27.1°. This pattern completely matches the JCPDS no. 71-0784 standard LTA (Na) zeolite diffraction pattern [39,40,41]. The presence and intensity of these peaks indicate a good crystallinity of LTA zeolite. The absence of additional peaks indicates that the sample is pure, without secondary phases.
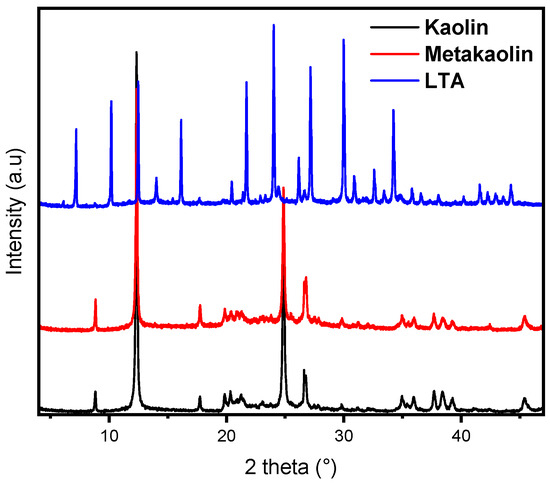
Figure 3.
XRD patterns of kaolin, metakaolin, and LTA zeolite.
3.3. SEM and EDS Measurement
Figure 4 presents the SEM images of the prepared LTA zeolite. At lower magnifications (40 μm and 20 μm scale bars), the images display aggregates of diminutive, irregularly shaped particles, indicating the preliminary phases of crystallisation and the presence of precursor material. With further magnification (5 μm scale bars), distinct cubic crystals became apparent inside the aggregates, indicating the successful nucleation and development of the LTA zeolite phase. These cubic crystals are indicative of LTA zeolite and exhibit a significant degree of homogeneity in both morphology and dimensions [42,43]. At maximum magnification (4 μm scale bars), distinct cubic crystals are observable, displaying smooth surfaces and sharp edges. The crystals measure roughly 2–3 μm, validating the successful conversion of kaolin into high-quality LTA zeolite. The uniform cubic morphology and limited size distribution shown in the photos suggest effective crystallization and the establishment of distinct zeolite structures, crucial for enhanced adsorption characteristics and efficacy in heavy metal remediation.
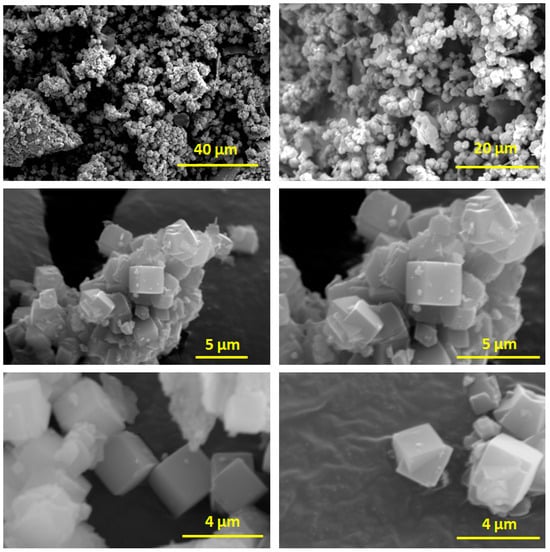
Figure 4.
SEM images of the prepared LTA zeolite.
Energy Dispersive Spectroscopy (EDS) provides detailed information about the elements present in the sample, such as silicon (Si), aluminium (Al), oxygen (O), sodium (Na), and other elements. Figure 5 shows EDS spectra of the prepared LTA zeolite. The SEM-EDS study validates the effective hydrothermal synthesis of zeolite LTA (Linde Type A) from kaolin precursors, as demonstrated by the distinctive element composition and morphological characteristics. The elemental mapping indicates a uniform distribution of framework components: oxygen (43.64 wt%, 55.68 at%) as the primary bridging element, silicon (20.18 wt%, 14.67 at%) and aluminum (18.86 wt%, 14.27 at%) constituting the tetrahedral framework, with a Si/Al molar ratio of approximately 1.03, aligning with the theoretical LTA structure that necessitates Si/Al ≈ 1.0 for optimal ion-exchange characteristics [44,45]. The red zone in the elemental mapping corresponds to regions where the concentration of the analyzed element is highest, indicating a uniform and locally intense distribution of key components such as oxygen, silicon, and aluminum within the LTA zeolite structure. The substantial sodium concentration (17.32 wt%, 15.38 at%) signifies the existence of charge-balancing Na+ cations within the zeolitic cavities, crucial for the cation exchange process that facilitates heavy metal adsorption applications. The SEM micrographs illustrate the development of distinct cubic crystallites characteristic of LTA topology, exhibiting a uniform particle size distribution and the absence of amorphous phases, thereby affirming the complete conversion of the clay aluminosilicate precursor into the intended zeolitic framework under alkaline hydrothermal conditions, essential for attaining elevated adsorption capacity and selectivity in wastewater treatment processes.
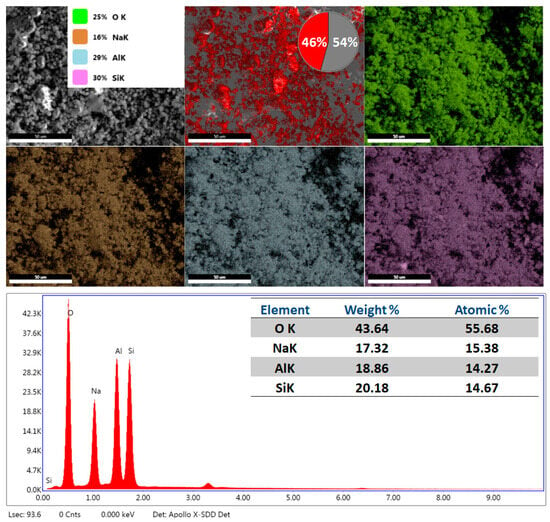
Figure 5.
EDS spectra and element mapping images of the prepared LTA zeolite.
3.4. Zeta Potential Measurement
The zeta potential measurement of LTA zeolite as a function of pH is illustrated in Figure S1 (see the Supplementary Data). From the figure, a clear transition from low (near-neutral) to highly negative values as the pH increases from 2 to 12. At acidic pH (2–4), the zeta potential is close to zero, indicating protonation of surface silanol (Si–OH) and aluminol (Al–OH) groups, which neutralize surface charges. As the pH increases, deprotonation of these surface hydroxyl groups occurs, resulting in the formation of negatively charged sites (Si–O−, Al–O−) that dominate at neutral and basic conditions [46]. This shift leads to strongly negative zeta potentials (up to −54 mV in this study), enhancing electrostatic dispersion and colloidal stability. These changes in surface charge directly affect the adsorption behavior of zeolite toward cationic contaminants, as electrostatic attraction is maximized above the point of zero charge (pHpzc), typically in the pH range 6–12 for zeolite A [46].
3.5. Adsorption Study
3.5.1. Impact of pH and Adsorbent Dosage
Solution pH, one key factor that significantly affected the sorption of heavy metals, determined the metal speciation and surface charge of the adsorbent [47]. Figure 6 depicts the influence of pH on the efficacy of Pb2+ and Cu2+ ion removal by synthetic zeolite. At a pH of 1, the removal efficiency for both metals was negligible, with values under 10%. As the pH rises, removal performance significantly enhances for Pb2+, efficiency surpasses 90% at pH 4, while, for Cu2+, it nears comparable values by pH 5. Beginning at a pH of 5, both metals are extracted with exceptional efficiency, maintaining a recovery rate of 95% or higher up to a pH of 11. This phenomenon can be elucidated by the conflict between hydronium ions (H3O+) and metal ions at low pH, which restricts the adsorption of Pb2+ and Cu2+ onto the zeolite [48]. At low pH, the zeolite surface exhibits a higher positive charge, and the prevalence of H3O+ ions further inhibits the metal adsorption. The main Pb2+ species were presented as Pb2+ at pH ≤ 5, and the competition from H+ decreased the Pb2+ removal via ion exchange [28]. As the pH rises, the dissociation of surface hydroxyl groups (Si-OH and Al-OH) generates additional negatively charged sites, hence augmenting the attraction and binding of metal cations. At elevated pH levels (above 8), the elimination of metals may furthermore entail the precipitation of metal hydroxides; however, within the specified pH range (2–11), the substantial removal efficacy is predominantly ascribed to adsorption, particularly beneath the precipitation threshold. As the pH rises, the dissociation of surface hydroxyl groups (Si-OH and Al-OH) on the zeolite A framework generates additional negatively charged sites, thereby enhancing the attraction and binding of metal cations such as Cu2+ and Pb2+. At elevated pH levels (above 8), metal removal can also involve precipitation of metal hydroxides; however, within the studied pH range (2–11), the dominant removal mechanism is adsorption, particularly below the precipitation threshold. The crystalline microporous structure of zeolite A, composed of negatively charged aluminosilicate frameworks balanced by exchangeable cations, provides numerous active sites for ion exchange and adsorption of heavy metals. Studies have demonstrated that increasing pH favors deprotonation of hydroxyl groups, thus increasing adsorption capacities for Cu2+ and Pb2+ ions. Additionally, the zeolite’s ion-exchange properties facilitate selective adsorption, making it effective for treating metal-contaminated water [49,50,51,52]. The data indicate that the best heavy metal removal using zeolite occurs at neutral to slightly alkaline pH, with markedly reduced efficacy in severely acidic environments.
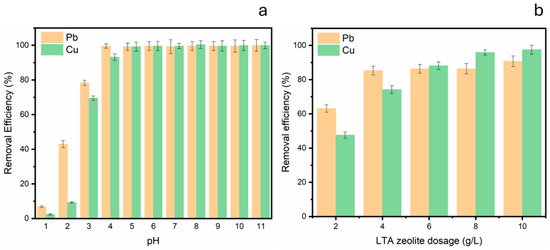
Figure 6.
(a) Influence of initial pH, conditions: [Cu2+]0 = [Pb2+]0 = 80 mg/L, LTA zeolite dosage 10 mg/L and room temperature. (b) Effect of LTA zeolite adsorbent dosage on the removal of heavy metals from wastewater, conditions: [Cu2+]0 = [Pb2+]0 = 80 mg/L, pH = 5 and room temperature.
Figure 6b depicts the impact of LTA zeolite dose on the efficacy of Pb2+ and Cu2+ ion removal from aqueous solution. Increasing the amount of the adsorbent within a certain range can achieve the desired removal effect and reduce costs. As the zeolite dosage escalates from 2 to 10 g/L, the removal effectiveness for both metals markedly enhances. The removal efficiency for Pb2+ increases from approximately 65% at 2 g/L to above 95% at 10 g/L. Likewise, with Cu2+, the efficiency escalates from roughly 45% to over 90% with increasing dosage. This pattern suggests that increased zeolite dosages offer additional adsorption sites, hence improving the extraction of heavy metals from the solution [53]. Moreover, at every dosage level, Pb2+ is eliminated more effectively than Cu2+, perhaps due to variances in their attraction for the zeolite surface. The findings indicate that augmenting the quantity of LTA zeolite effectively enhances the elimination of Pb2+ and Cu2+ ions from water.
3.5.2. Kinetic Studies
Figure 7 presents the adsorption kinetics of Pb2+ and Cu2+ ions onto the synthesized zeolite. The adsorption process for both metals occurs rapidly within the first 60 min, followed by a slower phase until equilibrium is reached at around 250 min. The experimental maximum adsorption capacity is 63 mg/g for Pb2+ and 56.2 mg/g for Cu2+. This indicates that most of the adsorption takes place quickly, and the equilibrium is established within four hours. The contact duration between the metal ions and the nanosorbent plays an important role in the cost-effective treatment of wastewater. By increasing the duration of contact between contaminants and adsorbent, the adsorption efficiency significantly enhances because of the increased interaction between metals and chelation active sites. Typically, at the start of the adsorption process, the removal efficiency starts off fast and then builds up progressively. This happens as a result of the accessibility of the initial free active sites available for adsorption, which are progressively taken up by chelated metals over time [54]. Adsorption kinetic modelling evaluates the effectiveness of the adsorbent through the adsorption rate and mechanism [55]. The kinetic data were modelled by applying three kinetic models: pseudo-first-order, pseudo-second-order, and Elovich. Equations of kinetic models and the model parameters of each of the isotherm models, with the value of R2, are presented in Table S1. The pseudo-first-order kinetic model is normally applied at the beginning stage of the adsorption process. Pseudo-second-order kinetic model predicts the chemisorption mechanism over time until the equilibrium state, while the Elovich model assumes the heterogeneous-based chemisorption process with the pure assessment of the kinetic behaviours [56]. The kinetic curves show that the pseudo-second-order model provides the best fit for both Pb2+ and Cu2+ adsorption, as evidenced by the close agreement between the experimental data (points) and the model predictions (R2 = 0.995 for Cu2+ and 0.994 for Pb2+). This suggests that chemisorption is the dominant mechanism governing the adsorption process [3]. The pseudo-first-order and Elovich models fit the data less accurately, particularly at later stages of adsorption. Overall, the figure demonstrates that the adsorption of Pb2+ and Cu2+ ions onto zeolite is characterised by a rapid initial uptake, followed by slower adsorption as equilibrium approaches, and that the process is best described by the pseudo-second-order kinetic model, indicating the importance of chemical interactions between the metal ions and the zeolite surface, involving valence forces and potential electron sharing or exchange between the adsorbate and adsorbent.
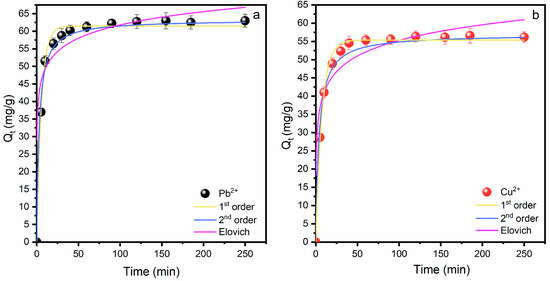
Figure 7.
Kinetic modelling of Pb2+ and Cu2+ removal: mechanistic insights from First-Order, Pseudo-Second-Order and Elovich models: (a) Pb2+ and (b) Cu2+. Conditions: [Cu2+]0 = [Pb2+]0 = 80 mg/L, LTA zeolite dosage 10 g/L, pH = 5 and room temperature.
Further, the Boyd kinetic model was used to determine the actual rate-controlling step involved in the heavy metals adsorption onto the zeolite LTA. The Boyd kinetics equation is represented by Equation (3) [57]:
where F = Qt/Qe. The plot of Bt vs. time is called a Boyd plot (Figure 8), which is employed to distinguish between external transport (film diffusion) and intraparticle diffusion. If the plot gives a straight line passing through the origin, then the adsorption process is governed by an intraparticle diffusion mechanism; otherwise, they are governed by film diffusion or external mass transport [58]. The curves of the Boyd model plot do not pass through the origin, which indicates that external mass transport mainly governs the rate-limiting process of adsorption of Cu2+ and Pb2+ on zeolite LTA.
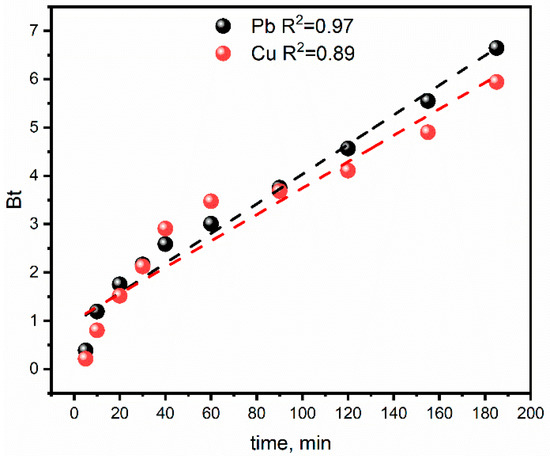
Figure 8.
Plot of Bt vs. t for the adsorption of copper and lead ions. Conditions: [Cu2+]0 = [Pb2+]0 = 80 mg/L, LTA zeolite dosage 10 g/L, pH = 5 and room temperature.
3.5.3. Phenomenological Mass Transfer Kinetics: Chemisorption and Physisorption
The adsorption process is known to be complex and can be governed by multiple processes, such as chemisorption and physisorption. The rates of physisorption and chemisorption can be evaluated by applying the adsorption onto the active sites (AOAS) model [59] expressed by Equation (4):
where kche [g/(mg·min)] and kphy (1/min) represent the chemisorption and physisorption rate coefficients, respectively. The left side term (dQt/dt) can be estimated by applying the second-order kinetic model (Equation (S2) in Supplementary Information) to obtain the first derivative of Qt with respect to time t. The rates of physisorption and chemisorption can be evaluated through Equations (5) and (6) (Figure 9):
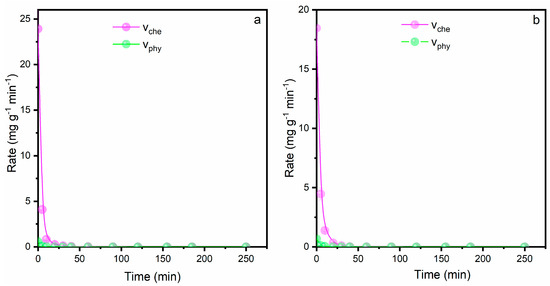
Figure 9.
Contributions of physisorption and chemisorption rates referred to (a) Pb2+ and (b) Cu2+ removal. Conditions: [Cu2+]0 = [Pb2+]0 = 80 mg/L, LTA zeolite dosage, pH = 5 and room temperature.
The AOAS model greatly describes the process with an R2 > 0.995. The fastest chemisorption and physisorption rates during the copper and lead removal were reached at the initial stage, before reducing sharply for a longer contact time until reaching equilibrium. As far as the Pb2+ removal is concerned, the chemisorption rate dominates the adsorption process in all the investigated time range since its contribution is higher than the physisorption rate.
3.5.4. Isothermal Studies
To define these adsorption behaviours, three conventional nonlinear isotherm models were employed: the Langmuir and the Freundlich models, in addition to the Temkin model for comparative analysis (Figure 10). The Langmuir isotherm posits monolayer adsorption on a uniform surface with equivalent adsorption sites and no interactions among adsorbed species, whereas the Freundlich isotherm is an empirical model that characterizes adsorption on heterogeneous surfaces with sites exhibiting diverse adsorption energies. The adsorption isotherms depicted in Figure 8 demonstrate the equilibrium relationship between the concentration of heavy metal ions (Pb2+ and Cu2+) in solution (Ce) and their respective adsorption capacity (Qe) on the produced LTA zeolite adsorbent. The maximum adsorption capacity values are 69.9 mg/g for Pb2+ and 59.2 mg/g for Cu2+. The experimental data points exhibit classic Type I isotherm behaviour, marked by swift initial absorption at low concentrations, succeeded by the establishment of a plateau at elevated equilibrium values. The fitted curves indicate that both the Langmuir and the Freundlich models adequately describe the experimental data; however, the Langmuir model demonstrates a superior fit quality, as evidenced by the smooth curve progression, signifying its superior fit to the metal adsorption data and affirming the surface homogeneity of the adsorbent. This observation aligns with literature that reports higher correlation coefficients (R2) for Langmuir isotherms in zeolite-based heavy metal adsorption systems [21,60]. The comparison indicates that Pb2+ attains a greater maximum adsorption capacity (Qm) than Cu2+, approximately 81.5 mg/g versus 67.5 mg/g for Cu2+, suggesting a preferential adsorption of lead ions, likely attributable to variations in ionic radius, electronegativity, and hydration energy that affect the interaction strength with the zeolite framework [61]. Particularly, the low-hydrated ionic radius of Pb2+ (4.01 Å) compared to the Cu2+ one (4.19 Å) may justify the reported data. Likewise, the Pauling electronegativity values followed as Pb2+ > Cu2+, reflecting that Pb2+ possessed the greatest ionic potential [61]. The effective fitting of these models indicates that the synthesized LTA zeolite demonstrates advantageous adsorption properties for heavy metal removal, with the Langmuir model implying a primary monolayer covering mechanism on the zeolite surface. The value of 1/n obtained from the Freundlich adsorption isotherm is < 1 and also indicates a favorable condition for adsorption [62].
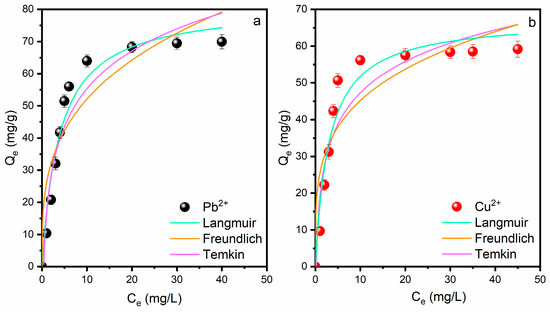
Figure 10.
Application of isothermal models to adsorption data: (a) Pb2+ and (b) Cu2+. Conditions: LTA zeolite dosage 10 g/L, pH = 5, and room temperature.
The separation factor (RL) is then calculated using Equation (7):
RL values were found to be 0.037 for Pb2+ ions and 0.029 for Cu2+ ions. The values range from 0 to 1, confirming favorable conditions [63] for the adsorption of Pb2+ and Cu2+ ions on the surface of zeolite LTA.
3.5.5. Adsorption Performance in Binary System (Cu2+/Pb2+)
In order to further evaluate the practical applicability of the synthesized LTA zeolite under realistic wastewater conditions, adsorption experiments were conducted using binary-metal systems. The kinetics of Cu2+ and Pb2+ ion adsorption are illustrated in Figure S2 (Supplementary Data). As shown in the figure, the adsorption capacity (Qt) for Pb2+ rapidly increases, reaching approximately 28.4 mg/g at equilibrium (after ~50 min), while the maximum adsorption for Cu2+ is significantly lower, plateauing around 19 mg/g under the same conditions. By comparison, in the single-ion systems, the adsorption capacities were significantly higher: 63 mg/g for Pb2+ and 56.16 mg/g for Cu2+. The presence of Pb2+ caused a pronounced decrease of about 65% in Cu2+ uptake, whereas Pb2+ removal was only moderately reduced (by roughly 55%) under competitive conditions. This distinct difference demonstrates competitive adsorption: the presence of Pb2+ markedly decreases the capacity for Cu2+ uptake, whereas Pb2+ removal efficiency remains high and only slightly reduced compared to single-ion conditions. These results indicate that the synthesized zeolite exhibits preferential adsorption of Pb2+ over Cu2+, attributed to the lower hydration energy of Pb2+ (−1481 kJ/mol vs. −1760 kJ/mol for Cu2+) and its higher polarizability [32,60,64]. Such properties enhance the ion exchange and surface complexation mechanisms, making Pb2+ more competitive for active sites. Previous reports confirm this selectivity, with zeolites showing Pb2+ > Cu2+ in adsorption order in multi-component solutions. The selectivity for Pb2+ ions in the presence of Cu2+ provides significant benefits for practical wastewater treatment, where multiple heavy metal contaminants frequently coexist. This preferential adsorption leads to higher removal efficiency for Pb2+ compared to other metals and enables effective purification even in competitive conditions. Mechanistic studies consistently highlight that factors such as hydrated ionic radius and hydration energy contribute to zeolite selectivity, with similar competitive performance and mechanistic explanations reported in the literature [65,66].
3.5.6. Regeneration and Reusability of the Adsorbent
The regeneration and reusability of zeolite A for the removal of Cu2+ and Pb2+ ions were evaluated under the following conditions: initial metal concentration ([Cu2+]0 = [Pb2+]0 = 80 mg/L), zeolite dosage of 10 g/L, pH 5, and room temperature. Figure 11 shows the reusability performance of zeolite A for Cu2+ and Pb2+ removal over four consecutive cycles. The adsorbent underwent multiple adsorption–desorption cycles, with metal desorption achieved using an acid solution (HNO3), allowing efficient recovery of adsorbed metals and restoration of active sites. This approach demonstrates the feasibility of chemical regeneration, enabling zeolite A to be reused effectively over successive cycles. After each cycle, the removal efficiency remained above 83% for both Cu2+ and Pb2+ ions, indicating high stability and resilience of the adsorbent. The gradual reduction in efficiency after four cycles reflects partial saturation or minor loss in available exchange sites, yet the material continues to exhibit practical applicability [28,29,67].
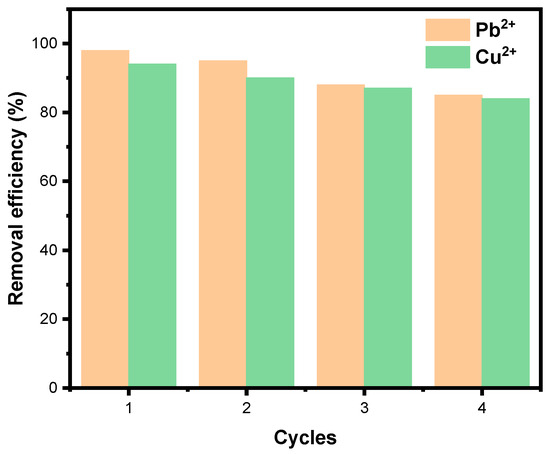
Figure 11.
Reusability performance of LTA zeolite for Cu2+ and Pb2+ removal over successive cycles. Conditions: [Cu]0 = [Pb]0 = 80 mg/L, LTA zeolite dosage = 10 g/L, pH = 5 and room Temperature.
3.5.7. Adsorption Mechanism
Because of their chemical nature, the dominant adsorption mechanism for natural zeolites is ion exchange since zeolite cations can exchange with other cations in liquid solutions [31,68]. Besides, other mechanisms may be considered, such as electrostatic attraction, intrapore adsorption, surface complexation, and surface precipitation [28] (Figure 12).
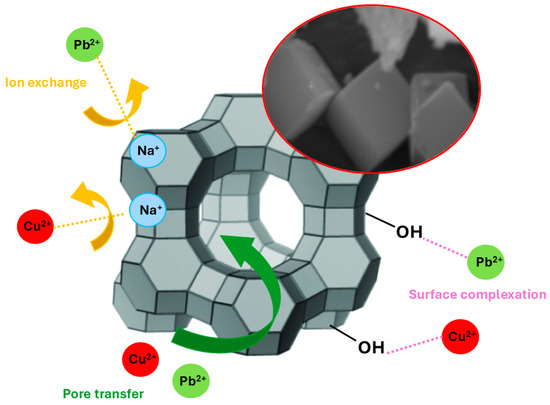
Figure 12.
Adsorption mechanism of Pb2+ and Cu2+ onto zeolite LTA.
Particularly, heavy metal ions may exchange with Na+ ions or react with the OH groups through surface complexation phenomena. Besides, due to the structure of zeolite-type materials, pore diffusion into existing cages may be considered. The adsorption kinetics and equilibrium isotherms confirmed that the removal of Cu2+ and Pb2+ ions by zeolite A is governed by physico-chemical adsorption mechanisms, predominantly ion-exchange processes. Detailed insights into the adsorption mechanism were obtained from the SEM and EDS analyses following sorption of the metal ions. SEM micrographs of ZeoliteA@Cu and ZeoliteA@Pb (see Figure S3 in the Supplementary Data) retained the characteristic crystalline morphology, indicating that the structural integrity of the zeolite remained stable after adsorption–desorption cycles. EDS elemental mapping demonstrated a significant reduction in the Na content concurrently with the appearance of strong Cu and Pb signals (see Figure S4 in the Supplementary Data), confirming the effective cationic exchange between the framework sodium ions and the adsorbed metal ions. The persistence of Al and Si peaks in the EDS spectra suggests that the zeolitic framework is preserved throughout repeated adsorption cycles. Moreover, after acid regeneration (desorption), the zeolite exhibited comparable SEM and EDS profiles to the initial material, signifying the reversibility of the exchange process and the resilience of the crystalline network. These results reveal that the adsorption and desorption of Cu2+ and Pb2+ do not compromise the chemical composition or microstructure of zeolite A, supporting its role as an effective and reusable adsorbent for the removal of toxic metals from aqueous solutions in sustainable water treatment applications.
3.6. Comparative Study
The performance of LTA zeolite synthesized from kaolin in adsorbing copper (Cu2+) and lead (Pb2+) ions from aqueous solutions was compared with various inorganic and composite adsorbents previously reported in the literature. Two primary considerations are emphasized: maximum adsorption capacity (Qm, mg/g) and preparation cost, both crucial for large-scale wastewater treatment. Table 3 presents the maximum adsorption capacities of these adsorbents. Advanced materials such as Fe3O4@SiO2 functionalized nanocomposites [3] and PAN-NaY-zeolite [69] frequently show high Qm values, particularly for Pb2+. Natural adsorbents like banana peel and diatomite composites offer ecological and cost advantages but generally deliver lower performance. Remarkably, the LTA zeolite derived from kaolin in this work demonstrates excellent adsorption capacities, with Qm values reaching 81.5 mg/g for Pb2+ and 67.5 mg/g for Cu2+. These results are competitive with or superior to many other materials in the table, including several complex nanocomposites, highlighting the practical efficiency of kaolin-based LTA zeolite for heavy metal removal. The synthesis of LTA zeolite from kaolin is also notable for its simplicity, scalability, and low cost, relying on abundant raw materials and straightforward calcination and hydrothermal steps. Unlike some high-capacity engineered adsorbents that require costly functionalization and multi-step processes, kaolin-derived LTA zeolite offers an optimal balance between performance and economic feasibility. With its high adsorption capacity Qm = 81.5 mg/g for Pb2+ and 67.5 mg/g for Cu2+ and cost-effective synthesis, LTA zeolite from kaolin emerges as a strong candidate for large-scale environmental remediation applications, especially for treating water contaminated by heavy metals. This new data reinforces its position as a practical alternative to both expensive engineered nanomaterials and less efficient natural adsorbents.

Table 3.
Qm value of adsorbents related to Cu2+ and Pb2+ removal.
4. Conclusions
Zeolite LTA cubic crystals were synthesised for the removal of Cu2+ and Pb2+ ions from wastewater, adopting a simple process based on the reutilization of natural clay such as kaolin. The results of characterisation analyses showed that a crystalline adsorbent with a mean crystal dimension of 2 μm. The adsorption process appears to be favoured at pH > 5 and higher adsorbent dosage. The zeolite LTA showed great adsorption capacity in the order Pb2+ > Cu2+, as proved by the Langmuir model. Then, the ability of zeolite LTA to adsorb the selected heavy metals could be better fitted by a pseudo-second-order model, indicating a chemisorption process. The effective chemical regeneration of zeolite A via acid desorption ensures its sustained adsorption capacity and operational durability for heavy metal removal. This reusability highlights the material’s potential as a cost-effective and environmentally friendly adsorbent for water treatment applications. Thus, according to the obtained results, the prepared adsorbent is able to reduce the concentrations of heavy metals in wastewater, opening the route to the application of novel, cheap materials for water remediation. Further research is needed to investigate the storage conditions and explore the feasibility of scaling up nanoparticle synthesis for commercial applications in pollutant removal.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13103060/s1.
Author Contributions
S.A.: resources, methodology, formal analysis and methodology writing—original draft. A.M.: conceptualization, methodology, formal analysis, investigation, writing—original draft. A.S.: Resources, methodology writing—original draft. B.A.: methodology, formal analysis, and investigation. M.H.: resources and methodology. B.B.: review & editing, methodology, validation. M.S.: review & editing, methodology, validation. G.V.: Formal analysis and investigation, formal analysis, review & editing, funding acquisition. Z.A.: conceptualization, formal analysis, supervision, review & editing. M.A.: conceptualization, validation, formal analysis, review & editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through the Large Research Project under grant number RGP2/132/46.
Institutional Review Board Statement
The authors declare that all procedures followed were in accordance with the ethical standards.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through the Large Research Project under grant number RGP2/132/46.
Conflicts of Interest
All the authors explicitly declared the absence of any conflict of interest.
References
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.-S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A Critical Review on Various Remediation Approaches for Heavy Metal Contaminants Removal from Contaminated Soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef]
- Topare, N.S.; Wadgaonkar, V.S. A Review on Application of Low-Cost Adsorbents for Heavy Metals Removal from Wastewater. Mater. Today Proc. 2023, 77, 8–18. [Google Scholar] [CrossRef]
- Liu, Z.; Lei, M.; Zeng, W.; Li, Y.; Li, B.; Liu, D.; Liu, C. Synthesis of Magnetic Fe3O4@SiO2-(-NH2/-COOH) Nanoparticles and Their Application for the Removal of Heavy Metals from Wastewater. Ceram. Int. 2023, 49, 20470–20479. [Google Scholar] [CrossRef]
- Sarker, A.; Kim, J.-E.; Islam, A.R.M.T.; Bilal, M.; Rakib, M.R.J.; Nandi, R.; Rahman, M.M.; Islam, T. Heavy Metals Contamination and Associated Health Risks in Food Webs—A Review Focuses on Food Safety and Environmental Sustainability in Bangladesh. Env. Sci Pollut. Res 2022, 29, 3230–3245. [Google Scholar] [CrossRef]
- Kao, R.T.; Dault, S.; Pichay, T. Understanding the Mercury Reduction Issue: The Impact of Mercury on the Environment and Human Health. J. Calif. Dent. Assoc. 2004, 32, 574–579. [Google Scholar] [CrossRef]
- Ferreira-Rodríguez, N.; Castro, A.J.; Tweedy, B.N.; Quintas-Soriano, C.; Vaughn, C.C. Mercury Consumption and Human Health: Linking Pollution and Social Risk Perception in the Southeastern United States. J. Environ. Manag. 2021, 282, 111528. [Google Scholar] [CrossRef]
- Xu, H.; Jia, Y.; Sun, Z.; Su, J.; Liu, Q.S.; Zhou, Q.; Jiang, G. Environmental Pollution, a Hidden Culprit for Health Issues. Eco-Environ. Health 2022, 1, 31–45. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Angaru, G.K.R.; Lingamdinne, L.P.; Choi, Y.-L.; Koduru, J.R.; Yang, J.-K.; Chang, Y.-Y. Encapsulated Zerovalent Iron/Nickel-Fly Ash Zeolite Foam for Treating Industrial Wastewater Contaminated by Heavy Metals. Mater. Today Chem. 2021, 22, 100577. [Google Scholar] [CrossRef]
- Sheth, Y.; Dharaskar, S.; Khalid, M.; Sonawane, S. An Environment Friendly Approach for Heavy Metal Removal from Industrial Wastewater Using Chitosan Based Biosorbent: A Review. Sustain. Energy Technol. Assess. 2021, 43, 100951. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.M. A Comprehensive Review on Conventional and Biological-Driven Heavy Metals Removal from Industrial Wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Viscusi, G.; Gorrasi, G. Fabrication of Novel Multifunctional Copper-Functionalized Hemp Fibers to Remove Anionic Dye and Non-Steroidal Anti-Inflammatory Drugs from Wastewaters. Chemosphere 2025, 372, 144039. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, H.; Lu, Y.-Y.; Ren, Z.-Q.; Gao, N.; Wang, J.-J.; Huang, B.-C.; Jin, R.-C. In-Situ Synthesis of Lanthanum-Coated Sludge Biochar for Advanced Phosphorus Adsorption. J. Environ. Manag. 2025, 373, 123607. [Google Scholar] [CrossRef]
- Mokhtar, A.; Abdelkrim, S.; Hachemaoui, M.; Boukoussa, B.; Djelad, A.; Sassi, M.; Issam, I.; Patole, S.P.; Viscusi, G.; Abboud, M. Optimization of Zeolite LTA Formation from Kaolin Using Fusion/Hydrothermal Method: Crystallization Parameters and Box-Behnken Experimental Design. Appl. Clay Sci. 2025, 275, 107877. [Google Scholar] [CrossRef]
- Nir, S.Z.; Salem, A.; Salem, S. Application of Clay-Based Waste Collected in Landfill of Vegetable Oil Refinery for Immobilization of Heavy Metal Ions from Wastewater of Zinc Industry through Fabrication of Zeolite LTA and Hydroxysodalite. Sustain. Chem. Pharm. 2024, 39, 101618. [Google Scholar] [CrossRef]
- El-Kordy, A.; Elgamouz, A.; Abdelhamid, A.; Kawde, A.-N.; Tijani, N.; Lemdek, E.M. Manufacturing of Novel Zeolite-Clay Composite Membrane from Natural Clay and Diatomite, an Electrochemical Study of the Surface and Application towards Heavy Metals Removal. J. Environ. Chem. Eng. 2024, 12, 112143. [Google Scholar] [CrossRef]
- Jiang, W.; Xing, Y.; Mo, L.; Liao, J.; Chen, W.; Wang, H.; Wang, T. Synthesis of Polyethylenimine Modified Sugarcane Bagasse Cellulose and Its Competitive Adsorption of Pb2+, Cu2+ and Zn2+ from Aqueous Solutions. Desalination Water Treat. 2022, 270, 172–184. [Google Scholar] [CrossRef]
- Mazouz, F.; Abdelkrim, S.; Mokhtar, A.; Zahraoui, M.; Abdelmoumène, B.; Fouatih, S.L.; Hasnaoui, M.A.; Bengueddach, A.; Sassi, M.; Djelad, A. Removal of Cu(II) Ions from Aqueous Solutions Using Chitosan/Zeolite Composites: Effects of the Size of the Beads and the Zeolitic Content. J Polym Environ. 2023, 31, 193–209. [Google Scholar] [CrossRef]
- Silva, M.C.; Crespo, L.H.S.; Cazetta, A.L.; Silva, T.L.; Spessato, L.; Almeida, V.C. Activated Carbon Fibers of High Surface Area from Corn Husk: Mono and Multicomponent Adsorption Studies of Pb2+ and Cu2+ Ions from Aqueous Solution. J. Mol. Liq. 2024, 405, 124919. [Google Scholar] [CrossRef]
- Marhoon, A.A.; Hasbullah, S.A.; Asikin-Mijan, N.; Mokhtar, W.N.A.W. Hydrothermal Synthesis of High-Purity Zeolite X from Coal Fly Ash for Heavy Metal Removal: Kinetic and Isotherm Analysis. Adv. Powder Technol. 2023, 34, 104242. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy Metal Adsorption with Zeolites: The Role of Hierarchical Pore Architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Payne, K.B.; Abdel-Fattah, T.M. Adsorption of Divalent Lead Ions by Zeolites and Activated Carbon: Effects of pH, Temperature, and Ionic Strength. J. Environ. Sci. Health Part A 2004, 39, 2275–2291. [Google Scholar] [CrossRef]
- Prepilková, V.; Poništ, J.; Ďuricová, A.; Salva, J.; Schwarz, M.; Samešová, D.; Mordačová, M. Adsorption of Cd and Mn from Neutral Mine Effluents Using Bentonite, Zeolite, and Stabilized Dewatered Sludge. Environ. Sci Eur 2024, 36, 100. [Google Scholar] [CrossRef]
- Kovo, A.S.; Holmes, S.M.; Rios, C.A.; Otaru, A.J.; Abdulkareem, A.S.; Eluwa, V.C. Synthesis of Zeolites from Different Kaolin Deposits Worldwide. Appl. Clay Sci. 2025, 269, 107757. [Google Scholar] [CrossRef]
- Zdretsov, I.M.; Gerasimov, A.M. Green and Low-Cost Synthesis of Zeolites from Kaolin: A Promising Technology or a Delusion? React. Chem. Eng. 2024, 9, 1994–2027. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, M.; Zhu, L.; Wu, Q.; Meng, X.; Xiao, F.-S. Recent Advances in Sustainable Synthesis of Zeolites. Mater. Today Sustain. 2025, 29, 101065. [Google Scholar] [CrossRef]
- Vogrin, J.; Santini, T.; Peng, H.; Zhao, L.; Vaughan, J. Synthesis of Zeolites Using Kaolin in Concentrated Sodium Hydroxide-Aluminate Solutions. Appl. Clay Sci. 2023, 244, 107106. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of Heavy Metals on Natural Zeolites: A Review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Senila, M.; Cadar, O. Modification of Natural Zeolites and Their Applications for Heavy Metal Removal from Polluted Environments: Challenges, Recent Advances, and Perspectives. Heliyon 2024, 10, e25303. [Google Scholar] [CrossRef]
- Karimi, F.; Khosravi, K. Removal of Pb(II), Cr(III), Co(II), Cu(II) Cations from Aqueous Solutions Using Tetraethylenepentamine-Functionalized HY Cubic Zeolite: Optimization, Characterization, and Mechanistic Insights. Environ. Sci. Pollut. Res. 2024, 31, 65676–65697. [Google Scholar] [CrossRef]
- Shah, K.J.; Yu, J.; Zhang, T.; You, Z.; Kim, H. Simultaneous Removal of Cu(II) And Pb(Ii) From Stormwater Runoff by Y-Type-Zeolite-Modified Bioretention System. Water Air Soil Pollut. 2024, 235, 395. [Google Scholar] [CrossRef]
- Kuldeyev, E.; Seitzhanova, M.; Tanirbergenova, S.; Tazhu, K.; Doszhanov, E.; Mansurov, Z.; Azat, S.; Nurlybaev, R.; Berndtsson, R. Modifying Natural Zeolites to Improve Heavy Metal Adsorption. Water 2023, 15, 2215. [Google Scholar] [CrossRef]
- Mokhtar, A.; Zaiter, K.; Abdelkrim, S.; Sardi, A.; Sassi, M.; Hachemaoui, M.; Boukoussa, B.; Viscusi, G.; Abboud, M. Bimetallic AgNi Doped LTA Zeolite from Kaolin: A Dual-Action Catalyst for Efficient Dye Degradation and Antibacterial Disinfection. Inorg. Chem. Commun. 2025, 180, 114943. [Google Scholar] [CrossRef]
- Celoria, G.; Begni, F.; Paul, G.; Boccaleri, E.; Merlo, V.; Marchese, L.; Bisio, C. Zeolites Derived from Natural Kaolinite for CO2 Adsorption. Processes 2024, 12, 194. [Google Scholar] [CrossRef]
- El Hassani, A.A.; Tanji, K.; El Mrabet, I.; Fahoul, Y.; El Gaidoumi, A.; Benjelloun, A.T.; Sfaira, M.; Zaitan, H.; Kherbeche, A. A Combined Molecular Dynamics Simulation, DFT Calculations, and Experimental Study of the Adsorption of Rhodamine B Dye on Kaolinite and Hydroxyapatite in Aqueous Solutions. Surf. Interfaces 2023, 36, 102647. [Google Scholar] [CrossRef]
- Żbik, M.S.; Raftery, N.A.; Smart, R.S.C.; Frost, R.L. Kaolinite Platelet Orientation for XRD and AFM Applications. Appl. Clay Sci. 2010, 50, 299–304. [Google Scholar] [CrossRef]
- Irfan Khan, M.; Khan, H.U.; Azizli, K.; Sufian, S.; Man, Z.; Siyal, A.A.; Muhammad, N.; Faiz ur Rehman, M. The Pyrolysis Kinetics of the Conversion of Malaysian Kaolin to Metakaolin. Appl. Clay Sci. 2017, 146, 152–161. [Google Scholar] [CrossRef]
- Khaled, Z.; Mohsen, A.; Soltan, A.; Kohail, M. Optimization of Kaolin into Metakaolin: Calcination Conditions, Mix Design and Curing Temperature to Develop Alkali Activated Binder. Ain Shams Eng. J. 2023, 14, 102142. [Google Scholar] [CrossRef]
- Moisés, M.P.; da Silva, C.T.P.; Meneguin, J.G.; Girotto, E.M.; Radovanovic, E. Synthesis of Zeolite NaA from Sugarcane Bagasse Ash. Mater. Lett. 2013, 108, 243–246. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Q. Synthesis and Characterisation of Zeolite LTA with Sheet Structure. Micro Nano Lett. 2020, 15, 433–436. [Google Scholar] [CrossRef]
- Horta-Fraijo, P.; Smolentseva, E.; Simakov, A.; José-Yacaman, M.; Acosta, B. Ag Nanoparticles in A4 Zeolite as Efficient Catalysts for the 4-Nitrophenol Reduction. Microporous Mesoporous Mater. 2021, 312, 110707. [Google Scholar] [CrossRef]
- Yu, S.; Kwon, S.; Na, K. Synthesis of LTA Zeolites with Controlled Crystal Sizes by Variation of Synthetic Parameters: Effect of Na+ Concentration, Aging Time, and Hydrothermal Conditions. J. Sol-Gel Sci. Technol. 2021, 98, 411–421. [Google Scholar] [CrossRef]
- Sharma, P.; Yeo, J.; Yu, J.; Han, M.H.; Cho, C.H. Effect of Ethanol as an Additive on the Morphology and Crystallinity of LTA Zeolite. J. Taiwan Inst. Chem. Eng. 2014, 45, 689–704. [Google Scholar] [CrossRef]
- da Silva, A.; Elias, E.B.C.D.; Cruz, T.J.T.; Pinto, F.G.H.S.; de Mello, M.I.S.; Bieseki, L.; Pergher, S.B.C. Synthesis and Cation Exchange of LTA Zeolites Synthesized from Different Silicon Sources Applied in CO2 Adsorption. Coatings 2024, 14, 680. [Google Scholar] [CrossRef]
- Painer, F.; Baldermann, A.; Gallien, F.; Eichinger, S.; Steindl, F.; Dohrmann, R.; Dietzel, M. Synthesis of Zeolites from Fine-Grained Perlite and Their Application as Sorbents. Materials 2022, 15, 4474. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.L.; Kieu, T.T.; Nguyen, T.T.T.; Truong, T.T.T.; Hoang, D.T.; Vu, T.L.C.; Nguyen, T.M.T.; Le, T.S.; Doan, T.H.Y.; Pham, T.D. Surface Modification of Zeolite by Cationic Surfactant and the Application on Adsorptive Removal of Azo Dye Ponceau 4R. J. Mol. Struct. 2024, 1304, 137619. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, Y.; Huang, C.; Huang, M.; Chen, J.; Rao, T.; Ran, X. Removal of the Heavy Metal Ion Nickel (II) via an Adsorption Method Using Flower Globular Magnesium Hydroxide. J. Hazard. Mater. 2019, 373, 131–140. [Google Scholar] [CrossRef]
- Kaleem, M.; Anjum Minhas, L.; Zaffar Hashmi, M.; Umer Farooqi, H.M.; Waqar, R.; Kamal, K.; Saad Aljaluod, R.; Alarjani, K.M.; Samad Mumtaz, A. Biogenic Synthesis of Iron Oxide Nanoparticles and Experimental Modeling Studies on the Removal of Heavy Metals from Wastewater. J. Saudi Chem. Soc. 2024, 28, 101777. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Bahmanzadegan, F.; Ghaemi, A. A Comprehensive Review on Novel Zeolite-Based Adsorbents for Environmental Pollutant. J. Hazard. Mater. Adv. 2025, 17, 100617. [Google Scholar] [CrossRef]
- Tao, H.; Liu, Y.; Li, J.; Zhang, C.; Zhao, C.; Yang, X.; Yang, R.T.; Li, Z. Oxidative Adsorption Mechanism-Based Screening of Zeolites for Deep Purification and Recycling of NOx from Humid Gases. Chem. Eng. J. 2023, 475, 146148. [Google Scholar] [CrossRef]
- Inglezakis, V.J. The Concept of “Capacity” in Zeolite Ion-Exchange Systems. J. Colloid Interface Sci. 2005, 281, 68–79. [Google Scholar] [CrossRef]
- Xiang, B.; Ling, D.; Gao, F.; Lou, H.; Gu, H.; Guo, Z. Hexavalent Chromium Induced Tunable Surface Functionalization of Graphite. RSC Adv. 2016, 6, 58354–58362. [Google Scholar] [CrossRef]
- Dubey, R.; Bajpai, J.; Bajpai, A.K. Chitosan-Alginate Nanoparticles (CANPs) as Potential Nanosorbent for Removal of Hg (II) Ions. Environ. Nanotechnol. Monit. Manag. 2016, 6, 32–44. [Google Scholar] [CrossRef]
- Hafeznezami, S.; Zimmer-Faust, A.G.; Dunne, A.; Tran, T.; Yang, C.; Lam, J.R.; Reynolds, M.D.; Davis, J.A.; Jay, J.A. Adsorption and Desorption of Arsenate on Sandy Sediments from Contaminated and Uncontaminated Saturated Zones: Kinetic and Equilibrium Modeling. Environ. Pollut. 2016, 215, 290–301. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. A General Kinetic Model for Adsorption: Theoretical Analysis and Modeling. J. Mol. Liq. 2019, 288, 111100. [Google Scholar] [CrossRef]
- Simonin, J.P.; Bouté, J.; Simonin, J.P.; Bouté, J. Intraparticle Diffusion-Adsorption Model to Describe Liquid/Solid Adsorption Kinetics. Rev. Mex. De Ing. Química 2016, 15, 161–173. [Google Scholar]
- Dotto, G.L.; Buriol, C.; Pinto, L.A.A. Diffusional Mass Transfer Model for the Adsorption of Food Dyes on Chitosan Films. Chem. Eng. Res. Des. 2014, 92, 2324–2332. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of Heavy Metals from Acid Mine Drainage by Natural Zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Yurekli, Y. Determination of Adsorption Characteristics of Synthetic NaX Nanoparticles. J. Hazard. Mater. 2019, 378, 120743. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, R.; Kondal, N.; Kumari, A. Alkaline Earth Metal Doped Nickel Ferrites as a Potential Material for Heavy Metal Removal from Waste Water. Mater. Chem. Phys. 2023, 301, 127582. [Google Scholar] [CrossRef]
- Al-Nayili, A.; Majdi, H.S.; Albayati, T.M.; Saady, N.M.C. Formic Acid Dehydrogenation Using Noble-Metal Nanoheterogeneous Catalysts: Towards Sustainable Hydrogen-Based Energy. Catalysts 2022, 12, 324. [Google Scholar] [CrossRef]
- He, K.; Chen, Y.; Tang, Z.; Hu, Y. Removal of Heavy Metal Ions from Aqueous Solution by Zeolite Synthesized from Fly Ash. Environ. Sci. Pollut. Res. 2016, 23, 2778–2788. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Hwang, H.; Seoung, D.; Kim, H.; Kim, P.; Lee, Y. Relationship Between Electronegativity of the Extra-Framework Cations and Adsorption Capacity for CO2 Gas on Mordenite Framework. Inorg. Chem. 2025, 64, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, Y. Potassium-Assisted Synthesis of SUZ-4 Zeolite as an Efficient Adsorbent for Pb2+ Removal from Wastewater. Sep. Purif. Technol. 2022, 286, 120438. [Google Scholar] [CrossRef]
- Ankrah, A.F.; Tokay, B.; Snape, C.E. Regenerability of Fly-Ash Derived Zeolite NaP1: Evaluation via Copper Recovery. Eng. Rep. 2023, 5, e12591. [Google Scholar] [CrossRef]
- Jiménez-Reyes, M.; Almazán-Sánchez, P.T.; Solache-Ríos, M. Radioactive Waste Treatments by Using Zeolites. A Short Review. J. Environ. Radioact. 2021, 233, 106610. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Bindary, A.A.; Kouta, E.Y.; Guibal, E. Functionalization of Polyacrylonitrile/Na-Y-Zeolite Composite with Amidoxime Groups for the Sorption of Cu(II), Cd(II) and Pb(II) Metal Ions. Chem. Eng. J. 2018, 332, 727–736. [Google Scholar] [CrossRef]
- Marszałek, A.; Puszczało, E. Removal of Copper and Lead Ions from Rainwater with an Alginate-Bentonite Composite: Batch and Column Studies. Desalination Water Treat. 2023, 311, 100–110. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Zhang, K.; Liu, T.; Hu, Y.; Chen, X.; Wang, C.; Huang, X.; Kong, L.; Liu, J. Super Rapid Removal of Copper, Cadmium and Lead Ions from Water by NTA-Silica Gel. RSC Adv. 2018, 9, 397–407. [Google Scholar] [CrossRef]
- Marszałek, A. Adsorption of Copper and Lead from Rainwater Using Adsorbents Based on Diatomite and Calcium Alginate. Desalination Water Treat. 2022, 275, 81–91. [Google Scholar] [CrossRef]
- Eftekhari, M.; Gheibi, M.; Azizi-Toupkanloo, H.; Hossein-Abadi, Z.; Khraisheh, M.; Fathollahi-Fard, A.M.; Tian, G. Statistical Optimization, Soft Computing Prediction, Mechanistic and Empirical Evaluation for Fundamental Appraisal of Copper, Lead and Malachite Green Adsorption. J. Ind. Inf. Integr. 2021, 23, 100219. [Google Scholar] [CrossRef]
- Goswami, A.; Singh, A.K. Silica Gel Functionalized with Resacetophenone: Synthesis of a New Chelating Matrix and Its Application as Metal Ion Collector for Their Flame Atomic Absorption Spectrometric Determination. Anal. Chim. Acta 2002, 454, 229–240. [Google Scholar] [CrossRef]
- El-Sawaf, A.K.; El-Dakkony, S.R.; Zayed, M.A.; Eldesoky, A.M.; Nassar, A.A.; El Shahawy, A.; Mubarak, M.F. Green Synthesis and Characterization of Magnetic Gamma Alumina Nanoparticlesfor Copper Ions Adsorption from Synthetic Wastewater. Results Eng. 2024, 22, 101971. [Google Scholar] [CrossRef]
- Sarı, A.; Tuzen, M. Kinetic and Equilibrium Studies of Biosorption of Pb(II) and Cd(II) from Aqueous Solution by Macrofungus (Amanita rubescens) Biomass. J. Hazard. Mater. 2009, 164, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, F.O.; Musonge, P.; Bakare, B.F. Bio-Sorption of a Bi-Solute System of Copper and Lead Ions onto Banana Peels: Characterization and Optimization. J. Environ. Health Sci. Eng. 2021, 19, 613–624. [Google Scholar] [CrossRef]
- Alswat, A.A.; Al-shorifi, F.T.; Ali, S.L. Preparation of Nanohybrid CuO-Fe3O4/Zeolite Nanocomposite as Potential Adsorbent for Toxic as (V) and Pb (II) from Water Solution. Iran. J. Mater. Sci. Eng. 2022, 19, 1–13. [Google Scholar]
- Lu, J.; Zhang, F. Novel Fe–Mn Oxide/Zeolite Composite Material for Rapid Removal of Toxic Copper Ions from Aqueous Solutions. J. Clean. Prod. 2023, 397, 136496. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Bindary, A.A.; Kouta, E.Y. Retention of Copper, Cadmium and Lead from Water by Na-Y-Zeolite Confined in Methyl Methacrylate Shell. J. Environ. Chem. Eng. 2017, 5, 3698–3710. [Google Scholar] [CrossRef]
- Pandey, P.K.; Sharma, S.K.; Sambi, S.S. Removal of Lead(II) from Waste Water on Zeolite-NaX. J. Environ. Chem. Eng. 2015, 3, 2604–2610. [Google Scholar] [CrossRef]
- Sarı, A.; Tuzen, M.; Cıtak, D.; Soylak, M. Adsorption Characteristics of Cu(II) and Pb(II) onto Expanded Perlite from Aqueous Solution. J. Hazard. Mater. 2007, 148, 387–394. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).