Abstract
The rising demand for sustainable aquaculture necessitates innovative solutions to environmental and operational challenges. Biofloc technology (BFT) has emerged as an effective method, leveraging microbial communities to enhance water quality, reduce feed costs, and improve fish health. However, traditional BFT systems are susceptible to water quality fluctuations, demanding precise monitoring and control. This review explores the integration of Artificial Intelligence (AI) and Internet of Things (IoT) technologies in smart BFT systems, highlighting their capacity to automate processes, optimize resource utilization, and boost system performance. IoT devices facilitate real-time monitoring, while AI-driven analytics provide actionable insights for predictive management. We present a comparative analysis of AI models, such as LSTM, Random Forest, and SVM, for various aquaculture prediction tasks, emphasizing the importance of performance metrics like RMSE and MAE. Furthermore, we discuss the environmental and economic impacts, including quantitative case studies on cost reduction and productivity increases. This paper also addresses critical aspects of AI model reliability, interpretability (SHAP/LIME), uncertainty quantification, and failure mode analysis, advocating for robust testing protocols and human-in-the-loop systems. By addressing these challenges and exploring future opportunities, this article underscores the transformative potential of AI and IoT in advancing BFT for sustainable aquaculture practices, offering a pathway to more resilient and efficient food production.
1. Introduction
Over the past three decades, aquaculture has undergone remarkable expansion. It has become a vital contributor to food security, income creation, and economic growth worldwide. The growing global population is driving increased demand for aquaculture products, requiring the augmentation of aquaculture production for seafood, as capture fisheries face limitations due to overfishing and environmental concerns. Intensive traditional aquaculture practices often lead to negative environmental effects, like water pollution, damage to aquatic ecosystem systems, and inefficient resource utilization [1]. Despite these advancements, a critical research gap exists in effectively addressing the inherent limitations of conventional aquaculture systems, particularly concerning scalability, precise disease prediction, and comprehensive environmental monitoring. These are areas where current methodologies often fall short, leading to inefficiencies and potential ecological harm. However, these conventional systems face challenges in scalability, disease prediction, and environmental monitoring—areas where AI and IoT can offer transformative solutions.
A new culture system has emerged to address the challenges of intensification, known as biofloc technology (BFT), which relies on heterotrophic bacteria that require specific conditions to thrive: (1) maintaining a high carbon-to-nitrogen (C:N) proportion through the supplementation of exogenous carbon sources, (2) ensuring appropriate aeration, and (3) operating under zero-water exchange conditions. By leveraging the microbial community to convert organic waste into valuable biomass, BFT reduces environmental impact while enhancing the overall productivity of aquaculture systems. Heterotrophic bacteria absorb ammonium, an important aquatic contaminant, and transform it into microbial protein, a continuous protein source for cultured organisms [2]. BFT provides a sustainable and cost-effective method for augmenting water quality, lowering food costs, and improving fish health [3].
Despite its advantages, biofloc technology is highly sensitive to fluctuations in water quality. Minor changes in water parameters can lead to significant fish mortality, highlighting the critical need for constant monitoring and precise control. Additionally, these parameters vary based on the type of fish species cultivated within the culture system. Moreover, the dynamic nature of biofloc systems, influenced by microbial activity and external factors, necessitates a data-driven approach to optimize system performance [4].
Incorporating Artificial Intelligence (AI) and the Internet of Things (IoT) has become essential to tackle these obstacles. AI and IoT can provide early warnings of potential water quality issues, allowing for timely intervention and reducing the likelihood of large-scale losses [5]. IoT devices, like sensors and actuators, facilitate instant monitoring and remote management of biofloc systems, reducing the need for manual intervention. These devices continuously collect data on critical parameters, giving aquaculture practitioners a comprehensive understanding of system dynamics. Meanwhile, AI algorithms analyze this data, identifying patterns, predicting trends, and offering actionable insights. For instance, machine learning models can forecast water quality fluctuations based on historical data, enabling proactive adjustments to maintain optimal conditions [6]. The fusion of aquaculture with AI has the potential to revolutionize aquatic food production, paving the way for sustainability, efficiency, and enhanced yields [7].
Integrating AI and IoT into biofloc technology not only enhances operational efficiency but also addresses key challenges, such as resource optimization, cost reduction, and sustainability Al [8]. By automating routine tasks and improving decision-making processes, these technologies have the potential to revolutionize aquaculture practices, making them more resilient and adaptable to changing environmental conditions [9]. This review seeks to explore the applications of AI and IoT in aquaculture systems, with a particular focus on biofloc technology, highlighting their transformative impact on sustainable aquaculture. It will explore how AI models are utilized for instant monitoring and predictive analytics of water criteria, the integration of IoT devices for system management and automation, and the impact of these technologies on enhancing sustainability, improving resource efficiency, and optimizing aquaculture production within biofloc systems.
2. Aquaculture
Aquaculture, the farming of aquatic plants and animals, has become a major contributor to the global supply of aquatic foods. Between 1990 and 2020, global production grew sixfold, making it the quickest expanding industry in food production. Within 2020 alone, aquaculture produced 122.6 million tonnes (live weight) across freshwater, brackish water, and marine systems [10]. Globally, around 424 aquatic species are farmed, supporting millions of people through nourishment, food security, long-term livelihoods, and poverty eradication. The rapid advancement of aquaculture and its vital role in seafood production has heightened concerns about its social and environmental implications. To satisfy the increasing requirement for safety and premium aquatic protein, particularly fish and prawn, aquaculture must employ suitable culture techniques that support advanced stocking densities, maintain adequate water criteria, promote supreme aquatic organism performance, and address biosecurity and ecological challenges, like uneaten feed, water contamination, the spread of diseases, and the overuse of marine pharmaceuticals [11]
Maintaining water quality in traditional intensive systems necessitates frequent water exchanges, and these systems are heavily dependent on feed inputs. Regrettably, the unprocessed effluent from these systems frequently holds elevated concentrations of pollutants, like nitrogen and phosphorus compounds, which can harm the surrounding environment. Once the ability of the local ecosystem is overwhelmed, densely stocked aquaculture areas are prone to disease outbreaks [12]. Given the issues with traditional aquaculture systems, BFT offers an encouraging alternative by enhancing water efficiency, minimizing environmental impact, and reducing feed costs [13].
3. Biofloc Technology (BFT)
Biofloc technology is an eco-sustainable culture technology that enables the continuous recycling and utilization of nutrients. The sustainability of this technology relies on microbial proliferation in the water, which is assisted by bare minimum or nil water replacement. The microbes (biofloc) provide two chief contributions: (i) augmenting water quality criteria by utilizing nitrogenous composites and generating microbial protein directly; and (ii) nourishment, augmenting production efficiency by lessening feed conversion ratios and food costs.
Microorganisms in BFT have a crucial contribution to the nutrition of farmed aquatic organisms. Macro-assemblies (biofloc) are natural resources with a high protein and lipid content that are accessible “in situ” throughout the day. A complex interaction arises through the water column among organic material and a wide variety of microorganisms, such as plankton, bacteria, agglomerates of organic material, and grazers, such as rotifers, ciliates, flagellates, protozoa, and copepods. It is a copious source of growth catalysts and bioactive aggregates, which augment each the digestive enzymes and the aquatic creatures’ condition, leading to accelerated growth [14]. Such natural productivity is critical for nutrient reuse and enhancing water quality.
Biological Effects of Biofloc Technology on Aquaculture
BFT has several biological effects on aquaculture systems, particularly in terms of water criteria management, nutrient cycling, and the general health of cultivated species (Table 1). BFT implies the propagation of microbial assemblages, such as bacteria, algae, and other microorganisms, that aggregate into “flocs” (small particles) in the water. These microorganisms utilize organic waste produced by the farmed species as their primary nutrient source, creating a self-sustaining ecosystem.
One critical parameter for optimal biofloc system performance is the carbon-to-nitrogen (C/N) ratio. Studies suggest that maintaining a C/N ratio between 10:1 and 20:1 promotes heterotrophic bacterial growth, which efficiently assimilates nitrogenous wastes. For example, molasses and starch are common carbon sources used to achieve and maintain this ratio, supporting effective nutrient cycling and microbial protein production [13,15,16], stated that a C/N proportion of 15/1 is the optimal because it allows for better ammonia-N elimination than a C/N proportion of 10/1. It showed no notable decrease in dissolved oxygen owing to too much BFT formation, as shown with a C/N proportion of 20/1. A C/N proportion of 15/1 is advisable for ideal BFT efficacy at any point, whereas a C/N ratio of 10 could be a preferable option when the bioflocs have reached maturity. The benefits of employing a low C/N proportion around 10 to 15 include lowering the operational costs and guarding the culture system from excessive oxygen demand, which could cause hypoxia. Yet, an increased C/N proportion (>15) may even remain desirable at the onset to help bioflocs proliferate more rapidly.
Optimal dissolved oxygen (DO) levels are also crucial for system stability and organism health. The literature recommends maintaining DO levels above 4 mg/L in biofloc systems to support aerobic microbial activity and fish [17,18]. Aeration strategies are therefore essential to avoid hypoxia and ensure the efficiency of biofloc processes.

Table 1.
The various impacts of biofloc technology on aquaculture systems.
Table 1.
The various impacts of biofloc technology on aquaculture systems.
| References | Carbon Source | C: N Ratio | Biological Effect |
|---|---|---|---|
| [19] | - | - | The results show that salinity influenced both the water criteria and the nutritional profile of the biofloc material. |
| [20] | - | - | The findings emphasized the importance of precise biofloc management, demonstrating that selectively removing unsettled bioflocs boosted fish growth and enhanced overall system health. |
| [21] | Jaggery | 15:1 | The jaggery-biofloc-based system demonstrated enhanced hematological, immunological, and antioxidative responses compared to other treatments. |
| [22] | - | ≥15:1–10:1 | The results show that the new strategy, combining early-stage heterotrophic and later-stage autotrophic bacteria in biofloc technology, is an effective and innovative approach for white-leg shrimp farming. |
| [23] | Molasses | 10:1–20:1 | The use of BFT systems could effectively increase the total bacterial count in water and gut microbiota, either individually or alongside probiotic supplementation. |
| [24] | - | - | Upgraded growth performance and upgraded gut microbiota and body composition of cultured fish were noted. |
| [25] | - | - | Overall, the highest performance was recorded in shrimp fed a diet supplemented with 10% wet biofloc. |
| [15] | Starch | 10:1–20:1 | Nile Tilapia reared with BFT with a C/N ratio of 10:1 had the best growth efficacy and feed utilization indicators. |
| [26] | Wheat meal | 10:1, 15:1, and 20:1 | The C/N 20 generated the most favorable growth parameters and the lowest feed conversion ratio (p < 0.05). |
| [27] | Molasses | 14:1, 17:1, and 20:1 | Ultimately, the C/N proportion could be lowered to 14 without altering growth efficiency or physiological responses in juvenile tilapia. |
| [28] | Molasses | 8:1, 12:1, and 16:1 | The C/N proportion had no noticeable impact on shrimp production efficiency. |
| [29] | Molasses | 15:1 | Probiotic inclusion in the BFT system considerably augmented the water criteria, floc volume, growth performance, and total bacterial number in the water. |
| [30] | Molasses | - | Probiotic addition at a dose of 1.08 × 105 CFU g−1 upgraded the culture performance of M. rosenbergii in BFT. |
| [31] | Molasses | - | The enrichment of BFT with probiotics upgraded the water quality, growth performance parameters, and disease resistance against A. hydrophila. |
| [32] | Molasses | 20:1 | The acquisition of a probiotic through BFT boosted the performance and hematological criteria of Oreochromus niloticus in the introductory stage with no notable changes in intestinal morphometry. |
| [33] | Molasses | 15:1, 20:1, and 25:1 | BFT (especially C/N 20) could improve Cyprinus carpio’s immunological and anti-oxidative condition. |
| [34] | Molasses | 11:1, 15:1, 19:1, and 23:1 | The BFT with C/N 19:1 enhanced water criteria and growth efficiency. |
| [35] | Molasses | Assuming 50% of the daily feed amount | The combination prompted the fastest method to sustain the water quality as optimally as possible for cultural operations. |
| [36] | A blend of carbohydrate sources | 12:1 | Symbiosis boosted shrimp production and immunity. |
| [37] | Molasses | 50, 100, 150, and 200 mL molasse/m3 | Optimal growth efficiency and feed utilization were recorded using BFT at a dose of 200 mL/m3 |
| [38] | Molasses, rice flour, wheat flour | Assuming 50% of the daily feed amount | The enrichment of BFT with probiotics upgraded the water quality and growth potentials. |
| [39] | Molasses | 15:1 20:1 | The introduction of probiotics to the BFT-15 could contribute to improved water quality, as well as boosted immunity. |
| [40] | Molasses | 12/1 | The addition of probiotics ultimately resulted in autochthonous bacteria exerting the most relevance on diversity. |
| [41] | Molasses | 20/1 | The inclusion of probiotics had no influence on water quality and growth parameters. |
| [42] | Molasses | 6:1 | Probiotic incorporation in BFT had a minor impact, but it can boost immunity in a conventional system. |
| [43] | Molasses | 15:1 | Probiotic administration to BFT ensured optimum water quality criteria. |
| [44] | Molasses | 6:1 | The acquisition of a probiotic through BFT seemed to have no effect on the water quality. |
| [45] | Molasses | 6:1 | The inclusion of BFT with a probiotic contributed to the decline of the Vibrio concentration and upsurge in the Bacillus in gut microbiota. |
| [46] | Molasses | 64% of the daily feed amount | The employment of probiotics with molasses increased the variety of the microbial population and substantially suppressed infections in L. vannamei. |
| [47] | Molasses | Application rates of 30% of the total daily feed | BFT supplemented with probiotics showed superior growth in shrimp and progressed gut morphology. |
| [48] | Molasses | BFT conc. were 60, 80, 100, 120, and 140% | BFT with probiotics boosted shrimp growth and immunological interaction. |
| [49] | Molasses | 15:1 | BFT including probiotics (106 CFU mL−1) provided the best catfish productive efficiency. |
4. Smart Aquaculture and Biofloc Technology
Smart aquaculture involves leveraging advanced technologies, comprising the Internet of Things (IoT), Artificial Intelligence (AI), robotics, and data analytics, to enhance and automate different components of aquaculture systems (Figure 1). This approach enhances efficiency, sustainability, and productivity while minimizing environmental impact and labor requirements.
Automation has revolutionized aquaculture, particularly in food practices and water criteria. The introduction of automation controllers has significantly improved effectiveness, accuracy, and sustainability in the industry [50]. These controllers utilize algorithms to regulate various system components, boost conditions, lower labor force, and enhance production efficiency. Key parameters, like water quality, temperature, dissolved oxygen, and feeding, are carefully managed by these systems [51]. Using sensors and actuators, automation controllers ensure ideal conditions to support shrimp growth, beneficial development, and reduce strain. As a result, automation controllers have become essential in fish culture, helping to optimize production, minimize ecological impact, and ensure the health of aquatic creatures [52].
Automated prawn culture systems leverage robotics and AI to optimize productivity, improve efficiency, and maintain accuracy and stability in operations [53]. Despite these advancements, challenges still exist, including the labor-intensive nature of traditional cleaning methods, the necessity for remote sensing technologies, the buildup of sludge that negatively impacts water criteria, and the demand for dependable automated systems in large-scale commercial settings.
The reviews correlated to aquaculture and their most relevant keywords are summarized in Table 2, emphasizing the adoption of revolutionary and modern technologies in the field. This table provides an overview of studies focusing on AI and IoT in culture systems, which were involved in the research review. These studies highlight the transformative potential of these technologies in optimizing aquaculture practices and advancing sustainability.
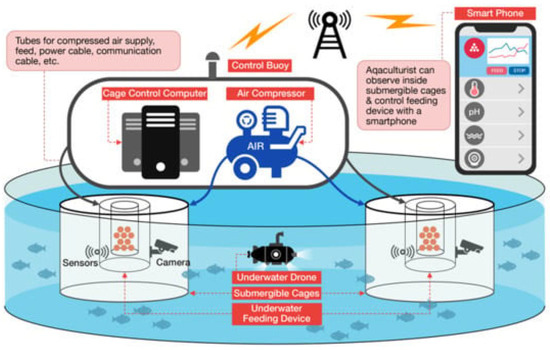
Figure 1.
Graph of smart fish culture system [52].

Table 2.
Smart aquaculture reviews and their corresponding key keywords and findings.
Table 2.
Smart aquaculture reviews and their corresponding key keywords and findings.
| Reference | Keywords | Findings |
|---|---|---|
| [54] | Artificial Intelligence, water quality | The findings demonstrate that AI-driven tools offer significant capacity to enhance sustainability, efficacy, and production in aquaculture through applications. |
| [55] | Machine learning, artificial vision | Enhancing aquaculture practices will enable effective management of environmental resources, fostering sustainable fishing and meeting nutritional demands. |
| [7] | AI, IOT | The fusion of aquaculture and Artificial Intelligence holds the potential to revolutionize aquatic food production, paving the way for sustainability, efficiency, and enhanced yields. |
| [56] | AI, IoT | The paper concluded by providing recommendations for stakeholders and proposing directions for future research, to guide the integration of AI technologies in sustainable vertical farming to promote a sustainable agricultural future. |
| [57] | Aquaculture robots | The paper explored the role of machine learning in aquaculture, focusing on the assessment of fish recognition and categorization, fish biomass, and the forecasting of water criteria. |
| [58] | Water quality, sensors, IoT | The study proposed a forward-looking solution for smart fisheries, enabling the monitoring of water quality parameters, data-driven decision-making, and faster adaptation to changing conditions. |
| [59] | Cloud computing, AI, IoT | Application of AI and IOT into the aquaculture value chain is crucial for optimizing feeding behavior, detecting diseases, predicting growth, supervising the environment, providing market insights, and more, ultimately boosting productivity and ensuring sustainability in aquaculture. |
| [60] | Aquaculture, IoT | The review provided a summary of existing research on monitoring water quality in culture systems. |
| [52] | Smart aquaculture, AI, machine learning | The paper discussed the incorporation of AI into smart culture systems, especially emphasizing machine learning and computer vision, and their applications within aquaculture systems. |
| [61] | Edge computing, IoT, Artificial Intelligence | The study examined the use of aquatic intelligent tools, IoT, edge computing, and AI in smart culture. |
| [62] | Automation, intelligence, machine learning | The study investigated the use of machine learning in aquaculture, encompassing the assessment of fish biomass, the detection and categorization techniques of fish, and the estimation of water criteria. |
| [63] | Smart aquaculture | The paper supported a comprehensive and structured knowledge resource for further inspection of the dynamic interactions among the water criteria changes and fish body characteristics and behavior. |
| [64] | Precision livestock farming modeling sensors | The trial established the concept of accurate fish culture, aimed at applying controlled engineering standards to aquaculture to boost farmers’ capacity to monitor, manage, and record biological operations in fish farms. |
4.1. Applications of AI and IOT in Aquaculture Systems
Artificial Intelligence (AI) and the Internet of Things (IOT) are advanced technologies that empower engines to carry out tasks traditionally demanding human intelligence. They involve the creation of computer systems and algorithms capable of processing data, acquiring wisdom through experience, deciding actions, and addressing challenges in ways that mimic human cognitive abilities, as shown in Figure 2. AI can be seen as “the future built upon the foundations of the past.” It covers various methods like machine learning, computer vision, and robotics. Machine learning, a branch of AI, concentrates on training algorithms utilizing extensive datasets to recognize patterns and generate predictions or decisions without explicit programming for each task [65]. AI is transforming culture systems through enabling analytical decision-making and automation. Its key applications are discussed in the following subsections.
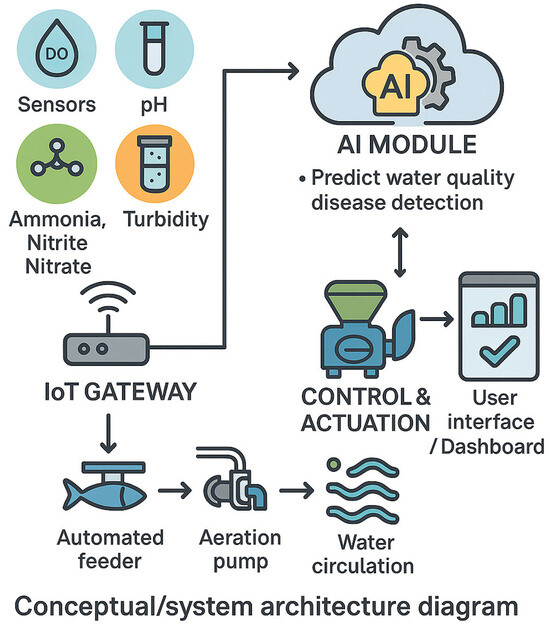
Figure 2.
Conceptual system architecture diagram (created by authors).
4.1.1. Predictive Analytics and Decision-Making
AI-driven predictive analytics has emerged as a linchpin of contemporary aquaculture. Machine learning models, including Random Forest and Genetic Algorithms, are applied for the prediction of fish productivity, mortality, and even environmental parameters. These predictions provide farmers with actionable insights to implement precautionary measures, such as a change in feeding schedules or altering water quality conditions, to potentially optimize fish growth or reduce losses [66,67]. A study researching tilapia farming utilizing genetic algorithms to optimize hydrochemical conditions in geothermal ponds attained excellent prediction accuracy for fish productivity. These examples illustrate the existing or future potential of artificial intelligence to build sustainability and efficiency in aquaculture practices [67].
Predictive analytics is essential in modern aquaculture by anticipating key factors, such as growth rates, water quality, and disease outbreaks. Leveraging historical data, AI-driven models uncover patterns and deliver precise predictions, allowing aquaculture professionals to optimize resources and boost production efficiency. Methods like regression analysis, time series forecasting, and neural networks are extensively utilized to forecast changes in aquatic ecosystems. Several studies demonstrate the diverse applications of predictive analytics in aquaculture. Ref. [68], highlighted the effectiveness of recurrent neural networks (RNNs) in accurately forecasting water temperature changes and enhancing planning and resource allocation. Similarly, Ref. [69] developed Random Forest models that achieved up to 95% accuracy in predicting disease outbreaks, facilitating early interventions. Ref. [70], showcased the ability of convolutional neural networks (CNNs) to analyze underwater imagery and environmental factors to estimate fish growth rates, enabling real-time feeding adjustments.
Hybrid AI systems also present significant benefits. Ref. [71], investigated the incorporation of decision trees and support vector machines (SVMs) for predicting oxygen levels in aquaculture ponds, ensuring optimal living conditions for aquatic species. Ref. [72], emphasized how big data analytics enhances the accuracy of predictive models, increasing productivity while reducing losses.
These advancements highlight the transformative potential of predictive analytics in aquaculture, driving more efficient, sustainable, and data-driven farming practices.
4.1.2. Optimized Feeding and Resource Management
Managing feed is important in aquaculture, as over-feeding can lead to waste and environmental problems. AI algorithms have recently been created to optimize feeding systems, including the adaptive neuro-fuzzy inference system (ANFIS) and particle swarm optimization (PSO). These systems look at characteristics, such as fish behavior, water quality and conditions, and fish nutritional needs, to provide the best feeding time for fish involved in the feeding process. This allows producers to reduce waste and improve feed conversion rates [73]. Deep learning models, such as convolution neural networks (CNNs), have also been used to analyze video streams of fish feeding behavior. These models can estimate fish density and feeding intensity for managers to fine-tune feeding in real-time. One example is fish feed, a method that uses density distribution analysis to assess feeding behavior to optimize feed distribution. This will allow for improved breeding efficiency and overall full resource utilization [73].
Feeding efficiency is a critical aspect of aquaculture, and machine learning (ML) has revolutionized feeding strategies by optimizing schedules and feeding quantities derived from fish behavior and ecological factors. ML algorithms analyze data from underwater cameras, acoustic sensors, and water quality monitors to develop tailored feeding protocols, minimizing waste and enhancing growth.
Ref. [74], demonstrated the successful application of deep reinforcement learning in refining feeding schedules, achieving a 20% reduction in feed waste. Similarly, Ref. [75] employed convolutional neural networks (CNNs) to interpret fish swimming patterns, leading to the expansion of automated feeding systems fit for instant response adjustments. Ref. [69] highlighted the effectiveness of decision tree algorithms in detecting overfeeding by analyzing fish movement patterns.
Ref. [76] reported that ML-powered feeding systems could reduce operational costs by 15% through precise predictions of fish appetite. Ref. [77] utilized unsupervised learning techniques to group fish behaviors, enabling the creation of customized feeding protocols that promote fish health. Additionally, Ref. [78] emphasized the potential of hybrid models combining ML with fuzzy logic to create adaptive feeding strategies tailored to the unique needs of varied aquaculture systems.
These advancements underscore the transformative role of machine learning in enhancing feeding efficiency, reducing waste, and supporting sustainable aquaculture practices.
4.1.3. Water Quality Monitoring and Management
Keeping aquatic organisms healthy and growing requires monitoring water quality conditions. The developed AI-infused IoT sensors monitor in situ water temperature, pH, dissolved oxygen, and salinity, among other variables, to provide real-time information on environmental conditions. These systems fully monitor water quality and can detect environmental conditions before they become serious issues, which allows the farmer to manage them proactively [79]. More advanced versions of machine learning models that are used in smart IoT systems analyze past and real-time data to predict trends in water quality. For example, deep learning algorithms have been utilized to predict water conditions, allowing farmers to change their response methods to reduce adverse conditions in aquaculture and the caregiving of fish farms [80].
4.1.4. Automated Aeration Systems
Artificial Intelligence (AI)-driven automated aeration systems are essential for maintaining optimal dissolved oxygen levels in aquaculture ponds, promoting healthy aquatic ecosystems and lessening the threat of fish death. By analyzing data from dissolved oxygen sensors, weather conditions, and fish activity, AI algorithms enable real-time regulation of aeration processes.
Ref. [71] highlighted the capabilities of AI-enhanced aeration systems employing long short-term memory (LSTM) networks to forecast oxygen depletion up to 24 h in advance, allowing for timely aeration adjustments. Similarly, Ref. [81] demonstrated the effectiveness of adaptive neuro-fuzzy inference systems (ANFIS) in dynamically controlling aerators, achieving a 30% reduction in energy usage. Ref. [82] emphasized the utility of Bayesian networks in predicting low-oxygen events, thereby preventing widespread fish fatalities.
Ref. [83] showcased the potential of combining AI with drone technology to enhance aeration efficiency by identifying oxygen-deficient areas and directing aeration efforts where needed. Furthermore, Ref. [84] revealed that deep learning models, by incorporating meteorological data, ensure that aeration processes align with weather conditions, thereby increasing resilience and improving productivity.
These advancements illustrate the critical role of AI-driven aeration systems in optimizing aquaculture operations, reducing energy consumption, and ensuring sustainable fish farming practices.
4.1.5. Decision Support Systems
Decision support systems (DSS) within the aquaculture domain amalgamate AI technologies to assist aquaculturists in making well-informed decisions about production planning, disease management, and resource optimization. DSS utilizes data obtained from diverse sources, thereby providing actionable insights and recommendations that enhance operational efficiency.
According to ref. [85] DSS platforms that are powered by AI leverage ensemble learning methodologies to forecast disease outbreaks, achieving a remarkable 92% success rate in mitigating prospective risks. Research by [86] demonstrated that DSS equipped with real-time analytics could optimize feeding schedules, leading to an 18% reduction in operational expenditures. Ref. [71] emphasized the application of reinforcement learning within DSS to perpetually refine stocking density decisions, thereby augmenting yield without jeopardizing fish health.
Additional studies by ref. [87] indicate that AI-driven DSS can incorporate environmental data to recommend optimal harvesting periods, ensuring maximum profitability.
Ref. [72] highlighted the comprehensive benefits of integrating AI, IoT, and DSS, presenting a unified approach to aquaculture management that enhances productivity while mitigating risks. Ref. [81] reported that AI-enhanced DSS could reduce disease-related mortality by 40% by enabling early detection and prompt intervention.
4.1.6. Automation and Sustainability in Aquaculture
AI has significantly automated aquaculture processes, increasing productivity and sustainability, respectively. The use of machine-learning algorithms for fish species classification, health monitoring, and water quality management is now common in practice. These systems enable real-time decisions based on data, minimize environmental damage, and manage resources more effectively [88]. Digital Twin technology, which integrates AI with IoT, has advanced intelligent aquaculture further. Digital Twin technology involves creating virtual models of real-world systems, like fish farms, to simulate and optimize processes such as feeding, environmental monitoring, and health management. Digital twins employ AI to predict future scenarios and analyze intricate functions, ensuring farm efficiency and maximizing profits [79].
4.1.7. Fish Health and Disease Detection
The use of AI represents a major advancement in monitoring fish health through the early detection of diseases and parasites. Computer vision and deep learning models, including convolutional neural networks (CNNs), can analyze imagery and video of fish for visible signs of illness or stress in fish. YOLOv8, a state-of-the-art object detection model, has been used to detect and classify fish diseases for early treatment and mitigate financial losses [80,89]. AI-based predictive health management (PHM) systems also leverage cameras and sensors to obtain fish behavior and health data. These systems can detect behavioral abnormalities and declining vitality, such as altered feeding behaviors or activity time. These predictive algorithms enable early treatment of health threats, reducing fish mortality and improving fish health [80].
4.1.8. Species Identification and Biomass Estimation
Effective management of aquaculture systems relies heavily on the proper identification of species and the estimation of fish biomass. Artificial Intelligence has been applied in aquaculture by employing deep learning techniques to classify fish species and estimate lengths or biomass. An example of this has been the use of sonar technology along with machine learning models to predict fish biomass in various visibility conditions, including murky water, which would be difficult with traditional means (“Improving Aquaculture Systems using AI: Employing predictive models for Biomass Estimation on Sonar Images”). Artificial Intelligence has also been applied in analyzing underwater imagery and video recording to estimate fish densities and growth. These systems are particularly useful in recirculating aquaculture systems (RAS), where high densities of fish and high turbidity can pose a challenge to manual identification and measuring [90].
4.2. AI Algorithm Performance Comparison in Biofloc Systems
To provide a more rigorous analysis of AI applications in biofloc aquaculture, this section compares the performance of various machine learning models for key parameters, such as dissolved oxygen (DO) prediction and disease detection. The evaluation focuses on commonly used metrics: Root Mean Square Error (RMSE), Mean Absolute Error (MAE), and the coefficient of determination (R2).
4.2.1. Dissolved Oxygen (DO) Prediction
Accurate prediction of dissolved oxygen levels is crucial for maintaining optimal conditions in biofloc systems and preventing fish mortality. Various machine learning models have been employed for this purpose, demonstrating varying degrees of accuracy as shown in Table 3 and Table 4.

Table 3.
Various applications of machine learning models on biofloc system.

Table 4.
Various applications of AI-driven image analysis and sensor data on biofloc system.
4.2.2. Disease Detection
Early and accurate disease detection is paramount in aquaculture to prevent widespread outbreaks and minimize economic losses. AI-driven image analysis and sensor data interpretation are increasingly being used for this purpose.
4.3. Water Criteria Monitoring in Smart Aquaculture
AI and IoT have numerous applications in aquaculture systems, revolutionizing the way operations are managed and enhancing their efficiency, sustainability, and profitability; the various applications of AI and IoT in monitoring the water quality in aquaculture systems are briefed in Table 5 and Table 6.

Table 5.
Various applications of AI and IOT on aquaculture systems.

Table 6.
Comparative analysis of AI/ML models in smart aquaculture.
4.4. Smart Feeding Control
In aquaculture, feed stands out as a primary cost, accounting for 50–60% of the total investment [106]. It contributes critically to determine both operational costs and water criteria. While artificial feeding offers a convenient method for providing nutrition to farmed aquatic species, it also presents certain challenges [107]. Overfeeding or incorrect feeding practices can lead to water pollution, as uneaten feed and waste accumulate, negatively impacting the aquatic environment [108]. Furthermore, inefficient feeding can result in significant economic losses.
Inefficient feeding practices can lead to significant economic losses due to feed wastage and higher production costs. Poor monitoring of feeding can result in overfeeding or underfeeding, both of which can adversely affect the robustness and growth performance of cultivated organisms [106]. Automatic feeding machines, while useful, often dispense feed at set times regardless of water conditions, which can trigger overfeeding and deterioration of water quality [109]. However, the incorporation of AI and IoT (sensor technology) offers a potential solution through regulation of the amount of feed and automation of the feeding process. By analyzing vibrations and acoustic signals produced by the cultured animals, it becomes possible to identify individual hungry animals within the group. Measuring appetite in this way could ensure that feed is provided in the right amount and at the optimal time [110].
Therefore, [111] developed a mobile food feeder designed to provide proper food maintenance for the species. This system automatically feeds the fish without the need for human interference, reducing labor requirements, enhancing feeding efficiency, and minimizing food waste.
They developed an automated feeding system running 24 h a day, which can reduce the need for manual feeding by 30% to 40%. Additionally, [112] introduced an innovative robotic feeding technique, which is composed of a mobile robot and a feed distribution container, including a server for facilitating computer-mediated interaction.
4.5. The Implementation of AI and IoT in Smart Biofloc Technology
BFT represents a sustainable culture system innovation that utilizes heterotrophic microbial assemblies to improve water criteria and provide additional nutrition for aquatic species. While conventional biofloc systems have demonstrated significant potential in reducing resource consumption and enhancing productivity, their manual operation and reliance on periodic monitoring pose challenges. The AI and IoT in biofloc systems, often referred to as “smart biofloc technology,” address these limitations by introducing automation, real-time monitoring, and advanced analytics. The collaboration of AI and IoT facilitates predictive maintenance, early detection of potential system failures, and adaptive feeding schedules. This leads to reduced operational costs, improved fish health, and enhanced environmental sustainability. Various applications of AI and IOT on biofloc technology are summarized in Table 7 and Table 8.

Table 7.
Various applications of AI and IOT on biofloc system.

Table 8.
Summary table of AI and IoT tools in aquaculture.
4.6. Economic and Environmental Impact: Quantitative Case Studies and Impact Assessment
BFT, particularly when integrated with smart systems leveraging AI and IoT, offers significant environmental and economic advantages over traditional aquaculture methods. While the descriptive benefits are well-established, a quantitative analysis reveals the tangible improvements in cost reduction, productivity increases, and sustainability outcomes. This section provides case studies and simulations to demonstrate the measurable impact of smart biofloc systems.
4.6.1. Economic Impact
BFT enhances feed utilization efficiency. In conventional systems, a significant portion of feed, particularly nitrogenous compounds, is not assimilated by the aquatic organisms and accumulates in the water, leading to pollution and wasted resources. BFT, however, converts these unutilized nutrients into microbial biomass (floc), which serves as a supplementary food source for the cultured species. This internal recycling of nutrients can increase the efficiency of protein assimilation in feed by almost two times [115], thereby reducing feed costs, which typically constitute the largest operational expense in aquaculture. For example, a study on Nile tilapia culture in a biofloc system demonstrated a significant reduction in the feed conversion ratio (FCR) compared to traditional systems, leading to improved profitability [116].
Productivity increases in smart biofloc systems are also evident through enhanced growth rates and survival rates of cultured species. Real-time monitoring and AI-driven predictive analytics enable optimal environmental conditions to be maintained, minimizing stress and disease outbreaks. This proactive management leads to healthier and more productive aquatic populations. For instance, in shrimp farming, smart BFT systems have reported higher stocking densities and improved final weights due to stable water parameters and reduced disease incidence [117].
One of the most impactful applications of AI and IoT in commercial aquaculture is in optimizing feeding strategies. Traditional feeding methods often lead to overfeeding, resulting in wasted feed, poor water quality, and increased production costs. Smart feeding systems leverage IoT sensors and AI algorithms to deliver feed precisely when and where it is needed, based on real-time data [118].
eFishery (Indonesia): A prominent example is eFishery, an Indonesian aquaculture technology company that provides smart feeding solutions for fish and shrimp farmers [119]. Their IoT-enabled feeders use sensors to detect fish appetite and biomass, automatically adjusting feed quantities and schedules. This not only reduces feed waste by up to 21% but also improves feed conversion ratios and overall farm profitability. eFishery’s platform also offers data analytics and financial services to farmers, demonstrating a comprehensive approach to smart aquaculture.
4.6.2. Environmental Impact: Sustainability Outcomes
The environmental benefits of smart biofloc systems are equally compelling, primarily revolving around reduced waste discharge and improved resource efficiency. The minimal water exchange in BFT systems significantly reduces the discharge of nutrient-rich wastewater into natural water bodies, thereby mitigating eutrophication and associated ecological damage [115]. This closed-loop approach aligns with sustainable aquaculture practices, minimizing the environmental footprint of production.
Moreover, the efficient nutrient cycling within BFT systems contributes to a reduction in the overall carbon footprint of aquaculture. By converting waste into valuable biomass, BFT reduces the reliance on external feed sources and minimizes the energy required for water treatment. Life cycle assessments of biofloc aquaponics systems have shown a reduction in environmental impacts compared to conventional systems, particularly in terms of greenhouse gas emissions and water pollution [120].
One of the most impactful applications of AI and IoT in commercial aquaculture is maintaining optimal water quality, which is paramount in aquaculture, especially in intensive systems like BFT. AI and IoT enable continuous, real-time monitoring of critical water parameters and provide predictive insights for proactive management [121].
Fishwell (Vietnam): Fishwell, in collaboration with Orient Software (https://www.orientsoftware.com/case-studies/fishwell/, accessed on 23 June 2025), developed an AI-powered, cloud-based monitoring system for aquaculture [122]. This system utilizes IoT sensors to collect data on water temperature, pH, dissolved oxygen, ammonia, and other parameters. AI algorithms analyze this data to detect anomalies, predict potential water quality issues, and provide alerts to farmers, allowing for timely intervention and preventing mass mortalities. This enhances operational efficiency and reduces environmental impact.
Flow Studios (Malaysia): Flow Studios offers FS-IOT Products, a smart aquaculture system that leverages IoT and advanced analytics for real-time insights into water conditions [123]. Their solutions help farmers monitor and manage water quality parameters, contributing to healthier aquatic environments and improved yields.
4.7. Technical Feasibility Matrix for AI Solutions
To systematically categorize AI solutions, a technical feasibility matrix can be developed based on key implementation factors (Table 9):

Table 9.
Technical feasibility matrix for implementing AI solutions in smart biofloc aquaculture systems.
This matrix highlights that simpler AI solutions, such as threshold-based alerts for water quality parameters, typically fall into the low complexity category. These often involve edge processing on local, near real-time data, and straightforward integration, making them more accessible for smaller farms.
Conversely, advanced AI solutions, particularly those leveraging deep learning for complex tasks like disease detection or precise feeding optimization, demand high computational power, ultra-low latency for real-time decision-making, and intricate integration with diverse farm systems. These often necessitate cloud-based processing and significant technical expertise, leading to higher initial investments [79].
4.8. Challenges and Limitations of AI and IoT Integration in Aquaculture
While the integration of Artificial Intelligence (AI) and Internet of Things (IoT) technologies holds immense promise for transforming aquaculture into a more sustainable and efficient industry, their real-world implementation is not without significant challenges, limitations, and trade-offs. Addressing these barriers is crucial for the successful and widespread adoption of smart aquaculture systems.
4.8.1. High Implementation Costs
One of the primary barriers to the adoption of AI and IoT in aquaculture is the substantial initial investment required [79]. This includes the cost of advanced sensors, data acquisition systems, communication infrastructure, and AI-powered analytical platforms. For small-to-medium-sized aquaculture farms, these costs can be prohibitive, making it difficult to justify the investment despite the long-term benefits. The return on investment (ROI) may not be immediately apparent, and the capital expenditure can be a significant deterrent, especially in regions with limited access to financing or government subsidies.
4.8.2. Data Management and Quality
Effective AI and IoT systems rely heavily on large volumes of high-quality data. However, collecting, storing, and processing such data in aquaculture environments presents several challenges. Sensors can be prone to fouling, calibration issues, and environmental interference, leading to inaccurate or incomplete data [127]. Data from various sources (e.g., water quality sensors, feeding systems, biomass cameras) often need to be integrated, which can be complex due to disparate formats and protocols. Furthermore, ensuring data privacy and security, especially when dealing with sensitive operational data, is a growing concern [79]. The lack of standardized data collection methods and interoperability between different systems can also hinder seamless data flow and analysis.
4.8.3. Connectivity and Infrastructure Limitations
Many aquaculture farms are located in remote or rural areas where internet connectivity is poor, unreliable, or non-existent. This poses a significant challenge for IoT devices that require constant communication to transmit real-time data to cloud-based AI platforms. The absence of robust and affordable communication infrastructure can severely limit the deployment and effectiveness of smart aquaculture solutions. Edge computing, which processes data closer to the source, can mitigate some of these issues but it also adds to the complexity and cost of the system [128].
4.8.4. Technical Expertise and Training
The successful operation and maintenance of AI and IoT systems require a certain level of technical expertise that may be lacking in traditional aquaculture workforces. Farmers and technicians need to be trained not only in operating the new technologies but also in interpreting the data and insights provided by AI algorithms. The digital literacy gap can be a significant barrier to adoption, and there is a need for comprehensive training programs and user-friendly interfaces to bridge this gap [129].
4.8.5. AI Implementation Complexity Analysis
The implementation of AI solutions in biofloc aquaculture systems involves varying degrees of complexity, influenced by computational requirements, real-time processing needs, and integration challenges with existing infrastructure [65,126]. This complexity can lead to challenges in troubleshooting, maintenance, and system integration. Understanding these complexities is crucial for practical deployment decisions, especially when considering the trade-offs between accuracy gains and implementation costs [130,131].
4.9. Trade-Offs: YOLOv8 for Fish Health Detection vs. Threshold-Based Systems
Comparing advanced computer vision models, like YOLOv8 (You Only Look Once, version 8), for fish health detection against simpler threshold-based systems provides a clear illustration of the trade-offs between accuracy gains and implementation costs.
4.9.1. Threshold-Based Systems
Mechanism: These systems rely on predefined thresholds for specific parameters (e.g., water quality, fish activity levels). If a parameter crosses a set threshold, an alert is triggered. For instance, if dissolved oxygen drops below a certain level, an alarm sounds [132].
Accuracy: Limited. They can detect deviations from normal but often fail to identify subtle changes or specific disease symptoms that do not immediately impact a measurable parameter. They are prone to false positives/negatives if the thresholds are not finely tuned [132].
Implementation Cost: Low. They typically require basic sensors and simple programming, making them cost-effective and easy to deploy.
Computational Requirements: Minimal. Processing is often conducted on edge devices.
Integration Complexity: Low. Easy to integrate with basic alert systems [133].
4.9.2. YOLOv8 for Fish Health Detection
Mechanism: YOLOv8 is a state-of-the-art object detection model capable of real-time analysis of video or image streams. In aquaculture, it can be trained to identify specific fish behaviors (e.g., lethargy, erratic swimming), physical anomalies (e.g., lesions, fin rot), or even count and size fish, all of which are indicators of health [134,135].
Accuracy: High. YOLOv8 can achieve high accuracy in detecting subtle visual cues associated with fish diseases, offering more precise and early detection capabilites than threshold-based systems. Improved versions of YOLOv8 have demonstrated high accuracy (e.g., 97.53% average accuracy) while being lightweight enough for real-time deployment [136,137].
Implementation Cost: High. This includes the cost of high-resolution cameras, powerful edge computing devices (GPUs for inference), data labeling for model training, and, potentially, cloud infrastructure for model development and deployment. Custom model development and continuous retraining add to the operational costs [134].
Therefore, the choice between YOLOv8 and simpler systems involves a clear trade-off. YOLOv8 offers significantly higher accuracy and more granular insights into fish health, enabling proactive interventions and potentially reducing disease-related losses. However, this comes at a substantially higher implementation and operational cost, along with increased technical complexity. For large-scale commercial operations where disease outbreaks can lead to massive financial losses, the investment in advanced AI solutions like YOLOv8 might be justified by the potential for significant accuracy gains and improved disease management. For smaller farms with limited budgets, simpler, more cost-effective threshold-based systems might be a more feasible starting point, even with their inherent limitations in detection capabilities.
5. AI Model Validation and Assessment
The robustness and reliability of AI models in biofloc aquaculture systems are critically dependent on rigorous validation and assessment methodologies. This section systematically reviews common practices in model validation, identifies gaps in current research, and highlights the importance of interpretability, uncertainty quantification, and failure mode analysis.
5.1. Training, Testing, and Cross-Validation Protocols
Effective model validation typically involves splitting data into training, validation, and testing sets to assess a model’s generalization capability to unseen data. Common approaches include the following:
- Holdout Validation: The dataset is split into a training set and a test set. The model is trained on the training set and evaluated on the test set. While simple, this method can be sensitive to the specific data split [65,126].
- K-Fold Cross-Validation: The dataset is divided into K equal folds. The model is trained on K-1 folds and validated on the remaining fold. This process is repeated K times, with each fold serving as the validation set once. The results are then averaged. This method provides a more robust estimate of model performance and reduces bias compared to a single holdout set [138,139].
- Leave-One-Out Cross-Validation (LOOCV): A special case of K-fold where K equals the number of data points. Each data point is used as a validation set once. This is computationally intensive but provides a nearly unbiased estimate of model performance.
- Time-Series Cross-Validation: For time-dependent data common in aquaculture (e.g., water quality parameters), traditional random splitting can lead to data leakage. Time-series cross-validation ensures that the training data always precedes the validation data, preserving the temporal order [140].
Gaps in Current Research: While many studies report using these standard protocols, there is often a lack of detailed justification for the chosen method, especially concerning the unique characteristics of aquaculture data (e.g., seasonality, sensor drift, sudden environmental changes). More emphasis is needed on how these protocols are adapted to ensure that models are robust to real-world aquaculture dynamics.
5.2. Long-Term Model Reliability
Long-term reliability is a critical yet often overlooked aspect of AI model assessment in dynamic biofloc environments. Models trained on historical data may degrade in performance over time due to the following reasons:
- Concept Drift: Changes in the underlying relationships between input features and target variables (e.g., changes in fish behavior due to new feed formulations, seasonal variations affecting water quality). This can lead to a decline in predictive accuracy.
- Data Drift: Changes in the distribution of input data over time (e.g., sensor degradation, changes in farming practices). This can cause the model to encounter data it was not adequately trained on.
Assessment Gaps: Few studies explicitly address long-term model monitoring, retraining strategies, or mechanisms for detecting and adapting to concept/data drift. Future research should focus on developing adaptive AI systems that can continuously learn and update themselves in response to evolving biofloc conditions, ensuring sustained performance and reliability over extended periods [141].
5.3. Interpretability Methods (SHAP/LIME Analysis)
As AI models become more complex (e.g., deep neural networks), their decision-making processes can become opaque, leading to a lack of trust and difficulty in debugging. Interpretability methods aim to make these black-box models more understandable.
- SHAP (SHapley Additive exPlanations): Based on game theory, SHAP values explain the contribution of each feature to a prediction. It provides a unified measure of feature importance and can explain individual predictions, as well as global model behavior [141].
- LIME (Local Interpretable Model-agnostic Explanations): LIME explains the predictions of any classifier or regressor by approximating it locally with an interpretable model. It focuses on understanding individual predictions rather than the entire model [141].
Gaps in Current Research: While SHAP and LIME are gaining traction in various AI applications, their systematic application and reporting in aquaculture AI research are still limited.
There is a critical need to
- Promote interpretability: Encourage researchers to use SHAP/LIME to explain why a model makes a particular prediction, especially for critical applications, like disease detection or water quality management. This can build trust among aquaculture practitioners and facilitate better decision-making.
- Identify critical features: Use interpretability methods to identify the most influential parameters for specific predictions (e.g., which water quality parameters are most indicative of an impending disease outbreak).
- Understand model biases: Interpretability can help uncover unintended biases in models, such as reliance on spurious correlations or over-dependence on certain features, which might lead to misclassifications [142].
5.4. Uncertainty Quantification Techniques
AI models often provide point predictions without indicating the confidence or uncertainty associated with those predictions. In dynamic and uncertain environments, like biofloc systems, understanding prediction uncertainty is crucial for risk assessment and robust decision-making.
Techniques:
- Bayesian Neural Networks: These networks provide a probability distribution over their weights, allowing for the quantification of epistemic uncertainty (uncertainty due to limited data).
- Ensemble Methods: By combining predictions from multiple models, ensemble methods can provide a measure of uncertainty based on the variance among individual model predictions.
- Conformal Prediction: This framework provides valid prediction intervals or sets for individual predictions, without making assumptions about the underlying data distribution [143].
Gaps in Current Research: Most aquaculture AI studies focus solely on predictive accuracy, with limited attention to quantifying uncertainty. This is a significant gap, as a model that predicts a water quality parameter with high accuracy but also high uncertainty might be less useful than a slightly less accurate model with well-quantified low uncertainty. Future research should emphasize reporting prediction intervals and confidence scores alongside point predictions [144].
5.5. Failure Mode Analysis for AI-Driven Automated Systems
Understanding potential failure modes is essential for deploying reliable and safe AI-driven automated systems in biofloc aquaculture. This involves anticipating scenarios where the AI system might fail, assessing the impact of such failures, and developing mitigation strategies.
Key Considerations:
- CNN Misclassification Risks: For image-based applications, like fish health detection, convolutional neural networks (CNNs) can misclassify due to poor image quality, occlusions, novel disease presentations, or adversarial attacks. The consequences of misclassification (e.g., missed disease outbreaks, unnecessary treatments) can be severe [137].
- Predictive Model Failures during Extreme Environmental Conditions: Models trained on normal operating conditions may fail under extreme environmental conditions (e.g., sudden temperature drops, power outages, unusual algal blooms) that could compromise biofloc stability. These unforeseen circumstances can lead to catastrophic system failures if not accounted for
Gaps in Current Research: There is a significant lack of systematic failure mode analysis, risk assessment, and development of robust fallback mechanisms for AI-driven systems in aquaculture. Future research should focus on the following:
- Developing robust testing protocols: Beyond standard validation, testing should include stress testing under simulated extreme conditions and adversarial scenarios [145].
- Implementing anomaly detection: AI systems should be able to detect when they are operating outside their trained distribution and flag potential failures.
- Designing human-in-the-loop systems: For critical applications, human oversight and intervention capabilities are essential to prevent and mitigate AI failures.
- Quantifying and reporting failure rates: Studies should transparently report not just accuracy but also different types of errors and their potential impacts.
By addressing these critical gaps in model validation, interpretability, uncertainty quantification, and failure mode analysis, the aquaculture industry can build more trustworthy, reliable, and resilient AI-driven systems, paving the way for truly sustainable and efficient practices.
6. Conclusions
Biofloc technology (BFT) presents a highly efficient and environmentally sustainable approach to aquaculture by promoting nutrient recycling, minimizing water use, and enabling intensive fish production in limited spaces. However, successful implementation requires precise control and continuous monitoring of water quality parameters, such as dissolved oxygen, pH, temperature, and ammonia—tasks that become increasingly complex and error-prone in large-scale operations.
The integration of Artificial Intelligence (AI) and Internet of Things (IoT) technologies offers transformative potential in addressing these challenges. Wireless sensor networks enable real-time environmental monitoring, while predictive AI models (e.g., LSTM, Random Forest) assist in forecasting water quality fluctuations and optimizing feeding schedules. These innovations reduce labor demands, enhance productivity, and contribute to more stable and resilient aquaculture ecosystems.
Despite these advances, several barriers remain. Limited internet infrastructure in rural aquaculture settings can constrain real-time data transmission, while concerns over data privacy, cybersecurity, and the interpretability of complex AI models may hinder adoption. Additionally, high upfront costs and a lack of standardized protocols for AI–IoT integration pose challenges for small-to-medium-scale producers.
Looking ahead, future developments may include edge-AI systems for decentralized, low-latency monitoring; blockchain technologies to enhance the transparency and security of IoT data; and adaptive control algorithms capable of learning from long-term operational data. Interdisciplinary collaboration across aquaculture, data science, and engineering will be essential to overcome existing challenges and unlock the full potential of smart biofloc systems.
In summary, the convergence of AI and IoT with biofloc aquaculture represents a significant step toward a more automated, cost-effective, and ecologically sustainable fish farming paradigm.
Author Contributions
M.A.: conceptualization, methodology, editing, data curation, visualization, and supervision. Y.G.H.: writing—original draft preparation, reviewing, and investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Custodio, M.; Villasante, S.; Calado, R.; Lillebø, A.I. Valuation of ecosystem services to promote sustainable aquaculture practices. Rev. Aquac. 2020, 12, 392–405. [Google Scholar] [CrossRef]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guidebook; World Aquaculture Society: Sorrento, LA, USA, 2012. [Google Scholar]
- Santaella, S.; Vale, M.; Cabral, C.; de-Araujo, W.; Pinto, A.; Viana, O. Biofloc production in activated sludge system treating shrimp farming effluent. Eng. Sanit. Ambient. 2018, 23, 1143–1152. [Google Scholar] [CrossRef]
- Olanubi, O.O.; Akano, T.T.; Asaolu, O.S. Design and development of an IoT-based intelligent water quality management system for aquaculture. J. Electr. Syst. Inf. Technol. 2024, 11, 15. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Li, W. Machine learning applications in aquaculture systems. Aquac. Res. 2019, 27, 567–580. [Google Scholar]
- Gokulnath, S.R.; Vasanthakumaran, K.; Thanga, A.; Naveen, S.K.; Iburahim, A.; Paul, N.T. Precision aquaculture: Empowering fish farming with AI and IoT. Aquac. Rep. 2024, 42, 102625. [Google Scholar]
- Al Mamun, M.R.; Ashik-E-Rabbani, M.; Haque, M.M.; Upoma, S.M. IoT-based real-time biofloc monitoring and controlling system. Smart Agric. Technol. 2024, 9, 100598. [Google Scholar] [CrossRef]
- Rashid, T.; Nayan, A.; Rahman, O.; Simi, S.; Kibria, M. IoT-based smart water quality prediction for biofloc aquaculture. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 470–477. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Da Silva, K.R.; Wasielesky, W.; Abreu, P. Nitrogen and phosphorus dynamics in the biofloc production of the Pacific white shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2013, 44, 30–41. [Google Scholar] [CrossRef]
- Diatin, I.; Shafruddin, D.; Hude, N.; Sholihah, M.; Mutsmir, I. Production performance and financial feasibility analysis of farming catfish (Clarias gariepinus) utilizing water exchange system, aquaponic, and biofloc technology. J. Saudi Soc. Agric. Sci. 2021, 20, 344–351. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Biofloc: A sustainable alternative for improving the production of farmed cyprinid species. Aquac. Rep. 2023, 33, 101748. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Bioflocs Production System for Aquaculture; SRAC Publication No. 4503; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2013. [Google Scholar]
- Aboseif, A.M.; Flefil, N.S.; Taha, M.K.; Tahoun, U.M.; Mola, H.R.; El-Haroun, E.; Van Doan, H.; Goda, A.M.S.-A. Influence of dietary C:N:P ratios on Nile tilapia Oreochromis niloticus growth performance and formation of water biotic communities within a biofloc system containment. Aquac. Rep. 2022, 24, 101136. [Google Scholar] [CrossRef]
- Daud, A.; Sulaiman, N.; Yusof, Y.; Kassim, M. An IoT-based smart aquarium monitoring system. In Proceedings of the 2020 IEEE 10th Symposium on Computer Applications & Industrial Electronics (ISCAIE), Penang, Malaysia, 18–19 April 2020; pp. 277–282. [Google Scholar]
- Crab, R.; Avnimelech, Y.; Defoirdt, T.; Bossier, P.; Verstraete, W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture 2012, 356–357, 351–356. [Google Scholar] [CrossRef]
- Saravanan, K.; Kumar, J.; Sundar, M. Effect of different carbon sources on water quality and microbial dynamics in biofloc based shrimp culture system. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 114–124. Available online: https://www.ijcmas.com (accessed on 20 June 2025).
- Al-Sayegh, S.Y.; Rosas, V.T.; Vethamony, P.; Kasan, N.A.; Liew, H.J.; Ikhwanuddin, M.; Al Disi, Z.A.; Elsayed, H.; Al-Khayat, J.A. Maturation of the biofloc system in Penaeus vannamei culture under different salinities and its effects on the microbial communities. Aquac. Rep. 2025, 40, 102568. [Google Scholar] [CrossRef]
- Zhu, Z.; Tan, J.; Abakari, G.; Hu, X.; Tan, H.; Liu, W.; Luo, G. Effects of settleable versus unsettled biofloc removal strategy on aquaculture system performance and microbial community. Aquaculture 2025, 595, 741553. [Google Scholar] [CrossRef]
- Singh, T.; Amit, M.; Shanthanagouda, A.; Sachin, O.; Anuj, T. Growth performance and physiological responses of striped catfish (Pangasianodon hypophthalmus) under different carbohydrates supplemented biofloc aquaculture systems. Aquaculture 2024, 579, 740252. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Dong, D.; Li, M.; Yang, X.; Song, X.; Li, X. Effects of carbon source addition strategies on water quality, growth performance, and microbial community in shrimp BFT aquaculture systems. Aquaculture 2024, 578, 740027. [Google Scholar] [CrossRef]
- Haraz, Y.G.; Shourbela, R.M.; El-Hawarry, W.N.; Mansour, A.M.; Elblehi, S.S. Performance of juvenile Oreochromis niloticus (Nile tilapia) raised in conventional and biofloc technology systems as influenced by probiotic water supplementation. Aquaculture 2023, 566, 739180. [Google Scholar] [CrossRef]
- Hersi, M.A.; Genc, E.; Pipilos, A.; Keskin, E. Effects of dietary synbiotics and biofloc meal on the growth, tissue histomorphology, whole-body composition and intestinal microbiota profile of Oreochromis niloticus cultured at different salinities. Aquaculture 2023, 570, 739391. [Google Scholar] [CrossRef]
- Barzamini, B.; Harsij, M.; Adineh, H.; Jafaryan, H. The effect of biofloc-supplemented diets on the Pacific white shrimp (Litopenaeus vannamei): Analysis of water quality, growth performance, and biochemical composition. Iran. J. Fish. Sci. 2021, 7, 30–43. [Google Scholar] [CrossRef]
- Azimi, A.; Shekarabi, S.P.H.; Paknejad, H.; Harsij, M.; Khorshidi, Z.; Zolfaghari, M.; Hatami, A.-S.; Dawood, M.A.O.; Mazloumi, N.; Zakariaee, H. Various carbon/nitrogen ratios in a biofloc-based rearing system of common carp (Cyprinus carpio) fingerlings: Effect on growth performance, immune response, and plasma biochemistry. Aquaculture 2022, 548, 737622. [Google Scholar] [CrossRef]
- Dilmi, A.; Refes, W.; Meknachi, A. Effects of C/N ratio on water quality, growth performance, digestive enzyme activity and antioxidant status of Nile tilapia Oreochromis niloticus (Linnaeus, 1758) in biofloc-based culture system. TrJFAS 2022, 22. [Google Scholar] [CrossRef]
- Xu, W.; Wen, G.; Su, H.; Xu, Y.; Hu, X.; Cao, Y. Effect of input C/N ratio on bacterial community of water biofloc and shrimp gut in a commercial zero-exchange system with intensive production of Penaeus vannamei. Microorganisms 2022, 10, 1060. [Google Scholar] [CrossRef]
- Amjad, K.; Dahms, H.U.; Ho, C.H.; Wu, Y.C.; Lin, F.Y.; Lai, H.T. Probiotic additions affect the biofloc nursery culture of white shrimp (Litopenaeus vannamei). Aquaculture 2022, 560, 738475. [Google Scholar] [CrossRef]
- Frozza, A.; Fiorini, A.; Vendruscolo, E.G.; Rosado, F.R.; Konrad, D.; Rodrigues, M.C.G.; Ballester, E.C. Probiotics in the rearing of freshwater prawn Macrobrachium rosenbergii (de Man, 1879) in a biofloc system. Aquac. Res. 2021, 52, 4269–4277. [Google Scholar] [CrossRef]
- Mohammadi, G.; Rafiee, G.; Tavabe, K.; Abdel-Latif, H.; Dawood, M. The enrichment of diet with beneficial bacteria (single- or multi-strain) in biofloc system enhanced the water quality, growth performance, immune responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 539, 736640. [Google Scholar] [CrossRef]
- Laice, L.; Corrêa Filho, R.; Ventura, A.; Farias, K.; do Silva, A.; Fernandes, C.; Silva, A.C.F.; Barbosa, P.T.L.; de Souza, A.I.; Emerenciano, M.G.C.; et al. Use of symbiotics in biofloc (BFT)-based Nile tilapia culture: Production performance, intestinal morphometry and hematological parameters. Aquaculture 2020, 530, 735715. [Google Scholar] [CrossRef]
- Haghparast, M.; Alishahi, M.M.; Ghorbanpour, M. Evaluation of hemato-immunological parameters and stress indicators of common carp (Cyprinus carpio) in different C/N ratio of biofloc system. Aquac. Int. 2020, 28, 2191–2206. [Google Scholar] [CrossRef]
- Minabi, K.; Sourinejad, I.; Alizadeh, M.; Ghatrami, E.R.; Khanjani, M.H. Effects of different carbon to nitrogen ratios in the biofloc system on water quality, growth, and body composition of common carp (Cyprinus carpio L.) fingerlings. Aquac. Int. 2020, 28, 1883–1898. [Google Scholar] [CrossRef]
- Naik, M.; Reddy, M. Performance of shrimp Litopenaeus vannamei with the addition of probiotics and bioflocs: A field study. Int. J. Fish. Aquat. Stud. 2020, 8, 286–291. [Google Scholar]
- Panigrahi, A.; Das, R.R.; Sivakumar, M.R.; Saravanan, A.; Saranya, C.; Sudheer, N.S.; Vasagam, K.P.K.; Mahalakshmi, P.; Kannappan, S.; Gopikrishna, G. Bio-augmentation of heterotrophic bacteria in biofloc system improves growth, survival, and immunity of Indian white shrimp Penaeus indicus. Fish. Shellfish Immunol. 2020, 98, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Putra, I.; Effendi, I.; Lukistyowati, I.; Tang, U.M. Growth and survival rate of red tilapia (Oreochromis sp.) cultivated in the brackish water tank under biofloc system. Adv. Eng. Res. 2020, 190, 96–99. [Google Scholar]
- Reddy, M.S.; Naik, M.K. Growth performance of Litopenaeus vannamei in the presence and absence of probiotics with bioflocs of different carbon sources. Int. J. Fish. Aquat. Stud. 2020, 8, 349–354. [Google Scholar]
- Dash, P.; Tandel, R.; Bhat, R.; Mallik, S.; Pandey, N.; Singh, A.; Sarma, D. The addition of probiotic bacteria to microbial floc: Water quality, growth, non-specific immune response and disease resistance of Cyprinus carpio in mid-Himalayan altitude. Aquaculture 2018, 495, 961–969. [Google Scholar] [CrossRef]
- Huerta-Rabago, J.A.; Martinez-Porchas, M.; Miranda-Baeza, A.; Nieves-Soto, M.; Rivas-Vega, M.E.; Martinez-Cordova, L.R. Addition of commercial probiotic in a biofloc shrimp farm of Litopenaeus vannamei during the nursery phase: Effect on bacterial diversity using massive sequencing 16S rRNA. Aquaculture 2019, 502, 391–399. [Google Scholar] [CrossRef]
- Kathia, C.; Carmen, M.; Aida, H.; José Félix, C.; Amadeo, M. Effect of two probiotics on bacterial community composition from biofloc system and their impact on survival and growth of tilapia (Oreochromis niloticus). Int. J. Fish. Aquat. Stud. 2018, 6, 525–533. [Google Scholar]
- Liu, G.; Ye, Z.; Liu, D.; Zhu, S. Inorganic nitrogen control, growth, and immunophysiological response of Litopenaeus vannamei (Boone, 1931) in a biofloc system and in clear water with or without commercial probiotic. Aquac. Int. 2018, 26, 981–999. [Google Scholar] [CrossRef]
- Pacheco-Vega, J.; Cadena-Roa, M.; Leyva-Flores, J.; Zavala-Leal, O.; Pérez-Bravo, E.; Ruiz-Velazco, J. Effect of isolated bacteria and microalgae on the biofloc characteristics in the Pacific white shrimp culture. Aquac. Rep. 2018, 11, 24–30. [Google Scholar] [CrossRef]
- Ferreira, M.G.; Melo, F.; Lima, J.V.; Andrade, H.A.; Severi, W.; Correia, E.S. Bioremediation and biocontrol of commercial probiotic in marine shrimp culture with biofloc. Lat. Am. J. Aquat. Res. 2017, 45, 167–176. [Google Scholar] [CrossRef]
- Hostins, B.; Lara, G.; Decamp, O.; Cesar, D.; Wasielesky, W. Efficacy and variations in bacterial density in the gut of Litopenaeus vannamei reared in a BFT system and in clear water supplemented with a commercial probiotic mixture. Aquaculture 2017, 480, 58–64. [Google Scholar] [CrossRef]
- Hu, X.; Cao, Y.; Wen, G.; Zhang, X.; Xu, Y.; Xu, L.; Xu, Y.; Li, Z. Effect of combined use of Bacillus and molasses on microbial communities in shrimp cultural enclosure systems. Aquac. Res. 2017, 48, 2691–2705. [Google Scholar] [CrossRef]
- Maia, E.d.P.; Alves Modesto, G.; Brito, L.O.; Vasconcelos-Gesteira, T.C. Intensive culture system of Litopenaeus vannamei in commercial ponds with zero water exchange and addition of molasses and probiotics. Rev. Biol. Mar. Oceanogr. 2016, 51, 61–67. [Google Scholar] [CrossRef]
- Kim, Y.; Mo, H.-h.; Son, J.; Lee, Y.-S.; Lee, S.-E.; Cho, K. Interactive effects of water pH and hardness levels on the growth and reproduction of Heterocypris incongruens (Crustacea: Ostracoda). Hydrobiologia 2015, 753, 97–109. [Google Scholar] [CrossRef]
- Yusuf, O.Y.; Adeshina, I.; Adewale, A.Y. Comparative studies of some semen physical characteristics of cultured and wild African catfish (Clarias gariepinus, Burchell, 1822) broodstock. Gashua J. Irrig. Desertif. Stud. 2015, 1, 173–180. [Google Scholar]
- Antonucci, F.; Costa, C. Precision aquaculture: A short review on engineering innovations. Aquacult. Int. 2020, 28, 41–57. [Google Scholar] [CrossRef]
- Simbye, D.S.; Yang, S.F. Water quality monitoring and control for aquaculture based on wireless sensor networks. J. Netw. 2014, 9, 840. [Google Scholar] [CrossRef]
- Vo, T.; Ko, H.; Huh, J.-H.; Kim, Y. Overview of smart aquaculture system: Focusing on applications of machine learning and computer vision. Electronics 2021, 10, 2882. [Google Scholar] [CrossRef]
- Jasmin, A.; Ramesh, P.; Tanveer, M. An intelligent framework for prediction and forecasting of dissolved oxygen level and biofloc amount in a shrimp culture system using machine learning techniques. Expert. Syst. Appl. 2022, 199, 117160. [Google Scholar] [CrossRef]
- Aung, T.; Abdul Razak, R.; Rahiman Bin Md Nor, A. Artificial intelligence methods used in various aquaculture applications: A systematic literature review. J. World Aquacult. Soc. 2024, 56, e13107. [Google Scholar] [CrossRef]
- Capetillo-Contreras, O.; Pérez-Cisneros, F.D.; González-Barbosa, J.J. Artificial Intelligence-Based Aquaculture System for Optimizing the Quality of Water: A Systematic Analysis. J. Mar. Sci. Eng. 2024, 12, 161. [Google Scholar] [CrossRef]
- Chowdhury, H.; Argha, D.B.P.; Ahmed, M.A. Artificial Intelligence in Sustainable Vertical Farming. arXiv 2023, arXiv:2312.00030. [Google Scholar]
- Wu, Y.; Duan, Y.; Wei, Y.; An, D.; Liu, J. Application of intelligent and unmanned equipment in aquaculture: A review. Comput. Electron. Agric. 2022, 199, 107201. [Google Scholar] [CrossRef]
- Akhter, F.; Siddiquei, H.; Alahi, E. Recent advancement of the sensors for monitoring the water quality parameters in smart fisheries farming. Computers 2021, 10, 26. [Google Scholar] [CrossRef]
- Mustapha, U.; Alhassan, A.; Neng Jiang, D.; Li, G. Sustainable aquaculture development: A review on the roles of cloud computing, internet of things and artificial intelligence (CIA). Rev. Aquac. 2021, 13, 2076–2091. [Google Scholar] [CrossRef]
- Prapti, D.; Shariff, A.; Che Man, H.; Ramli, H.; Shariff, M. Internet of Things (IoT)-based aquaculture: An overview of IoT application on water quality monitoring. Rev. Aquac. 2021, 14, 979–992. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Wang, T.; Xu, X.; Zhang, X.; Li, D. Intelligent fish farm—The future of aquaculture. Aquac. Int. 2021, 29, 2681–2711. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S.; Liu, J.; Wang, H.; Zhu, J.; Li, D.; Zhao, R. Application of machine learning in intelligent fish aquaculture: A review. Aquaculture 2021, 540, 736724. [Google Scholar] [CrossRef]
- Hu, W.; Wu, H.; Zhang, Y.; Zhang, S.; Lo, C. Shrimp recognition using ShrimpNet based on convolutional neural network. J. Ambient. Intell. Human. Comput. 2020, 11, 1133–1141. [Google Scholar] [CrossRef]
- Føre, M.; Frank, K.; Norton, T.; Svendsen, E.; Alfredsen, J.A.; Dempster, T.; Eguiraun, H.; Watson, W.; Stahl, A.; Sunde, L.M.; et al. Precision fish farming: A new framework to improve production in aquaculture. Biosyst. Eng. 2018, 173, 176–193. [Google Scholar] [CrossRef]
- Yang, H.; Feng, Q.; Xia, S.; Wu, Z.; Zhang, Y. AI-driven aquaculture: A review of technological innovations and their sustainable impacts. Artif. Intell. Agric. 2025, 15, 508–525. [Google Scholar] [CrossRef]
- Gkikas, M.C.; Gkikas, D.C.; Vonitsanos, G.; Theodorou, J.A.; Sioutas, S. Application of Machine Learning for Predictive Analysis and Management of Mediterranean-Farmed Fish Mortalities: A Risk Management Case Study Using Apache Spark. Appl. Sci. 2024, 14, 10112. [Google Scholar] [CrossRef]
- Тынченкo, B.C.; Kukartseva, O.; Tynchenko, Y.; Кукарцев, B.B.; Panfilova, T.; Kravtsov, K.; Wu, X.; Malashin, I. Predicting Tilapia Productivity in Geothermal Ponds: A Genetic Algorithm Approach for Sustainable Aquaculture Practices. Sustainability 2024, 16, 9276. [Google Scholar] [CrossRef]
- Smith, J. AI for predictive aquaculture systems. Aquac. Res. 2022, 54, 345–357. [Google Scholar]
- Lee, H.; Kim, T. Decision tree algorithms for overfeeding detection in aquaculture. Mar. Biol. J. 2021, 49, 211–223. [Google Scholar]
- Zhang, Y. CNN for fish growth prediction. Aquac. Int. 2023, 29, 112–124. [Google Scholar]
- Brown, D.; Zhang, H.; Kim, S.; Ahmed, M.; López, J.; Singh, P.; Chen, Y.; Alotaibi, R.; Wang, X.; Smith, A.; et al. Hybrid AI models in aquaculture. Fish. Sci. 2020, 65, 432–445. [Google Scholar]
- Williams, P. Big data in aquaculture. Aquac. Eng. 2021, 50, 601–615. [Google Scholar]
- Zhao, H.; Wu, J.; Liu, L.; Qu, B.; Yin, J.; Yu, H.; Jiang, Z.; Zhou, C. A real-time feeding decision method based on density estimation of farmed fish. Front. Mar. Sci. 2024, 11, 1358209. [Google Scholar] [CrossRef]
- Lopez, K. Deep reinforcement learning in feeding systems. Aquac. Technol. 2022, 55, 123–134. [Google Scholar]
- Patel, A. CNN for feeding optimization. Aquac. Syst. 2021, 33, 145–156. [Google Scholar]
- Gonzalez, M.; Smith, J.; Lee, A.; Martinez, R.; Patel, S.; Chen, L.; Kim, D.; Johnson, E.; Ramirez, T.; Nguyen, H. Machine learning for feeding optimization in fish farms. Aquac. Manag. 2020, 62, 301–315. [Google Scholar]
- Ahmed, S. Unsupervised learning for fish behavior analysis. Aquat. Technol. 2023, 58, 187–196. [Google Scholar]
- Nguyen, L.; Tran, P.; Le, T.; Pham, H.; Hoang, Q.; Vu, D.; Bui, N.; Do, A.; Dang, M.; Ho, T.; et al. Hybrid machine learning and fuzzy logic for adaptive feeding systems. Aquacult. Eng. J. 2021, 37, 521–534. [Google Scholar]
- Huang, Y.-P.; Khabusi, S.P. Artificial Intelligence of Things (AIoT) Advances in Aquaculture: A Review. Appl. Sci. 2025, 13, 73. [Google Scholar] [CrossRef]
- Yang, D.; Chen, X.; Sun, X.; Tan, G. Research Progress of Artificial Intelligence in Intelligent Fisheries Breeding in Prediction and Health Management Systems. Preprints 2024. [Google Scholar] [CrossRef]
- Zhang, S. AI, IoT, and DSS integration in aquaculture management. Aquac. Res. 2022, 61, 451–464. [Google Scholar]
- Chen, M.; Wang, J.; Liu, Y.; Zhang, X.; Huang, P.; Li, Q.; Zhao, R.; Sun, Y.; Xu, D.; Yang, F.; et al. Bayesian networks for predicting oxygen depletion in fish ponds. Mar. Technol. 2023, 40, 399–412. [Google Scholar]
- Smith, T.; Johnson, R.; Williams, A.; Brown, K.; Davis, M.; Wilson, S.; Taylor, J.; Anderson, L.; Thomas, E.; Jackson, P.; et al. Drones and AI for aeration management in aquaculture. Fish. Eng. 2021, 39, 321–334. [Google Scholar]
- Garcia, R.; Martinez, L.; Hernandez, D.; Lopez, F.; Perez, S.; Torres, C.; Ramirez, J.; Sanchez, M.; Cruz, V.; Morales, N.; et al. Deep learning and meteorological data for aeration systems. J. Aquacult. Innov. 2020, 44, 254–267. [Google Scholar]
- Martinez, D. Decision support systems for disease prediction in aquaculture. Aquacult. Inform. 2022, 55, 401–414. [Google Scholar]
- Wilson, H. Real-time analytics in DSS for feeding optimization. J. Aquacult. Eng. 2021, 47, 277–290. [Google Scholar]
- Johnson, L. Environmental data integration in DSS for aquaculture. Fish. Res. Manag. 2022, 38, 312–326. [Google Scholar]
- Roy, D.; Padhiary, M.; Roy, P.; Barbhuiya, J.A. Artificial Intelligence-Driven Smart Aquaculture: Revolutionizing Sustainability through Automation and Machine Learning. LatIA 2024, 2, 116. [Google Scholar] [CrossRef]
- Panduranga, D.; Kumar, R.; Das, M.S.; Narender, G.; Sreelaxmi, M.; Rajeswaran, N. Sustainable Aquaculture Management through IoT and Deep Learning-Driven Remote Monitoring. In Proceedings of the 2024 8th International Conference on Electronics, Communication and Aerospace Technology (ICECA), Coimbatore, India, 6–8 November 2024; pp. 338–344. [Google Scholar] [CrossRef]
- Steele, S.R.; Ranjan, R.; Sharrer, K.; Tsukuda, S.; Good, C. Computer-Simulated Virtual Image Datasets to Train Machine Learning Models for Non-Invasive Fish Detection in Recirculating Aquaculture. Sensors 2024, 24, 5816. [Google Scholar] [CrossRef]
- Othman, Z. Dissolved Oxygen Prediction in Rivers Based on RF-LSTM. Artif. Intell. Mach. Learn. 2025; preprint. Available online: https://www.preprints.org/frontend/manuscript/ff083cdda5a93aee168b4f72b9615105/download_pub (accessed on 20 June 2025).
- Yang, W.; Liu, W.; Gao, Q. Prediction of dissolved oxygen concentration in aquaculture based on attention mechanism and combined neural network. Math. Biosci. Eng. 2023, 20, 998–1017. [Google Scholar] [CrossRef]
- Li, T. Predicting Aquaculture Water Quality Using Machine Learning Approaches. Semantic Scholar. 2022. Available online: https://pdfs.semanticscholar.org/6b49/d9f91195739ef1cd37b7e33862a895f5799b.pdf (accessed on 20 June 2025).
- Nemade, B. IoT-based automated system for water-related disease detection in aquaculture. Nat. Sci. Rep. 2024, 14, 29479. Available online: https://www.nature.com/articles/s41598-024-79989-6 (accessed on 20 June 2025).
- Navarro, L.C. Predicting weight dispersion in seabass aquaculture using machine learning. Aquac. Rep. 2024, 35, 102021. Available online: https://www.sciencedirect.com/science/article/pii/S2352513424004034 (accessed on 20 June 2025).
- Al-Mutairi, A.; Al-Aubidy, K. IoT-based smart monitoring and management system for fish farming. J. Environ. Monit. 2023, 12, 1435–1446. [Google Scholar] [CrossRef]
- Jabbar, W.A.; Ting, T.M.; Hamidun, M.F.I.; Kamarudin, A.H.C.; Wu, W.; Sultan, J.; Alsewari, A.A.; Ali, M.A. Development of LoRaWAN-based IoT system for water quality monitoring in rural areas. Expert Syst. Appl. 2024, 242, 122862. [Google Scholar] [CrossRef]
- Chiu, C.H.; Tsai, M.F.; Chen, Y.L. Development of smart aquaculture farm management system using IoT-based intelligent fishpond monitoring. J. Mar. Sci. Eng. 2022, 10, 1105. [Google Scholar]
- Chen, M.; Sivaparthipan, C.; Muthu, B. Energy optimization using IoT in aquaculture. Mar. Technol. 2022, 42, 399–411. [Google Scholar]
- Haque, H.K.; Labeeb, R.; Riha, R.; Khan, M. IoT based water quality monitoring system by using Zigbee protocol. In Proceedings of the 2021 International Conference on Emerging Smart Computing and Informatics (ESCI), Pune, India, 5–7 March 2021; pp. 619–622. [Google Scholar]
- Tolentino, L.; De Pedro, C.P.; Icamina, J.D.; Madrigal, G.A.M.; Enriquez, L. Development of an IoT-based intensive aquaculture monitoring system with automatic water correction. Int. J. Comput. Digit. Syst. 2021, 10, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.; Kardile, A.; Kasar, K.; Gaikwad, S. E-monitoring system for biofloc fish farming. IJRAR 2020, 7, 653–657. [Google Scholar]
- Mayrawan, D.; Herwanto, B.; Widyatmoko, B. Development of Internet of Things for water quality monitoring system for gouramy cultivation. In Proceedings of the 4th Forum in Research, Science, and Technology (FIRST-T1-T2-2020), Palembang, Indonesia, 10–11 November 2020; Atlantis Press: Dordrecht, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Ragab, S.; Hoseinifar, S.H.; Van Doan, H.; Rossi, W.; Davies, S.; Ashour, M.; El-Haroun, E. Overview of aquaculture Artificial Intelligence (AAI) applications: Enhance sustainability and productivity, reduce labor costs, and increase the quality of aquatic products. Ann. Anim. Sci. 2024, 25, 441–453. [Google Scholar] [CrossRef]
- Rastegari, H.; Kim, Y. Internet of Things in aquaculture: A review of the challenges and potential solutions based on current and future trends. Aquac. Rep. 2023, 28, 101512. [Google Scholar] [CrossRef]
- Chrispin, L.; Jothiswaran, V.V.; Velumani, T.; Agnes, D.A.S.; Jayaraman, R. Application of artificial intelligence in fisheries and aquaculture. Biot. Res. Today 2020, 2, 499–502. [Google Scholar]
- Li, D.; Hao, Y.; Duan, Y. Nonintrusive methods for biomass estimation in aquaculture with emphasis on fish: A review. Rev. Aquac. 2020, 12, 1390–1411. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, D.; Hong, J. Design of automatic and uniform feeding system carried by workboat and effect test for raising river crab. Trans. Chin. Soc. Agric. Eng. 2015, 31, 31–39. [Google Scholar]
- Liu, Z.; Li, X.; Fan, L.; Lu, H.; Liu, L.; Liu, Y. Measuring feeding activity of fish in RAS using computer vision. Aquac. Eng. 2014, 60, 20–27. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, K.; Xu, D.M.; Chen, L.; Guo, Q.; Sun, C.H.; Yang, X.T. Near infrared computer vision and neuro-fuzzy model-based feeding decision system for fish in aquaculture. Comput. Electron. Agric. 2018, 146, 114–124. [Google Scholar] [CrossRef]
- Padmavathy, N.; Venu, M.; Valarmathi, B.; Chinnaiah, M.; Prasanthi, B.; Sharif, G. Smart peripatetic food feeding system for aquafarm. In Proceedings of the IEEE International Conference on Interdisciplinary Approaches in Technology and Management for Social Innovation (IATMSI), Gwalior, India, 14–16 March 2024; pp. 1–5. [Google Scholar] [CrossRef]
- Luna, F.D.V.B.; Aguilar, E.; Naranjo, J.S. Robotic system for automation of water quality monitoring and feeding in aquaculture shadehouse. IEEE Trans. Syst. Man Cybern. Syst. 2017, 47, 1575–1589. [Google Scholar] [CrossRef]
- Abid, M.A.; Mushtaq, M.F.; Akram, U.; Abbasi, M.A.; Rustam, F. Comparative analysis of TF-IDF and loglikelihood method for keywords extraction of Twitter data. Mehran Univ. Res. J. Eng. Technol. 2023, 42, 88. [Google Scholar] [CrossRef]
- Ahammed, M.B.; Momin, A.; Sultana, S.; Sarkar, A. pH and temperature monitoring with a GSM-based auto feeding system of biofloc technology. Int. J. Sci. Eng. Res. 2022, 13, 270–274. [Google Scholar]
- Matishov, G. Using BioFloc Technology to Improve Aquaculture Efficiency. Fishes 2025, 10, 144. [Google Scholar] [CrossRef]
- Helal, A.M.; Zaher, M.M.; Meshhal, D.T.; Ashour, M. Biofloc supplementation improves growth performances. Aquaculture 2024, 586, 739985. [Google Scholar] [CrossRef]
- Yu, Y.B. Biofloc Technology in Fish Aquaculture: A Review. J. Fish. Aquat. Sci. 2023, 18, 398. [Google Scholar] [CrossRef]
- Lee, K. Optimizing Feed Management in Aquaculture through IoT and AI: A Review. Aquac. Technol. Innov. 2022, 5, 45–58. [Google Scholar]
- Tan, S. The Rise of Smart Aquaculture Platforms: The eFishery Model. Asian Fish. Sci. J. 2023, 36, 22–35. [Google Scholar]
- Nisar, U. A Solution for Sustainable Utilization of Aquaculture Waste. Front. Nutr. 2022, 8, 791738. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Li, L.; Sun, R.; Wang, S. A novel deep learning framework for rolling bearing fault diagnosis enhancement using VAE-augmented CNN model. Heliyon 2024, 10, e35407. [Google Scholar] [CrossRef] [PubMed]
- Smith, J. AI-Powered Cloud Monitoring for Aquaculture: A Case Study of Fishwell Vietnam. J. Smart Farming Technol. 2023, 1, 1–10. [Google Scholar]
- Johnson, R. IoT and AI in Aquaculture: A Comprehensive Review of Applications and Future Trends. Rev. Aquac. 2023, 15, 1200–1215. [Google Scholar]
- Yu, Z. Research and design of an intelligent aquaculture system based on IoT and AI. In Proceedings of the 2024 IEEE 2nd International Conference on Electronic Technology, Communication and Control Engineering (ETCCE), Jilin, China, 26–28 April 2024; pp. 1–5. Available online: https://ieeexplore.ieee.org/abstract/document/10853673/ (accessed on 20 June 2025).
- Ficili, I.; Giacobbe, M.; Tricomi, G.; Puliafito, A. From Sensors to Data Intelligence: Leveraging IoT, Cloud, and Edge Computing with AI. Sensors 2025, 25, 1763. [Google Scholar] [CrossRef]
- Tina, F.W.; Afsarimanesh, N.; Nag, A.; Alahi, M.E.E. Integrating AIoT Technologies in Aquaculture: A Systematic Review. J. Mar. Sci. Eng. 2025, 17, 199. [Google Scholar] [CrossRef]
- Rastegari, H.; Nadi, F.; Lam, S.S.; Ikhwanuddin, M.; Kasan, N.A.; Rahmat, R.F.; Wan Mahari, W.A.W. Internet of things in aquaculture: A review of the challenges and potential solutions based on current and future trends. Smart Agric. Technol. 2023, 4, 100187. [Google Scholar] [CrossRef]
- Seafood.media. Leveraging Artificial Intelligence to Optimize Fish Farming. 2024. Available online: https://www.seafood.media/fis/worldnews/worldnews.asp?monthyear=10-2024&day=28&id=132395&l=e&country=0&special=&ndb=1&df=0 (accessed on 20 June 2025).
- WAS. Intelligent Aquaculture. Available online: https://www.was.org/article/Intelligent-aquaculture.aspx (accessed on 20 June 2025).
- Rabia, M. AI-Driven Aquafarming: Recent Innovations, Challenges, and Future Prospects. Pure Appl. Food Manag. Bull. 2025, 18, 46–53. Available online: https://academicstrive.com/PAFMB/PAFMB180046.pdf (accessed on 20 June 2025).
- Rather, M.A. Exploring opportunities of Artificial Intelligence in aquaculture: A review. Rev. Aquac. 2024, 16, 1459–1478. [Google Scholar] [CrossRef]
- Jais, N.A.M. Improved accuracy in IoT-Based water quality monitoring system for aquaculture. J. King Saud. Univ. Comput. Inf. Sci. 2024, 36, 101822. Available online: https://www.sciencedirect.com/science/article/pii/S2405844024050539 (accessed on 20 June 2025).
- Dayaday, M.G. An innovative real-time water quality monitoring system for aquaculture application. ARPN J. Eng. Appl. Sci. 2021, 16, 8798. Available online: http://www.arpnjournals.org/jeas/research_papers/rp_2021/jeas_1221_8798.pdf (accessed on 20 June 2025).
- Liu, M. SD-YOLOv8: An Accurate Seriola dumerili Detection Model Based on Improved YOLOv8. Sensors 2024, 24, 3400. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Sun, X.; Yu, G.; Wang, J.; Li, D.; Wang, Y. CBFW-YOLOv8: Automated recognition method for fish body surface diseases in recirculating aquaculture systems. Comput. Electron. Agric. 2025, 236, 110612. Available online: https://www.sciencedirect.com/science/article/pii/S0168169925007185 (accessed on 20 June 2025). [CrossRef]
- Zhang, Z. An Improved YOLOv8n Used for Fish Detection in Natural Waters. Animals 2024, 14, 2022. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H. Utilizing an Enhanced YOLOv8 Model for Fishery Detection. J. Mar. Sci. Eng. 2025, 10, 81. [Google Scholar] [CrossRef]
- Kumar, C. Random Cross-Validation Produces Biased Assessment of Machine Learning Performance in Regional Landslide Susceptibility Prediction. Remote Sens. 2025, 17, 213. [Google Scholar] [CrossRef]
- Keylabs.ai. Cross-Validation Techniques to Validate Precision. 2024. Available online: https://keylabs.ai/blog/cross-validation-techniques-to-validate-precision/ (accessed on 20 June 2025).
- Sweet, L.-b.; Müller, C.; Anand, M.; Zscheischler, J. Cross-Validation Strategy Impacts the Performance and Interpretation of Machine Learning Models. Artif. Intell. Earth Syst. 2023, 2, e230026. [Google Scholar] [CrossRef]
- Datacamp. Explainable AI, LIME & SHAP for Model Interpretability. 2023. Available online: https://www.datacamp.com/tutorial/explainable-ai-understanding-and-trusting-machine-learning-models (accessed on 20 June 2025).
- Ranjbaran, G.; Recupero, D.R.; Roy, C.K.; Schneider, K.A. C-SHAP: A Hybrid Method for Fast and Efficient Interpretability. Appl. Sci. 2025, 15, 672. [Google Scholar] [CrossRef]
- Abdar, M.; Pourpanah, F.; Hussain, S.; Rezazadegan, D.; Liu, L.; Ghavamzadeh, M.; Fieguth, P.; Cao, X.; Khosravi, A.; Acharya, U.R.; et al. A review of uncertainty quantification in deep learning: Techniques, applications and challenges. Inf. Fusion 2021, 76, 243–297. [Google Scholar] [CrossRef]
- Tim, G.; Toner, H. Key Concepts in AI Safety: Reliable Uncertainty Quantification in Machine Learning; Georgetown University: Washington, DC, USA, 2024; Available online: https://cset.georgetown.edu/wp-content/uploads/CSET-Key-Concepts-in-AI-Safety-Reliable-Uncertainty-Quantification-in-Machine-Learning.pdf (accessed on 20 June 2025).
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaría, J.; Fadhel, M.A.; Al-Amidie, M. Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions. J. Big Data 2021, 8, 53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).