1. Introduction
Nearly one-third of the food harvested is wasted due to improper post-harvest handling [
1]. However, the demands on infrastructure, energy, and resource management at every stage, from harvesting to waste management, place a considerable burden on farmers, producers, businesses, and governments. Although drying is a major and widely used method of food preservation worldwide [
2], low energy drying technology based on fossil fuels is the most suitable method for developing countries because they are rich in renewable energy sources [
3] and the application of these sustainable energy sources (solar, geothermal, biomass) in different drying technologies requires significant scientific studies [
4].
Papaya is one of the major fruit crops in the tropics and subtropics. The majority of production is held by Central and South American countries (mainly Brazil)—47%; Asia—30%; and Africa—20%. The total annual world papaya production in 2023 was 14,917,903.5 tons, of which Mexico accounts for 7.7% of this production, or 1,148,545.6 tons [
5]. Papaya fruits exhibit diverse shapes, ranging from melon-like and oval to nearly round, pear-shaped, or oblong-clubbed, with weights reaching up to 9 kg. However, smaller fruits, measuring, are characteristic of semi-wild (purebred) plants. Unripe papayas contain significant amounts of white resin, and their skin presents a rugged, green appearance. The skin transitions to a deep orange-yellow hue as the fruit matures and ripens. Concurrently, the dense, juicy flesh transforms color, shifting from green to a vibrant orange-yellow or various shades of salmon or red [
6]. Because of these special properties of papaya, it is important to preserve them during processing, in this case by solar drying. Several studies have been conducted to find the best conditions for the drying process. Abrol et al. (2014) studied the tunnel drying method under sunlight to evaluate the effect of drying on the physicochemical and antioxidant activities of papaya dried at 60 °C for 6 h. They found that antioxidant compounds, total phenolic content, and total carotenoid content increased, while heat-sensitive vitamin C decreased [
7]. Minuye et al. (2021) studied dried papaya using different drying methods such as oven drying, sunny herb greenhouse, outdoor drying, sunny tray, refractive window, and freeze drying and found that refractive window and sunny greenhouse produced higher quality dried papaya products than those dried in oven, outdoors, and on sunny tray [
8]. Irondi et al. (2013) studied the effects of drying methods such as air drying, freeze drying, oven drying, and sun drying on the phytochemical composition and antioxidant activity of papaya seeds (
Carica papaya L.), a medicinal plant [
9].
Due to uncontrolled solar irradiance and uneven drying conditions, traditional solar drying systems often fail to preserve the color and nutritional quality of photosensitive crops like papaya. These limitations result in significant color degradation, nutrient loss, and reduced market value.
Solar drying is the use of solar radiation to dry materials. However, humans can only see a small portion (less than 50%) of the solar spectrum. This radiation, invisible to us, contributes significantly to the heating of objects. Therefore, controlling the radiation reaching the material being dried is very important for temperature regulation. This control is achieved by exploiting the optical properties of various crystalline materials.
Windows are a significant source of energy loss and gain in buildings. Solar heat gain is estimated to be 37% in terms of cooling energy consumption, while energy lost through windows is 40%. Passive houses can meet heating needs during the cold months by using solar heat. Advanced solar panel control equipment is required to reduce energy loss and improve indoor air quality. Shades, curtains, and similar structures restrict daylight and open space because they have fixed optical properties [
10].
To meet the needs and development of these technologies, it is necessary to develop thin films that can cover windows but at the same time ensure visibility to the outside, the penetration of natural light (visible spectrum), and at the same time provide thermal insulation. These metal sheets must be completely transparent, and to ensure thermal insulation, it is necessary to use filters that only allow certain wavelengths to pass through, reflect others, and absorb others [
11]. During the development of these technologies, properties and characteristics were studied to compare conventional windows in different environments. These technologies include reflective glass, Low-E glass, and various coatings on conventional glass. When solar radiation strikes glass, it is transmitted, reflected, and absorbed by the glass depending on the optical properties of the glass.
Liquid crystal (LC) and polymer-dispersed (DP) layers, known as PDLC layers, have been the subject of interest to many research groups since studies demonstrating their mechanical, optical, and magnetic properties were recently published. The most common configuration for these films is that of LC droplets uniformly dispersed in a matrix consisting of a transparent and optically active polymer [
12]. The refractive index of this matrix varies spatially and is characterized by very efficient light scattering properties. These films can be produced at low cost, using simple methods, and can be used successfully in exhibitions and have many advantages over other types of films [
13]. PDLC liquid crystals are membranes of matrix materials based on liquid crystal materials, epoxy, and standard materials. PDLC membranes are useful as photovoltaic devices because they can be converted from the case of lighting (Off) to a transparent condition (liter). The polymer matrix is an optical measurement material with NP. On the other hand, the liquid crystal material in Sissue Micro is not equipped with the visual direction, and the traditional and unusual refractive indexes characterize the inequality. When the pdlc membrane is extinguished, the liquid crystal is unevenly distributed by the polymer.
Therefore, a series of refractive index values between NO and NE (La ≠ Ne) was found, as these clues differ from the NP with the polymer index, and the access light is spread by microphils. The effect of converting this device extends across the entire spectrum of sunlight. When turning off, the device looks white. Since the PDLC membrane is disabled in the spread method, it is distributed evenly. When applying an electric field, liquid crystals are aligned in the field, making the device transparent.
The control of emissivity by controlling the voltage of PDLC films has been studied previously; however, it has only been applied in the construction industry. Mangthong et al. (2020) [
11] studied the comparison of the light transmittance of commercial glass and PDLC-coated laminated glass used for thermal insulation in buildings and investigated their application with different power sources and found that commercial glass had higher light transmittance than PDLC-coated laminated glass film. PDLC film and permeability increased with higher voltage [
11]. Ghosh and Mallick (2018) also studied this voltage-dependent change in transmittance and found that there was modulation of transmittance in the visible and near-infrared (NIR) ranges at different applied voltages, as well as a decrease in transmittance in the UV range and a low contrast ratio between the semi-transparent and transparent states [
14].
While our previous work established the thermal performance and optical characteristics of the PDLC-controlled solar dryer [
15], the current study focuses specifically on the spectral-dependent quality parameters of dried papaya. The earlier publication demonstrated the technical feasibility of irradiance control but did not investigate the phytochemical preservation or detailed color degradation mechanisms reported here.
To address these challenges, this study introduces a novel Solar Dryer with Dynamic Irradiance Control (SDIC), which utilizes a PDLC (Polymer Dispersed Liquid Crystal) film to regulate solar irradiance dynamically. This innovative approach ensures consistent drying conditions, minimizing quality degradation in dried papaya. While previous studies have explored various drying methods and our group has demonstrated PDLC film applications in solar drying systems, none have investigated the spectral-specific effects on phytochemical preservation and color degradation mechanisms in photosensitive crops. This study aims to fill this gap by evaluating the impact of controlled irradiance on the drying kinetics and color preservation of papaya slices. This study aims to determine the impact of solar irradiance variations (both natural and simulated) on the color development of papaya during solar drying. Specifically, papaya was solar dried at 50 °C under three conditions: full solar irradiance, 700 W/m2 of solar irradiance, and 700 W/m2 of simulated solar irradiation. This research seeks to optimize drying conditions and ultimately improve the quality of the dried papaya product.
2. Materials and Methods
2.1. Raw Material
Papaya (Carica papaya L.) fruits were purchased fresh at an early ripening stage from a local market and stored at room temperature (25–30 °C) in Guadalupe, Zacatecas, Mexico, from May to June 2024.
When the fruits reached the desired maturity, they were peeled and cut into equal slices (average thickness: 5 mm), then 300 g of papaya were placed in 4 trays (75 g per tray) in each dryer and distributed between both solar dryers. For SSD, due to its capacity, 30 g of papaya samples were placed on the tray. The initial moisture content of papaya fruit was ~4.27 g H2O/g dry matter. with color parameters L*: 44.4; a*: 22.69; and b*: 20.05.
2.2. Solar Dryer Description
The drying kinetics and the effect of operational conditions on the color of papaya slices were investigated using three different dryers: a solar dryer with irradiance control (SDIC), a cylindrical solar dryer (CSD), and a solar simulator dryer (SSD).
2.2.1. Solar Dryers
The solar dryer with dynamic irradiance control (SDIC) and the cylindrical solar dryer (CSD) are two identical cylindrical solar dryers; the SDIC is equipped with a PDLC (Polymer Dispersed Liquid Crystal) coating that allows flexible control of solar radiation (
Figure 1). The SDIC system incorporates a PDLC film, which adjusts its transmittance based on input voltage, allowing dynamic control of solar irradiance. This feature enables the system to maintain consistent drying conditions and prevent overheating. The transparency of the PDLC film was adjusted to optimize the drying process. Dynamic control increases drying efficiency and product quality by preventing overheating and excessive moisture loss. Both dryers are 80 cm long and 45 cm in diameter, made of 0.6 cm thick acrylic, and equipped with a microcontroller-based control system to monitor various parameters, including weight loss, temperature, humidity, and solar radiation. Another dryer, the cylindrical solar dryer (CSD) without PDLC cover, was used as a reference. A drying temperature of 50 °C was selected to balance drying efficiency and product quality, as higher temperatures can cause thermal degradation of sensitive compounds like carotenoids. An irradiance level of 700 W/m
2 was chosen to simulate typical solar conditions while ensuring controlled drying.
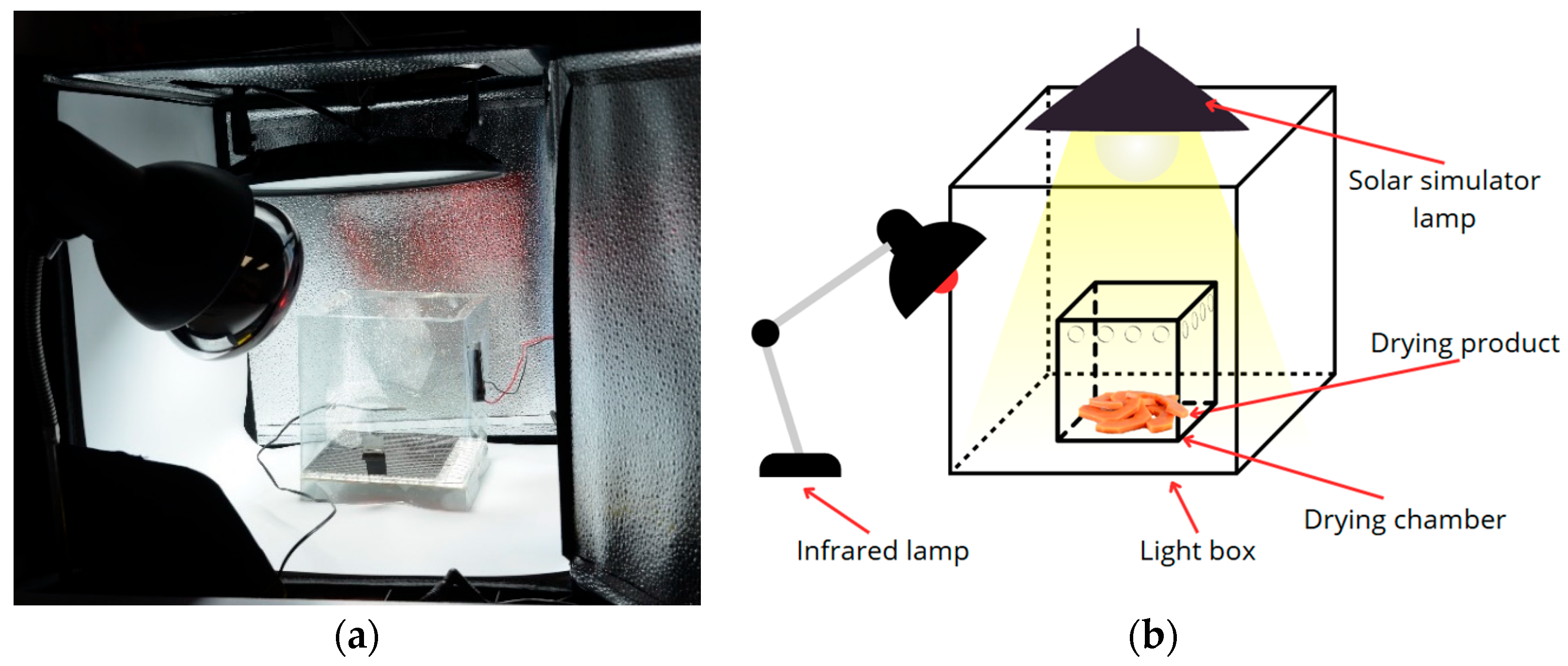
2.2.2. Solar Simulator Dryer
To evaluate the effect of controlled solar radiation, it was necessary to establish a prototype solar dryer with a lamp that simulates the spectrum of solar radiation to form a drying chamber with a solar simulator. The elements of this chamber can be seen in
Figure 2; the experimental setup was composed of a 200 W LED lamp at a height of 2.5 m. The lamp was positioned inside a lightbox (0.5 × 0.5 × 0.5 m) covered with a white surface and reflective areas to increase the power to simulate the sun irradiance. An infrared spotlight of 250 W supports it because the LED lamp mainly provides light within the visible spectrum, and an electronic control system maintains the drying cabin temperature at 50 °C. The drying cabin was a transparent box measuring 0.15 × 0.15 × 0.15 m with a fan of 0.05 × 0.05 m serving as an air extractor, where 25 g of papaya fruit slices were placed to dry.
2.3. Light Spectrum Measuring
To assess the optical characteristics, particularly wavelength distribution, within the solar dryers, an Ocean Optics spectrometer (USB-2000, VIS-NIR 350–1000 nm, 0.5 nm resolution) equipped with an integrating sphere (FOIS-1) was utilized. This sphere enhanced the collection of solar radiation by increasing the effective angle of incidence. The spectrometer measured solar radiation within the drying chambers of both the CSD and SDIC dryers. Optical measurements were conducted under a standard irradiance of 700 W/m2 for the SDIC, within the CSD, and under ambient solar conditions.
When solar radiation interacts with a PDLC film, it undergoes various optical phenomena, including scattering, diffraction, absorption, and transmission. The extent of these processes is influenced by the spectral composition of the light and the film’s inherent selectivity [
16]. The incident radiant intensity exhibits an inverse relationship with the enhanced intensity, which represents the power per unit area impinging on the surface. Moreover, the spectral gain power (
F) is wavelength-dependent, indicating the power density at specific wavelengths [
17]:
where
represents the spectral gain power per unit area incident on a surface,
is the fluence,
is the potency that is inversely related to a wavelength interval
.
2.4. Moisture Ratio
The following experimental procedure was employed to investigate drying kinetics under varying conditions for the three drying methods. Papaya slices, each approximately 75 g, with dimensions of 7 cm in length and 0.5 cm in thickness, were prepared. An amount of 0.6 kg of these slices was evenly distributed across four trays within the SDIC and CSD dryers. Throughout the drying process, the weight of the samples was monitored at 20 min intervals. The drying process was considered complete when the weight variation within a 20 min period did not exceed 0.2 g, indicating the attainment of equilibrium moisture content under the designated drying conditions. The moisture content coefficient was determined using the following equation [
18]:
In this equation, represents the moisture content at any given time during the drying process, signifies the equilibrium moisture content reached under the specific drying conditions, and denotes the initial moisture content of the sample.
2.5. Drying Rate
The drying rate represents the rate at which moisture is extracted or eliminated from the product at a specific moment. This parameter is defined as follows [
19]:
where
represents the drying rate at a given time
, and
is the moisture content.
2.6. Color Degradation
The color parameters of dried papaya were measured every 20 min during the drying process using a CR30 colorimeter, Hangzhou CHNSpec Technology Co., Ltd., Hangzhou, China. The parameters were calibrated in the CIELAB color space, and their parameters were expressed as L* (lightness–darkness), a* (red–green), and b* (yellow–blue). Likewise, to visually capture the color changes that occur during drying, photographs were taken at the same brightness and camera distance to maintain the most faithful color reproduction possible.
2.7. Preparation Method to Determine Antioxidant Capacity
The ultrasound-assisted extraction (UAE) method was used, for which a solution of methanol (J.T. Baker, Radnor, PA, USA), water, and formic acid (Sigma-Aldrich, St. Louis, MO, USA) (80:18:2 ratio) was prepared. Then, 3 g of material was added to 30 mL of the solution. The mixture was stirred for 30 s and subjected to ultrasound in five one-minute cycles at 60 Hz, maintaining a temperature of 4 °C between cycles. The supernatant was recovered via filtration and stored at room temperature [
20].
2.8. Antioxidant Capacity Analysis
For the ABTS Test, according to Patrón-Vázquez [
21], a solution of ABTS at 2 Mm was prepared, and potassium persulfate, 70 mM, was used to activate the radical. ABTS solution was adjusted to an absorbance of 0.700 ± 0.02 (λ = 734 nm), diluting the solution with phosphate buffer (pH = 7), then 10 µL of the papaya residue extract was added to 990 µL of the ABTS solution, and the absorbance was measured at room temperature after 6 min of incubation. All analyses were carried out in triplicate. We used Trolox to make a standard, and the results are expressed as Trolox equivalent (µmol Eq Trolox/g d.w.).
The antioxidant capacity obtained from the DPPH assay was measured according to the methodology by Brand-Williams et al. [
22] and Chew et al. [
23]. A solution of 1 mM DPPH in methanol was stirred. The absorbance of the solution was adjusted to 1 (<0.010) at 480 nm. Then, 20 μL of extract was placed on an ELISA microplate, followed by 280 μL of DPPH solution (Sigma-Aldrich, St. Louis, MO, USA), and incubated for 30 min in the dark, covered with aluminum foil. Then, decreasing absorbance was monitored at 480 nm. A calibration curve was plotted using standard solutions of Trolox–methanol with concentrations in the range of 0 to 0.0008 μM, and the results are expressed as Trolox equivalent (µmol Eq Trolox/g d.w.).
2.9. Phytochemical Characterization
Total phenolic content was determined using the Folin–Ciocalteu method [
24]. We mixed 50 μL samples with 250 μL of diluted Folin–Ciocalteu reagent (1:10 in water), allowed a 2 min reaction at room temperature, then added 200 μL sodium carbonate solution (75 mg/L). After two hours of dark incubation, we measured absorbance at 765 nm and quantified phenolics against a gallic acid standard curve, reporting results as mg gallic acid equivalents per mL [
25].
For flavonoid analysis, we sequentially reacted 100 μL samples with sodium nitrite (5%), aluminum chloride (10%), and sodium hydroxide (1 N), with specific incubation times between each step. Following dilution, we measured absorbance at 510 nm and calculated concentrations using a catechin standard curve (0–0.6 mg/mL) [
26].
Tannin content was assessed following Amarowicz and Pegg (2008) [
27] using a vanillin-HCl method. We reacted 50 μL samples with 250 μL of vanillin–HCl reagent (1:1 mixture of 8% HCl and 1% vanillin), then measured the developed color at 492 nm against a catechin standard curve (0.1–0.8 mg/mL). All spectrophotometric measurements were performed in triplicate using a Thermo Scientific microplate reader [
27].
2.10. Statistical Correlation
The Pearson correlation coefficient (r) is a statistical measure that quantifies the linear relationship between two continuous variables. It ranges from −1 to +1, where +1 is a perfect positive linear correlation (as one variable increases, the other increases proportionally), −1 is a perfect negative linear correlation (as one variable increases, the other decreases proportionally), and 0 is a no linear correlation (variables are independent). The analysis is measured with the following equation [
28]:
where
and
are data points, and
and
are means of X and Y.
3. Results and Discussion
3.1. Moisture Ratio
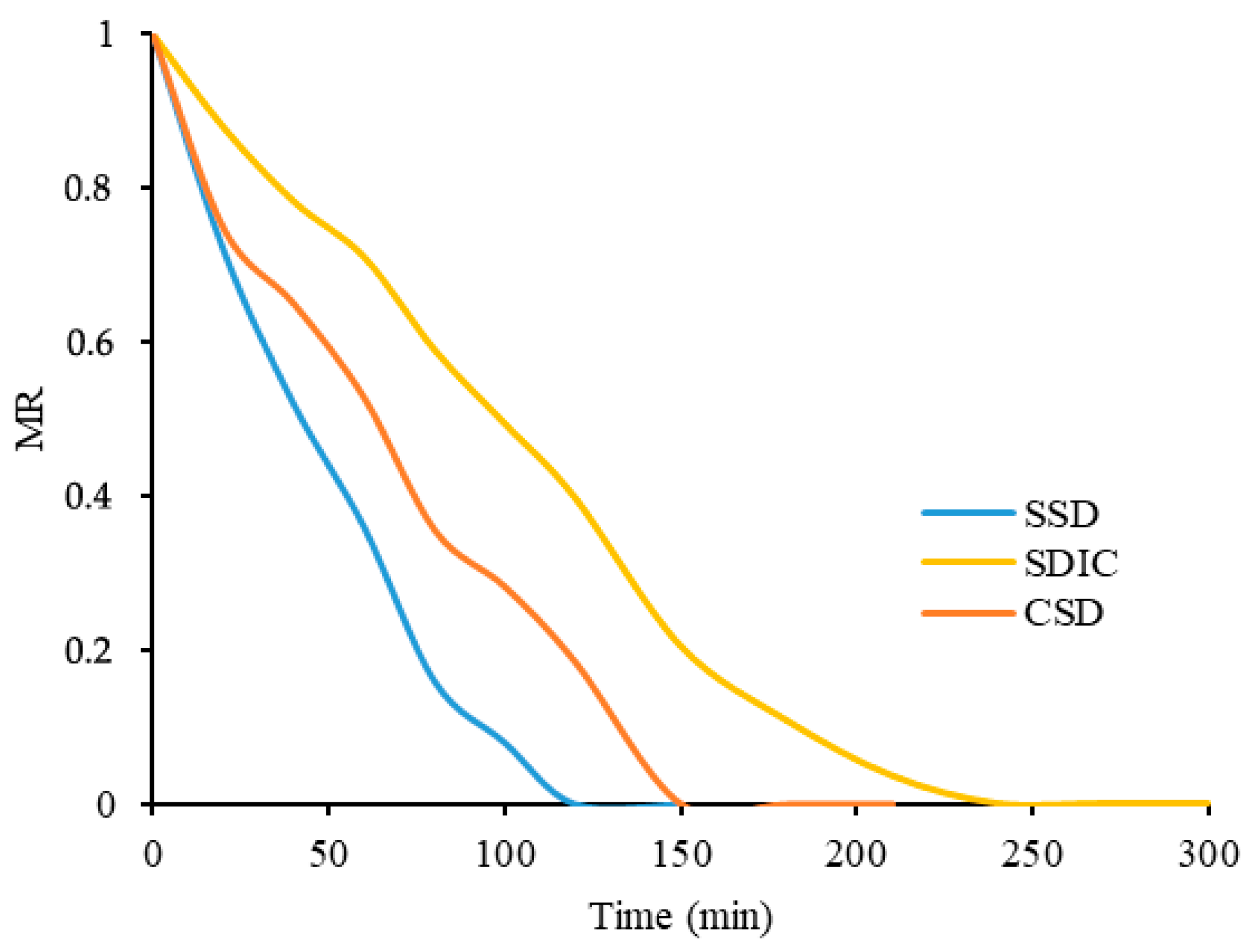
As shown in
Figure 3, the drying kinetics of papaya slices showed three different moisture content ratios (MR) behaviors corresponding to different drying methods: CSD, SDIC, and SSD. The initial moisture contents were 4.6, 4.63, and 6.67 g H
2O/g d.m. for SDIC, CSD, and SSD, respectively. For CSD, the moisture content decreased rapidly during the initial drying period, indicating that more moisture was lost with a drying time of 210 min. This may be due to the broad spectrum of sunlight, which increases the heat absorption and accelerates water evaporation. Over time, the slope of MR gradually decreases, indicating a decrease in moisture content and a decrease in the moisture removal rate due to water diffusing through the solid and water having difficulty reaching the surface for removal. In the SDIC case, the initial moisture content was at the same level as in the CSD case, but initially decreased slightly slower than in the CSD case, and the drying time was 300 min. However, the MR decreased gradually over time, although more slowly than in the CSD case. The limiting intensity of 700 W/m
2 can contribute to a moderate drying rate.
SSD requires 180 min to simulate solar radiation at 700 W/m
2. The short drying time is due to the low dry matter content; that is, the same drying temperature and the same irradiation power at low dry matter content lead to faster drying. This confirms the influence of solar radiation on the drying process. Compared with previous studies such as Hawlader et al. (2006) and Garcia et al. (2014), who dried papaya at 50, 60, and 70 °C by convection drying for 490 [
29], 390, and 300 min [
30], respectively. This study showed that radiation can significantly reduce the drying time. Although the drying temperature is equivalent, the solar or simulated solar drying method always gives shorter drying times.
3.2. Drying Rate
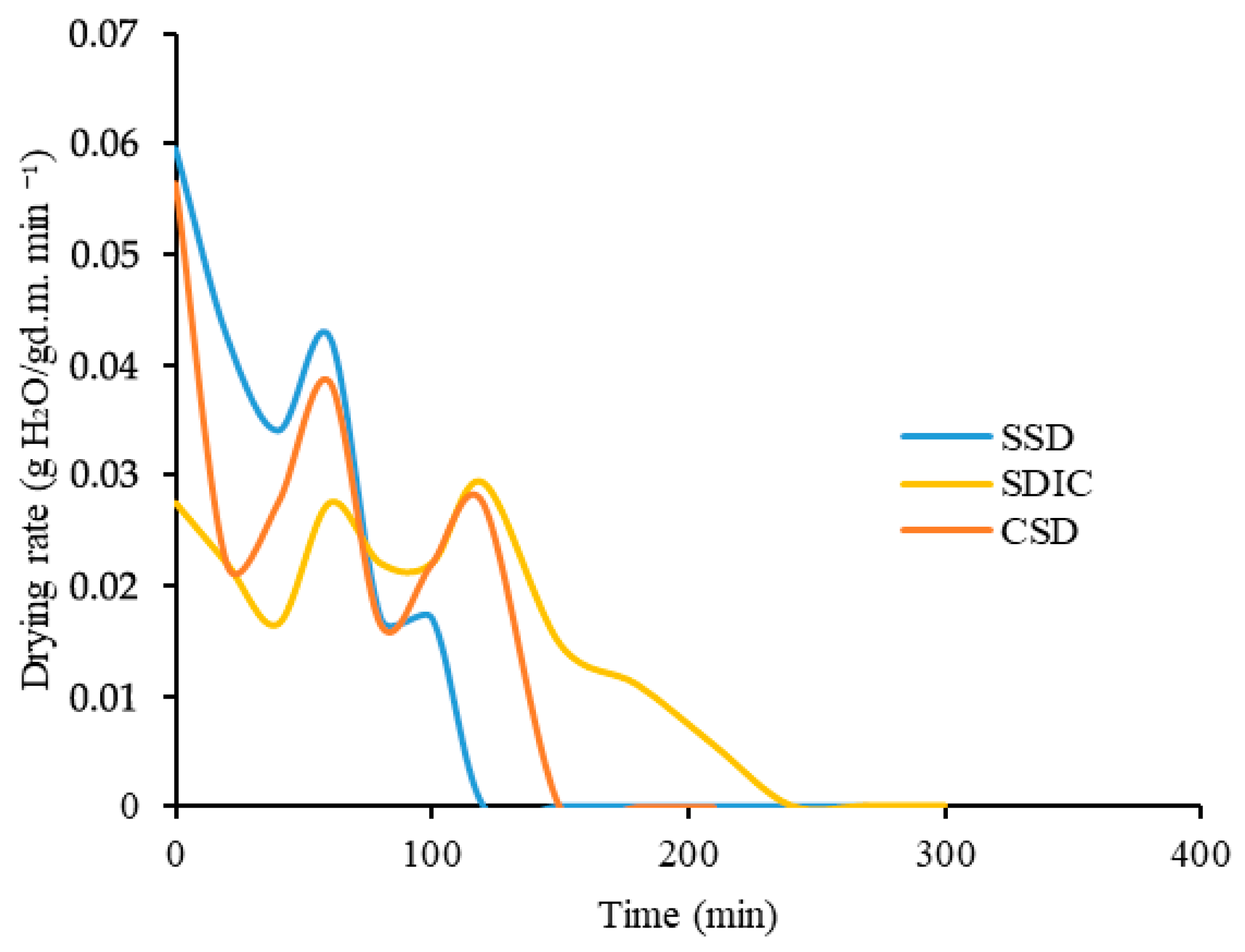
Regarding drying rate,
Figure 4 provides valuable information on the moisture removal efficiency of three different drying methods: CSD, SDIC, and SSD. In the case of CSD, the initial drying rate is the highest (0.056 g H
2O/g dry weight min
−1), reaching a peak value at the beginning of the drying process. At the beginning of the drying process, there is a large amount of available moisture or free water that can be easily removed, thus the drying rate is faster. Over time, the moisture content decreases, and the moisture in the food is bound, making the drying process difficult to remove due to difficulty moving and adhering to the food components or structure. In the SDIC dryer, the drying of papaya showed a drying rate of 0.027 g H
2O/g d.m. min
−1, which started slightly lower than the CSD. The initial drying rate was still significant, but not as pronounced as in the CSD case. However, although the drying rate of SSD is 0.06 g H
2O/g d.m. min
−1, it is not comparable because the drying matter is only 30 g due to the dryer size, so the drying rate is not comparable to SDIC and CSD. Over time, the drying rate stabilized at a lower level than that of CSD, reflecting the continuous evaporation of moisture. After 80 min of drying, the moisture contents of the SSD, SDIC, and CSD samples were 0.70 g H
2O/g d.m., 2.73 g H
2O/g d.m., and 1.74 g H
2O/g d.m., respectively. The drying rates of these samples were 0.017 g H
2O/g d.m. min
−1, 0.021 g H
2O/g d.m. min
−1, and 0.016 g H
2O/g d.m. min
−1, respectively. Although the drying rates were relatively similar for all samples, the moisture contents varied significantly, with a difference of approximately 1 g H
2O/g d.m. between them. The average drying rates were 0.017, 0.016, and 0.036 g H
2O/g d.m. min
−1 for CSD, SDIC, and SSD, respectively. Hawlader et al. (2006) found a higher point of drying rate, where they obtained 0.027 g H
2O/g d.m. min
−1 in convective drying at 50 °C [
29]. However, it should be noted that a constant drying rate or no thermal jumps can prevent the build-up of thermal stress in the product, which can affect the product’s structure and disrupt proper drying [
31].
3.3. Color Change
The following table (
Table 1) provides insights into the color changes induced by different drying methods. For the color shift of the SDIC dryer, there was an increase of 13 units in the a* coordinate (red color), an increase of 5 units in the b* (yellow color), and a decrease of 3 units in the L* coordinate (luminosity). This means that the papaya fruit slices did not get dark. For the CSD dryer, there was an increase of 6 units in the a* coordinate and 12 units in the b* coordinate, which shows a loss of its characteristic orange color. The color degradation presented an increase of 16 units in the a* coordinate for SSD, an increase of 4 units in the b* coordinate, and a decrease of 6 units in the L* coordinate (
Figure 5). This demonstrates the direct impact of solar irradiation on color in solar drying at the same temperature. These findings highlight the importance of optimizing drying conditions to minimize color deterioration and maintain the visual appeal of the dried product. Yi et al. (2017) studied the impact of papaya chips in convective drying at 65 °C, which showed a lower color difference than combined hot air drying and explosion puffing drying, combined freeze drying and explosion puffing drying methods [
32]. The results shown in color are strongly related to the irradiation spectrum, as Garcia et al. (2014) demonstrated in their study, where they dried under the convective method, and the samples did not change significantly after drying at either 60 or 70 °C [
30].
3.4. Light Spectrum
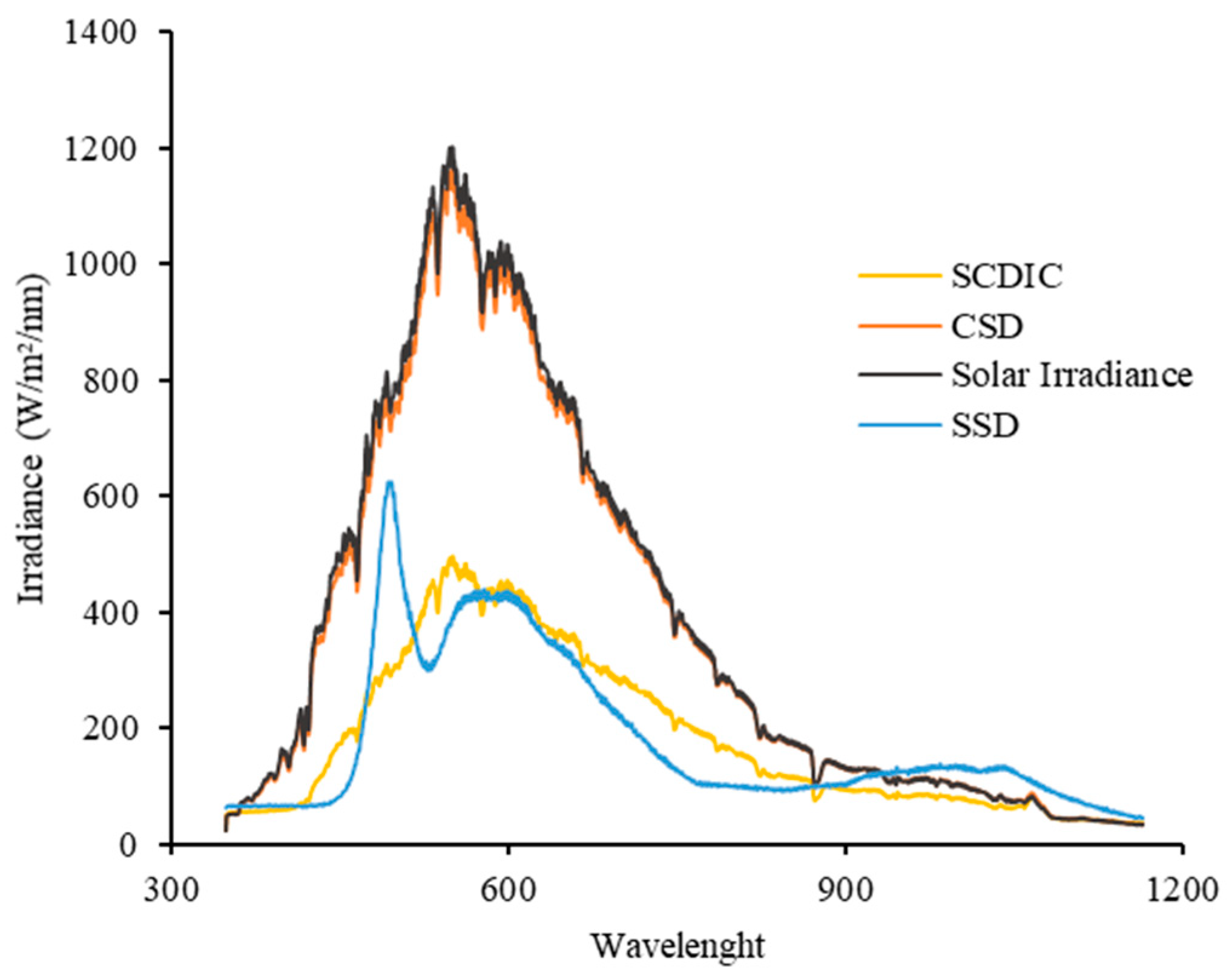
Figure 6 shows the radiation spectra to which the papaya slices were exposed during the different drying methods: CSD, SDIC, and SSD. These spectra and the colorimetric data obtained establish a direct relationship between the quality and quantity of incident radiation and the color changes observed in the final product. Visible light, ranging from 380 nm (violet) to 780 nm (red), interacts with matter differently, depending on its wavelength. Food pigments absorb specific wavelengths and reflect others, which determines the color we perceive.
When analyzing radiation spectra, the following trends can be observed: UV radiation (300–400 nm) is highly energetic and can cause degradation of pigments, especially carotenoids, which are responsible for the orange color of papaya. Visible radiation (400–700 nm) can induce photochemical reactions that alter the structure of pigments, leading to color changes, and infrared radiation (<700 nm), although not visible, contributes thermal energy to the product, which can accelerate chemical and biochemical reactions and increase the drying rate.
The results obtained show that exposure to different radiation spectra produces different degrees of color degradation in the dried papaya. For the SDIC dryer, the radiation spectrum presents a lower intensity in the UV and visible region compared to the other methods. This results in less color degradation, with more moderate increases in the a* and b* coordinates. The cylindrical solar dryer (CSD) exhibited the shortest drying time but caused the most significant color degradation. This can be attributed to the full solar spectrum, including high UV and visible radiation, which accelerates pigment degradation in papaya. In contrast, the SDIC system, with its controlled irradiance, minimized color changes by reducing exposure to harmful wavelengths. This explains the higher color degradation observed in the solar drying process, with significant increases in the a* and b* coordinates. Finally, for SSD, the radiation spectrum in the laboratory is different from that of the other methods, suggesting that the light sources used in the laboratory have a different spectral composition. This may explain the variations in color degradation observed in this method.
3.5. Antioxidant Capacity Analysis
The antioxidant profile revealed complex relationships between drying methods and phytochemical preservation. SSD samples showed 35% higher ABTS values (1432.91 ± 43.24 µmol TE/g dw), suggesting that controlled laboratory conditions may optimally preserve certain antioxidants (
Table 2). This contrasts with field conditions where environmental fluctuations occur. SDIC demonstrated intermediate antioxidant levels (1084.09 ABTS, 97.28 DPPH), significantly higher than CSD (924.81 ABTS, 96.46 DPPH), supporting the protective effect of irradiance control. The similar DPPH values across methods (94.74–97.28) suggest this assay may be less sensitive to drying-induced changes than ABTS, possibly because DPPH primarily measures hydrogen-donating antioxidants while ABTS detects both hydrophilic and lipophilic compounds.
3.6. Phytochemical Characterization
Phytochemical analysis revealed complex interactions between drying methods and compound classes (
Table 3). Total phenols showed an inverse relationship with drying control, with SSD (58.92 mg GAE/100 g) > CSD (49.15) > SDIC (39.53). This unexpected pattern may result from enhanced extractability where more intense drying breaks cell walls, releasing bound phenolics, a phenomenon observed by Abrol et al. [
7].
Tannins showed the most dramatic variation (1.43–2.83 mg/mL), suggesting harsher conditions promote phenolic polymerization. Flavonoids were most stable (0.008–0.016 mg/mL), possibly due to their thermal stability as reported by Amarowicz and Pegg [
27]. SDIC’s spectral filtering appears to protect carotenoid-related compounds while allowing moderate phenolic retention selectively, offering a compromise between visual and nutritional quality.
3.7. Correlation Analysis of Studied Parameters
A Pearson correlation analysis was performed on the measured parameters to elucidate further the relationships between drying conditions, color preservation, and phytochemical content (
Table 4).
A strong negative correlation was observed between drying time and ΔE (total color change) (r = −0.89, p < 0.05), indicating that shorter drying times (CSD) led to greater color degradation. This aligns with the higher irradiance exposure in CSD, which accelerated pigment oxidation.
The a* (redness) and b* (yellowness) coordinates correlated positively with UV irradiance (r = 0.82 and 0.76, respectively), confirming the role of UV light in carotenoid degradation.
For the antioxidant activity and spectral properties, ABTS antioxidant capacity showed a moderate negative correlation with UV irradiance (r = −0.65), suggesting that UV exposure reduced certain antioxidants. However, total phenols unexpectedly correlated positively with drying rate (r = 0.71), likely due to enhanced extractability from cell wall breakdown under faster drying.
Flavonoids exhibited weak correlations with all parameters, supporting their thermal stability. In contrast, tannins were strongly linked to irradiance intensity (r = 0.85), implying polymerization under harsher conditions.
3.8. Discussion
Color is one of the most important qualities of food and is closely related to the nutritional properties of the product, thus determining the final price in the market. The color of natural products comes from their natural compounds, such as carotenoids, anthocyanins, betalains, and chlorophyll. However, these compounds are sensitive to heat and generally suffer great reductions during the drying process, leading to a serious degradation of color in the product. The orange color, characteristic of papaya, is provided by carotenes that are part of antioxidant compounds; then, when color deterioration is presented, it is because the product presents an antioxidant degradation [
33]. In this work, the compounds’ content degraded more in CSD than in SDIC and SSD, considering the same drying temperature process; the principal factor for degradation was the radiation. Yang et al. [
33] studied the evolution of color changes in red pepper during hot air drying, performed under three temperatures: 60, 70, and 80 °C. The results indicate that the drying time did not impact the compounds’ degradation, with 5 h at 80 °C and 10 h at 60 °C. However, the antioxidant compounds were higher at 60 °C. Nevertheless, when the temperature factor is controlled, as it was in the present work, the only factor to consider is the radiation spectra.
The spectral measurements (
Figure 6) provide mechanistic explanations for the observed quality differences. CSD’s full spectrum, particularly its UV component (300–400 nm), correlates with the greatest color degradation, consistent with carotenoid photobleaching mechanisms. SDIC’s attenuated UV/visible transmission appears to protect chromophores while maintaining adequate drying efficiency. The SSD’s artificial spectrum, though matching total irradiance, differs in spectral composition, which may explain its superior phenolic preservation but poorer color outcomes.
The phytochemical results suggest competing degradation mechanisms: while controlled irradiance (SDIC) protects against photo-oxidation, moderate thermal stress may improve extractability of some compounds. This trade-off requires optimization based on target product characteristics, prioritizing visual appeal (SDIC) or maximum phytochemical retention (SSD).
4. Conclusions
The results of this study reveal that dynamic control of solar radiation during drying can make the difference between a quality product and one that loses its characteristic properties. Comparing the three drying methods, we found that each has its advantages and limitations. The solar dryer with dynamic irradiance control (SDIC), utilizing PDLC film technology, effectively balanced these competing demands. Compared to cylindrical solar drying (CSD) and solar simulator drying (SSD), SDIC achieved intermediate drying times (300 min) while minimizing color degradation, a critical factor for consumer acceptance. Spectral analysis revealed that UV and visible radiation (300–700 nm) were primary drivers of color degradation, particularly affecting carotenoid stability, whereas near-infrared radiation influenced drying rates. Notably, despite its laboratory-controlled conditions, the SSD method induced greater darkening than SDIC, suggesting that spectral composition (not just irradiance intensity) determines quality outcomes.
Phytochemical analysis further highlighted these trade-offs: SSD samples exhibited the highest antioxidant activity (1432.91 µmol TE/g dw) and phenolic content, likely due to precise temperature regulation, while SDIC better preserved color compounds. These findings underscore that irradiance control must account for spectral distribution and thermal effects to optimize specific quality parameters. This leads us to reflect that the preferred drying technique depends mainly on which characteristics we want to prioritise in the final product.
This study was constrained by its focus on a single temperature (50 °C) and limited spectral range (350–1000 nm), which may not capture the full effects of far-infrared radiation on drying kinetics. The small-scale solar simulator dryer (SSD) introduced potential biases due to its reduced sample capacity compared to the solar dryers (SDIC/CSD). While correlations between irradiance and quality parameters were identified, mechanistic insights into specific phytochemical degradation pathways were not investigated.
Future studies should expand spectral control to include far-infrared wavelengths and test multiple temperatures (40–70 °C) to optimize drying conditions. Research should also explore diverse papaya cultivars and other photosensitive crops, supported by advanced techniques to quantify degradation kinetics of key compounds like carotenoids. Finally, economic analyses of PDLC dryer adoption for small-scale producers would bridge the gap between technical innovation and commercial feasibility.