Abstract
A quantitative characterization of the change in coal molecular structures with different cyclical microwave modification parameters and a better understanding of the reaction mechanism of the modification are of great significance for the commercial extraction of coal bed methane (CBM). Therefore, long-flame coal samples obtained from the Ordos Basin, China, were modified by microwave radiation with different times, and the long-flame coal molecular structure parameters were determined by solid-state 13C nuclear magnetic resonance (ss13C NMR), Fourier transform infrared (FTIR) spectrometry, and X-ray photoelectron spectrometry (XPS). Atomistic representations of the raw long-flame coal molecular model and modified long-flame coal molecular models were established. The temperature rise, pore volume increase, mineral removal, and functional group changes after the modification have a negative effect on methane adsorption. After the modification, the decrease in surface area of the micropores reduced the adsorption site of methane in coal. As a result, the methane adsorption amount decreased linearly with the decreasing surface area. The CH4 adsorption isotherms of the long-flame models were dynamically simulated and analyzed. The results of this study can prove that after multiple cycles of microwave modifications, the functional groups in long-flame coal were fractured, and the number of micropores was reduced, which effectively decreased the methane adsorption performance in long-flame coal seams, thereby promoting methane extraction. Microwave modification is a promising method for enhancing CBM recovery.
1. Introduction
The CH4 adsorption in coal seams has long been of interest to the coal industry, from the prevention and control of coal and gas bursts to the exploitation of coal bed methane (CBM) as a high-quality clean energy source [1]. The practice of CBM exploration and development has proved that the coal seams in the Ordos Basin contain a large amount of high-quality CBM resources. The strong methane adsorption and low permeability of coal reservoirs are still big issues that affect the CBM commercial [2]. Scholars have proposed enhanced extraction methods for CBM based on coal seam regulation, such as hydraulic methods, to create fractures in coal seams to increase the transport of methane [3,4]. Heat injections to raise the temperature of the coal seam to weaken the adsorption of methane [5]. Electrochemical modification integrates enhanced methane desorption rates and electroosmotic flow to accelerate the CBM extraction efficiency [6,7]. These coal seam regulation processes have improved the efficiency of CBM development, but there are still limitations, such as increased water lock damage, complex operation, and unfriendliness to the environment. Considering the unique transient, strong penetrating, selective, and controllable characteristics of microwave radiation, microwave radiation modifications can effectively change the molecular structure parameters and pore characteristics of coal, affecting the adsorption of methane in coal [8].
How does microwave modification influence the pore structure and surface characteristics of coal, and how does methane adsorption capacity change after modification? This is necessary to achieve microwave-enhanced CBM extraction, and it is not accurately measured at the nanoscale by conventional methane adsorption and desorption experiments. Besides experimental and theoretical derivation, molecular dynamics simulation is a “third tool” for understanding the microscopic world, which can analyze the physicochemical properties, process performance, and reservoir physical characteristics of coal at the molecular level, thus guiding the safe and efficient exploitation of methane in coal [9]. Therefore, the main work of this paper is as follows: to investigate the changes in temperature, water content, microporosity, and the number of associated minerals of coal after cyclic microwave modification and their effects on methane adsorption; to construct atomic representations of coal, associated minerals, water, and methane; and to construct molecular structure models of coal, coal-associated minerals, and coal water. The molecular dynamics simulation of methane adsorption is carried out in the constructed model to obtain the response of the variation of coal’s structural parameters to the amount of methane in coal and to quantify the methane adsorption in coal through the variation in microscopic parameters, such as interaction energy and radial distribution function.
The pore wall properties and pore volume of the micropores in coal are important parameters affecting the adsorption/desorption characteristics of methane. Guo et al. [10] selected different modification parameters to modify the surface of anthracite coal and found that the seepage rate of methane in modified anthracite coal increased linearly with the increase in the modified potential gradient, while it changed in an inverted “U” shape with the increase in the modified electrolyte concentration; the modification could effectively improve the desorption capacity of methane. The desorption rate of anthracite methane increased by 20.44% compared to that of unmodified anthracite; the degree of influence of the surface modification parameters was as follows: pH of electrolyte > applied potential gradient > concentration of electrolyte. Kang [11] investigated the effect of different modification parameters on the adsorption capacity of methane in anthracite coal, and the experimental results found that electrochemical modification could effectively reduce the adsorption capacity of anthracite coal for methane, and the final desorption rate was increased. Zhang et al. [12] showed that acidification modification increases the amount of major acidic groups on the surface of activated carbon, which significantly inhibits the methane adsorption capacity while changing its specific surface area and pore volume. Hao et al. [13] found that the saturation adsorption of methane by coal was significantly negatively correlated with the oxygen-to-carbon ratio in coal when the surface of bituminous coal was modified by acidification; coal samples with high oxygen content and poor hydrophobicity had a lower adsorption capacity for methane when the number of micropores was similar. Cheng et al. [14] experimentally found that the adsorption capacity of methane in coal was mainly influenced by the pore volume of the micropores and the specific surface of the pores, without considering the influence of the pore surface branched chains.
Unlike traditional experimental and theoretical models of methane adsorption, the 3D molecular structure models of coal can demonstrate the nature of functional groups on the pore surface of coal [15,16] and also characterize the nanopore structure of coal composed of molecular interstices, such as pore volume and pore surface area. As a result, scholars have constructed diverse planar and 3D models of the molecular structure of coal and carried out molecular dynamics simulations under different adsorption conditions [15,17]. Molecular dynamics simulations can provide information on the heat of adsorption during methane adsorption, the interaction energy and radial distribution functions, and the expansion of coal volume after methane adsorption, making them an effective tool for analyzing methane adsorption in coal and minerals [18,19,20].
In this work, atomistic representations of long-flame coal, including different contents of water and minerals in the Ordos Basin, were constructed. Molecular dynamics simulations were performed to characterize methane adsorption considering temperature, pressure, moisture, pore volume, mineral content, and modification. The response characteristics of the different surface areas of the micropores to the adsorption characteristics of methane in coal from the molecular level were explained, and a micro mechanism for methane adsorption was provided.
2. Experiments and Simulation Details
2.1. Coal Samples
The long-flame coal samples used in the experiments were obtained from the Ordos Basin. The coal seams at the sampling location have retained their primary sedimentary structure; the primary bedding is complete and distinct, and only a few secondary fractures are developed. As indicated by the results of our prior tests, industrial and microcomponent analyses of coal samples are conducted; the results are shown in Table 1 [21]. Chemical composition testing of the minerals on the coal surface revealed that the mineral matters were dominated by calcite, kaolinite, and pyrite [22].

Table 1.
Industrial and microcomponent analysis results of natural coal samples [21].
2.2. Experimental Process
2.2.1. Cyclical Microwave Modification Process
The current microwave modification experiments were primarily conducted on selected long-flame coal samples through multiple repeated operations. As the detailed procedures have been comprehensively described in our previous study [8], they are not reiterated here.
2.2.2. Molecular Parameter Tests
A Nicolet iS5 FTIR spectrometer was used to test the surface groups of the coal samples. The attribution of chemical shifts of carbon elements in 13C solid-state nuclear magnetic resonance (13C NMR) spectra is an important basis for defining the distribution state of carbon elements in coal. As shown in Figure 1, the detailed data on molecular parameters and structures are shown in our previous study [17,21].
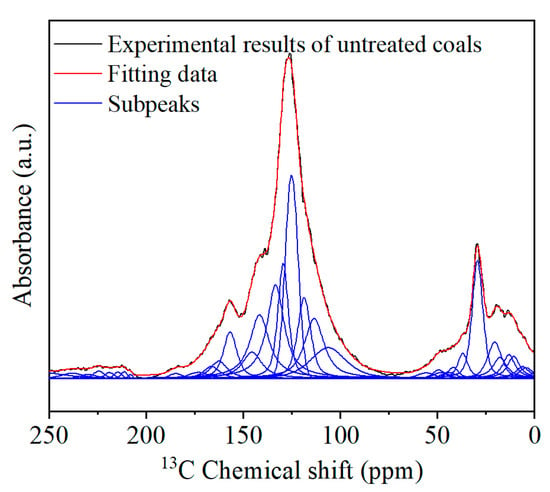
Figure 1.
13C NMR peak fit spectra of coal samples [21].
2.3. Molecular Model Construction and Simulation Details
The coal molecular model was constructed in the Amorphous Cell Calculation module of the Materials Studio 2019 software using a force field in the form of a Dreiding force field. The chemical formula for the molecular structure is C235H185O35N3S. The molecular model of coal was constructed utilizing the Amorphous Cell Calculation module from the Materials Studio, applying a Dreiding force field. The molecular formula that characterizes the structure of long-flame coal is represented as C235H185O35N3S. In order to simulate methane adsorption on coal, we executed both grand canonical Monte Carlo (GCMC) simulations and molecular dynamics (MD) simulations under varying dynamic and static conditions. These simulations were facilitated through the Sorption and Forcite modules in the Materials Studio software, incorporating the Compass force field with a truncation radius set at 1.25 nm. Within the GCMC simulation framework, the system comprised coal molecules, methane molecules, and water molecules. The simulations were capped at a maximum of 2 × 107 steps, with the initial 1 × 107 steps allocated for achieving equilibrium, ensuring the stabilization of the adsorption system, while the following 1×107 steps were dedicated to calculating thermodynamic parameters, including adsorption heat. Throughout the simulation process, potential errors may arise from both systematic and random sources. To minimize these inaccuracies, each simulation was performed more than five times. The isosteric heat associated with CH4 adsorption within the three coal models was computed using the Clausius–Clapeyron equation [23].
where Qst is the isosteric heat, K is the Henry coefficient, N is the amount of gas adsorbed, T is the temperature, C is the constant, and R is 8.314 J/(mol·K).
3. Results and Discussion
3.1. Molecular Model Changes After Modification
According to the 13C NMR tests described in Reference [22], Table 2 shows the relationship between the chemical shifts obtained from the 13C NMR tests in coal and the corresponding functional groups in the coal. The displays of the peak fitting spectra of the 13C NMR, before and after the microwave treatment, with fitting degrees of 0.998 and 0.998, indicate a good fit. Combining Table 2, it can be observed that the carbon skeleton of the organic molecular structure in the coal sample consists of three peak groups: 0–90 ppm, 90–165 ppm, and 165–240 ppm [21]. The area of aromatic carbon in the coal sample is the largest, followed by aliphatic carbon, with carbonyl carbon being the smallest. This indicates that aromatic carbon accounts for the highest proportion of the organic structure in coal, far exceeding that of aliphatic carbon and carbonyl carbon. After the microwave treatment, the proportion of aromatic carbon in the coal sample increases, while the proportion of aliphatic carbon decreases. The FTIR test results showed the breakage of self-conjugated hydrogen bonds, the breaking off of alkane side chains, such as methyl and methylene, and their release as gases, and the decomposition of some silicate minerals by the action of microwave cycles. The XPS test showed the limited change in the presence of N and S elements after the modification [24], and the coal molecular structure model based on chemical structure testing before and after the modification is shown in Figure 2 [25].

Table 2.
13C NMR chemical shift assignments in coal organic structures [22].
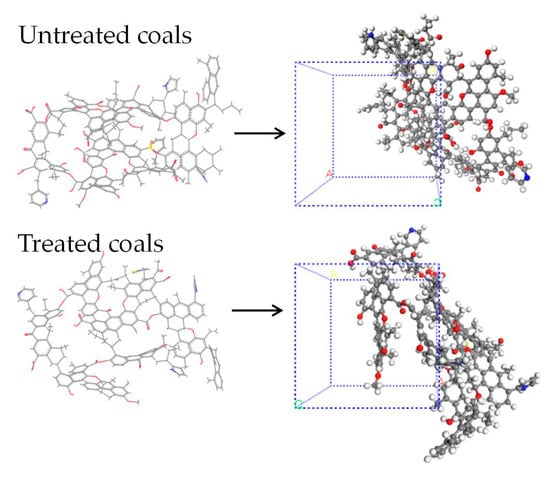
Figure 2.
Coal molecule structure models before and after treatment, Color code: C (gray), H (white), O (red), N (blue), S (yellow) [25].
3.2. CH4 Adsorption Isotherm Changes After Modification
3.2.1. Temperature Rise After Modification on Methane Adsorption
The most intuitive change in the coal samples under the effect of cyclic microwave modification is the increase in temperature. A Smart Sensor AS825B infrared thermometer from Sigma was used to measure the surface temperature of the modified coal, and it was found that the surface temperature of the coal samples generally increased, reaching 373.15 K at the highest point. To investigate the change in the law of methane adsorption in coal due to the microwave, methane adsorption isotherms were simulated by selecting 273.15–313.15 K. The simulation results of methane adsorption at different temperature conditions are shown in Figure 3. Figure 3a shows that the adsorption of methane in the coal model under the temperature range of 273.15 to 313.15 K conforms to the Langmuir adsorption model. Figure 3b shows the Langmuir fitting parameters, and Figure 3c shows the thermodynamic information of methane adsorption. It can be seen that the warming effect of the coal seam caused by the microwave modification reduces the adsorption performance of methane in coal, and the higher the temperature, the lower the adsorption. This means that more methane gas is present in the coal seam in the Free State, making the modified methane extraction more efficient.
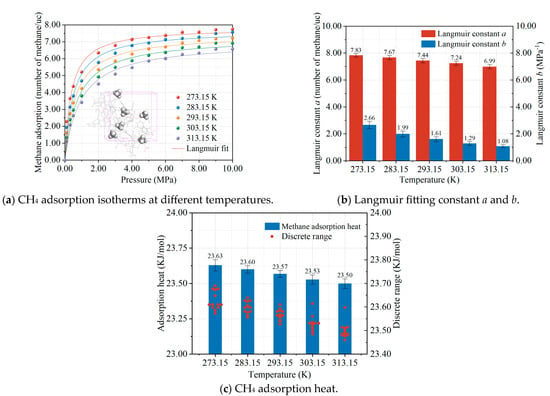
Figure 3.
Effect of temperature on methane adsorption isotherms (a); Langmuir constant (b); and adsorption heat (c). Each colored curve in the figure corresponds to its respective Langmuir fitting.
3.2.2. Effect of Pore Volume on Methane Adsorption
Coal samples in the cyclical microwave modification display “pore expansion”, which indicates that the number of micropores in the coal is reduced, while the pore volume and pore surface area of medium and large pores increased [8]. The effect of this “pore expansion” becomes more pronounced as the number of microwave modifications increases. Therefore, a model of the coal structure with different pore volumes was constructed in the Materials Studio software to simulate the changes in microporosity of the coal after different cycle times of the modification. A probe of the size of the diameter of the methane molecule (0.38 nm) was chosen to probe the structural model. The results of the pore volume and surface area changes of the micropores in the structural model of coal after different numbers of modifications are shown in Table 3.

Table 3.
Pore volume change after different modification times.
The results of methane adsorption under different micropore volume conditions are shown in Figure 4. The methane adsorption at different micropore volumes is shown in Figure 4a. After fitting the Langmuir formula, it was found that the methane adsorption under different micropore volume conditions was in accordance with the Langmuir adsorption model, and the results of fitting the Langmuir parameters are shown in Figure 4b. It can be seen that the amount of methane adsorbed decreases as the pore volume of the micropores in the coal model decreases, which is consistent with the theoretical calculation by Cheng et al. that concluded that methane in coal is mainly filled in the micropores of the coal [14]. Figure 4c shows the relationship between different surface areas and the amount of methane adsorbed in coal, which shows that the amount of methane adsorbed in coal increases linearly with the increasing surface area of the micropores. The simulation results confirm the conclusion that the microwave modification leads to a reduction in micropores and a decrease in methane adsorption in the coal.
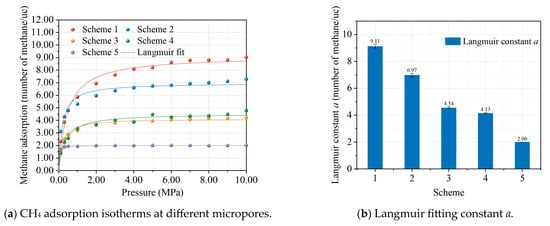
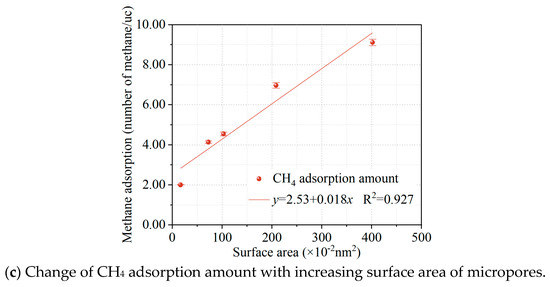
Figure 4.
Effect of micropores on methane adsorption isotherms (a); Langmuir constant (b); and methane adsorption amount vs. surface area (c). Each colored curve in the figure corresponds to its respective Langmuir fitting.
3.2.3. Effect of Moisture and Coassociated Minerals on Methane Adsorption
After the microwave modification, the water in the coal evaporates, and the moisture in the coal is greatly reduced. The presence of water in coal occupies the adsorption sites on the surface, leading to a decrease in methane adsorption, and the removal of water by microwaves leads to an increase in methane adsorption; the detailed results of these contents can be found in our previous paper results [22]. Also, pyrite may undergo chemical reactions under microwave catalytic conditions with surrounding small molecules, such as H2, O2, and adsorbed H2O, CO, and CO2, and release gases, such as H2S, SO2, and carbonyl sulfide (COS) [26]. Additionally, some minerals in the coal may dislocate and move into larger fractures [27]. We have added the removal of pyrite in coal to the Conclusion section of the revised manuscript. To investigate the effect of the removal of associated minerals, such as calcite and kaolinite, from the microwave-modified coal on methane adsorption, composite coal-rock compound structure models containing different minerals were constructed based on the pore volume of Scheme 3 as the raw coal.
The simulation results of the methane adsorption in the coal-rock compound models are shown in Figure 5. Figure 5a shows the methane adsorption isotherms, which were fitted by the Langmuir model, and the results are shown in Figure 5b. It can be seen that the associated minerals in the coal also adsorb a certain amount of methane, which is consistent with the experimental results of Feng et al. [28]. The variation in pore volume in the structural model of coal when minerals are present is shown in Figure 5c,d. It can be seen that the amount of adsorbed methane in the compound models increases linearly with increasing the pore area, implying that methane is also adsorbed in the micropores of the mineral.
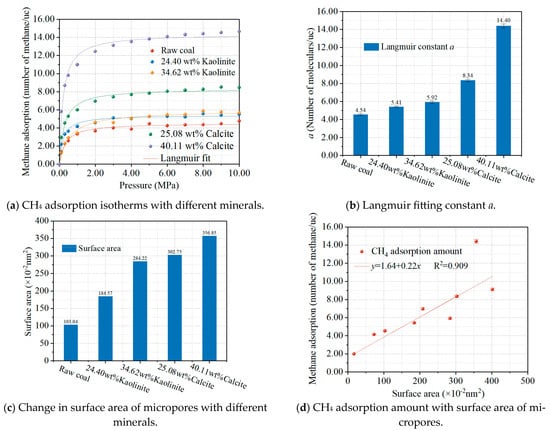
Figure 5.
Effect of minerals on methane adsorption isotherms (a); Langmuir constant (b); surface area (c); and methane adsorption amount vs. surface area (d). Each colored curve in the figure corresponds to its respective Langmuir fitting.
3.2.4. Effect of Functional Groups After Modification on Methane Adsorption
The microwave modification resulted in alterations to the functional groups on the coal surface. There was a noticeable decrease in the presence of alkane side chains and certain oxygen-containing groups, indicating a reduction in both oxygen and hydrogen content, alongside an increased ratio of aromatic bridge carbon to periplasmic carbon [24]. Following the in situ modification, the intensity of the absorption peaks associated with the hydroxyl groups and aliphatic structures saw a significant decline. These changes in functional groups suggest that the modification process disrupts the self-conjugated hydrogen bonds on the coal surface, leading to a reduction in oxygen-containing functional groups. Functional groups like methyl and methylene were cleaved and emitted as gases, which contributed to an increase in aromatic carbon content, enhanced aromaticity, and greater aromatic ring condensation, while the length and branching degree of the alkane side chains decreased.
Based on the changes in the molecular structure parameters of the coal after the modification, some oxygen-containing groups were removed to construct coal models after different cyclical times of modifications. The chemical formulae of the coal molecular structures after 0, 1, 2, and 3 time modifications are C235H185N3O35S, C232H181N3O28S, C232H179N3O24S, and C231H177N3O20S, respectively. The simulation results of methane adsorption under different molecular structure conditions after microwave modification are shown in Figure 6. The methane adsorption isotherms for different levels of functional groups are shown in Figure 6a, and the results of the Langmuir fit are presented in Figure 6b. The observed decrease in methane adsorption following the microwave treatment can be attributed to the reduction in oxygen-containing functional groups, with a more substantial decrease corresponding to further functional group loss. This finding is in agreement with the results of Zhou et al. [29], who reported that both oxygen-containing functional group depletion and alkyl side chain removal reduce the methane adsorption potential of coal, supporting our simulation-based conclusions.
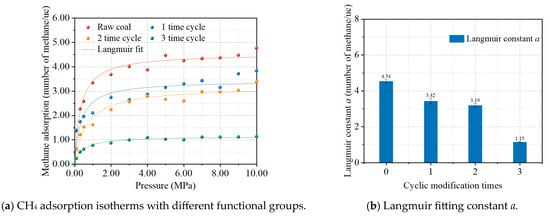
Figure 6.
Effect of functional groups after cyclic modification times on CH4 adsorption (a) and Langmuir fitting constant a (b). Each colored curve in the figure corresponds to its respective Langmuir fitting.
Most microwave modifications are conducted in the laboratory [30,31]. How to extend the experimental results of small block coal samples to large-scale coal seams is a question that must be considered. Research indicates that methane extraction from coal seams is influenced by the pore structure and surface properties of the coal. And the pore structure and surface roughness of coal can be analyzed and characterized using the fractal theory [32]. In our previous work, we attempted to use combined fractals to analyze the effects of modification technology on small block coal, providing reasonable process parameters for coal reservoir modification [33], and further work is the application of microwave modification in the field through fractal theory in the future. In addition, molecular dynamics simulations have only modeled the structural changes before and after modification. We will also explore more simulation methods [34], hoping to accurately characterize the structural evolution during the modification process.
4. Conclusions
- (1)
- The temperature rise, pore volume increase, mineral removal, and functional group changes after modification have a negative effect on methane adsorption, while the decrease in moisture will increase the methane adsorption capacity. The methane adsorption finally decreases after modification, and methane adsorption decreases further with increasing the cyclical modification times.
- (2)
- The results of molecular dynamics simulations further reveal the mechanism of methane adsorption and modification in coal. The surface properties and surface area of coal micropores affect the amount of monolayer adsorption on the coal surface; the pore volume of coal micropores fills a certain amount of methane molecules and is the main site of methane adsorption in coal seams.
- (3)
- The construction of molecular structure models and molecular dynamics simulations of long-flame coal before and after microwave modification were obtained in this work, but the dynamics changes during the modification process still need further studies. In addition, more coal rank coals, modification parameters, and dynamics simulations will be shown in our further work.
- (4)
- The tests on coal industrial and microcomponent analyses, microwave modification experiments, coal chemical structure tests, and molecular dynamics simulations of methane adsorption in this work were conducted only once, which may lead to a certain degree of uncertainty in the data. And the presented data concern small coal volumes, and the extension to greater, industrial volumes needs a significant volume of future work. We advise scholars to cite the data results from this work with caution.
Author Contributions
Conceptualization, G.Z.; methodology, G.Z. and Y.C.; text editing and correction, X.Z.; numerical simulation, L.W. and T.Z.; engineering experiments, X.Z.; normal analysis and writing—preparation of the original, T.Z. and X.Z.; writing—review and editing, T.Z., L.W., G.Y. and X.Z.; L.Z. and Y.W. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (52304088, 52204220, and 2023YFB3211005), the Shanxi Province Science and Technology Achievement Transformation Guidance Special Project (202304021301017), the Shanxi Province Central Guidance Local Science and Technology Development Fund (YDZJSX2024C036), the Natural Science Foundation of Shanxi Province (202403021221354), the Natural Science Foundation of Inner Mongolia (2023LHMS05052; 2025LHMS0523), the Basic Research Funds for Directly Affiliated Universities in Inner Mongolia (JY20240024; JY20250100), and the Research Support Projects for Talent Introduction at the Autonomous Region Level in 2022 (DC2300001429).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Guofei Zhao was employed by the company Shanxi Gowin Construction Technology of Coalbed Gas Co., Ltd. Author Guangtong Yang was employed by the company Deyang Haohua Qingping Phosphate Mine Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Xu, H.; Sang, S.; Yang, J.; Jin, J.; Hu, Y.; Liu, H.; Ren, P.; Gao, W. In-situ stress measurements by hydraulic fracturing and its implication on coalbed methane development in Western Guizhou, SW China. J. Unconv. Oil Gas Resour. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Mu, F.; Zhong, W.; Zhao, X.; Che, C.; Chen, Y.; Zhu, J.; Wang, B. Strategies for the development of CBM gas industry in China. Nat. Gas Ind. B 2015, 2, 383–389. [Google Scholar] [CrossRef]
- Hu, G.; Sun, C.; Sun, M.; Qin, W.; Linghu, J. The case for enhanced coalbed methane using hydraulic fracturing in the geostructural belt. Energy Explor. Exploit. 2018, 36, 1629–1644. [Google Scholar] [CrossRef]
- Zhi, S.; Elsworth, D.; Wang, J.; Gan, Q.; Liu, S. Hydraulic fracturing for improved nutrient delivery in microbially-enhanced coalbed-methane (MECBM) production. J. Nat. Gas Sci. Eng. 2018, 60, 294–311. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, C.; Xu, J.; Sun, Y.; Yu, X. Feasibility investigation of enhanced coalbed methane recovery by steam injection. Energy 2022, 255, 124473. [Google Scholar] [CrossRef]
- Guo, J.; Kang, T.; Kang, J.; Chai, Z.; Zhao, G. Accelerating methane desorption in lump anthracite modified by electrochemical treatment. Int. J. Coal Geol. 2014, 131, 392–399. [Google Scholar] [CrossRef]
- Guo, J.; Kang, T.; Kang, J.; Zhang, H.; Chai, Z.; Zhang, B.; Zhang, X. Research progress of electrokinetic dynamics of rock and fluid. Chin. J. Rock Mech. Eng. 2019, 38, 511–526. [Google Scholar]
- Zhang, L.; Kang, T.; Kang, J.; Zhang, X.; Zhang, B.; Chai, Z.; Zhang, R.; Wang, Y.; Kang, G.; Zhao, G. Effect of cyclical microwave modification on the apparent permeability of anthracite: A case study of methane extraction in Sihe mine, China. ACS Omega 2021, 6, 15001–15011. [Google Scholar] [CrossRef]
- Seyyedattar, M.; Zendehboudi, S.; Butt, S. Molecular dynamics simulations in reservoir analysis of offshore petroleum reserves: A systematic review of theory and applications. Earth-Sci. Rev. 2019, 192, 194–213. [Google Scholar] [CrossRef]
- Guo, J.; Kang, T.; Zhang, H. Property and its mechanism of enhanced gas desorption from anthracite under electrochemical treatment. J. China Coal Soc. 2018, 43, 210–218. [Google Scholar]
- Kang, J. Experimental Study on Modification of Anthracite and its Effect on Adsorption and Wettability; Taiyuan University of Technology: Taiyuan, China, 2018. [Google Scholar]
- Zhang, X.B.; Huan, X.; Zhang, H.; Zhang, Y. Microstructure and methane adsorption of coal-based activated carbons with different coal body structures. J. China Univ. Min. Technol. 2017, 46, 155–161. [Google Scholar]
- Hao, S.; Wen, J.; Yu, X.; Chu, W. Effect of the surface oxygen groups on methane adsorption on coals. Appl. Surf. Sci. 2013, 264, 433–442. [Google Scholar] [CrossRef]
- Cheng, Y.; Biao, H. Main occurrence form of methane in coal: Micropore filling. J. China Coal Soc. 2021, 46, 2933–2948. [Google Scholar]
- Mathews, J.P.; Van Duin, A.C.T.; Chaffee, A.L. The utility of coal molecular models. Fuel Process. Technol. 2011, 92, 718–728. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Zhong, Q.; Zhuo, J.; Mathews, J.P. A large-scale molecular model of Fenghuangshan anthracite coal. Fuel 2021, 295, 120616. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, T.; Zhang, B.; Kang, G.; Kang, J.; Zhang, R.; Zhang, L. Combined Molecular Simulations and Experimental Study of Methane Adsorption on Anthracite. Energy Fuels 2020, 34, 12118–12125. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, J.; Kang, T. Effect of water on methane adsorption on the kaolinite (0 0 1) surface based on molecular simulations. Appl. Surf. Sci. 2018, 439, 792–800. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, J.; Kang, T. Monte Carlo simulations of methane adsorption on kaolinite as a function of pore size. J. Nat. Gas Sci. Eng. 2018, 49, 410–416. [Google Scholar] [CrossRef]
- Zhang, J.; Clennell, M.B.; Dewhurst, D.N.; Liu, K. Combined Monte Carlo and molecular dynamics simulation of methane adsorption on dry and moist coal. Fuel 2014, 122, 186–197. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, Y.; Zhou, T.; Cheng, J.; Zhao, G.; Zhang, L.; Kang, J. Thermodynamic characteristics of methane adsorption on coals from China with selected metamorphism degrees: Considering the influence of temperature, moisture content, and in situ modification. Fuel 2023, 342, 127771. [Google Scholar] [CrossRef]
- Zhang, X. Research on the Influence of Electrode and Electrolyte Types on Anthracite Modification; Taiyuan University of Technology: Taiyuan, China, 2022. [Google Scholar]
- Zhu, H.; Zhang, Y.; Qu, B.; Liao, Q.; Wang, H.; Gao, R. Thermodynamic characteristics of methane adsorption about coking coal molecular with different sulfur components: Considering the influence of moisture contents. J. Nat. Gas Sci. Eng. 2021, 94, 104053. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, T.; Kang, J.; Zhang, X.; Zhang, B.; Guo, J.; Chai, Z. Response of Molecular Structures and Methane Adsorption Behaviors in Coals Subjected to Cyclical Microwave Exposure. ACS Omega 2021, 6, 31566–31577. [Google Scholar] [CrossRef]
- Zhang, L. Research on the Influence of Cyclical Microwave Treatment on Coal Structure and Methane Adsorption in Coal; Taiyuan University of Technology: Taiyuan, China, 2020. [Google Scholar]
- Weng, S. Exploration on the Mechanism of in-situ Microwave ChemicaReaction of Pyrite Intrinsic in Bituminous Coal. J. East China Norm. Univ. Nat. Sci. 1996, 3, 46–51. [Google Scholar]
- Kumar, H.; Lester, E.; Kingman, S.; Bourne, R.; Avila, C.; Jones, A.; Robinson, J.; Halleck, P.M.; Mathews, J.P. Inducing fractures and increasing cleat apertures in a bituminous coal under isotropic stress via application of microwave energy. Int. J. Coal Geol. 2011, 88, 75–82. [Google Scholar] [CrossRef]
- Feng, Z.C.; Cai, T.T.; Zhou, D.; Zhao, D.; Zhao, Y.S.; Wang, C. Temperature and deformation changes in anthracite coal after methane adsorption. Fuel 2017, 192, 27–34. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, S.; Pang, Y.; Li, J.; Xin, H. Effects of coal functional groups on adsorption microheat of coal bed methane. Energy Fuels 2015, 29, 1550–1557. [Google Scholar] [CrossRef]
- Li, H.; Lin, B.; Yang, W.; Zheng, C.; Hong, Y.; Gao, Y.; Liu, T.; Wu, S. Experimental study on the petrophysical variation of different rank coals with microwave treatment. Int. J. Coal Geol. 2016, 154–155, 82–91. [Google Scholar] [CrossRef]
- He, L.; Baiquan, L.; Zhongwei, C.; Hong, Y.-D. Evolution of Coal Petrophysical Properties under Microwave Irradiation Stimulation for Different Water Saturation Conditions. Energy Fuels 2017, 31, 8852–8864. [Google Scholar]
- Su, E.; Liang, Y.; Zou, Q. Structures and fractal characteristics of pores in long-flame coal after cyclical supercritical CO2 treatment. Fuel 2021, 286, 119305. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, J.; Zhang, L.; Zhou, T.; Kang, T.; Li, L. Pore fractal characteristics of Suancigou long-flame coal after electrochemical treatment: An experimental study through the implementation of N2 adsorption and mercury intrusion prosimetry techniques. ACS Omega 2021, 6, 27358–27367. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Li, W.; Du, F.; Ma, J.; Qian, R.; Huo, N. Research on abutment stress distribution of roof-cutting coalface: Numerical simulation and field measurement. Geomech. Geophys. Geo-Energy Geo-Resour. 2024, 10, 86. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).