Health Risk Assessment and Accumulation of Potentially Toxic Elements in Capsella bursa-pastoris (L.) Medik
Abstract
1. Introduction
2. Materials and Methods
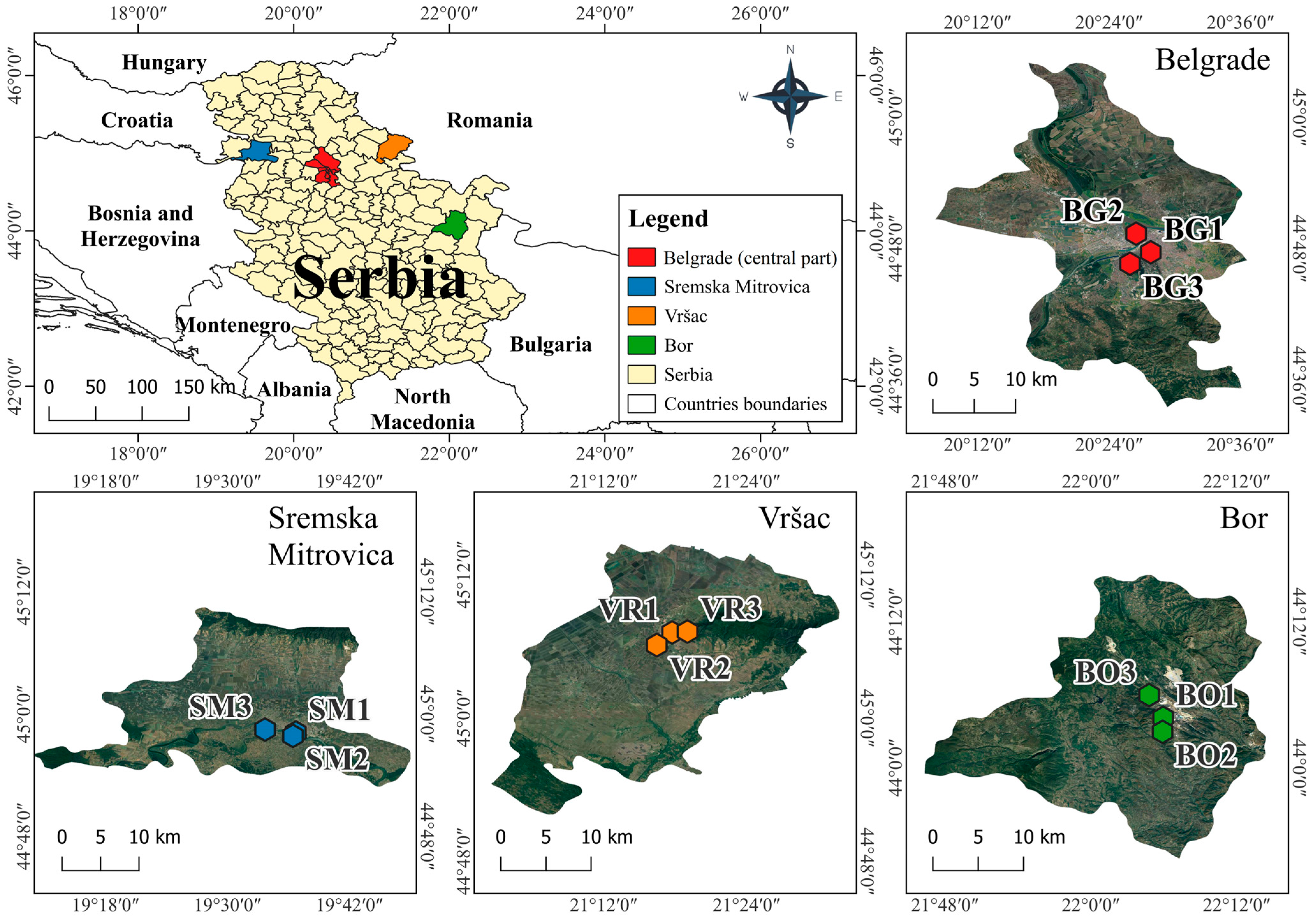
2.1. Sampling Site Description
2.2. Samples Preparation
2.2.1. Soils
2.2.2. Plant Material
2.2.3. Herbal Extracts
2.3. Determination of PTE Contents in Samples
2.4. Validation of the Method (QA/QC)
2.5. Health Risk Assessment
2.5.1. Non-Carcinogenic Risk
2.5.2. Carcinogenic Risk
2.6. Geo-Accumulation Index, Bioconcentration and Translocation Factors
2.7. Data Analysis
3. Results and Discussion
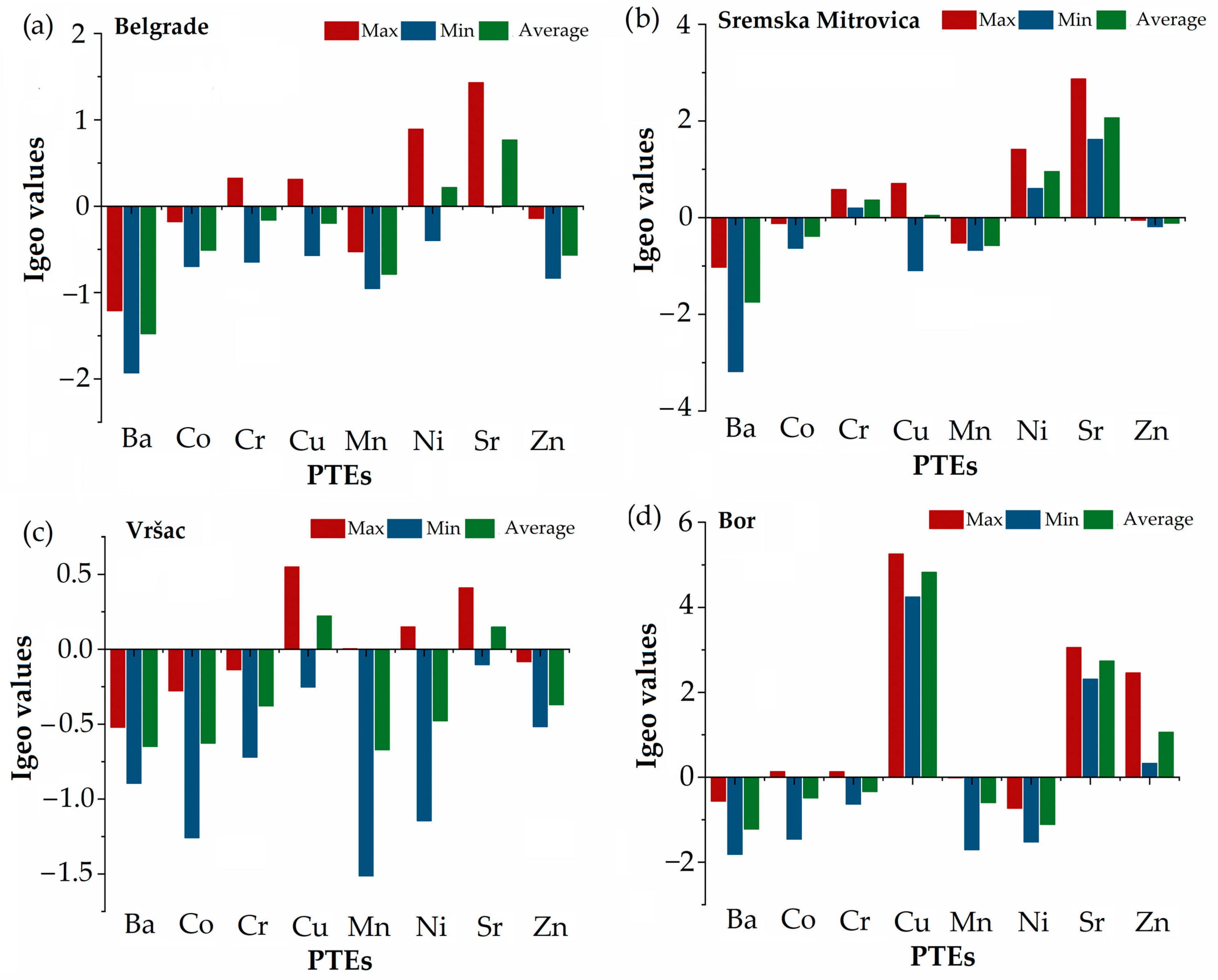
3.1. PTE Contents in Soils
3.2. PTE Contents in C. bursa-pastoris
3.3. Correlation Analysis
3.4. PCA Analysis
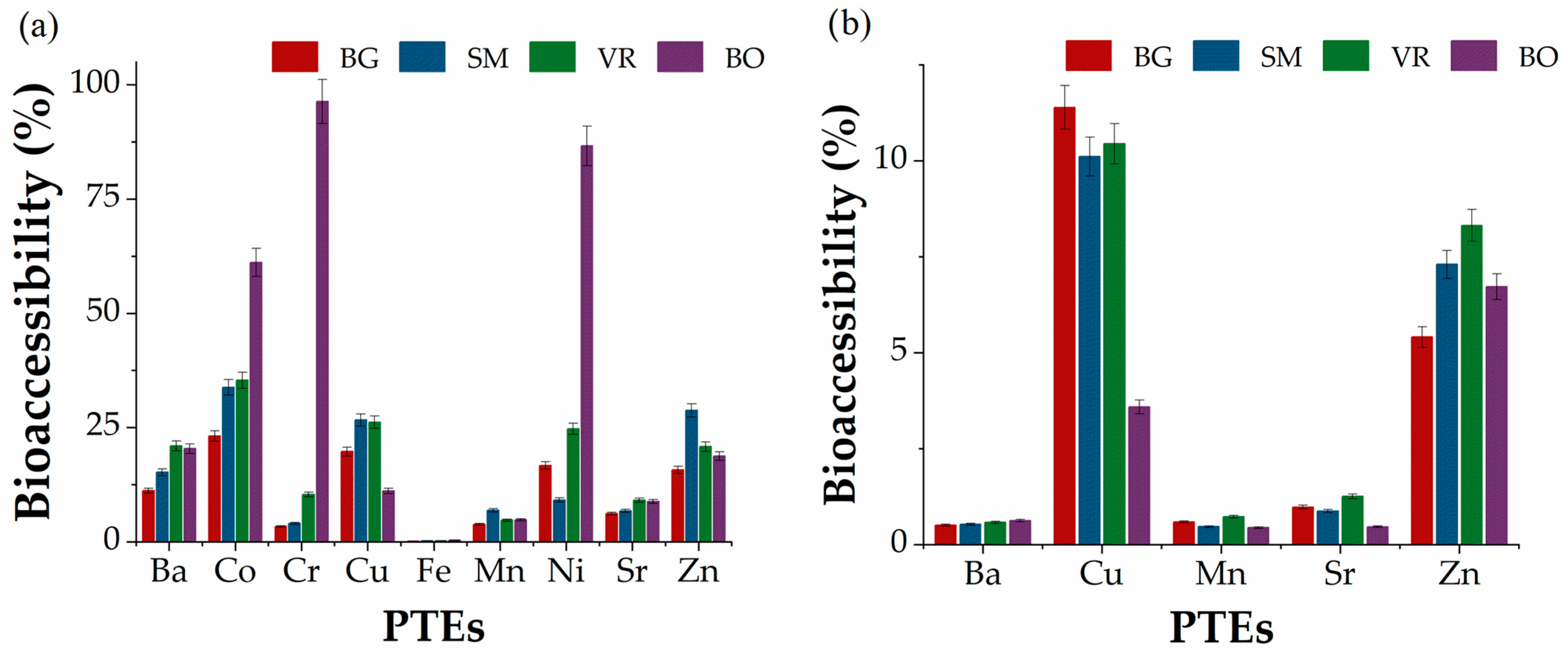
3.5. PTE Contents in Herbal Extracts
3.6. Health Risk Assessment
3.6.1. Risk Assessment of PTEs in Soils
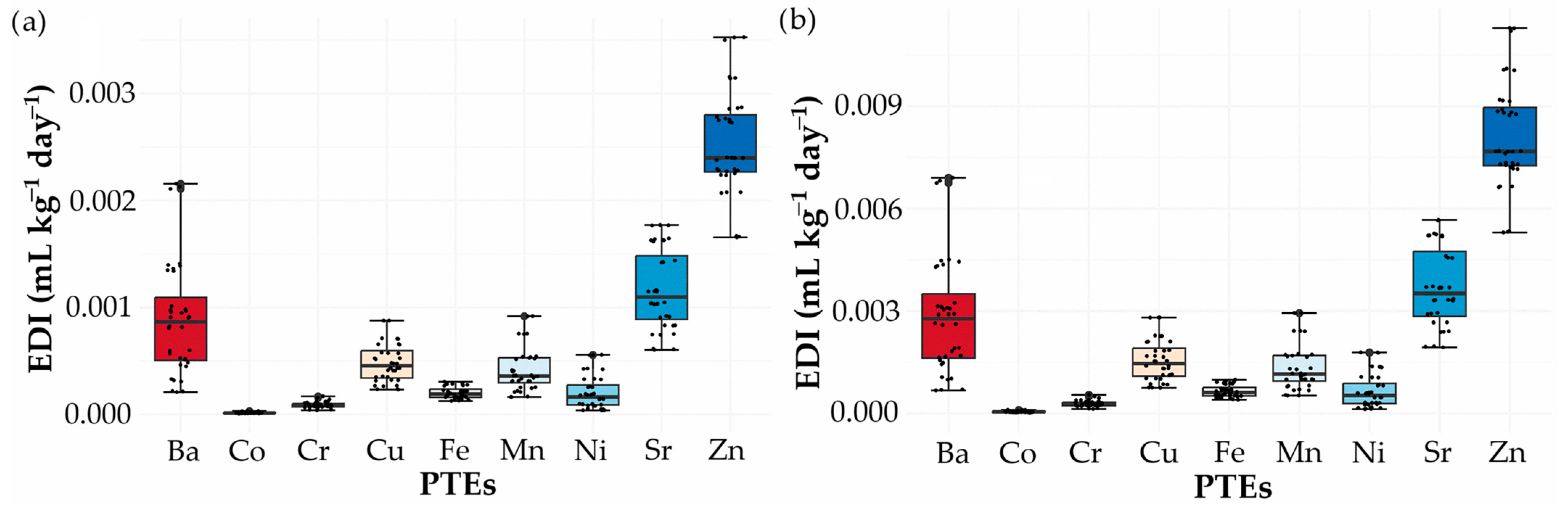
3.6.2. Risk Assessment of PTEs in Plants
3.6.3. Risk Assessment of PTEs in Herbal Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Keshvari, M.; Nedaeinia, R.; Nedaeinia, M.; Ferns, G.A.; Nia, S.N.; Asgary, S. Assessment of Heavy Metal Contamination in Herbal Medicinal Products Consumed in the Iranian Market. Environ. Sci. Pollut. Res. 2021, 28, 33208–33218. [Google Scholar] [CrossRef] [PubMed]
- Moniakowska, A.; Strumińska-Parulska, D. Assessment of Cancer Risk and Radiological Effects from 210Po and 210Pb with Consumption of Wild Medicinal Herbal Plants. J. Trace Elem. Med. Biol. 2024, 84, 127452. [Google Scholar] [CrossRef] [PubMed]
- Ivkovic, D.; Cvijetic, I.; Radoicic, A.; Stojkovic-Filipovic, J.; Trifkovic, J.; Krstic Ristivojevic, M.; Ristivojevic, P. NADES-Based Extracts of Selected Medicinal Herbs as Promising Formulations for Cosmetic Usage. Processes 2024, 12, 992. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Wu, H.; Lu, R.; Cui, J. Bioaccessibility and Bioavailability Evaluation of Heavy Metal(Loid)s in Ginger in Vitro: Relevance to Human Health Risk Assessment. Sci. Total Environ. 2023, 857, 159582. [Google Scholar] [CrossRef]
- Kong, D.; Li, X.; Yao, J.; He, Y.; Luo, J.; Yang, M. Health Risk Assessment and Bioaccessibility of Toxic Elements in Edible and Medicinal Plants under Different Consumption Methods. Microchem. J. 2020, 159, 105577. [Google Scholar] [CrossRef]
- Picking, D. PHARMACOGNOSY: Fundamentals, Applications and Strategies; ELSEVIER ACADEMIC PRESS: Cambridge, MA, USA, 2023; ISBN 978-0-443-18657-8. [Google Scholar]
- Kaur, H.; Garg, N. Zinc Toxicity in Plants: A Review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Štofejová, L.; Fazekaš, J.; Fazekašová, D. Transfer of Potentially Toxic Elements in the Soil-Plant System in Magnesite Mining and Processing Areas. Processes 2022, 10, 720. [Google Scholar] [CrossRef]
- Chen, L.; Fang, L.; Yang, X.; Luo, X.; Qiu, T.; Zeng, Y.; Huang, F.; Dong, F.; White, J.C.; Bolan, N.; et al. Sources and Human Health Risks Associated with Potentially Toxic Elements (PTEs) in Urban Dust: A Global Perspective. Environ. Int. 2024, 187, 108708. [Google Scholar] [CrossRef] [PubMed]
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent Developments in Essentiality of Trivalent Chromium and Toxicity of Hexavalent Chromium: Implications on Human Health and Remediation Strategies. J. Hazard. Mater. Adv. 2022, 7, 100113. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Chromium, Nickel and Welding. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 49; World Health Organization: Lyon, France, 1990; Available online: https://publications.iarc.fr/49 (accessed on 28 May 2025).
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Lison, D. Cobalt. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 221–242. ISBN 978-0-12-822946-0. [Google Scholar]
- Oskarsson, A. Barium. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 91–100. ISBN 978-0-12-822946-0. [Google Scholar]
- Marx, D.; Rahimnejad Yazdi, A.; Papini, M.; Towler, M. A Review of the Latest Insights into the Mechanism of Action of Strontium in Bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef]
- Augustsson, A.; Qvarforth, A.; Engström, E.; Paulukat, C.; Rodushkin, I. Trace and Major Elements in Food Supplements of Different Origin: Implications for Daily Intake Levels and Health Risks. Toxicol. Rep. 2021, 8, 1067–1080. [Google Scholar] [CrossRef]
- Jakovljević, K.; Mišljenović, T.; Savović, J.; Ranković, D.; Ranđelović, D.; Mihailović, N.; Jovanović, S. Accumulation of Trace Elements in Tussilago Farfara Colonizing Post-Flotation Tailing Sites in Serbia. Environ. Sci. Pollut. Res. 2020, 27, 4089–4103. [Google Scholar] [CrossRef]
- Dar, F.A.; Pirzadah, T.B.; Malik, B. Accumulation of Heavy Metals in Medicinal and Aromatic Plants. In Plant Micronutrients; Aftab, T., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 113–127. ISBN 978-3-030-49855-9. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Nahar, K.; Fujita, M. Selenium Toxicity in Plants and Environment: Biogeochemistry and Remediation Possibilities. Plants 2020, 9, 1711. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lee, S.-H.; Lee, J.; Kim, M.-S.; Lee, Y.-G.; Hwang, J.-T.; Choi, S.-Y.; Yoon, H.-G.; Lim, T.-G.; Lee, S.-H.; et al. Water Extract of Capsella Bursa-Pastoris Mitigates Doxorubicin-Induced Cardiotoxicity by Upregulating Antioxidant Enzymes. IJMS 2023, 24, 15912. [Google Scholar] [CrossRef]
- Ahmed, H.T.; Francis, A.; Clements, D.R.; Dyck, E.; Ross, N.; Upadhyaya, M.K.; Hall, L.M.; Martin, S.L. The Biology of Canadian Weeds. 159. Capsella Bursa-Pastoris(L.) Medik. Can. J. Plant Sci. 2022, 102, 529–552. [Google Scholar] [CrossRef]
- Mikavica, I.; Ranđelović, D.; Ilić, M.; Obradović, M.; Stojanović, J.; Mutić, J. Distribution of Microplastics in (Sub)Urban Soils of Serbia and Cd, As, and Pb Uptake by Capsella Bursa-Pastoris (L.) Medik. Chemosphere 2024, 363, 142891. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). U.S. Environmental Protection Agency (USEPA). Method 3050B: Acid Digestion of Sediments, Sludges, and Soils, Revision 2; Environmental Monitoring Systems Laboratory, Office of Research and Development, U.S. EPA: Cincinnati, OH, USA, 1996. Available online: https://www.epa.gov/esam/epa-method-3050b (accessed on 28 May 2025).
- McGrath, D. Application of Single and Sequential Extraction Procedures to Polluted and Unpolluted Soils. Sci. Total Environ. 1996, 178, 37–44. [Google Scholar] [CrossRef]
- USEPA Method 3051; Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils. Revision 1; EPA SW-846; U.S. Government Printing Office: Washington, DC, USA, 2007.
- Brasanac-Vukanovic, S.; Mutic, J.; Stankovic, D.M.; Arsic, I.; Blagojevic, N.; Vukasinovic-Pesic, V.; Tadic, V.M. Wild Bilberry (Vaccinium Myrtillus L., Ericaceae) from Montenegro as a Source of Antioxidants for Use in the Production of Nutraceuticals. Molecules 2018, 23, 1864. [Google Scholar] [CrossRef]
- USEPA. Regional Screening Levels (RSLs)—Equations. 2024. Available online: www.epa.gov/risk/regional-screening-levels-rsls-equations (accessed on 28 May 2025).
- USEPA. Regional Screening Levels (RSLs)—User’s Guide. 2024. Available online: www.epa.gov/risk/regional-screening-levels-rsls-users-guide (accessed on 28 May 2025).
- USEPA. Risk assessment guidance for superfund, Volume I: Human health evaluation manual (Part A), Interim Final, Office of Emergency and Remedial Response, EPA/540/1-89/0 02. 1989. Available online: https://www.epa.gov/sites/default/files/ (accessed on 28 May 2025).
- USEPA. Guidelines for Carcinogen Risk Assessment. 2024. Available online: https://www.epa.gov/risk/guidelines-carcinogen-risk-assessment (accessed on 28 May 2025).
- Müller, G. Index of Geoaccumulation in the Sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Yang, C.; Yu, G.; Liu, Y.; Shan, B.; Wang, L.; Sun, D.; Huang, Y. Heavy Metal Distribution in Surface Sediments of the Coastal Pearl Bay, South China Sea. Processes 2022, 10, 822. [Google Scholar] [CrossRef]
- Alloway, B.J. (Ed.) Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Environmental Pollution; Springer Netherlands: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-4469-1. [Google Scholar]
- Aničić Urošević, M.; Vuković, G.; Vasić, P.; Jakšić, T.; Nikolić, D.; Škrivanj, S.; Popović, A. Environmental Implication Indices from Elemental Characterisations of Collocated Topsoil and Moss Samples. Ecol. Indic. 2018, 90, 529–539. [Google Scholar] [CrossRef]
- Tarvainen, T.; Salminen, R.; Vos, W.D. Geochemical Atlas of Europe. Part 1: Background Information, Methodology and Maps; Geological Survey of Finland: Espoo, Finland, 2005; ISBN 978-951-690-913-7. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-0-429-19203-6. [Google Scholar]
- Chen, X.; Ren, Y.; Li, C.; Shang, Y.; Ji, R.; Yao, D.; He, Y. Pollution Characteristics and Ecological Risk Assessment of Typical Heavy Metals in the Soil of the Heavy Industrial City Baotou. Processes 2025, 13, 170. [Google Scholar] [CrossRef]
- Official Gazette of RS, No.30/2018 and 64/2019. Regulation on the Limit Values of Pollutant, Harmful and Dangerous Substances in the Soil (in Serbian: Uredba o Graničnim Vrednostima Zagađujućih, Štetnih i Opasnih Materija u Zemljištu). Available online:Https://Www.Ekologija.Gov.Rs/Sites/Default/Files/Old-Documents/Zemljiste/Uredbe/Uredba-o-Granicnim/Vrednostima.Pdf (accessed on 28 May 2025).
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shahid, M. Copper Uptake, Essentiality, Toxicity, Detoxification and Risk Assessment in Soil-Plant Environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- Djordjević, V.; Lakušić, D.; Novković, I.; Stevanović, V.; Tsiftsis, S. Factors Influencing Orchid Species Richness in the Central Balkans: The Importance of Belowground Organ Types. Plants 2025, 14, 443. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Lichtscheidl, I. Strontium in the Environment: Review about Reactions of Plants towards Stable and Radioactive Strontium Isotopes. Sci. Total Environ. 2019, 653, 1458–1512. [Google Scholar] [CrossRef]
- Filimon, M.N.; Caraba, I.V.; Popescu, R.; Dumitrescu, G.; Verdes, D.; Petculescu Ciochina, L.; Sinitean, A. Potential Ecological and Human Health Risks of Heavy Metals in Soils in Selected Copper Mining Areas—A Case Study: The Bor Area. Int. J. Environ. Res. Public Health 2021, 18, 1516. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Alvarez, J.M.; Orellana-Gallego, R.; Fernandez-Marcos, M.L. Potentially Toxic Elements in Urban Soils of Havana, Cuba. Environments 2020, 7, 43. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Ma, J.; Zhao, W.; Chen, H.; Qu, Y. Antimony, Beryllium, Cobalt, and Vanadium in Urban Park Soils in Beijing: Machine Learning-Based Source Identification and Health Risk-Based Soil Environmental Criteria. Environ. Pollut. 2022, 293, 118554. [Google Scholar] [CrossRef]
- Pavlović, D.; Pavlović, M.; Perović, V.; Mataruga, Z.; Čakmak, D.; Mitrović, M.; Pavlović, P. Chemical Fractionation, Environmental, and Human Health Risk Assessment of Potentially Toxic Elements in Soil of Industrialised Urban Areas in Serbia. Int. J. Environ. Res. Public Health 2021, 18, 9412. [Google Scholar] [CrossRef]
- Drozdova, I.; Alekseeva-Popova, N.; Dorofeyev, V.; Bech, J.; Belyaeva, A.; Roca, N. A Comparative Study of the Accumulation of Trace Elements in Brassicaceae Plant Species with Phytoremediation Potential. Appl. Geochem. 2019, 108, 104377. [Google Scholar] [CrossRef]
- Aksoy, A.; Hale, W.H.G.; Dixon, J.M. Capsella Bursa-Pastoris (L.) Medic. as a Biomonitor of Heavy Metals. Sci. Total Environ. 1999, 226, 177–186. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Food and Agriculture Organization/Word Health Organization (FAOP/WHO). FAO/WHO, Food Contaminants. In Codex Alimentarius, 1st ed.; FAO/WHO, Codex Alimentarius Commission: Rome, Italy, 1984; Volume XVII. [Google Scholar]
- Kohzadi, S.; Shahmoradi, B.; Ghaderi, E.; Loqmani, H.; Maleki, A. Concentration, Source, and Potential Human Health Risk of Heavy Metals in the Commonly Consumed Medicinal Plants. Biol. Trace Elem. Res. 2019, 187, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the Mechanisms of Plant Tolerance to Manganese Toxicity. IJMS 2019, 20, 5096. [Google Scholar] [CrossRef]
- Liang, X.; Wang, L.; Xu, L.; Chi, H.; Lin, W. Development of a Novel NIR-II Fluorescence Probe for Monitoring Serum Albumin Fluctuation in Cerebra Neurotoxicity Induced by Manganese Exposure. J. Hazard. Mater. 2025, 485, 136936. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium Bioaccumulation and Its Impacts on Plants: An Overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Belanović Simić, S.; Miljković, P.; Baumgertel, A.; Lukić, S.; Ljubičić, J.; Čakmak, D. Environmental and Health Risk Assessment Due to Potentially Toxic Elements in Soil near Former Antimony Mine in Western Serbia. Land 2023, 12, 421. [Google Scholar] [CrossRef]

| Ba | Co | Cr | Cu | Fe | Mn | Ni | Sr | Zn | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EDTA | BG | mean | 7.16 ab | 1.41 ab | 0.06 a | 7.63 b | 168.79 a | 156.92 a | 3.13 ab | 16.46 a | 15.82 b |
| min | 3.96 | 0.81 | 0.01 | 5.41 | 88.07 | 99.1 | 2.58 | 13.75 | 13.02 | ||
| max | 9.26 | 2.17 | 0.13 | 10.15 | 269.6 | 243.76 | 3.46 | 20.69 | 17.27 | ||
| stdev | 2.42 | 0.59 | 0.05 | 1.98 | 78.91 | 64.96 | 0.39 | 2.94 | 2.02 | ||
| SM | mean | 2.85 b | 0.55 ab | 0.04 a | 8.66 b | 154.65 a | 112.41 a | 3.11 a | 14.25 ab | 18.50 b | |
| min | 1.22 | 0.39 | 0 | 2.22 | 112.53 | 87.95 | 1.35 | 10.64 | 11.77 | ||
| max | 4.3 | 0.66 | 0.12 | 12.37 | 220.55 | 142.03 | 4.51 | 20.41 | 25.29 | ||
| stdev | 1.33 | 0.11 | 0.05 | 4.73 | 48.99 | 22.83 | 1.33 | 4.26 | 5.66 | ||
| VR | mean | 11.19 a | 1.80 a | 0.10 a | 11.97 ab | 234.10 a | 161.39 a | 2.73 ab | 12.02 ab | 23.72 ab | |
| min | 9.64 | 1.23 | 0.05 | 7.86 | 177.34 | 110.89 | 2.03 | 8.87 | 18.79 | ||
| max | 12.48 | 2.82 | 0.16 | 14.5 | 266.71 | 232.61 | 3.82 | 16.07 | 26.57 | ||
| stdev | 1.21 | 0.76 | 0.04 | 3.1 | 41.23 | 53.07 | 0.82 | 3.12 | 3.68 | ||
| BO | mean | 3.91 ab | 1.10 ab | 0.08 a | 279.74 a | 252.20 a | 140.88 a | 0.90 b | 10.68 b | 85.87 a | |
| min | 2.07 | 0.18 | 0 | 159.34 | 93.49 | 49.42 | 0.24 | 7.16 | 40.4 | ||
| max | 5.17 | 1.65 | 0.18 | 440.01 | 335.31 | 194.32 | 1.31 | 12.65 | 174.6 | ||
| stdev | 1.4 | 0.69 | 0.07 | 124.4 | 118.49 | 68.59 | 0.5 | 2.6 | 65.89 | ||
| p value | *** | ** | ns | *** | ns | ns | *** | * | *** | ||
| AR | BG | mean | 35.79 b | 7.45 a | 30.45 ab | 16.21 b | 14,902.54 a | 334.13 a | 26.02 ab | 41.30 ab | 49.70 b |
| min | 25.6 | 6.47 | 21.08 | 12.11 | 12,237.57 | 295.74 | 15.94 | 22.36 | 40.39 | ||
| max | 42.12 | 9.27 | 41.33 | 22.35 | 18,468.96 | 397.37 | 38.99 | 60.66 | 65.23 | ||
| stdev | 7.37 | 1.18 | 8.03 | 4.48 | 2735.76 | 42.21 | 9.98 | 16.2 | 11.63 | ||
| SM | mean | 34.85 ab | 8.10 a | 42.69 a | 21.11 ab | 16,613.58 a | 384.13 a | 41.86 a | 101.84 ab | 66.50 ab | |
| min | 10.72 | 6.77 | 37.91 | 8.42 | 14,773.04 | 358.53 | 31.92 | 69.16 | 63.2 | ||
| max | 47.82 | 9.64 | 49.46 | 29.42 | 17,681.63 | 397.36 | 56.01 | 164.67 | 69.38 | ||
| stdev | 17.77 | 1.15 | 4.49 | 9.46 | 1241.56 | 15.18 | 10.7 | 44.78 | 2.48 | ||
| VR | mean | 62.43 a | 7.05 a | 25.69 b | 21.55 ab | 14,568.10 a | 391.37 a | 16.06 ab | 25.18 b | 56.23 ab | |
| min | 52.43 | 4.39 | 20.03 | 15.11 | 9677.42 | 200.75 | 9.49 | 20.94 | 50.31 | ||
| max | 67.93 | 8.66 | 30 | 26.35 | 18,839.49 | 574.74 | 23.29 | 29.91 | 67.94 | ||
| stdev | 5.89 | 1.87 | 4.06 | 4.9 | 3876.18 | 156.76 | 5.86 | 3.61 | 8.58 | ||
| BO | mean | 44.40 ab | 8.18 a | 26.77 ab | 533.16 a | 14,189.69 a | 428.17 a | 9.92 b | 153.48 a | 193.47 a | |
| min | 27.7 | 3.82 | 21.26 | 341.51 | 11,391.85 | 175.56 | 7.3 | 111.8 | 90.52 | ||
| max | 65.93 | 11.54 | 36.17 | 689.95 | 16,409.97 | 566.82 | 12.65 | 187.39 | 396.04 | ||
| stdev | 16.23 | 3.25 | 6.57 | 151.53 | 2083.09 | 188.18 | 2.13 | 32.26 | 151.1 | ||
| p value | *** | ns | *** | *** | ns | ns | *** | *** | *** | ||
| European average | 85.2 | 8.91 | 32.6 | 16.4 | / | 524 | 30.7 | 130 | 60.9 | ||
| World average | 19–2368 | 25 | 54 | 13–24 | 5% | 437 | 22 | 87–210 | 64 | ||
| Remediation values | 625 | 240 | 30 | 75 | / | / | 210 | / | 720 | ||
| Ba | Co | Cr | Cu | Fe | Mn | Ni | Sr | Zn | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roots | BG | mean | 26.33 a | 0.43 a | 12.85 a | 5.10 ab | 792.52 a | 35.91 a | 7.90 a | 40.03 ab | 39.24 ab |
| min | 14.6 | 0.05 | 2.37 | 3.23 | 201.85 | 14.88 | 3.68 | 17.97 | 30.49 | ||
| max | 35.19 | 0.79 | 18.59 | 6.51 | 1387.47 | 54.34 | 10.89 | 52.9 | 44.21 | ||
| stdev | 9.05 | 0.32 | 7.84 | 1.43 | 510.56 | 17.02 | 3.22 | 16.54 | 6.49 | ||
| SM | mean | 17.48 a | 0.30 a | 9.04 a | 5.99 ab | 566.93 a | 29.74 a | 5.90 a | 34.21 ab | 37.46 ab | |
| min | 11.58 | 0.12 | 6.07 | 4.22 | 271.39 | 18.76 | 4.61 | 30.96 | 32.82 | ||
| max | 21.02 | 0.42 | 14.33 | 7.94 | 799.19 | 36.72 | 7.46 | 37.89 | 45.96 | ||
| stdev | 4.39 | 0.13 | 3.87 | 1.57 | 229.49 | 8.2 | 1.17 | 2.82 | 6.34 | ||
| VR | mean | 20.89 a | 0.16 ab | 5.58 ab | 4.57 b | 547.11 a | 22.47 a | 3.25 ab | 27.18 b | 30.39 b | |
| min | 12.42 | 0.1 | 3.72 | 4.14 | 330.89 | 17.18 | 2.16 | 23 | 23.54 | ||
| max | 25.54 | 0.22 | 7.53 | 5.35 | 728.52 | 26.23 | 4.28 | 31.37 | 39.92 | ||
| stdev | 6.27 | 0.04 | 1.5 | 0.52 | 171.65 | 4.01 | 0.89 | 3.51 | 7.17 | ||
| BO | mean | 17.64 a | 0.09 b | 2.54 b | 23.36 a | 353.93 a | 24.32 a | 1.68 b | 57.04 a | 68.98 a | |
| min | 15.84 | 0.02 | 1.53 | 19 | 158.93 | 13.85 | 0.45 | 36.18 | 37.12 | ||
| max | 19.75 | 0.14 | 4.1 | 30.74 | 512.1 | 32.72 | 3.22 | 69.22 | 86.37 | ||
| stdev | 1.6 | 0.05 | 1.12 | 5.53 | 152.44 | 8.16 | 1.2 | 15.2 | 23.84 | ||
| p value | ns | * | ** | *** | ns | ns | *** | *** | ** | ||
| Shoots | BG | mean | 20.62 ab | 0.27 a | 9.27 a | 5.57 ab | 480.00 a | 30.75 a | 6.26 a | 49.56 ab | 45.48 a |
| min | 19.57 | 0.19 | 5.07 | 4.79 | 330.31 | 27.18 | 4.64 | 30.85 | 30.41 | ||
| max | 21.81 | 0.38 | 13.9 | 7.02 | 604.98 | 33.01 | 9.17 | 59.58 | 55.98 | ||
| stdev | 0.88 | 0.07 | 3.76 | 1.08 | 119.46 | 2.58 | 2.16 | 13.69 | 11.37 | ||
| SM | mean | 14.97 ab | 0.15 ab | 5.19 a | 5.47 ab | 343.61 ab | 29.24 ab | 6.03 a | 42.67 ab | 30.74 a | |
| min | 13.98 | 0.03 | 4.63 | 3.79 | 309.7 | 21.53 | 2.06 | 29.06 | 22.94 | ||
| max | 16.89 | 0.23 | 5.98 | 6.58 | 400.51 | 34.2 | 13.15 | 52.72 | 36.69 | ||
| stdev | 1.36 | 0.09 | 0.58 | 1.25 | 40.66 | 5.74 | 5.29 | 10.23 | 6 | ||
| VR | mean | 19.60 a | 0.14 ab | 3.54 ab | 5.40 b | 364.11 ab | 26.12 ab | 1.87 ab | 39.21 b | 33.84 a | |
| min | 12.5 | 0.09 | 2.94 | 3.71 | 302.29 | 22.35 | 1.15 | 34.28 | 30.98 | ||
| max | 23.58 | 0.18 | 4.33 | 6.97 | 486.55 | 31.3 | 2.76 | 45.64 | 35.87 | ||
| stdev | 5.21 | 0.03 | 0.56 | 1.37 | 89.61 | 3.88 | 0.69 | 4.91 | 2.11 | ||
| BO | mean | 13.11 b | 0.05 b | 0.27 b | 20.21 a | 175.28 b | 21.61 b | 0.63 b | 60.78 a | 49.99 a | |
| min | 6.97 | 0.02 | 0.09 | 10.48 | 123.66 | 15.71 | 0.34 | 39.51 | 32.81 | ||
| max | 22.02 | 0.1 | 0.58 | 34.05 | 215.31 | 24.91 | 0.94 | 81.59 | 67.81 | ||
| stdev | 6.71 | 0.04 | 0.22 | 10.56 | 39.46 | 4.31 | 0.23 | 17.78 | 15.04 | ||
| p value | * | *** | *** | *** | *** | ** | *** | * | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikavica, I.; Ranđelović, D.; Ilić, M.; Simić, M.; Petrović, J.; Koprivica, M.; Mutić, J. Health Risk Assessment and Accumulation of Potentially Toxic Elements in Capsella bursa-pastoris (L.) Medik. Processes 2025, 13, 2222. https://doi.org/10.3390/pr13072222
Mikavica I, Ranđelović D, Ilić M, Simić M, Petrović J, Koprivica M, Mutić J. Health Risk Assessment and Accumulation of Potentially Toxic Elements in Capsella bursa-pastoris (L.) Medik. Processes. 2025; 13(7):2222. https://doi.org/10.3390/pr13072222
Chicago/Turabian StyleMikavica, Ivana, Dragana Ranđelović, Miloš Ilić, Marija Simić, Jelena Petrović, Marija Koprivica, and Jelena Mutić. 2025. "Health Risk Assessment and Accumulation of Potentially Toxic Elements in Capsella bursa-pastoris (L.) Medik" Processes 13, no. 7: 2222. https://doi.org/10.3390/pr13072222
APA StyleMikavica, I., Ranđelović, D., Ilić, M., Simić, M., Petrović, J., Koprivica, M., & Mutić, J. (2025). Health Risk Assessment and Accumulation of Potentially Toxic Elements in Capsella bursa-pastoris (L.) Medik. Processes, 13(7), 2222. https://doi.org/10.3390/pr13072222











