A Green and Simple Analytical Method for the Evaluation of the Effects of Zn Fertilization on Pecan Crops Using EDXRF
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples’ Origin and Preparation
2.2. Zinc Determination by FAAS
2.3. Zinc Determination by EDXRF
3. Results
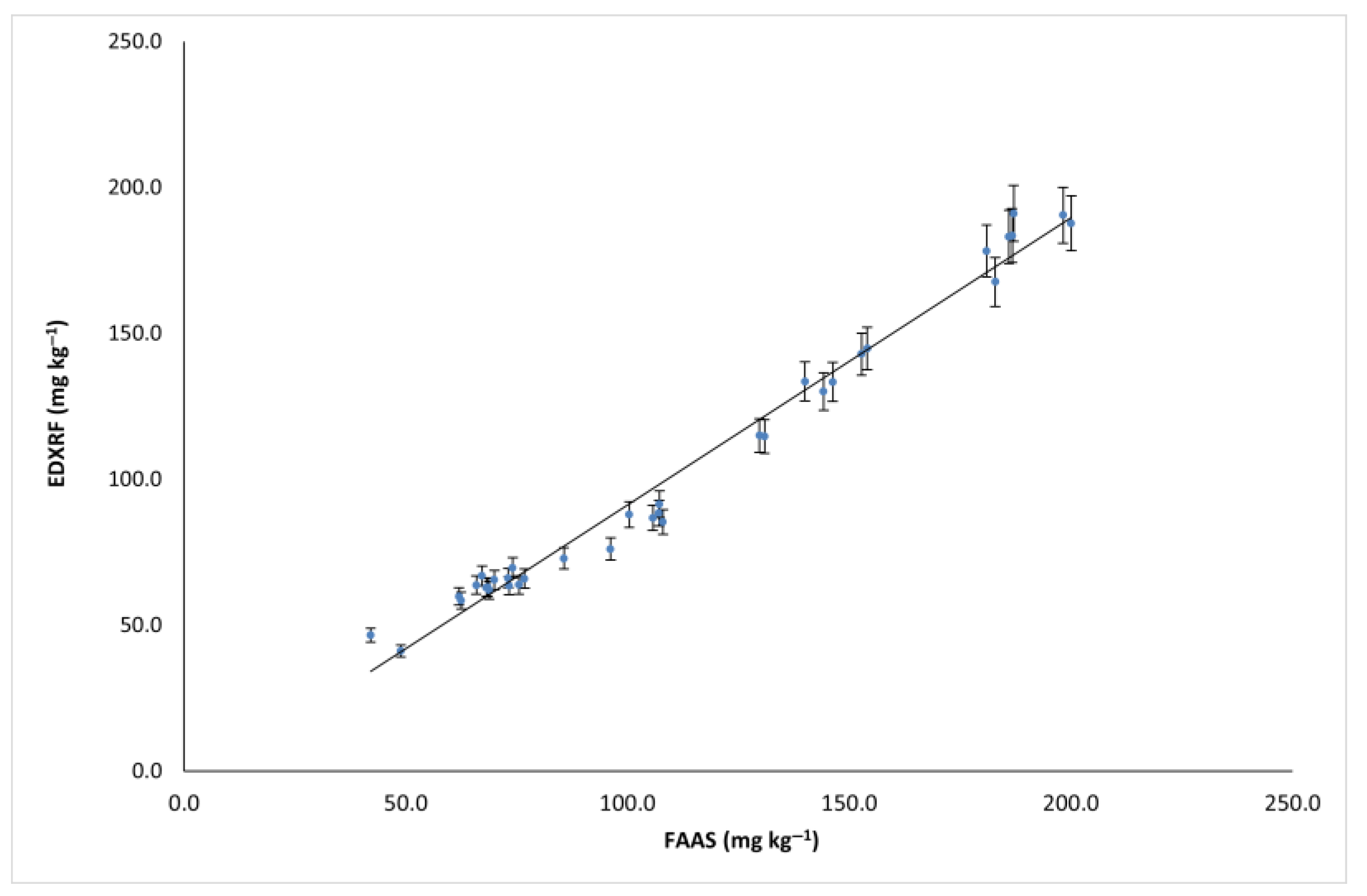
3.1. Validation
3.2. Analytical Determinations and Statistical Comparison
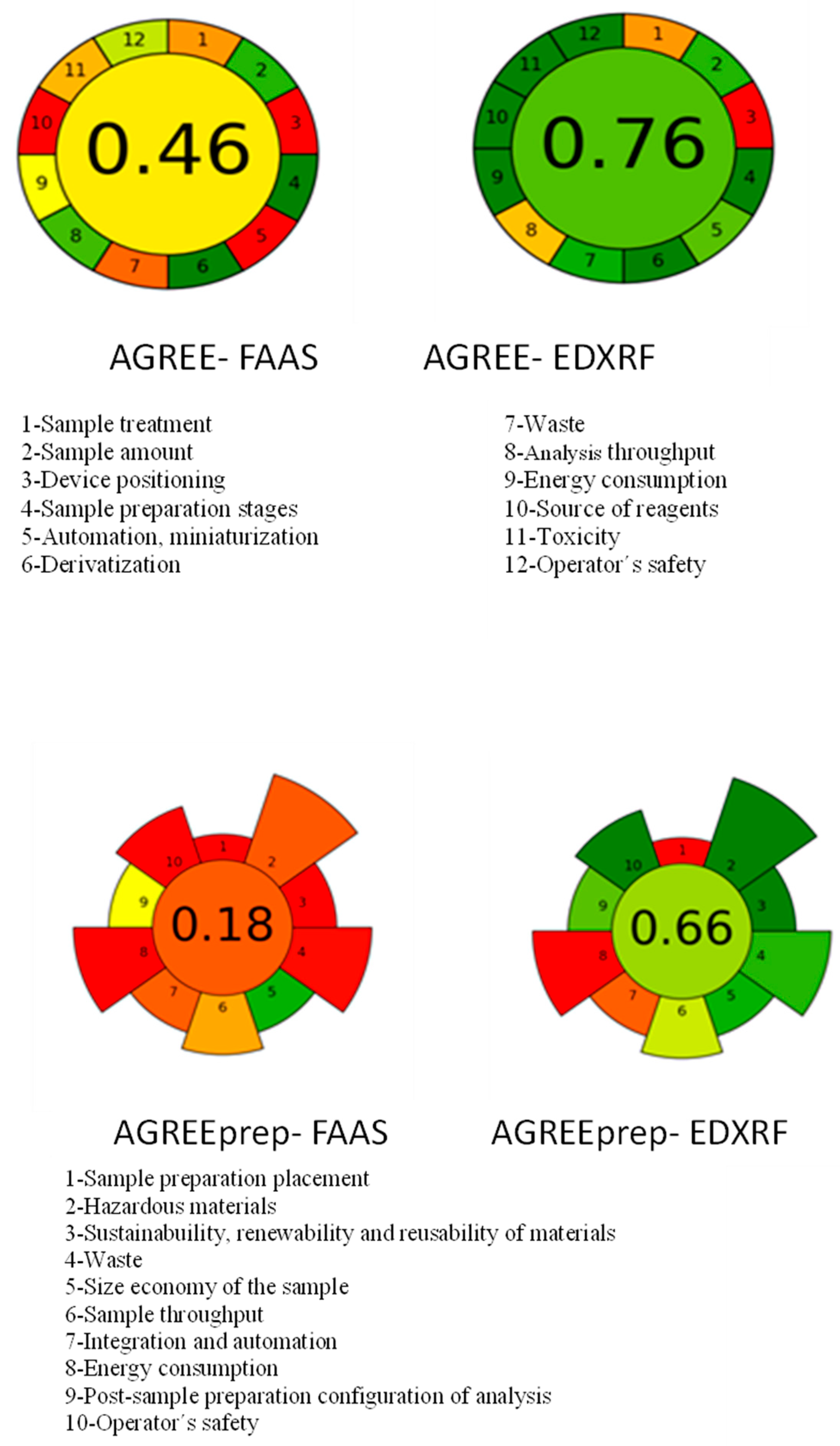
3.3. Green Analytical Chemistry Approach
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAAS | Flame Atomic Absorption Spectrometry |
| EDXRF | Energy Dispersive X-ray Spectrometry |
| AGREE | Analytical Greenness Metric Approach (also AGREEprep) |
| CRM | Certified Reference Material |
| SRM | Standard Reference Material |
| RSD | Relative Standard Deviation |
| INIA | Agricultural Research Institute |
| HCL | Hollow-Cathode Lamp |
| NIST | National Institute of Standards and Technology |
| AOAC | Association of Official Analytical Chemists |
References
- Vargas Piedra, G.; Arriola Ávila, J. Response of Pecan (Carya illinoensis K. Koch) to foliar applications of nutrients. In Revista Chapingo Serie Zonas Áridas; Universidad Autónoma Chapingo: Durango, Mexico, 2008; Volume VII, pp. 7–14. [Google Scholar]
- Cambareri, G.; Frusso, E.; Herrera-Aguirre, E.; Zoppolo, R.; Figueiredo Granja Dorileo Leite, F.; Beltrán, M.; Martins, C.; Mendoza, C. Contribution of pecan (Carya illinoinensis [Wangenh K. Koch]) to Sustainable Development Goal 2 under the dual perspective of carbon storage and human nutrition. Front. Soil Sci. 2023, 3, 1092003. [Google Scholar] [CrossRef]
- Ferrari, V.; Gil, G.; Heinze, H.; Zoppolo, R.; Ibáñez, F. Influence of Cultivar on Nutritional Composition and Nutraceutical Potential of Pecan Growing in Uruguay. Front. Nutr. 2022, 9, 868054. [Google Scholar] [CrossRef] [PubMed]
- Fasiolo, C.; Zoppolo, R. Alternativa para la producción frutícola: Nuez pecán. In Revista INIA Uruguay n° 38; Instituto Nacional de Investigación Agropecuaria: Canelones, Uruguay, 2014; pp. 37–42. [Google Scholar]
- Varela, V.; Takata, V.; Camussi, G.; Zoppolo, R. Pecan: Viability of a new crop in Uruguay. Acta Hort. 2015, 1070, 245–251. [Google Scholar] [CrossRef]
- Madrigal-Soteno, N.; Ojeda-Barrios, D.; Guerrero-Prieto, V.; Ávila-Quezada, G.; Parra-Quezada, R. Bioavailable zinc in soil for pecan tree nutrition. In Revista Chapingo Serie Zonas Áridas; Universidad Autónoma Chapingo: Durango, Mexico, 2016; Volume XV, pp. 1–7. [Google Scholar] [CrossRef]
- Villamil, J.; Conde, P.; Bianchi, D.; Leoni, C.; Zoppolo, R. Cultivares de nuez pecán, manejo y parámetros productivos. In Revista INIA Uruguay n° 67; Instituto Nacional de Investigación Agropecuaria: Canelones, Uruguay, 2017; pp. 53–58. [Google Scholar]
- Ojeda-Barrios, D.; Hernández-Rodríguez, O.; Martínez-Téllez, J.; Núñez-Barrios, A.; Perea-Portillo, E. Foliar application of zinc chelates on pecan. Rev. Chapingo Ser. Hortic. 2009, 15, 205–210. [Google Scholar] [CrossRef]
- Nuñez, H.; Walworth, J.; Pond, A.; Kilby, M. Soil Zinc fertilization of ‘Wichita’ Pecan trees growing under alkaline soil conditions. HortScience 2009, 44, 1736–1740. [Google Scholar] [CrossRef]
- Roholla Musavi, S.; Galavi, M.; Rezaei, M. Zinc importance for crop production-a review. Int. J. Plant Prod. 2013, 4, 64–68. [Google Scholar]
- Bonilla, I. Introducción a la nutrición mineral de las plantas. Los elementos minerales. In Fundamentos De Fisiología Vegetal, 2nd ed.; McGraw-Hill Interamericana de España, S.L., Ed.; Publicacions i Edicions de la Universitat de Barcelona: Barcelona, Spain, 2013; pp. 103–122. [Google Scholar]
- Ojeda-Barrios, D.; Perea-Portillo, E.; Hernández-Rodríguez, A.; Ávila-Quezada, G.; Abadía, J.; Lombardini, L. Foliar Fertilization with zinc in Pecan trees. HortScience 2014, 49, 562–566. [Google Scholar] [CrossRef]
- Smith, M.; Rohla, C.; Goff, W. Pecan leaf elemental sufficiency ranges and fertilizer recommendations. HortTechnology 2012, 22, 594–599. [Google Scholar] [CrossRef]
- Machado, I.; Dol, I.; Rodríguez-Arce, E.; Cesio, M.; Pistón, M. Comparison of different sample treatments for the determination of As, Cd, Cu, Ni, Pb and Zn in globe artichoke (Cynara cardunculus L. subsp. Cardunculus). Microchem. J. 2016, 128, 128–133. [Google Scholar] [CrossRef]
- Soylak, M.; Tuzen, M.; Santos Souza, A.; Andrade Korn, M.; Costa Ferreira, S. Optimization of microwave assisted digestion procedure for the determination of zinc, copper and nickel in tea samples employing flame atomic absorption spectrometry. J. Hazard. Mater. 2007, 149, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Bühl, V.; Iaquinta, F.; Pistón, M. Should we think about green or white analytical chemistry? Case study: Accelerated sample preparation using an ultrasonic bath for the simultaneous determination of Mn and Fe in beef. Heliyon 2023, 9, e20967. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2025; Available online: https://doi.org/10.1093/oso/9780198506980.001.0001 (accessed on 25 May 2025).
- Keith, L.; Gron, L.; Young, J. Green Analytical Methodologies. Chem. Rev. 2007, 107, 2695–2708. [Google Scholar] [CrossRef] [PubMed]
- De La Guardia, M.; Garrigues, S. Past, present and future of Green Analytical Chemistry. In Challenges in Green Analytical Chemistry, 2nd ed.; The Royal Society of Chemistry: London, UK, 2020; pp. 1–18. [Google Scholar]
- Manju Singh, R.M.; Sonali Mishra, N.G.; Karuna Shanker, N.G.; Birendra, K. Ultra performance liquid chromatography coupled with principal component and cluster analysis of Swertia chirayita for adulteration check. J. Pharm. Biomed. Anal. 2019, 164, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists—AOAC International. Metals and Other Elements at Trace Levels in Foods; Chapter 9. In AOAC Official Methods of Analysis; Association of Official Analytical Chemists—AOAC International: Rockville, MD, USA, 2016; pp. 16–17. [Google Scholar]
- Thomsen, V. Basic Fundamental Parameters in X-Ray Fluorescence. Spectroscopy 2007, 22, 46–50. [Google Scholar]
- Eurachem. Eurachem. Eurachem Guide. In The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Magnusson, B., Örnemark, U., Eds.; LGC: London, UK, 2014; Available online: https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf (accessed on 25 May 2025).
- Association of Official Analytical Chemists—AOAC International. Guidelines for Standard Method Performance Requirements (Appendix F). In AOAC Official Methods of Analysis; Association of Official Analytical Chemists—AOAC International: Rockville, MD, USA, 2016; pp. 1–18. [Google Scholar]
- Miller, J.; Miller, J. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Limited 2000: Edinburg, UK, 2010; pp. 39–43. [Google Scholar]
| Parameter | FAAS | EDXRF | |
|---|---|---|---|
| Linear range (mg L−1) | 0.049–1.5 | Not applicable | |
| Limit of detection (mg kg−1), n = 10 | 1.3 | 1.7 | |
| Limit of quantification (mg kg−1), n = 10 | 4.2 | 5.8 | |
| Intermediate precision (RSD%) | <7% (n = 5) | <13% (n = 10) | |
| Zn * (mg kg−1) | 11.4–13.5 | 11.7–13.1 | |
| Trueness ** | 91.4–108.2 (n = 5) | 94.0–105.2 (n = 7) | |
| Sample Code | AAS | EDXRF | Sample Code | AAS | EDXRF |
|---|---|---|---|---|---|
| 1 | 67.2 ± 2.8 | 66.8 ± 3.3 | 19 | 198.2 ± 1.7 | 190.4 ± 5.9 |
| 2 | 68.5 ± 3.0 | 63.0 ± 3.6 | 20 | 186.7 ± 1.9 | 183.4 ± 6.3 |
| 3 | 73.1 ± 3.6 | 66.1 ± 1.9 | 21 | 154.1 ± 2.1 | 144.7 ± 6.8 |
| 4 | 70.0 ± 3.4 | 65.5 ± 2.3 | 22 | 182.9 ± 1.1 | 168 ± 14 |
| 5 | 68.8 ± 2.9 | 61.9 ± 2.9 | 23 | 152.8 ± 2.7 | 143 ± 19 |
| 6 | 62.0 ± 2.8 | 59.8 ± 4.3 | 24 | 186.0 ± 2.5 | 183 ± 20 |
| 7 | 68.2 ± 3.8 | 62.8 ± 2.5 | 25 | 105.7 ± 2.7 | 86.7 ± 7.1 |
| 8 | 48.9 ± 3.3 | 41.1 ± 3.7 | 26 | 107.1 ± 2.7 | 88.4 ± 3.3 |
| 9 | 42.1 ± 2.3 | 46.5 ± 5.4 | 27 | 107.2 ± 1.9 | 91.4 ± 3.1 |
| 10 | 74.1 ± 2.4 | 69.6 ± 3.3 | 28 | 130.9 ± 2.1 | 114.6 ± 5.3 |
| 11 | 65.9 ± 3.0 | 63.7 ± 3.0 | 29 | 96.2 ± 1.7 | 76.0 ± 3.6 |
| 12 | 62.5 ± 3.1 | 58.4 ± 1.3 | 30 | 100.4 ± 2.1 | 87.8 ± 3.6 |
| 13 | 144.2 ± 2.7 | 130 ± 11 | 31 | 76.8 ± 1.2 | 65.9 ± 1.8 |
| 14 | 181.0 ± 5.5 | 178.1 ± 6.2 | 32 | 75.5 ± 1.9 | 63.7 ± 1.5 |
| 15 | 146.3 ± 2.5 | 133.3 ± 4.6 | 33 | 85.65 ± 0.76 | 72.8 ± 2.2 |
| 16 | 140.0 ± 2.6 | 133.4 ± 4.2 | 34 | 108.0 ± 4.5 | 85.3 ± 2.8 |
| 17 | 200.1 ± 3.3 | 187.6 ± 9.1 | 35 | 129.8 ± 1.8 | 114.9 ± 3.3 |
| 18 | 187.1 ± 3.2 | 191.0 ± 5.4 | 36 | 73.3 ± 1.2 | 63.5 ± 1.2 |
| Treatment | Method | n | Mean ± SD (mg kg−1) | CV (%) | Range | ANOVA | Tukey HSD |
|---|---|---|---|---|---|---|---|
| Control (T0) | AAS | 4 | 63.8 ± 0.7 a | 1.1 | 62.7–64.4 | F = 1.02 | All p > 0.05 |
| EDXRF | 4 | 65.4 ± 1.5 a | 2.3 | 63.0–66.8 | p = 0.40 | (NS) | |
| Fertigation (T1) | AAS | 4 | 60.5 ± 1.1 a | 1.8 | 59.0–61.8 | df = 2.9 | |
| EDXRF | 4 | 56.4 ± 9.4 a | 16.7 | 41.1–62.8 | |||
| Foliar (T2) | AAS | 4 | 63.9 ± 2.6 a | 4.1 | 61.4–67.6 | ||
| EDXRF | 4 | 59.6 ± 9.7 a | 16.3 | 46.5–69.6 |
| Treatment | Method | n | Mean ± SD | CV% | Min–Max | ANOVA | Tukey HSD |
|---|---|---|---|---|---|---|---|
| T0 (Control) | AAS | 8 | 136.8 ± 30.7 b | 22.4 | 105.7–181.0 | F = 4.25 (p = 0.023 *) | T1 > T0 * |
| EDXRF | 8 | 128.3 ± 32.1 b | 25.0 | 86.7–178.1 | T1 > T2 * | ||
| T1 (Fertigation) | AAS | 8 | 162.1 ± 50.3 a | 31.0 | 96.2–200.1 | df = 2.21 | |
| EDXRF | 8 | 151.9 ± 53.8 a | 35.4 | 76.0–191.0 | |||
| T2 (Foliar) | AAS | 8 | 129.6 ± 38.2 b | 29.5 | 73.3–186.0 | ||
| EDXRF | 8 | 121.8 ± 42.6 b | 35.0 | 63.5–183.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belluzzi Muiños, M.; Silva, J.; Conde, P.; Ibáñez, F.; Bühl, V.; Pistón, M. A Green and Simple Analytical Method for the Evaluation of the Effects of Zn Fertilization on Pecan Crops Using EDXRF. Processes 2025, 13, 2218. https://doi.org/10.3390/pr13072218
Belluzzi Muiños M, Silva J, Conde P, Ibáñez F, Bühl V, Pistón M. A Green and Simple Analytical Method for the Evaluation of the Effects of Zn Fertilization on Pecan Crops Using EDXRF. Processes. 2025; 13(7):2218. https://doi.org/10.3390/pr13072218
Chicago/Turabian StyleBelluzzi Muiños, Marcelo, Javier Silva, Paula Conde, Facundo Ibáñez, Valery Bühl, and Mariela Pistón. 2025. "A Green and Simple Analytical Method for the Evaluation of the Effects of Zn Fertilization on Pecan Crops Using EDXRF" Processes 13, no. 7: 2218. https://doi.org/10.3390/pr13072218
APA StyleBelluzzi Muiños, M., Silva, J., Conde, P., Ibáñez, F., Bühl, V., & Pistón, M. (2025). A Green and Simple Analytical Method for the Evaluation of the Effects of Zn Fertilization on Pecan Crops Using EDXRF. Processes, 13(7), 2218. https://doi.org/10.3390/pr13072218







