From Shale to Value: Dual Oxidative Route for Kukersite Conversion
Abstract
1. Introduction
2. Materials and Methods
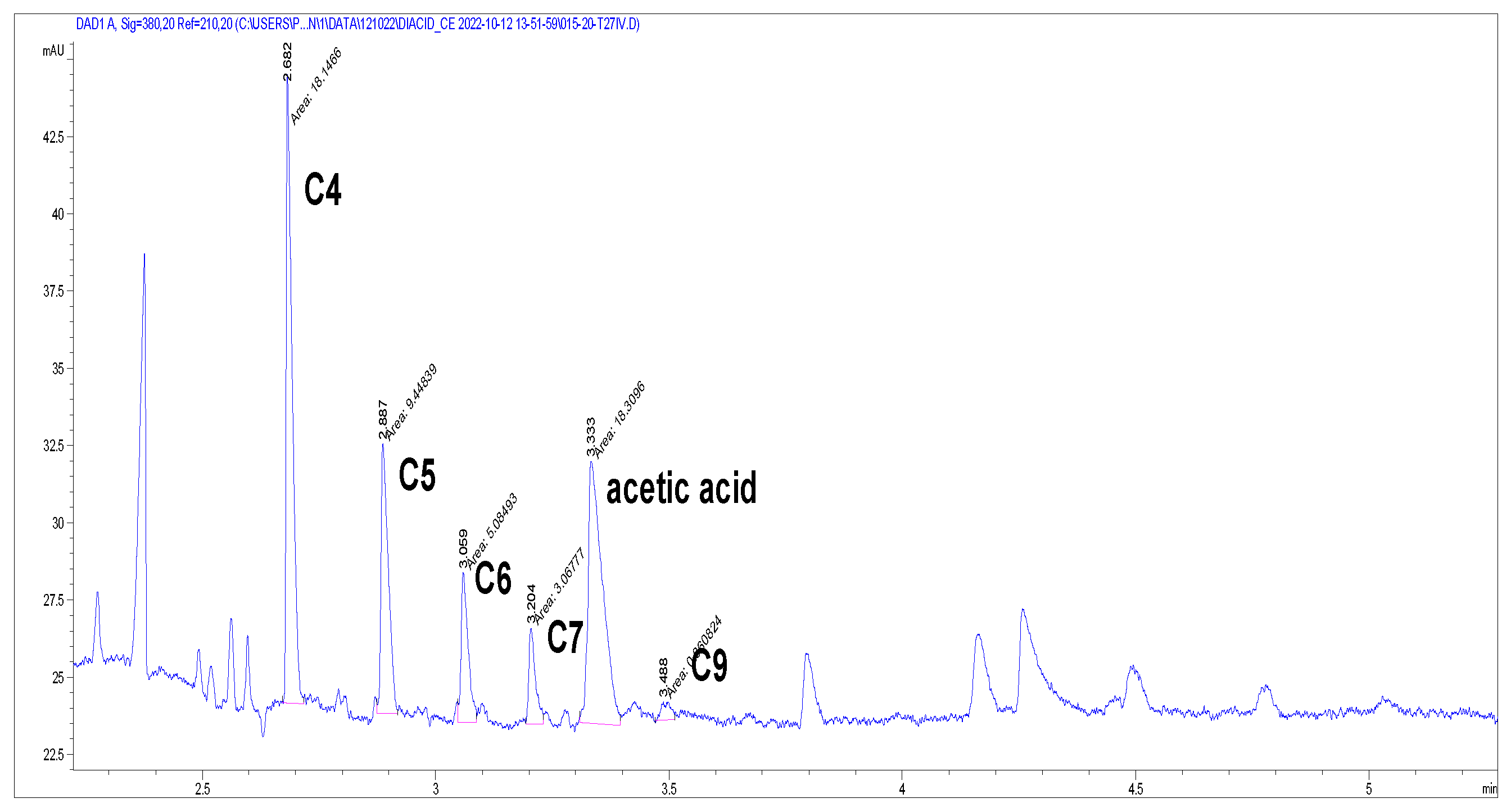
3. Results and Discussion
3.1. Step 1—Partial Dissolution of Organic Matter by WAO
3.1.1. C, N, and S Balance in Solution Without Added Base
3.1.2. C, N, S Balance in Basic Solution
3.2. Step 2—Further Oxidation of Organic Matter by Nitric Acid
3.2.1. Oxidation of Dissolved Organics from WAO
3.2.2. Oxidation of Whole Reaction Mixture (Solid + Dissolved) from WAO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acetic acid |
| C4 | Succinic acid |
| C5 | Glutaric acid |
| C6 | Adipic acid |
| C7 | Pimelic acid |
| C8 | Suberic acid |
| C9 | Azelaic acid |
| Cresidual OS | Carbon in residual oil shale mass after oxidation |
| Cdiss.organics | Carbon in dissolved organics after oxidation |
| Cmolarmassratio | Carbon content based on the molar mass ratio of the compound |
| Ctotal | Total carbon measured using an elemental analyzer |
| C conv. | Carbon conversion |
| DCA | Dicarboxylic acid |
| FA | Formic acid |
| md | Dry mass |
| mfeed OS | Mass of initial oil shale (reaction feed) |
| mdiss.organics | Mass of dissolved organics |
| OM | Organic matter |
| OS | Oil shale |
| TOC | Total organic carbon |
| TIC | Total inorganic carbon |
| WAO | Wet air oxidation |
References
- Dyni, J.R. Geology and resources of some world oil-shale deposits. Oil Shale 2003, 20, 193–252. [Google Scholar] [CrossRef]
- World Energy Council. World Energy Resources. 2016. Available online: https://www.worldenergy.org/assets/images/imported/2016/10/World-Energy-Resources-Full-report-2016.10.03.pdf (accessed on 1 June 2025).
- Raukas, A.; Punning, J.M. Environmental problems in the Estonian oil shale industry. Energy Environ. Sci. 2009, 2, 723–728. [Google Scholar] [CrossRef]
- Shu, Y.; Liu, B.; Zhang, H.; Zhou, A.; Zhang, S.; Shi, Z.; Hu, Z.; Wang, X. Process design and optimization on upgrading and utilization of ultra-high-quality oil shale by pyrolysis. Fuel 2025, 390, 134675. [Google Scholar] [CrossRef]
- Han, X.; Kulaots, I.; Jiang, X.; Suuberg, E.M. Review of oil shale semicoke and its combustion utilization. Fuel 2014, 126, 143–161. [Google Scholar] [CrossRef]
- Liu, K.; Qi, H.; Wang, Z.; Liu, B.; Wang, Y.; Zhang, Z.; Zhang, H.; Yang, R.; Jiang, L.; Ostadhassan, M. The organic matrix: An in-depth review of kerogen and its significance. Fuel 2025, 397, 135493. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Wang, Q.; Xu, C.; Zhang, R.-Z.; Hou, C.-X.; Hu, W.-J.; Lu, T.; Liang, Y.-H. Exploring the mechanism of alkali-oxygen oxidation of Yilan oil shale to carboxylic acids using a combined experimental and theoretical method. Chem. Phys. 2025, 595, 112737. [Google Scholar] [CrossRef]
- Mets, B.; Kaldas, K.; Uustalu, J.M.; Lopp, M. The Lille-Blokker model—An excellent tool to describe the structure of kukersite. Oil Shale 2023, 40, 234–243. [Google Scholar] [CrossRef]
- Bajc, S.; Amblès, A.; Largeau, C.; Derenne, S.; Vitorović, D. Precursor biostructures in kerogen matrix revealed by oxidative degradation: Oxidation of kerogen from estonian kukersite. Org. Geochem. 2001, 32, 773–784. [Google Scholar] [CrossRef]
- Lille, Ü.; Heinmaa, I.; Pehk, T. Molecular model of Estonian kukersite kerogen evaluated by 13C MAS NMR spectra. Fuel 2003, 82, 799–804. [Google Scholar] [CrossRef]
- Mets, B.; Lopp, M.; Uustalu, J.M.; Muldma, K.; Niidu, A.; Kaldas, K. A two-step model for assessing the potential of shale-derived chemicals by oxidation of kukersite. Oil Shale 2023, 40, 344–362. [Google Scholar] [CrossRef]
- Vandenbroucke, M.; Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Blokker, P.; van Bergen, P.; Pancost, R.; Collinson, M.E.; de Leeuw, J.W.; Damste, J.S.S. Sinninghe Damste. The chemical structure of gloeocapsomorpha prisca microfossils: Implications for their origin. Geochim. Cosmochim. Acta 2001, 65, 885–900. [Google Scholar] [CrossRef]
- Barakat, A.O.; Yen, T.F. Kerogen structure by stepwise oxidation. Use of sodium dichromate in glacial acetic acid. Fuel 1987, 66, 587–593. [Google Scholar] [CrossRef]
- Robinson, W.E. Origin and Characteristics of Green River Oil Shale. In Developments in Petroleum Science; Yen, T.F., Chilingarian, G.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1976; Volume 3, Chapter 4; pp. 61–79. [Google Scholar]
- Durand, B. Sedimentary organic matter and kerogen. Definition and quantitative importance of kerogen. In Kerogen; Edistions Technip: Paris, France, 1980. [Google Scholar]
- Veski, R.; Veski, S. Aliphatic dicarboxylic acids from oil shale organic matter–historic review. Oil Shale 2019, 36, 76–95. [Google Scholar] [CrossRef]
- Fomina, A.; Pobul, L.; Degtereva, Z.; Veski, R.; Kirret, O.; Nikopensius, I.; Männik, A.; Pärn, A.; Poom, A.; Murumets, K.; et al. Method for Processing Caustobilites of Sapropelite Type with Oxidizer. Australian Patent Application No. 1973052367, 22 August 1974. Available online: https://ipsearch.ipaustralia.gov.au/patents/1973052367 (accessed on 1 June 2025).
- Gobin, M.; Loulergue, P.; Audic, J.-L.; Lemiègre, L. Synthesis and characterisation of bio-based polyester materials from vegetable oil and short to long chain dicarboxylic acids. Ind. Crops Prod. 2015, 70, 213–220. [Google Scholar] [CrossRef]
- Nakayama, Y.; Matsu-Ura, T.; Tanaka, R.; Shiono, T.; Hino, S.; Kawasaki, N.; Yamano, N.; Nakayama, A.; Tezuka, R.; Tanaka, K. Biodegradable thermoplastic elastomers synthesized from C7–C10 aliphatic dicarboxylic acids, 2-methyl-1,3-propanediol, and L-lactide. Polym. Degrad. Stab. 2024, 229, 110978. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, J.; Wang, T.; Zai, Y.; Qiu, L.; Wang, Q. Fabrication and properties of a bio-based biodegradable thermoplastic polyurethane elastomer. Polymers 2019, 11, 1121. [Google Scholar] [CrossRef]
- Proskurjakov, V.A.; Soloveichik, Z.V. Oxidation of oil shale by atmospheric oxygen. 1. Oxidation of an aqueous-alkaline suspension of Gdov shale in an autoclave. Tr. Vsesoj. N.I.Instituta po Pererab. i Issl. Topl. 1961, 10, 64–80. (In Russian) [Google Scholar]
- Proskurjakov, V.A.; Soloveichik, Z.V. Oxidation of oil shale by atmospheric oxygen. 2. Oxidation of Gdov shales with continuous air supply. Tr. Vsesoj. N.I.Instituta po Pererab. i Issl. Topl. 1961, 10, 81–90. (In Russian) [Google Scholar]
- Proskurjakov, V.A.; Yakovlev, V.I.; Kudrjukov, O.I. Oxidation of oil shale by atmospheric oxygen. 3. Oxidation of common oil shales. Tr. Vsesoj. N.I.Instituta po Pererab. i Issl. Topl. 1962, 11, 20–27. (In Russian) [Google Scholar]
- Fomina, A.S.; Pobul, L. The deriving of dibasic aliphatic acids from a new raw material. Acad. Sci. Est. SSR 1957, 6, 190–198. [Google Scholar]
- Degtereva, Z.; Fomina, A. Production of Dibasic Acids C4-C10 from Oil Shale Kukersite. Acad. Sci. Est. SSR 1959, 8, 122–136. [Google Scholar]
- Kaldas, K. Wet Air Oxidation of Oil Shale. Ph.D. Thesis, Tallinn University of Technology, Tallinn, Estonia, 2021. [Google Scholar]
- Kaldas, K.; Preegel, G.; Muldma, K.; Lopp, M. Wet Air Oxidation of Oil Shales: Kerogen Dissolution and Dicarboxylic Acid Formation. ACS Omega 2020, 5, 22021–22030. [Google Scholar] [CrossRef]
- Kaldas, K.; Niidu, A.; Preegel, G.; Uustalu, J.M.; Lopp, M. Aspects of kerogen oxidative dissolution in subcritical water using oxygen from air. Oil Shale 2021, 38, 199–214. [Google Scholar] [CrossRef]
- Kann, J.; Raukas, A.; Siirde, A. About the gasification of kukersite oil shale. Oil Shale 2013, 30, 283–293. [Google Scholar] [CrossRef]
- Debellefontaine, H.; Crispel, S.; Reilhac, P.; Périé, F.; Foussard, J.N. Wet air oxidation (WAO) for the treatment of industrial wastewater and domestic sludge. Design of bubble column reactors. Chem. Eng. Sci. 1999, 54, 4953–4959. [Google Scholar] [CrossRef]
- Debellefontaine, H.; Foussard, J.N. Wet air oxidation for the treatment of industrial wastes. Chemical aspects, reactor design and industrial applications in Europe. Waste Manag. 2000, 20, 15–25. [Google Scholar] [CrossRef]
- Van De Vyver, S.; Román-Leshkov, Y. Emerging catalytic processes for the production of adipic acid. Catal. Sci. Technol. 2013, 3, 1465–1479. [Google Scholar] [CrossRef]
- Pohorecki, R.; Bałdyga, J.; Moniuk, W.; Podgórska, W.; Zdrójkowski, A.; Wierzchowski, P.T. Kinetic model of cyclohexane oxidation. Chem. Eng. Sci. 2001, 56, 1285–1291. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Dou, X.; Wei, H.; Shang, M.; Luo, Z.; Su, Y. Synthesis of adipic acid through oxidation of K/A oil and its kinetic study in a microreactor system. AIChE J. 2020, 66, e16289. [Google Scholar] [CrossRef]
- Castellan, A.; Bart, J.C.J.; Cavallaro, S. Industrial production and use of adipic acid. Catal. Today 1991, 9, 237–254. [Google Scholar] [CrossRef]
- Castellan, A.; Bart, J.C.J.; Cavallaro, S. Synthesis of adipic acid via the nitric acid oxidation of cyclohexanol in a two-step batch process. Catal. Today 1991, 9, 285–299. [Google Scholar] [CrossRef]
- Väli, E.; Valgma, I.; Reinsalu, E. Usage of Estonian Oil Shale. Oil Shale 2008, 25, 101. [Google Scholar] [CrossRef]
- Mishra, V.S.; Mahajani, V.V.; Joshi, J.B. Wet Air Oxidation. Ind. Eng. Chem. Res. 1995, 34, 2–48. [Google Scholar] [CrossRef]
- Oliviero, L.; Barbier, J.; Duprez, D. Wet air oxidation of nitrogen-containing organic compounds and ammonia in aqueous media. Appl. Catal. B Environ. 2003, 40, 163–184. [Google Scholar] [CrossRef]
- Arena, F.; Italiano, C.; Raneri, A.; Saja, C. Mechanistic and kinetic insights into the wet air oxidation of phenol with oxygen (CWAO) by homogeneous and heterogeneous transition-metal catalysts. Appl. Catal. B Environ. 2010, 99, 321–328. [Google Scholar] [CrossRef]
- Sales, F.G.; Maranhão, L.C.A.; Filho, N.M.L.; Abreu, C.A.M. Kinetic evaluation and modeling of lignin catalytic wet oxidation to selective production of aromatic aldehydes. Ind. Eng. Chem. Res. 2006, 45, 6627–6631. [Google Scholar] [CrossRef]
- Luo, X.; Zhan, J.; Mei, Q.; Zhang, S. Selective oxidative upgrade of waste polystyrene plastics by nitric acid to produce benzoic acid. Green Chem. 2023, 25, 6717–6727. [Google Scholar] [CrossRef]

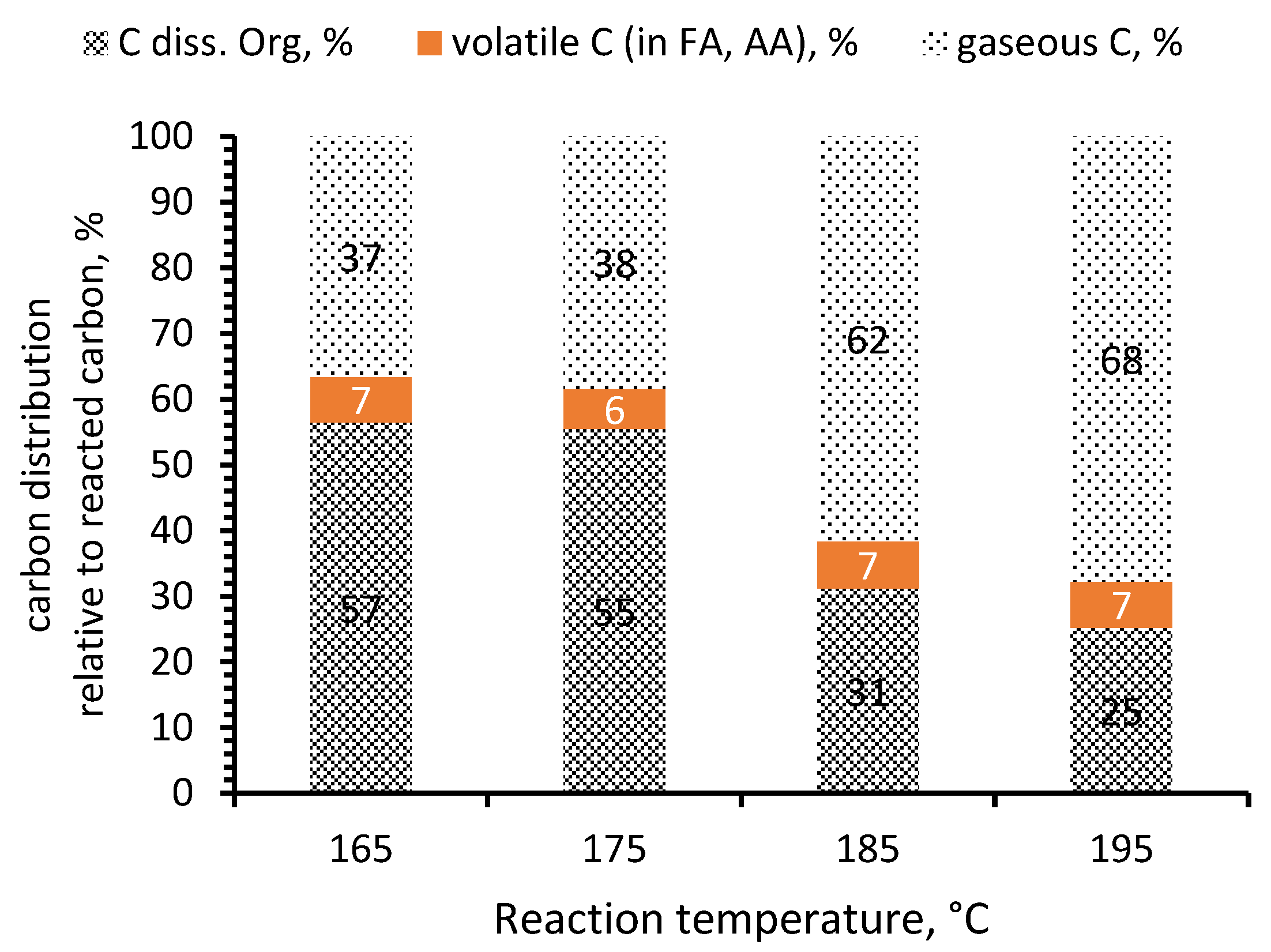
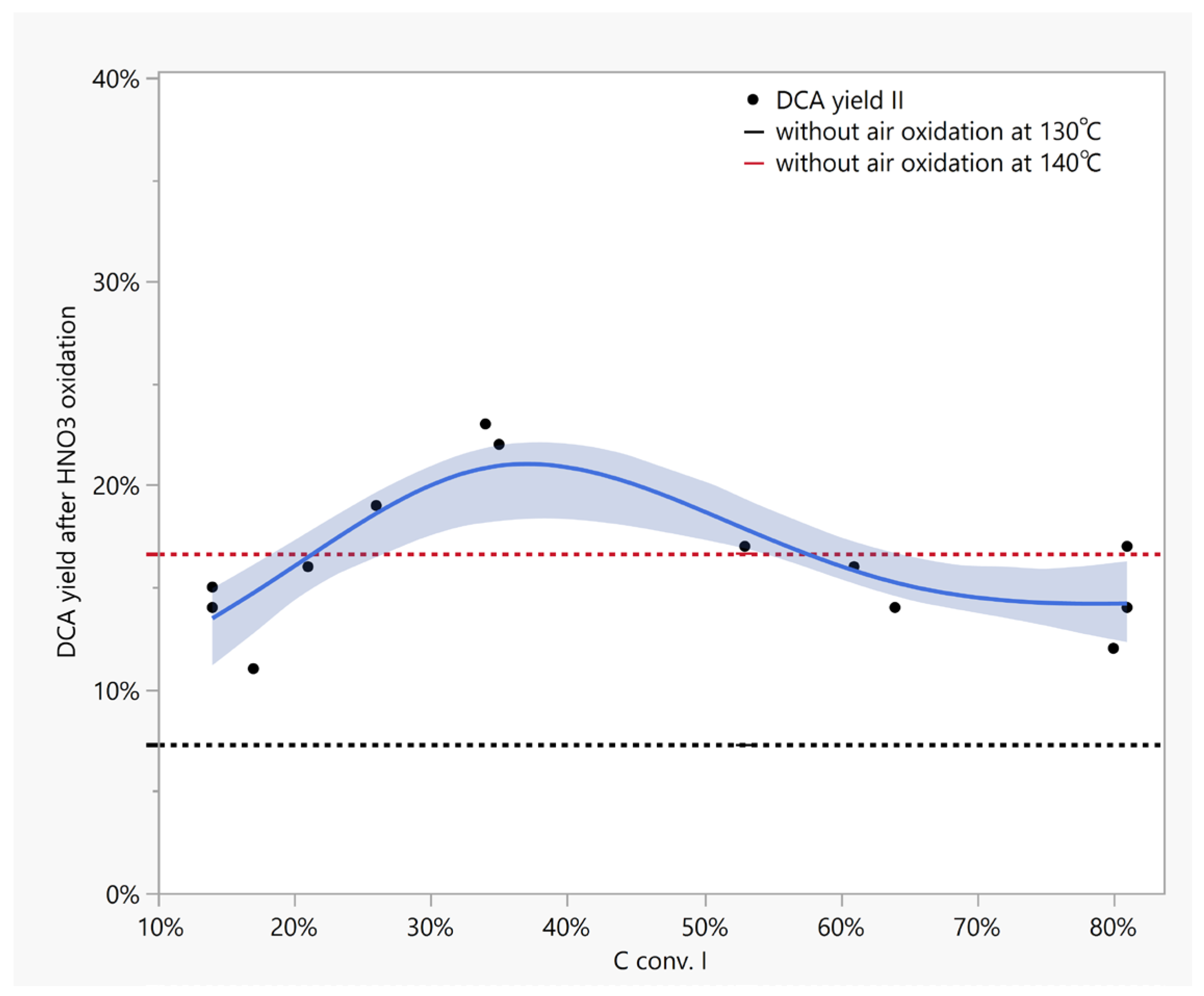
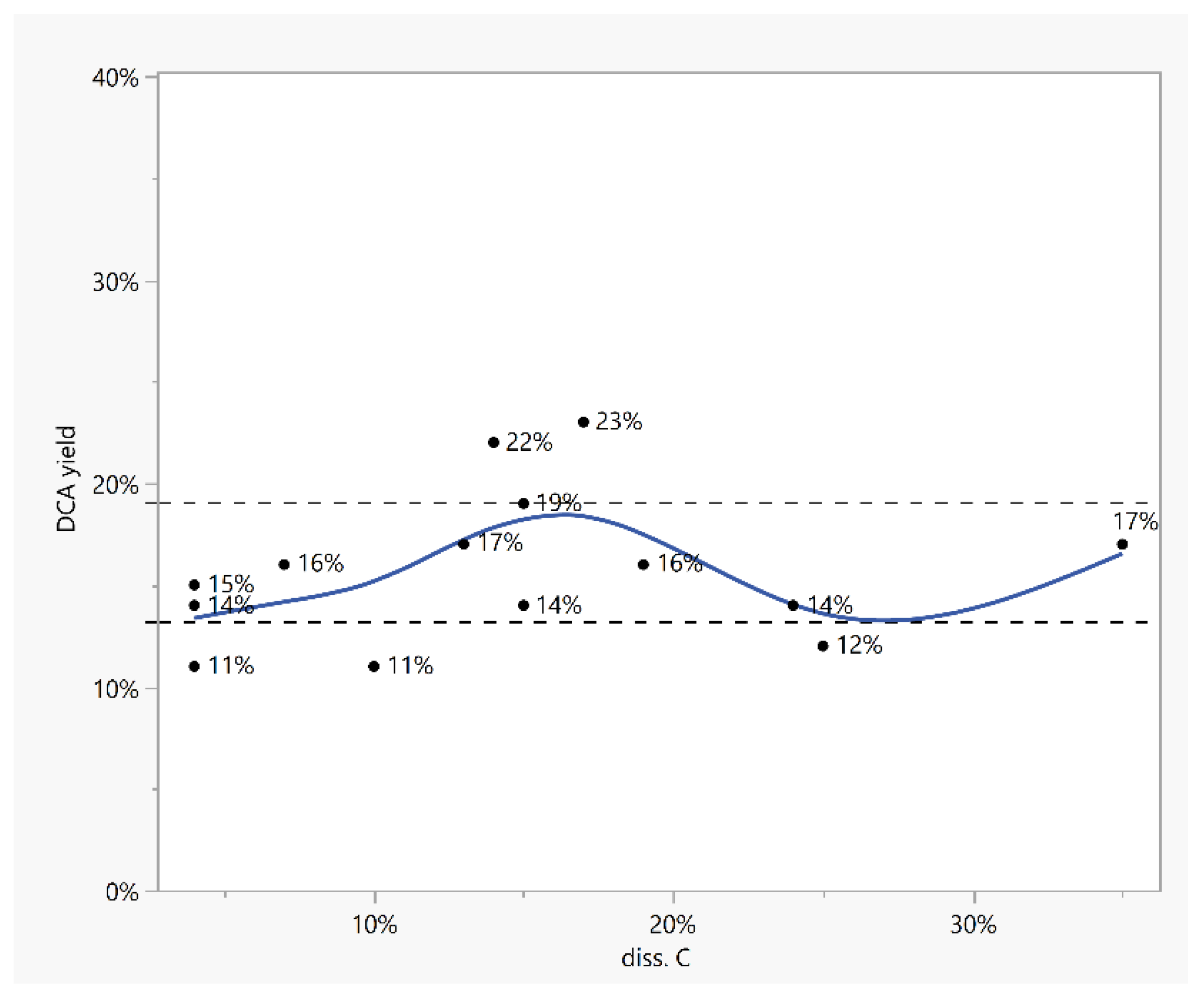
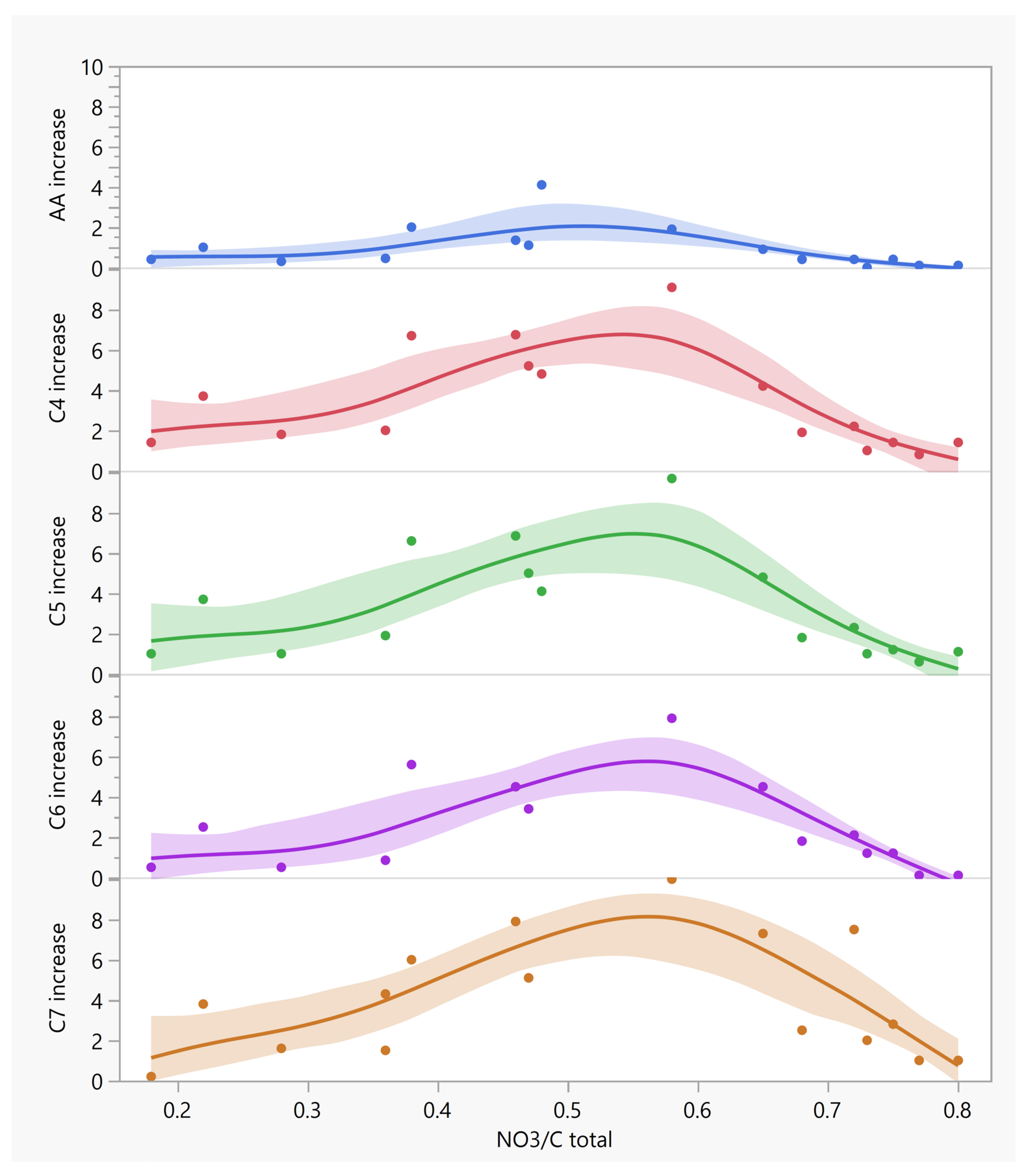
| Oxidation Method | DCA wt% Per Kerogen | Other Acids a |
|---|---|---|
| KMnO4 [9,25] | [25] 60% of DCA raw mixture [9] 21% of C4–C10 (max at C8) | [25] Volatile, viscous, and solid acids [9] 40% total acids; 1% CA C14–C18; 10% tri- and tetracarboxylic acids C6–C18; 2.4% aromatic acids |
| RuO4 [13] | C5–C14 (max at C9); no yield determined | CA and oxo-CA C7–C18; 2-methyl DCA C5–C19 and tri-CA C12–C20 (max at C17) |
| HNO3 [17,18,26] | [26] Up to 43% C4–C10 with 65% of HNO3 (lab); [17,18] 26% C4–C10 in pilot plant (max at C5–C7) | ≈80% of kerogen was soluble in acid; 1:1 water-soluble and solid oxygenated products |
| O2 [22,23,24] | Up to 20% C4–C10 | 0.5–5% volatile acids and 10–50% solid high-molecular-weight acids |
| OM 1 | Ctotal | H | N | S | TIC 2 | TOC 3 |
|---|---|---|---|---|---|---|
| 58 | 43.8 | 5.6 | 0.1 | 2.8 | 0.7 | 43.0 |
| No. | T, °C | pO2, bar | Time, h | C in Residual, %wt | C in Diss. Organics, %wt | C in Volatiles, %wt | Gaseous C 2, % |
|---|---|---|---|---|---|---|---|
| 1 | 185 | 40 | 0.25 | 56 | 5 | 11 | 27 |
| 2 | 185 | 40 | 0.5 | 40 | 7 | 14 | 39 |
| 3 | 185 | 40 | 1 | 6 | 7 | 22 | 65 |
| 4 | 195 | 40 | 0.25 | 56 | 5 | 10 | 29 |
| 5 | 195 | 40 | 0.5 | 31 | 5 | 9 | 54 |
| 6 | 195 | 40 | 1 | 6 | 5 | 22 | 67 |
| No. | T, °C | pO2, bar | Time, h | S in Residual, %wt | S in Diss. Organics, %wt |
|---|---|---|---|---|---|
| 1 | 185 | 40 | 0.25 | 23 | 70 |
| 2 | 185 | 40 | 0.5 | 10 | 81 |
| 3 | 185 | 40 | 1 | 4 | 77 |
| 4 | 195 | 40 | 0.25 | 17 | 73 |
| 5 | 195 | 40 | 0.5 | 12 | 78 |
| 6 | 195 | 40 | 1 | 8 | 87 |
| No | T, °C | pO2, bar | C in Residual, %wt | C in Diss. Organics, %wt | C in Volatiles, %wt | Gaseous C, % 2 |
|---|---|---|---|---|---|---|
| 1 | 185 | 20 | 70 | 7.4 | 1.5 | 12 |
| 2 | 185 | 30 | 57 | 15.5 | 2.7 | 25 |
| 3 | 185 | 40 | 29 | 22.0 | 5.1 | 44 |
| 4 | 175 | 40 | 68 | 17.9 | 2.0 | 13 |
| 5 | 165 | 40 | 74 | 15.0 | 1.8 | 10 |
| No. | T, °C | pO2, bar | S in Residual, %wt | S in Diss. Organics, %wt | N in Residual, %wt | N in Diss. Organics, %wt |
|---|---|---|---|---|---|---|
| 1 | 185 | 20 | 44 | 56 | 33 | 39 |
| 2 | 185 | 30 | 36 | 57 | 43 | 44 |
| 3 | 185 | 40 | 20 | 82 | 22 | 64 |
| 4 | 175 | 40 | 32 | 51 | 39 | 52 |
| 5 | 165 | 40 | 40 | 49 | 46 | 45 |
| Feed from Step 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tbl/No | Dry Matter 1, %wt | C in Dry Matter, % | Yield 2, % | C4, mg/g | C5, mg/g | C6, mg/g | C7, mg/g | Σ DCA, mg/g | |
| 4./1 | 22 | 22 | 1.4 | 2.4 | 1.5 | 0.8 | 0 | 4.7 | |
| 4./3 | 23 | 32 | 7.2 | 9.9 | 5.3 | 2.3 | 1.4 | 18.9 | |
| Step 2 i.e., HNO3 oxidation | |||||||||
| Tbl/No-Exp. | HNO3 ox. T, °C/t, h | HNO3, mol eq. 3 | yield, % | C4, mg/g | C5, mg/g | C6, mg/g | C7, mg/g | Σ DCA, mg/g | Final 4 HNO3, mg/g |
| 4./1-A | 120/0.25 | 0.5 | 3.1 | 6.0 | 2.8 | 1.1 | 0.5 | 10.4 | 62.4 |
| 4./1-B | 120/0.5 | 0.5 | 3.3 | 6.4 | 2.8 | 1.1 | 0.4 | 10.7 | 48.0 |
| 4./3-C | 140/0.25 | 0.35 | 12.1 | 15.6 | 6.6 | 3.2 | 1.1 | 27.1 | 58.4 |
| 4./3-D | 140/0.5 | 0.35 | 15.4 | 19.2 | 8.4 | 3.3 | 1.2 | 32.5 | 45.4 |
| E | F | G | H | I | ||
|---|---|---|---|---|---|---|
| Feed from Step 1 | Tbl/No | 4./2 | 4./2 | 4./4 | 4./4 | 4./3 |
| DCA yield, %wt | 4 | 4 | 2.6 | 2.6 | 6 | |
| C conv., % | 43 | 43 | 32.4 | 32.4 | 71 | |
| Step 2 i.e., HNO3 ox. | HNO3, mol eq. 1 | 0.3 | 0.7 | 0.3 | 0.5 | 0.7 |
| c(HNO3)initial, % | 5 | 8 | 5 | 8 | 8 | |
| c(HNO3)end, % | 0.6 | 2.4 | 0.6 | 1.1 | 4.4 | |
| DCA yield 2, %wt | 12 | 22 | 8 | 23 | 14 | |
| DCA yield 3, %wt | 18 | 28 | 10 | 28 | 28 | |
| (C4-C5)/DCA, % | 91 | 76 | 85 | 81 | 87 | |
| AA/C, % | 7 | 3 | 5 | 4 | 5 | |
| C conv. 4, % | 59 | 84 | 59 | 88 | 74 |
| 1. Step Tbl./No | 2. Step HNO3/C | c(HNO3), % | C in Residual, %wt | C in Diss. Organics, %wt | C in Volatiles, %wt | Gaseous C 2, % |
|---|---|---|---|---|---|---|
| 4./4 | 0.5 | 8 | 18 | 23 | 4 | 55 |
| 4./5 | 0.5 | 8 | 29 | 19 | 3 | 49 |
| 4./3 | 0.7 | 8 | 16 | 22 | 3 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaldas, K.; Muldma, K.; Simm, A.; Mets, B.; Kontson, T.; Silm, E.; Kimm, M.; Koern, V.Ö.; Uustalu, J.M.; Lopp, M. From Shale to Value: Dual Oxidative Route for Kukersite Conversion. Processes 2025, 13, 2421. https://doi.org/10.3390/pr13082421
Kaldas K, Muldma K, Simm A, Mets B, Kontson T, Silm E, Kimm M, Koern VÖ, Uustalu JM, Lopp M. From Shale to Value: Dual Oxidative Route for Kukersite Conversion. Processes. 2025; 13(8):2421. https://doi.org/10.3390/pr13082421
Chicago/Turabian StyleKaldas, Kristiina, Kati Muldma, Aia Simm, Birgit Mets, Tiina Kontson, Estelle Silm, Mariliis Kimm, Villem Ödner Koern, Jaan Mihkel Uustalu, and Margus Lopp. 2025. "From Shale to Value: Dual Oxidative Route for Kukersite Conversion" Processes 13, no. 8: 2421. https://doi.org/10.3390/pr13082421
APA StyleKaldas, K., Muldma, K., Simm, A., Mets, B., Kontson, T., Silm, E., Kimm, M., Koern, V. Ö., Uustalu, J. M., & Lopp, M. (2025). From Shale to Value: Dual Oxidative Route for Kukersite Conversion. Processes, 13(8), 2421. https://doi.org/10.3390/pr13082421








