New Efficient High-Energy Materials Based on 4,6-Dinitrobenzimidazol-2-one Core: Simulations of Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
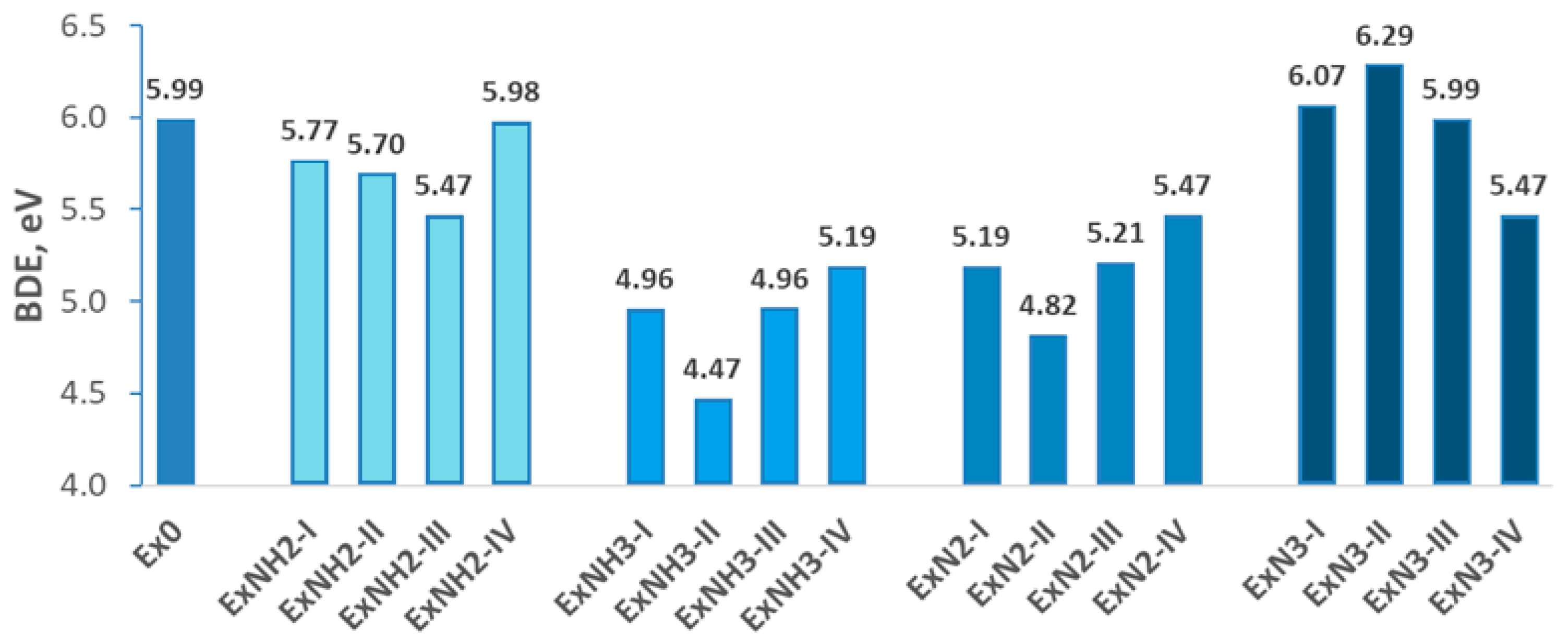
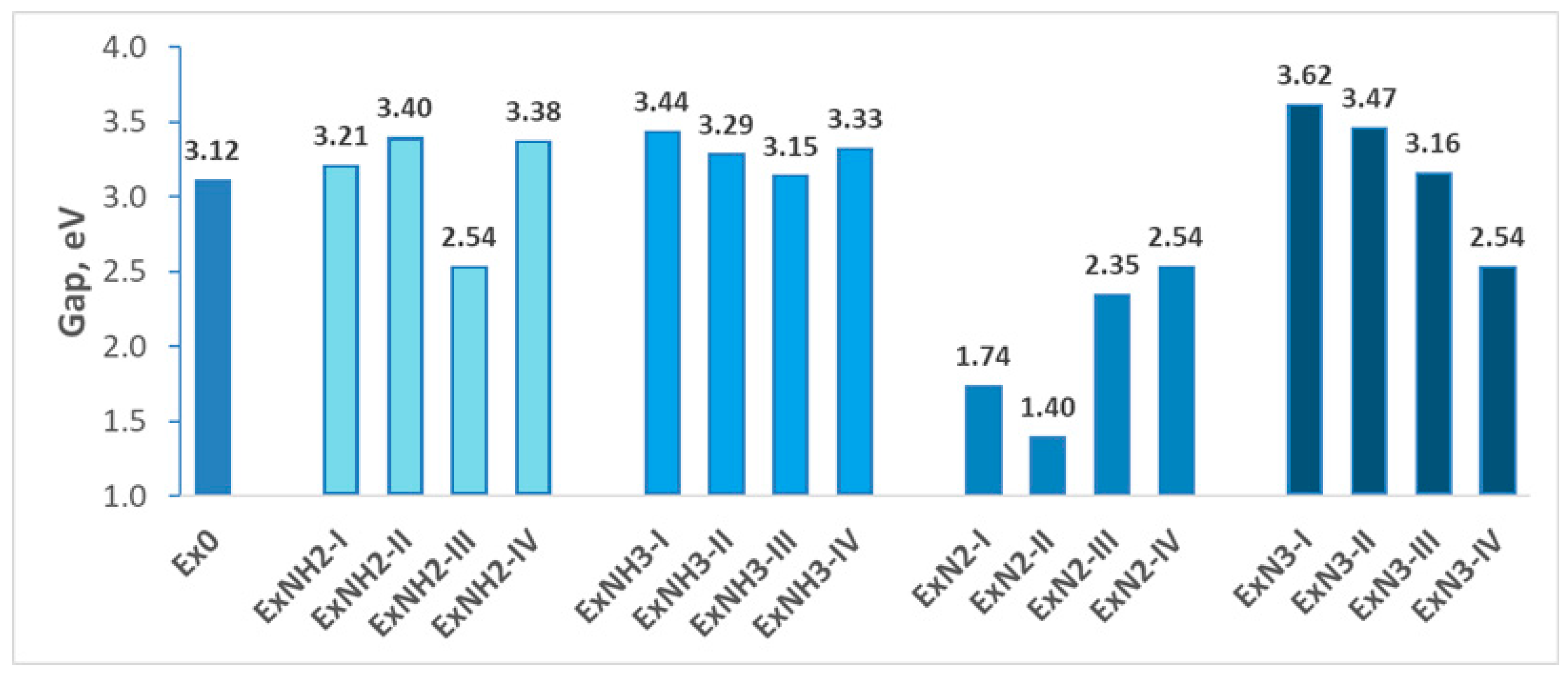
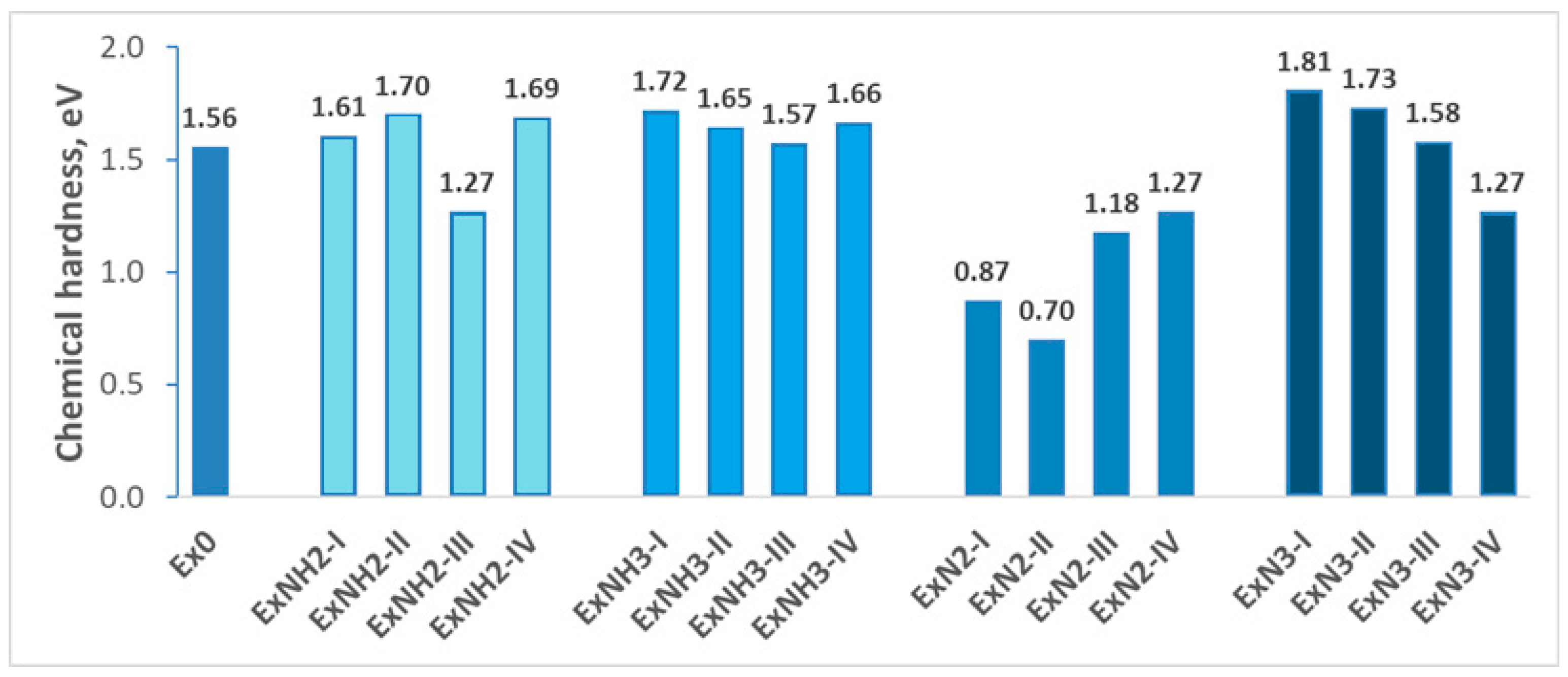
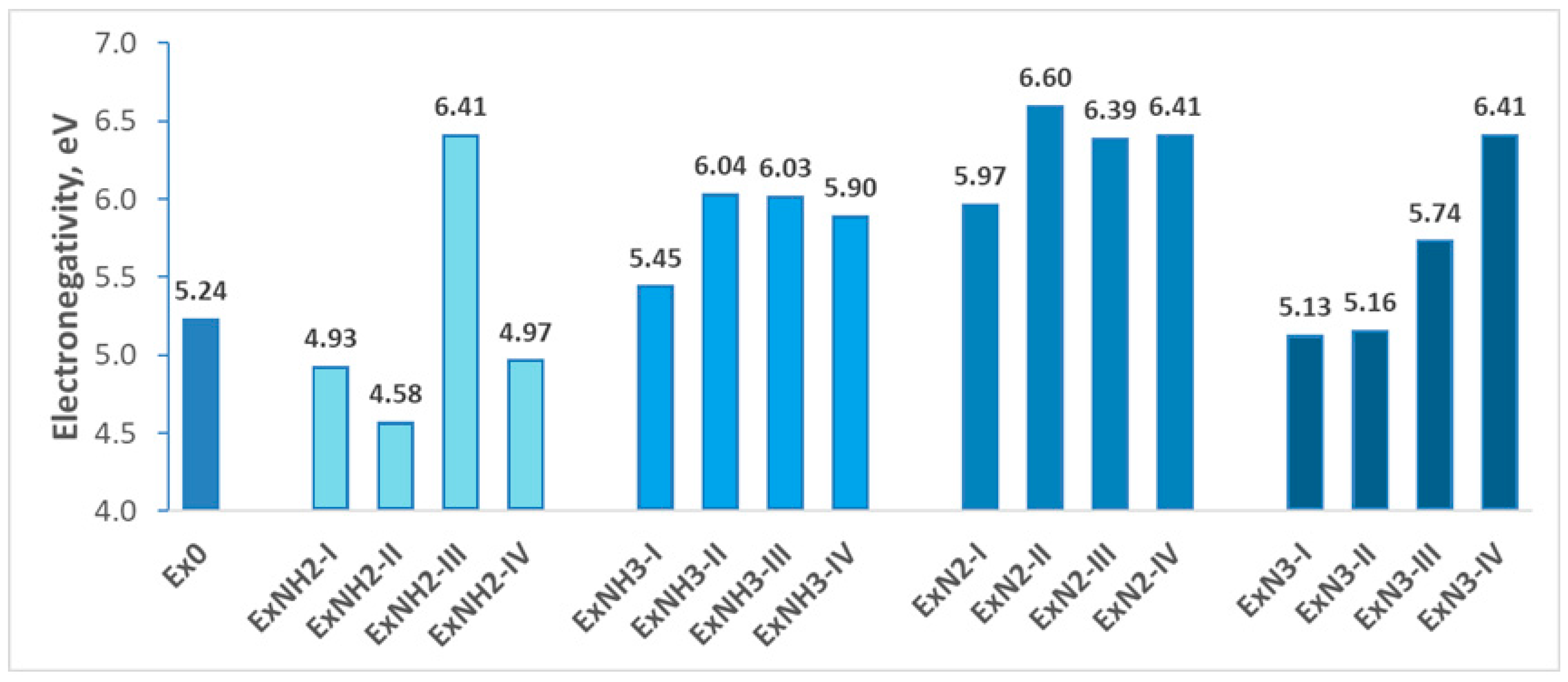
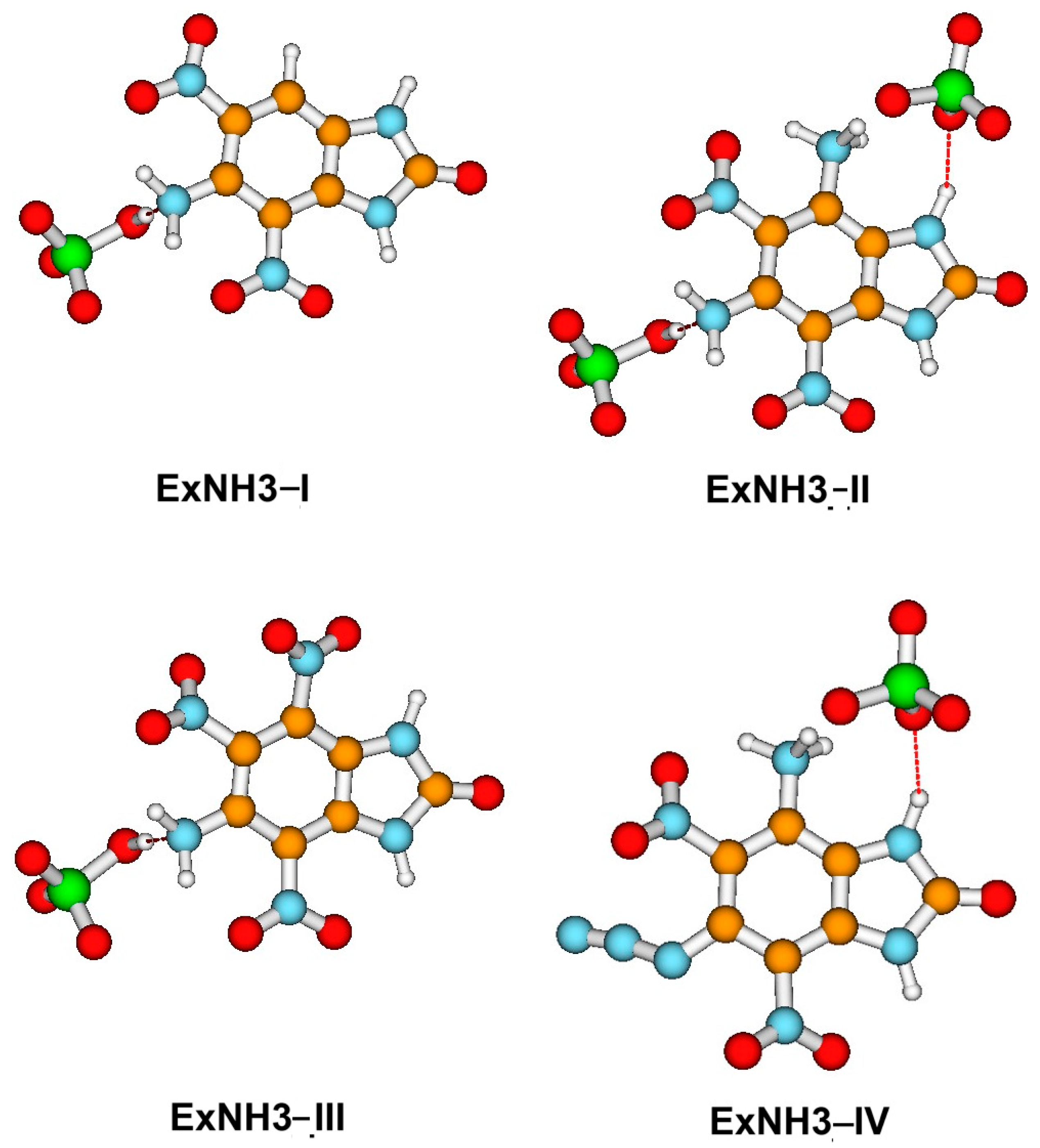
4.1. Stability
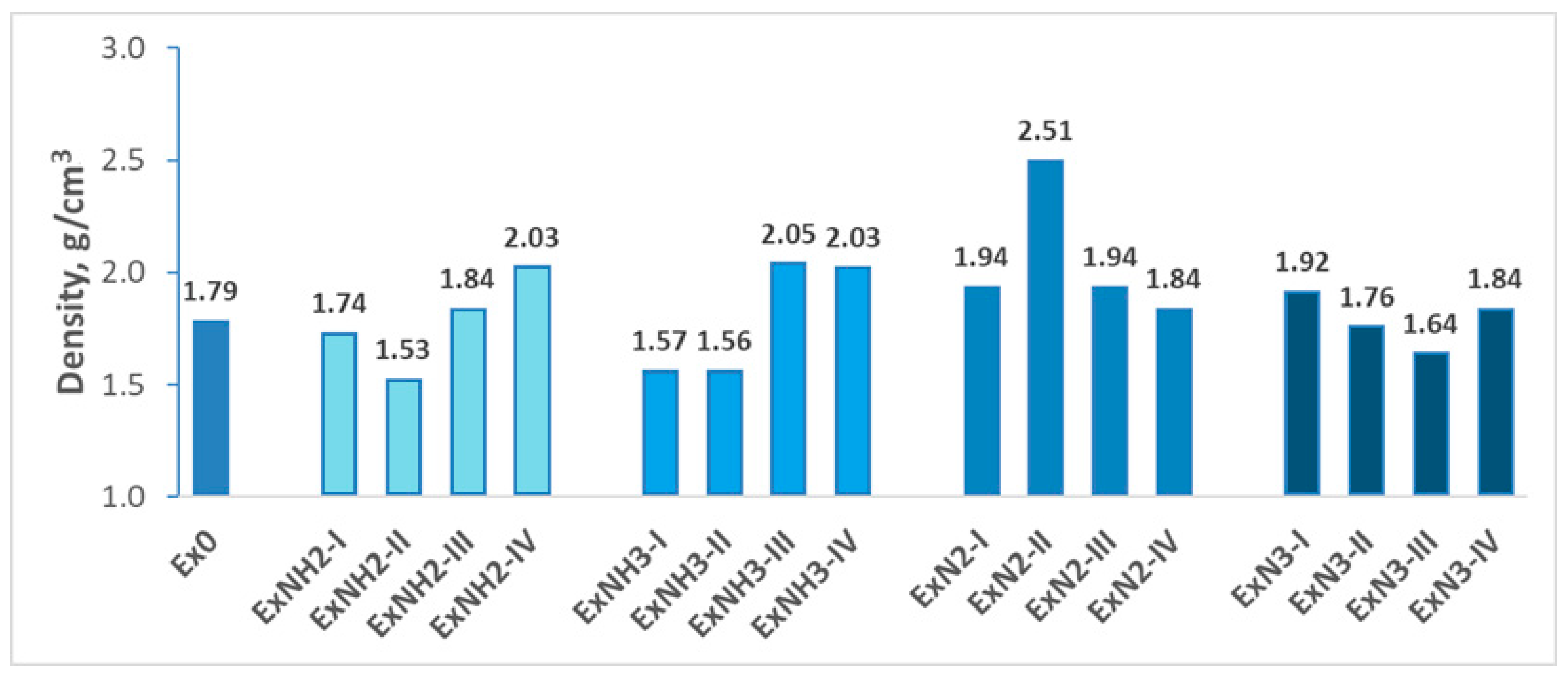
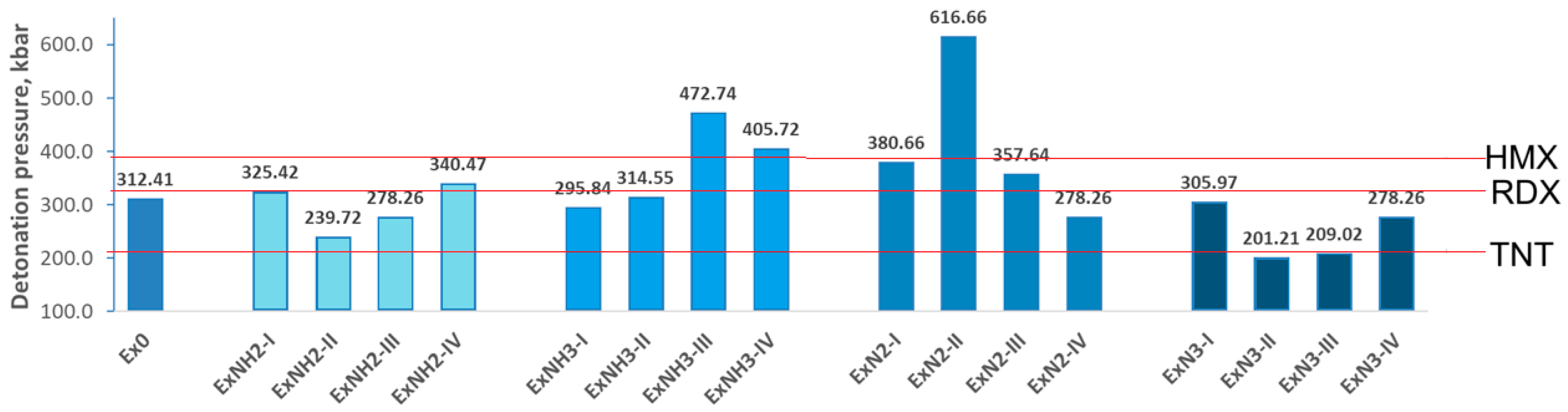
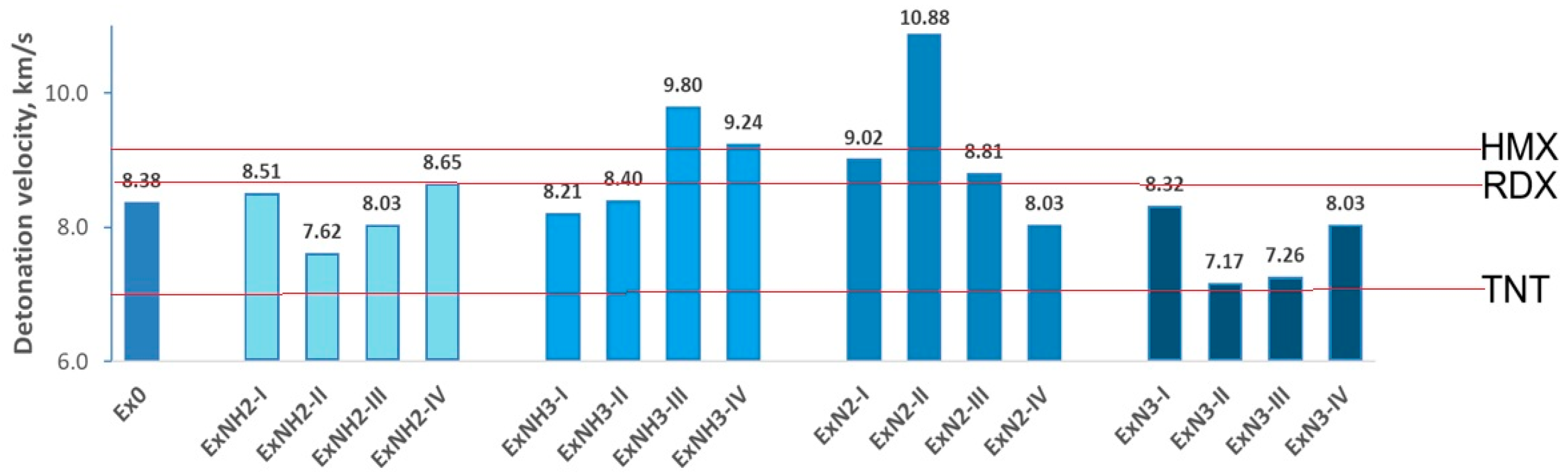
4.2. Energetic Properties
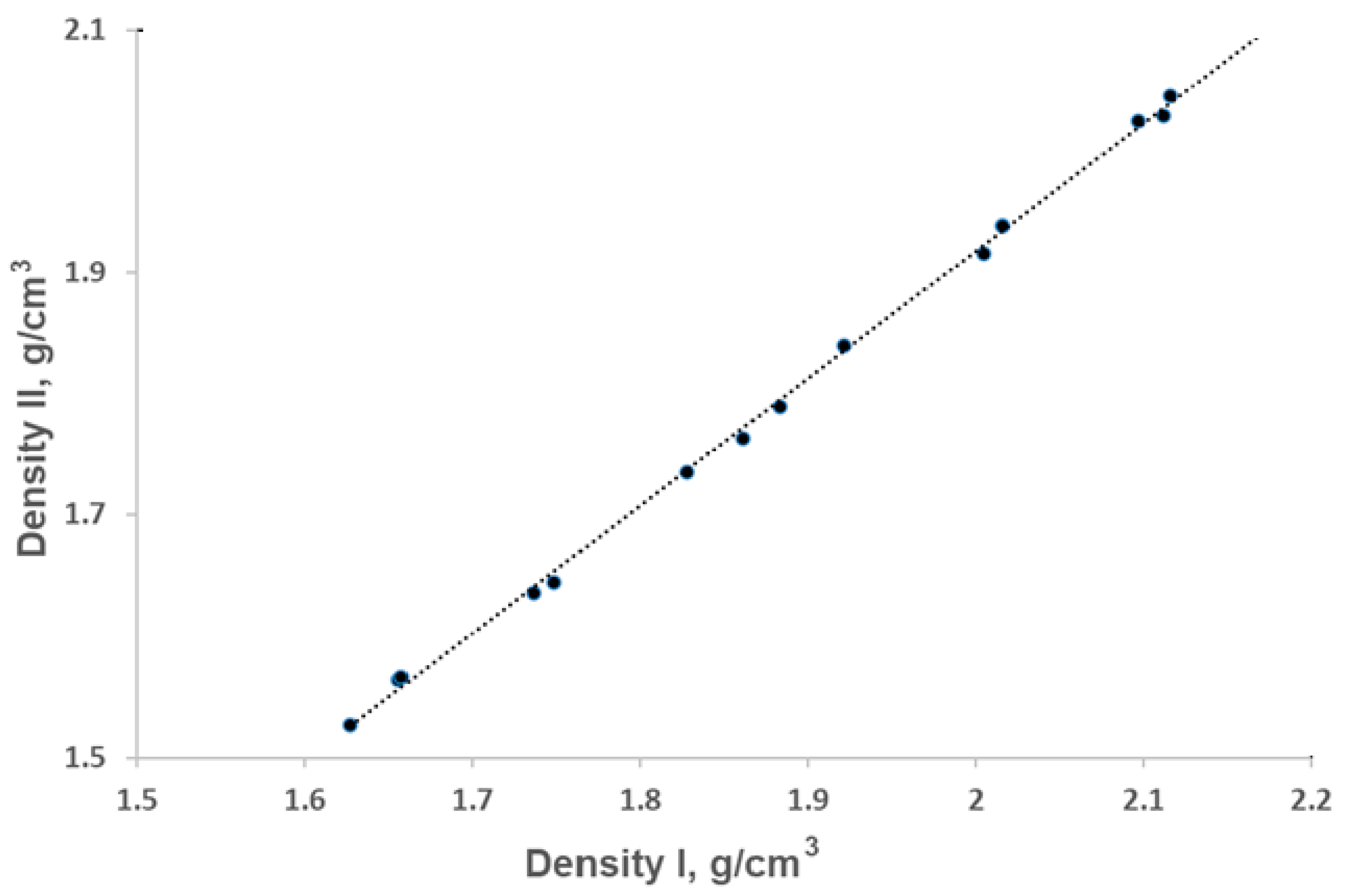
- In combination with -NH2, -NH3, or -N2 (the last two along with the ClO4− anion), the density of Ex0 increases.
- In combination with -N3, the density of Ex0 decreases.
- Incorporation of it in compounds with -NH2 or -NH3 (along with the ClO4− anion) leads to an increase in their densities.
- Incorporation of it in the compound with -N2 (along with the ClO4− anion) does not influence its density.
- Incorporation of it in the compound with -N3 reduces its density.
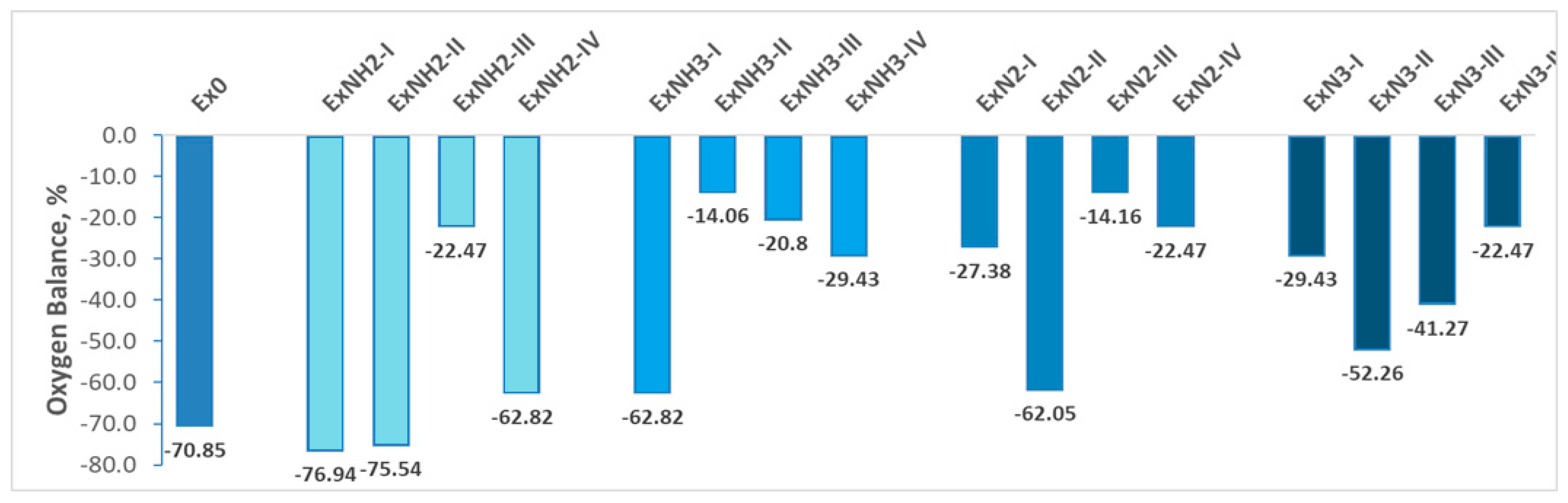
4.3. Oxygen Balance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDE | Cohesive energy per atom | |
| HOMO-LUMO gap | Difference between the highest occupied and the lowest unoccupied orbitals | |
| OB | Oxygen balance (calc. in relation to CO2) | |
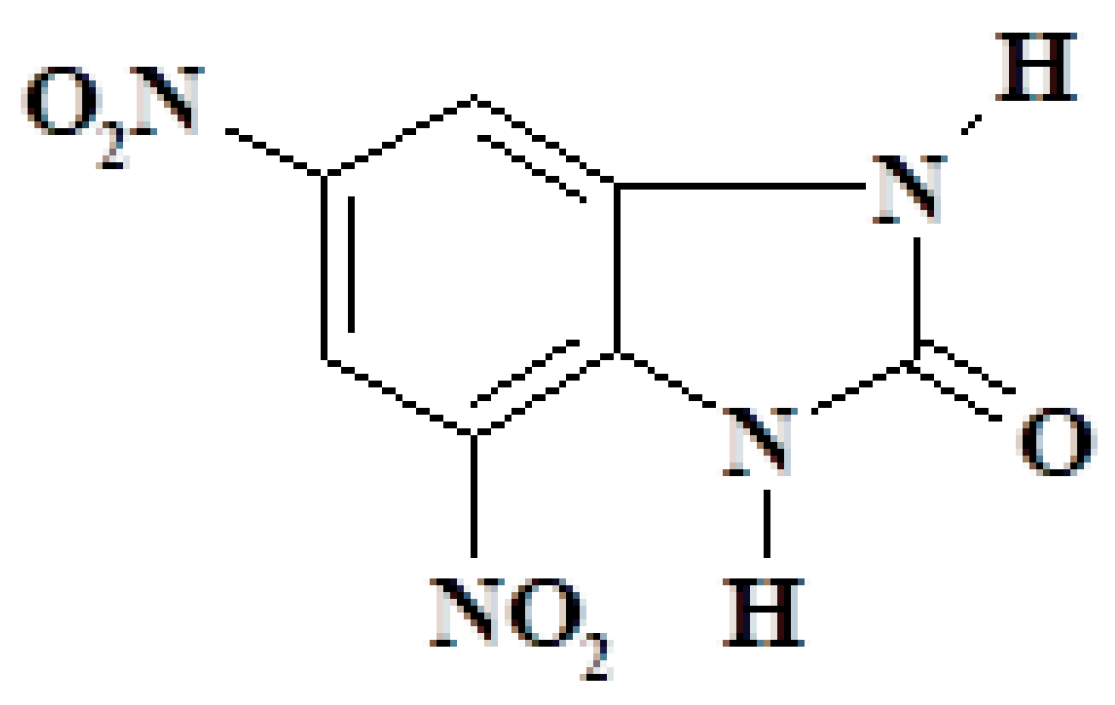
| Ex0 | C7H3N5O5 | 4,6-Dinitrobenzimidazol-2-one |
| ExNH2-I | C7H5N5O5 | 5-Amino-4,6-dinitrobenzimidazol-2-one |
| ExNH3-I | C7H6ClN5O9 | 5-Ammonium-4,6-dinitrobenzimidazol-2-one perchlorate |
| ExN2-I | C7H3ClN6O9 | [(4,6-Dinitrobenzimidazol-2-one) 5-diazonium] perchlorate |
| ExN3-I | C7H3N7O5 | 5-Azido-4,6-dinitrobenzimidazol-2-one |
| ExNH2-II | C7H6N6O5 | 5,7-Diamino-4,6-dinitrobenzimidazol-2-one |
| ExNH3-II | C7H8Cl2N6O13 | 5,7-Diammonium-4,6-dinitrobenzimidazol-2-one diperchlorate |
| ExN2-II | C7H2Cl2N8O13 | [(4,6-Dinitrobenzimidazol-2-one bis-(5,7-diazonium)] diperchlorate |
| ExN3-II | C7H2N10O5 | 5,7-Diazido-4,6-dinitrobenzimidazol-2-one |
| ExNH2-III | C7H2ClN9O9 | 5-Amino-4,6,7-trinitrobenzimidazol-2-one |
| ExNH3-III | C7H5ClN6O11 | 5-Ammonium-4,6,7-trinitrobenzimidazol-2-one perchlorate |
| ExN2-III | C7H2ClN7O11 | (4,6,7-Trinitrobenzimidazol-2-one 5-diazonium) perchlorate |
| ExN3-III | C7H2N8O7 | 5-Azido-4,6,7-trinitrobenzimidazol-2-one |
| ExNH2-IV | C7H4N8O5 | 5-Azido-7-amino-4,6-dinitrobenzimidazol-2-one |
| ExNH3-IV | C7H5ClN8O9 | (5-Azido-4,6-dinitrobenzimidazol-2-one 7-ammonium) perchlorate |
| ExN2-IV/ExN3-IV | C7H2ClN9O9 | (5-Azido-4,6-dinitrobenzimidazol-2-one 7-diazonium) perchlorate |
Appendix A
| Substitution | Structure | Mol. Formula, Abbreviation | MW | Calculated Elemental Composition Data | ||||
|---|---|---|---|---|---|---|---|---|
| C, % | H, % | Cl, % | N, % | O, % | ||||
| -NH2 |  | C7H5N5O5 ExNH2-I | 239.15 | 35.16 | 2.11 | 0 | 29.28 | 33.45 |
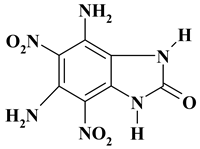 | C7H6N6O5 ExNH2-II | 254.16 | 33.08 | 2.38 | 0 | 33.07 | 31.47 | |
 | C7H4N6O7 ExNH2-III | 284.15 | 29.59 | 1.42 | 0 | 29.58 | 39.41 | |
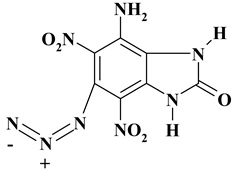 | C7H4N8O5 ExNH2-IV | 280.16 | 30.01 | 1.44 | 0 | 40.00 | 28.55 | |
| -NH3 with perchlorate | 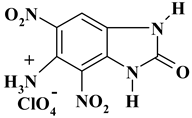 | C7H5ClN5O9 ExNH3-I | 338.60 | 24.83 | 1.49 | 10.47 | 20.68 | 42.53 |
 | C7H8Cl2N6O13 ExNH3-II | 455.08 | 18.48 | 1.77 | 15.58 | 18.47 | 45.70 | |
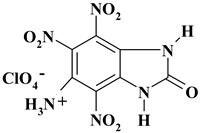 | C7H5ClN6O11 ExNH3-III | 384.60 | 21.86 | 1.31 | 9.22 | 21.85 | 45.76 | |
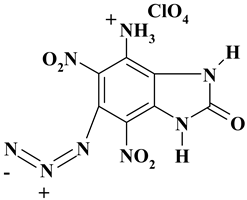 | C7H5ClN8O9 ExNH3-IV | 380.62 | 22.09 | 1.32 | 9.31 | 29.44 | 37.83 | |
| -N2 with perchlorate | 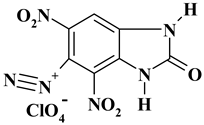 | C7H3ClN6O9 ExN2-I | 350.59 | 23.98 | 0.86 | 10.11 | 23.97 | 41.07 |
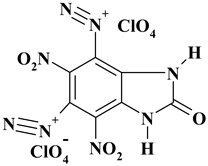 | C7H2Cl2N8O13 ExN2-II | 477.05 | 17.62 | 0.42 | 14.86 | 23.49 | 43.60 | |
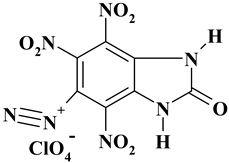 | C7H2ClN7O11 ExN2-III | 395.59 | 21.20 | 0.76 | 8.94 | 24.72 | 44.38 | |
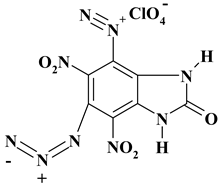 | C7H2ClN9O9 ExN2-IV/ExN3-IV | 391.60 | 21.47 | 0.51 | 9.05 | 32.19 | 36.77 | |
| -N3 | 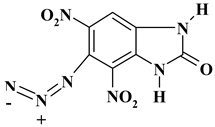 | C7H3N7O5 ExN3-I | 265.15 | 31.71 | 1.14 | 0 | 36.98 | 30.17 |
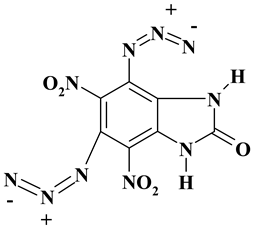 | C7H2N10O5 ExN3-II | 306.16 | 27.46 | 0.66 | 0 | 45.75 | 26.13 | |
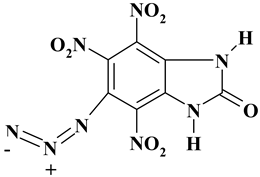 | C7H2N8O7 ExN3-III | 310.14 | 21.25 | 0.51 | 8.96 | 24.79 | 44.49 | |
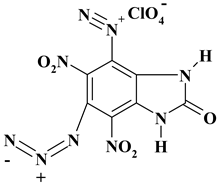 | C7H2ClN9O9 ExN3-IV/ExN2-IV | 391.60 | 21.47 | 0.51 | 9.05 | 32.19 | 36.77 | |
References
- Nguyen, J.; Nguyen, T.Q.; Han, B.; Hoang, B.X. Oral Fenbendazole for Cancer Therapy in Humans and Animals. Anticancer Res. 2024, 44, 3725–3735. [Google Scholar] [CrossRef]
- Ebenezer, O.; Oyetunde-Joshua, F.; Omotoso, O.D.; Shapi, M. Benzimidazole and its derivatives: Recent Advances (2020–2022). Results Chem. 2023, 5, 100925. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Preimesser, A.; Stierstorfer, J. Energetic Derivatives of 2-Nitrimino-5,6-dinitro-benzimidazole. Propellants Explos. Pyrotech. 2015, 40, 60–66. [Google Scholar] [CrossRef]
- Szala, M.; Gutowski, Ł.; Trzcinski, W. New thermally stable and insensitive energetic compound: 5,5′,6,6′-tetranitro-2,2′-bibenzimidazole. ChemPlusChem 2018, 83, 87–91. [Google Scholar]
- Politzer, P.A.; Murray, J.S. (Eds.) Theoretical and Computational Chemistry, Energetic Materials. Part 1. Decomposition, Crystal and Molecular Properties; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2003; Volume 12, pp. 1–466. [Google Scholar]
- Politzer, P.A.; Murray, J.S. (Eds.) Theoretical and Computational Chemistry, Energetic Materials. Part 2. Detonation, Combustion; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2003; Volume 13, pp. 1–474. [Google Scholar]
- Pang, W.; DeLuca, L.T.; Gromov, A.A.; Cumming, A.S. (Eds.) Innovative Energetic Materials. Properties, Combustion Performance and Application; Springer Nature: Singapore, 2020; pp. 1–569. [Google Scholar]
- Sarlauskas, J.; Tamuliene, J.; Bekesiene, S.; Kravcov, A. Benzimidazole Derivatives as Energetic Materials: A Theoretical Study. Materials 2021, 14, 4112. [Google Scholar] [CrossRef] [PubMed]
- Drake, G.W.; Bova, S.J.; Shreeve, J.M. Energetic, low-melting salts of simple heterocycles. Propellants Explos. Pyrotech. 2003, 28, 174–180. [Google Scholar] [CrossRef]
- Drake, G.W.; Hawkins, T.W.; Boatz, J.; Hall, L.; Vij, A. Experimental and theoretical study of 1,5-diamino-4-H-1,2,3,4-tetrazolium perchlorate. Propellants Explos. Pyrotech. 2005, 30, 156–163. [Google Scholar] [CrossRef]
- Darwich, C.; Klapötke, T.M.; Sabaté, C.M. 1,2,4-Triazolium-cation-based energetic salts. Chem. Eur. J. 2008, 14, 5756–5771. [Google Scholar] [CrossRef] [PubMed]
- Smiglak, M.; Hines, C.C.; Reichert, W.M.; Vincek, A.S.; Katritzky, A.R.; Thrasher, J.S.; Sun, L.; McCrary, P.D.; Beasley, P.A.; Kelley, S.P.; et al. Synthesis, limitations, and thermal properties of energetically-substituted, protonated imidazolium picrate and nitrate salts and further comparison with their methylated analogs. New J. Chem. 2012, 36, 702–772. [Google Scholar] [CrossRef]
- Tao, G.H.; Tang, M.; He, L.; Ji, S.P.; Nie, F.D.; Huang, M. Synthesis, structure and property of 5-aminotetrazolate room-temperature ionic liquids. Eur. J. Inorg. Chem. 2012, 18, 3070–3078. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, M.; He, J.; Yu, P.; Guo, X.; Liu, Y.; Gao, H.; Yin, P. Exchanging of NH2/NHNH2/NHOH groups: An effective strategy for balancing the energy and safety of fused-ring energetic materials. Chem. Eng. J. 2023, 466, 143333. [Google Scholar] [CrossRef]
- Man, T.; Wang, K.; Zhang, J.; Niu, X.; Zhang, T. Theoretical Studies of High-nitrogen-containing Energetic Compounds Based on the s-Tetrazine Unit. Cent. Eur. J. Energ. Mater. 2013, 10, 171–189. [Google Scholar]
- Zhang, Y.; Parrish, D.; Shreeve, J. Derivatives of 5-nitro-1,2,3-2H-triazole—High performance energetic materials. J. Mater. Chem. A 2013, 1, 585–593. [Google Scholar] [CrossRef]
- Cao, W.; Qin, J.; Zhang, J.; Sinditskii, V. 4,5-Dicyano-1,2,3-Triazole—A Promising Precursor for a New Family of Energetic Compounds and Its Nitrogen-Rich Derivatives: Synthesis and Crystal Structures. Molecules 2021, 26, 6735. [Google Scholar] [CrossRef]
- Gu, H.; Xiong, H.; Yang, H.; Cheng, G. Tricyclic nitrogen-rich cation salts based on 1,2,3-triazole: Chemically stable and insensitive candidates for novel gas generant. Chem. Eng. J. 2021, 408, 128021. [Google Scholar] [CrossRef]
- Feng, S.; Yin, P.; He, C.; Pang, S.; Shreeve, J. Tunable Dimroth rearrangement of versatile 1,2,3-triazoles towards high performance energetic materials. J. Mater. Chem. A 2021, 9, 12291–12298. [Google Scholar] [CrossRef]
- Lai, Q.; Fei, T.; Yin, P.; Shreeve, J. 1,2,3-Triazole with linear and branched catenated nitrogen chains—The role of regiochemistry in Energetic Materials. Chem. Eng. J. 2020, 410, 128148. [Google Scholar] [CrossRef]
- Yao, W.; Xue, Y.; Qian, L.; Yang, H.; Cheng, G. Combination of 1,2,3-triazole and 1,2,4-triazole frameworks for new high-energy and low-sensitivity compounds. Energ. Mater. Front. 2021, 2, 131–138. [Google Scholar] [CrossRef]
- Chen, S.; Gao, Y.; Dong, C.; Shen, L.; Zeng, Y.; Bao, P.; Li, Y.; Yi, Z.; Chen, H.; Zhu, S.; et al. Tetrahedral nitrogen atoms arrangement in A-site cations: A new approach for regulating sensitivity and energy of perovskite energetic materials. Adv. Sci. 2025, 12, 2415680. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, M.; Liu, D.; Zhang, H.; Wang, J.; He, G.; Yan, Q.-L. Synergistically enhanced safety and energy density of energetic materials via interfacial constraint. Adv. Compos. Hybrid. Mater. 2025, 8, 275. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R. Computational studies on energetic properties of nitrogen-rich energetic materials with ditetrazoles. J. Chem. Sci. 2014, 126, 1753–1762. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, Q.; Yin, P.; Pang, S. Construction of adaptive deformation block: Rational molecular editing of the N-rich host molecule to remove water from the energetic hydrogen-bonded organic frameworks. ACS Appl. Mater. Interfaces 2024, 16, 21849–21856. [Google Scholar] [CrossRef]
- Hisaki, I.; Suzuki, Y.; Gomez, E.; Ji, Q.; Tohnai, N.; Nakamura, T.; Douhal, A. Acid responsive hydrogen-bonded organic frameworks. J. Am. Chem. Soc. 2019, 141, 2111–2121. [Google Scholar] [CrossRef]
- Han, B.; Wang, H.; Wang, C.; Wu, H.; Zhou, W.; Chen, B.; Jiang, J. Postsynthetic metalation of a robust hydrogen-bonded organic framework for heterogeneous catalysis. J. Am. Chem. Soc. 2019, 141, 8737–8740. [Google Scholar] [CrossRef]
- Sukhanov, G.T.; Filippova, Y.; Gatilov, Y.; Sukhanova, A.; Krupnova, I.; Bosov, K.; Pivovarova, E.; Krasnov, V. Energetic Materials Based on N-substituted 4(5)-nitro-1,2,3-triazoles. Materials 2022, 15, 1119. [Google Scholar] [CrossRef]
- Lai, Q.; Pei, L.; Fei, T.; Yin, P.; Pang, S.; Shreeve, J. Size-matched hydrogen bonded hydroxylammonium frameworks for regulation of energetic materials. Nat. Commun. 2022, 13, 6937. [Google Scholar] [CrossRef]
- Du, Y.; Su, H.; Fei, T.; Hu, B.; Zhang, J.; Li, S.; Pang, S.; Nie, F. Structure-Property Relationship in Energetic Cationic Metal–Organic Frameworks: New Insight for Design of Advanced Energetic Materials. Cryst. Growth Des. 2018, 18, 1–16. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Garcia-Santos, I.; Fernández-Vazquez, R.; Gargari, M.S.; Gomila, R.M.; Castiñeiras, A.; Frontera, A.; Safin, D.A. Anion driven tetrel bonding dictated supramolecular architectures of lead(II) with a zwitterionic form of polydentate N′-(piperidine-1 carbonothioyl)picolinohydrazonamide. CrystEngComm 2024, 26, 435. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Zhao, X.; Yang, Z. Dye decorated ammonium perchlorate with fast decomposition and high safety performance. Adv. Mater. Interfaces 2025, 35, 2418301. [Google Scholar] [CrossRef]
- Liang, Y.; Hu, X.; Yang, Z.; Liu, M.; Zhang, Y.; Wu, J.; Zhang, J.; Zhao, T.; Sun, S.; Wang, S. Benzimidazole-based low-sensitivity and heat-resistant energetic materials: Design and synthesis. New J. Chem. 2025, 49, 257. [Google Scholar] [CrossRef]
- Becke, A. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 56. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.2; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Cardia, R.; Malloci, G.; Mattoni, A.; Cappellini, G. Effects of TIPS-Functionalization and Perhalogenation on the Electronic, Optical, and Transport Properties of Angular and Compact Dibenzochrysene. J. Phys. Chem. A 2014, 118, 5170–5177. [Google Scholar] [CrossRef] [PubMed]
- Cardia, R.; Malloci, G.; Rignanese, G.; Blasé, X.; Molteni, E.; Cappellini, G. Electronic and optical properties of hexathiapentacene in the gas and crystal phases. Phys. Rev. B 2016, 93, 235132. [Google Scholar] [CrossRef]
- Dardenne, N.; Cardia, R.; Li, J.; Malloci, G.; Cappellini, G.; Blasé, X.; Charlier, J.; Rignanese, G. Tuning Optical Properties of Dibenzochrysenes by Functionalization: A Many-Body Perturbation Theory Study. J. Phys. Chem. C 2017, 121, 24480–24488. [Google Scholar] [CrossRef]
- Antidormi, A.; Aprile, G.; Cappellini, G.; Cara, E.; Cardia, R.; Colombo, L.; Farris, R.; d’Ischia, M.; Mehrabanian, M.; Melis, C. Physical and Chemical Control of Interface Stability in Porous Si–Eumelanin Hybrids. J. Phys. Chem. C 2018, 122, 28405–28415. [Google Scholar] [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Inclusions of Si-atoms in Graphene nanostructures: A computational study on the ground-state electronic properties of Coronene and Ovalene. J. Phys. Conf. Ser. 2018, 956, 012020. [Google Scholar] [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Si-atoms substitutions effects on the electronic and optical properties of coronene and ovalene. New J. Phys. 2018, 20, 113008. [Google Scholar] [CrossRef]
- Kumar, A.; Cardia, R.; Cappellini, G. Electronic and optical properties of chromophores from bacterial cellulose. Cellulose 2018, 25, 2191–2203. [Google Scholar] [CrossRef]
- Szafran, M.; Koput, J. Ab initio and DFT calculations of structure and vibrational spectra of pyridine and its isotopomers. J. Mol. Struct. 2001, 565, 439–448. [Google Scholar] [CrossRef]
- Begue, D.; Carbonniere, P.; Pouchan, C. Calculations of Vibrational Energy Levels by Using a Hybrid ab Initio and DFT Quartic Force Field: Application to Acetonitrile. J. Phys. Chem. A 2005, 109, 4611–4616. [Google Scholar] [CrossRef]
- Kaya, S.; Kaya, C. New equation based on ionization energies and electron affinities of atoms for calculating of group electronegativity. Comput. Theor. Chem. 2015, 1052, 42–46. [Google Scholar] [CrossRef]
- Politzer, P.; Martinez, J.; Murray, J.; Concha, M.; Toro-Labbé, A. An electrostatic interaction correction for improved crystal density prediction. Mol. Phys. 2009, 107, 2095–2101. [Google Scholar] [CrossRef]
- Keshavarz, M.; Zamani, A. A simple and reliable method for predicting the detonation velocity of CHNOFCl and aluminized explosives. Cent. Eur. Energ. Mater. 2015, 12, 13–33. [Google Scholar]
- Keshavarz, M.; Pouretedal, H. An empirical method for predicting detonation pressure of CHNOFCl explosives. Thermochim. Acta 2004, 414, 203–208. [Google Scholar] [CrossRef]
- Zahari, N.; Montazeri, M.; Hoseini, S. Estimation of the Detonation Pressure of Co-crystal Explosives through a Novel, Simple and Reliable Model. Cent. Eur. J. Energ. Mater. 2020, 17, 492–505. Available online: https://bibliotekanauki.pl/articles/1062768 (accessed on 5 June 2025). [CrossRef] [PubMed]
- Meyer, R.; Köhler, J.; Homburg, A. Explosives, 6th ed.; Wiley-VCH: Weinheim, Germany, 2007; ISBN 978-3-527-31656-4. [Google Scholar]
- Rajakumar, B.; Arathala, P.; Muthiah, B. Thermal Decomposition of 2-Methyltetrahydrofuran behind Reflected Shock Waves over the Temperature Range of 1179–1361 K. J. Phys. Chem. A 2021, 125, 5406–5423. [Google Scholar] [CrossRef] [PubMed]
- Šarlauskas, J.; Stankevičiūtė, J.; Tamulienė, J. An Efficient Synthesis and Preliminary Investigation of Novel 1,3-Dihydro-2H-benzimidazol-2-one Nitro and Nitramino Derivatives. Materials 2022, 15, 8330. [Google Scholar] [CrossRef]
- Christe, K.O.; Wilson, W.W.; Sheehy, J.A.; Boatz, J.A. Thermal Stability of Energetic Ionic Liquids. Inorg. Chem. 2007, 46, 6299–6303. [Google Scholar]
- Džingalašević, V.; Antić, G.; Mlađenović, D. Ratio of detonation pressure and critical pressure of high explosives with different compounds. Sci. Tech. Rev. 2004, 5, 72–76. Available online: http://www.vti.mod.gov.rs/ntp/rad2004/34-04/dzin/dzin.pdf (accessed on 8 February 2025).
- Kumar, P.; Ghule, V.D.; Dharavath, S. Advancing energetic chemistry: The first synthesis of sulfur-based C–C bonded thiadiazole-pyrazine compounds with a nitrimino moiety. Dalton Trans. 2024, 53, 19112–19115. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.; Ferguson, R.E.; Forbes, J.; Kuhl, A.L.; Oppenheim, A.K.; Spektor, R. Confined combustion of TNT explosion products in air. In Proceedings of the 8th International Colloquium on Dust Explosions, Schaumburg, IL, USA, 21–25 September 1998; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 1998. Available online: https://www.osti.gov/biblio/3648 (accessed on 8 February 2025).
- Rice, B.M.; Byrd, E.F.C. Relationships with oxygen balance and bond dissociation energies. Theor. Comput. Chem. 2022, 22, 67–75. [Google Scholar] [CrossRef]
- Zeman, S.; Jungova, M. Sensitivity and Performance of Energetic Materials. Propellants Explos. Pyrotech. 2016, 41, 426–451. [Google Scholar] [CrossRef]
- Lia, G.; Zhang, C. Review of the molecular and crystal correlations on sensitivities of energetic materials. J. Hazard. Mater. 2020, 398, 122910. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. High Performance, Low Sensitivity: Conflicting or Compatible? Propellants Explos. Pyrotech. 2016, 41, 414–425. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamuliene, J.; Sarlauskas, J. New Efficient High-Energy Materials Based on 4,6-Dinitrobenzimidazol-2-one Core: Simulations of Properties. Processes 2025, 13, 2386. https://doi.org/10.3390/pr13082386
Tamuliene J, Sarlauskas J. New Efficient High-Energy Materials Based on 4,6-Dinitrobenzimidazol-2-one Core: Simulations of Properties. Processes. 2025; 13(8):2386. https://doi.org/10.3390/pr13082386
Chicago/Turabian StyleTamuliene, Jelena, and Jonas Sarlauskas. 2025. "New Efficient High-Energy Materials Based on 4,6-Dinitrobenzimidazol-2-one Core: Simulations of Properties" Processes 13, no. 8: 2386. https://doi.org/10.3390/pr13082386
APA StyleTamuliene, J., & Sarlauskas, J. (2025). New Efficient High-Energy Materials Based on 4,6-Dinitrobenzimidazol-2-one Core: Simulations of Properties. Processes, 13(8), 2386. https://doi.org/10.3390/pr13082386









