Ultrasonic-Assisted Enzymatic Extraction: An Innovative Technique for the Obtention of Betalains and Polyphenols from Dragon Fruit Peel
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Ultrasonic-Assisted Enzymatic Extraction
2.3. Box–Behnken Experimental Design
2.4. Determination of Total Betalains
2.5. Determination of Total Polyphenols
2.6. Comparison with Other Extraction Techniques
2.6.1. Ultrasound Extraction
2.6.2. Microwave Extraction
2.6.3. Enzymatic Hydrolysis
2.6.4. Statistical Analysis
3. Results and Discussion
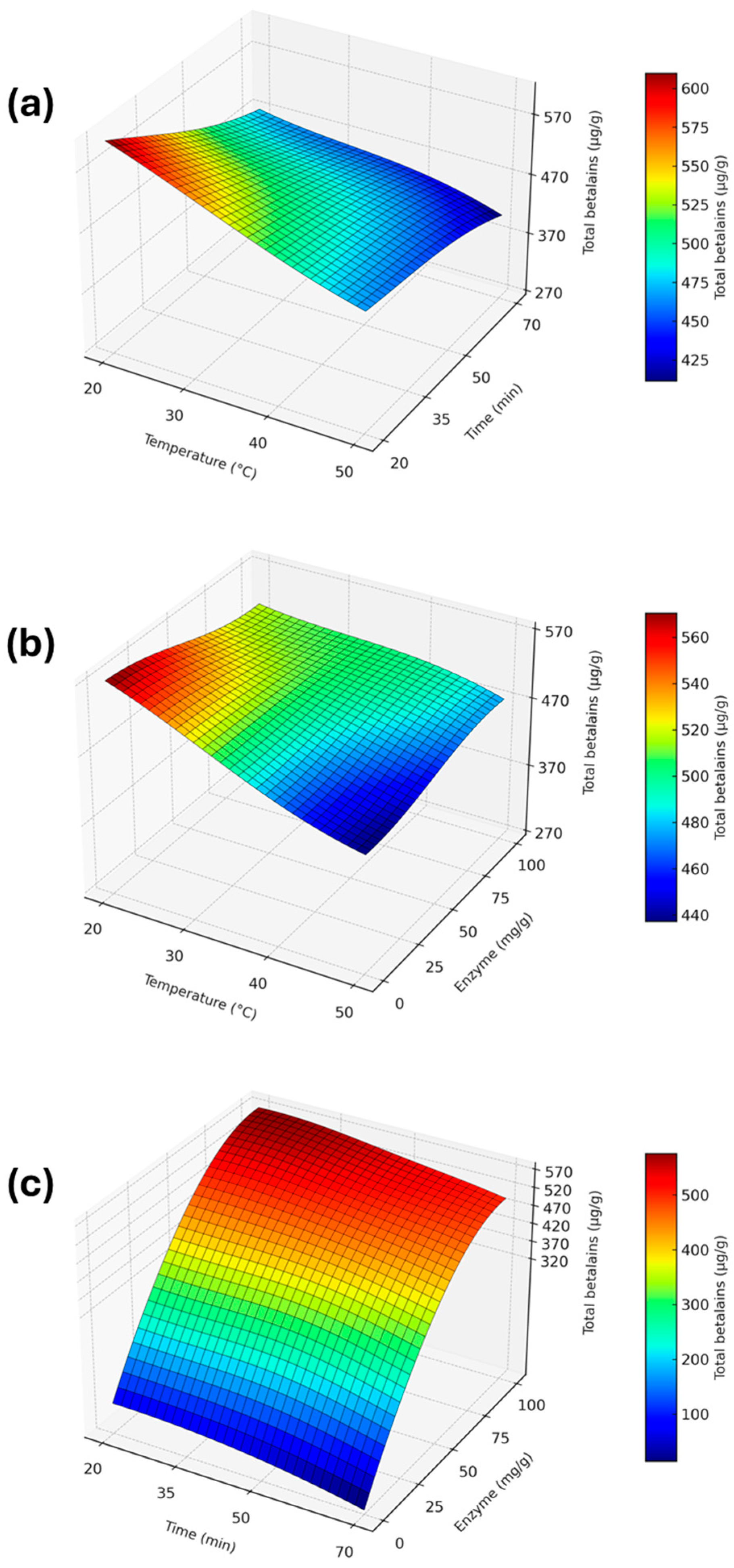
3.1. Results of the Box–Behnken Experimental Design
3.2. Analysis of the Effect of Factors
3.3. Optimization of Ultrasonic-Assisted Enzymatic Extraction
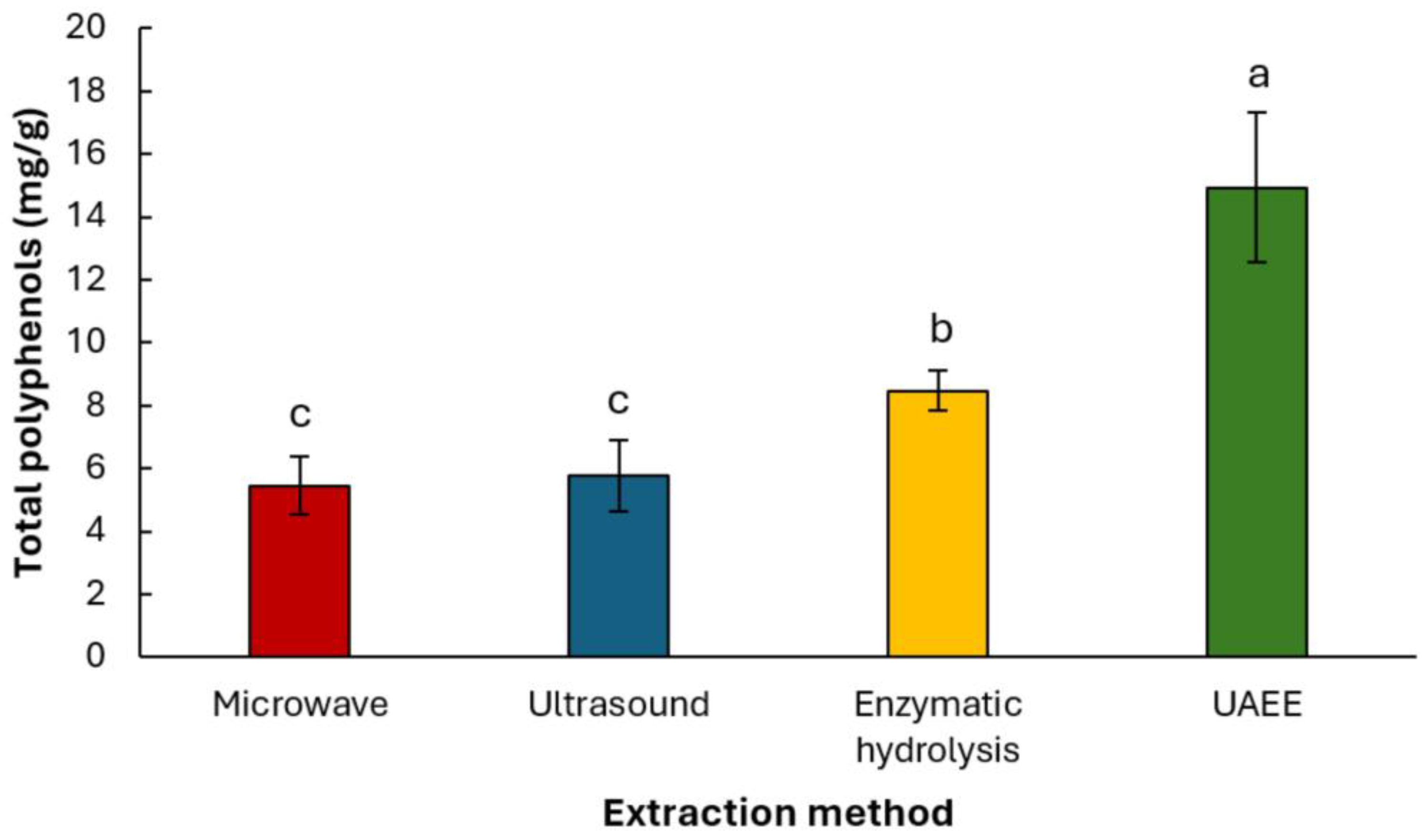
3.4. Comparison of Extraction Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verona-Ruiz, A.; Urcia-Cerna, J.; Paucar-Menacho, L.M. Pitahaya (Hylocereus spp.): Culture, physicochemical characteristics, nutritional composition, and bioactive compounds. Sci. Agropecu. 2020, 11, 439–453. [Google Scholar] [CrossRef]
- Le, N.L. Functional compounds in dragon fruit peels and their potential health benefits: A review. Int. J. Food Sci. Technol. 2022, 57, 2571–2580. [Google Scholar] [CrossRef]
- Chen, J.Y.; Xie, F.F.; Cui, Y.Z.; Chen, C.B.; Lu, W.J.; Hu, X.D.; Hua, Q.Z.; Zhao, J.; Wu, Z.J.; Gao, D.; et al. A chromosome-scale genome sequence of pitaya (Hylocereus undatus) provides novel insights into the genome evolution and regulation of betalain biosynthesis. Hortic. Res. 2021, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandham, K.; Sivakumar, V. Microwave assisted extraction process of betalain from dragon fruit and its antioxidant activities. J. Saudi. Soc. Agric. Sci. 2017, 16, 41–48. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Montoya Vizuete, S.N.; Castillo Mendoza, B.E.; Cajas Palacios, M.P.; Garcia Larreta, F.S. Actividad antioxidante, fenoles totales y tamizaje fitoquímico de Dragón Fruit roja y amarilla. Reciamuc 2022, 6, 408–417. [Google Scholar] [CrossRef]
- Mogaddam, M.R.A.; Ghareaghajlou, N.; Eyshi, S.; Ghasempour, Z. Red beet betalains extraction process: A comprehensive review of methods, applications, and physicochemical properties. Food Sci. Nutr. 2024, 12, 8540–8558. [Google Scholar] [CrossRef]
- Bettaieb, E.; Hamdi, M.; Smirani, N.; Bouazizi, S.; Torkhani, R. Effect of Environmentally Friendly Betalain Extraction Methods on Antioxidant Compounds of Tunisian Opuntia stricta Fruit. Foods 2025, 14, 851. [Google Scholar] [CrossRef]
- Righi Pessoa da Silva, H.; da Silva, C.; Bolanho, B.C. Ultrasonic-assisted extraction of betalains from red beet (Beta vulgaris L.). J. Food Process Eng. 2018, 41, e12833. [Google Scholar] [CrossRef]
- Lombardelli, C.; Benucci, I.; Mazzocchi, C.; Esti, M. A novel process for the recovery of betalains from unsold red beets by low-temperature enzyme-assisted extraction. Foods 2021, 10, 236. [Google Scholar] [CrossRef]
- Liao, N.; Zhong, J.; Ye, X.; Lu, S.; Wang, W.; Zhang, R.; Xu, J.; Chen, S.; Liu, D. Ultrasonic-assisted enzymatic extraction of polysaccharide from Corbicula fluminea: Characterization and antioxidant activity. LWT 2015, 60, 1113–1121. [Google Scholar] [CrossRef]
- Córdova, A.; Henríquez, P.; Nuñez, H.; Rico-Rodriguez, F.; Guerrero, C.; Astudillo-Castro, C.; Illanes, A. Recent advances in the application of enzyme processing assisted by ultrasound in agri-foods: A review. Catalysts 2022, 12, 107. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-assisted extraction of plants for sustainable and functional applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Li, F.; Mao, Y.D.; Wang, Y.F.; Raza, A.; Qiu, L.P.; Xu, X.Q. Optimization of ultrasonic-Assist. Enzym. extraction conditions for improving total phenolic content, antioxidant and antitumor activities in vitro from Trapa quadrispinosa Roxb. residues. Mol. 2017, 22, 396. [Google Scholar] [CrossRef]
- Huynh, T.D.; Nguyen, H.T.; Nguyen, T.K.N.; Dang, T.N.A.; Le, T.T.; Duong, T.N.D.; Phan, T.H. Optimization of ultrasound-assisted enzymatic extraction of betalains from red beetroot (Beta vulgaris L.). J. Agric. Dev. 2023, 22, 65–77. [Google Scholar] [CrossRef]
- Fernando, G.S.N.; Wood, K.; Papaioannou, E.H.; Marshall, L.J.; Sergeeva, N.N.; Boesch, C. Application of an ultrasound-assisted extraction method to recover betalains and polyphenols from red beetroot waste. ACS Sustain. Chem. Eng. 2021, 9, 8736–8747. [Google Scholar] [CrossRef]
- Shakir, B.K.; Simone, V. Estimation of betalain content in beetroot peel powder. Ital. J. Food Sci. 2024, 36, 53–57. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Omre, P.K.; Chand, K.; Kumar, A.; Awasthi, P. Process optimization of ultrasonic assisted extraction of betalains from red beet, Beta vulgaris L. waste stalks. Indian J. Exp. Biol. 2022, 59, 858–866. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Nivetha, C.V. Optimization of ultrasound-assisted extraction of natural pigments from Bougainvillea glabra flowers. Ind. Crops Prod. 2015, 63, 182–189. [Google Scholar] [CrossRef]
- Jayachandran, L.; Jat, K.; Rao, P. Impact of temperature assisted ultrasonication on the quality attributes of beetroot (Beta vulgaris L.) juice. J. Food Process Eng. 2023, 46, e14329. [Google Scholar] [CrossRef]
- Mehta, D.; Zhang, Y.; Kuksal, K.; Nile, S.H.; Yadav, S.K.; Yadav, K. Ultrasound-assisted extraction and encapsulation of betalain from prickly pear: Process optimization, in-vitro digestive stability, and development of functional gummies. Ultrason. Sonochem. 2024, 108, 106975. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Juárez-Onofre, J.; Del-Toro-Sánchez, C.; Cárdenas-López, J.; Carvajal-Millan, E.; Montaño-Leyva, B.; Tapia-Hernández, J. Optimization of the Extraction of Betalains from the Pulp of Pitaya (Stenocereus thurberi) and its Antioxidant Capacity. Food Anal. Methods 2023, 16, 1252–1260. [Google Scholar] [CrossRef]
- Chew, Y.M.; King, V.A.E.; Hung, C.H. Accelerated storage test of betalains extracted from the peel of pitaya (Hylocereus cacti) fruit. J. Food Sci. Technol. 2019, 56, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Kalita, N.K.; Hakkarainen, M. Triggering Degradation of Cellulose Acetate by Embedded Enzymes: Accelerated Enzymatic Degradation and Biodegradation under Simulated Composting Conditions. Biomacromolecules 2023, 24, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Lombardelli, C.; Benucci, I.; Mazzocchi, C.; Esti, M. Betalain Extracts from Beetroot as Food Colorants: Effect of Temperature and UV-Light on Storability. Plant Foods Hum. Nutr. 2021, 76, 347–353. [Google Scholar] [CrossRef]
- Silva, J.P.P.; Bolanho, B.C.; Stevanato, N.; Massa, T.B.; da Silva, C. Ultrasound-assisted extraction of red beet pigments (Beta vulgaris L.): Influence of operational parameters and kinetic modeling. J. Food Process. Preserv. 2020, 44, e14762. [Google Scholar] [CrossRef]
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols During Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants, 2 nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 243–259. [Google Scholar] [CrossRef]
- Heemann, A.C.W.; Heemann, R.; Kalegari, P.; Spier, M.R.; Santin, E. Enzyme-assisted extraction of polyphenols from green yerba mate. Braz. J. Food Technol. 2019, 22, 1–10. [Google Scholar] [CrossRef]
- Vahid, A.; Kelebek, H.; Odouaro, O.; Oussou, K.; Goksen, G.; Selli, S.; Galanakis, C. A comprehensive review of recent development in extraction and encapsulation techniques of betalains. Crit. Rev. Food Sci. Nutr. 2023, 64, 11263–11280. [Google Scholar] [CrossRef]
- Ran, J.; Fan, M.; Li, Y.; Li, G.; Zhao, Z.; Liang, J. Optimisation of ultrasonic-assisted extraction of polyphenols from apple peel employing cellulase enzymolysis. Int. J. Food Sci. Technol. 2013, 48, 910–917. [Google Scholar] [CrossRef]


| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Actual Value | |||
| Temperature (°C) | 20 | 35 | 50 |
| Time (min) | 20 | 45 | 70 |
| Enzyme/substrate (mg/g) | 0 | 50 | 100 |
| Exp | Temperature (°C) | Time (min) | E/S Ratio (mg/g) | Total Betalains (µg/g) | Total Polyphenols (mg/g) |
|---|---|---|---|---|---|
| 1 | 20 | 20 | 200 | 556.9 | 9.68 |
| 2 | 50 | 20 | 200 | 368.6 | 5.13 |
| 3 | 20 | 70 | 200 | 512.0 | 9.12 |
| 4 | 50 | 70 | 200 | 313.2 | 8.31 |
| 5 | 20 | 45 | 0 | 569.8 | 4.75 |
| 6 | 50 | 45 | 0 | 250.1 | 5.90 |
| 7 | 20 | 45 | 400 | 500.7 | 10.92 |
| 8 | 50 | 45 | 400 | 299.8 | 10.02 |
| 9 | 35 | 20 | 0 | 435.0 | 4.87 |
| 10 | 35 | 70 | 0 | 442.8 | 6.48 |
| 11 | 35 | 20 | 400 | 559.4 | 15.90 |
| 12 | 35 | 70 | 400 | 456.2 | 12.22 |
| 13 | 35 | 45 | 200 | 517.9 | 9.67 |
| 14 | 35 | 45 | 200 | 395.0 | 7.90 |
| 15 | 35 | 45 | 200 | 412.2 | 8.43 |
| Source | Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A: Temperature | 102,990 | 1 | 102,990 | 91.89 | 0.0002 * |
| B: Time | 4787.31 | 1 | 4787.31 | 4.27 | 0.0936 |
| C: Enzyme | 1752.32 | 1 | 1752.32 | 1.56 | 0.2665 |
| AA | 1399.80 | 1 | 1399.80 | 1.25 | 0.3146 |
| AB | 27.56 | 1 | 27.56 | 0.02 | 0.8815 |
| AC | 3528.36 | 1 | 3528.36 | 3.15 | 0.1362 |
| BB | 8785.50 | 1 | 8785.50 | 7.84 | 0.0380 |
| BC | 3080.25 | 1 | 3080.25 | 2.75 | 0.1583 |
| CC | 969.51 | 1 | 969.51 | 0.87 | 0.3950 |
| Total error | 5604.01 | 5 | 1120.80 | ||
| Total (Corr.) | 133,302 | 14 |
| Source | Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A: Temperature | 3.26 | 1 | 3.26 | 1.33 | 0.3004 |
| B: Time | 0.04 | 1 | 0.04 | 0.02 | 0.9059 |
| C: Enzyme | 91.53 | 1 | 91.53 | 37.38 | 0.0017 * |
| AA | 4.64 | 1 | 4.64 | 1.90 | 0.2269 |
| AB | 3.49 | 1 | 3.49 | 1.43 | 0.2857 |
| AC | 1.05 | 1 | 1.05 | 0.43 | 0.5414 |
| BB | 2.66 | 1 | 2.66 | 1.09 | 0.3453 |
| BC | 6.99 | 1 | 6.99 | 2.86 | 0.1518 |
| CC | 1.74 | 1 | 1.74 | 0.71 | 0.4381 |
| Total error | 12.24 | 5 | 2.45 | ||
| Total (Corr.) | 128.37 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puc-Santamaria, C.G.; Us-Camas, R.; Hernández-Núñez, E.; Can-Herrera, L.A.; Dzib-Cauich, D.A.; Cabal-Prieto, A.; Pensupa, N.; Oney-Montalvo, J.E. Ultrasonic-Assisted Enzymatic Extraction: An Innovative Technique for the Obtention of Betalains and Polyphenols from Dragon Fruit Peel. Processes 2025, 13, 2307. https://doi.org/10.3390/pr13072307
Puc-Santamaria CG, Us-Camas R, Hernández-Núñez E, Can-Herrera LA, Dzib-Cauich DA, Cabal-Prieto A, Pensupa N, Oney-Montalvo JE. Ultrasonic-Assisted Enzymatic Extraction: An Innovative Technique for the Obtention of Betalains and Polyphenols from Dragon Fruit Peel. Processes. 2025; 13(7):2307. https://doi.org/10.3390/pr13072307
Chicago/Turabian StylePuc-Santamaria, Cristhel Guadalupe, Rosa Us-Camas, Emanuel Hernández-Núñez, Luis Alfonso Can-Herrera, Dany Alejandro Dzib-Cauich, Adán Cabal-Prieto, Nattha Pensupa, and Julio Enrique Oney-Montalvo. 2025. "Ultrasonic-Assisted Enzymatic Extraction: An Innovative Technique for the Obtention of Betalains and Polyphenols from Dragon Fruit Peel" Processes 13, no. 7: 2307. https://doi.org/10.3390/pr13072307
APA StylePuc-Santamaria, C. G., Us-Camas, R., Hernández-Núñez, E., Can-Herrera, L. A., Dzib-Cauich, D. A., Cabal-Prieto, A., Pensupa, N., & Oney-Montalvo, J. E. (2025). Ultrasonic-Assisted Enzymatic Extraction: An Innovative Technique for the Obtention of Betalains and Polyphenols from Dragon Fruit Peel. Processes, 13(7), 2307. https://doi.org/10.3390/pr13072307








