Effect of Additives on Properties of Phase-Change Solidified Plugging Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Slurry Fluidity Test
2.2.2. Setting Time
2.2.3. Thickening Time
2.2.4. Compressive Strength
2.2.5. Characterizations
2.2.6. Heat of Hydration Test
2.2.7. Krstulovic–Dabic Model Construct
3. Results and Discussions
3.1. Effects of Additives on Engineering Properties of PSPM
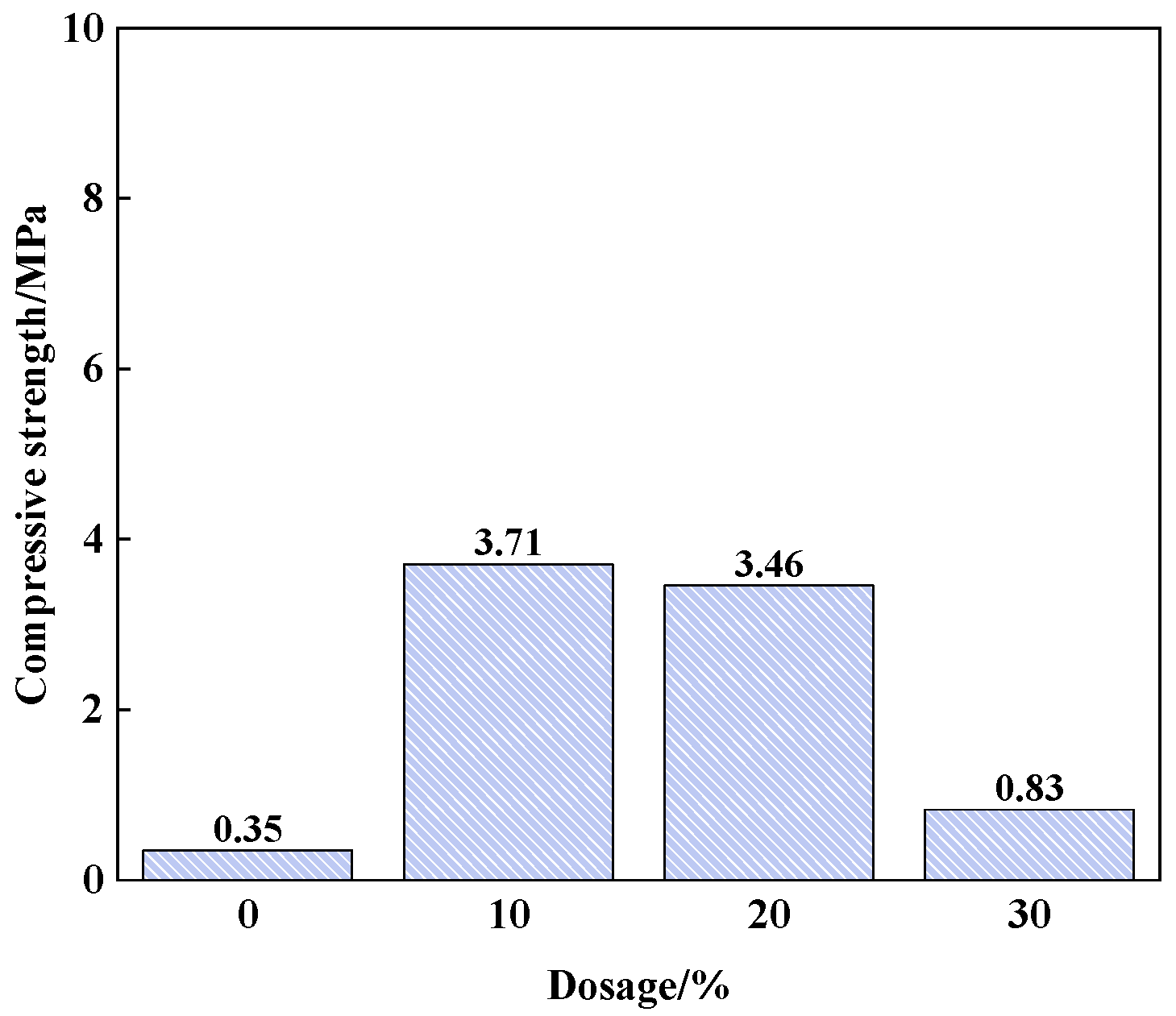
3.1.1. Effects of Structure Additives on Properties of PSPM
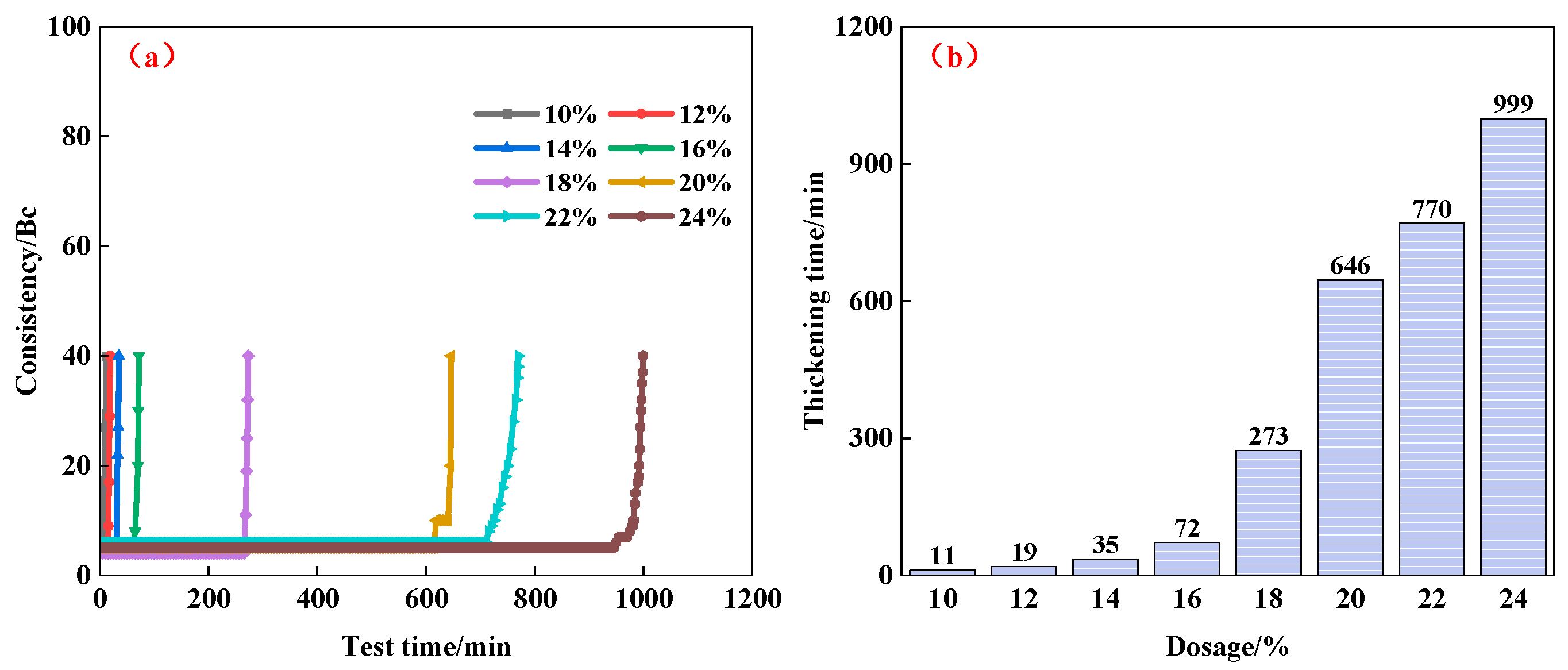
3.1.2. Effects of Flow Pattern Regulator on Properties of PSPM
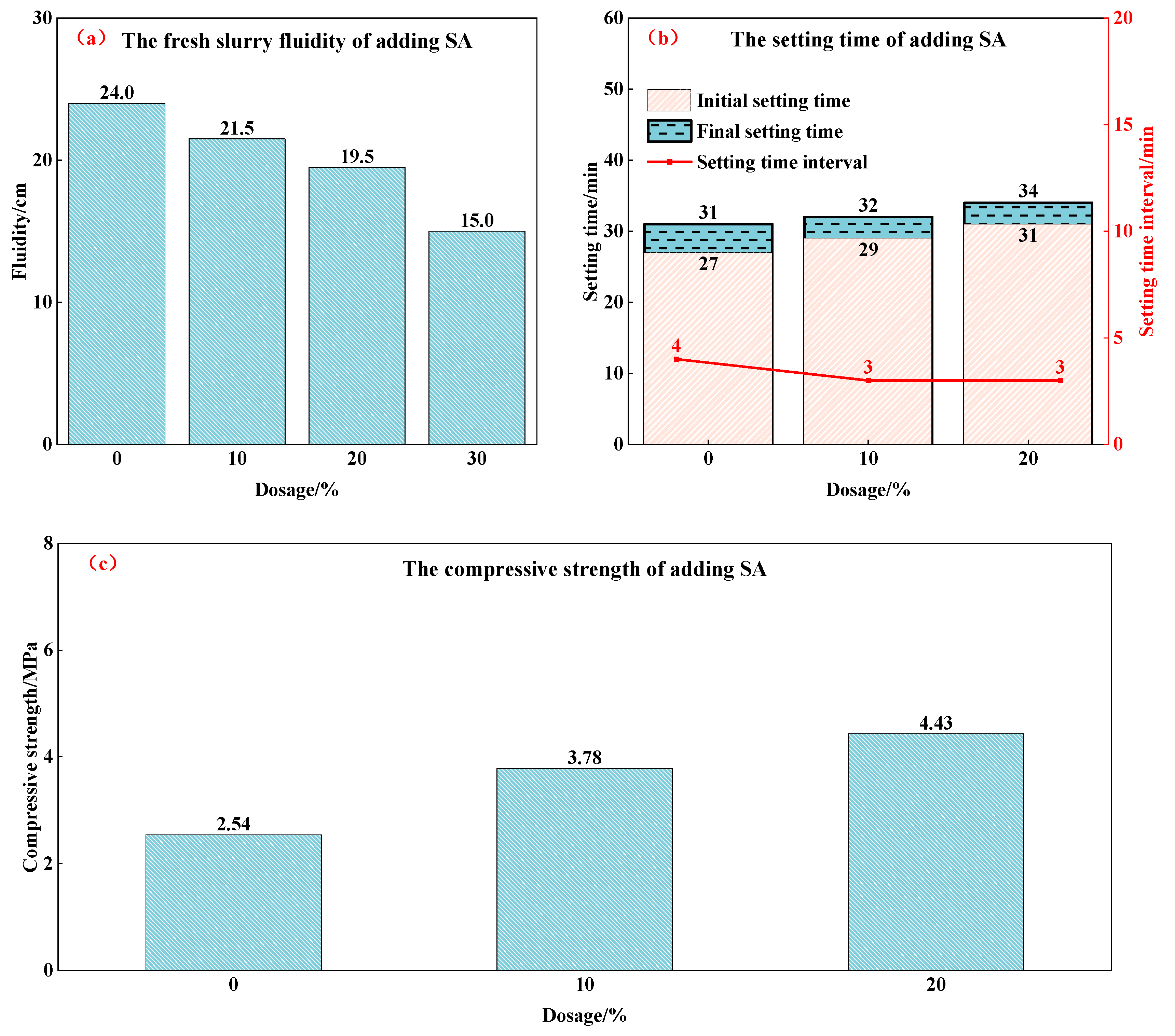
3.1.3. Effects of Phase Regulator on Properties of PSPM
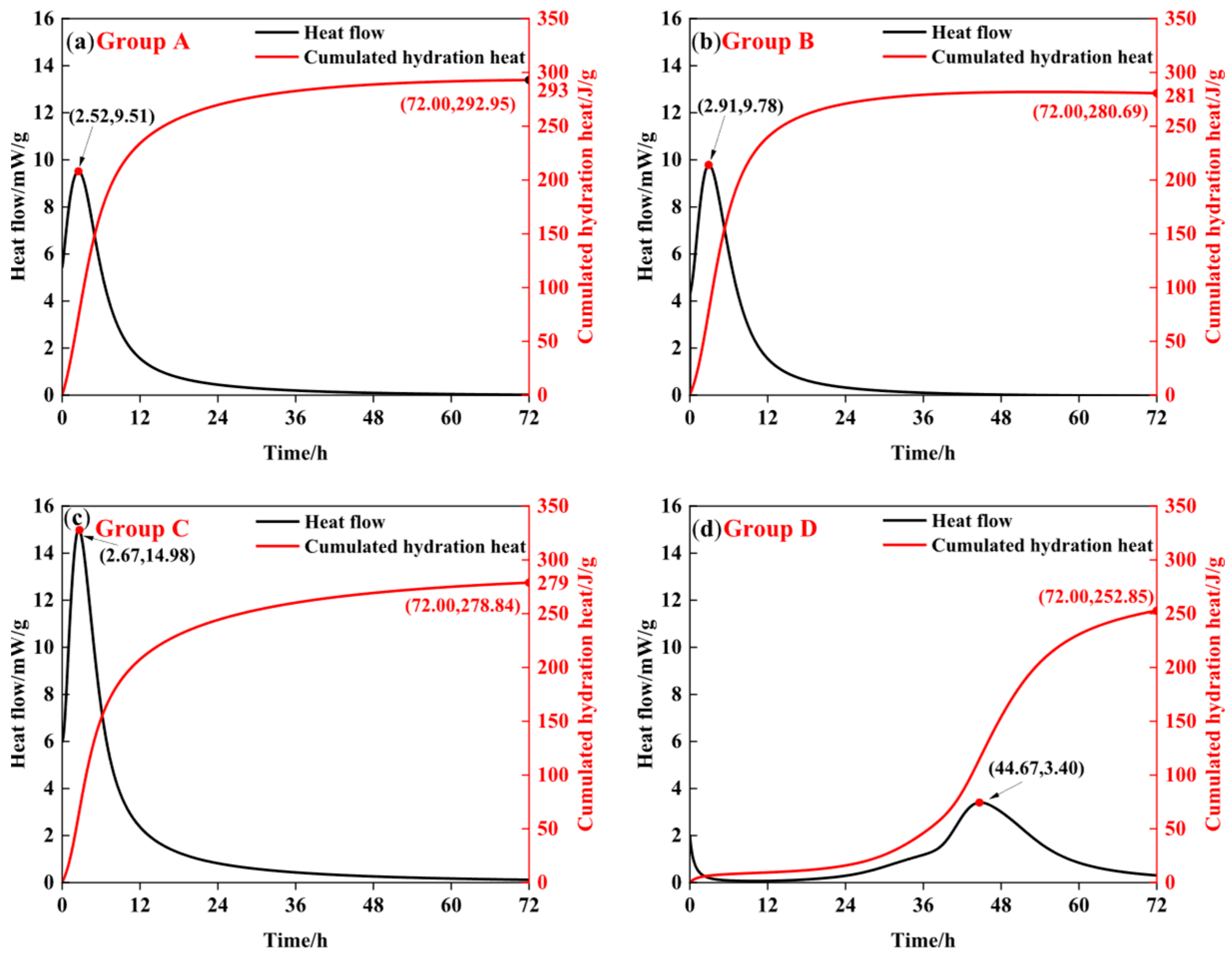
3.2. Effects of Additives on the Hydration Reaction Process of PSPM
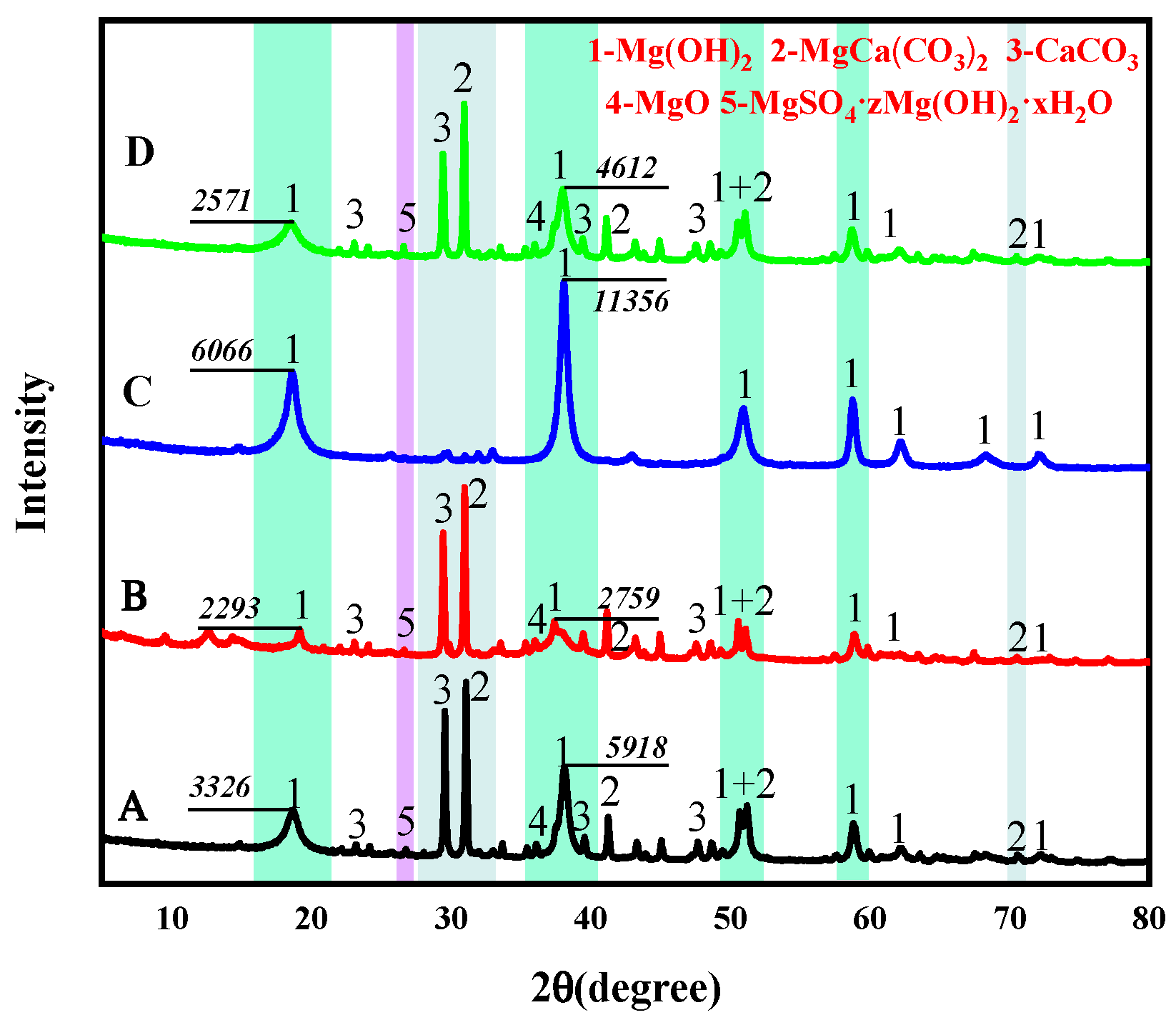
3.3. Effects of Additives on Hydration Products of PSPM
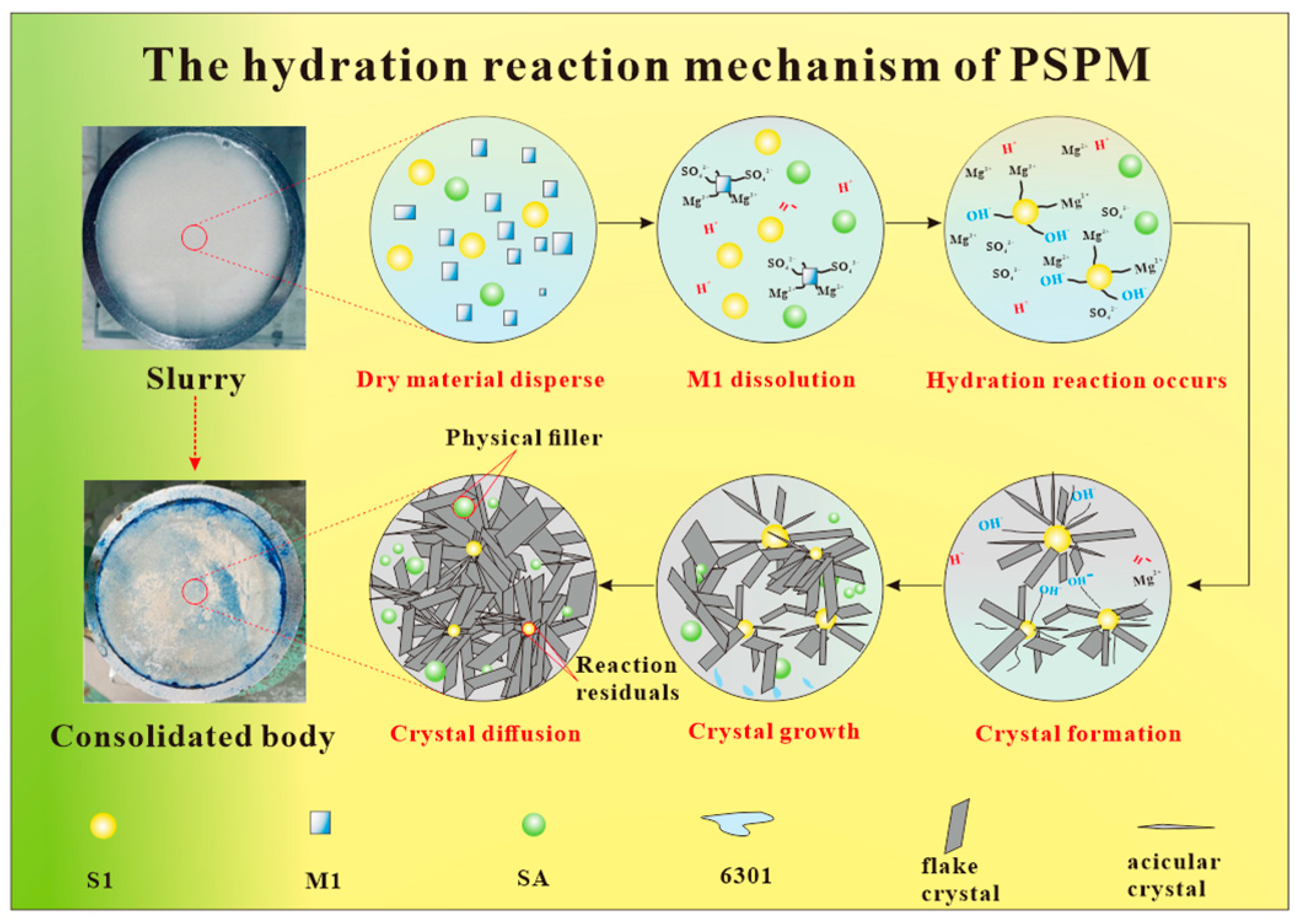
3.4. The Hydration Reaction Mechanism of PSPM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pu, L.; Xu, P.; Xu, M.B.; Song, J.J.; He, M. Lost circulation materials for deep and ultra-deep wells: A review. J. Pet. Sci. Eng. 2022, 214, 110404. [Google Scholar] [CrossRef]
- Yang, J.B.; Sun, J.S.; Bai, Y.R.; Lv, K.H.; Zhang, G.D.; Li, Y.H. Status and prospect of drilling fluid loss and lost circulation control technology in fractured formation. Gels 2022, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.S.; Bai, Y.R.; Cheng, R.C.; Lv, K.H.; Liu, F.; Feng, J.; Lei, S.F.; Zhang, J.; Hao, H.J. Research progress and prospect of plugging technologies for fractured formation with severe lost circulation. Pet. Explor. Dev. 2021, 48, 732–743. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, Y.; Zhang, Y.Q.; Wang, Z.F.; Wang, Z.H.; Liu, C. Successful Applications of a Novel Compound Lost Circulation Additive with Variable Structure. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 23–26 April 2018. [Google Scholar] [CrossRef]
- Kang, Y.L.; Wang, K.C.; Xu, C.Y.; You, L.J.; Wang, L.M.; Li, N.; Li, J.X. High-temperature aging property evaluation of lost circulation materials in deep and ultra-deep well drilling. Acta Pet. Sin. 2019, 40, 215–223. [Google Scholar] [CrossRef]
- Su, X.M.; Lian, Z.H.; Xiong, H.Q.; Yuan, Y.; Fang, J.W. Laboratory Study on a New Composite Plugging Material with High Bearing Strength and High-Temperature Resistance. J. Chem. 2019, 2019, 1874617. [Google Scholar] [CrossRef]
- Zhu, Q. Applications of a Novel Lost Circulation Additive. In Proceedings of the International Petroleum Technology Conference, Virtual, 23 March–2 April 2021. [Google Scholar] [CrossRef]
- Liu, P.D.; Wei, F.L.; Zhang, S.; Zhu, X.Y.; Wang, L.F.; Xiong, C.M. A bull-heading water control technique of thermo-sensitive temporary plugging agent. Pet. Explor. Dev. 2018, 45, 536–543. [Google Scholar] [CrossRef]
- Li, C.P.; Yang, S.C.; Zheng, W.; Pan, Y.; Zeng, F.Z.; Zhao, C.L. The research of new type gel plugging agent for deep well. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 46, 7485–7499. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, J.T.; Kong, X.J.; Qian, F.; Li, W.J.; Zhong, Z.; Jing, N.; Feng, J.; Zheng, Q.; Gu, Y.X. Development of responsive well plugging material in carbonate reservoir. J. Phys. Conf. Ser. 2021, 1777, 012016. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Liu, Y.J.; Qi, Z.L.; Tian, J.; Luo, T.T.; Hu, S.B.; Li, J. Laboratory Evaluation of the Plugging Performance of an Inorganic Profile Control Agent for Thermal Oil Recovery. Energies 2022, 15, 5452. [Google Scholar] [CrossRef]
- Cui, K.X.; Jiang, G.C.; Yang, L.L.; Deng, Z.Q.; Zhou, L. Preparation and properties of magnesium oxysulfate cement and its application as lost circulation materials. Pet. Sci. 2021, 18, 1492–1506. [Google Scholar] [CrossRef]
- Tian, L.; Bu, Y.; Liu, H.; Guo, S. Study on the penetration of strengthening material for deep-water weakly consolidated shallow formation. J. Pet. Sci. Eng. 2022, 210, 109862. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Liu, S.H.; Liu, J.H.; Zhang, F.Y.; Cheng, Z.W. Development and application of chemical consolidation plugging agent HDL-1. Pet. Drill. Tech. 2014, 42, 69–74. [Google Scholar] [CrossRef]
- Chen, Z.W.; Wang, Y.J.; Li, D.Q.; Liu, S.H.; Lin, Y.X. Application of high temperature downhole crosslinking solidification technology for mud loss control in tahe oilfield. Drill. Fluid Complet. Fluid 2015, 32, 42–46. [Google Scholar] [CrossRef]
- Fang, J.W.; Zhang, X.; Li Liang Zhang, J.J.; Shi, X.; Hu, G.Q. Research progress of high-temperature resistant functional gel materials and their application in oil and gas drilling. Gels 2022, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.B.; Bai, Y.R.; Sun, J.S.; Lv, K.H. High-Temperature Salt-Resistant High-Strength-Controlled Consolidated Resin Slurry for Fracture Plugging during Oil Gas Well Drilling. SPE J. 2024, 29, 4619–4632. [Google Scholar] [CrossRef]
- Ashok, S.; Patel, H.; Thaemlitz, C. Expandable, acid soluble and settable LCM for severe losses. In Proceedings of the International Petroleum Technology Conference, Dhahran, Kingdom of Saudi Arabia, 13–15 January 2020. [Google Scholar] [CrossRef]
- Jiang, G.C.; Cui, K.X.; Dong, T.F.; Yang, L.L.; Quan, X.H. Magnesium oxysulfate cement as a fast curing agent in drilling fluids to solve the severe loss. Pet. Sci. Technol. 2021, 39, 216–234. [Google Scholar] [CrossRef]
- Yang, J.B.; Bai, Y.R.; Sun, J.S.; Lv, K.H. Curing kinetics and plugging mechanism of high strength curable resin plugging material. Pet. Sci. 2024, 21, 3446–3463. [Google Scholar] [CrossRef]
- Pang, X.Y.; Sun, L.J.; Sun, F.; Zhang, G.; Guo, S.L.; Bu, Y.H. Cement hydration kinetics study in the temperature range from 15 °C to 95 °C. Cem. Concr. Res. 2021, 148, 106552. [Google Scholar] [CrossRef]
- Guo, X.Y.; Deng, Z.Z.; Li, Z.Y.; Zhang, X.G.; Zhang, L.H.; Zhao, Q.Y. The effect of different activators on the performance of an inorganic solidifiable gel plugging fluid. J. Nat. Gas Sci. Eng. 2016, 34, 253–264. [Google Scholar] [CrossRef]
- Sun, Y.X.; Zhang, W.J.; He, Y.; Kong, S.Q.; Liu, Y.L.; Han, R.F. A novel application of new high-strength plugging agent in Baikouquan oilfield. Appl. Sci. 2022, 12, 8150. [Google Scholar] [CrossRef]
- Han, F.; He, X.; Zhang, Z.; Liu, J. Hydration heat of slag or fly ash in the composite binder at different temperatures. Thermochim. Acta 2017, 655, 202–210. [Google Scholar] [CrossRef]
- He, J.H.; Long, G.C.; Chang, Z.Y.; Yang, Z.H.; Xie, Y.J. Effect of SAP on the Hydration Kinetics of Cement Paste at Different Curing Temperature. Mater. Rev. 2022, 36, 223–229. [Google Scholar] [CrossRef]
- Dai, L.H.; Xie, G.C.; Gong, J.W. Comparative study on the effect of fly ash on early hydration of low-heat cement and ordinary Portland cement. Concrete 2023, 2, 75–79+84. [Google Scholar] [CrossRef]
- Zhang, H.; She, J.P.; Yang, Y.; Ni, J.J.; Han, K. Plugging and Formation Damage Control Performance of a Lost Circulation Material. Oilfield Chem. 2020, 37, 581–586. [Google Scholar] [CrossRef]
- Knudsen, T. On particle size distribution in cement hydration. In Proceedings of the 7th International Congress on the Chemistry of Cement, Paris, France, 5–11 July 1980; pp. 170–175. [Google Scholar]
- Krstulović, R.; Dabić, P. A conceptual model of the cement hydration process. Cem. Concr. Res. 2000, 30, 693–698. [Google Scholar] [CrossRef]
- Dabić, P.; Krstulović, R.; Rušić, D. A new approach in mathematical modelling of cement hydration development. Cem. Concr. Res. 2000, 30, 1017–1021. [Google Scholar] [CrossRef]
- GB/T 8077; Technical Specification for Methods for Testing Uniformity of Concrete Admixture. China Standard Press: Beijing, China, 2000.
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. China Standard Press: Beijing, China, 2011.
- Qingdao Haitongda Special Instruments Co., Ltd. Available online: https://www.haitongda.com/htd1250.html (accessed on 1 November 2024).
- GB/T 19139-2012; Test Method for Oil Well Cement. China Standard Press: Beijing, China, 2012.
- GB/T 12959-2008; Test Methods for Heat of Hydration of Cement. China Standard Press: Beijing, China, 2008.
- Teng, G.G. Study on the Mechanism of Retarding Agent Inhibiting the Hydration Reaction of Phase Change Solidified Plugging Materials. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2024. [Google Scholar]





| Group | S1 + M1 | SA | 6301 | BA |
|---|---|---|---|---|
| A | √ | √ | √ | --- |
| B | √ | √ | --- | --- |
| C | √ | --- | √ | --- |
| D | √ | √ | √ | √ |
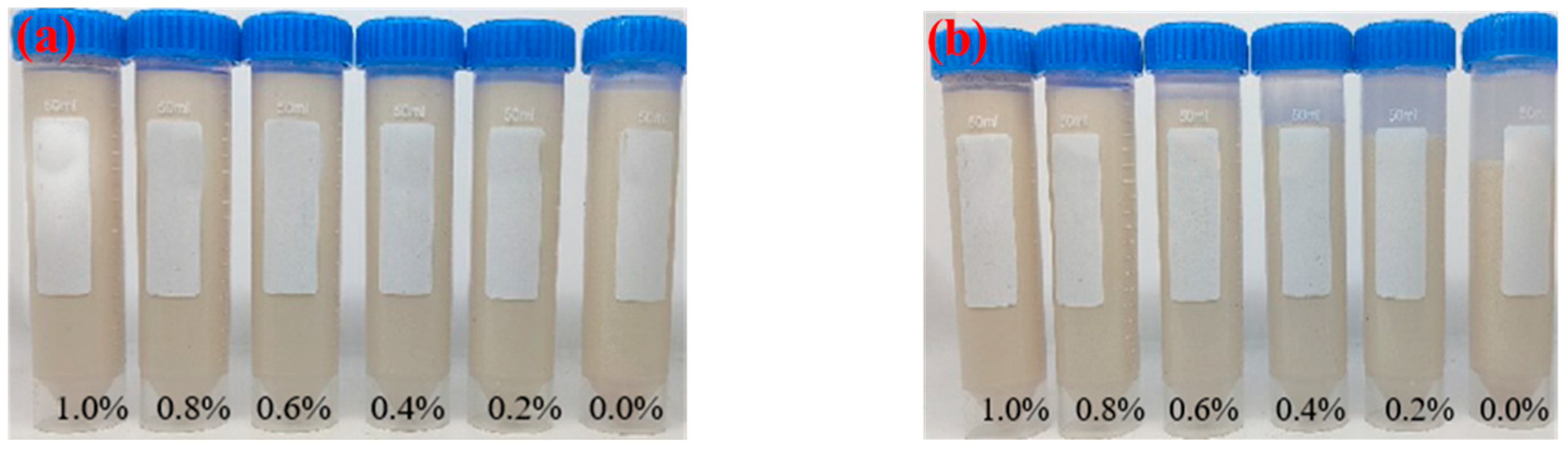
| 6301 | 6301 Mass Concentration/% | |||||
|---|---|---|---|---|---|---|
| 1.0 | 0.8 | 0.6 | 0.4 | 0.2 | 0 | |
| Supernatant volume/mL | <1 | <1 | 1 | 4 | 9 | 12 |
| Group | Qmax (J/g) | t50 (h) | Knudsen Equations |
|---|---|---|---|
| A | 292.95 | 7.5 | 1/Q = 0.003414 + 0.025602/(t − t0) |
| B | 280.69 | 6.2 | 1/Q = 003563 + 0.022088/(t − t0) |
| C | 275.74 | 6.8 | 1/Q = 0.003627 + 0.024661/(t − t0) |
| D | 266.17 | 46.2 | 1/Q = 0.003757 + 0.173573/(t − t0) |
| Group | Qmax (J/g) | t0 (h) | t50 (h) | n | K1′ | K2′ | K3′ |
|---|---|---|---|---|---|---|---|
| A | 292.95 | 0.241 | 7.5 | 1.4516 | 0.0347 | 0.0063 | 0.0015 |
| B | 280.69 | 0.254 | 6.2 | 1.3688 | 0.0335 | 0.0063 | 0.0016 |
| C | 275.74 | 0.512 | 6.8 | 1.3997 | 0.0344 | 0.0064 | 0.0016 |
| D | 266.17 | 0.314 | 46.2 | 1.3813 | 0.0185 | 0.0029 | 0.0079 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Feng, Y.; Teng, G.; Ni, J.; She, J. Effect of Additives on Properties of Phase-Change Solidified Plugging Materials. Processes 2025, 13, 2160. https://doi.org/10.3390/pr13072160
Zhang H, Feng Y, Teng G, Ni J, She J. Effect of Additives on Properties of Phase-Change Solidified Plugging Materials. Processes. 2025; 13(7):2160. https://doi.org/10.3390/pr13072160
Chicago/Turabian StyleZhang, Hui, Yongchao Feng, Gege Teng, Jianjun Ni, and Jiping She. 2025. "Effect of Additives on Properties of Phase-Change Solidified Plugging Materials" Processes 13, no. 7: 2160. https://doi.org/10.3390/pr13072160
APA StyleZhang, H., Feng, Y., Teng, G., Ni, J., & She, J. (2025). Effect of Additives on Properties of Phase-Change Solidified Plugging Materials. Processes, 13(7), 2160. https://doi.org/10.3390/pr13072160





