Purification of the Selenium Vapor Phase from Droplet Suspensions in Vacuum Distillation Refining
Abstract
1. Introduction
2. Potential Composition of the Vapor–Droplet Phase
3. Selection of Structural Materials for Process Equipment Involving Liquid and Vapor Selenium
4. Development of a Method and Device for Cleaning the Vapor Phase
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Varaksin, A.Y. Two-Phase Flows with Solid Particles, Droplets, and Bubbles: Problems and Research Results (Review). High Temp. 2020, 58, 595–614. [Google Scholar] [CrossRef]
- Volokitina, I.E.; Volokitin, A.V.; Latypova, M.A.; Chigirinsky, V.V.; Kolesnikov, A.S. Effect of Controlled Rolling on the Structural and Phase Transformations. Prog. Phys. Met. 2023, 24, 132–156. [Google Scholar] [CrossRef]
- Kolesnikov, A.S.; Sapargaliyeva, B.O.; Bychkov, A.Y.; Alferyeva, Y.O.; Syrlybekkyzy, S.; Altybaeva, Z.K.; Nurshakhanova, L.K.; Seidaliyeva, L.K.; Suleimenova, B.S.; Zhidebayeva, A.E.; et al. Thermodynamic modeling of the formation of the main minerals of cement clinker and zinc fumes in the processing of toxic technogenic waste of the metallurgical industry. Rasayan J. Chem. 2022, 15, 2181–2187. [Google Scholar] [CrossRef]
- Volokitina, I.; Bychkov, A.; Volokitin, A.; Kolesnikov, A. Natural Aging of Aluminum Alloy 2024 After Severe Plastic Deformation. Metallogr. Microstruct. Anal. 2023, 12, 564–566. [Google Scholar] [CrossRef]
- Khudyakova, T.M.; Kolesnikova, O.G.; Zhanikulov, N.N.; Ashirbaev, H.A.; Kolesnikova, V.A. Low-Basicity Cement, Problems and Advantages of its Utilization. Refract. Ind. Ceram. 2021, 62, 369–374. [Google Scholar] [CrossRef]
- Donayev, A.; Kolesnikov, A.; Shapalov, S.; Sapargaliyeva, B.; Ivakhniyuk, G. Studies of waste from the mining and metallurgical industry, with the determination of its impact on the life of the population. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Geol. Tech. Sci. 2022, 454, 55–68. [Google Scholar] [CrossRef]
- Nadirov, K.S.; Zhantasov, M.K.; Bimbetova, G.Z.; Sadyrbayeva, A.S.; Orynbasarov, A.K.; Sakybayev, B.A. The study of the gossypol resin impact on adhesive properties of the intermediate layer of the pipeline three-layer rust protection coating. Inter. J. Adhes. Adhesiv. 2017, 78, 195–199. [Google Scholar] [CrossRef]
- Zhantasov, M.K.; Bimbetova, G.Z.; Sadyrbayeva, A.S.; Orynbasarov, A.K.; Kutzhanova, A.N.; Turemuratov, R.S.; Botabaev, N.E.; Zhantasova, D. Examination of optimal parameters of oxy-ethylation of fatty acids with a view to obtaining demulsifiers for deliquefaction in the system of skimming and treatment of oil: A method to obtain demulsifier from fatty acids. Chem. Today 2016, 34, 72–77. [Google Scholar]
- Zhangabay, N.; Sapargaliyeva, B.; Suleimenov, U.; Abshenov, K.; Utelbayeva, A.; Baibolov, K.; Fediuk, R.; Arinova, D.; Duissenbekov, B.; Kolesnikov, A.; et al. Analysis of Stress-Strain State for a Cylindrical Tank Wall Defected Zone. Materials 2022, 15, 5732. [Google Scholar] [CrossRef] [PubMed]
- Zhangabay, N.; Baidilla, I.; Tagybayev, A.; Ibraimbayeva, G.; Abshenov, K.; Nsanbayev, B.; Anarbayev, Y.; Kozlov, P. Thermophysical indicators of elaborated sandwich cladding constructions with heat-reflective coverings and air gaps. Case Stud. Constr. Mater. 2023, 18, e02161. [Google Scholar] [CrossRef]
- Golubev, V.G.; Filin, A.E.; Agabekova, A.B.; Taimasov, B.T.; Janpaizova, V.M.; Kenzhibayeva, G.S.; Suigenbayeva, A.Z. Mathematical description of the process of film condensation of vapors from steam-gas mixtures. Rasayan J. Chem. 2022, 15, 1905–1915. [Google Scholar] [CrossRef]
- Legros, J.C.; Lutoshkina, O.; Piskunov, M. Vaporization of water droplets with non-metallic inclusions of different sizes in a high-temperature gas. Int. J. Therm. Sci. 2018, 127, 360–372. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Strizhak, P.A.; Shlegel, N.E. Interaction of Water ad Suspension Droplets during Their Collisions in a Gas Medium. Theor. Found. Chem. Eng. 2019, 53, 769–780. [Google Scholar] [CrossRef]
- Majhy, B.; Sen, A.K. Evaporation-induced transport of a pure aqueous droplet by an aqueous mixture droplet. Phys. Fluids 2020, 32, 032003. [Google Scholar] [CrossRef]
- Wang, Z.; Orejon, D.; Takata, Y.; Sefiane, K. Wetting and evaporation of multi-component droplets. Phys. Rep. 2022, 960, 1–37. [Google Scholar] [CrossRef]
- Miliauskas, G.; Puida, E.; Poškas, R.; Ragaišis, V.; Paukštaitis, L.; Jouhara, H.; Mingilaitẻ, L. Experimental investigations of water droplet transient phase changes in flue gas flow in the rage of temperatures characteristic of condensing economizer technologies. Energy 2022, 256, 124643. [Google Scholar] [CrossRef]
- Isakova, R.A.; Reznikov, A.A.; Spivak, M.M. Selenium refining. Alma-Ata Sci. In Distillation Processes of Selenium Extraction and Refining; Almaty Tengri Ltd.: Almaty, Kazakhstan, 2017. [Google Scholar]
- Khrapunov, V.E.; Kenzhaliev, B.K.; Isakova, R.A.; Volodin, V.N.; Chelokhsaev, L.S.; Trebukhov, S.A.; Sadvakasov, D.A. A Method of Refining Volatile Chemical Elements by Vacuum Distillation and an Apparatus for Its Implementation. Patent KZ No.12098, 15 October 2002. Available online: https://gosreestr.kazpatent.kz/Invention/Details?docNumber=133450 (accessed on 25 March 2025).
- Volodin, V.N.; Trebukhov, S.A. Distillation Processes of Selenium Extraction and Refining; Almaty Tengri Ltd.: Almaty, Kazakhstan, 2017; 222p. [Google Scholar] [CrossRef]
- Trebukhov, S.A.; Volodin, V.N.; Nitcenko, A.V.; Burabaeva, N.M.; Trebukhov, A.A. Integration of alloying metals of construction steels with liquid and vaporous selenium. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2017, 301, 16–21. Available online: https://kims-imio.kz/portfolio-item/%e2%84%962-2017/ (accessed on 31 March 2025).
- Trebukhov, S.A.; Kenzhaliyev, B.K.; Volodin, V.N.; Nitsenko, A.V.; Linnik, X.A.; Mishra, B. Liquid Selenium Granulation. Processes 2025, 13, 2094. [Google Scholar] [CrossRef]
- Goldfinger, P.; Jeunehomme, M. Mass spectrometric and knudsen-cell vaporization studies of group 2B–6B compounds. Trans. Faraday Soc. 1963, 59, 2851–2867. [Google Scholar] [CrossRef]
- Berkowitz, I.; Chupka, W.A. Equilibrium composition of selenium vapor the Termodinamics of Vaporication of HgSe, CdSe, SrSe. J. Chem. Phys. 1966, 45, 4289–4302. [Google Scholar] [CrossRef]
- Rao, Y.K. Composition of liquid-Saturated selenium vapor. Met. Trans. B 1983, 14, 308–311. [Google Scholar] [CrossRef]
- Ustyugov, G.P.; Vigdorovich, E.N.; Kudryavtsev, A.A. Molecular composition of steam in the tellurium–selenium system. News USSR Acad. Sci. Inorg. Mater. 1968, 4, 1796–1797. [Google Scholar]
- Zobac, O.; Novak, D.; Pavlu, J.; Friak, M.; Kroupa, A. Thermodynamic study of binary phase diagram iron-selenium. Calphad 2025, 88, 102774. [Google Scholar] [CrossRef]
- Kireev, V.A. Methods of practical calculations in thermodynamics of chemical reactions. Moscow Khimiya. In Distillation Processes of Selenium Extraction and Refining; Almaty Tengri Ltd.: Almaty, Kazakhstan, 2017. [Google Scholar]
- Lyakishev, N.P. Diagrams of the State of Double Metal Systems: Handbook; Mechanical Engineering: Moscow, Russia, 1997; Volume 2, p. 1024. Available online: https://f.eruditor.link/file/4033970/ (accessed on 27 March 2025).
- Lyakishev, N.P. Diagrams of the State of Double Metal Systems: Handbook; Mashinostroenie: Moscow, Russia, 2000; Volume 3, p. 448. Available online: https://h.twirpx.link/file/4033972/ (accessed on 10 March 2025).
- Pankratova, O.Y.; Undusk, E.P.; Vladimirova, V.A. and others. Thermochemistry of titanium selenides of variable composition TiSe1,5–2,0. J. Inorg. Chem. 1991, 36, 1249–1253. [Google Scholar]
- Kenzhaliev, B.K.; Volodin, V.N.; Trebukhov, S.A.; Ospanov, E.A.; Shakhalov, A.A. A Method for Refining Substandard Selenium by Vacuum Distillation and an Apparatus for Its Implementation. Patent KZ No. 37275, Application No. 2023/0906.1 Dated 27 December 2023, Published on 11 April 2025. Available online: https://gosreestr.kazpatent.kz/Invention/Details?docNumber=388600 (accessed on 28 April 2025).
- Goncharuk, L.V.; Lukashenko, G.M. Thermodynamic properties of chromium selenide Cr2Se3. J. Phys. Chem. 1986, 60, 1810–1811. [Google Scholar]
- Zha, G.; Wang, Y.; Cheng, M.; Huang, D.; Jiang, W.; Xu, B.; Yang, B. Purification of crude selenium by vacuum distillation and analysis. J. Mater. Res. Technol. 2020, 9, 2926–2933. [Google Scholar] [CrossRef]
- Makarychev, Y.B. Vapor–Gas Deposition of Polymer Coatings on Metals from Azeotropic Solutions of Organosilanes. Surfaces 2023, 6, 291–303. [Google Scholar] [CrossRef]
- Zhang, D. Significant Progress of Initiated Chemical Vapor Deposition in Manufacturing Soft Non-spherical Nanoparticles: Upgrading to the Condensed Droplet Polymerization Approach and Key Technological Aspects. ChemEngineering 2024, 8, 2. [Google Scholar] [CrossRef]
- Gubaidullin, D.A.; Gubaidullina, D.D.; Fedorov, Y.V. Mathematical Modeling of the Wave Dynamics of an Encapsulated Perfluorocarbon Droplet in a Viscoelastic Liquid. Mathematics 2023, 11, 1083. [Google Scholar] [CrossRef]
- Najafi, L.; Oropesa-Nuñez, R.; Bellani, S.; Martín-García, B.; Pasquale, L.; Serri, M.; Drago, F.; Luxa, J.; Sofer, Z.; Sedmidubský, D.; et al. Topochemical Transformation of Two-Dimensional VSe2 into Metallic Nonlayered VO2 for Water Splitting Reactions in Acidic and Alkaline Media. ACS Nano 2022, 16, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Papavasileiou, A.V.; Antonatos, N.; Luxa, J.; Děkanovský, L.; Ashtiani, S.; Fomekong, R.L.; Sofer, Z. Two-dimensional VSe2 nanoflakes as a promising sensing electrocatalyst for nitrobenzene determination in water samples. Electrochim. Acta 2024, 475, 143653. [Google Scholar] [CrossRef]
- Song, D.; Zhou, Y.; Zhang, M.; He, X.; Li, X. Structural and Transport Properties of 1T-VSe2 Single Crystal Under High Pressures. Front. Mater. 2021, 8, 710849. [Google Scholar] [CrossRef]
- Yoon, H.; Hong, S. Highly improved photocurrent of a flexible MoS2 photodetector via a backside Al metal mirror and its in- and outward folding states. RSC Adv. 2024, 14, 34979–34984. [Google Scholar] [CrossRef] [PubMed]
- GOST 1050-88; Carbon Structural Quality Steel Gauged Bars with Special Surface Finish. General Specifications. Standartinform: Moscow, Russia, 1989. Available online: https://online.zakon.kz/Document/?doc_id=30116621 (accessed on 14 April 2025).
- GOST 10298-2018; Technical Selenium. Specifications. Standartinform: Moscow, Russia, 2018. Available online: https://online.zakon.kz/Document/?doc_id=38422958 (accessed on 14 April 2025).


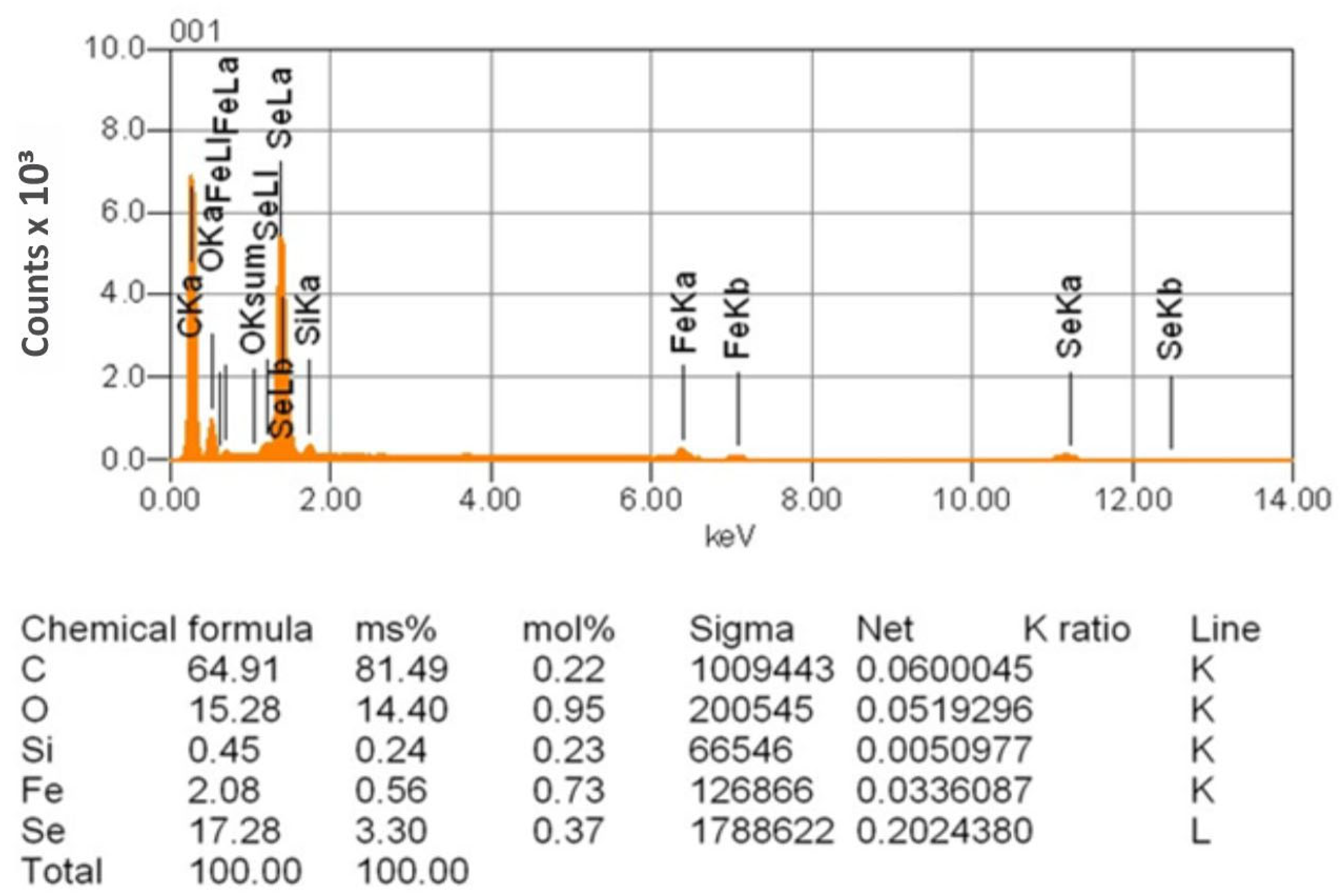
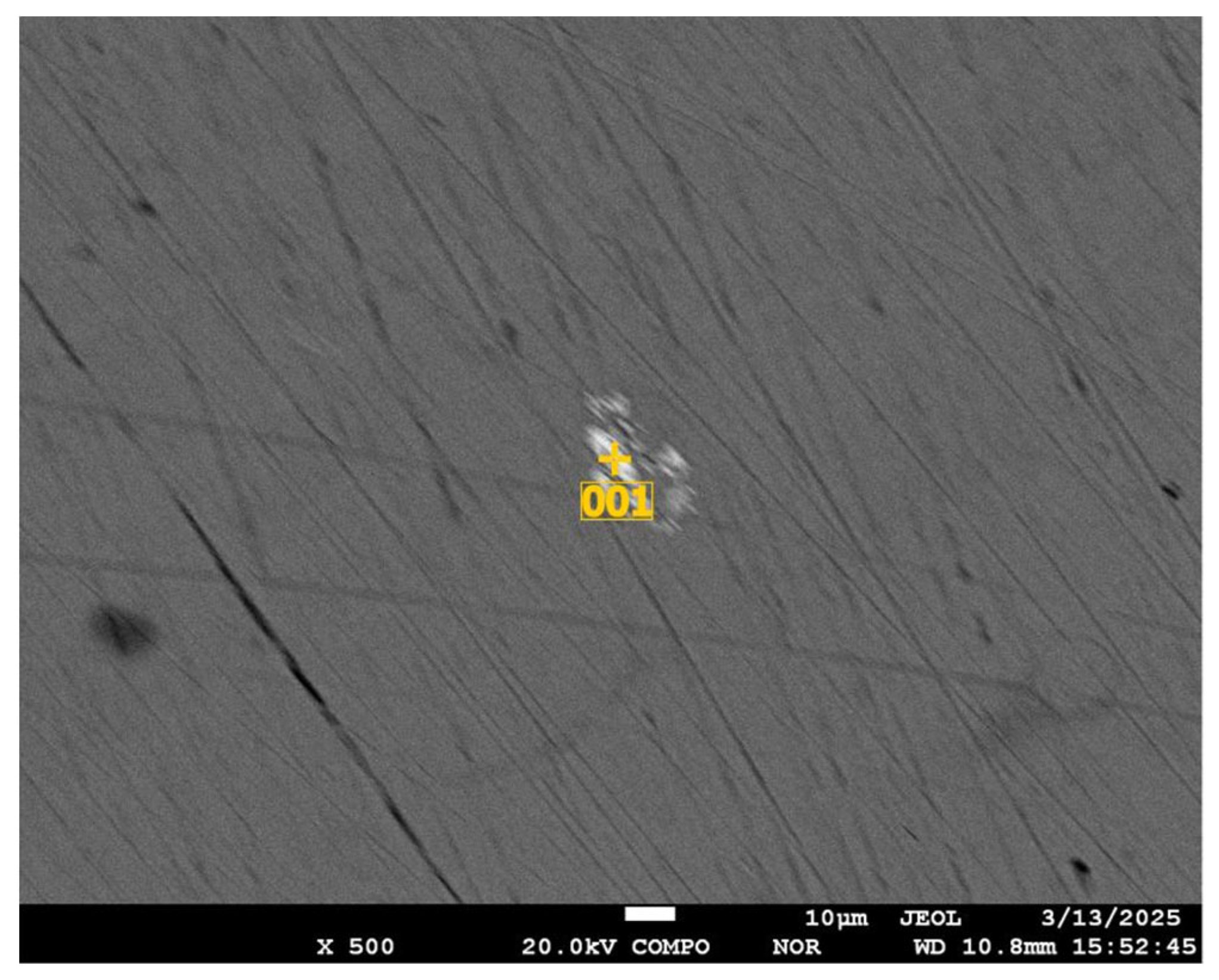
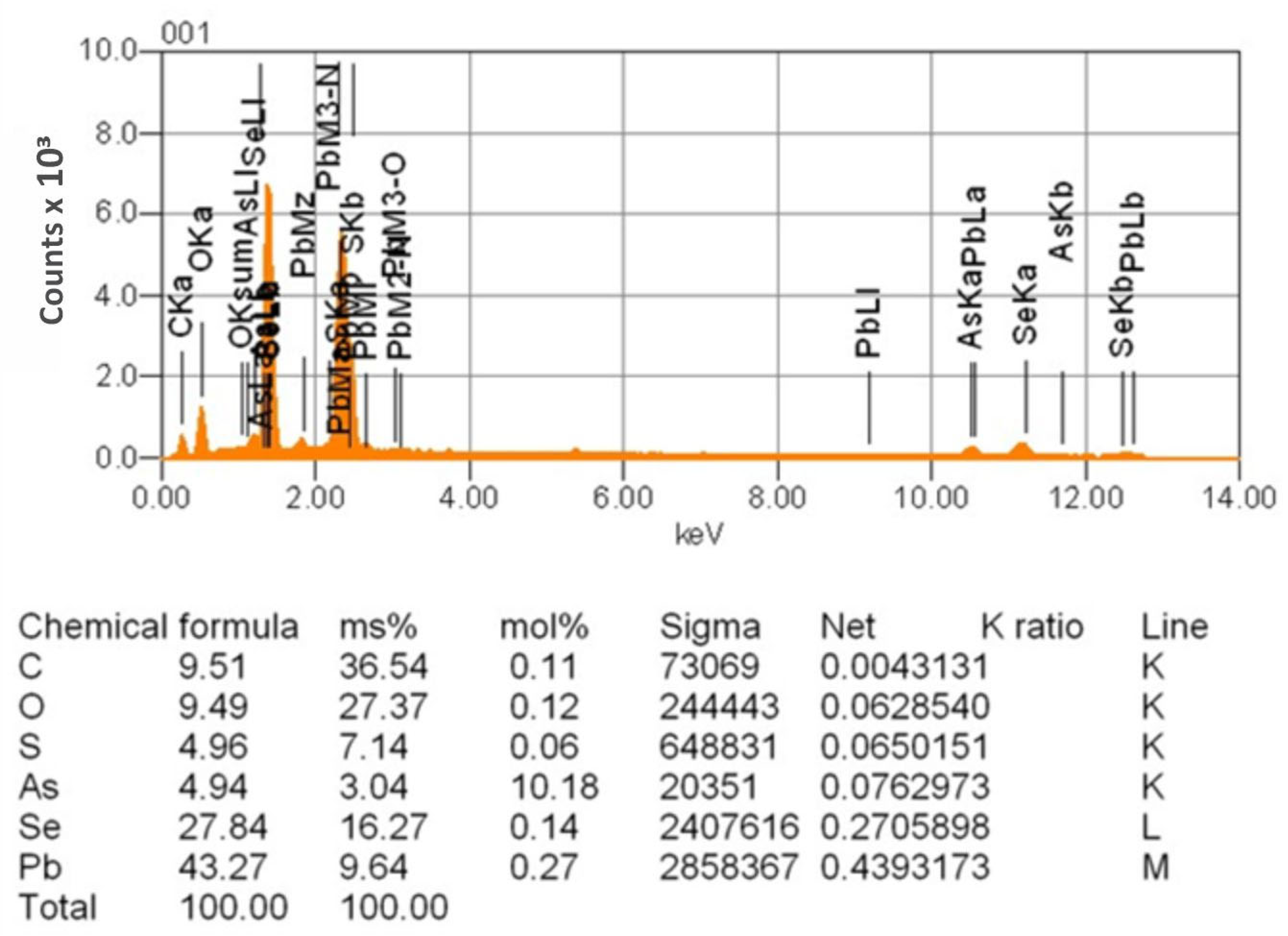
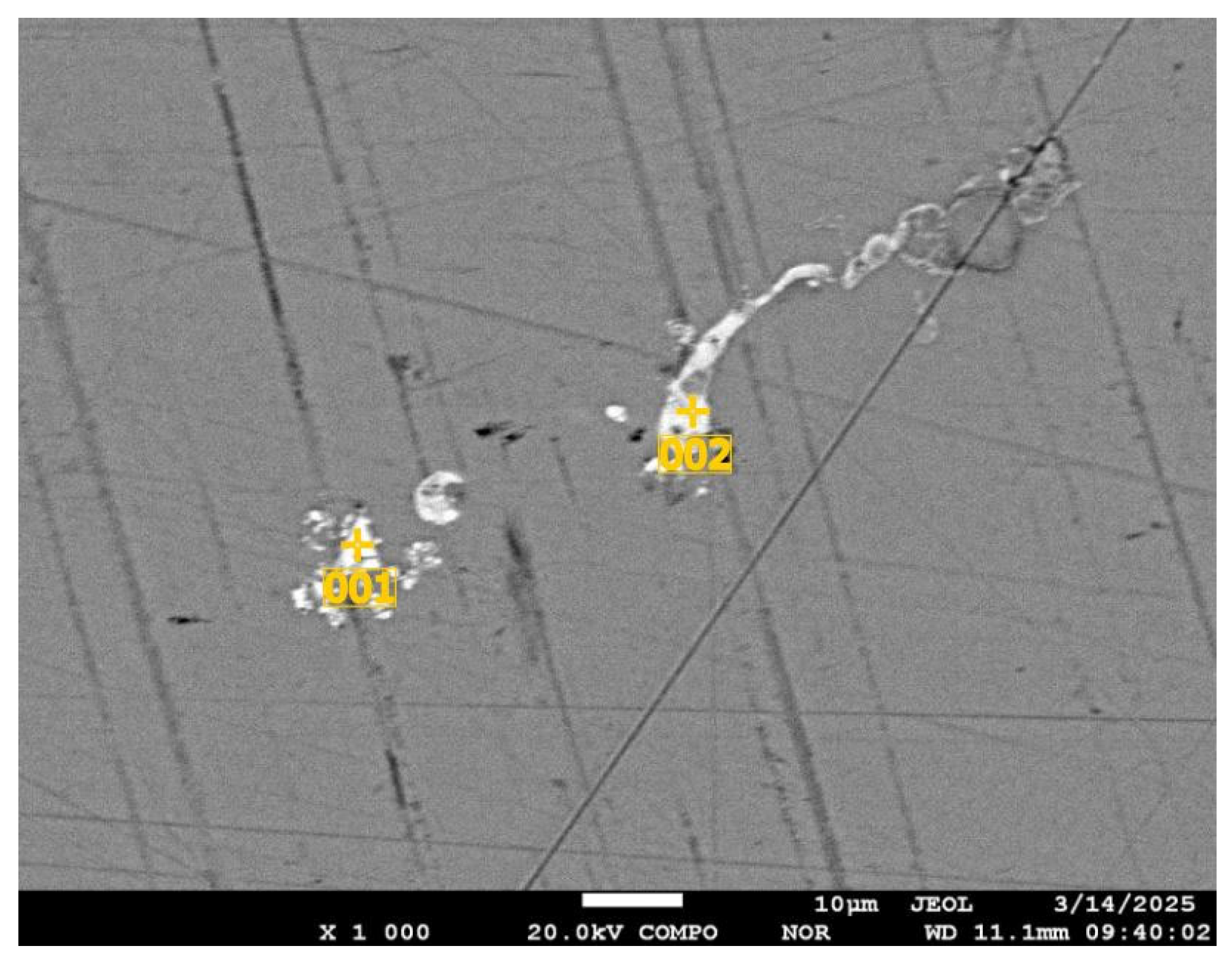
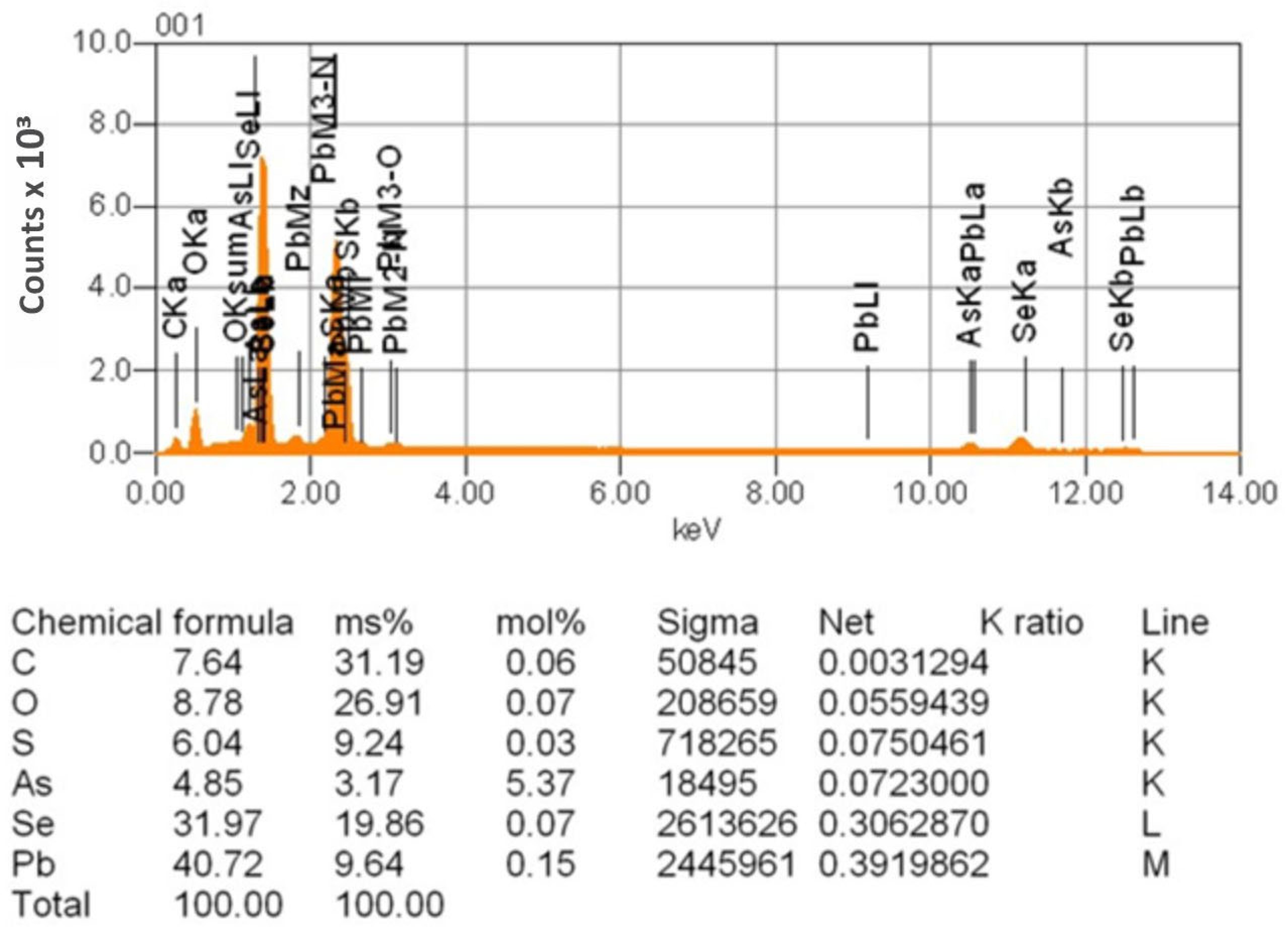
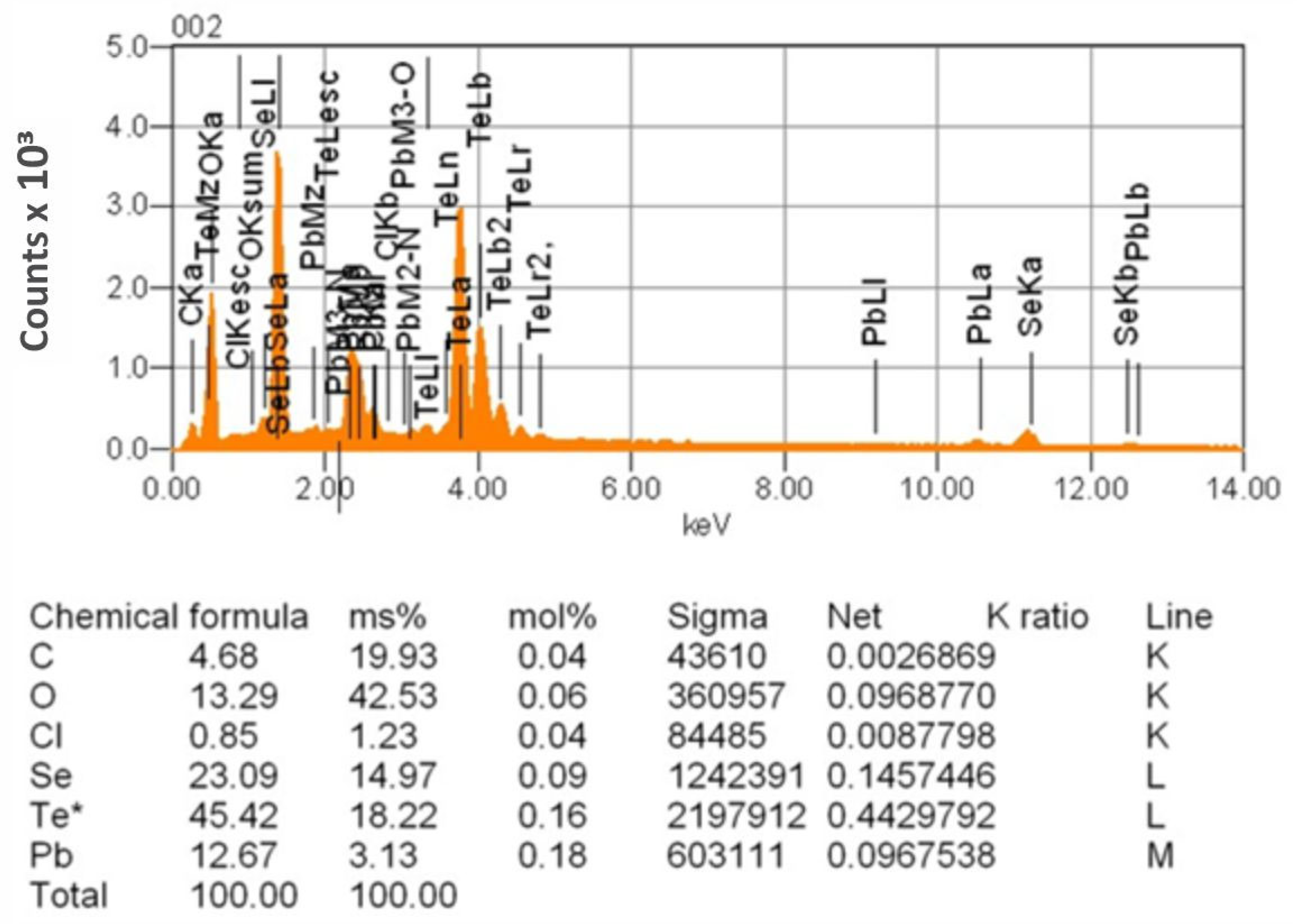
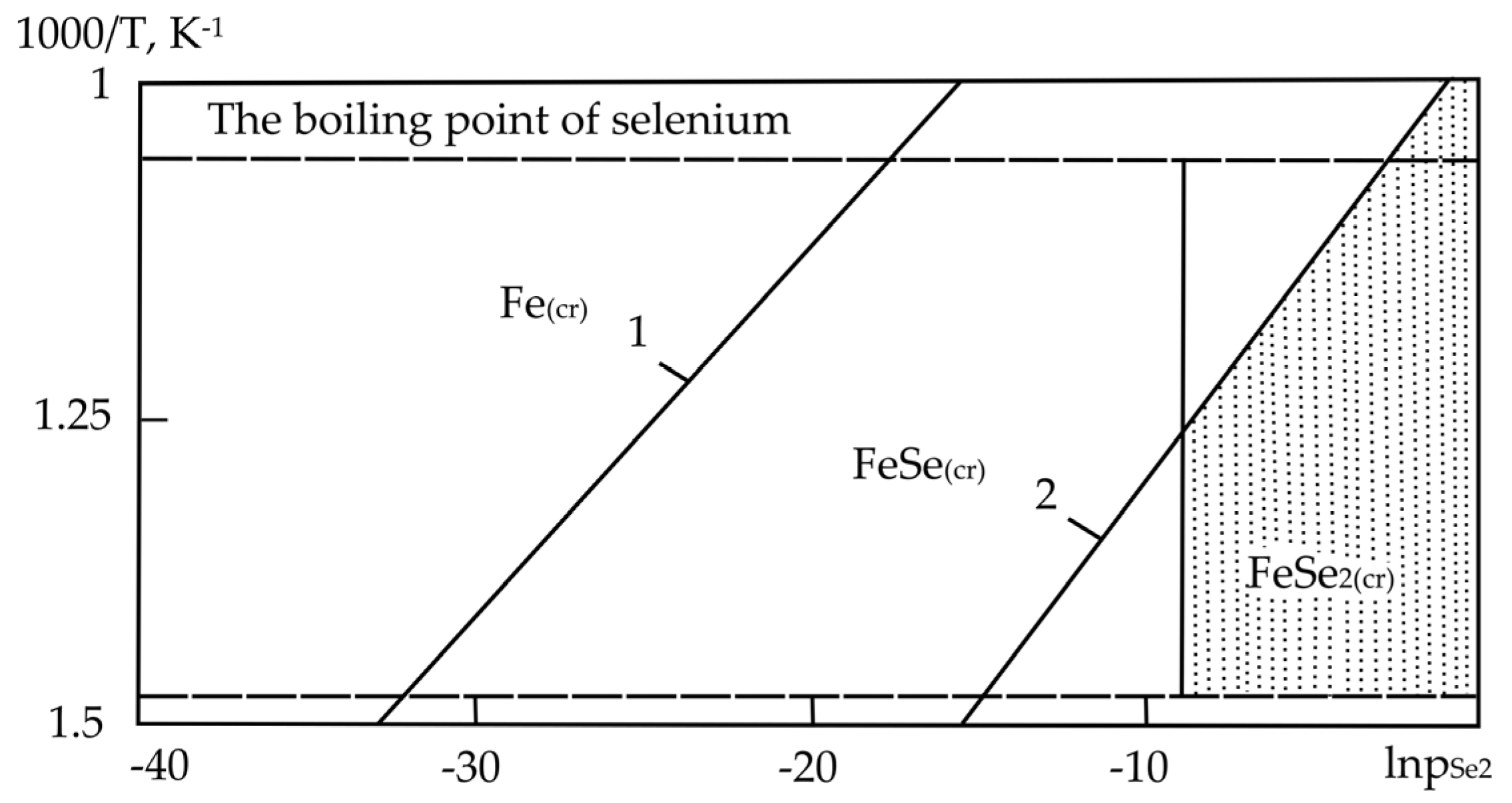
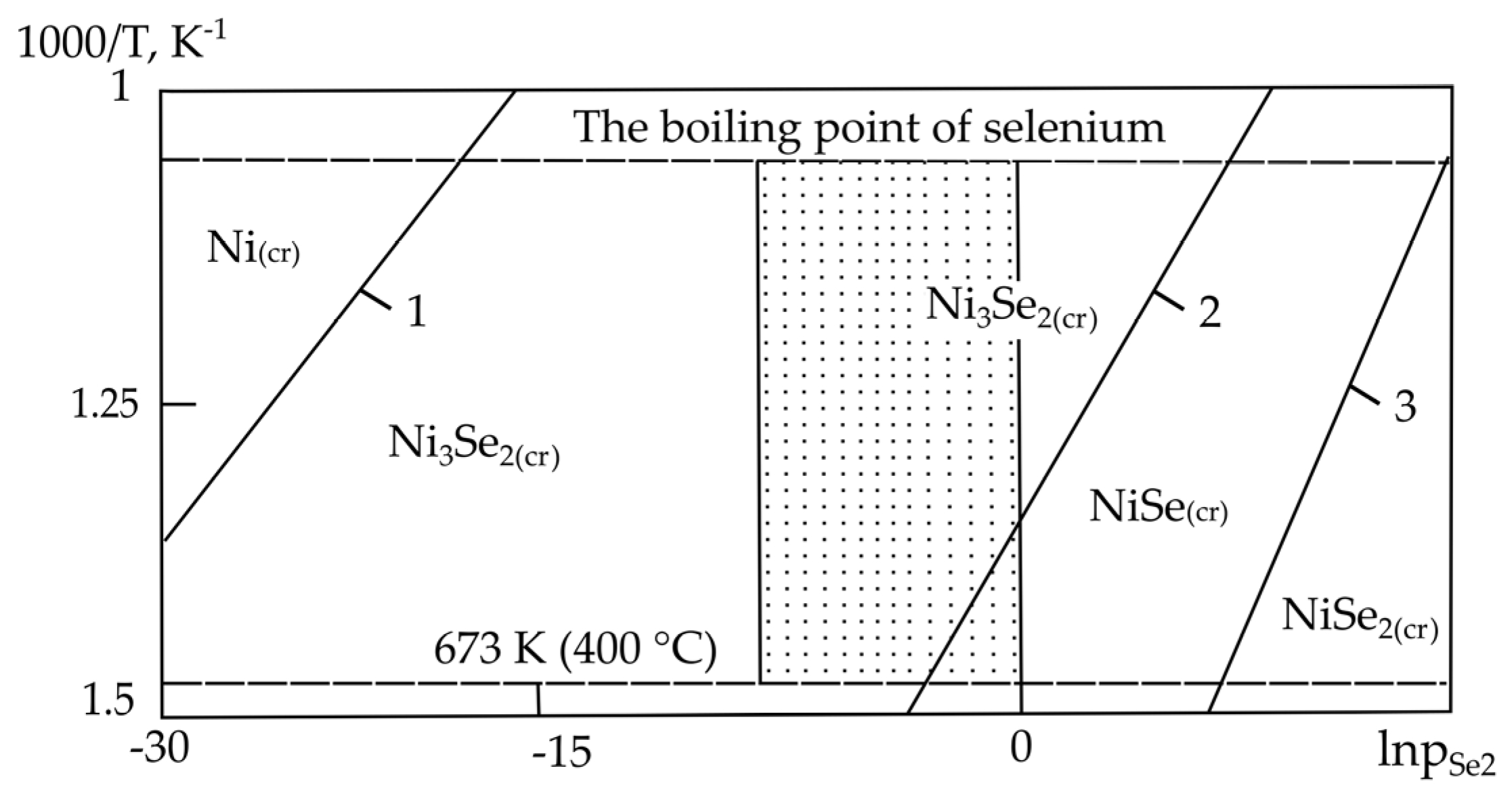
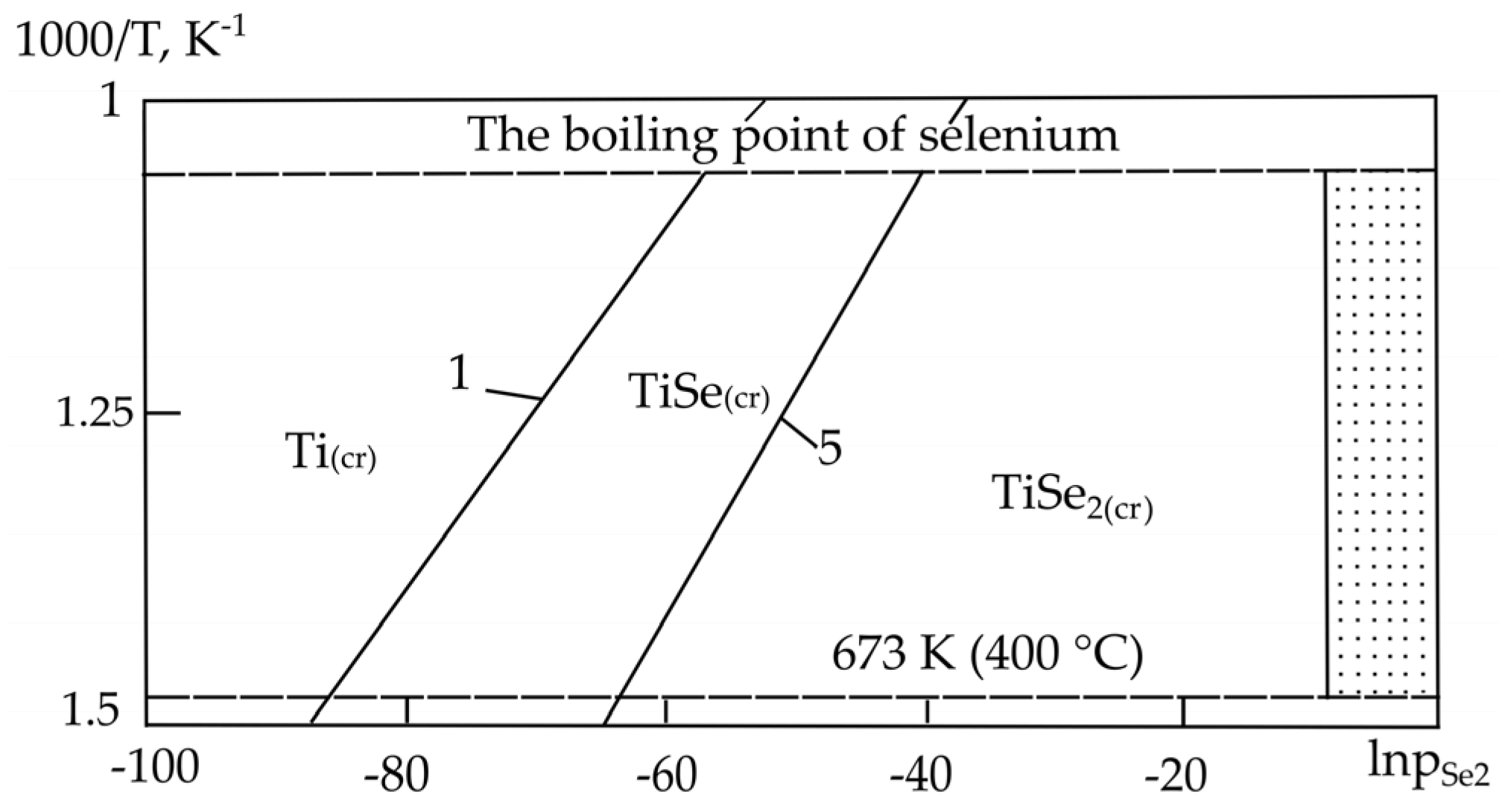
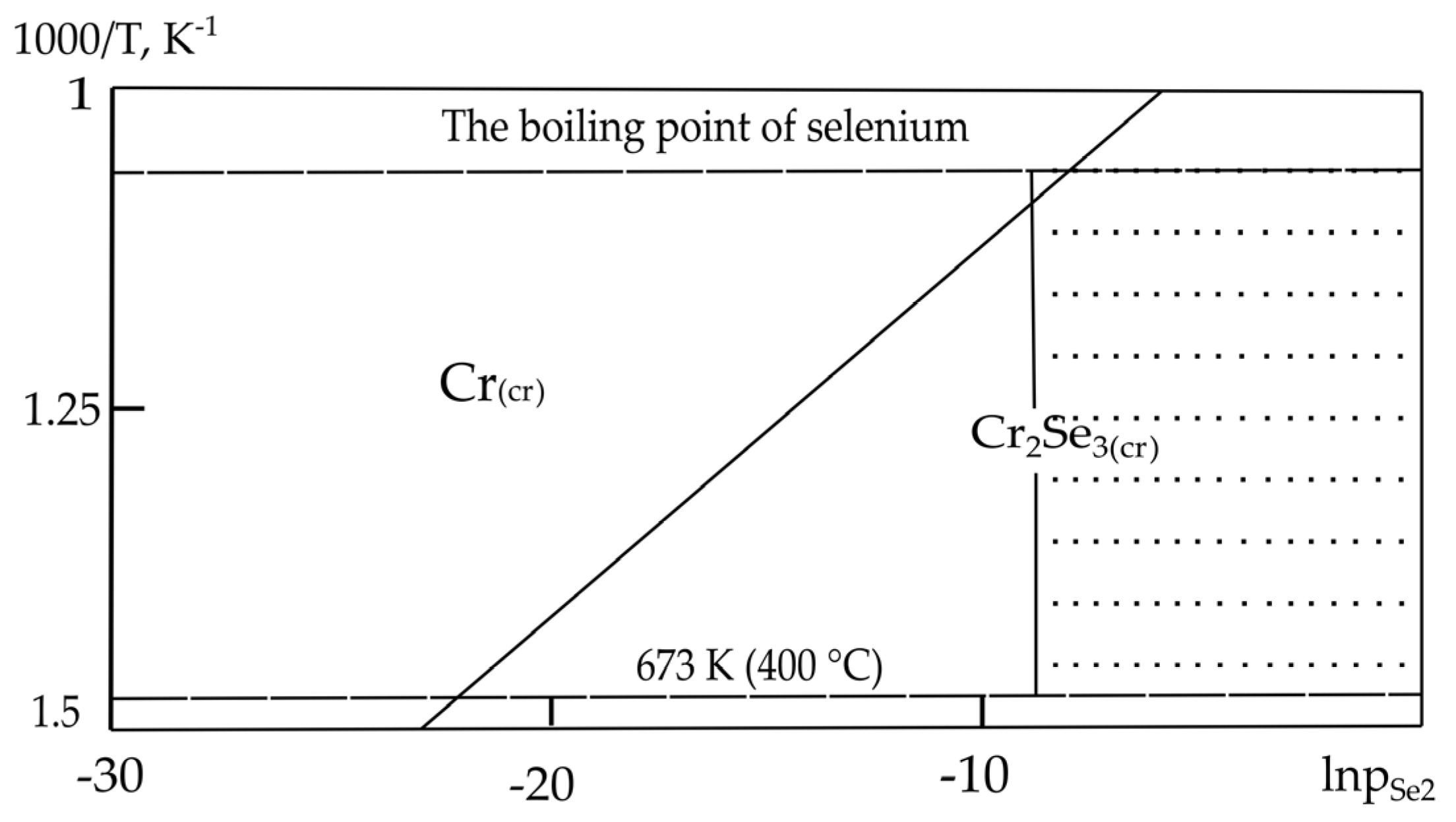


| Sr. No. | System | Selenide | , kJ/mol | , J/(mol·K) |
|---|---|---|---|---|
| 1. | Fe–Se | FeSe(cr) | −75.31 [27] | 72.00 [27] |
| FeSe2(cr) | −122.38 * | 86.77 [27] | ||
| 2. | Ni–Se | Ni3Se2(cr) | −146.40 * | 204.0 * |
| NiSe(cr) | −58.58 [27] | 65.70 [27] | ||
| Ni2Se3(cr) | −64.64 [27] | 103.50 [27] | ||
| 3. | Ti–Se | TiSe | −220.00 [28] | 86.60 * |
| Ti3Se4 | −271.00 [28] | 315.80 * | ||
| TiSe2 | −376.00 [28] | 142.60 * | ||
| 4. | Cr–Se | Cr2Se3 | −209.5 [29] | 86.20 [29] |
| Sr. No. | System | Decomposition Reaction of Selenides | Value ln Kp at Temperature, K: | |
|---|---|---|---|---|
| 673 | 945 | |||
| 1. | Fe–Se | FeSe2(cr) = FeSe(cr) + Se(l) | −4.649 | −1.884 |
| FeSe(cr) = Fe(cr) + Se(l) | −13.314 | −9.096 | ||
| FeSe2(cr) = Fe(cr) + 2Se(l) | −17.964 | −10.980 | ||
| 2. | Ni–Se | NiSe2(cr) = NiSe(cr) + Se(l) | −0.090 | +0.566 |
| 3NiSe(cr) = Ni3Se2(cr) + Se(l) | +1.125 | +2.979 | ||
| Ni3Se2(cr) = 3Ni(cr) + 2Se(l) | −28.833 | −20.614 | ||
| NiSe2(cr) = Ni(cr) + 2Se(l) | −9.326 | −5.312 | ||
| NiSe(cr) = Ni(cr) + Se(l) | −9.236 | −5.867 | ||
| 3. | Ti–Se | TiSe(cr) = Ti(cr) + Se(l) | −40.261 | −28.600 |
| Ti3Se4(cr) = 3TiSe(cr) + Se(l) | +67.323 | +48.658 | ||
| 3TiSe2(cr) = Ti3Se4(cr) + 2Se(l) | −155.55 | −110.78 | ||
| Ti3Se4(cr) = 3Ti(cr) + 4Se(l) | −53.217 | −37.889 | ||
| TiSe2(cr) = Ti(cr) + 2Se(l) | −69.589 | −49.559 | ||
| 2TiSe2(cr) = 2TiSe(cr) + Se(l) | −62.686 | −39.692 | ||
| 4. | Cr–Se | Cr2Se3(cr) = 2Cr(cr) + 3Se(l) | −25.514 | −13.705 |
| Sr. No. | Reactions | Value ln Kp at Temperature, K: | |
|---|---|---|---|
| 673 | 945 | ||
| 1. | 2 FeSe(cr)=2 Fe(cr) + Se2(g) | −32.119 | −17.221 |
| 2. | 2 FeSe2(cr)= 2 FeSe(cr) + Se2(g) | −14.796 | −2.803 |
| 3. | FeSe2(cr)= Fe(cr) + Se2(g) | −18.063 | −4.618 |
| 4. | 3 FeSe2(cr)= 2 FeSe(cr) + Fe(cr) + 2Se2(g) | −61.236 | −36.107 |
| Sr. No. | Reactions | Value ln Kp at Temperature, K: | |
|---|---|---|---|
| 673 | 945 | ||
| 1. | Ni3Se2(cr)= 3 Ni(cr) + Se2(g) | −34.335 | −19.654 |
| 2. | 6 NiSe(cr)= 2 Ni3Se2(cr) + Se2(g) | −3.218 | +6.945 |
| 3. | 2 NiSe2(cr)= 2 NiSe(cr) + Se2(g) | +6.849 | +14.602 |
| 4. | 2 NiSe(cr)= 2 Ni(cr) + Se2(g) | −24.033 | −10.836 |
| 5. | NiSe2(cr)= Ni(cr) + Se2(g) | −14.816 | −4.341 |
| 6. | 4 NiSe(cr)= NiSe2(cr) + 3 Ni(cr) + Se2(g) | −33.104 | −17.231 |
| Sr. No. | Reactions | Value ln Kp at Temperature, K: | |
|---|---|---|---|
| 673 | 945 | ||
| 1. | 2TiSe(cr) = 2 Ti(cr) + Se2(g) | −86.517 | −56.733 |
| 2. | Ti3Se4(cr) = 2TiSe(cr) + Ti(cr) + Se2(g) | +22.320 | +20.777 |
| 3. | 2Ti3Se4(cr) = 6TiSe(cr) + Se2(g) | +131.157 | +98.287 |
| 4. | 3TiSe2(cr) = Ti3Se4(cr) + Se2(g) | −161.041 | −109.807 |
| 5. | 2TiSe2(cr) = 2TiSe(cr) + Se2(g) | −63.641 | −40.442 |
| 6. | Ti3Se4(cr) = 3Ti(cr) + 2Se2(g) | −64.197 | −35.956 |
| 7. | TiSe2(cr) = Ti(cr) + Se2(g) | −75.079 | −48.588 |
| Sr. No. | System | Phase and Temperature Range of Existence (K) at Pressure, kPa (mm Hg): | ||
|---|---|---|---|---|
| 0.13 (1) | 1.33 (10) | 6.67 (50) | ||
| 1. | Fe–Se | FeSe2 (673–839) | FeSe2 (673–900) | FeSe2 (673–945) |
| FeSe (839–945) | FeSe (900–945) | - | ||
| 2. | Ni–Se | Ni3Se2 (673–945) | Ni3Se2 (673–945) | Ni3Se2 (746–945) |
| – | – | NiSe (673–746) | ||
| 3. | Ti–Se | TiSe2 (673–945) | TiSe2 (673–945) | TiSe2 (673–945) |
| 4. | Cr–Se | Cr2Se3 (673–945) | Cr2Se3 (673–945) | Cr2Se3 (673–945) |
| Impurity Content, Mass% | |||||||
|---|---|---|---|---|---|---|---|
| Fe | Cu | Pb | Hg | Te | As | S | Al |
| Grade ST 1 according to GOST 10298-2018 | |||||||
| 0.010 | 0.0050 | 0.005 | 0.0050 | 0.100 | 0.005 | 0.020 | 0.005 |
| Raw selenium | |||||||
| 0.001 | 0.001 | 0.002 | 0.003 | 0.09 | 0.005 | 0.02 | 0.001 |
| Refined selenium | |||||||
| 0.0001 | 0.0002 | 0.0001 | 0.002 | 0.07 | 0.003 | 0.01 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volodin, V.; Trebukhov, S.; Kenzhaliyev, B.; Nitsenko, A.; Mishra, B.; Kolesnikova, O.; Linnik, X.; Sukurov, B. Purification of the Selenium Vapor Phase from Droplet Suspensions in Vacuum Distillation Refining. Processes 2025, 13, 2397. https://doi.org/10.3390/pr13082397
Volodin V, Trebukhov S, Kenzhaliyev B, Nitsenko A, Mishra B, Kolesnikova O, Linnik X, Sukurov B. Purification of the Selenium Vapor Phase from Droplet Suspensions in Vacuum Distillation Refining. Processes. 2025; 13(8):2397. https://doi.org/10.3390/pr13082397
Chicago/Turabian StyleVolodin, Valeriy, Sergey Trebukhov, Bagdaulet Kenzhaliyev, Alina Nitsenko, Brajendra Mishra, Olga Kolesnikova, Xeniya Linnik, and Bulat Sukurov. 2025. "Purification of the Selenium Vapor Phase from Droplet Suspensions in Vacuum Distillation Refining" Processes 13, no. 8: 2397. https://doi.org/10.3390/pr13082397
APA StyleVolodin, V., Trebukhov, S., Kenzhaliyev, B., Nitsenko, A., Mishra, B., Kolesnikova, O., Linnik, X., & Sukurov, B. (2025). Purification of the Selenium Vapor Phase from Droplet Suspensions in Vacuum Distillation Refining. Processes, 13(8), 2397. https://doi.org/10.3390/pr13082397







