Catalytic Combustion Hydrogen Sensors for Vehicles: Hydrogen-Sensitive Performance Optimization Strategies and Key Technical Challenges
Abstract
1. Introduction
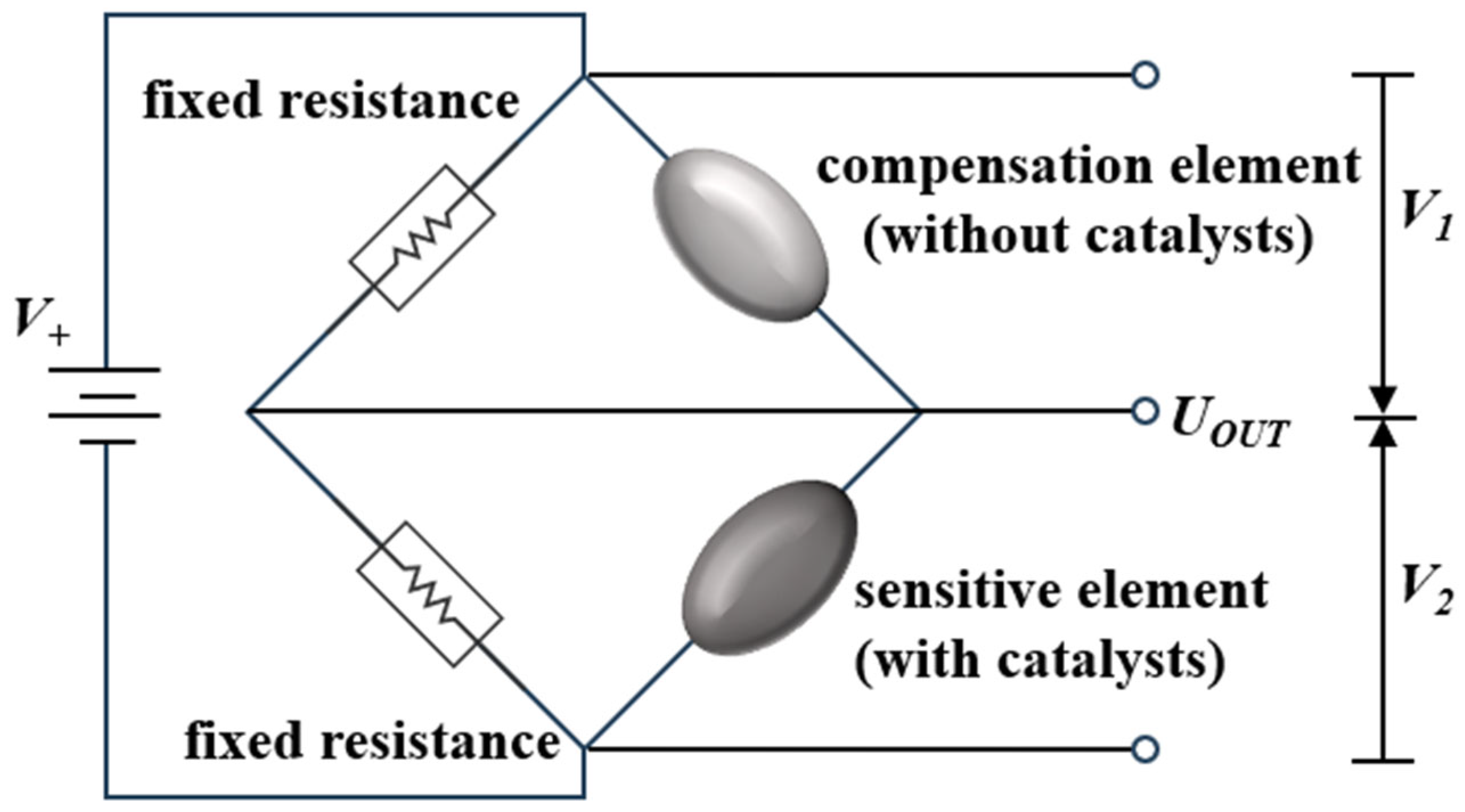
2. Working Principle of Catalytic Combustion Hydrogen Sensors
3. Optimization of Hydrogen-Sensitive Materials
3.1. Design Methods for High-Performance Hydrogen Oxidation Catalysts
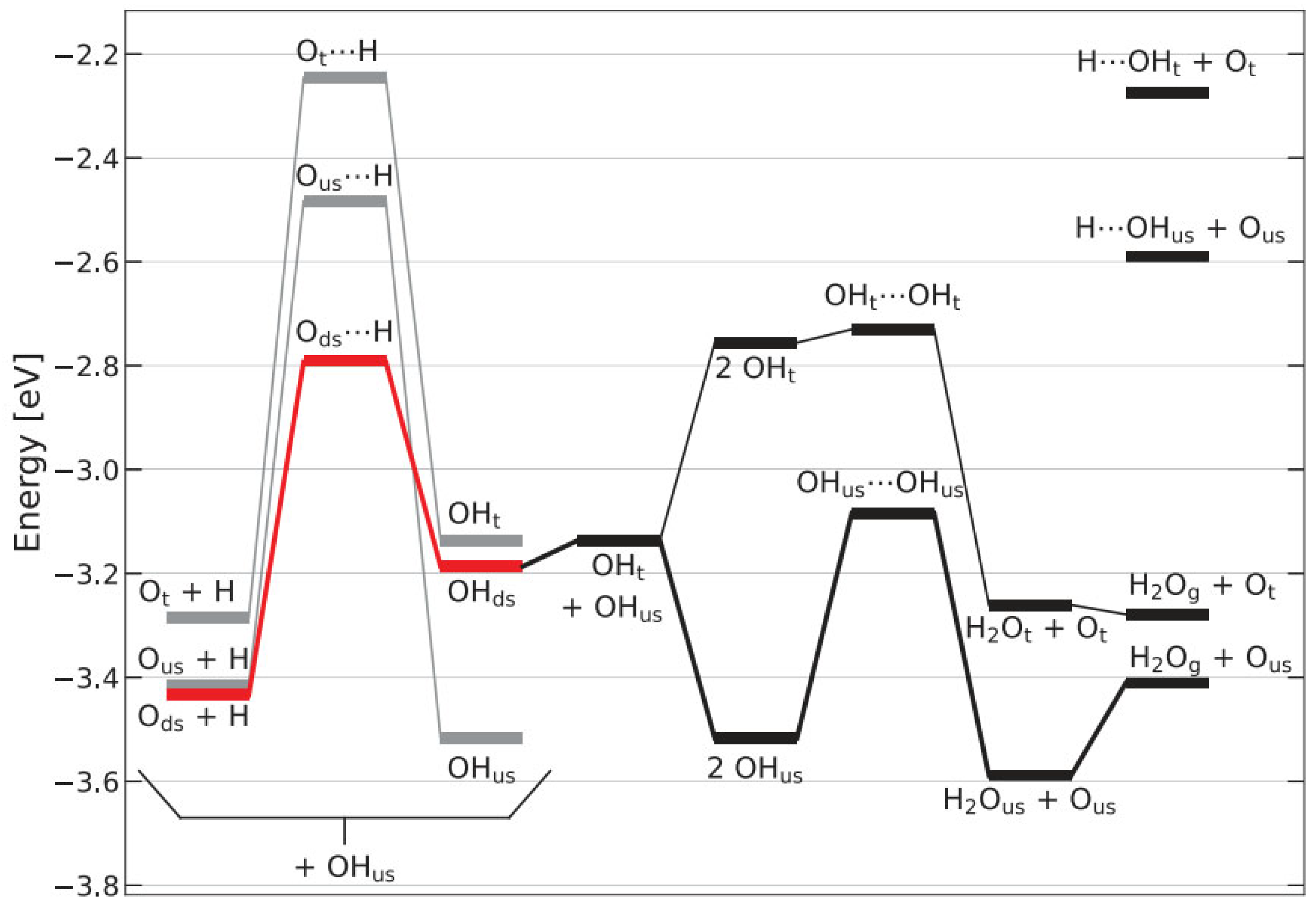
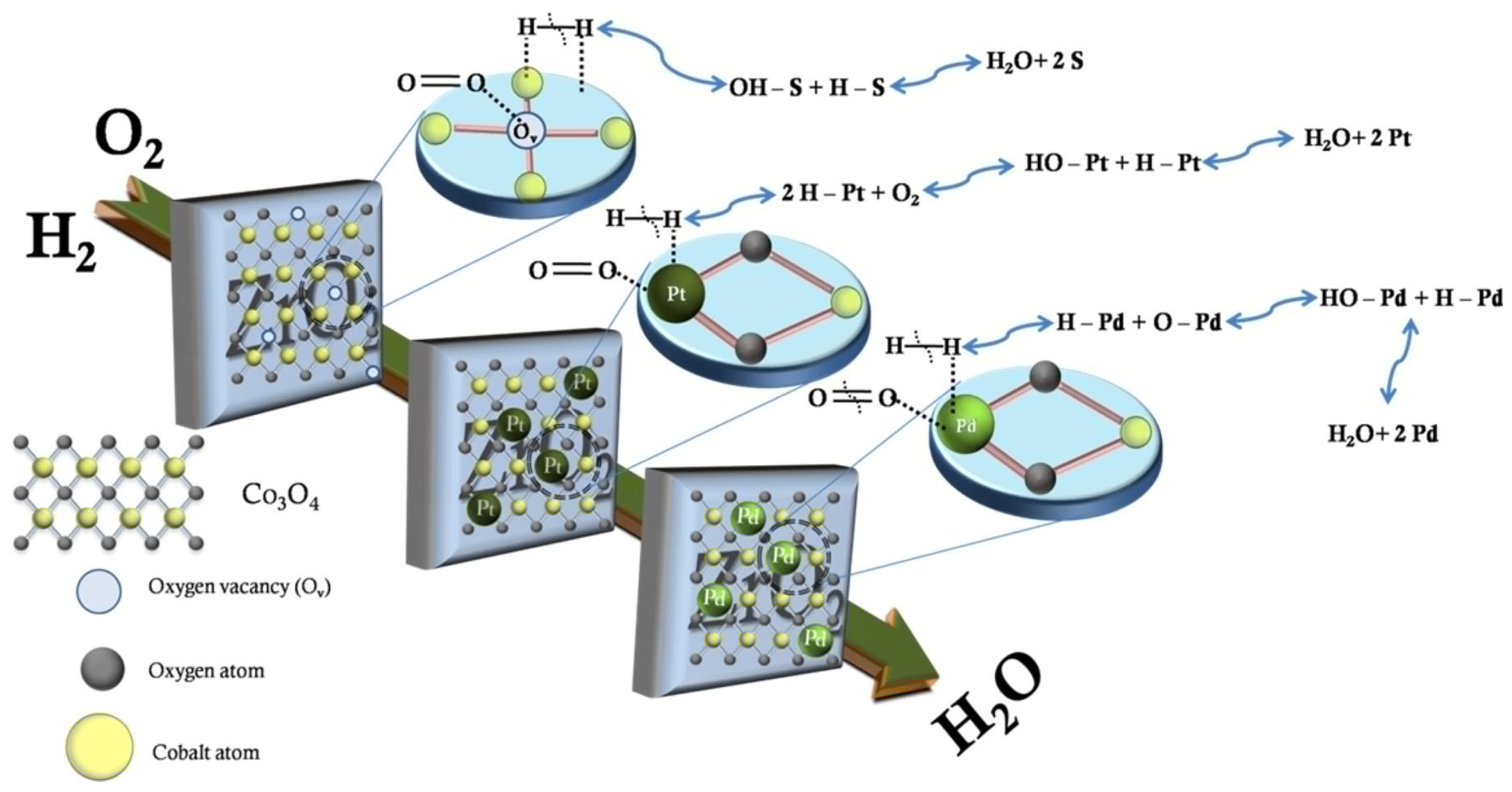
3.1.1. Reaction Mechanism
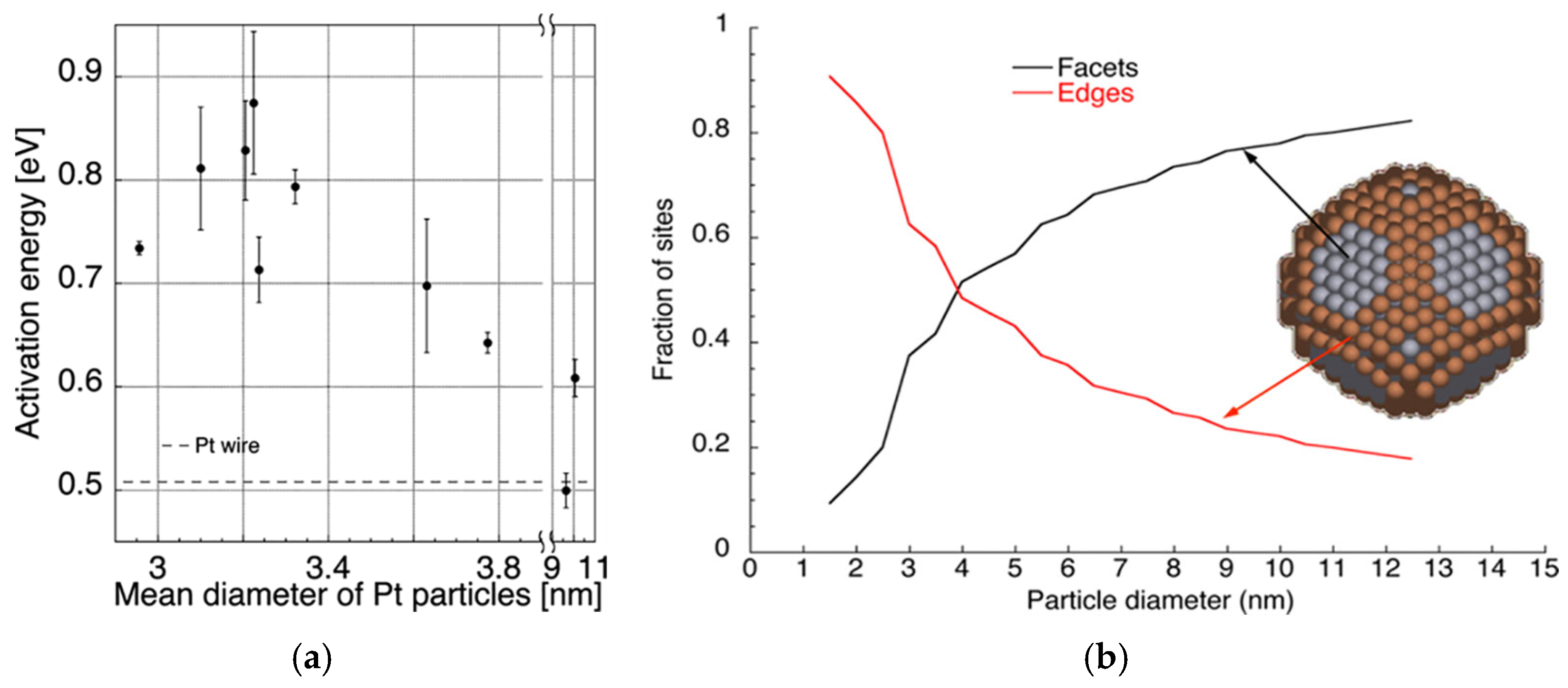
3.1.2. Enhancement of the Intrinsic Activity of Catalyst
3.1.3. Improvement in Catalyst Stability
3.1.4. Improvement in Catalyst Mass Transfer Performance
3.2. Feasible Methods to Improve Hydrogen Selectivity
3.3. Research on Si-Poisoning and Regeneration of Catalysts
4. Device Integration
4.1. Catalytic Layer Integration Method
4.2. Considerations for Device Integration
4.2.1. Weaken the Impact of Environmental Humidity
4.2.2. Optimization of Mechanical Stability
4.2.3. Simplification of the Preparation Process
4.2.4. Development of Integrated Sensors
5. Conclusions
- From the perspective of the reaction mechanism of hydrogen oxidation, promoting the supply of oxygen during the reaction is an effective means to improve device performance. It can be improved by designing reducible supports such as CeO2 and TiO2 or increasing O2 adsorption activation sites;
- The development of low-temperature hydrogen oxidation catalysts can effectively reduce the sensor’s response to other combustible gases, but the cross-response to CO remains a difficult problem to solve;
- In situ generation of catalytic materials on the substrate will greatly reduce the thermal loss during the operation of the sensor and improve the stability of the sensitive element. Developing catalysts with special morphological structures can further enhance the response rate and sensitivity of the device;
- In the vehicle environment, pollutants such as organosilicon are prone to exist, leading to catalyst poisoning. Catalyst modification and coating of filter layers can alleviate the process of silicon poisoning to a certain extent, but permanent deactivation under long-term poisoning cannot be avoided. Studying the catalyst regeneration mechanism and developing regeneration procedures may be another feasible solution to improve the service life of hydrogen sensors;
- Due to the scaling law, the significant reduction in the size of the micro-heater also correspondingly shortens the thermal response time, thereby achieving low duty cycle operation and further reducing power consumption. For vehicle sensors, the seismic performance cannot be ignored, but there are few studies in the literature in this regard. Although the reported sensors generally have ideal performance, from the perspective of the convenience of the preparation process and manufacturing cost, it is unfavorable for both large-scale industrial production and simplification of the production process;
- The future development of onboard catalytic combustion hydrogen sensors will focus on breakthroughs in intelligence and self-maintenance systems, such as dynamically compensating for signal drift through artificial intelligence algorithms to achieve high-precision self-calibration; developing multi-gas sensing fusion technology to enhance the anti-interference ability in complex environments; and exploring self-repairing catalytic materials to extend the sensor lifespan to the vehicle’s service period. These innovative directions will drive the hydrogen detection process to evolve from a single function to an integrated perception-diagnosis-protection intelligent safety system, ultimately meeting the inherent safety requirements of hydrogen fuel cell vehicles.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hydrogen Council. Hydrogen Scaling Up: A Sustainable Pathway for the Global Energy Transition; Hydrogen Council: Brussels, Belgium, 2017; Available online: https://hydrogencouncil.com/en/study-hydrogen-scaling-up/ (accessed on 11 June 2025).
- Song, K.; Hou, T.; Jiang, J.; Grigoriev, S.A.; Fan, F.; Qin, J.; Wang, Z.; Sun, C. Thermal management of liquid-cooled proton exchange membrane fuel cell: A review. J. Power Sources 2025, 648, 237227. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Y.; Yan, X.; Malekian, R.; Li, Z. A study on a numerical simulation of the leakage and diffusion of hydrogen in a fuel cell ship. Renew. Sustain. Energy Rev. 2018, 97, 177–185. [Google Scholar] [CrossRef]
- Boon-Brett, L.; Bousek, J.; Black, G.; Moretto, P.; Castello, P.; Huebert, T.; Banach, U. Identifying performance gaps in hydrogen safety sensor technology for automotive and stationary applications. Int. J. Hydrog. Energy 2010, 35, 373–384. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Mohseni-Dargah, M.; Falahati, Z.; Abbassi, R.; Razmjou, A.; Asadnia, M. Sensing advancement towards safety assessment of hydrogen fuel cell vehicles. J. Power Sources 2021, 489, 229450. [Google Scholar] [CrossRef]
- Boon-Brett, L.; Bousek, J.; Moretto, P. Reliability of commercially available hydrogen sensors for detection of hydrogen at critical concentrations: Part II—Selected sensor test results. Int. J. Hydrog. Energy 2009, 34, 562–571. [Google Scholar] [CrossRef]
- NISSHA FIS Inc. FH2-HY11 [Product Information]. Available online: https://www.fisinc.co.jp/products/products_search.html?Cat=Cat1 (accessed on 11 June 2025).
- Niu, G.; Wang, F. A review of MEMS-based metal oxide semiconductors gas sensor in Mainland China. J. Micromech. Microeng. 2022, 32, 054003. [Google Scholar] [CrossRef]
- Ladacki, M.; Houser, T.J.; Roberts, R.W. Catalyzed Low-Temperature Hydrogen-Oxygen Reaction. J. Catal. 1965, 4, 239–247. [Google Scholar] [CrossRef]
- Lee, J.-S.; An, J.W.; Bae, S.; Lee, S.-K. Review of Hydrogen Gas Sensors for Future Hydrogen Mobility Infrastructure. Appl. Sci. Converg. Technol. 2022, 31, 79–84. [Google Scholar] [CrossRef]
- Oigawa, H.; Shimojima, M.; Tsuno, T.; Ueda, T. Stable Heating Technology for Catalytic Combustion Hydrogen Gas Sensor Using Quartz Resonators. Sens. Mater. 2018, 30, 1103–1114. [Google Scholar]
- Henriquez, D.D.O.; Cho, I.; Yang, H.; Choi, J.; Kang, M.; Chang, K.S.; Jeong, C.B.; Han, S.W.; Park, I. Pt Nanostructures Fabricated by Local Hydrothermal Synthesis for Low-Power Catalytic-Combustion Hydrogen Sensors. ACS Appl. Nano Mater. 2021, 4, 7–12. [Google Scholar] [CrossRef]
- Kalinin, I.A.; Roslyakov, I.V.; Bograchev, D.; Kushnir, S.E.; Ivanov, I.I.; Dyakov, A.V.; Napolskii, K.S. High performance microheater-based catalytic hydrogen sensors fabricated on porous anodic alumina substrates. Sens. Actuators B 2024, 404, 135270. [Google Scholar] [CrossRef]
- Lee, E.-B.; Hwang, I.-S.; Cha, J.-H.; Lee, H.-J.; Lee, W.-B.; Pak, J.J.; Lee, J.-H.; Ju, B.-K. Micromachined catalytic combustible hydrogen gas sensor. Sens. Actuators B 2011, 153, 392–397. [Google Scholar] [CrossRef]
- Wettergren, K.; Hellman, A.; Cavalca, F.; Zhdanov, V.P.; Langhammer, C. Unravelling the Dependence of Hydrogen Oxidation Kinetics on the Size of Pt Nanoparticles by in Operando Nanoplasmonic Temperature Sensing. Nano Lett. 2015, 15, 574–580. [Google Scholar] [CrossRef]
- Fassihi, M.; Zhdanov, V.P.; Rinnemo, M.; Keck, K.E.; Kasemo, B. A Theoretical and Experimental-Study of Catalytic Ignition in the Hydrogen Oxygen Reaction on Platinum. J. Catal. 1993, 141, 438–452. [Google Scholar] [CrossRef]
- Schwarzer, M.; Borodin, D.; Wang, Y.; Fingerhut, J.; Kitsopoulos, T.N.; Auerbach, D.J.; Guo, H.; Wodtke, A.M. Cooperative adsorbate binding catalyzes high-temperature hydrogen oxidation on palladium. Science 2024, 386, 511–516. [Google Scholar] [CrossRef]
- Liu, Y.; Koo, K.; Mao, Z.; Fu, X.; Hu, X.; Dravid, V.P. Unraveling the adsorption- limited hydrogen oxidation reaction at palladium surface via in situ electron microscopy. Proc. Natl. Acad. Sci. USA 2024, 121, e2408277121. [Google Scholar] [CrossRef]
- Singh, S.A.; Vishwanath, K.; Madras, G. Role of Hydrogen and Oxygen Activation over Pt and Pd-Doped Composites for Catalytic Hydrogen Combustion. ACS Appl. Mater. Interfaces 2017, 9, 19380–19388. [Google Scholar] [CrossRef]
- Kim, J.; Tahmasebi, A.; Lee, J.M.; Lee, S.; Jeon, C.-H.; Yu, J. Low-temperature catalytic hydrogen combustion over Pd-Cu/Al2O3: Catalyst optimization and rate law determination. Korean J. Chem. Eng. 2023, 40, 1317–1330. [Google Scholar] [CrossRef]
- Owais, T.; Khater, M.; Al-Qahtani, H. Graphene-based MEMS devices for gas sensing applications: A review. Micro Nanostruct. 2024, 195, 207954. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, H.; Chen, M.; Wu, K.; Zhang, Y.; Long, D. Unveiling the Nature of Room-Temperature O2 Activation and O2•-Enrichment on MgO-Loaded Porous Carbons with Efficient H2S Oxidation. ACS Catal. 2021, 11, 5974–5983. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, W.; Huang, Z.; Yan, Z.; Jia, H.; Pei, W.; Tu, Y. Oxygen dissociation on the C3N monolayer: A first-principles study. Appl. Surf. Sci. 2023, 613, 155912. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Xie, X.; Tao, K.; Liu, C.; Khor, K.A.; Miao, J.; Norford, L.K. 3D superhydrophobic reduced graphene oxide for activated NO2 sensing with enhanced immunity to humidity. J. Mater. Chem. A 2018, 6, 478–488. [Google Scholar] [CrossRef]
- Harley-Trochimczyk, A.; Chang, J.; Zhou, Q.; Dong, J.; Pham, T.; Worsley, M.A.; Maboudian, R.; Zettl, A.; Mickelson, W. Catalytic hydrogen sensing using microheated platinum nanoparticle-loaded graphene aerogel. Sens. Actuators B 2015, 206, 399–406. [Google Scholar] [CrossRef]
- Aasmundtveit, K.E.; Roy, A.; Ta, B.Q. Direct Integration of Carbon Nanotubes in CMOS, Towards an Industrially Feasible Process: A Review. IEEE Trans. Nanotechnol. 2020, 19, 113–122. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, H.; Li, H.; Zhao, Z.; Wang, K.; Zhou, Y.; Zhao, X.; Dubal, D.P. CxNy-based materials as gas sensors: Structure, performance, mechanism and perspective. Coord. Chem. Rev. 2024, 503, 215653. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.; Yang, S.; Yang, X.; Meng, F.; Zhai, L.; Li, Z.; Zhao, S.; Zhang, G.; Qin, Y. Dual Pd2+and Pd0 sites on CeO2 for benzyl alcohol selective oxidation. J. Catal. 2022, 414, 385–393. [Google Scholar] [CrossRef]
- Deshpande, P.A.; Madras, G. Catalytic hydrogen combustion for treatment of combustible gases from fuel cell processors. Appl. Catal. B 2010, 100, 481–490. [Google Scholar] [CrossRef]
- Kim, J.; Yu, J.; Lee, S.; Tahmasebi, A.; Jeon, C.-H.; Lucas, J. Advances in catalytic hydrogen combustion research: Catalysts, mechanism, kinetics, and reactor designs. Int. J. Hydrog. Energy 2021, 46, 40073–40104. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Zhang, M.; Song, B.; Han, Y.; Wang, S.; Ren, Z.; Wang, L.; Yin, P.; Zheng, L.; et al. Highly Active MnCoOx Catalyst toward CO Preferential Oxidation. ACS Catal. 2024, 14, 1281–1291. [Google Scholar] [CrossRef]
- Han, C.-H.; Hong, D.-W.; Kim, I.-J.; Gwak, J.; Han, S.-D.; Singh, K.C. Synthesis of Pd or Pt/titanate nanotube and its application to catalytic type hydrogen gas sensor. Sens. Actuators B 2007, 128, 320–325. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Sun, Y.; Li, P.; Ji, J.; Chen, Y.; Zhang, W.; Hu, J. Fabrication and gas sensing properties of Au-loaded SnO2 composite nanoparticles for highly sensitive hydrogen detection. Sens. Actuators B 2017, 240, 664–673. [Google Scholar] [CrossRef]
- Brauns, E.; Morsbach, E.; Kunz, S.; Baeumer, M.; Lang, W. A fast and sensitive catalytic gas sensors for hydrogen detection based on stabilized nanoparticles as catalytic layer. Sens. Actuators B 2014, 193, 895–903. [Google Scholar] [CrossRef]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S.; et al. Nanoparticle decoration with surfactants: Molecular intercations, assmbly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Nan, H.Y.; Ni, Z.H.; Wang, J.; Zafar, Z.; Shi, Z.X.; Wang, Y.Y. The thermal stability of graphene in air investigated by Raman spectroscopy. J. Raman Spectrosc. 2013, 44, 1018–1021. [Google Scholar] [CrossRef]
- Yazawa, Y.; Yoshida, H.; Komai, S.; Hattori, T. The additive effect on propane combustion over platinum catalyst: Control of the oxidation-resistance of platinum by the electronegativity of additives. Appl. Catal. A 2002, 233, 113–124. [Google Scholar] [CrossRef]
- Del Orbe, D.V.; Yang, H.; Cho, I.; Park, J.; Choi, J.; Han, S.W.; Park, I. Low-power thermocatalytic hydrogen sensor based on electrodeposited cauliflower-like nanostructured Pt black. Sens. Actuators B 2021, 329, 129129. [Google Scholar] [CrossRef]
- Rao, A.; Long, H.; Harley-Trochimczyk, A.; Thang, P.; Zettl, A.; Carraro, C.; Mahoudian, R. In Situ Localized Growth of Ordered Metal Oxide Hollow Sphere Array on Microheater Platform for Sensitive, Ultra-Fast Gas Sensing. ACS Appl. Mater. Interfaces 2017, 9, 2634–2641. [Google Scholar] [CrossRef]
- Wang, P.; Tian, X.; Yan, M.; Yang, B.; Hua, Z. A low temperature catalytic-type combustible gas sensor based on Pt supported zeolite catalyst films. J. Mater. Sci. 2021, 56, 4666–4676. [Google Scholar] [CrossRef]
- Liu, X.; Dong, H.; Xia, S. Micromachined Catalytic Combustible Hydrogen Gas Sensor Based on Nano-structured SnO2. Acta Chim. Sinica 2013, 71, 657–662. [Google Scholar] [CrossRef]
- Baranov, A.; Ivanov, I.; Karpova, E. Effects of Flameless Catalytic Combustion of Hydrogen on the Parameters of Catalytic Sensors with Platinum-group Catalysts. Sens. Mater. 2023, 35, 3585–3594. [Google Scholar] [CrossRef]
- Ivanov, I.; Baranov, A.; Mironov, S.; Talipov, V.; Karami, H.; Gharehpetian, G.B. Development of an Approach to Increase Hydrogen Measurement Selectivity. Sens. Mater. 2023, 35, 1045. [Google Scholar] [CrossRef]
- Chen, W.; Cao, J.; Fu, W.; Zhang, J.; Qian, G.; Yang, J.; Chen, D.; Zhou, X.; Yuan, W.; Duan, X. Molecular-Level Insights into the Notorious CO Poisoning of Platinum Catalyst. Angew. Chem. Int. Ed. 2022, 61, e202200190. [Google Scholar] [CrossRef]
- Bao, S.; Yang, L.; Fu, H.; Qu, X.; Zheng, S. Fine Ru-Ru2P Heterostructure Enables Highly Active and Selective CO2 Hydrogenation to CO. ACS Catal. 2024, 14, 18134–18144. [Google Scholar] [CrossRef]
- Yue, L.; Zhao, W.; Li, J.; Wu, R.; Wang, Y.; Zhang, H.; Zhao, Y. Low-temperature CO preferential oxidation in H2-rich stream over Indium modified Pd-Cu/Al2O3 catalyst. J. Colloid Interface Sci. 2024, 662, 109–118. [Google Scholar] [CrossRef]
- Zhao, W.; Yue, L.; Li, J.; Kong, L.; Chen, Z.; Zheng, K.; Zhang, L.; Wang, C.; Wu, R.; Wang, Y.; et al. Pd species aggregation state regulation of Pd-Cu/Al2O3 for low-temperature CO preferential oxidation. Chem. Eng. J. 2025, 518, 164668. [Google Scholar] [CrossRef]
- Liu, S.; Gong, S.; Feng, H.; Ma, Z.; Yang, L.; Li, P. Selective hydrogen combustion in the presence of propylene and propane over Pt/A-zeolite catalysts. Int. J. Hydrog. Energy 2020, 45, 12347–12359. [Google Scholar] [CrossRef]
- Matsumiya, M.; Shin, W.S.; Qiu, F.B.; Izu, N.; Matsubara, I.; Murayama, N. Poisoning of platinum thin film catalyst by hexamethyldisiloxane (HMDS) for thermoelectric hydrogen gas sensor. Sens. Actuators B 2003, 96, 516–522. [Google Scholar] [CrossRef]
- Gentry, S.J.; Jones, A. Poisoning and inhibition of catalytic oxidations: 1. Effect of silicone vapor on the gas-phase oxidations of methane, propene, carbon-monoxide and hydrogen over platinum and palladium catalysts. J. Appl. Chem. Biotechnol. 1978, 28, 727–732. [Google Scholar] [CrossRef]
- Rahmani, M.; Sohrabi, M. Si-tolerance of iron doped Pt/alumina catalyst in the total oxidation of VOC. React. Kinet. Catal. Lett. 2005, 86, 397–405. [Google Scholar] [CrossRef]
- Porras, S.; Sippula, O.; Mesceriakove, S.-M.; Maunula, T.; Kallinen, K.; Suvanto, M.; Kinnunen, N.M. Poisoning of automotive methane oxidation catalysts by silicon compounds. Chem. Eng. Sci. 2023, 282, 119268. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Fu, Y.; Luo, W.; Tian, Y. Deactivation mechanism and anti-deactivation modification of SnO2-based catalysts for methane gas sensors. Sens. Actuators B 2019, 299, 126939. [Google Scholar] [CrossRef]
- Chao, Y.T.; Yao, S.; Buttner, W.J.; Stetter, J.R. Amperometric sensor for selective and stable hydrogen measurement. Sens. Actuators B 2005, 106, 784–790. [Google Scholar]
- Kwon, Y.M.; Kim, H.-J.; Ye, B.; Kim, H.-D.; Lee, Y.S.; Shin, H.; Baik, J.M. Ce oxide nanoparticles on porous reduced graphene oxides for stable hydrogen detection in air/HMDSO environment. Sens. Actuators B 2020, 321, 128529. [Google Scholar] [CrossRef]
- Moon, Y.K.; Jeong, S.-Y.; Kang, Y.C.; Lee, J.-H. Metal Oxide Gas Sensors with Au Nanocluster Catalytic Overlayer: Toward Tuning Gas Selectivity and Response Using a Novel Bilayer Sensor Design. ACS Appl. Mater. Interfaces 2019, 11, 32169–32177. [Google Scholar] [CrossRef]
- Liu, W.; Zou, J.; Li, S.; Li, J.; Li, F.; Zhan, Z.; Zhang, Y. Pd/In2O3-based bilayer H2 sensor with high resistance to silicone toxicity and ultra-fast response. Int. J. Hydrogen Energy 2023, 48, 5743–5753. [Google Scholar] [CrossRef]
- Ehrhardt, J.J.; Colin, L.; Jamois, D. Poisoning of platinum surfaces by hexamethyldisiloxane (HMDS): Application to catalytic methane sensors. Sens. Actuators B 1997, 40, 117–124. [Google Scholar] [CrossRef]
- Arnby, K.; Rahmani, M.; Sanati, M.; Cruise, N.; Carlsson, A.A.; Skoglundh, M. Characterization of Pt/-γ-Al2O3 catalysts deactivated by hexamethyldisiloxane. Appl. Catal. B 2004, 54, 1–7. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; Kustov, A.; Skotte, J.; Siret, B.; Tabaries, F.; Fehrmann, R. Characterization and regeneration of Pt-catalysts deactivated in municipal waste flue gas. Appl. Catal. B 2006, 69, 10–16. [Google Scholar] [CrossRef]
- Fernandez, A.; Arzac, G.M.; Vogt, U.F.; Hosoglu, F.; Borgschulte, A.; Jimenez de Haro, M.C.; Montes, O.; Zuettel, A. Investigation of a Pt containing washcoat on SiC foam for hydrogen combustion applications. Appl. Catal. B 2016, 180, 336–343. [Google Scholar] [CrossRef]
- Li, H.; Wu, R.; Liu, H.-B.; Han, L.-Y.; Yuan, W.-J.; Hua, Z.-Q.; Fan, S.-R.; Wu, Y. A novel catalytic-type gas sensor based on alumina ceramic substrates loaded with catalysts and printed electrodes. Chin. J. Anal. Chem. 2021, 49, 93–101. [Google Scholar] [CrossRef]
- Xu, D.; Xu, P.; Wang, X.; Chen, Y.; Yu, H.; Zheng, D.; Li, X. Pentagram-Shaped Ag@Pt Core-Shell Nanostructures as High-Performance Catalysts for Formaldehyde Detection. ACS Appl. Mater. Interfaces 2020, 12, 8091–8097. [Google Scholar] [CrossRef]
- Choi, K.-W.; Lee, J.-S.; Seo, M.-H.; Jo, M.-S.; Yoo, J.-Y.; Sim, G.S.; Yoon, J.-B. Batch-fabricated CO gas sensor in large-area (8-inch) with sub-10 mW power operation. Sens. Actuators B 2019, 289, 153–159. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, P.; Li, X.; Ren, Y.; Deng, Y. High-performance H2 sensors with selectively hydrophobic micro-plate for self-aligned upload of Pd nanodots modified mesoporous In2O3 sensing-material. Sens. Actuators B 2018, 267, 83–92. [Google Scholar] [CrossRef]
- Jo, M.-S.; Kim, K.-H.; Lee, J.-S.; Kim, S.-H.; Yoo, J.-Y.; Choi, K.-W.; Kim, B.-J.; Kwon, D.-S.; Yoo, I.; Yang, J.-S.; et al. Ultrafast (∼0.6 s), Robust, and Highly Linear Hydrogen Detection up to 10% Using Fully Suspended Pure Pd Nanowire. ACS Nano 2023, 17, 23649–23658. [Google Scholar] [CrossRef]
- Watanabe, M.; Inoue, R.; Ichikawa, D.; Furusaki, K. Development of Thermal Conductivity Type Hydrogen Sensor. In Proceedings of the Symposium on Sensors, Actuators, and Microsystems General Session Held During the 217th Meeting of the Electrochemical-Society (ECS), Vancouver, BC, Canada, 25–30 April 2010; pp. 31–42. [Google Scholar]
- Chang, H.S.; Son, G.G.; Park, J.Y.; Hyung, S.J.; Park, J.S. Development and Application of Hydrogen Sensor Evaluation Rig for FCEV. Int. J. Automot. Technol. 2025. [Google Scholar] [CrossRef]
- Xie, B.; Liu, Y.; Lei, Y.; Qian, H.; Li, Y.; Yan, W.; Zhou, C.; Wen, H.-M.; Xia, S.; Mao, P.; et al. Innovative Thermocatalytic H2 Sensor with Double-Sided Pd Nanocluster Films on an Ultrathin Mica Substrate. ACS Sens. 2024, 9, 2529–2539. [Google Scholar] [CrossRef]
- Girma, H.G.; Park, K.H.; Ji, D.; Kim, Y.; Lee, H.M.; Jeon, S.; Jung, S.-H.; Kim, J.Y.; Noh, Y.-Y.; Lim, B. Room-Temperature Hydrogen Sensor with High Sensitivity and Selectivity using Chemically Immobilized Monolayer Single-Walled Carbon Nanotubes. Adv. Funct. Mater. 2023, 33, 2213381. [Google Scholar] [CrossRef]
- Huebert, T.; Boon-Brett, L.; Palmisano, V.; Bader, M.A. Developments in gas sensor technology for hydrogen safety. Int. J. Hydrog. Energy 2014, 39, 20474–20483. [Google Scholar] [CrossRef]
- Kondalkar, V.V.; Park, J.; Lee, K. MEMS hydrogen gas sensor for in-situ monitoring of hydrogen gas in transformer oil. Sens. Actuators B 2021, 326, 128989. [Google Scholar] [CrossRef]
- Saxena, G.; Paily, R. Analytical Modeling of Square Microhotplate for Gas Sensing Application. IEEE Sens. J. 2013, 13, 4851–4859. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, J.; Wu, Y.; Wang, Y.; Ding, G. Two operating modes of palladium film hydrogen sensor based on suspended micro hotplate. Int. J. Hydrog. Energy 2019, 44, 11259–11265. [Google Scholar] [CrossRef]
- Samaeifar, F.; Afifi, A.; Abdollahi, H. Simple Fabrication and Characterization of a Platinum Microhotplate Based on Suspended Membrane Structure. Exp. Tech. 2016, 40, 755–763. [Google Scholar] [CrossRef]
- Spannhake, J.; Schulz, O.; Helwig, A.; Krenkow, A.; Muller, G.; Doll, T. High-temperature MEMS heater platforms:: Long-term performance of metal and semiconductor heater materials. Sensors 2006, 6, 405–419. [Google Scholar] [CrossRef]
- Coppola, G.; Striano, V.; Ferraro, P.; De Nicola, S.; Finizio, A.; Pierattini, G.; Maccagnani, P. A nondestructive dynamic characterization of a microheater through digital holographic microscopy. J. Microelectromech. Syst. 2007, 16, 659–667. [Google Scholar] [CrossRef]
- Behera, B.; Chandra, S. An innovative gas sensor incorporating ZnO-CuO nanoflakes in planar MEMS technology. Sens. Actuators B 2016, 229, 414–424. [Google Scholar] [CrossRef]
- Urasinska-Wojcik, B.; Vincent, T.A.; Chowdhury, M.F.; Gardner, J.W. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuators B 2017, 239, 1051–1059. [Google Scholar] [CrossRef]
- Xie, D.; Liu, R.; Adedokun, G.; Xu, L.; Wu, F. A Novel Low Power Hexagonal Gas Sensor Cell for Multi-Channel Gas Detection. In Proceedings of the 34th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Electr Network, Virtual, 25–29 January 2021; pp. 430–433. [Google Scholar]
- Liu, Q.; Ding, G.; Wang, Y.; Yao, J. Thermal Performance of Micro Hotplates with Novel Shapes Based on Single-Layer SiO2 Suspended Film. Micromachines 2018, 9, 514. [Google Scholar] [CrossRef]
- Coppeta, R.; Lahlalia, A.; Kozic, D.; Hammer, R.; Riedler, J.; Toschkoff, G.; Singulani, A.; Ali, Z.; Sagmeister, M.; Carniello, S.; et al. Electro-Thermal-Mechanical Modeling of Gas Sensor Hotplates. In Sensor Systems Simulations: From Concept to Solution; van Driel, W.D., Pyper, O., Schumann, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–72. [Google Scholar]
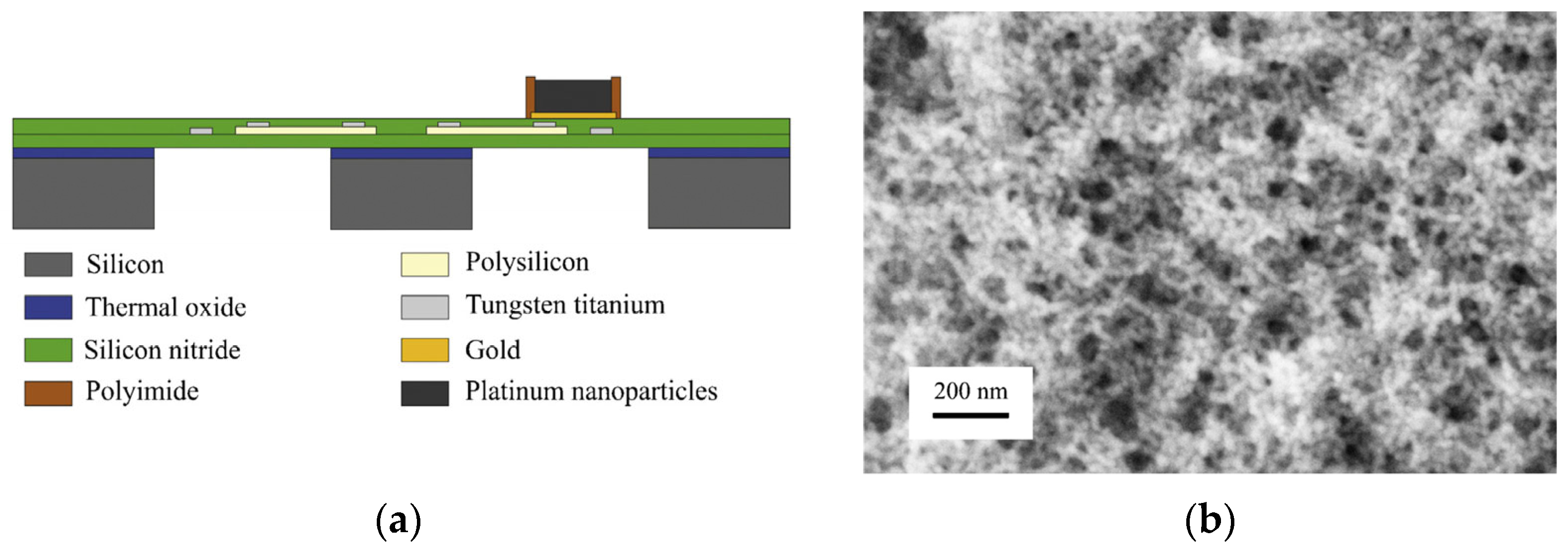
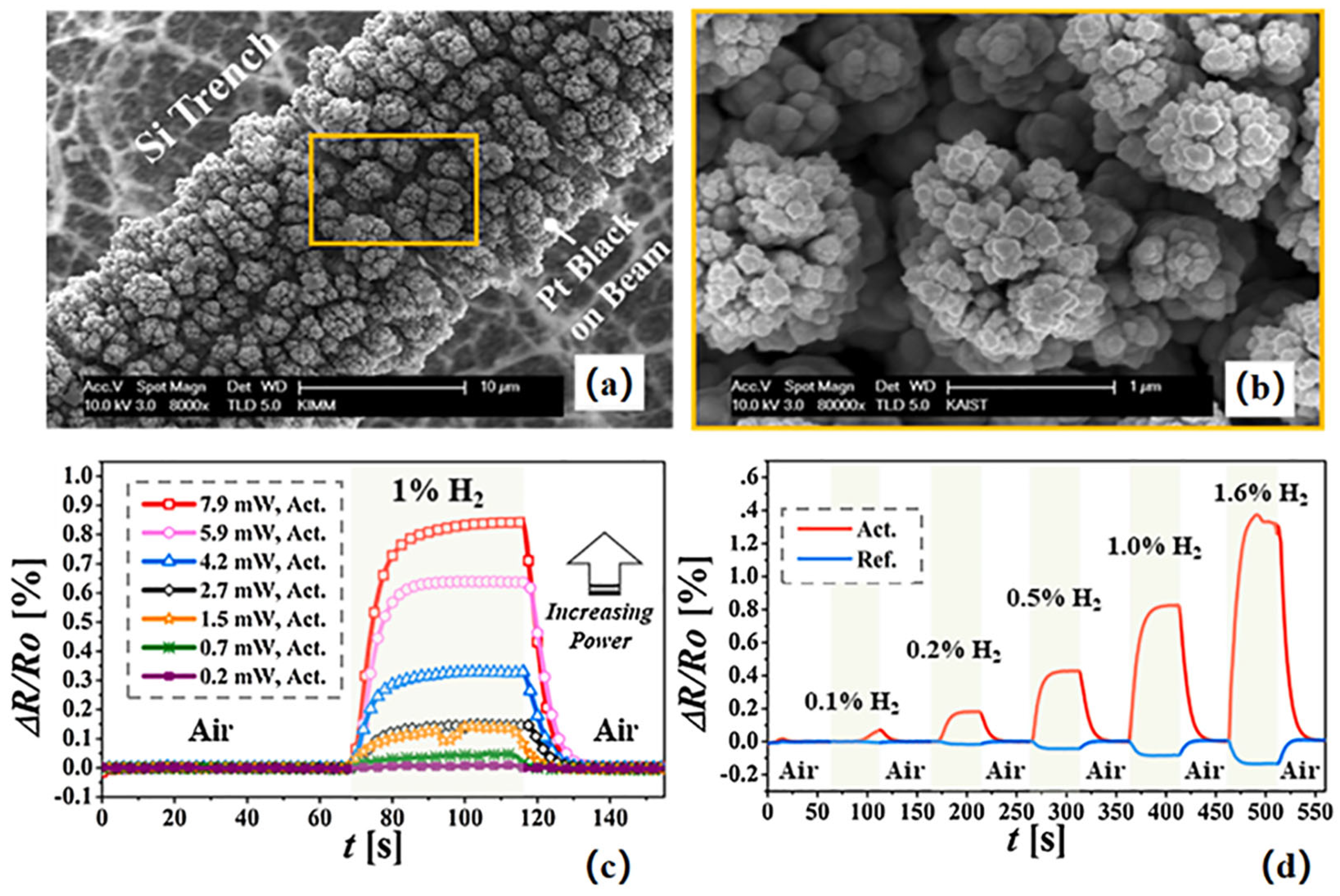


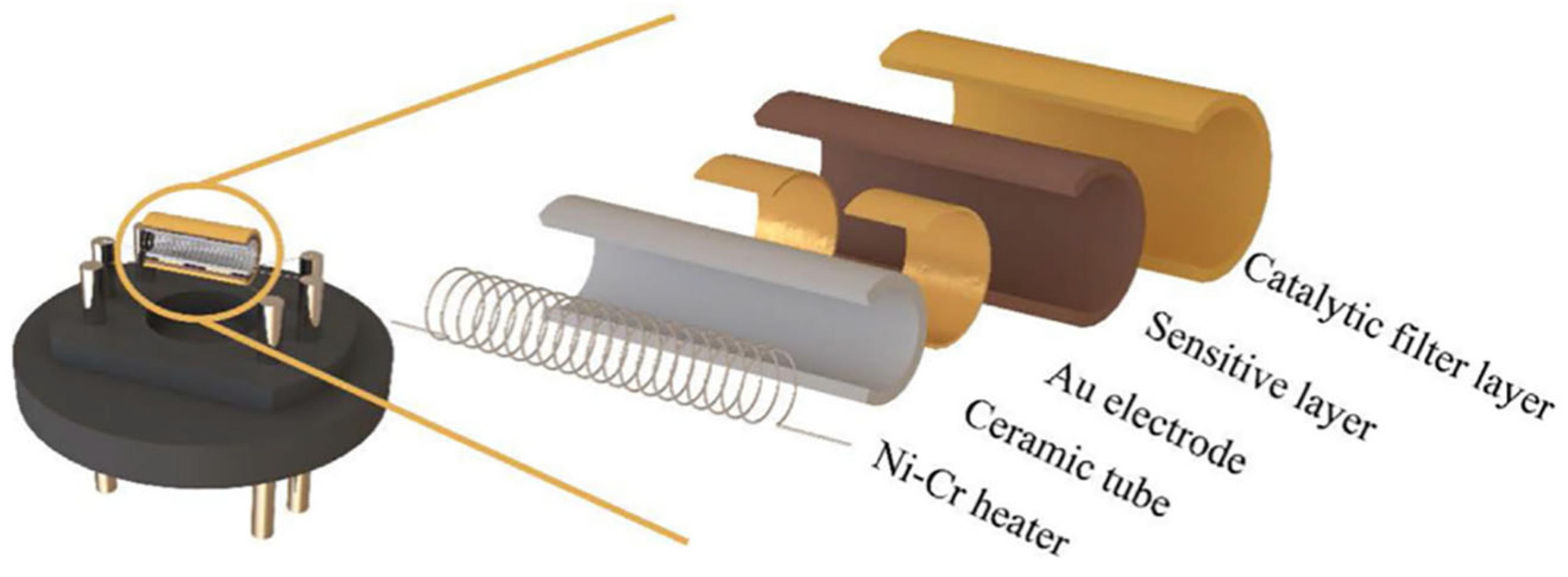
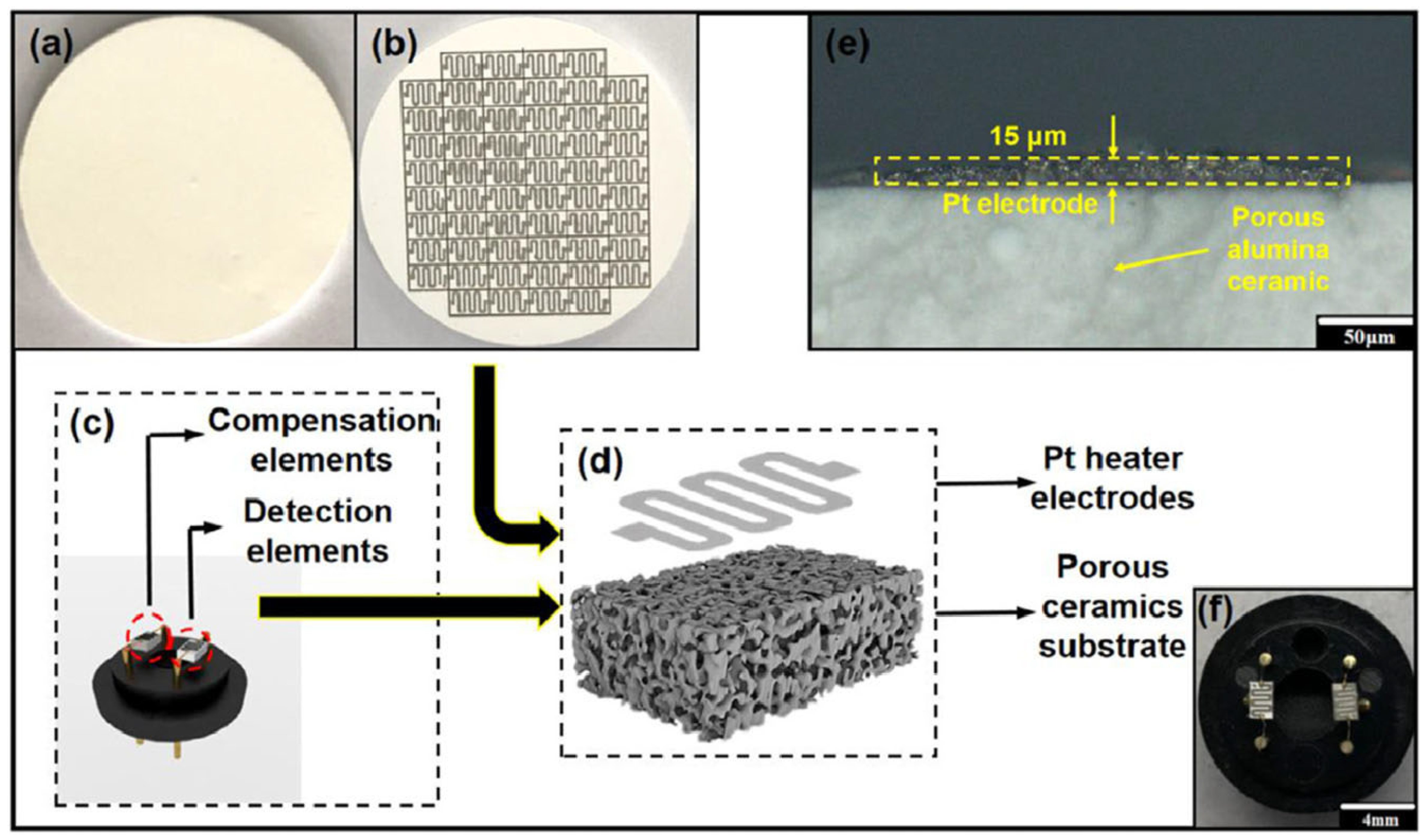
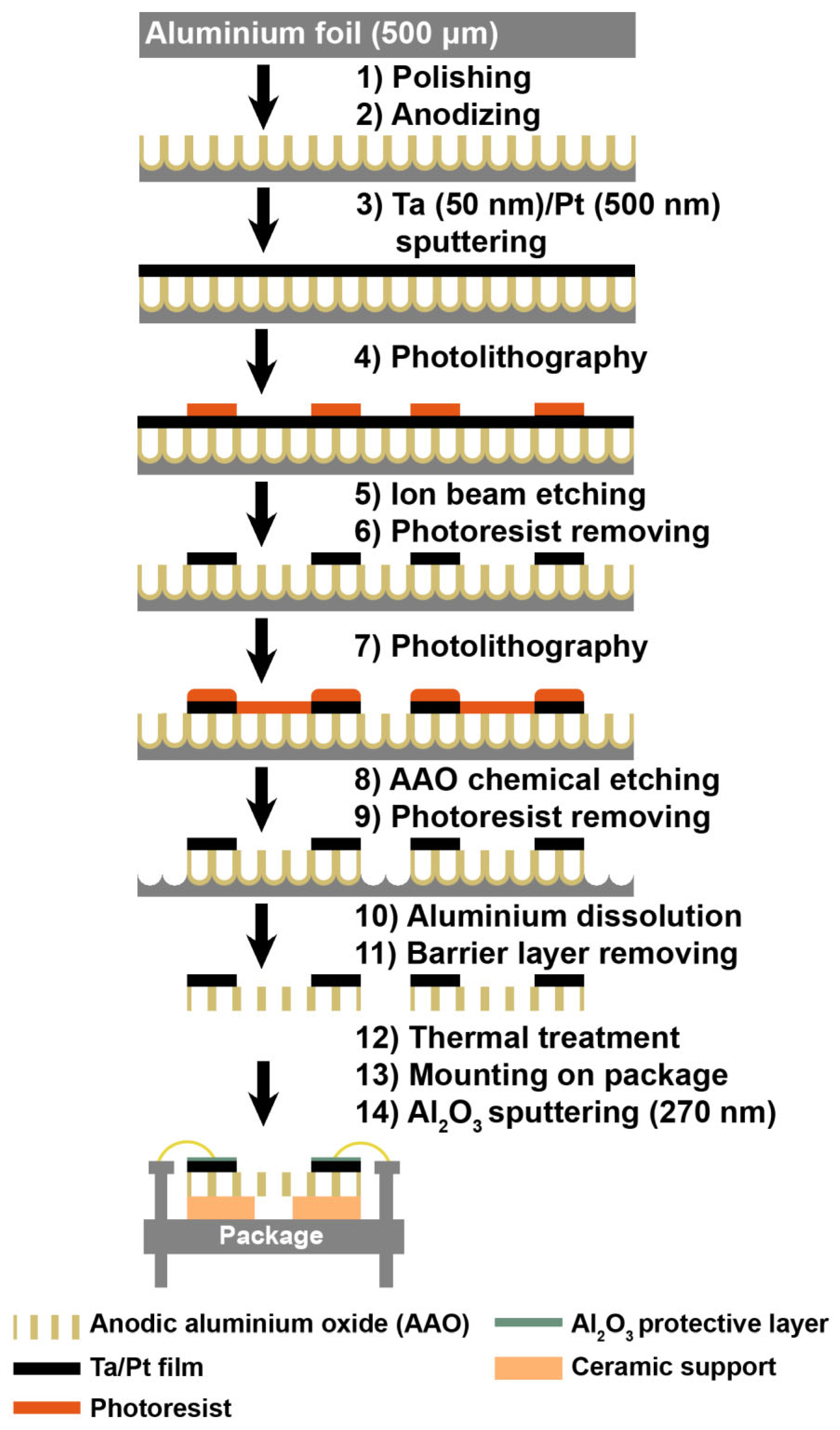
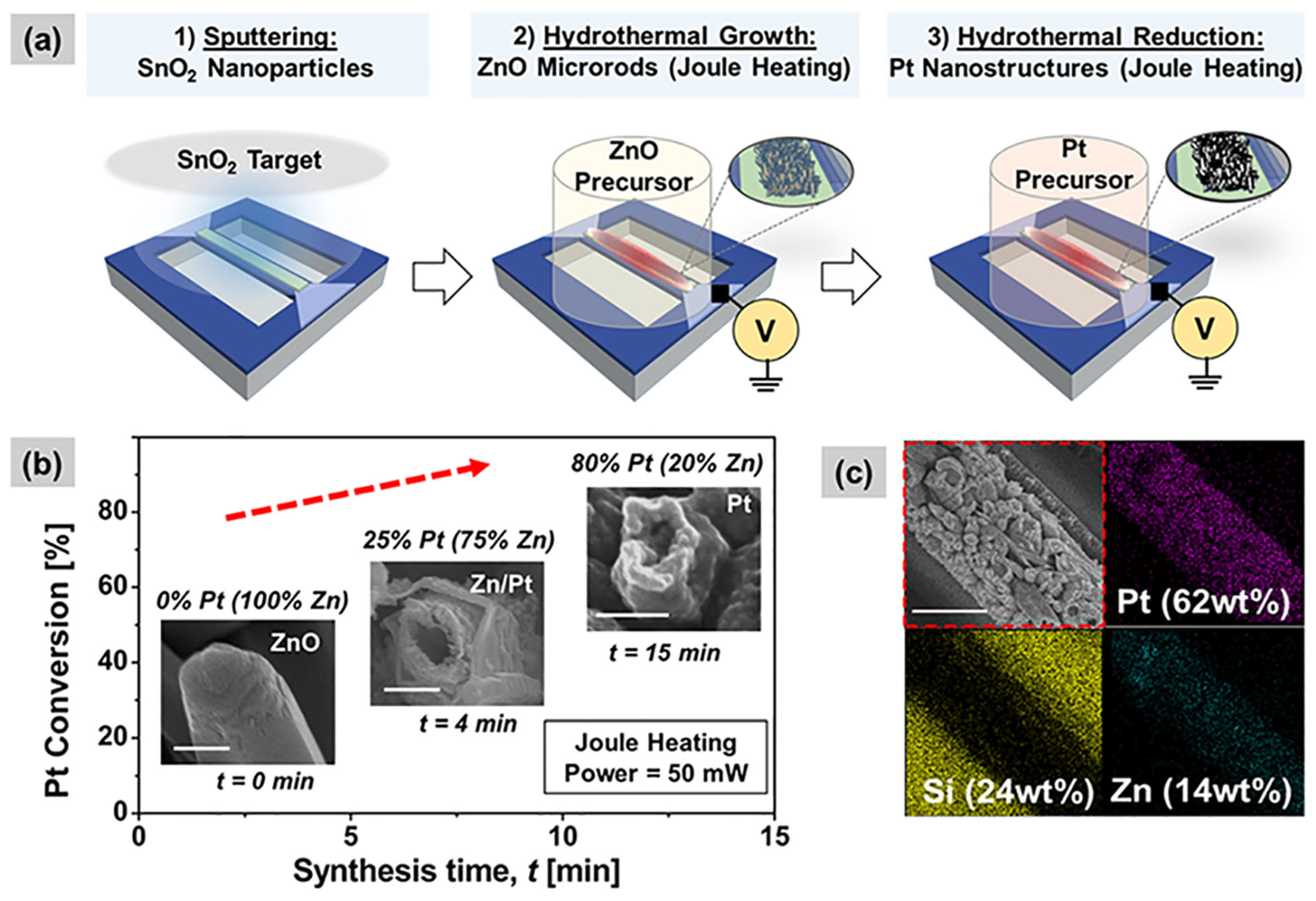
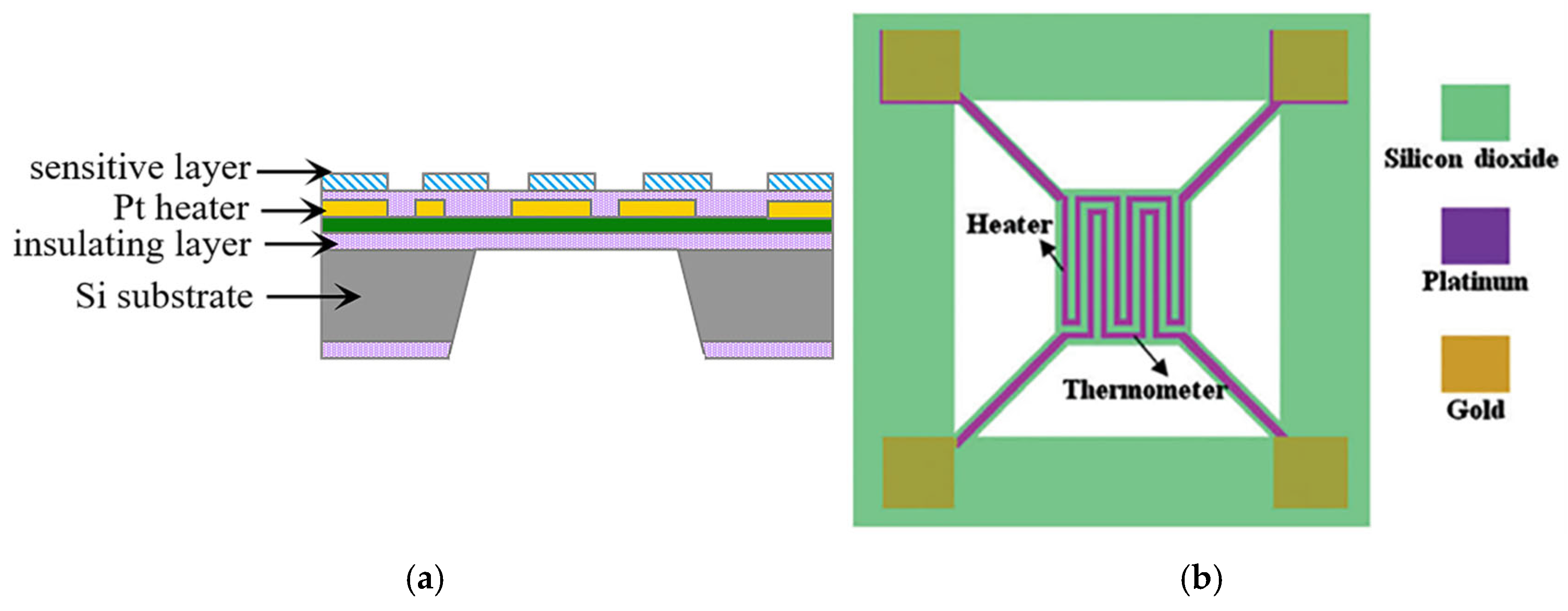
| Parameter | Performance Requirements |
|---|---|
| Detection Range | 0~4%H2 |
| Response Time (t90) | <3 s |
| Recovery Time (t10) | <3 s |
| Ambient Temperature | −40~125 °C |
| Ambient Humidity | 0~100%RH |
| Sensing Technologies | Lifestime (Year) | Response Time (s) | Power Consumption (mW) | Defect |
|---|---|---|---|---|
| Thermal conductivity | >5 | <15 | <500 | High power consumption; cross-interference from other gases (e.g., He); low precision; high detection limit; susceptible to temperature influence. |
| Electrochemical | ~2 | <30 | 2~700 | High cost; short service life; cross-sensitivity; requirement for special electrolytes; need for regular calibration; poor low-temperature performance; catalyst poisoning and aging; sensitivity to temperature changes. |
| Metal Oxide | 2~4 | <30 | <800 | High power consumption; low precision; cross-sensitivity to other gases and humidity; poor selectivity; requirement for O2 participation; memory effect; long non-linear response time. |
| Optical | <2 | <60 | ~1000 | Short service life; cross-sensitivity; sensitivity to ambient light interference and temperature changes; high cost. |
| Catalytic combustion | >5 | <20 | ~1000 | High power consumption; cross-interference from combustible gases; high detection limit; requirement for O2 participation; need for regular calibration. |
| Sensor Structure | Catalyst | Active Area Size | Power Consumption in the Constant Potential Mode (mW) | Response Time | Sensitivity | Stability | Reference |
|---|---|---|---|---|---|---|---|
| The micro-heating platform based on anodized aluminum | Local Joule heating decomposition for the preparation of 3Pd-Pt | 150 μm × 150 μm | 116 | 0.4 s | 76 mV/vol.% H2 | <4% response deviation after 14 days of operation | [14] |
| Floating micro-heat platform | Electrodeposited microrod-like Pt nanostructures | 9 μm × 110 μm | 4 | <12 s | ΔR/R0~0.46% per percent of H2 | Not mentioned | [13] |
| Floating micro-heat platform | Electrodeposited cauliflower-like Pt nanostructures | 9 μm × 110 μm | 8 | 1.8 s | ΔR/R0~0.75% per percent of H2 | Not mentioned | [40] |
| Floating micro-heat platform | 5 wt% Pt/γ-Al2O3 | 200 μm × 200 μm | 55.68 | 0.36 s | ~37 mV/vol.% H2 | No response drift after 248 days of operation | [15] |
| Microheat platform | Pt nanoparticl-loaded graphene aerogel | 10 μm × 100 μm | 11 | 0.97 s | ΔR/R0~1.5% per percent of H2 | Not mentioned | [26] |
| micro-heating platform, fabricated on an alumina plate through a printing process | Pd and Pt/titanate nanotubes | 1720 μm × 2000 μm | Not mentioned | <20 s | ~77.5 mV/vol.% H2 | Not mentioned | [33] |
| Micro-heating platform based on Al2O3 | 2%Pt-HZSM5 | 3000 μm × 1500 μm | Not mentioned | Not mentioned | ~18 mV/vol.% H2 | Not mentioned | [42] |
| Microheat platform | para-phenylendiamine-linked platinum nanoparticles | 2000 μm × 2800 μm | 30 | 0.15 s | ~220 mV/vol.% H2 | Remain inactive for a week under a pressure of 10,000 ppm H2 | [35] |
| Microheat platform | Preparation of SnO2 porous nanomembrane by CVD method | 60 μm × 100 μm | 35 | 0.65 s | 75.4 mV/vol.% H2 | Maintain a stability of 95% within 200 days | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Wang, Y.; Wang, C.; Wang, L.; Yan, S. Catalytic Combustion Hydrogen Sensors for Vehicles: Hydrogen-Sensitive Performance Optimization Strategies and Key Technical Challenges. Processes 2025, 13, 2384. https://doi.org/10.3390/pr13082384
Huang B, Wang Y, Wang C, Wang L, Yan S. Catalytic Combustion Hydrogen Sensors for Vehicles: Hydrogen-Sensitive Performance Optimization Strategies and Key Technical Challenges. Processes. 2025; 13(8):2384. https://doi.org/10.3390/pr13082384
Chicago/Turabian StyleHuang, Biyi, Yi Wang, Chao Wang, Lijian Wang, and Shubin Yan. 2025. "Catalytic Combustion Hydrogen Sensors for Vehicles: Hydrogen-Sensitive Performance Optimization Strategies and Key Technical Challenges" Processes 13, no. 8: 2384. https://doi.org/10.3390/pr13082384
APA StyleHuang, B., Wang, Y., Wang, C., Wang, L., & Yan, S. (2025). Catalytic Combustion Hydrogen Sensors for Vehicles: Hydrogen-Sensitive Performance Optimization Strategies and Key Technical Challenges. Processes, 13(8), 2384. https://doi.org/10.3390/pr13082384







