1. Introduction
In China’s energy consumption structure, coal has consistently played an indispensable role, making substantial contributions to national economic development. However, methane emissions generated during coal mining processes pose dual threats—endangering miners’ safety and exacerbating greenhouse gas emissions [
1]. Annual methane emissions from coal mining in China reach 37 billion cubic meters, of which 53% comprises coal mine methane (CMM) with concentrations ≥3%. If effectively utilized through combined heat and power (CHP) generation, this recoverable CMM could yield an annual economic value of RMB 43.3 billion while mitigating greenhouse gas emissions by 330 million tons of CO
2 equivalent (CO
2e) [
2,
3,
4]. The impending inclusion of methane emissions reduction within China’s national carbon trading framework is poised to significantly amplify the economic value of resource utilization for coal mine methane (CMM). In modern coal mining operations, high-concentration CMM (methane concentrations exceeding 30%) has already been directly utilized. In comparison, medium-concentration CMM (methane concentrations ranging from 9% to 30%) is primarily harnessed through internal combustion engine (ICE) power generation technologies [
5]. However, utilizing low-concentration coal mine methane (CMM) with methane concentrations between 3% and 9% remains a persistent technical challenge in the industry [
6]. While existing ventilation air methane (VAM) oxidation technologies can process low-concentration CMM (3–9% methane), they face critical limitations including difficulties in sustaining requisite high-temperature environments, unstable continuous operation, and suboptimal thermal efficiency [
7]. Concurrently, CMM blending technologies (mixing high- and low-concentration streams) demonstrate improved utilization rates but remain constrained by the limited availability of high-concentration CMM sources, hindering large-scale implementation.
The direct utilization of low-concentration coal mine methane (CMM, 3–9% methane) faces two primary technical barriers: difficult ignition and heightened explosion risks. Under standard temperature and pressure (STP) conditions, methane exhibits an explosion limit range of 5–16% (by volume) in air. Notably, this explosive range expands significantly with increasing temperature—a thermodynamic behavior that intensifies safety challenges during energy recovery processes [
8]. Explosion characteristics are constrained by multiple experimental conditions, primarily including the geometric parameters of the reaction vessel, configuration characteristics of the ignition device, energy density, and the initial thermodynamic state of the gas. Therefore, in-depth research on gas explosion mechanisms and mastering the explosion characteristics of gas under different conditions have significant practical importance for developing technologies for the direct utilization of low-concentration gas. Under standard atmospheric pressure and room temperature conditions (25 °C, 101.325 kPa), the explosive characteristics of methane–air mixtures have clearly defined concentration boundaries. When the volume fraction of methane in the mixture is below 5%, the mixture cannot maintain stable combustion; when the methane concentration exceeds 16%, the mixture cannot explode due to insufficient oxygen concentration. This 5–16% concentration range is defined as the explosive limit range of methane in air. As temperature increases, this explosive limit range widens, meaning the upper limit increases while the lower limit decreases [
9]. A large number of scholars have already conducted systematic research on the explosive limits of methane under high-temperature conditions. Kondo et al. [
10] constructed a 12 L spherical explosion vessel to study the explosive limits of various combustible gases at different temperatures. The temperature range tested was 5–100 °C, and they found that the lower explosive limit exhibited an almost linear relationship with temperature. Li et al. [
11] used a self-developed apparatus to determine the explosive limit values of coal bed methane under initial conditions of 20, 60, 100, 150, and 200 °C. They found that as the initial temperature increased, the lower explosive limit decreased.
It is worth noting that in actual industrial environments and coal mining processes, gas is not only mixed with pure air but also exists in complex gaseous environments. Coal mining production processes generate gases such as CO2, NH3, etc., ventilation systems may introduce O2 at different concentrations, while high-humidity environments produce large amounts of water vapor. The presence of these gas components significantly alters the explosive characteristics of methane, thereby affecting the safety boundaries and process parameter design for the utilization of low-concentration gas. However, existing research has mainly focused on the explosive characteristics of pure methane–air mixtures, with notably insufficient systematic research on methane explosion characteristics in complex atmospheres containing components such as CO2, NH3, O2, H2O, etc. Particularly under high-temperature conditions, the mechanisms for the influence of these gas components on methane explosion characteristics may undergo significant changes, but currently, there is a lack of systematic, quantitative experimental data to support this. Therefore, studying the lower explosive limit of methane in different mixed atmospheres and its coupling effect with temperature has important theoretical and practical significance for accurately assessing explosion risks in industrial environments, optimizing low-concentration gas utilization processes, and improving coal mine safety production levels. At the same time, these research findings also provide a scientific basis for the formulation of relevant safety standards and the design of explosion-proof equipment.
Through a systematic review of the existing literature, it has been found that the majority of experimental conditions in previous studies on methane explosive limit characteristics have been limited to temperatures below 200 °C, while research on methane explosion characteristics under high-temperature conditions above 200 °C is relatively scarce [
12]. Therefore, this paper reports the design of experimental detection equipment to test the effect of methane–air atmosphere on the lower explosive limit at higher temperatures, as well as to study the lower explosive limits of air/gas with components such as CO
2, N
2, O
2, and H
2O.
2. Experimental Setup and Testing Process
This research designed a comprehensive experimental system for studying the explosive characteristics of combustible gases. The core component of the system is a stainless steel reaction vessel with an internal volume of 1 L and a design pressure of 10 MPa, which far exceeds the maximum explosion pressure values that might occur during experiments. The system is equipped with comprehensive temperature monitoring and control devices. Heating bands are installed on the exterior of the reaction vessel, integrated with a specialized temperature regulation system. To precisely measure temperature changes during the reaction process, high-performance thermocouples are positioned inside the vessel. The experimental setup also integrates multiple functional modules, including gas cylinders, a gas distribution system, ignition devices, a vacuum pump system, mixing equipment, and data acquisition devices. These modules collectively form a complete testing platform, ensuring precise control of gas ratios during the experiment, thorough mixing of the mixture, effective removal of residual gases, and real-time recording and acquisition of experimental data.
The experimental design employs a partial pressure method to precisely configure combustible gas mixtures. The gas preparation process strictly follows a specific procedure; methane is first introduced into the explosion vessel to a preset partial pressure value, followed by the introduction of air to the target pressure. A high-precision pressure gauge with an accuracy grade of 0.02 is used to monitor the initial pressure of the combustible gas mixture during the experiment. The ignition system uses an electric spark discharge method, with two electrodes installed inside the explosion vessel, maintained at a distance of 2 mm apart. The discharge energy of the system is designed to remain constant at 20 J (
Figure 1).
This gas preparation and ignition scheme offers the following technical advantages. The partial pressure method ensures precise control of the mixed gas components, the high-precision pressure gauge ensures the accuracy of initial pressure measurements, while the fixed electrode gap and sufficient ignition energy provide stable and reliable initiation conditions for explosion experiments. The reasonable configuration of these technical parameters guarantees the reproducibility of experimental data. During the experimental process, a high-performance sensor system is used to monitor the explosion process. The measurement of explosion pressure utilizes piezoelectric sensors with a sensing frequency of 500 kHz, ensuring accurate capture of pressure changes throughout the entire explosion process. Meanwhile, to monitor explosion temperature changes, the experiment employs exceptionally fast-response thermocouples capable of measuring temperatures from –10 to 1700 °C, withstanding instantaneous high temperatures of 2100 °C and pressure environments of 30 MPa. Their response speed of 10 μs enables precise recording of temperature data at the moment of explosion. To ensure the accuracy and reliability of experimental data, a strict experimental procedure has been established. After each experiment, a vacuum pump must be activated to evacuate the explosion vessel, eliminating the interference of residual gases on subsequent experiments. Before igniting the mixed gases, thorough mixing is performed for 180 s to ensure the gas mixture reaches a uniform state. Furthermore, considering the scientific nature and reproducibility of the experimental results, each set of experimental conditions undergoes three repeated tests.
3. Results and Discussion
To calculate the lower explosive limit of methane at high temperatures, it is first necessary to determine the critical temperature of methane explosion, which is the adiabatic flame temperature of methane combustion. According to the lower explosive limit values of methane–air mixtures obtained from experiments at temperatures of 5–100 °C reported in the literature, the lower explosive limits of methane–air mixtures at temperatures of 5, 21, 35, 50, 75, and 100 °C are 5%, 4.95%, 4.94%, 4.88%, 4.74%, and 4.70%, respectively [
13]. Zhang et al. [
14] provided the adiabatic combustion temperatures for methane combustion at initial temperatures of 5, 21, 35, 50, 75, and 100 °C. It was observed that the adiabatic combustion temperatures of methane at different temperatures all fell within the range of 1445–1480 K. By calculating the average value, the critical explosion temperature of methane was determined to be 1471.4 K.
3.1. Temperature Effects on the Lower Explosive Limit of Air–Methane Mixtures
Based on the critical temperature of 1471.4 K, the lower explosion limits (LELs) of methane–air mixtures in the temperature range of 25–600 °C were calculated using the adiabatic flame temperature theory. The corresponding data are presented in
Table 1.
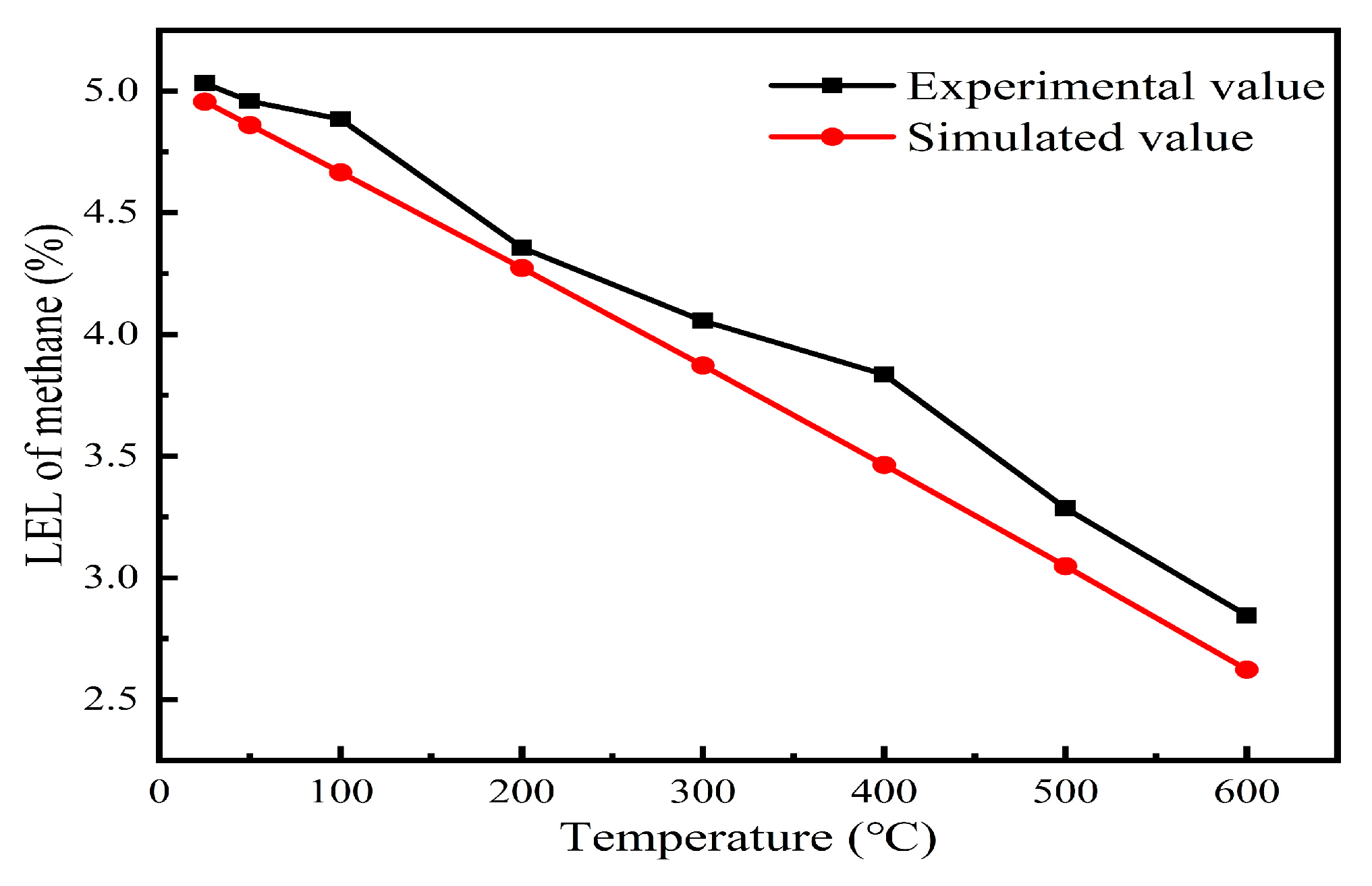
Experimental validation of the lower explosion limits (LELs) for methane–air mixtures was conducted at initial temperatures ranging from 25 °C to 600 °C; detailed data are presented in
Table 1. The measured LEL values under the tested temperatures (25 °C, 50 °C, 100 °C, 200 °C, 300 °C, 400 °C, 500 °C, and 600 °C) were 5.03%, 4.95%, 4.88%, 4.35%, 4.05%, 3.53%, 3.28%, and 2.84%, respectively. The simulated and experimental results both exhibited identical trends, with deviations less than 5%, confirming that the LELs of methane–air mixtures decreased progressively with increasing temperature. Specifically, as the initial temperature rose from 25 °C to 600 °C, the LELs of the methane–air mixtures declined from 5.03% to 2.84%, demonstrating that elevated temperatures significantly increase the explosion risk of methane–air mixtures (
Figure 2).
3.2. Effects of CO2, H2O, O2, and NH3 on the Lower Explosion Limits (LELs) of Coal Mine Methane–Air Mixtures
3.2.1. Effect of CO2 on the Lower Explosion Limits (LELs) of Coal Mine Methane–Air Mixtures
According to the molecular collision theory, the introduction of CO
2 reduces the energy of the activated free radicals or free atoms involved in the collision process by transferring it to CO
2, thus lowering their reactivity and interrupting the combustion process, which inhibits the propagation of explosion energy [
15]. The introduction of CO
2 decreases the relative concentration of O
2 in the reaction system, and the resulting dilution effect directly reduces the probability of effective collisions between CH
4 molecules and O
2 molecules, thereby reducing the reaction rate. Additionally, CO
2 molecules form a physical barrier in the reaction space, isolating the combustible CH
4 from the oxidizer O
2 at the microscopic scale. This spatial blocking effect further limits the contact opportunities between the reactants. Since chain reactions require adequate O
2 and O radicals, the blockage caused by CO
2 significantly reduces reactivity. From the perspective of the elementary equations of methane oxidation reactions, the main reactions responsible for CH4 consumption are OH + CH
4 → CH
3 + H
2O, H + CH
4 → CH
3 + H
2, and O + CH
4 → OH + CH
3. The addition of CO
2 significantly inhibits these three elementary reactions, reducing the consumption of methane [
16]. Furthermore, CO
2 itself is an important component in the CH
4/O
2 reaction, and its addition can suppress the forward reactions of CH
4/O
2, thus inhibiting methane explosions. Therefore, it is essential to study the effect of CO
2 on the lower explosion limits of gas mixtures.
The experiment verified the lower explosion limits (LELs) of CH
4/5% CO
2–air mixtures at initial temperatures ranging from 25 °C to 600 °C, and compared them with the LELs of methane–air mixtures. The results are shown in
Figure 3. At temperatures of 25 °C, 50 °C, 100 °C, 200 °C, 300 °C, 400 °C, 500 °C, and 600 °C, the LELs of methane–5% CO
2–air mixtures were found to be 5.09%, 4.99%, 4.89%, 4.39%, 4.12%, 3.95%, 3.48%, and 2.95%, respectively. As the temperature increased from 25 °C to 600 °C, the LEL of the CH
4–5% CO
2–air mixture decreased from 5.09% to 2.95%. Notably, under the same temperature conditions, the LEL of the methane/5% CO
2/air mixture was higher than that of the methane–air mixture. This result indicates that CO
2 can suppress the explosion of methane.
3.2.2. The Effect of H2O on the Lower Explosion Limits (LELs) of Gas Mixtures
Water primarily suppresses the detonation of methane–air mixtures in the form of water mist. Additionally, water particles collide with active species (such as free radicals), leading to the destruction of these active species and interrupting the initial chain reactions. This process effectively prevents the detonation of methane–air mixtures [
12]. The evaporation of water mist particles absorbs heat, leading to a decrease in the temperature of the gas mixture during the ignition process. As a result, the heat available during ignition is insufficient, preventing the ignition process from sustaining itself and inhibiting the occurrence of deflagration during the ignition process [
17]. Due to the absorption of heat by water mist particles during their vaporization, the oxygen concentration near the ignition source of the gas premixed mixture decreases, which prevents the continuation of the combustion reaction and thus prevents explosions. Therefore, it is necessary to study the effect of H
2O on the lower explosion limits (LELs) of gas mixtures.
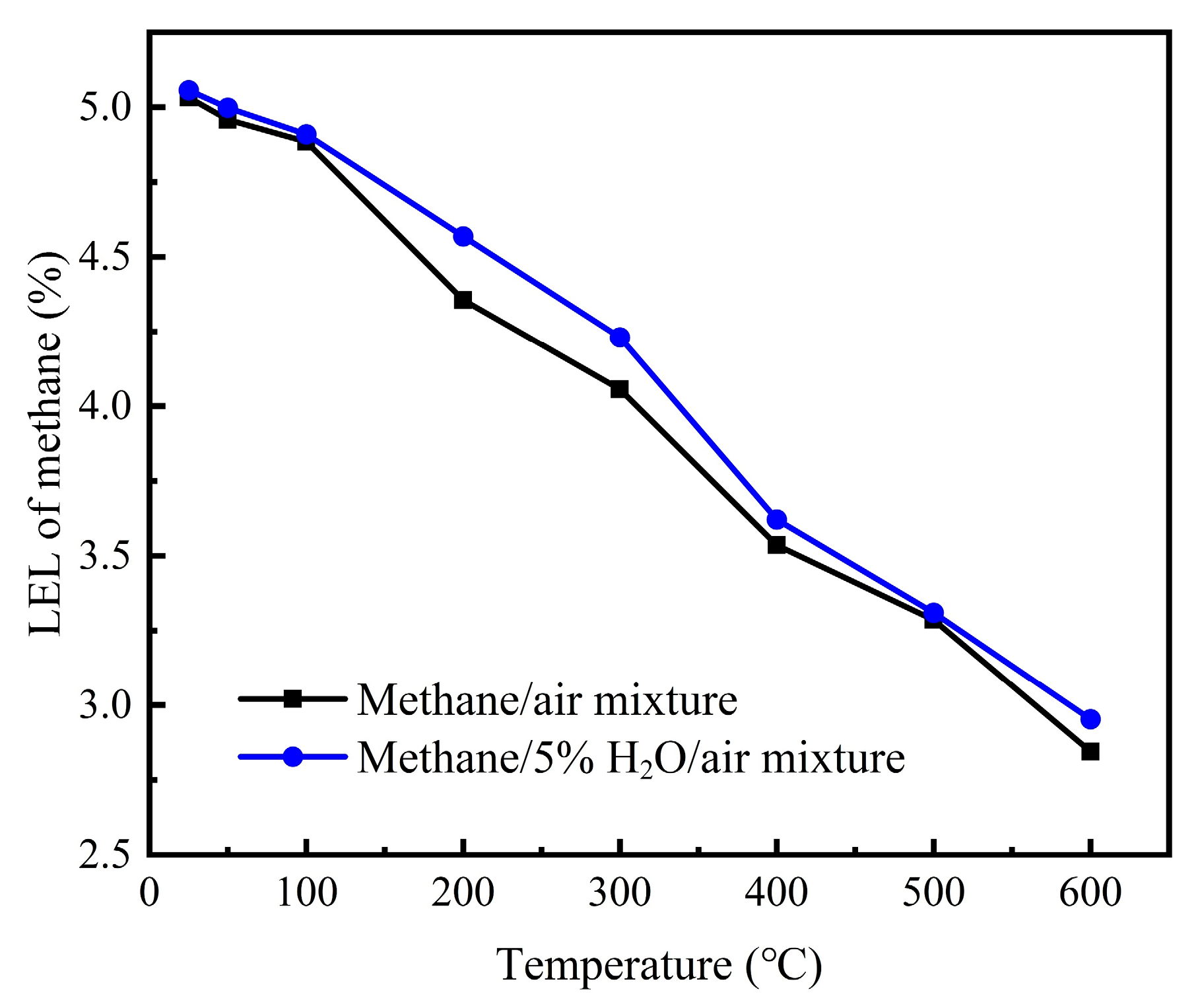
The experiment measured the lower explosion limits (LELs) of methane–5% H
2O–air mixtures at temperatures ranging from 25 °C to 600 °C and compared these with the LELs of methane–air mixtures. The results are shown in
Figure 4. At temperatures of 25 °C, 50 °C, 100 °C, 200 °C, 300 °C, 400 °C, 500 °C, and 600 °C, the LELs of methane–5% H
2O–air mixtures were found to be 5.05%, 4.99%, 4.90%, 4.56%, 4.23%, 3.62%, 3.31%, and 2.95%, respectively. As the temperature increased from 25 °C to 600 °C, the LEL of the methane–5% H
2O–air mixture decreased from 5.05% to 2.95%. Under the same initial temperature conditions, the LEL of the methane/5% H
2O/air mixture was higher than that of the methane–air mixture, indicating that H
2O can suppress methane explosions.
In the study of combustion reaction mechanisms, the chain reaction process plays a central role. Active species, particularly H, O free radicals, and OH radicals, serve as key intermediates in the reaction and play a decisive role in the oxidation of methane. When the addition of water vapor leads to a significant reduction in the concentration of highly reactive free radicals such as H, O, and OH, it results in the interruption and disruption of the chain reactions involved in methane combustion and explosion [
18]. If the chain reaction is blocked or broken, it slows down or even stops the oxidation reaction of methane (combustion and explosion), which means that the suppression of combustion and explosion can be achieved through the chemical quenching effect of water vapor. The addition of water vapor leads to a decrease in the system’s combustion temperature because compared with other gases, water vapor has a higher heat capacity. This enables water vapor to efficiently absorb the radiative heat from the unreacted gases in the flame reaction zone, thereby lowering the temperature of the flame reaction zone.
3.2.3. The Effect of NH3 on the Lower Explosion Limits (LELs) of Gas Mixtures
In recent years, China has been vigorously promoting renewable energy, and the hydrogen production industry has seen rapid development. However, the current bottleneck in this industry is that, due to the high reactivity of hydrogen, the produced hydrogen is difficult to utilize effectively [
19]. Japan has proposed a technology to convert hydrogen into NH
3 and implement it in conventional fuel direct combustion power generation [
20]. This technology not only significantly reduces carbon emissions but also effectively addresses the issue of renewable energy storage, providing a practical technical pathway for China to achieve its “dual carbon” goals. The addition of NH
3 to low-concentration gas mixtures offers two main advantages. First, NH
3 can reduce the methane oxidation rate, significantly improving the safety of its utilization. There are two reasons for NH
3’s effect on lowering the methane oxidation rate. The first of these is competition for oxygen; NH
3 and methane compete during the reaction process and NH
3 captures O
2, thereby reducing the methane oxidation rate. The second reason is the scavenging of active free radicals: NH
3 consumes active free radicals such as ·O and ·OH in the system. NH
3 effectively inhibits the generation of important free radicals, such as ·H and ·OH, involved in the CH
4 chain reaction. Second, the addition of NH
3 also increases the heating value of the mixture, providing a foundation for stable direct combustion in the subsequent process. The direct combustion of low-concentration gas by mixing NH
3 is a technically feasible and reasonable solution. Therefore, it is necessary to study the effect of NH
3 on the lower explosion limits (LELs) of gas mixtures.
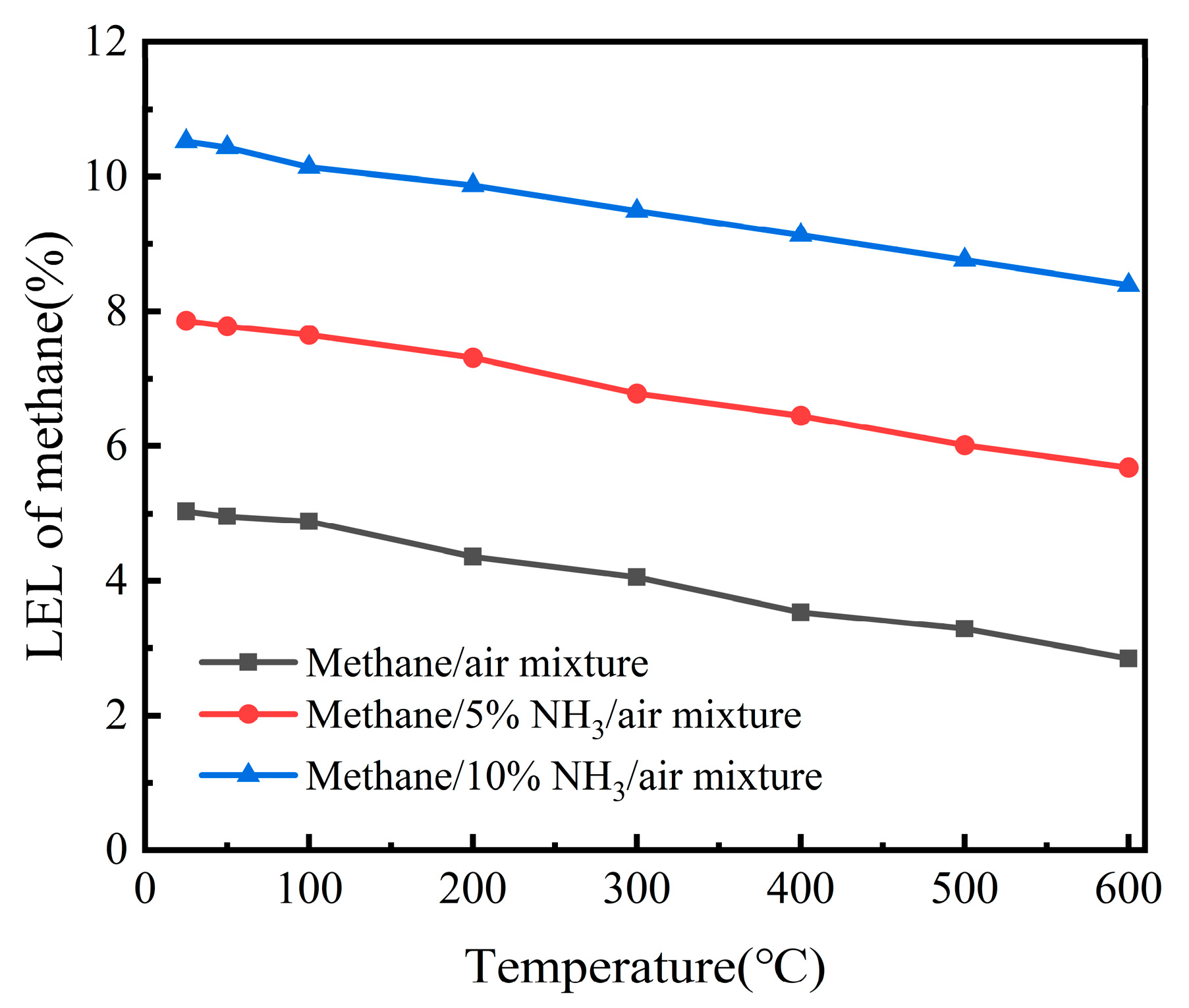
First, experiments were conducted to determine the lower explosion limit (LEL) of methane–5% NH
3–air mixtures at temperatures ranging from 25 °C to 600 °C, and the results were compared with the LELs of methane–air mixtures. The results are shown in
Figure 5. At temperatures of 25, 50, 100, 200, 300, 400, 500, and 600 °C, the LELs of the methane–5% NH
3–air mixtures were found to be 7.86%, 7.78%, 7.65%, 7.31%, 6.78%, 6.45%, 6.01%, and 5.68%, respectively. As the initial temperature increased from 25 °C to 600 °C, the LEL of the methane–5% NH
3–air mixture decreased from 7.86% to 5.68%. Clearly, under the same temperature conditions, the LEL of the methane–5% NH
3–air mixture was significantly higher than that of the methane/air mixture. The results indicate that NH
3 can suppress methane explosions.
To investigate the effect of ammonia concentration on the lower explosion limits (LELs) of gas mixtures,
Figure 5 shows the LELs of methane–air and methane–10% NH
3–air mixtures at different temperatures. The experiments at 25, 50, 100, 200, 300, 400, 500, and 600 °C yielded the following LEL values for the methane–10% NH
3–air mixture: 10.52%, 10.43%, 10.14%, 9.87%, 9.49%, 9.13%, 8.76%, and 8.39%, respectively. As the initial temperature increased from 25 °C to 600 °C, the LEL of the methane–10% NH
3–air mixture decreased from 10.52% to 8.39%.
It is noteworthy that under the same temperature conditions, the LEL of the methane–10% NH3–air mixture was significantly higher than that of the methane–5% NH3–air mixture. The results indicate that the LEL of the methane–ammonia–air mixture increased with the concentration of ammonia. As the ammonia concentration increased, the effect of suppressing the explosion of the mixture became more pronounced.
3.2.4. The Effect of O2 on the Lower Explosion Limits (LELs) of Gas Mixtures
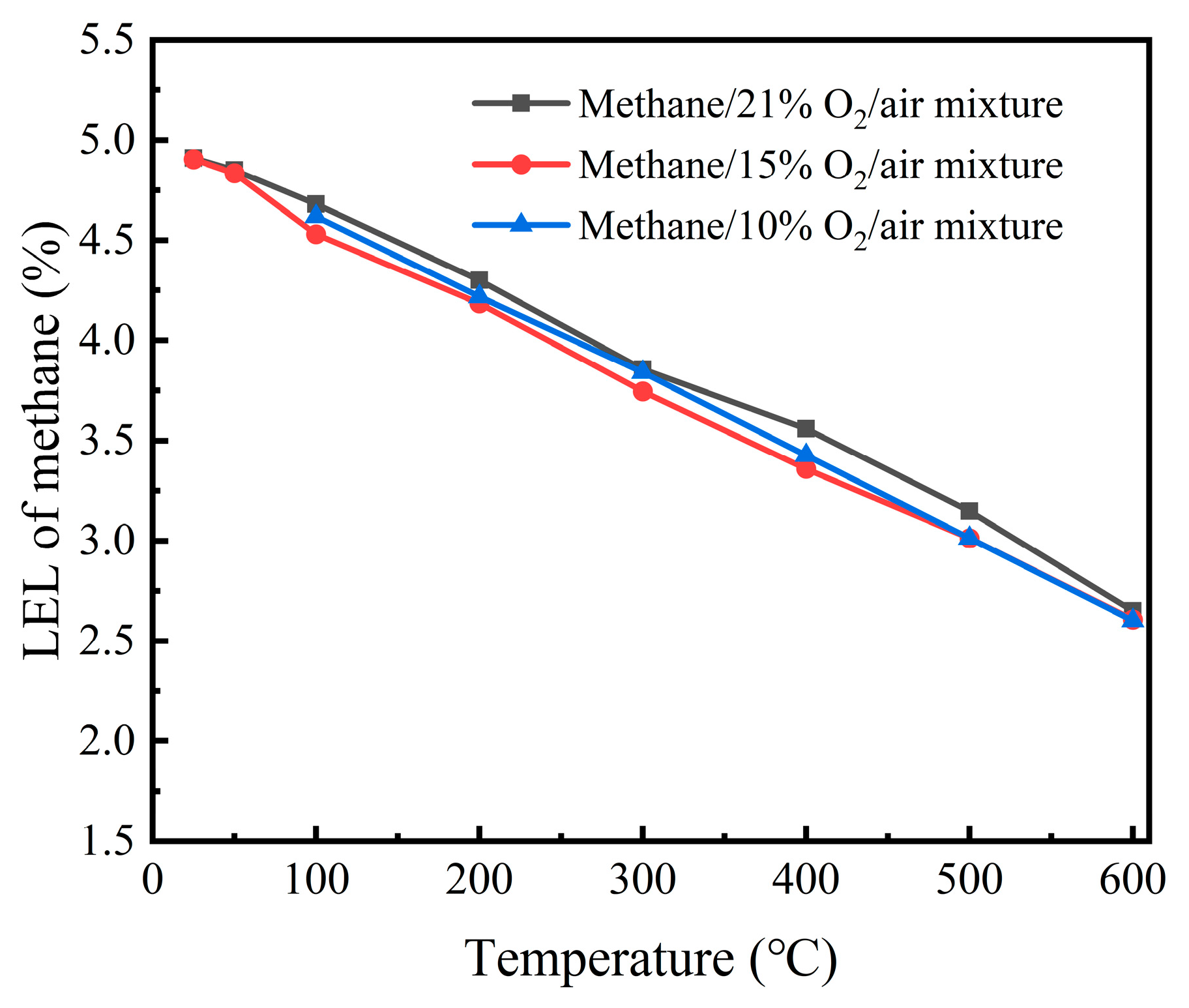
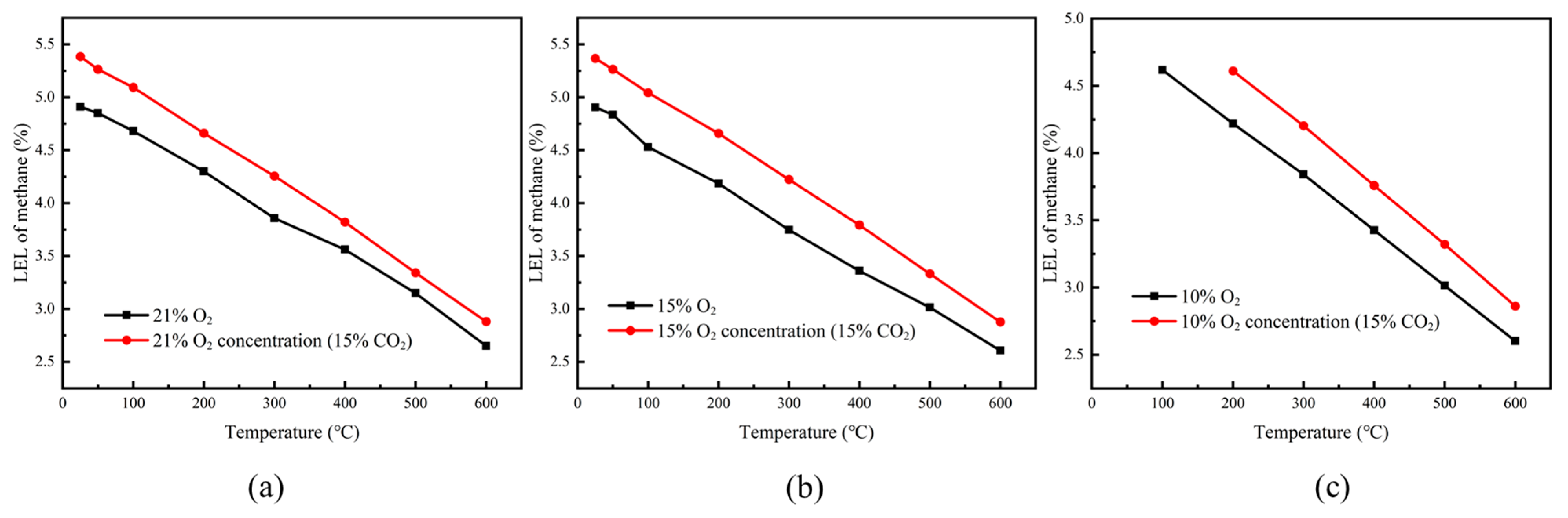
Experiments were conducted to determine the lower explosion limits (LELs) of methane–air mixtures at O
2 concentrations of 21%, 15%, and 10% over a temperature range of 25–600 °C. The results are shown in
Figure 6. The findings indicate that, at the same initial temperature, the O
2 concentration had little effect on the LEL of the methane–air mixture.
However, it is noteworthy that under 10% O2 concentration, when the initial temperature is below 66 °C, the adiabatic combustion temperature of the methane–air mixture does not reach the critical temperature, meaning that the methane–air mixture will not explode. Therefore, although lowering the O2 concentration has little impact on the LEL of the methane–air mixture, it can prevent explosions within a certain initial temperature range under low O2 concentrations (10%).
Experiments were conducted to determine the lower explosion limits (LELs) of methane–air mixtures at 15% CO
2 concentration and O
2 concentrations of 21%, 15%, and 10%, across a temperature range of 25–600 °C. The results are shown in
Figure 7. The findings indicate that at all O
2 concentrations, the addition of 15% CO
2 significantly lowered the LEL of the methane–air mixture. After adding 15% CO
2, under 10% O
2 concentration, when the temperature is below 167 °C, the adiabatic combustion temperature of the methane–air mixture does not reach the critical temperature, meaning that the methane–air mixture will not explode. Thus, the addition of CO
2 increases the temperature required for the methane–air mixture to explode, effectively suppressing the occurrence of explosions.
3.2.5. Comparison of the Effects of Single Components (CO2, NH3, O2, and H2O) on Methane Explosion
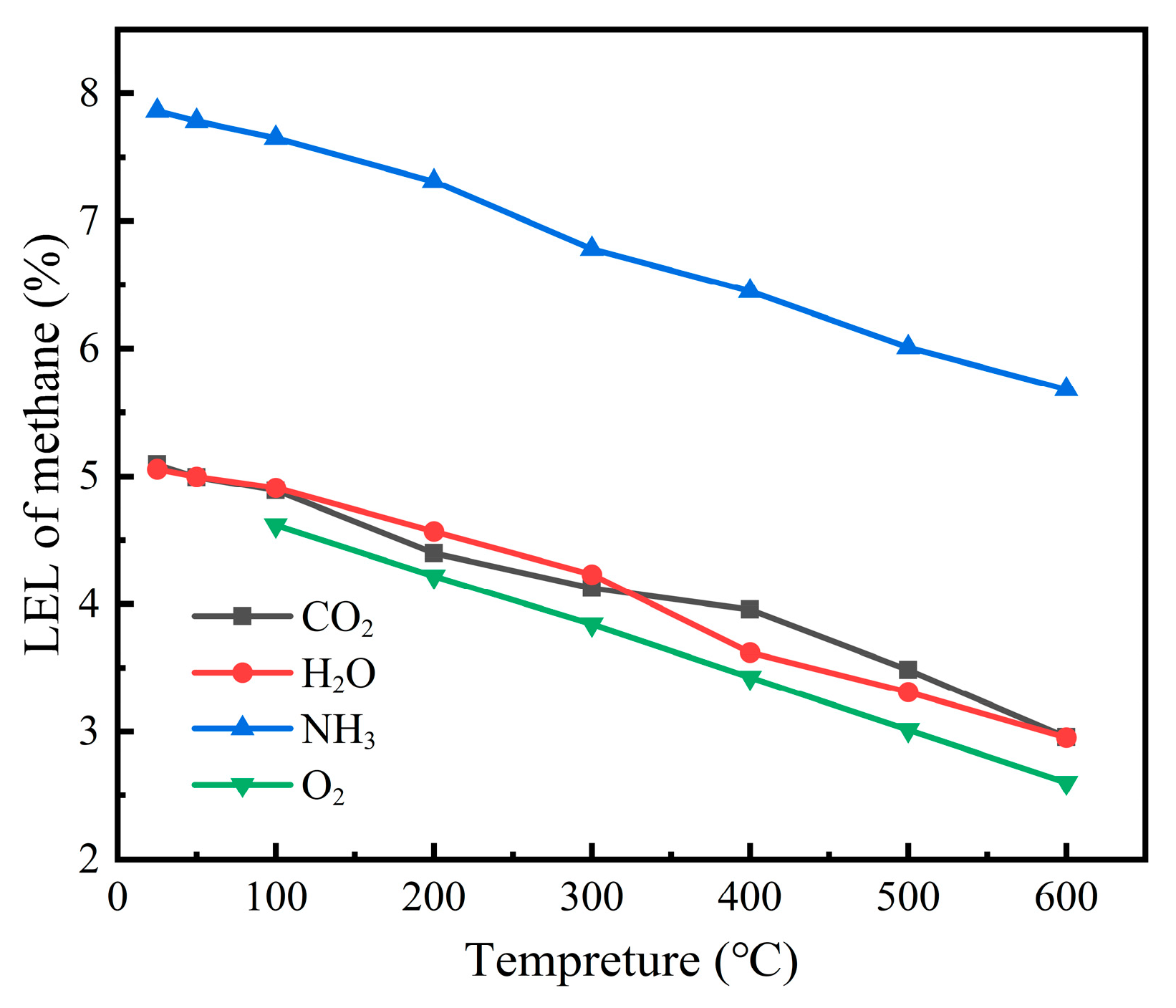
As can be seen from
Figure 8, NH
3 demonstrated the most pronounced inhibitory effect on methane explosibility, maintaining substantially elevated LEL values (approximately 7.8–5.7%) throughout the entire temperature range. This marked increase in LEL suggests that NH
3 functions as a highly effective chemical inhibitor, probably through radical scavenging mechanisms that interrupt chain-branching reactions critical to flame propagation. CO
2 and H
2O exhibited moderate inhibition characteristics with similar performance profiles. Both additives maintained the LEL around 5% at ambient temperature, gradually decreasing to approximately 3% at 600 °C. Their inhibitory action can primarily be attributed to thermal dilution effects, where their relatively high heat capacities absorb combustion energy and reduce flame temperature. O
2 addition produced the lowest LEL values across all temperatures, confirming its role as a combustion promoter rather than an inhibitor. This behavior aligns with fundamental combustion principles, as increased oxygen availability enhances oxidation reaction rates and broadens flammability limits. Universal temperature dependence was evident across all additive types, with LEL values consistently decreasing as temperature increased. This relationship underscores the heightened explosion risk in elevated temperature environments, where progressively lower methane concentrations become sufficient to form explosive mixtures. The varying slopes of these curves provide additional insights. NH
3 exhibited a relatively moderate gradient, indicating that its inhibitory efficiency remained relatively stable across the temperature spectrum. In contrast, the steeper gradient observed with O
2 suggests a more pronounced temperature sensitivity.
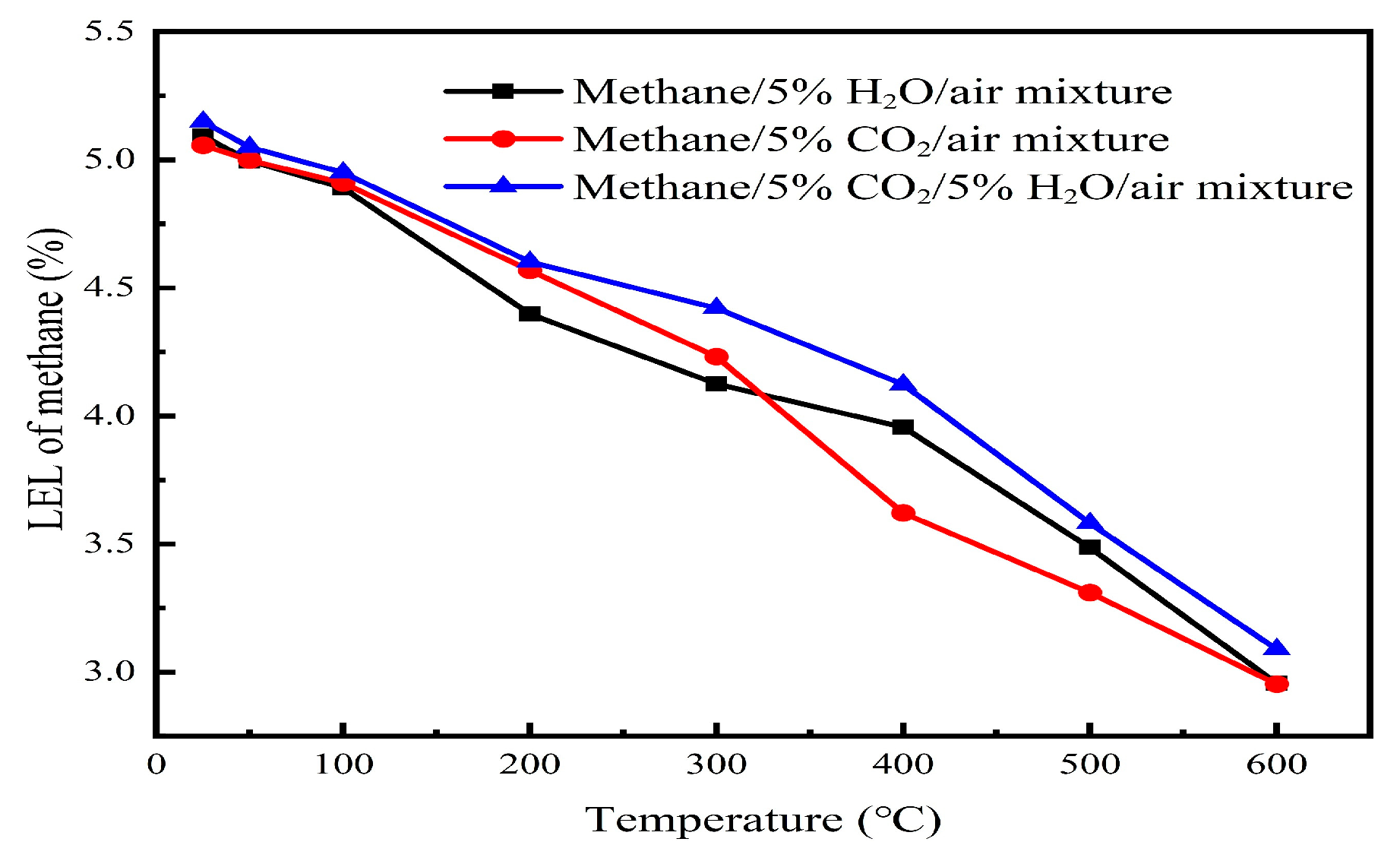
3.2.6. The Effect of Multi-Component Mixtures on the Lower Explosion Limit (LEL) of Gas Mixtures
In industrial environments, combustible gases are typically not single components but are instead gas mixtures that may include methane, ethane, propane, CO2, and other substances. The flammability, reactivity, and interactions of these different components can significantly affect the lower explosion limit (LEL) of the gas mixture.
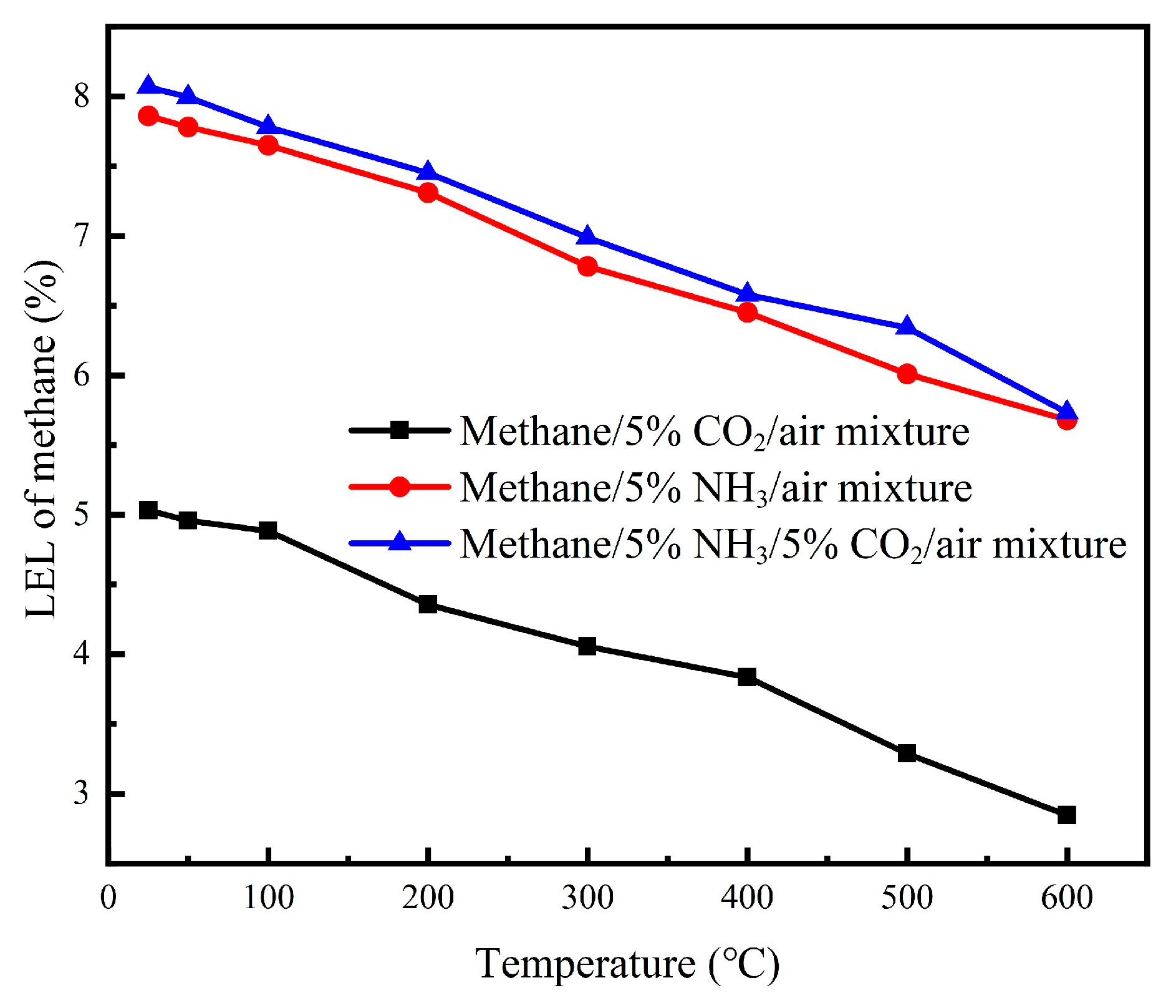
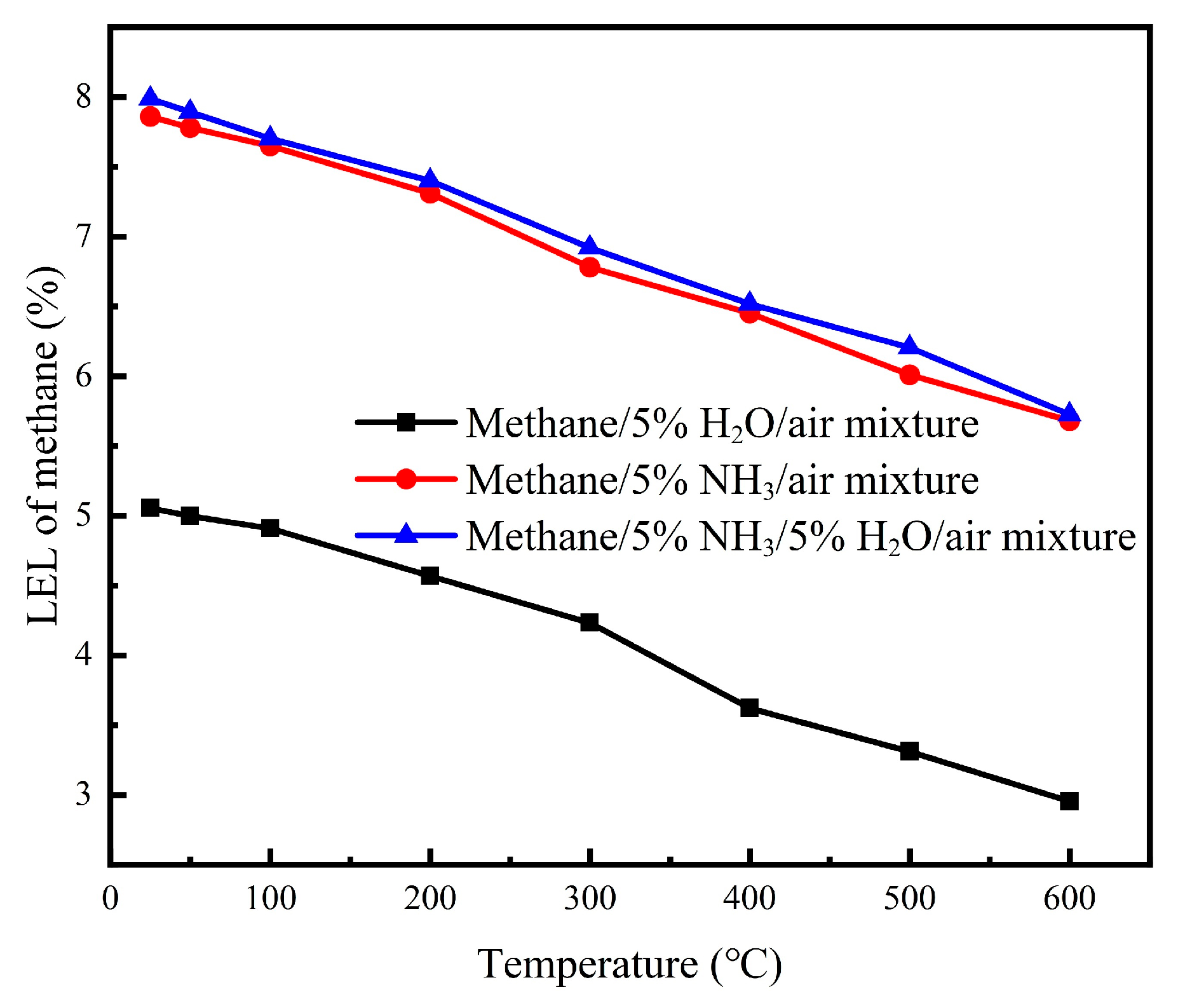
Our comprehensive investigation of multi-component inhibitor systems (NH
3/CO
2, NH
3/H
2O, and CO
2/H
2O) at 5% concentrations each demonstrated that all binary combinations provided enhanced suppression of explosion compared with single additives across the 25–600 °C temperature range, as shown in
Figure 9,
Figure 10 and
Figure 11. This synergistic effect is attributed to complementary inhibition mechanisms; NH
3 primarily contributes chemical inhibition through radical scavenging, while CO
2 and H
2O provide thermal inhibition via high heat capacities. Among these combinations, NH
3-containing mixtures exhibited the most significant LEL increases (18–24% above baseline), with the NH
3/CO
2 system showing particularly promising performance for industrial safety applications where maximum prevention of explosion is required.
4. Conclusions
Temperature is a key factor affecting the explosion limits of methane. Experimental results indicate that as the temperature increases from 25 °C to 600 °C, the explosion range of methane significantly expands. This phenomenon is primarily attributed to the increased frequency of molecular collisions and the lowered activation energy barrier in high-temperature environments, which promote the oxidation process of methane.
This study found that different reaction atmospheres had significant but varied effects on methane oxidation reactions. CO2, as an inert gas, suppresses the methane explosion reaction through dilution and heat capacity effects. The addition of NH3 reduces the concentration of methane and oxygen in the mixture through the dilution effect, thereby increasing the lower explosion limit (LEL). Additionally, NH3 itself has a certain degree of combustibility and decomposes under high-temperature conditions to generate active radicals, which may participate in the oxidation process of methane and affect the propagation of the reaction chain. The increase in O2 concentration significantly broadens the explosion limit range, while the presence of H2O not only has a dilution effect but may also influence the oxidation mechanism of methane by participating in the reaction process. The influence of gas component concentrations on the LEL of methane–air mixtures exhibits a nonlinear trend. Experimental data show that as the concentration of inert gases increases, the LEL gradually rises, but the growth rate exhibits a decreasing trend. This finding provides important insights for optimizing safety parameters in industrial production.
The results of this study have significant implications for industrial safety and can be directly applied to risk assessment and safety management in industries such as chemical engineering and coal mining. At the same time, the temperature and component impact patterns derived from this research provide a theoretical foundation for further development and improvement of gas explosion early warning systems. It is recommended that, in practical applications, the combined effects of temperature and environmental gas components be fully considered to formulate more precise safety management strategies. Future research could further explore the explosion characteristics under different pressure conditions and the synergistic effects of multi-component gases, thereby establishing a more comprehensive theoretical framework for methane explosions.
Author Contributions
Conceptualization, J.Z.; Methodology, Z.M.; Formal analysis, B.W.; Investigation, J.Y.; validation, S.L.; Resources, H.G.; Writing—original draft, Q.B. All authors have read and agreed to the published version of the manuscript.
Funding
China Coal Major Science and Technology Project: Research and Demonstration of Efficient Development and Cascade Utilization Technology for Mine Gas Resources (20221BY001); Development of specialized regenerative thermal oxidation (RTO) technology and equipment for the treatment of ultra-low concentration methane in coal mines (2023-JZSZ-ZB-007-FB-001).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Jida Zhang, Junhui Yang, Zhongcheng Ma were employed by the company China Coal (Tianjin) Underground Engineering Intelligent Research Institute Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The China Coal (Tianjin) Underground Engineering Intelligent Research Institute Co., Ltd. had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Junbo, L.; Xiangyang, X.; Zhijia, H.; Ying, L. The impact and causes of changes in China’s coal industry pattern on methane emissions from coal mines. Ecol. Econ. 2021, 37, 176–182. [Google Scholar]
- Azatyan, V.V.; Prokopenko, V.M.; Abramov, S.K. Inhibition of Combustion and Explosion of Methane–Air Mixtures in the Presence of Coal Dust. Russ. J. Phys. Chem. A 2024, 98, 514–552. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Ding, J. Chemical Effect of NO on CH4 Oxidation during Combustion in O2/NO Environments. Chem. Phys. Lett. 2019, 727, 59–65. [Google Scholar] [CrossRef]
- Le, P.A.; Trung, V.D.; Nguyen, P.L.; Phung, T.V.B.; Natsuki, J.; Natsuki, T. The current status of hydrogen energy: An overview. RSC Adv. 2023, 13, 28262–28287. [Google Scholar] [CrossRef] [PubMed]
- Pikai, Z. Study on the influence of environmental factors on the explosion limit concentration of methane. Coal Technol. 2019, 38, 108–111. [Google Scholar]
- Shaoran, R.; Lijuan, H.; Liang, Z.; Yu, W.; Shufeng, P.; Yong, W.; Yu, X.; Jia, C. Explosion limit test of high-pressure high-temperature methane-air mixture. J. China Univ. Pet. (Ed. Nat. Sci.) 2019, 43, 98–103. [Google Scholar]
- Fei, L. Study on the variation law of methane explosion limit under temperature and pressure coupling. Firef. World (Electron. Ed.) 2017, 4, 98. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, X.; Huang, R.; Wang, Z.; Wang, Z. Research on the resistance characteristics of corrugated flame-retardant system for hydrogen/methane explosion. Int. J. Hydrogen Energy 2024, 88, 228–241. [Google Scholar] [CrossRef]
- He, Y.; Deng, J.; Yi, X.; Chen, W.; Xiao, Y.; Deng, Y.; Zhu, X.; Yin, L. Effect of fluorine-containing explosion suppressants on methane explosions. J. Therm. Anal. Calorim. 2024, 149, 3711–3722. [Google Scholar] [CrossRef]
- Kondo, S.; Takizawa, K.; Takahashi, A.; Tokuhashi, K. On the temperature dependence of flammability limits of gases. J. Hazard. Mater. 2011, 187, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Aziz, M. Perspective of staged hydrogen economy in Japan: A case study based on the data-driven method. Renew. Sustain. Energy Rev. 2023, 189, 113907. [Google Scholar] [CrossRef]
- Huang, C.Y.; Liu, F.; Xin, K.; Gao, Y.H.; Duan, Y.P. Research on shock wave driving technology of methane explosion. Sci. Rep. 2024, 14, 14897. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Gao, W.; Huang, Z.; Bi, M.; Jiang, H.; Si, R.; Wen, G. Suppression characteristics of methane/coal dust explosions by active explosion suppression system in the large mining tunnel. Fire Saf. J. 2024, 150, 10425. [Google Scholar] [CrossRef]
- Jida, Z.; Junhui, Y.; Changsheng, M.; Dikun, H. Thermodynamic and reaction molecular dynamics study on the influence of ammonia addition on methane explosion. Clean Coal Technol. 2024, 30, 342–347. [Google Scholar]
- Haiyang, J.; Guobin, Z. Microscopic reaction mechanism of CO and H2O in inhibiting gas explosion. Coal Convers. 2019, 42, 77–87. [Google Scholar]
- Fangming, C.; Fan, N.; Yang, X.; Zhenmin, L.; Qiaoxia, N. Experimental study on the suppression of methane-air explosion by CF3I and CO2. Explos. Shock Waves 2022, 42, 158–166. [Google Scholar]
- Liwei, C.; Le, B.; Dongjie, W.; Yuehan, Y.; Zhongqiu, L. Experimental study on the suppression of gas explosion by additive-containing dual-fluid fine water mist. China Min. 2024, 33, 202–208. [Google Scholar]
- Dikun, H.; Xin, G. A reactive molecular dynamics study of CH4 combustion in O2/CO2/H2O environments. Fuel Process. Technol. 2017, 167, 416–424. [Google Scholar]
- Zhenmin, L.; Kai, K.; Junying, R. Microscopic mechanism of NH3 on methane chain explosion. J. China Coal Soc. 2016, 41, 876–883. [Google Scholar]
- Guoliang, L.; Wei, G.; Haipeng, J.; Weichen, W.; Fengyu, Z.; Zichao, H.; Rongjun, S.; Guangcai, W. On the lower explosion limit of ventilation air methane under high temperatures. Int. J. Hydrogen Energy 2024, 77, 1457–1466. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).