Graphene Oxide Aerogels: From Synthesis Pathways to Mechanical Performance and Applications
Abstract
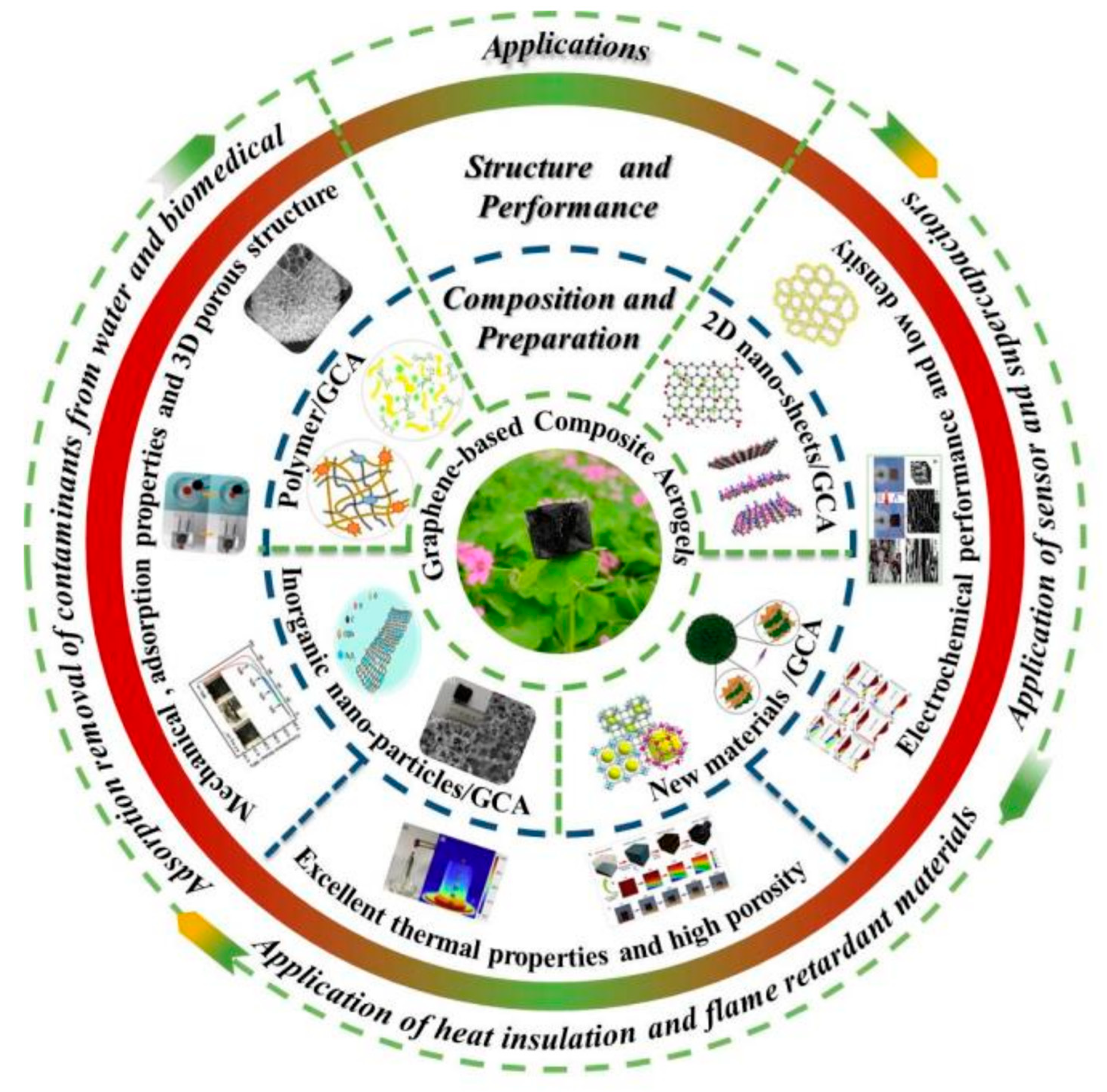
1. Introduction
2. Synthesis Methods of GO Aerogels
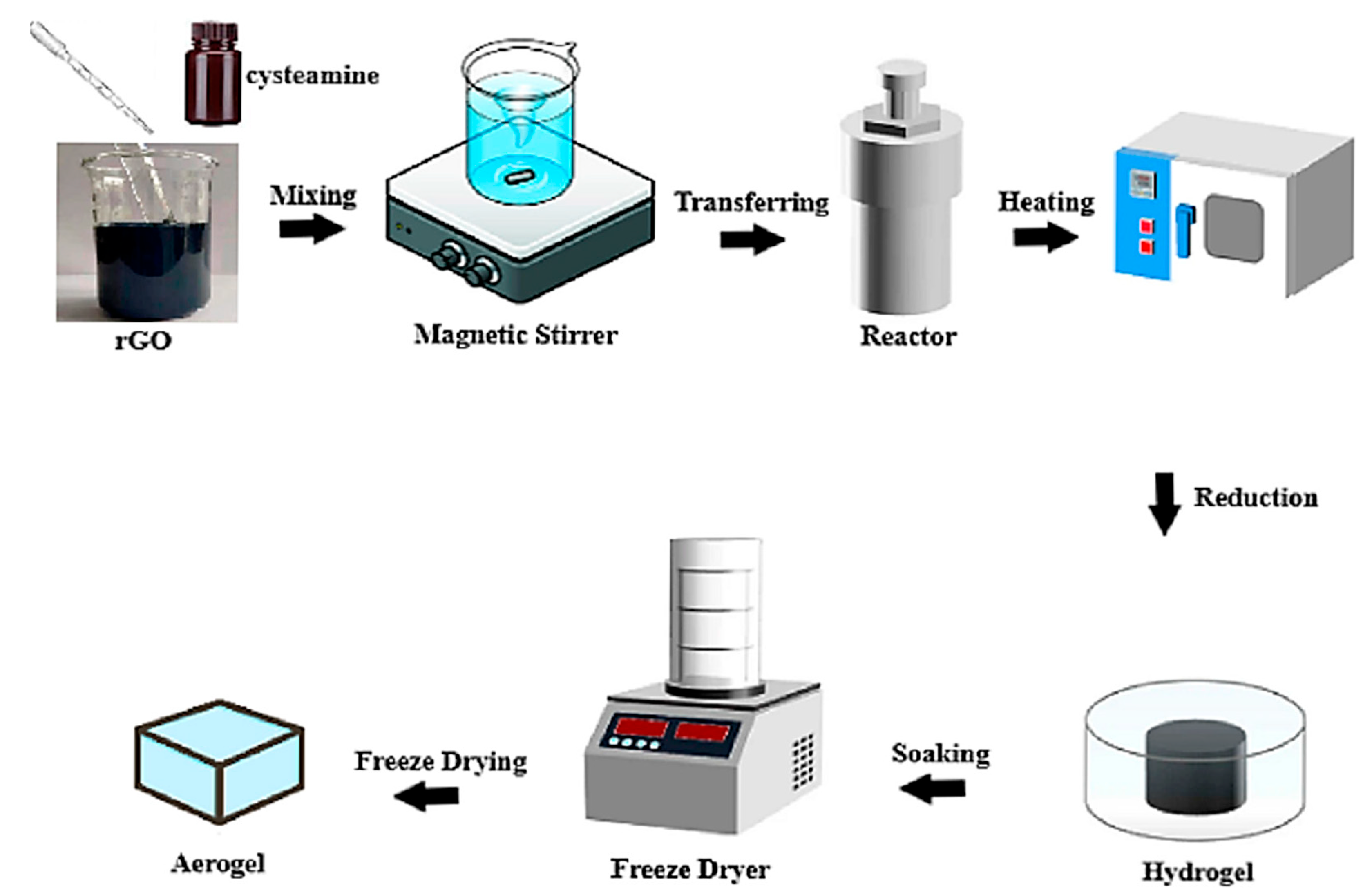
2.1. Hydrothermal Reduction of GO Aerogels
2.2. Chemical Reduction
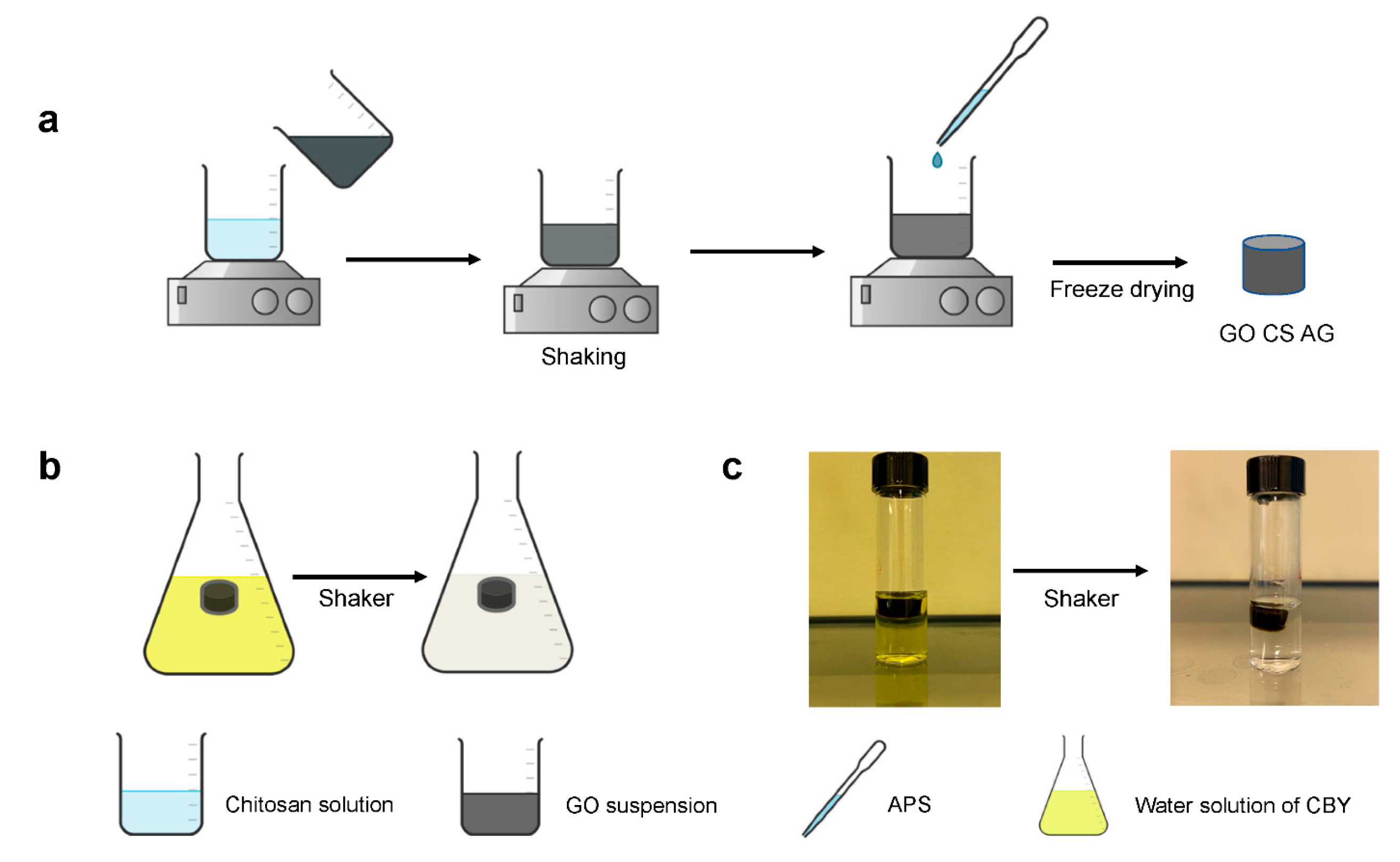
2.3. Crosslinking Method
2.4. 3D Printing
3. Factors Affecting Mechanical Properties
3.1. Density and Porosity
3.2. Crosslinking Agents
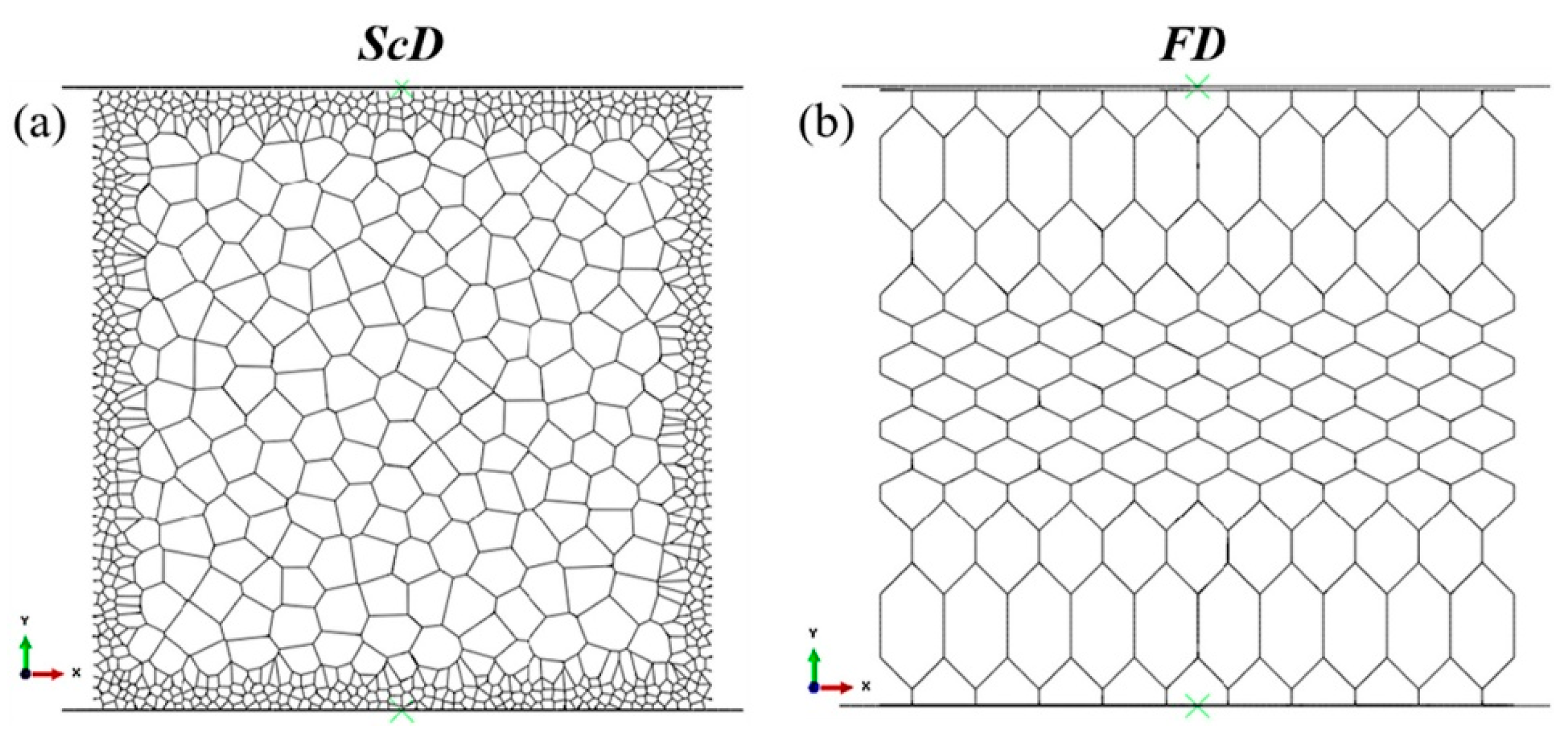
3.3. Freezing and Drying Methods
3.4. GO Concentration
3.5. Additives and Reinforcements
3.6. Temperature and Pressure During Synthesis
3.7. Structural Anisotropy
3.8. Functionalization and Reduction
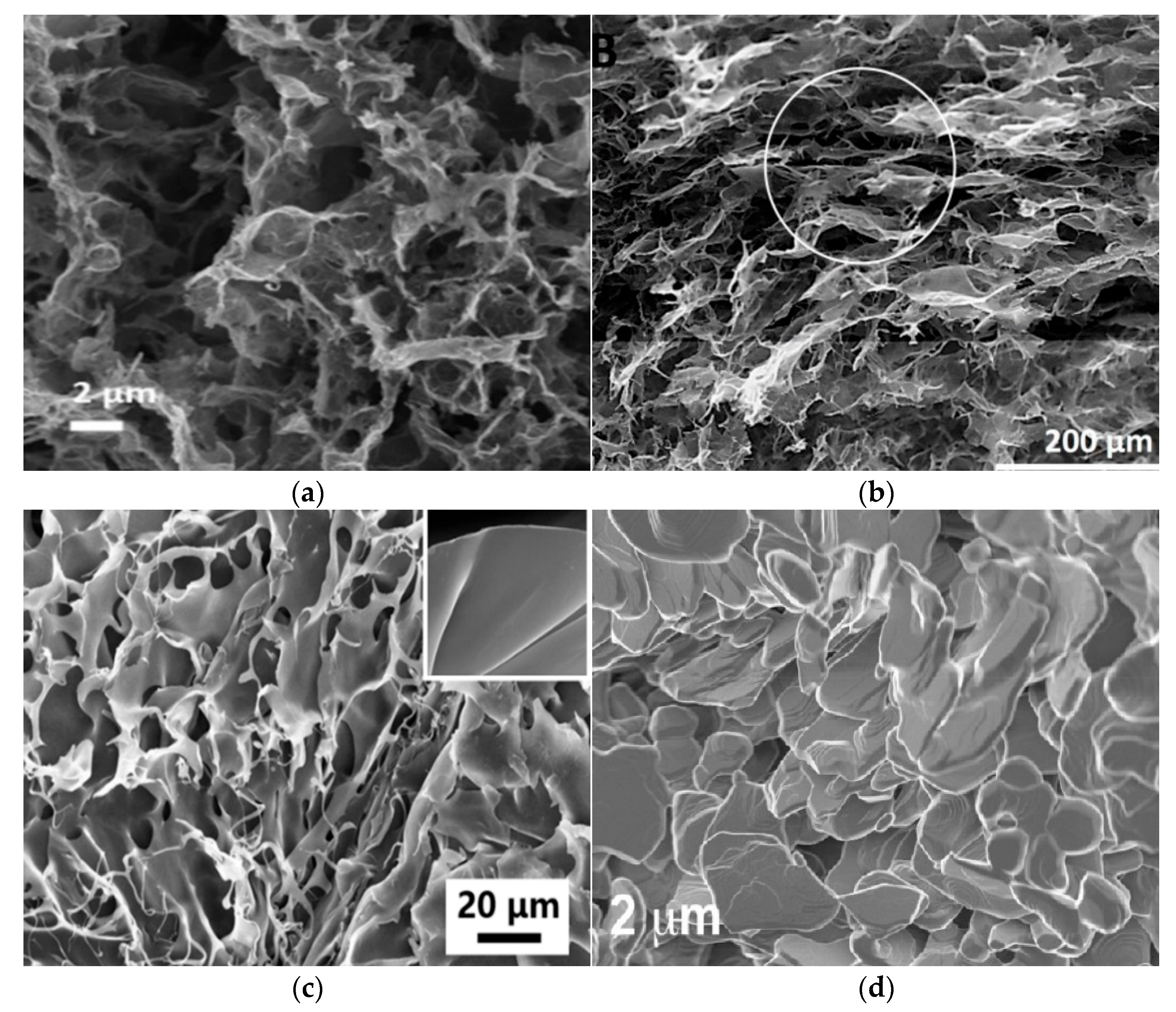
4. Characterization of Mechanical Properties
5. Applications
| Application | Relevance to Mechanical Properties | Key Features Utilized | Examples/Use Cases | Refs. |
|---|---|---|---|---|
| Energy Storage Devices | High porosity, lightweight structure, and mechanical stability ensure long-term performance under cyclic loads. | High surface area, low density, excellent compressive strength. | Supercapacitors, lithium-ion batteries, fuel cells. | [54] |
| Thermal Insulation | Low thermal conductivity with adequate compressive strength to maintain structural integrity. | Low density, resilience under thermal expansion. | Heat shields, cryogenic insulators in space technology. | [136] |
| Environmental Applications | Good mechanical stability supports repeated use in adsorption and filtration processes. | Compressive strength, chemical resilience. | Oil spill cleanup, heavy metal adsorption, water purification filters. | [137] |
| Biomedical Scaffolds | Biocompatibility combined with structural support for tissue engineering and drug delivery. | Elasticity, porosity, and compressive strength suitable for cellular growth. | Bone tissue scaffolds, slow-release drug carriers. | [138] |
| Lightweight Structural Materials | High compressive modulus and toughness ensure performance under load without significant deformation. | Superior strength-to-weight ratio. | Aerospace components, lightweight automobile parts. | [139] |
| Shock Absorption | Energy dissipation is under impact due to elastic deformation of the aerogel matrix. | Viscoelastic behavior, reversible compressive deformation. | Impact-resistant packaging, vibration dampers in machinery. | [140] |
| Catalysis Support | Strong, stable structures maintain integrity during catalytic reactions under extreme conditions. | Mechanical durability under high temperature and pressure. | Catalyst carriers in chemical reactors, photocatalysis applications. | [7] |
| Flexible Electronics | Elastic properties and flexibility enable integration into wearable devices and stretchable sensors. | High elasticity, durability under repetitive deformation. | Wearable health monitors, pressure sensors, strain gauges. | [141] |
6. Future Directions
7. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GO | Graphene Oxide |
| rGO | Reduced Graphene Oxide |
| GA | Graphene Aerogel |
| GOA | Graphene Oxide Aerogel |
| RTFG | Room-Temperature Freeze Gelation |
| GAFD | Graphene Aerogel Freeze-Dried |
| GAScD | Graphene Aerogel Supercritical Dried |
| CNT | Carbon Nanotubes |
| PVA | Polyvinyl Alcohol |
| APS | Ammonium Persulfate |
| DMA | Dynamic Mechanical Analysis |
| SEM | Scanning Electron Microscopy |
| XCT | X-ray Computed Tomography |
| ScD | Supercritical Drying |
| K_IC | Mode I Fracture toughness |
References
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Kasar, A.K.; Tian, S.; Xiong, G.; Menezes, P.L. Graphene Aerogel and Its Composites: Synthesis, Properties and Applications. J. Porous Mater. 2022, 29, 1011–1025. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Xin, X.; Zhang, N.; Tang, Z.-R.; Xu, Y.-J. Photoredox Catalysis over Graphene Aerogel-Supported Composites. J. Mater. Chem. A 2018, 6, 4590–4604. [Google Scholar] [CrossRef]
- Sato, S. Graphene for Nanoelectronics. Jpn. J. Appl. Phys. 2015, 54, 040102. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, D.; Yin, Z.; Yan, Q.; Zhang, H. Graphene and Graphene-Based Materials for Energy Storage Applications. Small 2014, 10, 3480–3498. [Google Scholar] [CrossRef]
- Hill, E.W.; Vijayaragahvan, A.; Novoselov, K. Graphene Sensors. IEEE Sens. J. 2011, 11, 3161–3170. [Google Scholar] [CrossRef]
- Qiu, B.; Xing, M.; Zhang, J. Recent Advances in Three-Dimensional Graphene Based Materials for Catalysis Applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Z. Graphene in Biomedicine: Opportunities and Challenges. Nanomedicine 2011, 6, 317–324. [Google Scholar] [CrossRef]
- Björk, J.; Hanke, F.; Palma, C.-A.; Samori, P.; Cecchini, M.; Persson, M. Adsorption of Aromatic and Anti-Aromatic Systems on Graphene through Π−π Stacking. J. Phys. Chem. Lett. 2010, 1, 3407–3412. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H., Jr.; Baumann, T.F. Synthesis of Graphene Aerogel with High Electrical Conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Huang, X.; Wang, Y.; Huang, Y.; Duan, X. Functionalized Graphene Hydrogel-Based High-Performance Supercapacitors. Adv. Mater. 2013, 25, 5779–5784. [Google Scholar] [CrossRef]
- Sun, J.; Memon, M.A.; Bai, W.; Xiao, L.; Zhang, B.; Jin, Y.; Huang, Y.; Geng, J. Controllable Fabrication of Transparent Macroporous Graphene Thin Films and Versatile Applications as a Conducting Platform. Adv. Funct. Mater. 2015, 25, 4334–4343. [Google Scholar] [CrossRef]
- Nassar, G.; Daou, E.; Najjar, R.; Bassil, M.; Habchi, R. A Review on the Current Research on Graphene-Based Aerogels and Their Applications. Carbon Trends 2021, 4, 100065. [Google Scholar] [CrossRef]
- Lim, M.B.; Hu, M.; Manandhar, S.; Sakshaug, A.; Strong, A.; Riley, L.; Pauzauskie, P.J. Ultrafast Sol–Gel Synthesis of Graphene Aerogel Materials. Carbon 2015, 95, 616–624. [Google Scholar] [CrossRef]
- Yuan, Z.; Xiao, X.; Li, J.; Zhao, Z.; Yu, D.; Li, Q. Self-Assembled Graphene-Based Architectures and Their Applications. Adv. Sci. 2018, 5, 1700626. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, M.; Ling, X.; Jiao, Z.; Chen, L.; Chen, L.; Hu, P.; Zhao, B. One-Step Hydrothermal Synthesis of Three-Dimensional Porous Graphene Aerogels/Sulfur Nanocrystals for Lithium–Sulfur Batteries. J. Alloys Compd. 2015, 645, 509–516. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Liu, M.; Zhang, C.; Chen, W. Three-Dimensional Mesoporous Graphene Aerogel-Supported SnO2 Nanocrystals for High-Performance NO2 Gas Sensing at Low Temperature. Anal. Chem. 2015, 87, 1638–1645. [Google Scholar] [CrossRef]
- Xiong, X.; Ji, N.; Song, C.; Liu, Q. Preparation Functionalized Graphene Aerogels as Air Cleaner Filter. Procedia Eng. 2015, 121, 957–960. [Google Scholar] [CrossRef]
- Long, S.; Wang, H.; He, K.; Zhou, C.; Zeng, G.; Lu, Y.; Cheng, M.; Song, B.; Yang, Y.; Wang, Z.; et al. 3D Graphene Aerogel Based Photocatalysts: Synthesized, Properties, and Applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124666. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Zhang, Y.; Huang, Y.; Liu, J.; Zhang, X.; Liu, X.; Teng, H.; Zhang, X.; Zhang, J.; et al. A Review of Graphene-Based Materials/Polymer Composite Aerogels. Polymers 2023, 15, 1888. [Google Scholar] [CrossRef]
- Zhou, D.; Song, W.-L.; Li, X.; Fan, L.-Z. Hierarchical Porous Reduced Graphene Oxide/SnO2 Networks as Highly Stable Anodes for Lithium-Ion Batteries. Electrochim. Acta 2016, 207, 9–15. [Google Scholar] [CrossRef]
- Gholipour, A.; Roudini, G.; Yaghoubinezhad, Y. Investigation on Corrosion Behavior of Reduced Graphene Oxide/Zirconia Nanocomposite Coated by Pulse Electrodeposition Technique on API-X65 Steel. arXiv 2022. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, J. Copper Nanomaterials and Assemblies for Soft Electronics. Sci. China Mater. 2019, 62, 1679–1708. [Google Scholar] [CrossRef]
- Tian, H.; Xu, Z.; Liu, K.; Wang, D.; Ren, L.; Wei, Y.; Chen, L.; Chen, Y.; Liu, S.; Yang, H. Heterogeneous Bimetallic Selenides Encapsulated within Graphene Aerogel as Advanced Anodes for Sodium Ion Batteries. J. Colloid Interface Sci. 2024, 670, 152–162. [Google Scholar] [CrossRef]
- Yan, D.-X.; Pang, H.; Li, B.; Vajtai, R.; Xu, L.; Ren, P.-G.; Wang, J.-H.; Li, Z.-M. Structured Reduced Graphene Oxide/Polymer Composites for Ultra-Efficient Electromagnetic Interference Shielding. Adv. Funct. Mater. 2015, 25, 559–566. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K. 3D Nitrogen-Doped Graphene Aerogel-Supported Fe3O4 Nanoparticles as Efficient Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pham, T.T.; Yan, A.; Shin, S.J.; Lee, J.R.I.; Bagge-Hansen, M.; Mickelson, W.; Zettl, A. Synthesis and Characterization of Highly Crystalline Graphene Aerogels. ACS Nano 2014, 8, 11013–11022. [Google Scholar] [CrossRef]
- Worsley, M.A.; Olson, T.Y.; Lee, J.R.I.; Willey, T.M.; Nielsen, M.H.; Roberts, S.K.; Pauzauskie, P.J.; Biener, J.; Satcher, J.H., Jr.; Baumann, T.F. High Surface Area, Sp2-Cross-Linked Three-Dimensional Graphene Monoliths. J. Phys. Chem. Lett. 2011, 2, 921–925. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Víctor-Román, S.; Sanahuja-Parejo, O.; Benito, A.M.; Maser, W.K. Control of the Microstructure and Surface Chemistry of Graphene Aerogels via pH and Time Manipulation by a Hydrothermal Method. Nanoscale 2018, 10, 3526–3539. [Google Scholar] [CrossRef]
- Goldstein, A.P.; Mickelson, W.; Machness, A.; Lee, G.; Worsley, M.A.; Woo, L.; Zettl, A. Simultaneous Sheet Cross-Linking and Deoxygenation in the Graphene Oxide Sol–Gel Transition. J. Phys. Chem. C 2014, 118, 28855–28860. [Google Scholar] [CrossRef]
- Mi Jung, S.; Lopes Mafra, D.; Lin, C.-T.; Young Jung, H.; Kong, J. Controlled Porous Structures of Graphene Aerogels and Their Effect on Supercapacitor Performance. Nanoscale 2015, 7, 4386–4393. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, C.; Cheng, D.; Zhou, H.; Liu, X.; Xie, P.; Zhao, Q.; Zhang, D.; Fan, T. Architectured Leaf-Inspired Ni0.33Co0.66S2/Graphene Aerogels via 3D Printing for High-Performance Energy Storage. Adv. Funct. Mater. 2018, 28, 1805057. [Google Scholar] [CrossRef]
- Madduri, S.B.; Kommalapati, R.R. Harnessing Novel Reduced Graphene Oxide-Based Aerogel for Efficient Organic Contaminant and Heavy Metal Removal in Aqueous Environments. Nanomaterials 2024, 14, 1708. [Google Scholar] [CrossRef]
- Sheng, K.; Xu, Y.; Li, C.; Shi, G. High-Performance Self-Assembled Graphene Hydrogels Prepared by Chemical Reduction of Graphene Oxide. New Carbon Mater. 2011, 26, 9–15. [Google Scholar] [CrossRef]
- Pei, S.; Zhao, J.; Du, J.; Ren, W.; Cheng, H.-M. Direct Reduction of Graphene Oxide Films into Highly Conductive and Flexible Graphene Films by Hydrohalic Acids. Carbon 2010, 48, 4466–4474. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced Graphene Oxide by Chemical Graphitization. Nat. Commun. 2010, 1, 73. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-Reduction of Graphite- and Graphene Oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, G.; Nejib Hedhili, M.; Zhang, H.; Wang, P. Three-Dimensional Assemblies of Graphene Prepared by a Novel Chemical Reduction-Induced Self-Assembly Method. Nanoscale 2012, 4, 7038–7045. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, C.; Li, X.; Zhang, L.; Ma, Y.; Zhang, L.; Xu, X.; Xia, F.; Wang, W.; Gao, J. A One-Step Method for Reduction and Self-Assembling of Graphene Oxide into Reduced Graphene Oxide Aerogels. J. Mater. Chem. A 2013, 1, 2869–2877. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Jae Wie, J.; Xu, Y.; Seok Park, H. Chemical Modification of Graphene Aerogels for Electrochemical Capacitor Applications. Phys. Chem. Chem. Phys. 2015, 17, 30946–30962. [Google Scholar] [CrossRef]
- Garcia-Bordejé, E.; Benito, A.M.; Maser, W.K. Graphene Aerogels via Hydrothermal Gelation of Graphene Oxide Colloids: Fine-Tuning of Its Porous and Chemical Properties and Catalytic Applications. Adv. Colloid Interface Sci. 2021, 292, 102420. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, X.; Chen, B. Covalently Cross-Linked Graphene Oxide Aerogel with Stable Structure for High-Efficiency Water Purification. Chem. Eng. J. 2018, 354, 896–904. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Y.; Chen, S.; Duan, X.; Gao, J.; Zhang, N.; He, L.; Wang, X.; Huang, J.; Chen, X.; et al. Iron-calcium Dual Crosslinked Graphene Oxide/Alginate Aerogel Microspheres for Extraordinary Elimination of Tetracycline in Complex Wastewater: Performance, Mechanism, and Applications. Int. J. Biol. Macromol. 2024, 264, 130554. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Song, P.; You, B.; Sun, G. Double-Cross-Linking Strategy for Preparing Flexible, Robust, and Multifunctional Polyimide Aerogel. Chem. Eng. J. 2020, 381, 122784. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, L.; Liu, C.; Zhen, Y.; Zhang, W.; Ma, J. A Novel 3D Adsorbent of Reduced Graphene Oxide-β-Cyclodextrin Aerogel Coupled Hardness with Softness for Efficient Removal of Bisphenol A. Chem. Eng. J. 2019, 372, 896–904. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Wang, W.; Ji, Q.; Xia, Y. Temperature-Sensitive Poly(N-Isopropylacrylamide)/Graphene Oxide Nanocomposite Hydrogels by in Situ Polymerization with Improved Swelling Capability and Mechanical Behavior. Eur. Polym. J. 2013, 49, 389–396. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Wang, X.; Shi, G. On the Gelation of Graphene Oxide. J. Phys. Chem. C 2011, 115, 5545–5551. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, Z.; Wan, W.; Gogotsi, Y.; Qiu, J. Ultralight and Highly Compressible Graphene Aerogels. Adv. Mater. 2013, 25, 2219–2223. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, Y.; Li, J.; Fan, Q.; Huang, W. Self-Assembly of Reduced Graphene Oxide into Three-Dimensional Architecture by Divalent Ion Linkage. J. Phys. Chem. C 2010, 114, 22462–22465. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Nguyen, H.T.; Rinaldi, A.; Nguyen, N.P.V.; Fan, Z.; Duong, H.M. Morphology Control and Thermal Stability of Binderless-Graphene Aerogels from Graphite for Energy Storage Applications. Colloids Surf. A Physicochem. Eng. Asp. 2012, 414, 352–358. [Google Scholar] [CrossRef]
- Pinelli, F.; Nespoli, T.; Rossi, F. Graphene Oxide-Chitosan Aerogels: Synthesis, Characterization, and Use as Adsorbent Material for Water Contaminants. Gels 2021, 7, 149. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, İ.A. Graphene and Graphene Oxide Based Aerogels: Synthesis, Characteristics and Supercapacitor Applications. J. Energy Storage 2020, 27, 101038. [Google Scholar] [CrossRef]
- Mao, J.; Iocozzia, J.; Huang, J.; Meng, K.; Lai, Y.; Lin, Z. Graphene Aerogels for Efficient Energy Storage and Conversion. Energy Environ. Sci. 2018, 11, 772–799. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, H.; Cai, Z.; Cheng, D.; He, P.; Xie, P.; Zhang, D.; Fan, T. Generalized 3D Printing of Graphene-Based Mixed-Dimensional Hybrid Aerogels. ACS Nano 2018, 12, 3502–3511. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, F.; Casano, G.; Bhavsar, R.; Kinloch, I.A.; Derby, B. Pristine Graphene Aerogels by Room-Temperature Freeze Gelation. Adv. Mater. 2016, 28, 7993–8000. [Google Scholar] [CrossRef]
- Zhu, C.; Han, T.Y.-J.; Duoss, E.B.; Golobic, A.M.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A. Highly Compressible 3D Periodic Graphene Aerogel Microlattices. Nat. Commun. 2015, 6, 6962. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Z.; Gong, J.; Zhou, X.; Li, J.; Lyu, Z. 3D Printing of Graphene-Based Aerogels and Their Applications. FlatChem 2024, 47, 100731. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Wang, M.; Shi, S.; Jing, J. Fused Deposition Modeling 3D Printing of Novel Poly(Vinyl Alcohol)/Graphene Nanocomposite with Enhanced Mechanical and Electromagnetic Interference Shielding Properties. Ind. Eng. Chem. Res. 2020, 59, 8066–8077. [Google Scholar] [CrossRef]
- Tetik, H.; Orangi, J.; Yang, G.; Zhao, K.; Bin Mujib, S.; Singh, G.; Beidaghi, M.; Lin, D. 3D Printed MXene Aerogels with Truly 3D Macrostructure and Highly Engineered Microstructure for Enhanced Electrical and Electrochemical Performance. Adv. Mater. 2022, 34, 2104980. [Google Scholar] [CrossRef]
- Li, Z.; Guo, F.; Pang, K.; Lin, J.; Gao, Q.; Chen, Y.; Chang, D.; Wang, Y.; Liu, S.; Han, Y.; et al. Precise Thermoplastic Processing of Graphene Oxide Layered Solid by Polymer Intercalation. Nano-Micro Lett. 2021, 14, 12. [Google Scholar] [CrossRef]
- Serrapede, M.; Fontana, M.; Gigot, A.; Armandi, M.; Biasotto, G.; Tresso, E.; Rivolo, P. A Facile and Green Synthesis of a MoO2-Reduced Graphene Oxide Aerogel for Energy Storage Devices. Materials 2020, 13, 594. [Google Scholar] [CrossRef]
- Pruna, A.; Cárcel, A.C.; Barjola, A.; Benedito, A.; Giménez, E. Tailoring the Performance of Graphene Aerogels for Oil/Organic Solvent Separation by 1-Step Solvothermal Approach. Nanomaterials 2019, 9, 1077. [Google Scholar] [CrossRef]
- Gong, Y.; Yu, Y.; Kang, H.; Chen, X.; Liu, H.; Zhang, Y.; Sun, Y.; Song, H. Synthesis and Characterization of Graphene Oxide/Chitosan Composite Aerogels with High Mechanical Performance. Polymers 2019, 11, 777. [Google Scholar] [CrossRef]
- García-Tuñón, E.; Feilden, E.; Zheng, H.; D’Elia, E.; Leong, A.; Saiz, E. Graphene Oxide: An All-in-One Processing Additive for 3D Printing. ACS Appl. Mater. Interfaces 2017, 9, 32977–32989. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption Characteristics and Behaviors of Graphene Oxide for Zn(II) Removal from Aqueous Solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Adhikary, S.K.; Ashish, D.K.; Rudžionis, Ž. Aerogel Based Thermal Insulating Cementitious Composites: A Review. Energy Build. 2021, 245, 111058. [Google Scholar] [CrossRef]
- Luo, H.; Malakooti, S.; Churu, H.G.; Leventis, N.; Lu, H. Mechanical Characterization of Aerogels. In Springer Handbook of Aerogels; Aegerter, M.A., Leventis, N., Koebel, M., Steiner, S.A., III, Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 197–229. ISBN 978-3-030-27322-4. [Google Scholar]
- Zhang, Q.; Xu, X.; Li, H.; Xiong, G.; Hu, H.; Fisher, T.S. Mechanically Robust Honeycomb Graphene Aerogel Multifunctional Polymer Composites. Carbon 2015, 93, 659–670. [Google Scholar] [CrossRef]
- Nardecchia, S.; Carriazo, D.; Luisa Ferrer, M.; Gutiérrez, M.C.; del Monte, F. Three Dimensional Macroporous Architectures and Aerogels Built of Carbon Nanotubes and/or Graphene: Synthesis and Applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, H.-B.; Liu, Z.; Xu, S.; Wang, Y.-Z. On Controlling Aerogel Microstructure by Freeze Casting. Compos. Part. B Eng. 2019, 173, 107036. [Google Scholar] [CrossRef]
- Ye, S.; Feng, J.; Wu, P. Highly Elastic Graphene Oxide–Epoxy Composite Aerogels via Simple Freeze-Drying and Subsequent Routine Curing. J. Mater. Chem. A 2013, 1, 3495–3502. [Google Scholar] [CrossRef]
- Gorgolis, G.; Galiotis, C. Graphene aerogels: A review. 2D Mater. 2017, 4, 032001. [Google Scholar] [CrossRef]
- Li, F.; Xie, L.; Sun, G.; Kong, Q.; Su, F.; Cao, Y.; Wei, J.; Ahmad, A.; Guo, X.; Chen, C.-M. Resorcinol-Formaldehyde Based Carbon Aerogel: Preparation, Structure and Applications in Energy Storage Devices. Microporous Mesoporous Mater. 2019, 279, 293–315. [Google Scholar] [CrossRef]
- Wang, X.; Lu, L.-L.; Yu, Z.-L.; Xu, X.-W.; Zheng, Y.-R.; Yu, S.-H. Scalable Template Synthesis of Resorcinol–Formaldehyde/Graphene Oxide Composite Aerogels with Tunable Densities and Mechanical Properties. Angew. Chem. Int. Ed. 2015, 54, 2397–2401. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B. Self-Assembly of Graphene Oxide Aerogels by Layered Double Hydroxides Cross-Linking and Their Application in Water Purification. J. Mater. Chem. A 2014, 2, 8941–8951. [Google Scholar] [CrossRef]
- Joshi, D.N.; Han, S.; Kim, D. Freeze-Casting Mold-Based Scalable Synthesis of Directional Graphene Aerogels with Long-Range Pore Alignment for Energy Applications. Small Methods 2024, 9, 2400856. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Politowicz, A.L.; Chen, E.; Huang, H.-X.; Turng, L.-S. Highly Compressible Ultra-Light Anisotropic Cellulose/Graphene Aerogel Fabricated by Bidirectional Freeze Drying for Selective Oil Absorption. Carbon 2018, 132, 199–209. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Wang, B.; Huang, M.; Wang, B.; Jiang, Y.; Ruoff, R.S. Freeze-Casting Produces a Graphene Oxide Aerogel with a Radial and Centrosymmetric Structure. ACS Nano 2018, 12, 5816–5825. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, L.; Chen, L.; Duan, G.; Mei, C.; Huang, C.; Han, J.; Jiang, S. Anisotropic Nanocellulose Aerogels with Ordered Structures Fabricated by Directional Freeze-Drying for Fast Liquid Transport. Cellulose 2019, 26, 6653–6667. [Google Scholar] [CrossRef]
- Xie, J.; Niu, L.; Qiao, Y.; Lei, Y.; Li, G.; Zhang, X.; Chen, P. The Influence of the Drying Method on the Microstructure and the Compression Behavior of Graphene Aerogel. Diam. Relat. Mater. 2022, 121, 108772. [Google Scholar] [CrossRef]
- Zhou, L.; Zhai, S.; Chen, Y.; Xu, Z. Anisotropic Cellulose Nanofibers/Polyvinyl Alcohol/Graphene Aerogels Fabricated by Directional Freeze-Drying as Effective Oil Adsorbents. Polymers 2019, 11, 712. [Google Scholar] [CrossRef]
- Yılmaz, P.; Öztürk Er, E.; Bakırdere, S.; Ülgen, K.; Özbek, B. Application of Supercritical Gel Drying Method on Fabrication of Mechanically Improved and Biologically Safe Three-Component Scaffold Composed of Graphene Oxide/Chitosan/Hydroxyapatite and Characterization Studies. J. Mater. Res. Technol. 2019, 8, 5201–5216. [Google Scholar] [CrossRef]
- Liu, X.; Pang, K.; Yang, H.; Guo, X. Intrinsically Microstructured Graphene Aerogel Exhibiting Excellent Mechanical Performance and Super-High Adsorption Capacity. Carbon 2020, 161, 146–152. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, X.; Akbari Garakani, M.; Lin, X.; Wu, Y.; Liu, X.; Sun, X.; Kim, J.-K. Graphene Aerogel/Epoxy Composites with Exceptional Anisotropic Structure and Properties. ACS Appl. Mater. Interfaces 2015, 7, 5538–5549. [Google Scholar] [CrossRef]
- Guo, K.; Song, H.; Chen, X.; Du, X.; Zhong, L. Graphene Oxide as an Anti-Shrinkage Additive for Resorcinol–Formaldehyde Composite Aerogels. Phys. Chem. Chem. Phys. 2014, 16, 11603–11608. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Ma, Q.; Jia, X.; Ma, P.-C. Preparation of Carbon Nanotubes/Graphene Hybrid Aerogel and Its Application for the Adsorption of Organic Compounds. Carbon 2017, 118, 765–771. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Bak, B.M.; Wie, J.J.; Kong, J.; Park, H.S. Reversibly Compressible, Highly Elastic, and Durable Graphene Aerogels for Energy Storage Devices under Limiting Conditions. Adv. Funct. Mater. 2015, 25, 1053–1062. [Google Scholar] [CrossRef]
- Javadi, A.; Zheng, Q.; Payen, F.; Javadi, A.; Altin, Y.; Cai, Z.; Sabo, R.; Gong, S. Polyvinyl Alcohol-Cellulose Nanofibrils-Graphene Oxide Hybrid Organic Aerogels. ACS Appl. Mater. Interfaces 2013, 5, 5969–5975. [Google Scholar] [CrossRef]
- Huo, J.; Yu, G.; Wang, J. Adsorptive Removal of Sr(II) from Aqueous Solution by Polyvinyl Alcohol/Graphene Oxide Aerogel. Chemosphere 2021, 278, 130492. [Google Scholar] [CrossRef]
- Pan, L.; Liu, S.; Oderinde, O.; Li, K.; Yao, F.; Fu, G. Facile Fabrication of Graphene-Based Aerogel with Rare Earth Metal Oxide for Water Purification. Appl. Surf. Sci. 2018, 427, 779–786. [Google Scholar] [CrossRef]
- Li, X.-H.; Liu, P.; Li, X.; An, F.; Min, P.; Liao, K.-N.; Yu, Z.-Z. Vertically Aligned, Ultralight and Highly Compressive All-Graphitized Graphene Aerogels for Highly Thermally Conductive Polymer Composites. Carbon 2018, 140, 624–633. [Google Scholar] [CrossRef]
- Lee, S.P.; Ali, G.A.M.; Hegazy, H.H.; Lim, H.N.; Chong, K.F. Optimizing Reduced Graphene Oxide Aerogel for a Supercapacitor. Energy Fuels 2021, 35, 4559–4569. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, S.; Hu, P.; Zhao, G.; Li, Y.; Zhang, X.; Han, W. Enhanced Mechanical, Thermal, and Electric Properties of Graphene Aerogels via Supercritical Ethanol Drying and High-Temperature Thermal Reduction. Sci. Rep. 2017, 7, 1439. [Google Scholar] [CrossRef]
- Yeon, J.S.; Gupta, N.; Bhattacharya, P.; Park, H.S. A New Era of Integrative Ice Frozen Assembly into Multiscale Architecturing of Energy Materials. Adv. Funct. Mater. 2022, 32, 2112509. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Yu, Z.; Zou, H.; Liu, P. Anisotropic Polyimide Aerogels Fabricated by Directional Freezing. J. Appl. Polym. Sci. 2019, 136, 47179. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Wu, X.; Wang, J.; Yang, S. Poly(Vinyl Alcohol)-Mediated Graphene Aerogels with Tailorable Architectures and Advanced Properties for Anisotropic Sensing. J. Phys. Chem. C 2019, 123, 3781–3789. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Wang, X.; Ye, X.; Zhou, S.; Wang, W.; Zhao, Z.; Qiu, J. Anisotropic 3D Aerogel of Graphene Oxide and Poly(Vinyl Alcohol) for Desalination by Interfacial Solar Vapor Generation. ACS Appl. Nano Mater. 2024, 7, 18870–18880. [Google Scholar] [CrossRef]
- Trinh, T.T.P.N.X.; Nguyet, D.M.; Quan, T.H.; Anh, T.N.M.; Thinh, D.B.; Tai, L.T.; Lan, N.T.; Trinh, D.N.; Dat, N.M.; Nam, H.M.; et al. Preparing Three-Dimensional Graphene Aerogels by Chemical Reducing Method: Investigation of Synthesis Condition and Optimization of Adsorption Capacity of Organic Dye. Surf. Interfaces 2021, 23, 101023. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.-W.; Wang, J. Graphene Aerogel–Metal–Organic Framework-Based Electrochemical Method for Simultaneous Detection of Multiple Heavy-Metal Ions. Anal. Chem. 2019, 91, 888–895. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Patel, J.; Sreevidya, S.; Carabineiro, S.A.C. Facile Preparation of Methionine-Functionalized Graphene Oxide/Chitosan Polymer Nanocomposite Aerogel for the Efficient Removal of Dyes and Metal Ions from Aqueous Solutions. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100743. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y. Three-Dimensional Graphene Networks: Synthesis, Properties and Applications. Natl. Sci. Rev. 2015, 2, 40–53. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L. In Situ Self-Assembly of Mild Chemical Reduction Graphene for Three-Dimensional Architectures. Nanoscale 2011, 3, 3132–3137. [Google Scholar] [CrossRef]
- Borrás, A.; Gonçalves, G.; Marbán, G.; Sandoval, S.; Pinto, S.; Marques, P.A.A.P.; Fraile, J.; Tobias, G.; López-Periago, A.M.; Domingo, C. Preparation and Characterization of Graphene Oxide Aerogels: Exploring the Limits of Supercritical CO2 Fabrication Methods. Chem. A Eur. J. 2018, 24, 15903–15911. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Ghalkhani, M.; Maleki, H. Directional Freeze-Casting: A Bioinspired Method to Assemble Multifunctional Aligned Porous Structures for Advanced Applications. Adv. Eng. Mater. 2020, 22, 2000033. [Google Scholar] [CrossRef]
- Rajasekar, R.; Kim, N.H.; Jung, D.; Kuila, T.; Lim, J.K.; Park, M.J.; Lee, J.H. Electrostatically Assembled Layer-by-Layer Composites Containing Graphene Oxide for Enhanced Hydrogen Gas Barrier Application. Compos. Sci. Technol. 2013, 89, 167–174. [Google Scholar] [CrossRef]
- Ge, X.; Shan, Y.; Wu, L.; Mu, X.; Peng, H.; Jiang, Y. High-Strength and Morphology-Controlled Aerogel Based on Carboxymethyl Cellulose and Graphene Oxide. Carbohydr. Polym. 2018, 197, 277–283. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, N.; Cui, Y.; Gao, W.; Zhao, Q.; Gao, C.; Bai, H.; Xie, T. Biomimetic Architectured Graphene Aerogel with Exceptional Strength and Resilience. ACS Nano 2017, 11, 6817–6824. [Google Scholar] [CrossRef]
- Li, C.; Qiu, L.; Zhang, B.; Li, D.; Liu, C.-Y. Robust Vacuum-/Air-Dried Graphene Aerogels and Fast Recoverable Shape-Memory Hybrid Foams. Adv. Mater. 2016, 28, 1510–1516. [Google Scholar] [CrossRef]
- Xia, Y.; Gao, C.; Gao, W. A Review on Elastic Graphene Aerogels: Design, Preparation, and Applications. J. Polym. Sci. 2022, 60, 2239–2261. [Google Scholar] [CrossRef]
- Xiao, J.; Tan, Y.; Song, Y.; Zheng, Q. A Flyweight and Superelastic Graphene Aerogel as a High-Capacity Adsorbent and Highly Sensitive Pressure Sensor. J. Mater. Chem. A 2018, 6, 9074–9080. [Google Scholar] [CrossRef]
- Huang, H.; Chen, P.; Zhang, X.; Lu, Y.; Zhan, W. Edge-to-Edge Assembled Graphene Oxide Aerogels with Outstanding Mechanical Performance and Superhigh Chemical Activity. Small 2013, 9, 1397–1404. [Google Scholar] [CrossRef]
- Jiao, C.; Xiong, J.; Tao, J.; Xu, S.; Zhang, D.; Lin, H.; Chen, Y. Sodium Alginate/Graphene Oxide Aerogel with Enhanced Strength–Toughness and Its Heavy Metal Adsorption Study. Int. J. Biol. Macromol. 2016, 83, 133–141. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.; Min, P.; Chang, X.; Shu, C.; Ding, Y.; Yu, Z.-Z. 3D Lamellar-Structured Graphene Aerogels for Thermal Interface Composites with High Through-Plane Thermal Conductivity and Fracture Toughness. Nano-Micro Lett. 2020, 13, 22. [Google Scholar] [CrossRef]
- Kim, J.; Han, N.M.; Kim, J.; Lee, J.; Kim, J.-K.; Jeon, S. Highly Conductive and Fracture-Resistant Epoxy Composite Based on Non-Oxidized Graphene Flake Aerogel. ACS Appl. Mater. Interfaces 2018, 10, 37507–37516. [Google Scholar] [CrossRef]
- Hou, X.; Chen, J.; Chen, Z.; Yu, D.; Zhu, S.; Liu, T.; Chen, L. Flexible Aerogel Materials: A Review on Revolutionary Flexibility Strategies and the Multifunctional Applications. ACS Nano 2024, 18, 11525–11559. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, Y.; Hu, J.; Wang, H. Flexible, Strong, Multifunctional Graphene Oxide/Silica-Based Composite Aerogels via a Double-Cross-Linked Network Approach. ACS Appl. Mater. Interfaces 2020, 12, 47854–47864. [Google Scholar] [CrossRef]
- Sun, K.; Dong, H.; Kou, Y.; Yang, H.; Liu, H.; Li, Y.; Shi, Q. Flexible Graphene Aerogel-Based Phase Change Film for Solar-Thermal Energy Conversion and Storage in Personal Thermal Management Applications. Chem. Eng. J. 2021, 419, 129637. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Chen, Z.-Y.; Tang, Y.-H.; Li, Y.-T.; Ma, D.; Zhang, G.-D.; Boukherroub, R.; Cao, C.-F.; Gong, L.-X.; Song, P.; et al. Silicone/Graphene Oxide Co-Cross-Linked Aerogels with Wide-Temperature Mechanical Flexibility, Super-Hydrophobicity and Flame Resistance for Exceptional Thermal Insulation and Oil/Water Separation. J. Mater. Sci. Technol. 2022, 114, 131–142. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Wang, Z.; Ren, Z.; Yan, S.; Duan, Y.; Zhang, J. Ultralight, Superelastic, and Fatigue-Resistant Graphene Aerogel Templated by Graphene Oxide Liquid Crystal Stabilized Air Bubbles. ACS Appl. Mater. Interfaces 2019, 11, 1303–1310. [Google Scholar] [CrossRef]
- Huang, J.; Zeng, J.; Liang, B.; Wu, J.; Li, T.; Li, Q.; Feng, F.; Feng, Q.; Rood, M.J.; Yan, Z. Multi-Arch-Structured All-Carbon Aerogels with Superelasticity and High Fatigue Resistance as Wearable Sensors. ACS Appl. Mater. Interfaces 2020, 12, 16822–16830. [Google Scholar] [CrossRef]
- Xie, M.; Qian, G.; Ye, Q.; Zhang, Y.; Wang, M.; Deng, Z.; Yu, Y.; Chen, C.; Li, H.; Li, D. Dual-Crosslinked Reduced Graphene Oxide/Polyimide Aerogels Possessing Regulable Superelasticity, Fatigue Resistance, and Rigidity for Thermal Insulation and Flame Retardant Protection in Harsh Conditions. J. Colloid Interface Sci. 2024, 676, 1011–1022. [Google Scholar] [CrossRef]
- Kim, K.H.; Tsui, M.N.; Islam, M.F. Graphene-Coated Carbon Nanotube Aerogels Remain Superelastic While Resisting Fatigue and Creep over −100 to +500 °C. Chem. Mater. 2017, 29, 2748–2755. [Google Scholar] [CrossRef]
- Zhang, C.; Tong, W.; Qin, H.; Ming, X.; Wang, L.; Yu, Y.; Mao, Y.; Lu, J.; Li, P.; Shi, T.; et al. Multi-Modal Resonance of Topological Hybrid Graphene Foam for Enhanced Acoustic Absorption. Chem. Eng. J. 2025, 503, 158560. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, W.; Xiao, Z.; Yao, Q.; Xia, X.; Mei, J.; Zhang, D.; Chen, P.; Li, S.; Wang, Y.; et al. A High-Temperature Accelerometer with Excellent Performance Based on the Improved Graphene Aerogel. ACS Appl. Mater. Interfaces 2023, 15, 19337–19348. [Google Scholar] [CrossRef]
- Divyah, N.; Devasena, M. Enhanced Self-Healing of Micro-Cracks in Concrete Using Graphene Oxide Encapsulated Sodium Alginate Microcapsules: Microstructure, Elemental, and Oxide Composition Analysis. J. Build. Rehabil. 2024, 9, 70. [Google Scholar] [CrossRef]
- Obeid, E.; Younes, K. Uncovering Key Factors in Graphene Aerogel-Based Electrocatalysts for Sustainable Hydrogen Production: An Unsupervised Machine Learning Approach. Gels 2024, 10, 57. [Google Scholar] [CrossRef]
- Castaldo, R.; Avolio, R.; Cocca, M.; Errico, M.E.; Lavorgna, M.; Šalplachta, J.; Santillo, C.; Gentile, G. Hierarchically Porous Hydrogels and Aerogels Based on Reduced Graphene Oxide, Montmorillonite and Hyper-Crosslinked Resins for Water and Air Remediation. Chem. Eng. J. 2022, 430, 133162. [Google Scholar] [CrossRef]
- Ahmadi, H.; Jahanshahi, M.; Khoei, A.R.; Bordas, S. Mechanical Behavior of Multilayer Graphene Reinforced Epoxy Nano-Composites via a Hierarchical Multi-Scale Technique. Carbon Trends 2021, 4, 100048. [Google Scholar] [CrossRef]
- Han, N.M.; Wang, Z.; Shen, X.; Wu, Y.; Liu, X.; Zheng, Q.; Kim, T.-H.; Yang, J.; Kim, J.-K. Graphene Size-Dependent Multifunctional Properties of Unidirectional Graphene Aerogel/Epoxy Nanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 6580–6592. [Google Scholar] [CrossRef]
- Jin, R.; Zhou, Z.; Liu, J.; Shi, B.; Zhou, N.; Wang, X.; Jia, X.; Guo, D.; Xu, B. Aerogels for Thermal Protection and Their Application in Aerospace. Gels 2023, 9, 606. [Google Scholar] [CrossRef]
- Sambucci, M.; Savoni, F.; Valente, M. Aerogel Technology for Thermal Insulation of Cryogenic Tanks—Numerical Analysis for Comparison with Traditional Insulating Materials. Gels 2023, 9, 307. [Google Scholar] [CrossRef]
- Wu, W.; Du, M.; Shi, H.; Zheng, Q.; Bai, Z. Application of Graphene Aerogels in Oil Spill Recovery: A Review. Sci. Total. Environ. 2023, 856, 159107. [Google Scholar] [CrossRef]
- Berrio, M.E.; Oñate, A.; Salas, A.; Fernández, K.; Meléndrez, M.F. Synthesis and Applications of Graphene Oxide Aerogels in Bone Tissue Regeneration: A Review. Mater. Today Chem. 2021, 20, 100422. [Google Scholar] [CrossRef]
- Wei, D.; Liu, X.; Lv, S.; Liu, L.; Wu, L.; Li, Z.; Hou, Y. Fabrication, Structure, Performance, and Application of Graphene-Based Composite Aerogel. Materials 2022, 15, 299. [Google Scholar] [CrossRef]
- Hu, L.; He, R.; Lei, H.; Fang, D. Carbon Aerogel for Insulation Applications: A Review. Int. J. Thermophys. 2019, 40, 39. [Google Scholar] [CrossRef]
- Maleki, H. Recent Advances in Aerogels for Environmental Remediation Applications: A Review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Tetik, H.; Wang, Y.; Sun, X.; Cao, D.; Shah, N.; Zhu, H.; Qian, F.; Lin, D. Additive Manufacturing of 3D Aerogels and Porous Scaffolds: A Review. Adv. Funct. Mater. 2021, 31, 2103410. [Google Scholar] [CrossRef]
- Yeo, S.J.; Oh, M.J.; Yoo, P.J. Structurally Controlled Cellular Architectures for High-Performance Ultra-Lightweight Materials. Adv. Mater. 2019, 31, 1803670. [Google Scholar] [CrossRef]
- Patil, S.P.; Kulkarni, A.; Markert, B. Shockwave Response of Graphene Aerogels: An All-Atom Simulation Study. Comput. Mater. Sci. 2021, 189, 110252. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, H.; Duan, Y.; Xu, T.; Xie, H.; Du, H.; Si, C. Nanocellulose-Graphene Composites: Preparation and Applications in Flexible Electronics. Int. J. Biol. Macromol. 2023, 253, 126903. [Google Scholar] [CrossRef]
- Ijaz, U.; Siyar, M.; Park, C. The power of pores: Review on porous thermoelectric materials. RSC Sustain. 2024, 2, 852–870. [Google Scholar] [CrossRef]
- Khan, H.; Siyar, M.; Park, C.; Javaid, F.; Umer, M.A.; Jin, W.; Saleem, M.; Adnan, A. Tuning the electronic properties in facile in-situ solution synthesis of SnSe2/rGO nanocomposites with enhanced thermoelectric performance. J. Mater. Res. 2023, 38, 3913–3922. [Google Scholar] [CrossRef]
- Siyar, M.; Maqsood, A.; Khan, S.B. Synthesis of mono layer graphene oxide from sonicated graphite flakes and their Hall effect measurements. Mater. Sci. Pol. 2014, 32, 292–296. [Google Scholar] [CrossRef]


| Composite Material | Properties | Applications | Refs. |
|---|---|---|---|
| Graphene oxide/Polymer aerogel | High porosity, lightweight, improved mechanical strength | Thermal insulation, energy storage | [20] |
| Graphene oxide/SnO2 | Superior anode performance, high specific capacity | Lithium-ion batteries | [21] |
| Reduced graphene oxide/Fe2O3 | Enhanced supercapacitive performance, high porosity | Supercapacitors | [22] |
| Copper nanowires/Graphene aerogel | Improved thermal conductivity, EMI shielding | Electromagnetic interference shielding | [23] |
| MoSe2-Cu1.82Se@GA | High capacity, excellent cycle stability | Sodium-ion batteries | [24] |
| Graphene oxide/Polymer composite | High mechanical strength, flexibility, low density | Adsorption, separation, sensors | [25] |
| Process Sequence | Description | Porosity | Mechanical Properties | Advantages | Challenges | Refs. |
|---|---|---|---|---|---|---|
| 1. GO Dispersion → Hydrothermal Treatment → Freeze-Drying | GO is dispersed in water, heated in an autoclave to induce gelation, then freeze-dried to form an aerogel. | High porosity (80–99%) | Moderate compressive strength, ~0.2–0.8 MPa | Simple method, preserves 3D structure | Long processing time, high energy requirement | [102] |
| 2. GO Dispersion → Chemical Reduction → Freeze-Drying | GO dispersion is chemically reduced (e.g., with hydrazine), then freeze-dried to form an aerogel. | High porosity (80–98%) | Improved strength, ~1–5 MPa | Good reduction of GO, lightweight aerogel | Toxic reductants (e.g., hydrazine), requires freeze-drying | [103] |
| 3. GO Dispersion → Self-Assembly → Supercritical Drying | GO forms a gel via self-assembly, which is then supercritically dried to form an aerogel. | Very high porosity (>95%) | Moderate mechanical strength, ~0.1–0.5 MPa | Preserves high porosity and surface area | Requires expensive supercritical drying equipment | [104] |
| 4. GO Dispersion → Freeze-Casting → Freeze-Drying | GO solution is directionally frozen, followed by sublimation and reduction to form an anisotropic aerogel. | Anisotropic porosity (~99%) | Low compressive strength (up to ~0.02 MPa) | Tailored anisotropic pore structure, Low mechanical properties | Requires control over freezing conditions | [105] |
| 7. GO Dispersion → Electrostatic Layer-by-Layer Assembly → Drying | GO is assembled layer-by-layer using electrostatic interactions, then dried to form an aerogel. | High porosity (~90–95%) | Tunable mechanical properties (~0.2–0.4 MPa) | Controlled structure, layer thickness | Time-consuming, not highly scalable | [106] |
| 8. GO Dispersion → Crosslinking (Polymer) → Freeze-Drying | GO is crosslinked using a polymer, followed by freeze-drying to form an aerogel. | Moderate porosity (~75–90%) | Enhanced mechanical properties (~0.02–0.2 MPa) | Tailored mechanical properties | Requires additional crosslinking agent | [72] |
| 9. GO Dispersion → 3D Printing → Drying (Freeze/Supercritical) | Direct ink writing or extrusion of GO/polymer inks into designed lattices, followed by drying and reduction. | Customizable; ~75–95% | High flexibility, fatigue-resistant, shape recovery >80% | Precise architecture, scalable, multifunctional | Requires rheological tuning, limited resolution | [57,58] |
| Mechanical Property | Example (Aerogel Type) | Typical Range/Value | References |
|---|---|---|---|
| Compressive Strength | Hydrothermally reduced GO aerogel | 0.5–1 MPa | [107,108,109] |
| Elastic Modulus | Chemically crosslinked GO aerogel | 10–100 kPa | [72,110,111,112] |
| Toughness | Polymer-GO composite aerogel | 50–200 J/m3 | [113,114,115] |
| Flexibility and Recoverability | Freeze-casted/directionally frozen GO aerogel | >80% shape recovery | [116,117,118,119] |
| Fatigue Resistance | 3D-printed GO aerogel | >1000 cycles with minimal degradation | [120,121,122,123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakchaure, M.B.; Menezes, P.L. Graphene Oxide Aerogels: From Synthesis Pathways to Mechanical Performance and Applications. Processes 2025, 13, 2375. https://doi.org/10.3390/pr13082375
Wakchaure MB, Menezes PL. Graphene Oxide Aerogels: From Synthesis Pathways to Mechanical Performance and Applications. Processes. 2025; 13(8):2375. https://doi.org/10.3390/pr13082375
Chicago/Turabian StyleWakchaure, Mayur B., and Pradeep L. Menezes. 2025. "Graphene Oxide Aerogels: From Synthesis Pathways to Mechanical Performance and Applications" Processes 13, no. 8: 2375. https://doi.org/10.3390/pr13082375
APA StyleWakchaure, M. B., & Menezes, P. L. (2025). Graphene Oxide Aerogels: From Synthesis Pathways to Mechanical Performance and Applications. Processes, 13(8), 2375. https://doi.org/10.3390/pr13082375








