A Novel Fluorescent Probe AP for Highly Selective and Sensitive Detection of Hg2+ and Its Application in Environmental Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
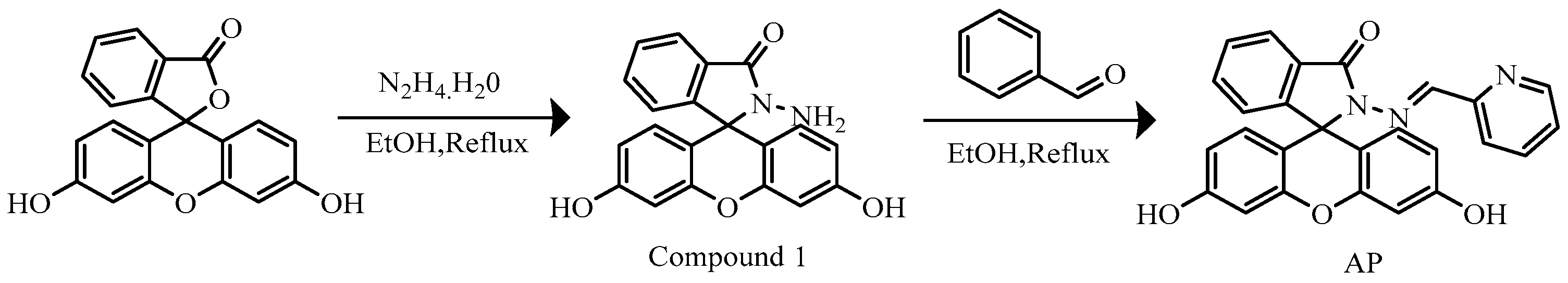
2.2. Synthesis of Probe AP
2.2.1. Synthesis of Compound 1
2.2.2. Preparation of Probe AP
2.3. Preparation of Solutions for Spectroscopic Testing
2.3.1. HEPES Buffer Solution Preparation
2.3.2. Preparation of Stock Solutions
2.4. Detection Limit Calculation
2.5. Detection in Real Water Samples
3. Results
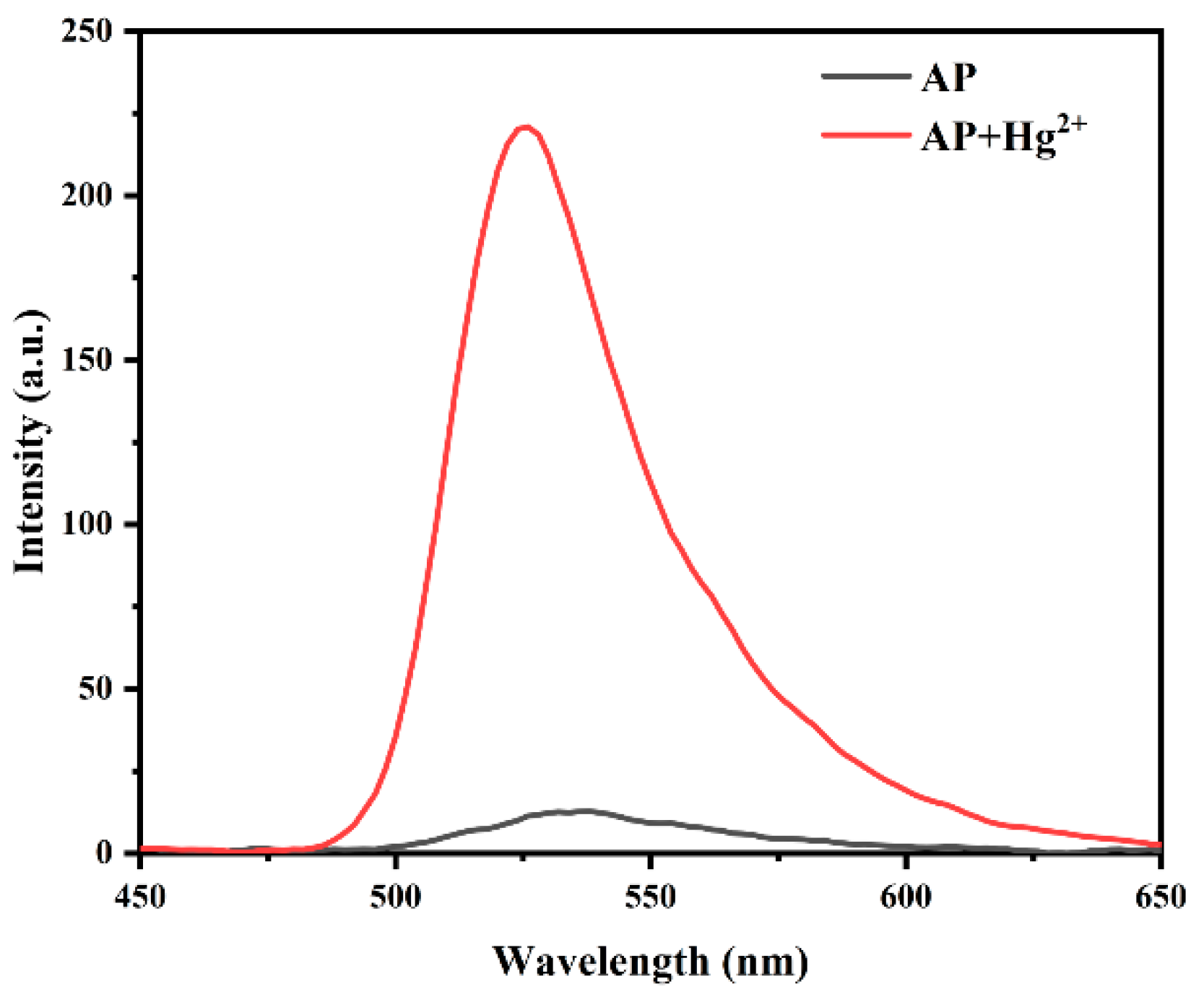
3.1. Feasibility Study of Probe AP
3.2. Optimization of Detection Conditions
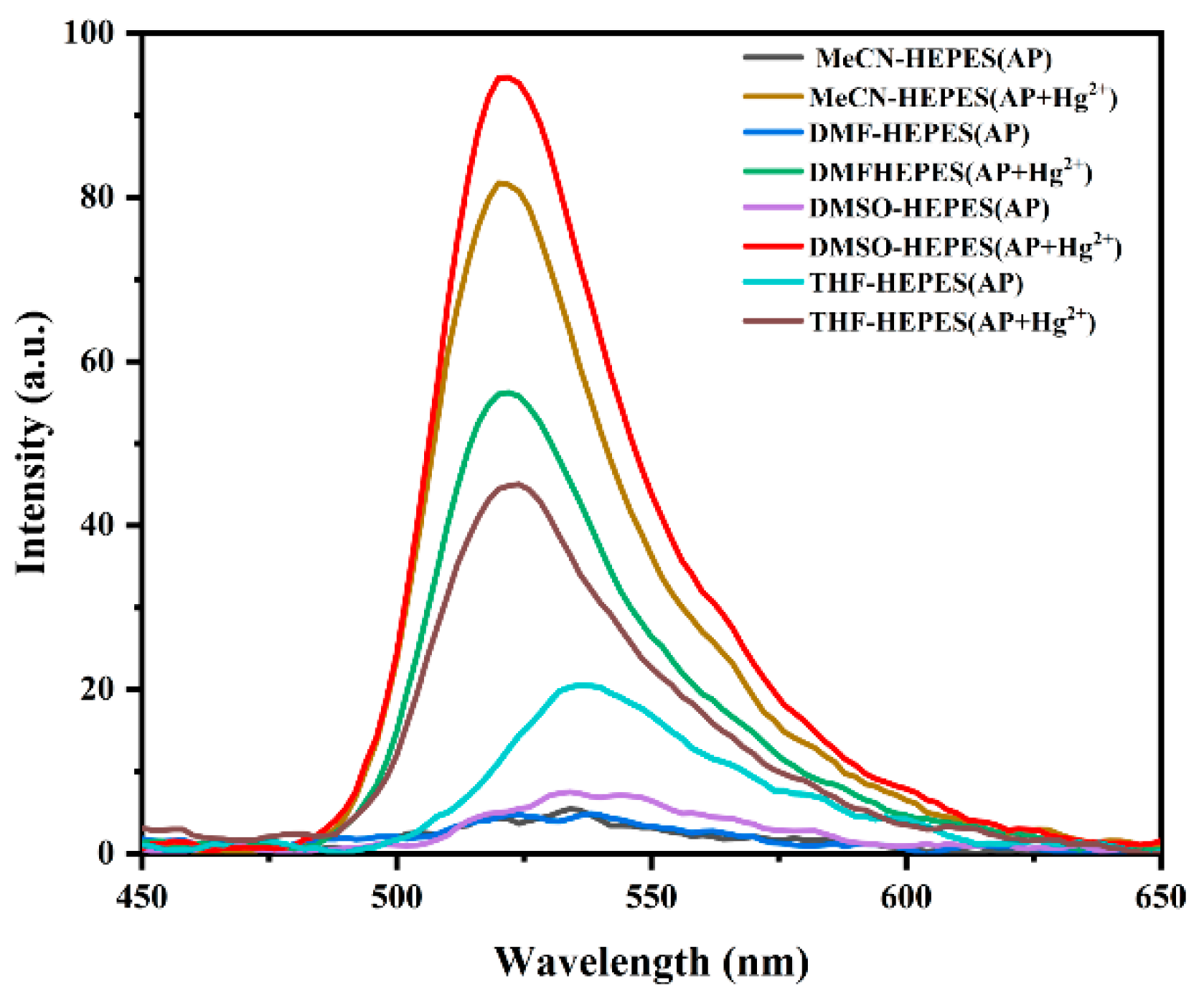
3.2.1. Solvent Type Effects on Fluorescence Properties of Probe AP
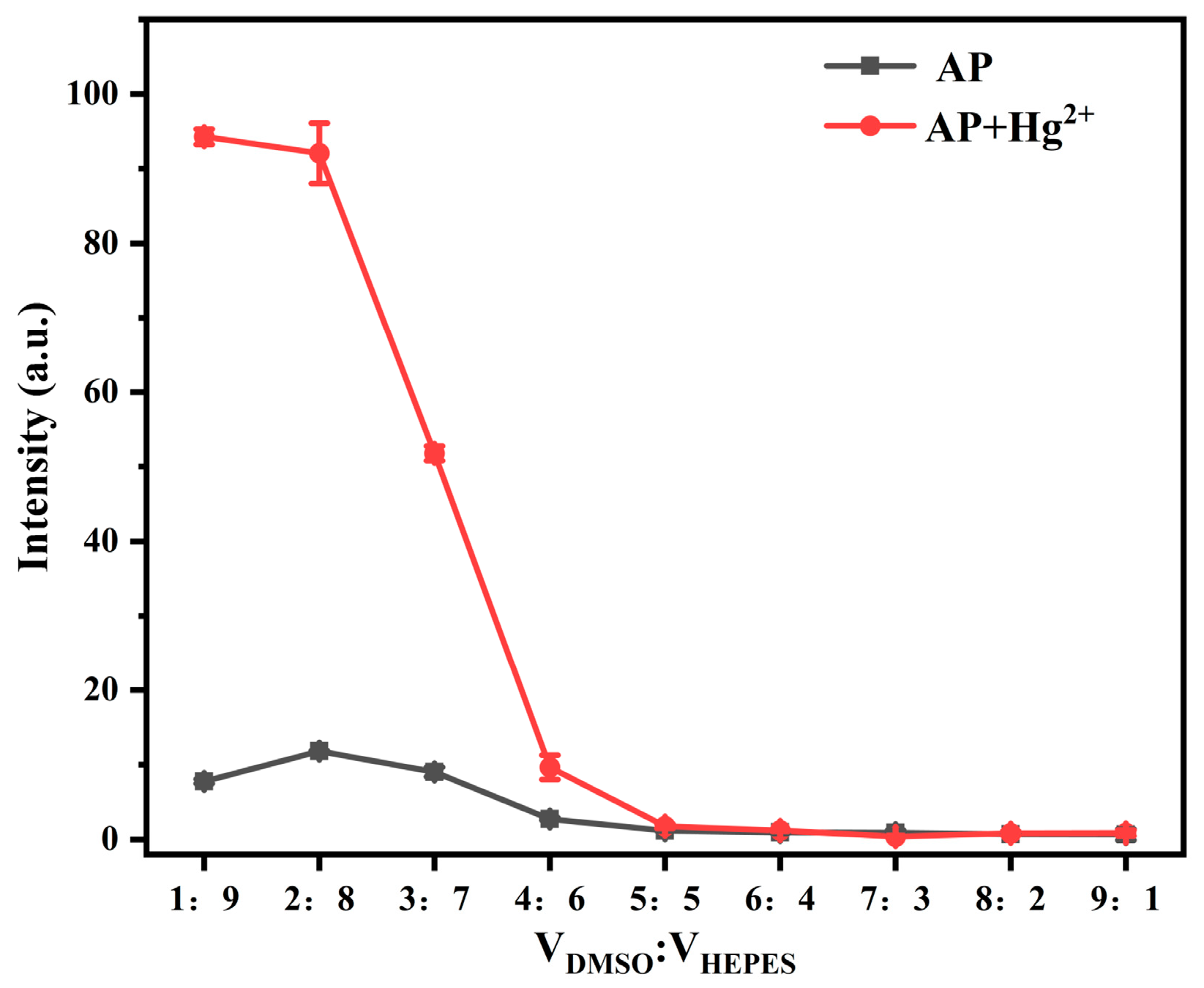
3.2.2. Effect of Solvent Ratio on Fluorescence Properties of Probe AP
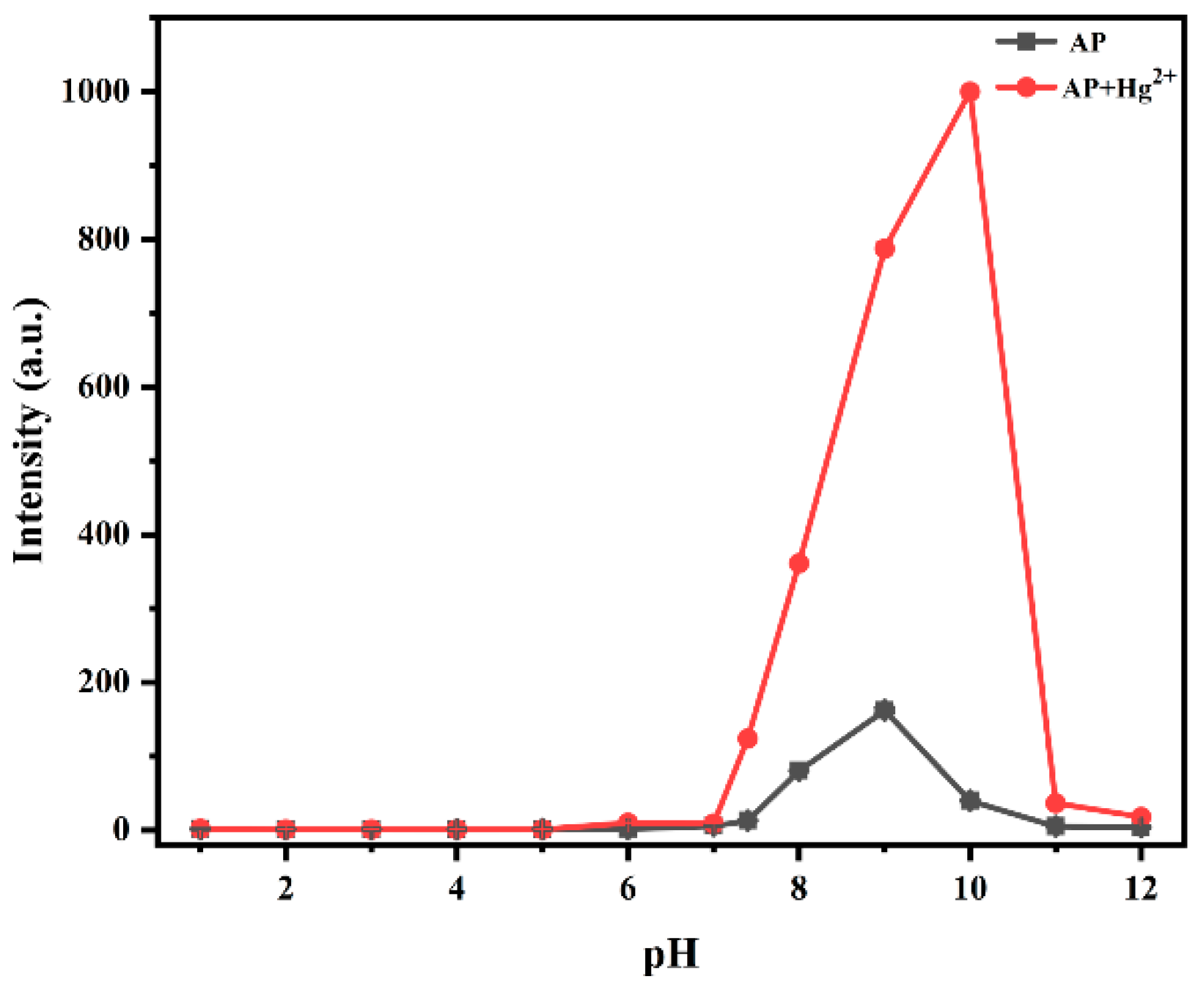
3.2.3. Effect of pH on Fluorescence Properties of Probe AP
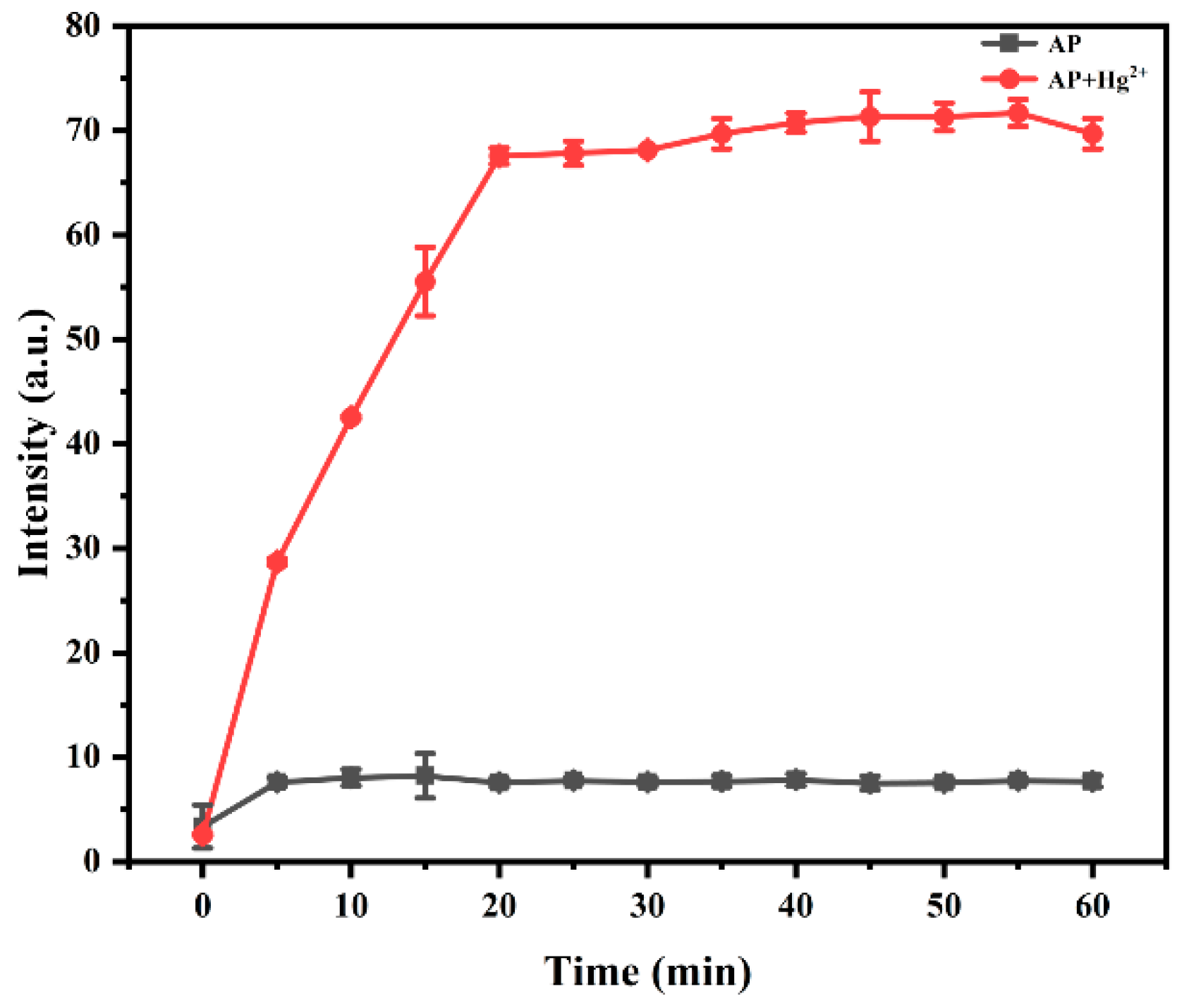
3.2.4. Response Time of Probe AP Toward Hg2+
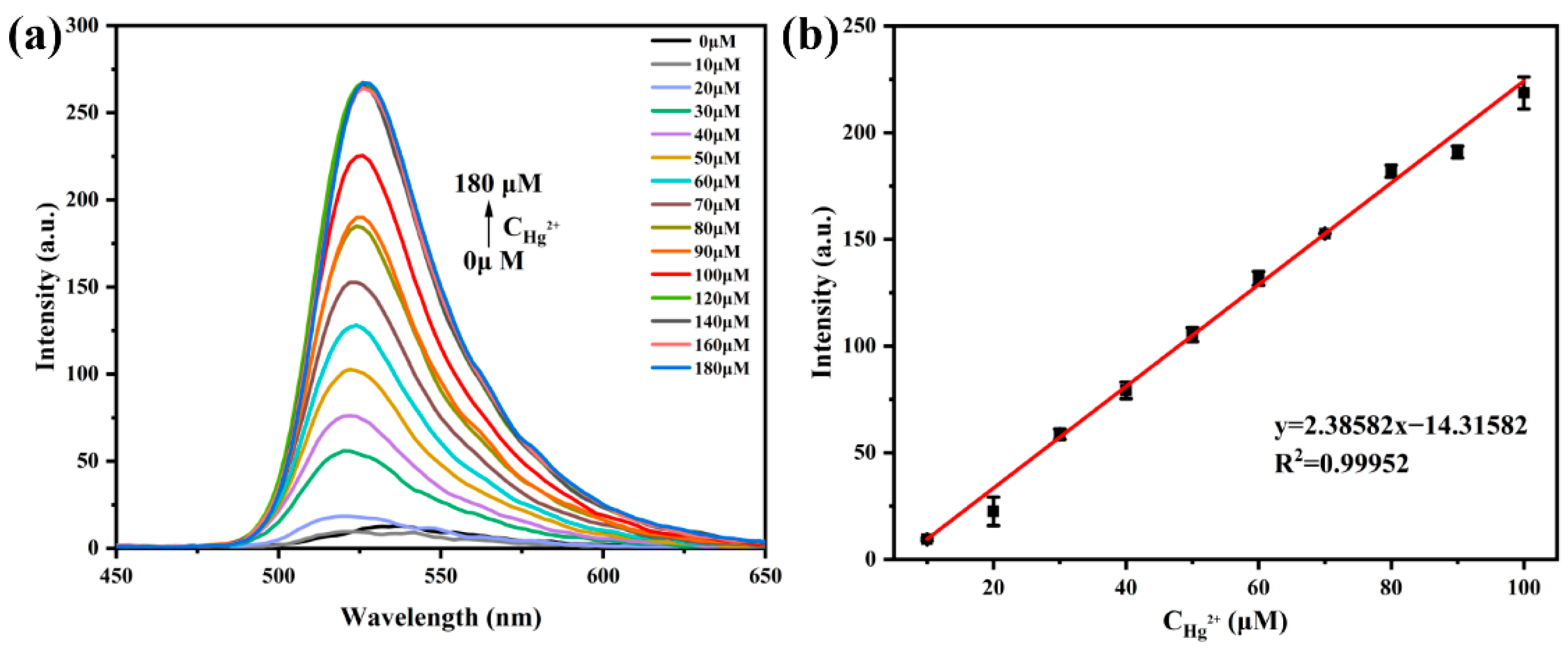
3.3. Fluorescence Titration Experiment of Probe AP with Hg2+
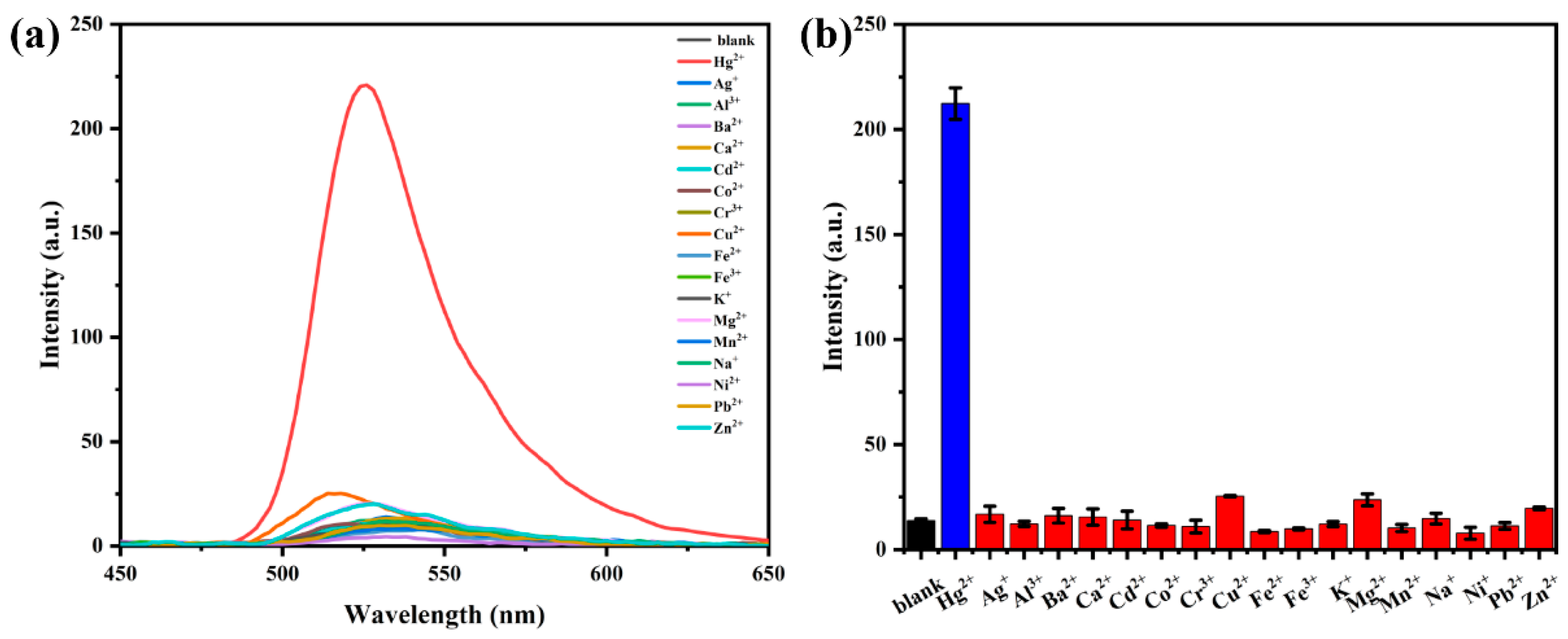
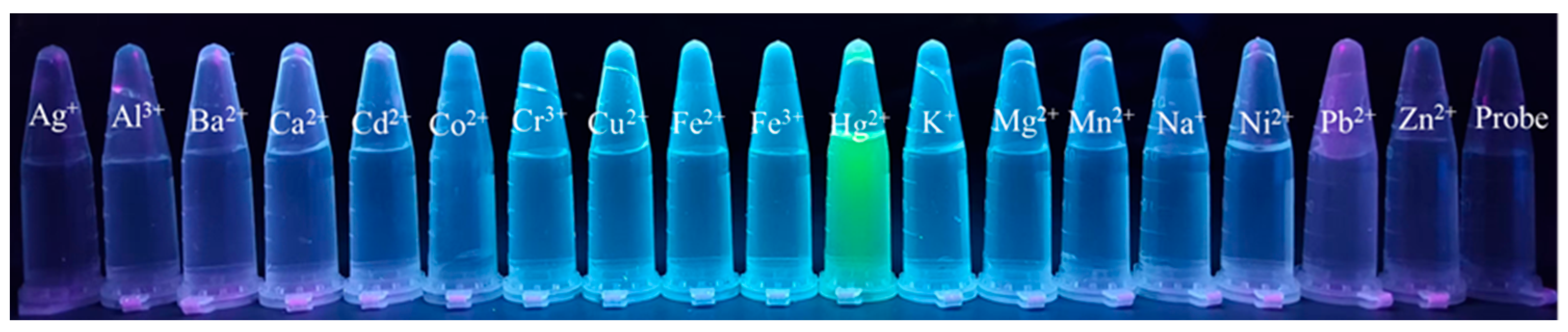
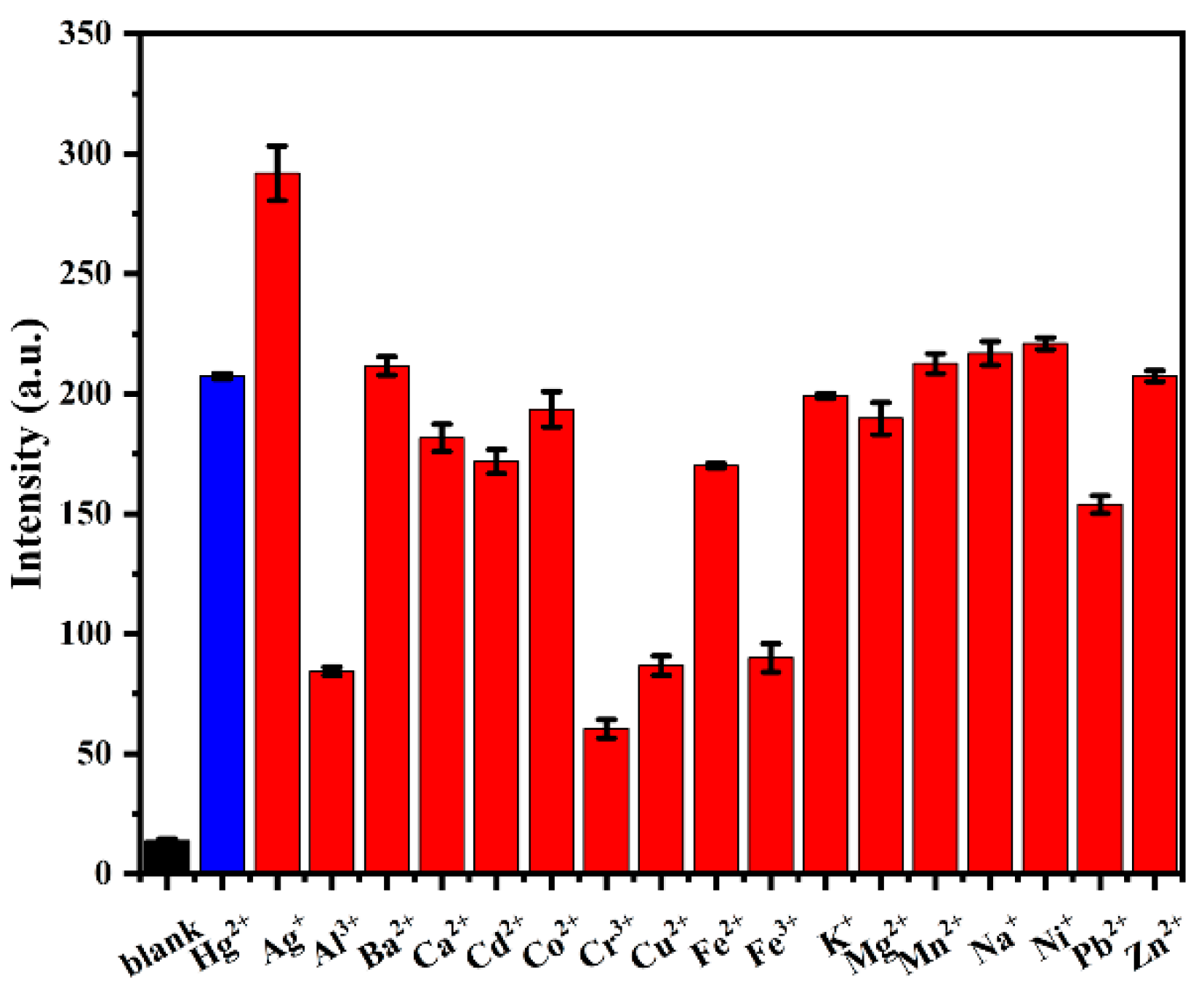
3.4. Selectivity Study of Probe AP for Hg2+
3.5. Interference Study of Probe AP for Hg2+ Detection
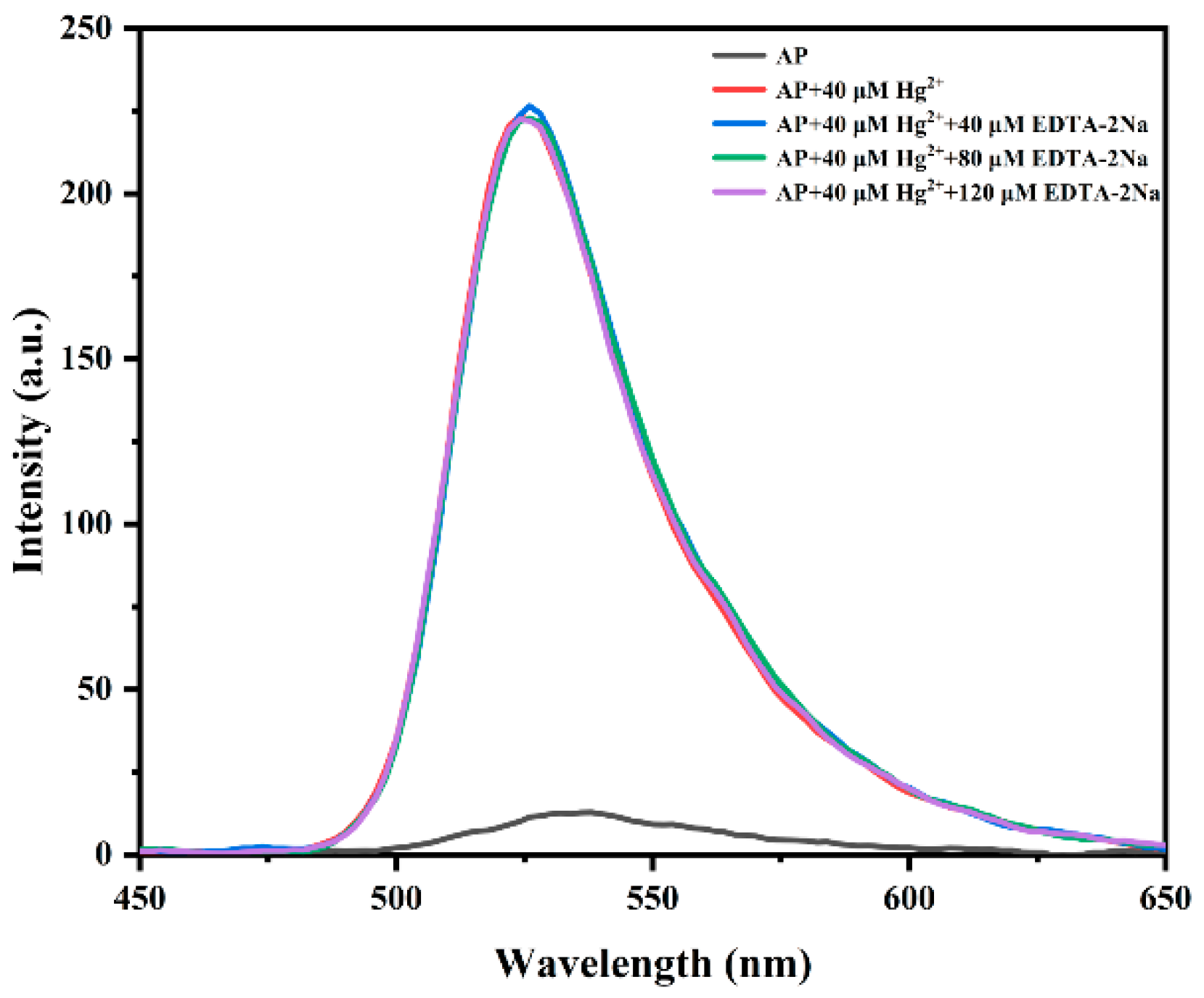
3.6. Reversibility Investigation of Probe AP
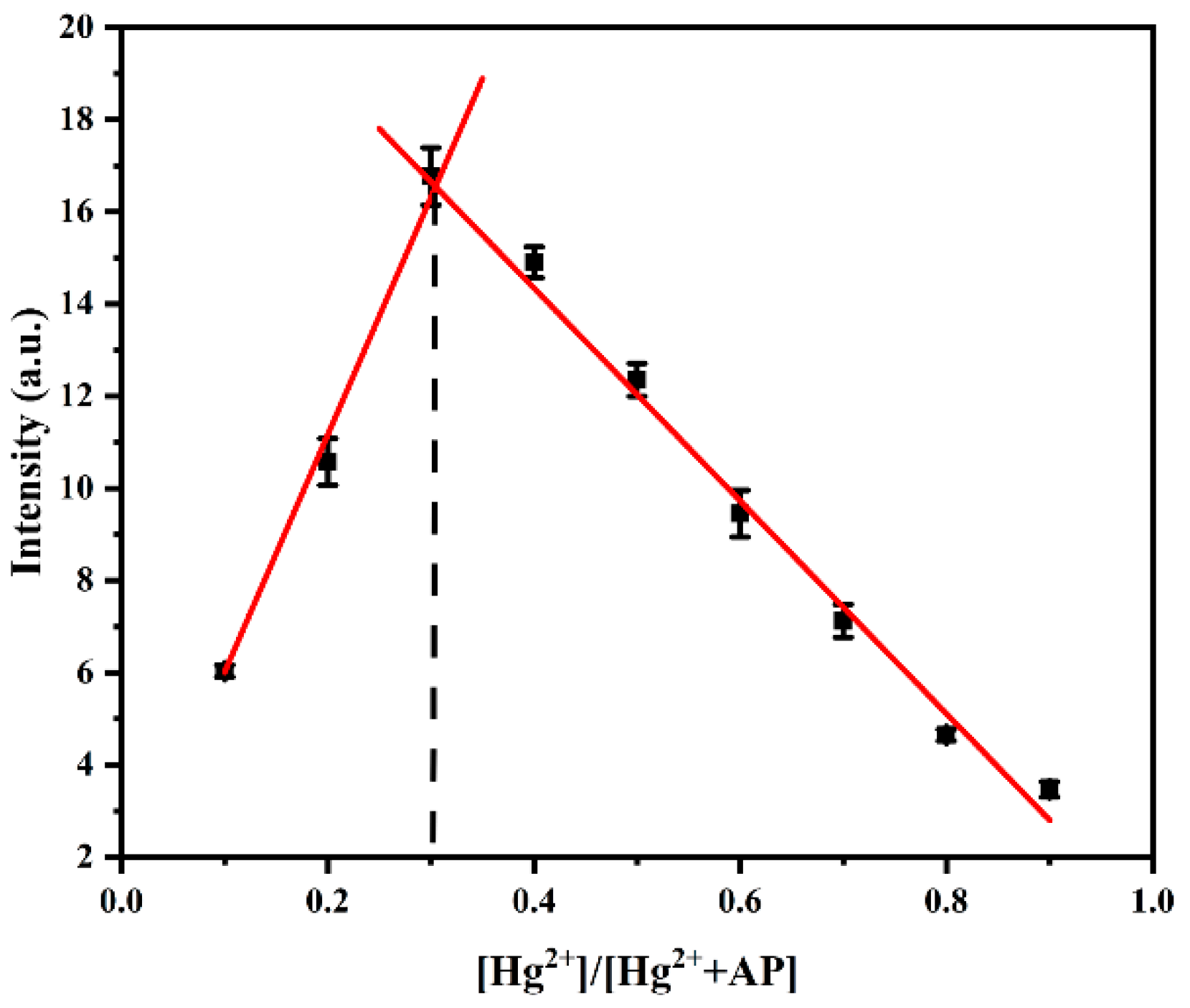
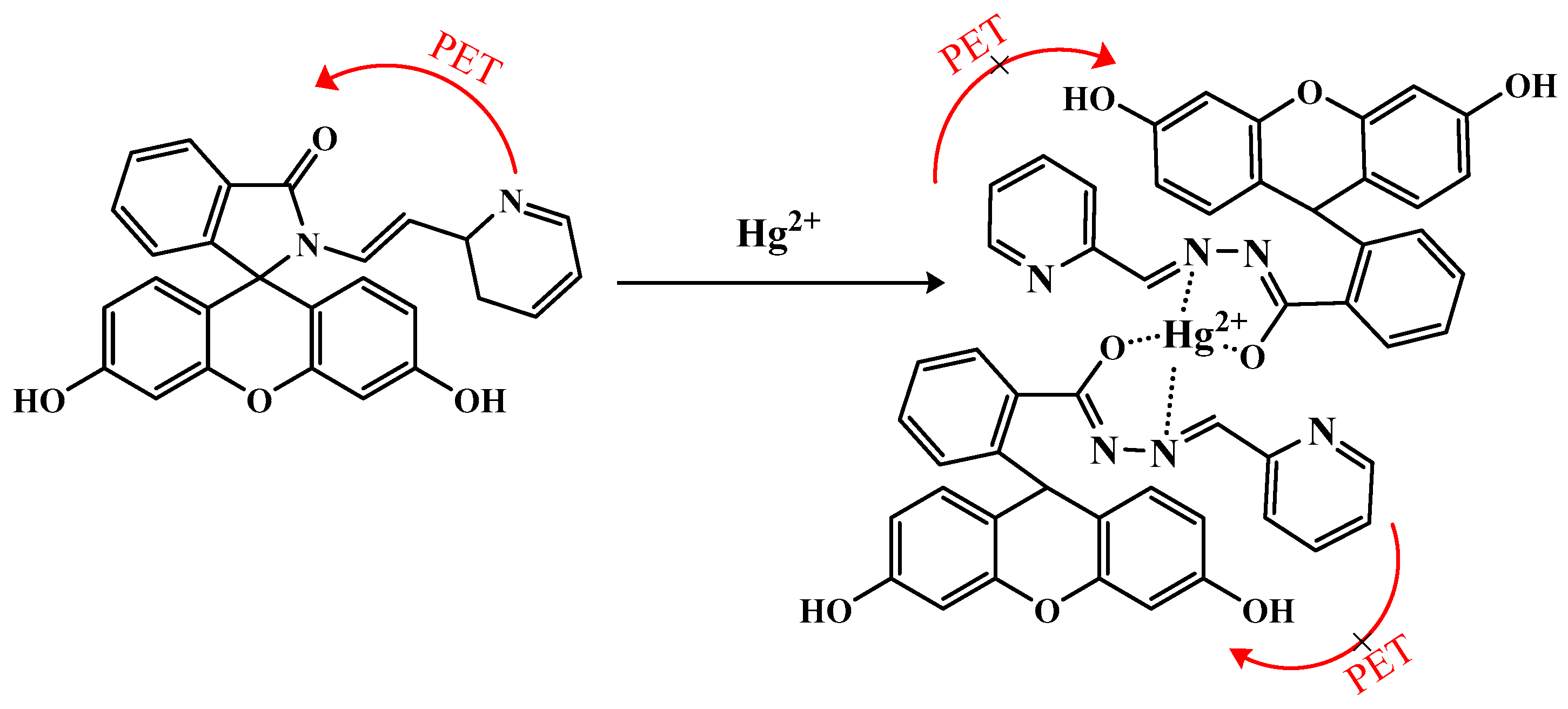
3.7. Mechanism Study of Probe AP for Hg2+ Recognition
3.8. Detection in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gauthama, B.U.; Narayana, B.; Sarojini, B.K.; Suresh, N.K.; Sangappa, Y.; Kudva, A.K.; Satyanarayana, G.; Raghu, S.V. Colorimetric “off–on” fluorescent probe for selective detection of toxic Hg2+ based on rhodamine and its application for in-vivo bioimaging. Microchem. J. 2021, 166, 106233. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, W.; Tan, Z.; Zhang, L.; Pan, R. A rapid response optical fiber sensor with highly selective probe for trace detection of mercury (II) ions. Microchem. J. 2024, 206, 111534. [Google Scholar] [CrossRef]
- Isaad, J.; Malek, F.; Achari, A.E. Colorimetric and fluorescent probe based on coumarin/ thiophene derivative for sequential detection of mercury (II) and cyanide ions in an aqueous medium. J. Mol. Struct. 2022, 1270, 133838. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, M.; Wang, D.; Chen, K.; Lin, P.; Ge, Y.; Liu, W.; Wu, J. Highly selective fluorescence probe with peptide backbone for imaging mercury ions in living cells based on aggregation-induced emission effect. J. Hazard. Mater. 2021, 415, 125712. [Google Scholar] [CrossRef] [PubMed]
- Ramki, K.; Thiruppathi, G.; Ramasamy, S.K.; Sundararaj, P.; Sakthivel, P. An aggregation-induced emission-based ratiometric fluorescent chemosensor for Hg (II) and its application in Caenorhabditis elegans imaging. Methods 2024, 221, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tamizhselvi, R.; Gangatharan Vinoth Kumar, G.; Anbarasu, G.; Arumugam Napoleon, A. Dual-channel fluorescent probe utilizing hydrazone for Zn2+ and Hg2+ detection: Exploring distinct signaling mechanisms and applications in bioimaging and latent fingerprint analysis. J. Photochem. Photobiol. A Chem. 2024, 452, 115609. [Google Scholar] [CrossRef]
- Patthana, P.; Zhong, H.-C.; Wu, Q.; Ren, T.-B.; Yuan, L. Engineering a far-red fluorescent probe for rapid detection of Hg (II) ions in both cells and zebrafish. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2024, 318, 124469. [Google Scholar] [CrossRef] [PubMed]
- Kaewnok, N.; Kraithong, S.; Mahaveero, T.; Maitarad, P.; Sirirak, J.; Wanichacheva, N.; Swanglap, P. Silver nanoparticle incorporated colorimetric/fluorescence sensor for sub-ppb detection of mercury ion via plasmon-enhanced fluorescence strategy. J. Photochem. Photobiol. A Chem. 2022, 433, 114140. [Google Scholar] [CrossRef]
- Erdemir, E.; Suna, G.; Gunduz, S.; Şahin, M.; Eğlence-Bakır, S.; Karakuş, E. Rapid, ultrasensitive, highly selective detection of toxic Hg (II) ions in seabass, swordfish and water samples. Food Chem. 2022, 371, 131309. [Google Scholar] [CrossRef] [PubMed]
- Ramki, K.; Selva Kumar, R.; Thiruppathi, G.; Sundararaj, P.; Sakthivel, P. Imidazole-based chromone Schiff base for highly selective detection of Hg (II) and bio-capability in Caenorhabditis elegans imaging. J. Photochem. Photobiol. A Chem. 2023, 444, 115022. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, H.; Wang, Y.; Fan, C.; Tu, Y.; Liu, G.; Pu, S. A ratiometric and colorimetric fluorescent probe for the detection of mercury ion based on rhodamine and quinoline–benzothiazole conjugated dyad. J. Photochem. Photobiol. A Chem. 2020, 400, 112657. [Google Scholar] [CrossRef]
- Chan, C.; Li, J.; Wu, J.; Zi, Y.; Xue, Z.; Ledwaba, M.; Mack, J.; Nyokong, T. An imidazole-based fluorescent probe for the Mercury (II) Ion with rapid response in vitro. Dye Pigment. 2023, 213, 111172. [Google Scholar] [CrossRef]
- Mohamed, M.B.I.; El-Sedik, M.S.; Youssef, Y.A.; Mohamed, N.A.; Aysha, T.S. New stilbene-biscarbothioamide based colorimetric chemosensor and turn on fluorescent probe for recognition of Hg2+ cation. J. Photochem. Photobiol. A Chem. 2022, 433, 114206. [Google Scholar] [CrossRef]
- Wang, G.; Xu, W.; Liu, Y.; Fu, N. Ultrasensitive detection and high-contrast bioimaging of Hg2+ using monothiosquaraine-based fluorescent probe via hydrogen bond promoted desulfurization. Microchem. J. 2022, 179, 107481. [Google Scholar] [CrossRef]
- Mishra, T.; Guria, S.; Sadhukhan, J.; Das, D.; Das, M.K.; Adhikari, S.S.; Maity, S.; Maiti, P. A naphthalimide appended rhodamine based biocompatible fluorescent probe: Chemosensor for selective detection of Hg2+ ion, live cell imaging and DFT study. J. Photochem. Photobiol. A Chem. 2024, 446, 115168. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Liu, L.; Li, D.; Huo, Y.; Liu, H. A novel carbazole-based highly sensitive and selective turn-on fluorescent probe for mercury (II) ions in aqueous THF. J. Photochem. Photobiol. A Chem. 2021, 416, 113322. [Google Scholar] [CrossRef]
- Giri, D.; Bankura, A.; Patra, S.K. Poly(benzodithieno-imidazole-alt-carbazole) based π-conjugated copolymers: Highly selective and sensitive turn-off fluorescent probes for Hg2+. Polymer 2018, 158, 338–353. [Google Scholar] [CrossRef]
- Wang, X.; Hu, C.; Wang, X.; Luo, Z.; Zhen, S.; Zhan, L.; Huang, C.; Li, Y. Facile synthesis of dual-ligand terbium-organic gels as ratiometric fluorescence probes for efficient mercury detection. J. Hazard. Mater. 2022, 436, 129080. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, Y.-F.; Li, C.-C.; Wang, M.-H.; Li, L.; Chen, G.-Y.; Zhang, L.-M.; Wang, L.-Q.; Wang, J.-Y. A novel 1,3,5-Triazine-based fluorescent probe with large Stokes shift for the detection of Hg (II) and its application in cell, zebrafish and tobacco imaging. J. Photochem. Photobiol. A Chem. 2024, 447, 115171. [Google Scholar] [CrossRef]
- Zhang, K.; Peng, L.; Tian, X.; Guang, S.; Xu, H. Based on theoretical calculations designed a novel dual-channel chemo-sensor for Mg2+ and Zn2+ detection and bioimaging applications. Microchem. J. 2023, 189, 108328. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Yang, Y. A novel flavonol-based fluorescent probe for rapid detection of Hg2+ and its multi-functional applications. Dye Pigment. 2023, 216, 111353. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, L.; Liu, X.; Pang, X.; Fan, F.; Yang, X.; Hua, R.; Wang, Y. A reversible CHEF-based NIR fluorescent probe for sensing Hg2+ and its multiple application in environmental media and biological systems. Sci. Total Environ. 2023, 874, 162460. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, X.; Ma, X.; Zhu, Y.; Liu, Y.; Zeng, H. Novel near-infrared frequency up-conversion luminescence probe for monitoring biothiols in vitro and in vivo. Sens. Actuators B Chem. 2023, 385, 133705. [Google Scholar] [CrossRef]
- Chan, C.; Liu, H.; Xue, Z. Chromogenic and fluorescent probe for detection of mercury (II) ion based on mono-pyrrolyl substituted BODIPY. Microchem. J. 2021, 166, 106247. [Google Scholar] [CrossRef]
- Liu, H.; Xing, H.; Gao, Z.; You, M.; Li, B.; Feng, X.; Zhou, B.; Cong, Z.; Zhu, J.; Jin, M. A single-wavelength excited NIR fluorescence probe for distinguishing GSH/H2S and Cys/Hcy in living cells and zebrafish through separated dual-channels. Talanta 2023, 254, 124153. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Wang, Q.; Guo, Z.; Chen, B.; Liu, Y.; Wang, P.; Yang, X.; An, Y. A rapid “on-off-on” peptide-based fluorescent probe for selective and consecutive detection of mercury and sulfide ions in aqueous systems and live cells. J. Photochem. Photobiol. A Chem. 2021, 417, 113354. [Google Scholar] [CrossRef]
- Khan, J. Optical Chemosensors Synthesis and Appplication for Trace Level Metal Ions Detection in Aqueous Media: A Review. J. Fluoresc. 2025, 35, 561–582. [Google Scholar] [CrossRef] [PubMed]
- Alhamami, M.A.M.; Algethami, J.S.; Khan, S. A Review on Thiazole Based Colorimetric and Fluorimetric Chemosensors for the Detection of Heavy Metal Ions. Crit. Rev. Anal. Chem. 2024, 54, 2689–2713. [Google Scholar] [CrossRef] [PubMed]
- Algethami, J.S.; Al-Saidic, H.M.; Alosaimi, E.H.; Yaser, A.A.; Mohammed, A.-A.K.; Khan, S. Recent Advancements in Fluorometric and Colorimetric Detection of Cd2+ Using Organic Chemosensors: A Review (2019–2024). Crit. Rev. Anal. Chem. 2024, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, W.; Chen, X.; Xie, Z. Isocyano-functionalized, 1,8-naphthalimide-based chromophore as efficient ratiometric fluorescence probe for Hg2+ in aqueous medium. Sens. Actuators B Chem. 2018, 255, 3074–3084. [Google Scholar] [CrossRef]
- Aydin, D.; Yilmaz, I. Simple synthesis and sensing applications of a new and low cost fluorescent chemosensor for selective recognition and facile removal of Hg2+. J. Photochem. Photobiol. A Chem. 2021, 414, 113280. [Google Scholar] [CrossRef]
- Wen, D.; Deng, X.; Xu, G.; Wu, H.; Yu, Y. A novel FRET fluorescent probe based on BODIPY-rhodamine system for Hg2+ imaging in living cells. J. Mol. Struct. 2021, 1236, 130323. [Google Scholar] [CrossRef]
- Elbayoumy, E.; Elhendawy, M.; Gaafar, M.M.; Moawed, E.A.; Aboelnga, M.M. Novel fluorescent sensor based on triazole-pyridine derivative for selective detection of mercury (II) ions in different real water samples: Experimental and DFT calculations. J. Mol. Liq. 2024, 401, 124589. [Google Scholar] [CrossRef]
- Tang, L.; Li, P.; Han, Y.; Yang, G.; Xin, H.; Zhao, S.; Guan, R.; Liu, Z.; Cao, D. A fluorescein-based fluorescent probe for real-time monitoring hypochlorite. J. Photochem. Photobiol. A Chem. 2023, 438, 114511. [Google Scholar] [CrossRef]
- Aruna; Verma, V.P.; Singh, A.P.; Shrivastava, R. Recent advancement in development of fluorescein based molecular probes for analytes sensing. J. Mol. Struct. 2024, 1295, 136549. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Song, X.; Xia, Y.; Yu, H.; Zhang, X.; Xiao, Y.; Song, Y. Ratiometric imaging of mitochondrial pH in living cells with a colorimetric fluorescent probe based on fluorescein derivative. Sens. Actuators B Chem. 2017, 253, 58–68. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, B.; Ye, R.; Zhu, J.; Bao, X. Synthesis and evaluation of a new fluorescein and rhodamine B-based chemosensor for highly sensitive and selective detection of cysteine over other amino acids and its application in living cell imaging. Sens. Actuators B Chem. 2017, 251, 481–489. [Google Scholar] [CrossRef]
- Bao, X.; Cao, Q.; Wu, X.; Shu, H.; Zhou, B.; Geng, Y.; Zhu, J. Design and synthesis of a new selective fluorescent chemical sensor for Cu2+ based on a Pyrrole moiety and a Fluorescein conjugate. Tetrahedron Lett. 2016, 57, 942–948. [Google Scholar] [CrossRef]
- Yang, G.; Meng, X.; Fang, S.; Wang, L.; Wang, Z.; Wang, F.; Duan, H.; Hao, A. Two novel pyrazole-based chemosensors: “Naked-eye” colorimetric recognition of Ni2+ and Al3+ in alcohol and aqueous DMF media. New J. Chem. 2018, 42, 14630–14641. [Google Scholar] [CrossRef]
- Erdemir, S.; Yuksekogul, M.; Karakurt, S.; Kocyigit, O. Dual-channel fluorescent probe based on bisphenol A-rhodamine for Zn2+ and Hg2+ through different signaling mechanisms and its bioimaging studies. Sens. Actuators B Chem. 2017, 241, 230–238. [Google Scholar] [CrossRef]
- Joshi, S.; Kumari, S.; Sarmah, A.; Pant, D.D.; Sakhuja, R. Detection of Hg2+ ions in aqueous medium using an indole-based fluorescent probe: Experimental and theoretical investigations. J. Mol. Liq. 2017, 248, 668–677. [Google Scholar] [CrossRef]
- Chen, C.-G.; Vijay, N.; Thirumalaivasan, N.; Velmathi, S.; Wu, S.-P. Coumarin-based Hg2+ fluorescent probe: Fluorescence turn-on detection for Hg2+ bioimaging in living cells and zebrafish. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, Y.; Lin, W. A coumarin-based fluorescent probe for highly selective detection of hazardous mercury ions in living organisms. J. Hazard. Mater. 2024, 461, 132604. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Xu, K.; Gu, Y.; Meng, Z.; Gong, S.; Wang, Z.; Wang, S. A ratiometric fluorescent probe with a large Stokes shift for rapid and sensitive detection of Hg2+ in environmental water samples and its applications in living cells and zebrafish. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 319, 124522. [Google Scholar] [CrossRef] [PubMed]

| Reference | Solvent Medium | Stokes Shift (nm) | LOD (mol/L) |
|---|---|---|---|
| [33] | Not mentioned | 42.6 | 7.5 × 10−5 |
| [40] | MeCN/H2O (8:2) | Not mentioned | 2.24 × 10−6 |
| [41] | DMF/H2O (3:7) | 125 | 1.43 × 10−7 |
| [42] | MeCN/H2O (3:7) | 79 | 1.08 × 10−6 |
| [43] | DMF/PBS (1;9) | 130 | 6.3 × 10−7 |
| [44] | MeOH/H2O (2:8) | 200 | 2.36 × 10−7 |
| This work | DMSO/HEPES (1:9) | 273 | 9.75 × 10−8 |
| Sample Type | Spiked (μM) | Detected (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap water | 0 | - | ||
| 30.0 | 28.5695 | 95.23 | 1.24 | |
| 60.0 | 57.1808 | 95.30 | 2.19 | |
| 90.0 | 93.0635 | 103.40 | 1.31 | |
| River water | 0 | - | ||
| 30.0 | 28.6254 | 95.42 | 3.07 | |
| 60.0 | 57.5835 | 95.97 | 0.78 | |
| 90.0 | 88.9210 | 98.80 | 1.22 | |
| Lake water | 0 | - | ||
| 30.0 | 28.8827 | 96.28 | 1.31 | |
| 60.0 | 58.4151 | 97.36 | 1.66 | |
| 90.0 | 91.0609 | 101.18 | 2.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Lei, C.; Wang, Q.; He, Y.; Tian, S. A Novel Fluorescent Probe AP for Highly Selective and Sensitive Detection of Hg2+ and Its Application in Environmental Monitoring. Processes 2025, 13, 2306. https://doi.org/10.3390/pr13072306
Yang Z, Lei C, Wang Q, He Y, Tian S. A Novel Fluorescent Probe AP for Highly Selective and Sensitive Detection of Hg2+ and Its Application in Environmental Monitoring. Processes. 2025; 13(7):2306. https://doi.org/10.3390/pr13072306
Chicago/Turabian StyleYang, Zhi, Chaojie Lei, Qian Wang, Yonghui He, and Senlin Tian. 2025. "A Novel Fluorescent Probe AP for Highly Selective and Sensitive Detection of Hg2+ and Its Application in Environmental Monitoring" Processes 13, no. 7: 2306. https://doi.org/10.3390/pr13072306
APA StyleYang, Z., Lei, C., Wang, Q., He, Y., & Tian, S. (2025). A Novel Fluorescent Probe AP for Highly Selective and Sensitive Detection of Hg2+ and Its Application in Environmental Monitoring. Processes, 13(7), 2306. https://doi.org/10.3390/pr13072306







