From Probiotic to Postbiotic: Conversion of Acerola Juice by Thermosonication
Abstract
1. Introduction
2. Materials and Methods
2.1. Juice Preparation
2.2. Lacticaseibacillus casei Inoculum Preparation and Enumeration
2.3. Acerola Juice Fermentation
2.4. Thermosonication (TS)
2.5. Thermal Processing (TH)
2.6. Storage Stability
2.7. Determination of Sugars and Organic Acids
2.8. Total Phenolic Compounds
2.9. Antioxidant Activity
2.10. Microbiological Quality
2.11. Antibacterial Activity Assay
- -
- 24 h sample: 100 μL of probiotic or postbiotic juice + 100 μL of bacterial suspension (E. coli or S. typhimurium), incubated for 24 h at 30 °C;
- -
- 0 h sample: 100 μL of probiotic or postbiotic juice + 100 μL of bacterial suspension (E. coli or S. typhimurium), measured immediately (0 h) at 30 °C;
- -
- 24 h negative control: 100 μL of nutrient broth + 100 μL of bacterial suspension (E. coli or S. typhimurium), incubated for 24 h at 30 °C;
- -
- 0 h negative control: 100 μL of nutrient broth + 100 μL of bacterial suspension (E. coli or S. typhimurium), measured immediately (0 h) at 30 °C.
2.12. Statistics
3. Results and Discussion
3.1. Acerola Juice Fermentation by L. casei
Probiotic Stability During Cold Storage
3.2. Postbiotic Production by Thermosonication (TS) and Thermal Processing (TH)
Postbiotic Stability During Cold Storage
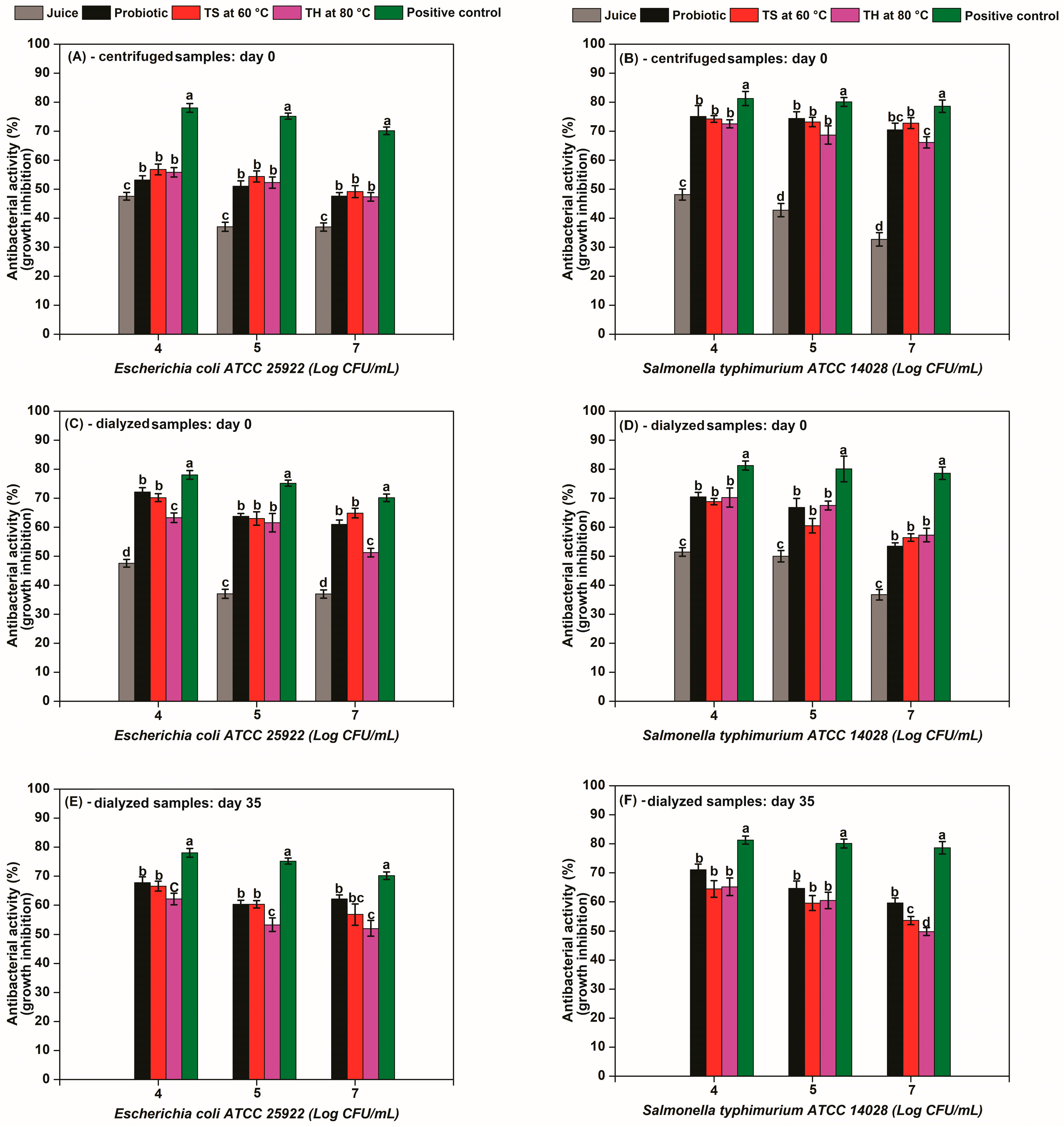
3.3. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Barros, C.P.; Guimarães, J.T.; Esmerino, E.A.; Duarte, M.C.K.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.S.; Freitas, M.Q.; Cruz, A.G. Paraprobiotics and Postbiotics: Concepts and Potential Applications in Dairy Products. Curr. Opin. Food Sci. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and Paraprobiotics: From Concepts to Applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Yeşilyurt, N.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Involvement of Probiotics and Postbiotics in the Immune System Modulation. Biologics 2021, 1, 89–110. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Kadum, H.; Meor Hussin, A.S. Metabolomics Profiling of Fermented Cantaloupe Juice and the Potential Application to Extend the Shelf Life of Fresh Cantaloupe Juice for Six Months at 8 °C. Food Control 2021, 120, 107555. [Google Scholar] [CrossRef]
- Algboory, H.L.; Muhialdin, B.J. Novel Peptides Contribute to the Antimicrobial Activity of Camel Milk Fermented with Lactobacillus plantarum IS10. Food Control 2021, 126, 108057. [Google Scholar] [CrossRef]
- İncili, G.K.; Karatepe, P.; Akgöl, M.; Güngören, A.; Koluman, A.; İlhak, O.İ.; Kanmaz, H.; Kaya, B.; Hayaloğlu, A.A. Characterization of Lactic Acid Bacteria Postbiotics, Evaluation in-Vitro Antibacterial Effect, Microbial and Chemical Quality on Chicken Drumsticks. Food Microbiol. 2022, 104, 104001. [Google Scholar] [CrossRef]
- Shirkhan, F.; Mirdamadi, S.; Mirzaei, M.; Akbari-adergani, B.; Nasoohi, N. The Role of Lactic Acid Bacteria in Production of Bioactive Peptides in Fermented Milk with Antioxidant and Antidiabetic Properties. J. Food Meas. Charact. 2023, 17, 4727–4738. [Google Scholar] [CrossRef]
- Nunes, B.V.; da Silva, C.N.; Bastos, S.C.; de Souza, V.R. Microbiological Inactivation by Ultrasound in Liquid Products. Food Bioproc. Tech. 2022, 15, 2185–2209. [Google Scholar] [CrossRef]
- Oliveira, G.A.R.; Guimarães, J.T.; Ramos, G.L.P.A.; Esmerino, E.A.; Pimentel, T.C.; Neto, R.P.C.; Tavares, M.I.B.; Sobral, L.A.; Souto, F.; Freitas, M.Q.; et al. Benefits of Thermosonication in Orange Juice Whey Drink Processing. Innov. Food Sci. Emerg. Technol. 2022, 75, 102876. [Google Scholar] [CrossRef]
- Fan, L.; Ismail, B.B.; Gao, L.; Liu, D. Comparison of High- and Low- Frequency Thermosonication and Carvacrol Treatments of Carrot Juice: Microbial Inactivation and Quality Retention. Appl. Food Res. 2022, 2, 100162. [Google Scholar] [CrossRef]
- Jafarpour, D. The Effect of Heat Treatment and Thermosonication on the Microbial and Quality Properties of Green Olive. J. Food Meas. Charact. 2022, 16, 2172–2180. [Google Scholar] [CrossRef]
- Rodrigues, V.C.d.C.; da Silva, L.G.S.; Simabuco, F.M.; Venema, K.; Antunes, A.E.C. Survival, Metabolic Status and Cellular Morphology of Probiotics in Dairy Products and Dietary Supplement after Simulated Digestion. J. Funct. Foods 2019, 55, 126–134. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Feitosa, W.S.C.; Abreu, V.K.G.; Lemos, T.d.O.; Gomes, W.F.; Narain, N.; Rodrigues, S. Impact of Fermentation Conditions on the Quality and Sensory Properties of a Probiotic Cupuassu (Theobroma grandiflorum) Beverage. Food Res. Int. 2017, 100, 603–611. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Maciel, T.C.; Rodrigues, S. Probiotic Beverage from Cashew Apple Juice Fermented with Lactobacillus casei. Food Res. Int. 2011, 44, 1276–1283. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Costa, M.G.M.; de Jesus, A.L.T.; Fontes, C.P.M.L.; Fernandes, F.A.N.; Rodrigues, S. Stability and Quality Parameters of Probiotic Cantaloupe Melon Juice Produced with Sonicated Juice. Food Bioproc Tech. 2013, 6, 2860–2869. [Google Scholar] [CrossRef]
- de Godoy Alves Filho, E.; Rodrigues, T.H.S.; Fernandes, F.A.N.; Pereira, A.L.F.; Narain, N.; de Brito, E.S.; Rodrigues, S. Chemometric Evaluation of the Volatile Profile of Probiotic Melon and Probiotic Cashew Juice. Food Res. Int. 2017, 99, 461–468. [Google Scholar] [CrossRef]
- dos Santos Filho, A.L.; Freitas, H.V.; Rodrigues, S.; Abreu, V.K.G.; Lemos, T.d.O.; Gomes, W.F.; Narain, N.; Pereira, A.L.F. Production and Stability of Probiotic Cocoa Juice with Sucralose as Sugar Substitute during Refrigerated Storage. LWT 2019, 99, 371–378. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to Optimize the Drop Plate Method for Enumerating Bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Silva do Nascimento, C.; Santos, B.N.; Rodrigues, S. High-intensity Ultrasound Processed Acerola Juice Containing Oligosaccharides and Dextran Promotes Lacticaseibacillus casei NRRL B-442 Growth. Int. J. Food Sci. Technol. 2022, 57, 5186–5194. [Google Scholar] [CrossRef]
- Almada, C.N.; Almada-Érix, C.N.; Bonatto, M.S.; Pradella, F.; dos Santos, P.; Abud, Y.K.D.; Farias, A.S.; Martínez, J.; Sant’Anna Filho, C.B.; Lollo, P.C.; et al. Obtaining Paraprobiotics from Lactobacilus acidophilus, Lacticaseibacillus casei and Bifidobacterium animalis Using Six Inactivation Methods: Impacts on the Cultivability, Integrity, Physiology, and Morphology. J. Funct. Foods 2021, 87, 104826. [Google Scholar] [CrossRef]
- Oliveira, A.F.A.; Mar, J.M.; Santos, S.F.; da Silva Júnior, J.L.; Kluczkovski, A.M.; Bakry, A.M.; Bezerra, J.d.A.; Nunomura, R.d.C.S.; Sanches, E.A.; Campelo, P.H. Non-Thermal Combined Treatments in the Processing of Açai (Euterpe oleracea) Juice. Food Chem. 2018, 265, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Fonteles, T.V.; dos Santos, A.Y.S.; Linhares, M.d.F.D.; Miguel, T.B.A.R.; Miguel, E.d.C.; Rodrigues, S. Metabolic Responses of Kombucha Consortium Fermentation upon Ultrasound-Processing. Food Chem. Adv. 2024, 4, 100646. [Google Scholar] [CrossRef]
- Tournas, V.H.; Heeres, J.; Burgess, L. Moulds and Yeasts in Fruit Salads and Fruit Juices. Food Microbiol. 2006, 23, 684–688. [Google Scholar] [CrossRef]
- Fernandes Pereira, A.L.; Rodrigues, S. Turning Fruit Juice Into Probiotic Beverages. In Fruit Juices: Extraction, Composition, Quality and Analysis; Academic Press: San Diego, CA, USA, 2018; pp. 279–287. [Google Scholar] [CrossRef]
- Rasika, D.M.; Vidanarachchi, J.K.; Rocha, R.S.; Balthazar, C.F.; Cruz, A.G.; Sant’Ana, A.S.; Ranadheera, C.S. Plant-Based Milk Substitutes as Emerging Probiotic Carriers. Curr. Opin. Food Sci. 2021, 38, 8–20. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation Transforms the Phenolic Profiles and Bioactivities of Plant-Based Foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Xu, H.; Feng, L.; Deng, Y.; Chen, L.; Li, Y.; Lin, L.; Liang, M.; Jia, X.; Wang, F.; Zhang, X.; et al. Change of Phytochemicals and Bioactive Substances in Lactobacillus Fermented Citrus Juice during the Fermentation Process. LWT 2023, 180, 114715. [Google Scholar] [CrossRef]
- Tran, A.M.; Nguyen, T.B.; Nguyen, V.D.; Bujna, E.; Dam, M.S.; Nguyen, Q.D. Changes in Bitterness, Antioxidant Activity and Total Phenolic Content of Grapefruit Juice Fermented by Lactobacillus and Bifidobacterium Strains. Acta Aliment. 2020, 49, 103–110. [Google Scholar] [CrossRef]
- Dzandu, B.; Chotiko, A.; Sathivel, S. Antioxidant Activity and Viability of Lacticaseibacillus rhamnosus, Lacticaseibacillus casei, and Co-Culture in Fermented Tomato Juice during Refrigerated Storage. Food Biosci. 2022, 50, 102085. [Google Scholar] [CrossRef]
- Maciel da Silva, R.; Henrique Campelo, P.; Rodrigues, S. In Vitro Viability of L. Casei B-442 and Fructooligosaccharides Integrity in Amazonian Sapota-Do-Solimões Functional Juice. Food Res. Int. 2022, 154, 111036. [Google Scholar] [CrossRef]
- Adebo, O.A.; Medina-Meza, I.G. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains: A Mini Review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef] [PubMed]
- Adebayo-Tayo, B.C.; Olomitutu, F.O.; Adebami, G.E. Production and Evaluation of Probioticated Mango Juice Using Pediococcus pentosaceus and Pediococcus acidilactici during Storage at Different Temperature. J. Agric. Food Res. 2021, 6, 100202. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, L.; Liu, X.; Hasan, K.M.F.; Li, H.; Zhou, S.; Zhang, Q.; Zhou, Y. Effect of Thermosonication Treatment on Blueberry Juice Quality: Total Phenolics, Flavonoids, Anthocyanin, and Antioxidant Activity. LWT 2021, 150, 112021. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D.; Soto, E.R.; Barba, F.J. Ultrasound-Assisted Lactic Acid Fermentation of Bakraei (Citrus reticulata cv. Bakraei) Juice: Physicochemical and Bioactive Properties. Fermentation 2023, 9, 37. [Google Scholar] [CrossRef]
- Alcántara-Zavala, A.E.; Figueroa-Cárdenas, J.d.D.; Pérez-Robles, J.F.; Arámbula-Villa, G.; Miranda-Castilleja, D.E. Thermosonication as an Alternative Method for Processing, Extending the Shelf Life, and Conserving the Quality of Pulque: A Non-Dairy Mexican Fermented Beverage. Ultrason. Sonochem. 2021, 70, 105290. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Velázquez-Estrada, R.M.; Roig, A.X.; García-Galindo, H.S.; Sayago-Ayerdi, S.G.; Montalvo-González, E. Thermosonication: An Alternative Processing for Fruit and Vegetable Juices. Trends Food Sci. Technol. 2017, 61, 26–37. [Google Scholar] [CrossRef]
- Evelyn; Milani, E.; Silva, F.V.M. Comparing High Pressure Thermal Processing and Thermosonication with Thermal Processing for the Inactivation of Bacteria, Moulds, and Yeasts Spores in Foods. J. Food Eng. 2017, 214, 90–96. [Google Scholar] [CrossRef]
- Peluzio, M.d.C.G.; Martinez, J.A.; Milagro, F.I. Postbiotics: Metabolites and Mechanisms Involved in Microbiota-Host Interactions. Trends Food Sci. Technol. 2021, 108, 11–26. [Google Scholar] [CrossRef]
- Prajapati, N.; Patel, J.; Singh, S.; Yadav, V.K.; Joshi, C.; Patani, A.; Prajapati, D.; Sahoo, D.K.; Patel, A. Postbiotic Production: Harnessing the Power of Microbial Metabolites for Health Applications. Front. Microbiol. 2023, 14, 1306192. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Jiang, A. Effect of Ultrasound Treatment on Quality and Microbial Load of Carrot Juice. Food Sci. Technol. 2016, 36, 111–115. [Google Scholar] [CrossRef]
- Muñoz, A.; Caminiti, I.M.; Palgan, I.; Pataro, G.; Noci, F.; Morgan, D.J.; Cronin, D.A.; Whyte, P.; Ferrari, G.; Lyng, J.G. Effects on Escherichia Coli Inactivation and Quality Attributes in Apple Juice Treated by Combinations of Pulsed Light and Thermosonication. Food Res. Int. 2012, 45, 299–305. [Google Scholar] [CrossRef]
- Qiu, X.; Su, J.; Nie, J.; Zhang, Z.; Ren, J.; Wang, S.; Pei, Y.; Li, X. Effects of Thermosonication on the Antioxidant Capacity and Physicochemical, Bioactive, Microbiological, and Sensory Qualities of Blackcurrant Juice. Foods 2024, 13, 809. [Google Scholar] [CrossRef]
- Xia, M.; LI, C.; Wu, D.; Wu, F.; Kong, L.; Jia, Z.; Han, W.; Chen, S.; Fang, W.; Liu, Y.; et al. Benefits of Heat-Killed Lactobacillus acidophilus on Growth Performance, Nutrient Digestibility, Antioxidant Status, Immunity, and Cecal Microbiota of Rabbits. Front. Vet. Sci. 2024, 11, 1361908. [Google Scholar] [CrossRef]
- Scudino, H.; Pimentel, T.C.; Guimarães, J.T.; Moura, R.S.; Mársico, E.T.; Esmerino, E.A.; Freitas, M.Q.; Souza, A.A.; Nogueira, F.C.S.; Cruz, A.G. Effect of Thermosonication on the Bioactive Peptide Profile, Volatile Compounds, and Fatty Acid Profile of Minas Frescal Cheese. Int. Dairy. J. 2024, 157, 106009. [Google Scholar] [CrossRef]
- Pavlova, A.S.; Ozhegov, G.D.; Arapidi, G.P.; Butenko, I.O.; Fomin, E.S.; Alemasov, N.A.; Afonnikov, D.A.; Yarullina, D.R.; Ivanov, V.T.; Govorun, V.M.; et al. Identification of Antimicrobial Peptides from Novel Lactobacillus fermentum Strain. Protein J. 2020, 39, 73–84. [Google Scholar] [CrossRef]
- He, J.F.; Jin, D.X.; Luo, X.G.; Zhang, T.C. LHH1, a Novel Antimicrobial Peptide with Anti-Cancer Cell Activity Identified from Lactobacillus casei HZ1. AMB Express 2020, 10, 204. [Google Scholar] [CrossRef]
- Tian, X.; Hu, W.; Chen, J.; Zhang, W.; Li, W. The Supplement of Vitamin C Facilitates L-Lactic Acid Biosynthesis in Lactobacillus thermophilus A69 from Sweet Sorghum Juice Coupled with Soybean Hydrolysate as Feedstocks. Ind. Crops Prod. 2020, 146, 112159. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R. Acerola, an Untapped Functional Superfruit: A Review on Latest Frontiers. J. Food Sci. Technol. 2018, 55, 3373–3384. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Peng, Z.; Zhao, Y.; Wu, K.; Zhou, N.; Yan, Y.; Ramaswamy, H.S.; Sun, J.; Bai, W. The Impact of Ultrasonic Treatment on Blueberry Wine Anthocyanin Color and Its In-Vitro Anti-Oxidant Capacity. Food Chem. 2020, 333, 127455. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Sackey, A.S.; Wu, M.; Xiao, L. Impact of Ultrasonication and Pulsed Light Treatments on Phenolics Concentration and Antioxidant Activities of Lactic-Acid-Fermented Mulberry Juice. LWT 2018, 92, 61–66. [Google Scholar] [CrossRef]
- Wang, J.; Vanga, S.K.; Raghavan, V. High-Intensity Ultrasound Processing of Kiwifruit Juice: Effects on the Ascorbic Acid, Total Phenolics, Flavonoids and Antioxidant Capacity. LWT 2019, 107, 299–307. [Google Scholar] [CrossRef]
- Aguilar, K.; Garvín, A.; Ibarz, A.; Augusto, P.E.D. Ascorbic Acid Stability in Fruit Juices during Thermosonication. Ultrason. Sonochem. 2017, 37, 375–381. [Google Scholar] [CrossRef]
- Szutowska, J. Functional Properties of Lactic Acid Bacteria in Fermented Fruit and Vegetable Juices: A Systematic Literature Review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Yang, E.; Fan, L.; Yan, J.; Jiang, Y.; Doucette, C.; Fillmore, S.; Walker, B. Influence of Culture Media, PH and Temperature on Growth and Bacteriocin Production of Bacteriocinogenic Lactic Acid Bacteria. AMB Express 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Qu, X. Genetic Mechanisms of Prebiotic Carbohydrate Metabolism in Lactic Acid Bacteria: Emphasis on Lacticaseibacillus casei and Lacticaseibacillus paracasei as Flexible, Diverse and Outstanding Prebiotic Carbohydrate Starters. Trends Food Sci. Technol. 2021, 115, 486–499. [Google Scholar] [CrossRef]
- Aneja, K.R.; Dhiman, R.; Aggarwal, N.K.; Kumar, V.; Kaur, M. Microbes Associated with Freshly Prepared Juices of Citrus and Carrots. Int. J. Food Sci. 2014, 2014, 408085. [Google Scholar] [CrossRef]
- Li, P.; Xia, J.; Nie, Z.; Shan, Y. Pectic Oligosaccharides Hydrolyzed from Orange Peel by Fungal Multi-Enzyme Complexes and Their Prebiotic and Antibacterial Potentials. LWT-Food Sci. Technol. 2016, 69, 203–210. [Google Scholar] [CrossRef]
- Majkut, M.; Kwiecińska-Piróg, J.; Wszelaczyńska, E.; Pobereżny, J.; Gospodarek-Komkowska, E.; Wojtacki, K.; Barczak, T. Antimicrobial Activity of Heat-Treated Polish Honeys. Food Chem. 2021, 343, 128561. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Wang, X.; Wu, J.; Guo, Y.; Ge, H.; Li, T.; Khan, S.; Li, Z.; Feng, X. Purification and Primary Characterization of a Novel Bacteriocin, LiN333, from Lactobacillus casei, an Isolate from a Chinese Fermented Food. LWT 2017, 84, 867–875. [Google Scholar] [CrossRef]
- Xue, X.; Gao, Y.; Liu, F.; Du, P.; Li, C.; Liu, Y.; Yu, W.; Liu, L. Purification, Characterization, and Identification of a Novel Bacteriocin Produced by Lacticaseibacillus casei KLS1, and Its Antimicrobial Mechanism against Staphylococcus aureus. LWT 2024, 200, 116207. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, Z.; Liang, Z.; Fu, X.; Li, D.; Zhu, C.; Kong, Q.; Mou, H. Current Challenges and Development Strategies of Bacteriocins Produced by Lactic Acid Bacteria Applied in the Food Industry. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70038. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and Potential Use as Antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Barbosa, A.A.T.; Mantovani, H.C.; Jain, S. Bacteriocins from Lactic Acid Bacteria and Their Potential in the Preservation of Fruit Products. Crit. Rev. Biotechnol. 2017, 37, 852–864. [Google Scholar] [CrossRef]
 ) glucose consumption (g/L) (
) glucose consumption (g/L) ( ), pH (
), pH ( ) and lactic acid (g/L) (
) and lactic acid (g/L) ( ) formation during fermentation.
) formation during fermentation.
 ) glucose consumption (g/L) (
) glucose consumption (g/L) ( ), pH (
), pH ( ) and lactic acid (g/L) (
) and lactic acid (g/L) ( ) formation during fermentation.
) formation during fermentation.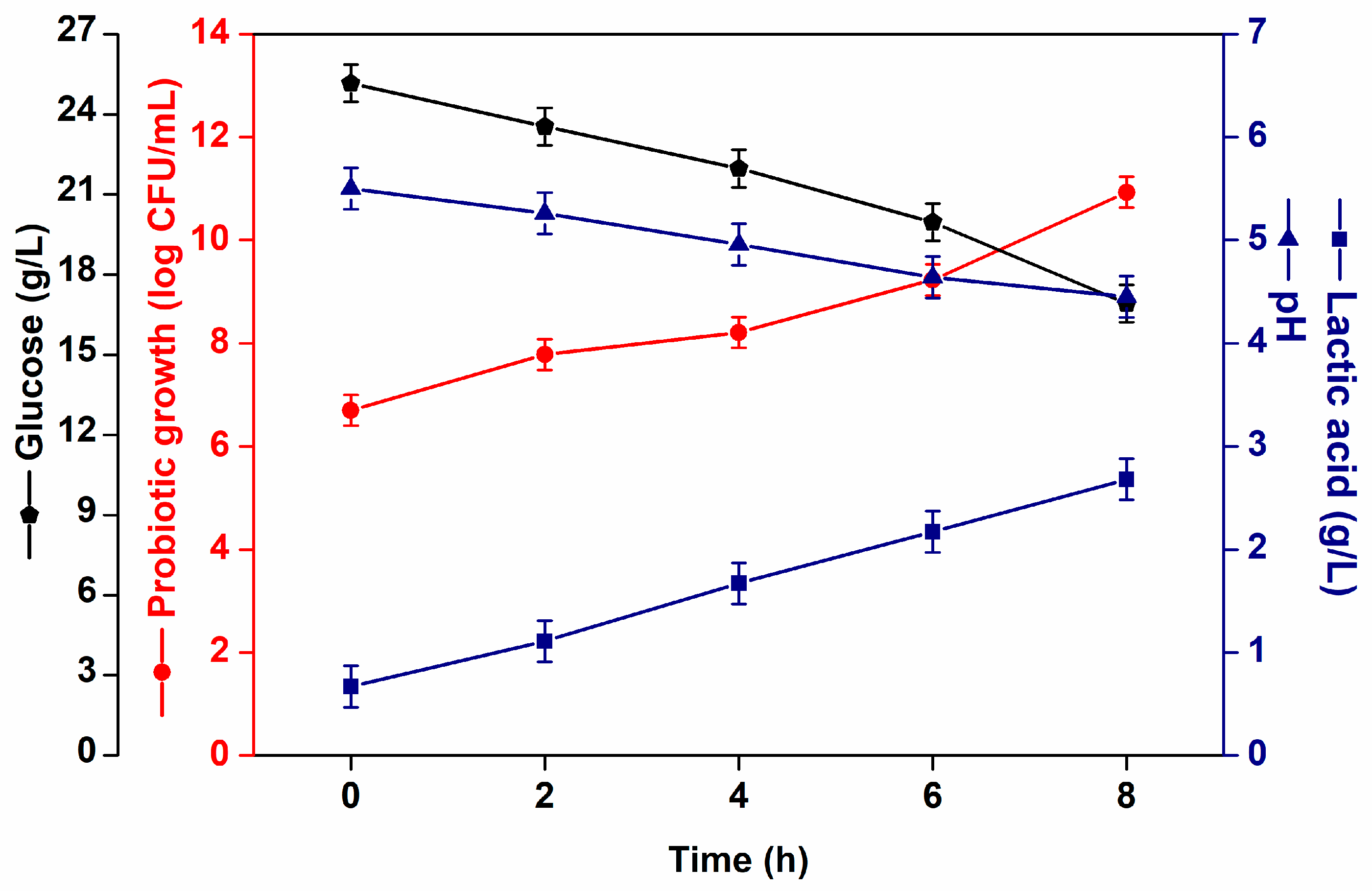
 ) glucose consumption (g/L) (
) glucose consumption (g/L) ( ), pH (
), pH ( ) and lactic acid (g/L) (
) and lactic acid (g/L) ( ) formation in probiotic acerola juice throughout storage at 4 °C.
) formation in probiotic acerola juice throughout storage at 4 °C.
 ) glucose consumption (g/L) (
) glucose consumption (g/L) ( ), pH (
), pH ( ) and lactic acid (g/L) (
) and lactic acid (g/L) ( ) formation in probiotic acerola juice throughout storage at 4 °C.
) formation in probiotic acerola juice throughout storage at 4 °C.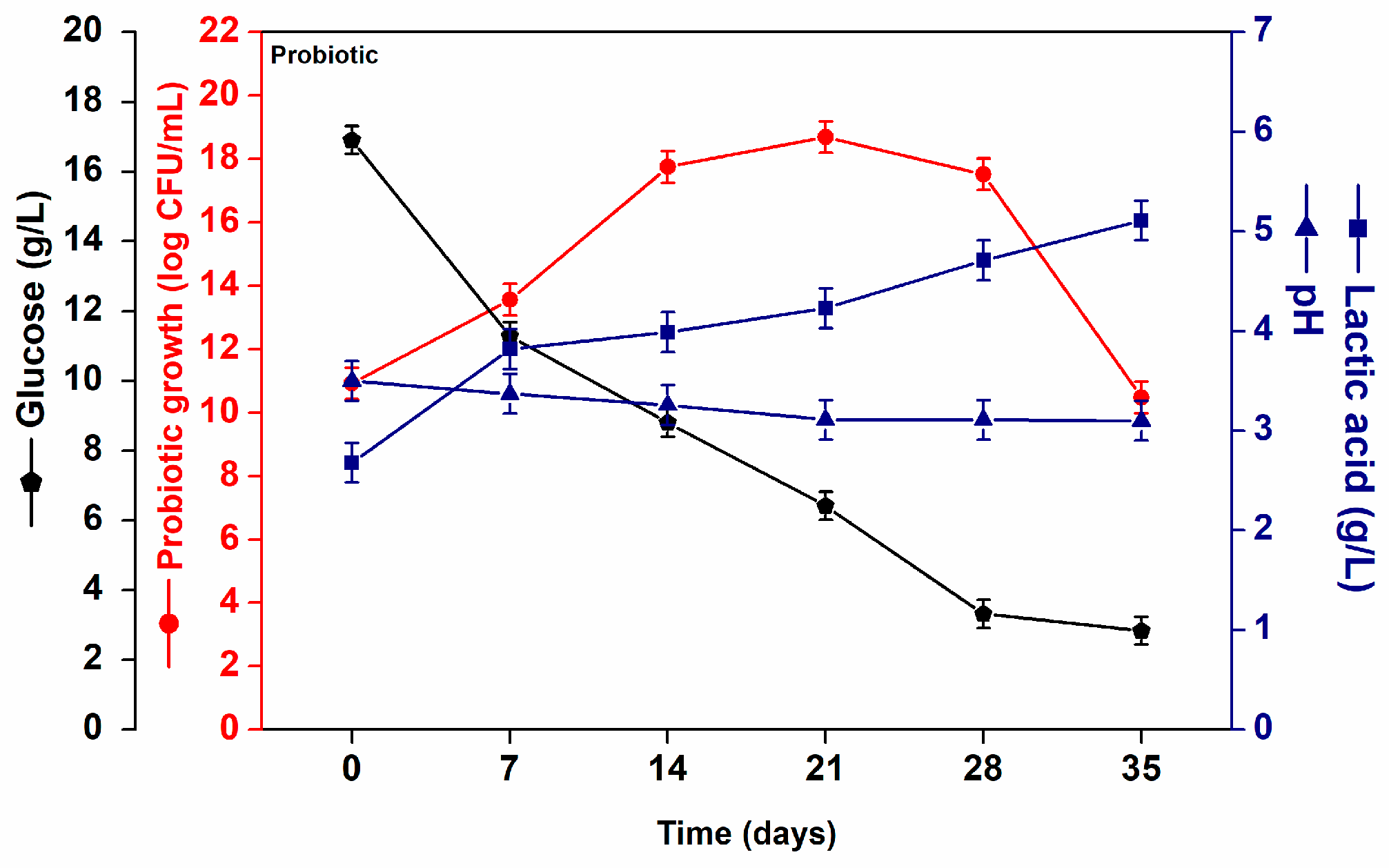
| Sample Codification | Description |
|---|---|
| NF | Natural acerola juice (non-fermented juice) |
| PRO | Probiotic acerola juice (fermented non-processed) |
| TS50 | Postbiotic acerola juice (fermented and subjected to ultrasound processing at 50 °C) |
| TS60 | Postbiotic acerola juice (fermented and subjected to ultrasound processing at 60 °C) |
| TS65 | Postbiotic acerola juice (fermented and subjected to ultrasound processing at 65 °C) |
| TH80 | Postbiotic acerola juice (fermented and subjected to heat processing at 80 °C) |
| Parameter | Acerola Fermented Juice | |
|---|---|---|
| 0 h | 8 h | |
| Phenolic Compounds (g/L) | 1.35 ± 0.1 a | 1.44 ± 0.1 a |
| Ascorbic Acid (g/L) | 1.88 ± 0.1 b | 2.40 ± 0.1 a |
| ABTS (μmol trolox/L) | 17,316 ± 261 b | 19,196 ± 272 a |
| FRAP (μg Fe2+/mL) | 3090 ± 150 a | 3170 ± 150 a |
| Parameter | Cold Storage | |
|---|---|---|
| Day 0 | Day 35 | |
| Phenolic compounds (g/L) | 1.40 ± 0.1 a | 1.17 ± 0.1 b |
| Ascorbic acid (g/L) | 2.45 ± 0.1 a | 2.55 ± 0.1 a |
| ABTS (μmol trolox/L) | 19,290 ± 272 a | 16,289 ± 261 b |
| FRAP (μg Fe2+/mL) | 3150 ± 150 a | 3250 ± 150 a |
| Parameter | TS50 | TS60 | TS65 | TH80 |
|---|---|---|---|---|
| Probiotic inactivation (%) | 63.17 a | 100 b | 100 b | 100 b |
| Yeasts and molds (CFU/mL) | 0 a | 0 a | 0 a | 0 a |
| Ascorbic acid (g/L) | 2.78 ± 0.1 a | 2.60 ± 0.1 a | 2.60 ± 0.1 a | 2.36 ± 0.1 b |
| Lactic acid (g/L) | 2.38 ± 0.1 a | 2.25 ± 0.1 a | 2.29 ± 0.1 a | 2.22 ± 0.1 a |
| Phenolic compounds (g/L) | 1.42 ± 0.05 a | 1.41 ± 0.05 a | 1.46 ± 0.05 a | 1.32 ± 0.05 b |
| ABTS (μmol trolox/L) | 16,889 ± 281 a | 16,516 ± 246 a | 16,689 ± 249 a | 13,382 ± 273 b |
| FRAP (μg Fe2+/mL) | 2900 ± 180 a | 2910 ± 160 a | 2860 ± 130 a | 2700 ± 180 b |
| Cold Storage | Parameter | TS60 | TS65 | TH80 |
|---|---|---|---|---|
| Day 0 | Probiotic inactivation (%) | 100 a | 100 a | 100 a |
| Yeasts and molds (CFU/mL) | 0 a | 0 a | 0 a | |
| Glucose (g/L) | 17.31 ± 0.5 a | 16.19 ± 0.5 a | 16.06 ± 0.5 a | |
| Lactic acid (g/L) | 2.25 ± 0.1 a | 2.29 ± 0.1 a | 2.22 ± 0.1 a | |
| Day 35 | Probiotic inactivation (%) | 100 a | 100 a | 100 a |
| Yeasts and molds (CFU/mL) | 0 a | 0 a | 0 a | |
| Glucose (g/L) | 16.95 ± 0.5 a | 15.88 ± 0.5 a | 15.95 ± 0.5 a | |
| Lactic acid (g/L) | 2.30 ± 0.1 a | 2.22 ± 0.1 a | 2.22 ± 0.1 a |
| Cold Storage | Parameter | TS60 | TS65 | TH80 |
|---|---|---|---|---|
| Day 0 | Phenolic compounds (g/L) | 1.41 ± 0.05 a | 1.46 ± 0.05 a | 1.32 ± 0.05 a |
| Ascorbic acid (g/L) | 2.62 ± 0.05 b | 2.63 ± 0.05 b | 2.36 ± 0.05 b | |
| ABTS (μmol trolox/L) | 16,516 ± 247 a | 16,689 ± 249 b | 13,382 ± 261 a | |
| FRAP (μg Fe2+/mL) | 2910 ± 129 b | 2860 ± 128 b | 2850 ± 132 b | |
| Day 35 | Phenolic compounds (g/L) | 1.30 ± 0.05 b | 1.48 ± 0.05 a | 1.34 ± 0.05 a |
| Ascorbic acid (g/L) | 2.85 ± 0.05 a | 2.89 ± 0.05 a | 2.59 ± 0.05 a | |
| ABTS (μmol trolox/L) | 16,896 ± 247 a | 17,976 ± 249 a | 13,956 ± 223 a | |
| FRAP (μg Fe2+/mL) | 3180 ± 129 a | 3200 ± 128 a | 3090 ± 132 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, C.S.d.; Santos, B.N.; Fonteles, T.V.; Rodrigues, S. From Probiotic to Postbiotic: Conversion of Acerola Juice by Thermosonication. Processes 2025, 13, 2122. https://doi.org/10.3390/pr13072122
Nascimento CSd, Santos BN, Fonteles TV, Rodrigues S. From Probiotic to Postbiotic: Conversion of Acerola Juice by Thermosonication. Processes. 2025; 13(7):2122. https://doi.org/10.3390/pr13072122
Chicago/Turabian StyleNascimento, Cristiano Silva do, Brenda Novais Santos, Thatyane Vidal Fonteles, and Sueli Rodrigues. 2025. "From Probiotic to Postbiotic: Conversion of Acerola Juice by Thermosonication" Processes 13, no. 7: 2122. https://doi.org/10.3390/pr13072122
APA StyleNascimento, C. S. d., Santos, B. N., Fonteles, T. V., & Rodrigues, S. (2025). From Probiotic to Postbiotic: Conversion of Acerola Juice by Thermosonication. Processes, 13(7), 2122. https://doi.org/10.3390/pr13072122







