Investigation of the Distribution of 5-Hydroxymethylfurfural in Black Garlic from Different Regions and Its Correlation with Key Process-Related Biochemical Components
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Samples
2.3. Determination of 5-HMF in Black Garlic
2.3.1. Pretreatment of the Samples
2.3.2. Purification of the Samples and Chromatography Conditions
2.4. Measurement of Reducing Sugar Contents in Black Garlic
2.5. Measurement of Amino Acids in Black Garlic
2.6. Measurement of Organic Acids in Black Garlic
2.7. Measurement of Total Polyphenols in Black Garlic
2.8. Statistical Analysis
3. Results and Discussion
3.1. Distribution of 5-HMF in Black Garlic from Different Regions
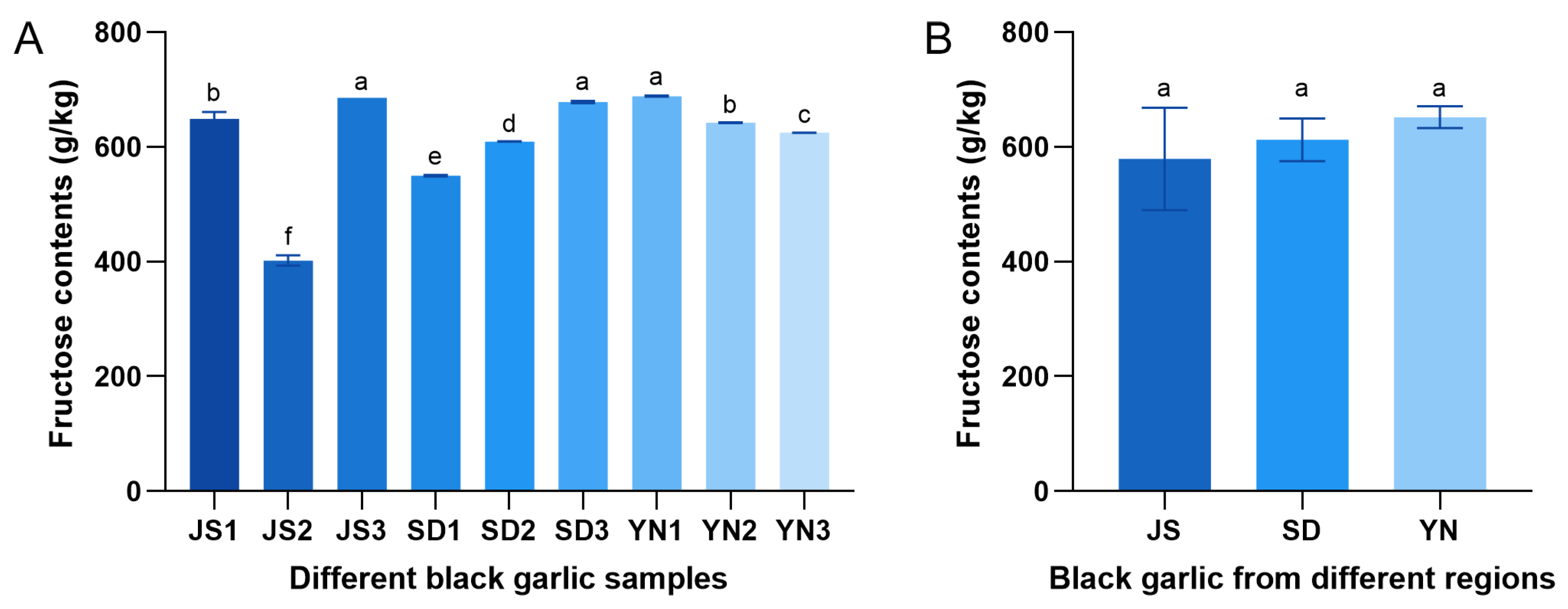
3.2. Changes in Reducing Sugar Content in Black Garlic from Different Regions
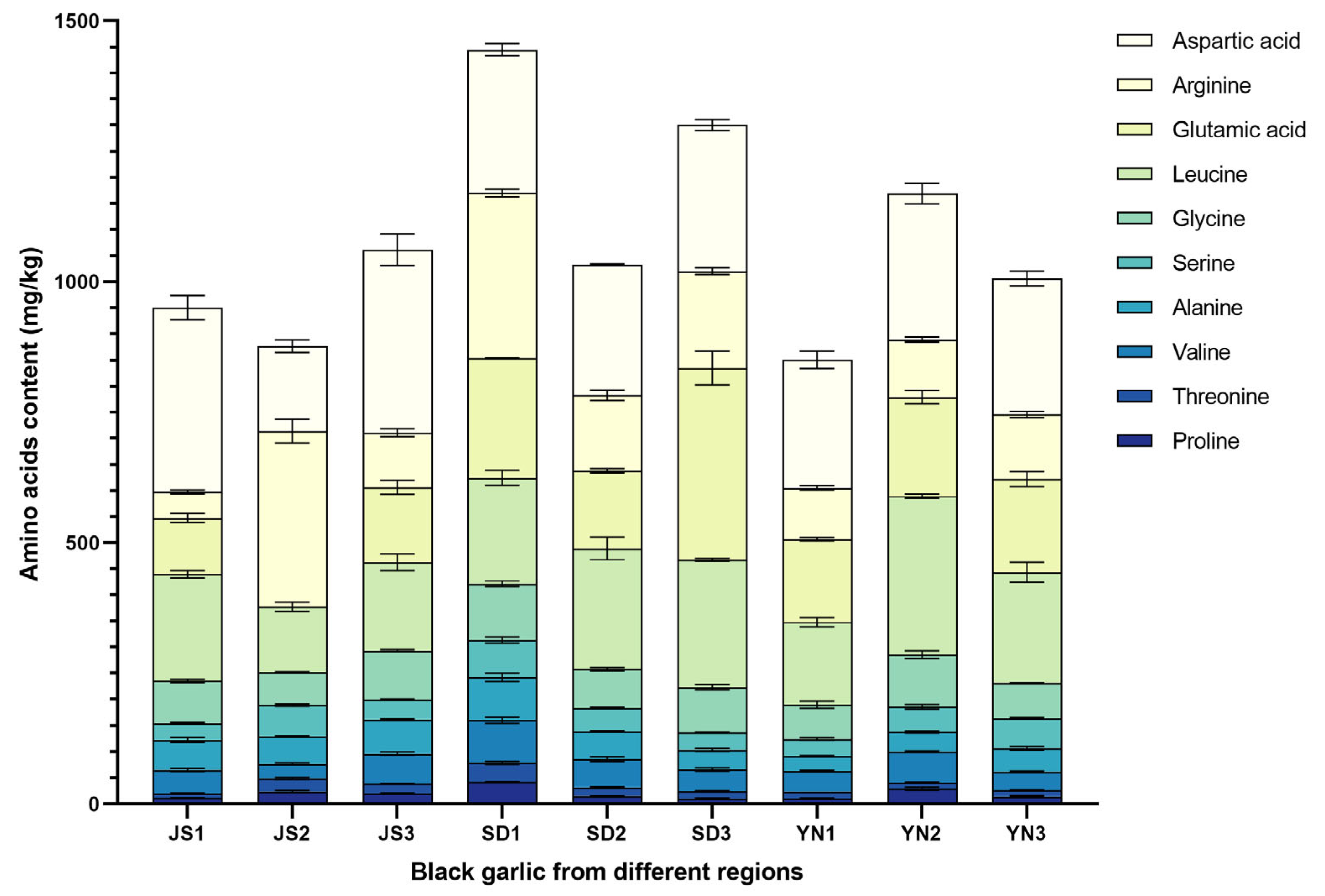
3.3. Analysis of Amino Acids Contents in Black Garlic from Different Regions
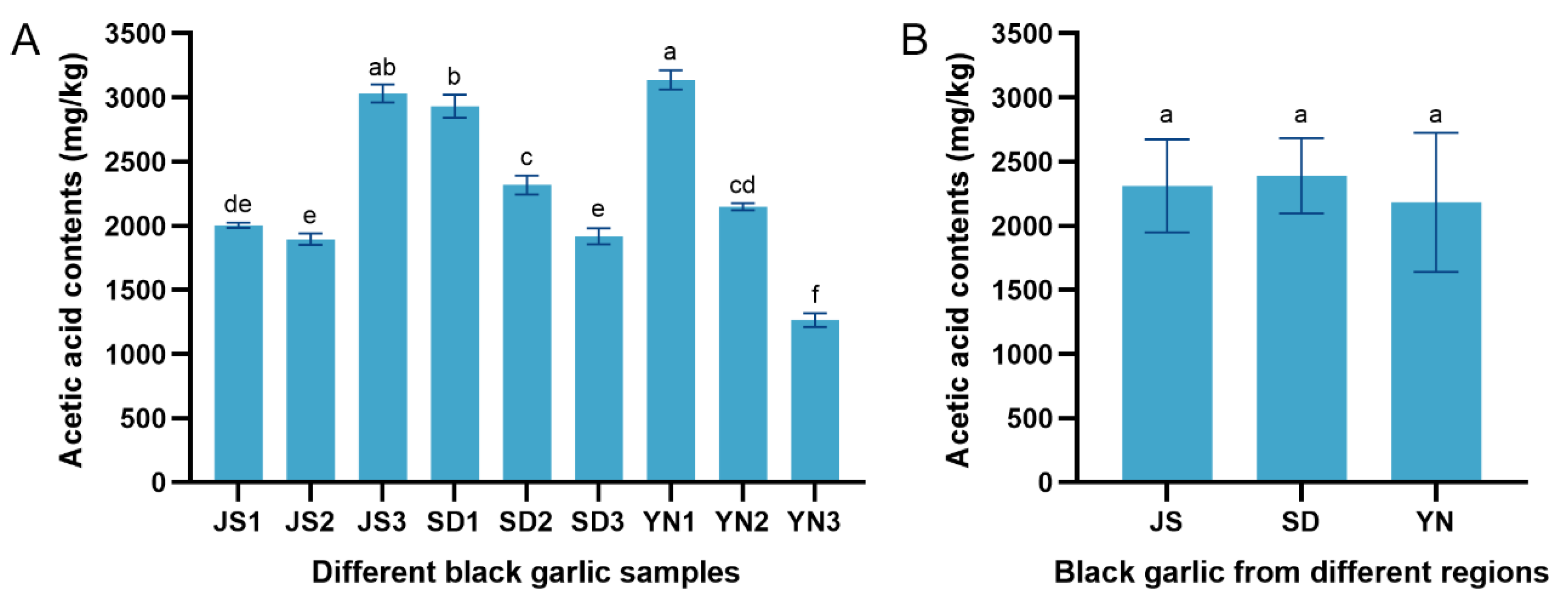
3.4. Distribution of Organic Acids in Black Garlic from Different Regions
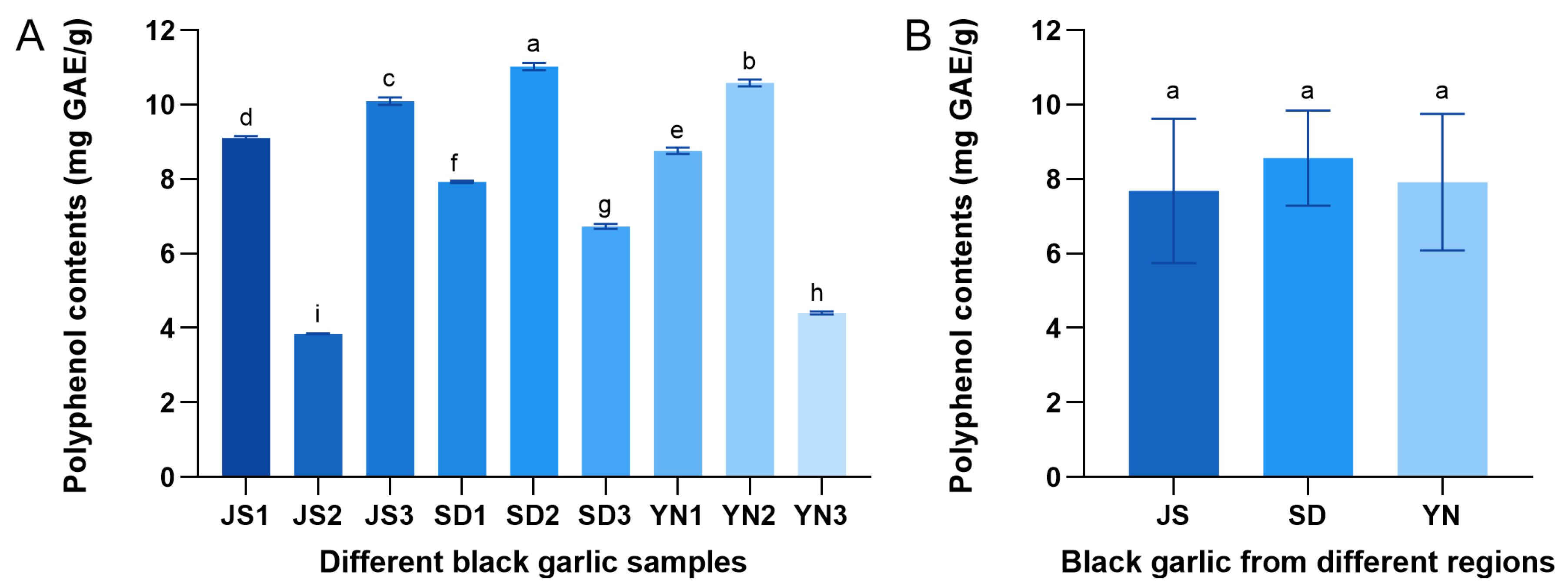
3.5. Distribution of Polyphenols in Black Garlic from Different Regions
3.6. PLSR-Based Quantification of Component Effects on 5-HMF Accumulation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HMF | 5-Hydroxymethylfurfural |
| PLSR | Partial least squares regression |
| SPE | Solid phase extraction |
| DAD | Diode array detector |
References
- Ahmed, T.; Wang, C.-K. Black garlic and its bioactive compounds on human health diseases: A review. Molecules 2021, 26, 5028. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Y.; Zhang, M. Evaluation on the physicochemical and digestive properties of melanoidin from black garlic and their antioxidant activities in vitro. Food Chem. 2021, 340, 127934. [Google Scholar] [CrossRef]
- Toledano Medina, M.Á.; Merinas-Amo, T.; Fernández-Bedmar, Z.; Font, R.; Del Río-Celestino, M.; Pérez-Aparicio, J.; Moreno-Ortega, A.; Alonso-Moraga, Á.; Moreno-Rojas, R. Physicochemical characterization and biological activities of black and white garlic: In vivo and in vitro assays. Foods 2019, 8, 220. [Google Scholar] [CrossRef]
- Martins, F.C.O.L.; Alcantara, G.M.R.N.; Silva, A.F.S.; Melchert, W.R.; Rocha, F.R.P. The role of 5-hydroxymethylfurfural in food and recent advances in analytical methods. Food Chem. 2022, 395, 133539. [Google Scholar] [CrossRef]
- Tsai, J.-C.; Chen, Y.-A.; Wu, J.-T.; Cheng, K.-C.; Lai, P.-S.; Liu, K.-F.; Lin, Y.-K.; Huang, Y.-T.; Hsieh, C.-W. Extracts from fermented black garlic exhibit a hepatoprotective effect on acute hepatic injury. Molecules 2019, 24, 1112. [Google Scholar] [CrossRef]
- Liu, P.; Lu, X.; Li, N.; Zheng, Z.; Zhao, R.; Tang, X.; Qiao, X. Effects and mechanism of free amino acids on browning in the processing of black garlic. J. Sci. Food Agric. 2019, 99, 4670–4676. [Google Scholar] [CrossRef]
- Francisquini, J.D.A.; Rocha, J.; Martins, E.; Stephani, R.; Henrique Fonseca Da Silva, P.; Toledo Renhe, I.R.; Tuler Perrone, Í.; Fernandes De Carvalho, A. 5-Hydroxymethylfurfural formation and color change in lactose-hydrolyzed Dulce de leche. J. Dairy Res. 2019, 86, 477–482. [Google Scholar] [CrossRef]
- Giovanelli, G.; Cappa, C. 5-Hydroxymethylfurfural formation in bread as a function of heat treatment intensity: Correlations with browning indices. Foods 2021, 10, 417. [Google Scholar] [CrossRef]
- Abrantes, T.; Moura-Nunes, N.; Perrone, D. Gallic acid mitigates 5-hydroxymethylfurfural formation while enhancing or preserving browning and antioxidant activity development in glucose/arginine and sucrose/arginine maillard model systems. Molecules 2022, 27, 848. [Google Scholar] [CrossRef]
- Nyarko, K.; Greenlief, C.M. Investigations of major alpha-dicarbonyl content in U.S. honey of different geographical origins. Molecules 2024, 29, 1588. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X.; Shi, L.; Liu, W. Comprehensive analyses of advanced glycation end products and heterocyclic amines in peanuts during the roasting process. Molecules 2023, 28, 7012. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, Z.; Liu, S.; Zhu, S.; Li, G.; Zhong, F.; Mao, J. Formation pathways and precursors of furfural during Zhenjiang aromatic vinegar production. Food Chem. 2021, 354, 129503. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, G.; Zheng, J.; Zhou, H.; Wang, Y.; Huang, C.; Hu, W.; Ou, S. Formation and identification of two hydroxmethylfurfural-glycine adducts and their cytotoxicity and absorption in Caco-2 Cells. J. Agric. Food Chem. 2020, 68, 384–389. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2016, 96, 2366–2372. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, X.; Zhong, Y.; Wang, D.; Dai, B.; Deng, Y. Metabolite, volatile and antioxidant profiles of black garlic stored in different packaging materials. Food Control. 2021, 127, 108131. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Li, Y.; Lin, M.; Chen, J. Non-enzymatic browning and the kinetic model of 5-hydroxymethylfurfural formation in residual solution of vinegar soaked-soybean. Ind. Crop. Prod. 2019, 135, 146–152. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, L.L.; Sun, Y.; Zhang, Y.Y.; Sun, B.G.; Chen, H.T. Determination of the free amino acid, organic Acid, and nucleotide in commercial vinegars. J. Food Sci. 2017, 82, 1116–1123. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, Z.; Yu, Y.; Liu, S.; Zhu, S.; Jian, D.; Cui, P.; Zhong, F.; Mao, J. Investigation of the 5-hydroxymethylfurfural and furfural content of Chinese traditional fermented vinegars from different regions and its correlation with the saccharide and amino acid content. LWT Food Sci. Technol. 2020, 124, 109175. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Song, Y.; Hu, X.-S.; Liao, X.-J.; Ni, Y.-Y.; Li, Q.-H. Effects of sugars in batter formula and baking conditions on 5-hydroxymethylfurfural and furfural formation in sponge cake models. Food Res. Int. 2012, 49, 439–445. [Google Scholar] [CrossRef]
- Sasmaz, H.K.; Kadiroglu, P.; Adal, E.; Sevindik, O.; Aksay, O.; Erkin, O.C.; Selli, S.; Kelebek, H. Optimization of black garlic production parameters using response surface methodology: Assessment and characterization of bioactive properties. J. Appl. Res. Med. Aromat. Plant 2023, 34, 100477. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, Y.-T.; Hsieh, H.-J.; Chen, K.-T.; Chen, Y.-A.; Wu, J.-T.; Tsai, M.-S.; Lin, J.-A.; Hsieh, C.-W. Exploring epigallocatechin gallate impregnation to inhibit 5-hydroxymethylfurfural formation and the effect on antioxidant ability of black garlic. LWT Food Sci. Technol. 2020, 117, 108628. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Ge, S.; Fu, X.; Zhu, J.; Wang, M.; Wang, Y.; Huang, X.; Qin, X.; Tu, Y.; et al. Characterization of a short-term processing technology of black garlic with low 5-HMF content. Food Control. 2024, 165, 110650. [Google Scholar] [CrossRef]
- Prakash, P.; Prakash, K. Quality assessment of promising garlic (Allium sativum L.) varieties based on principal component analysis. Int. Food Res. J. 2023, 30, 1540–1552. [Google Scholar] [CrossRef]
- Lee, J.; Harnly, J.M. Free amino acid and cysteine sulfoxide composition of 11 garlic (Allium sativum L.) cultivars by gas chromatography with flame ionization and mass selective detection. J. Agric. Food Chem. 2005, 52, 9100–9104. [Google Scholar] [CrossRef]
- Martínez-Casas, L.; Lage-Yusty, M.; López-Hernández, J. Changes in the aromatic profile, sugars, and bioactive compounds when purple garlic is transformed into black garlic. J. Agric. Food Chem. 2017, 65, 10804–10811. [Google Scholar] [CrossRef]
- Mi, S.; Zhang, X.; Wang, Y.; Yan, F.; Sang, Y.; Gong, H.; Wang, X. Geographical discrimination and authentication of Chinese garlic based on multi-element, volatile and metabolomics profiling combined with chemometrics. Food Control. 2021, 130, 108328. [Google Scholar] [CrossRef]
- Nie, J.; Weng, R.; Li, C.; Liu, X.; Wang, F.; Rogers, K.M.; Qian, Y.; Zhang, Y.; Yuan, Y. Chemometric origin classification of Chinese garlic using sulfur-containing compounds, assisted by stable isotopes and bioelements. Food Chem. 2022, 394, 133557. [Google Scholar] [CrossRef]
- Chang, W.; Lin, W.; Wu, S. Optimization of the black garlic processing method and development of black garlic jam using high-pressure processing. Foods 2023, 12, 1584. [Google Scholar] [CrossRef]
- Dursun Capar, T.; Inanir, C.; Cimen, F.; Ekici, L.; Yalcin, H. Black garlic fermentation with green tea extract reduced HMF and improved bioactive properties: Optimization study with response surface methodology. J. Food Meas. Charact. 2022, 16, 1340–1353. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An analysis of the changes on intermediate products during the thermal processing of black garlic. Food Chem. 2018, 239, 56–61. [Google Scholar] [CrossRef]
- KlikarovÁ, J.; ČEslovÁ, L.; Fischer, J. Rapid analysis of phenyl isothiocyanate derivatives of amino acids present in Czech meads. J. Chromatogr. A 2021, 1644, 462134. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Hammam, A.R.A.; El-Fatah, A.; Ali; Alsaleem, K.A.; Elfaruk, M.S.; Kamel, D.G.; Moneeb, A.H.M. Measurement of carbohydrates and organic acids in varieties of cheese using high-performance liquid chromatography. Food Sci. Nutr. 2023, 11, 2081–2085. [Google Scholar] [CrossRef]
- Kang, O.-J. Evaluation of melanoidins formed from black garlic after different thermal processing steps. Prev. Nutr. Food Sci. 2016, 21, 398–405. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Effects of thermal treatment on polysaccharide degradation during black garlic processing. LWT Food Sci. Technol. 2018, 95, 223–229. [Google Scholar] [CrossRef]
- González-Ramírez, P.J.; Pascual-Mathey, L.I.; García-Rodríguez, R.V.; Jiménez, M.; Beristain, C.I.; Sanchez-Medina, A.; Pascual-Pineda, L.A. Effect of relative humidity on the metabolite profiles, antioxidant activity and sensory acceptance of black garlic processing. Food Biosci. 2022, 48, 101827. [Google Scholar] [CrossRef]
- Liang, T.; Wei, F.; Lu, Y.; Kodani, Y.; Nakada, M.; Miyakawa, T.; Tanokura, M. Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. J. Agric. Food Chem. 2015, 63, 683–691. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Arena, E.; Fallico, B.; Maccarone, E. Thermal damage in blood orange juice kinetics of 5-hydroxymethyl-2-furancarboxaldehyde formation. Int. J. Food Sci. Technol. 2001, 36, 145–151. [Google Scholar] [CrossRef]
- Gökmen, V.; Şenyuva, H.Z. Effects of some cations on the formation of acrylamide and furfurals in glucose–asparagine model system. Eur. Food Res. Technol. 2006, 225, 815–820. [Google Scholar] [CrossRef]
- Qiu, Z.; Zheng, Z.; Zhang, B.; Sun-Waterhouse, D.; Qiao, X. Formation, nutritional value, and enhancement of characteristic components in black garlic: A review for maximizing the goodness to humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 801–834. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. The comparison of the contents of sugar, Amadori, and Heyns compounds in fresh and black garlic. J. Food Sci. 2016, 81, C1662–C1668. [Google Scholar] [CrossRef]
- Choi, I.; Cha, H.; Lee, Y. Physicochemical and antioxidant properties of black garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; He, C.; Song, H.; Chen, F. Effect of thermal treatment on the flavor generation from Maillard reaction of xylose and chicken peptide. LWT Food Sci. Technol. 2015, 64, 316–325. [Google Scholar] [CrossRef]
- Chua, L.S.; Abdullah, F.I.; Lim, S.H. Physiochemical changes and nutritional content of black garlic during fermentation. Appl. Food Res. 2022, 2, 100216. [Google Scholar] [CrossRef]
- Zhao, C.; Xia, T.; Du, P.; Duan, W.; Zhang, B.; Zhang, J.; Zhu, S.; Zheng, Y.; Wang, M.; Yu, Y. Chemical composition and antioxidant characteristic of traditional and industrial Zhenjiang aromatic vinegars during the aging process. Molecules 2018, 23, 2949. [Google Scholar] [CrossRef]
- Davídek, T.; Robert, F.; Devaud, S.; Vera, F.A.; Blank, I. Sugar fragmentation in the Maillard reaction cascade: Formation of short-chain carboxylic acids by a new oxidativer-dicarbonyl cleavage pathway. J. Agric. Food Chem. 2006, 54, 6677–6684. [Google Scholar] [CrossRef]
- Davídek, T.; Devaud, S.; Robert, F.; Blank, I. Sugar fragmentation in the Maillard reaction cascade: Isotope labeling studies on the formation of acetic acid by a hydrolytic β-dicarbonyl cleavage mechanism. J. Agric. Food Chem. 2006, 54, 6667–6676. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, Y.; Beeftink, R.; Ooijkaas, L.; Rinzema, A.; Chen, J.; Tramper, J. Chinese vinegar and its solid-state fermentation process. Food Rev. Int. 2004, 20, 407–424. [Google Scholar] [CrossRef]
- Kim, J.S.; Cuong, D.M.; Bae, Y.B.; Cho, S.K. Antioxidant and antiproliferative activities of solvent fractions of broccoli (Brassica oleracea L.) sprout. Appl Biol Chem. 2022, 65, 34. [Google Scholar] [CrossRef]
- Li, F.; Cao, J.; Liu, Q.; Hu, X.; Liao, X.; Zhang, Y. Acceleration of the Maillard reaction and achievement of product quality by high pressure pretreatment during black garlic processing. Food Chem. 2020, 318, 126517. [Google Scholar] [CrossRef]
- Nam, S.-H.; Han, Y.-S.; Yang, K.-H.S.S.-O.; Kim, M.-H. Changes in the physicochemical properties, antioxidant activity and metabolite analysis of black elephant garlic (Allium ampeloprasum L.) during aging period. Foods 2022, 12, 43. [Google Scholar] [CrossRef]
- Kimura, S.; Tung, Y.-C.; Pan, M.-H.; Su, N.-W.; Lai, Y.-J.; Cheng, K.-C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef]
- Bae, S.E.; Cho, S.Y.; Won, Y.D.; Lee, S.H.; Park, H.J. Changes in S-allyl cysteine contents and physicochemical properties of black garlic during heat treatment. LWT Food Sci. Technol. 2014, 55, 397–402. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Van Boekel, M.A.J.S. Kinetics of the glucose/glycine Maillard reaction pathways: Influences of pH and reactant initial concentrations. Food Chem. 2005, 92, 437–448. [Google Scholar] [CrossRef]
- Wen, C.; Shi, X.; Wang, Z.; Gao, W.; Jiang, L.; Xiao, Q.; Liu, X.; Deng, F. Effects of metal ions on formation of acrylamide and 5-hydroxymethylfurfural in asparagine-glucose model system. Int. J. Food Sci. Tech. 2015, 51, 279–285. [Google Scholar] [CrossRef]
- Verma, V.; Yadav, N. Inhibition of acrylamide and 5-hydroxymethylfurfural formation in French fries by additives in model reaction. J. Food Process Eng. 2022, 45, e14178. [Google Scholar] [CrossRef]
- Lim, S.I.; Kwak, E.J.; Lee, O.H.; Lee, B.Y. Effect of antibrowning agents on browning and intermediate formation in the glucose–glutamic acid model. J. Food Sci. 2010, 75, C678–C683. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, W.; Zhang, C.; Li, L.; Hu, W.; Guo, Y.; Lu, G. Simultaneous mitigation of acrylamide, 5-HMF, and browning formation by combining three additives in commercial crackers. J. Food Nutr. Res. 2022, 10, 536–545. [Google Scholar] [CrossRef]
- Liu, Q.-L.; Yi, Y.; Wang, S.-Q.; Wang, H.-X.; Xu, W.; Min, T.; Wang, L.-M. Non-enzymatic browning of lotus root during boiling. LWT Food Sci. Technol. 2022, 173, 114191. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Zhang, X.; Wu, X.; Zhang, Y. Addition of antioxidant of bamboo leaves (AOB) effectively reduces acrylamide formation in potato crisps and French fries. J. Agric. Food Chem. 2007, 55, 523–528. [Google Scholar] [CrossRef]







| No. | Code | Place of Origin | Moisture Content | Processing Parameters |
|---|---|---|---|---|
| 1 | JS1 | Jiangsu Province | 32% | 75–80 °C, 25–30 d, 55% |
| 2 | JS2 | Jiangsu Province | 35% | 70–75 °C, 20–25 d, 65% |
| 3 | JS3 | Jiangsu Province | 34% | 70–80 °C, 20–25 d, 60% |
| 4 | SD1 | Shandong Province | 42% | 60–65 °C, 15–25 d, 70% |
| 5 | SD2 | Shandong Province | 41% | 65–70 °C, 15–20 d, 65–70% |
| 6 | SD3 | Shandong Province | 42% | 60–65 °C, 15–20 d, 70–75% |
| 7 | YN1 | Yunnan Province | 37% | 70–75 °C, 15–25 d, 65–70% |
| 8 | YN2 | Yunnan Province | 36% | 70–80 °C, 15–25 d, 70% |
| 9 | YN3 | Yunnan Province | 37% | 70–75 °C, 15–25 d, 65–70% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Zhang, S.; Sun, Y.; Gong, H.; Wang, S.; Wang, J. Investigation of the Distribution of 5-Hydroxymethylfurfural in Black Garlic from Different Regions and Its Correlation with Key Process-Related Biochemical Components. Processes 2025, 13, 2133. https://doi.org/10.3390/pr13072133
Yuan H, Zhang S, Sun Y, Gong H, Wang S, Wang J. Investigation of the Distribution of 5-Hydroxymethylfurfural in Black Garlic from Different Regions and Its Correlation with Key Process-Related Biochemical Components. Processes. 2025; 13(7):2133. https://doi.org/10.3390/pr13072133
Chicago/Turabian StyleYuan, Heng, Simin Zhang, Yuee Sun, Hao Gong, Shuai Wang, and Jun Wang. 2025. "Investigation of the Distribution of 5-Hydroxymethylfurfural in Black Garlic from Different Regions and Its Correlation with Key Process-Related Biochemical Components" Processes 13, no. 7: 2133. https://doi.org/10.3390/pr13072133
APA StyleYuan, H., Zhang, S., Sun, Y., Gong, H., Wang, S., & Wang, J. (2025). Investigation of the Distribution of 5-Hydroxymethylfurfural in Black Garlic from Different Regions and Its Correlation with Key Process-Related Biochemical Components. Processes, 13(7), 2133. https://doi.org/10.3390/pr13072133





