Abstract
The increasing global demand for energy and the urgent need to mitigate climate change have driven the search for sustainable alternatives to fossil fuels, with biofuels emerging as a promising solution. However, the low yields and inefficiencies in biofuel production processes necessitate advanced strategies to enhance their commercial viability. Metabolic engineering has become a pivotal tool in optimizing microbial pathways to improve biofuel production, addressing these challenges through innovative genetic and synthetic biology approaches. This review highlights the role of metabolic engineering in enhancing biofuel production by focusing on microbial engineering for lignocellulosic biomass utilization, strategies to overcome inhibitor effects, and pathway optimization for biofuels like n-butanol and iso-butanol. It also explores the production of advanced biofuels from fatty acid and isoprenoid pathways, emphasizing the use of model organisms such as Escherichia coli and Saccharomyces cerevisiae. Key insights include the application of CRISPR/Cas9 and multiplex automated genome engineering for precise genetic modifications, as well as metabolic flux analysis to optimize pathway efficiency. Additionally, the review discusses synthetic biology methodologies to rewire metabolic networks and improve biofuel yields, providing a comprehensive overview of current advancements and their implications for industrial-scale production.
1. Introduction
Globally, the petrochemical industry is one of the main sources of current energy, which involves fossil fuels. The uncontrolled usages of these fossil fuels over the years led to the inequities in the carbon cycle, as the consumption of these fuels accumulated additional carbon to the atmosphere. With the mounting global population, the dependency on these fuels is in turn increasing, leading to their over-usage. This created catastrophic changes in nature, leading to global warming, and raised questions on energy security [1]. One way to handle this imminent demand of energy as well as the climatic concerns is to focus more on renewable sources of energy and develop efficient technologies to ensure maximum utilization. According to the International Energy Agency, to avoid further increases in world-wide temperatures, the carbon dioxide (CO2) emissions must be reduced. It also stated that by 2060, 70% of CO2 emission (with respect to 2017) should be reduced to control the global warming issue in future [2]. To achieve this target, along with measures to reduce the current CO2 levels in the atmosphere, there should be emphasis on sectors which are creating the CO2 emissions, like the automobile sector. Blending fossil fuels with biofuels and designing machinery that runs with biofuel alone can act as key factors for achieving this target. Consequently, these steps will eventually reduce the harmful effects of fossil fuels on the global environment. One such almost-commercialized biofuel is bio-ethanol. Nevertheless, there are few drawbacks, like that it produces less energy (30% less) and vapour pressure when compared to gasoline, its toxicity and corrosiveness, etc. Ethanol is hygroscopic and blends with gasoline before it can be used, a limiting factor for its use as an alternate fuel. Moreover, as stated above, for using these biofuel blends, automobile engines need to be modified accordingly. For instance, automobiles which use 10% and 5% ethanol blends (E10 and E5) must follow the regulations like ASTM D5798 and EN228 in North America and Europe, respectively [3]. With these drawbacks, there is a need for more dense energy sources similar to current fossil fuels, unlike low-density ethanol.
To resolve the present density, storage, and transportation issues, new biofuels like iso-butanol, n-butanol, isoprenoid, and fatty-acid-derived biofuels came into existence. These biofuels possess relatively similar energy content when compared to fossil fuels like diesel, petrol, gasoline, and jet fuels [4]. Even though many microorganisms possess inbuilt metabolic pathways to produce these compounds, for them the requirements in the system are much less and hence they produce very minimal quantities, which are insufficient to meet the commercial needs. There are several factors like metabolic flux, transcriptional regulators, inhibitors, cofactor imbalances, etc., which influence these minimal quantities. Most of these tasks can be handled by metabolic engineering approaches [3,5,6,7]. Metabolic engineering is a developing area of research which reconstructs the cellular pathways for the enhanced production of metabolites of interest. It has been applied to develop cell factories for scheming new metabolic frameworks for producing compounds that act as advanced biofuels in the required host [7]. The metabolic engineering approaches used to enhance the production of the target metabolites are heterologous gene expression, constructing orthogonal pathways, engineering co-factors, blocking the inhibitors and other competitor pathways, and overexpressing the required enzymes [8]. As there is a huge amount of omics-related data generated on hosts like Saccharomyces cerevisiae and Escherichia coli, these are most often chosen for metabolic engineering. As the genome editing approaches are established for these hosts, it becomes easy for engineering the genes associated with the target metabolites.
Apart from conventional hosts, microbes with special characteristics like high tolerance to target metabolites, high production of targeted compounds, and the capability of feeding on various substrates to produce target metabolites are also preferred [9]. With the help of novel research tools like clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 and multiplex automated genome engineering (MAGE)/eukaryotic MAGE, the genes of both conventional and unconventional hosts have become less arduous. CRISPR/Cas9 technology uses the most precise RNA of 20 nucleotide size to guide the Cas9 to the required site. Due to this, the effects of off-target engineering are reduced, making the gene engineering process easier. This approach (20-nucleotide RNA) gives it an edge over all the other existing genetic engineering techniques [10]. Few studies have already been published where CRISPR-based methods were employed for enhancing biofuels [11,12]. Due to the reduction in the sequencing costs, there has been a massive amount of data that has been generated. It gives an opportunity for scientists to study microbial systems and engineer them according to the requirements, with the help of reverse genetics [13]. To improvise and optimize the pathways, in silico methods like flux analyses and genome-scale models can be of good help.
2. Various Strategies for Enhancing the Production of Biofuel
2.1. Microbial Engineering for Enhanced Lignocellulosic Biomass Utility
Being one of the most abundant polysaccharides on the earth, cellulose can be an apt substrate for biofuel production. Most of the plant biomass is made up of lignocellulose. Lignocellulosic biomass is composed of lignin, cellulose, and hemicellulose. There are numerous cultivable and uncultivable microbes that have the capability to degrade lignocellulosic biomass and produce fermentable sugars [14]. These microbes produce lignolytic enzymes to degrade lignin, cellulases to degrade cellulose, and hemi-cellulases to degrade hemicellulose. Cellulose is a polymer of β-D glucose with β1→4 linkages. Cellulolytic enzymes can be broadly classified into three types, endoglucanses, exoglucanases, and β-glucosidases [15]. Endoglucanases hydrolyse the glycosidic linkages in an erratic manner, generating saccharides of varying size, whereas exoglucanases hydrolyse the non-reducing/reducing ends of cellulose to generate disaccharides (cellobiose) and glucose. β- glucosidases convert the cello-biose generated by exoglucanases into glucose. These three different types of cellulases hydrolyse cellulose in a collective manner to generate sugars required for the microbial growth and survival [15]. As hemicellulose is chiefly composed of sugars like xylan, mannan, and arabinose, hemi-cellulases like β-xylosidases, β-mannanases, xylanases, etc., are required for its degradation into simple sugars [16]. A humongous amount of research has been performed on lignocellulolytic microbes in the past few decades, and now, with the help of metabolic engineering, there is a viable chance to enhance their cellulolytic potential [17].
It was observed in few microbes that these enzymes, which were usually produced individually, were produced in a cluster complex called cellulosome. In cellulosomes, along with the catalytic enzymes, there are other proteins like carbohydrate-binding modules to hold the substrate [18]. For the more efficient degradation of cellulose, scientists have been designing artificial cellulosomes, too. In a study by Hu et al., the β-glucosidase and cello-biose transporter genes were over expressed in S. cerevisiae; this resulted in enhanced utilization of cello-biose followed by fermentation [19]. Kim et al. cultivated a consortium of yeast, which were cellulolytic in nature and were producing either mini CipA, a scaffoldin, or different cellulases. This engineered consortia, when supplemented with cellulose substrate, produced ethanol directly [20]. Morais et al. inferred that enhanced cello-biose degradation was achieved when enzymes were used in combination with thermophilic scaffoldin [21].
2.2. Approaches to Evade Effects of Lignocellulose-Derived Inhibitors
In the process of the pre-treatment of lignocellulosic biomass, numerous inhibitors like hydroxybenzaldehyde, furfural, acetic acid, tannic acid, hydroxymethyl furfural, etc., are formed, which hinders the cellulolysis and fermentation processes [22]. The amount of inhibitor generated directly depends on the method of pre-treatment used and the composition of biomass [23]. Hence there is a need to design proper purification method to detoxify inhibitors from the media on a regular basis or engineer the microbes to make them tolerant to these inhibitors [24]. Genetic engineering is a reliable tool to develop tolerance towards inhibitors in microbes. One of the major inhibitors produced during the pre-treatment process is furfural. It is a more potent inhibitor when compared to the others. The presence of furfural in the microbial cells instigates the reactive oxygen species levels which in turn affects mitochondria [22,25,26]. Particularly, in E. coli, furfural presence depletes the NADPH levels because of an enzyme NADPH-dependent oxidoreductase (YqhD). This drop in NADPH levels eventually affects the assimilation of sulfate, leading to growth inhibition [27]. Expression of the transhydrogenase gene (pntAB) to interconvert NADH and NADPH, and supplementing cysteine, along with YqhD gene deletion, can enhance the tolerance of E. coli towards furfural as well as hydroxymethyl furfural [27,28]. Even oxidoreductases like FucO when overexpressed can enhance the furfural tolerance in E. coli [29]. Seo et al. reported that overexpression of YqhD and FucO genes in E. coli enhanced the production of iso-butanol and even increased the glucose utilization [30]. Most of the NADPH is generated from the pentose phosphate pathway. As the presence of furfural inhibits the pentose phosphate pathway, overexpressing glucose-6-phophate dehydrogenase (which catalyzes the initial step of the pentose phosphate pathway) can enhance the furfural tolerance in E. coli [31].
In E. coli, with the help of new tools like genome mapping, novel genes like lpcA and thyA, associated with tolerance towards furfural, were discovered [32]. Numerous methods to remove inhibitors from the hydrolysate were already reported and some of those detoxification methods are liquid–solid extraction, chemical detoxification, vaporization, biological methods, liquid–liquid extraction, heating, etc. Chemical methods involve the addition of reducing agents like dithionite, sulphite, dithiothreitol, etc., and alkaline compounds like NaOH, NaBH4, Ca(OH)2, NH4OH, etc. [33,34,35]. In a study, a super critical liquid extraction strategy was employed to detoxify hydrolysate [36]. In a biological approach, enzymes like peroxidases can be employed to oxidize both non-phenolic and phenolic compounds, whereas laccases can be employed to oxidize phenolic compounds alone [37]. Apart from enzymes, microbes like Pseudomonas putida Fu1, Ureibacillus thermosphaericus, Candida guilliermondii, and Coniochaeta ligniaria can also be employed for detoxification [38,39,40]. Depending on the type of biomass and its composition, the detoxification method has to be chosen. With the increase in the number of steps, the economic viability of the production decreases [41]. The microbial potential of inhibitor tolerance and inhibitor detoxification varies depending on their habitat. It is not necessary that the microbes that have high inhibitor tolerance also have high productivity. In a study by Favaro et al., a S. cerevisiae strain isolated from a vineyard displayed resistance towards potent inhibitors like furan aldehyde and aliphatic carboxylic acids [42]. Emerging omics-related approaches, along with molecular adaptations, have the potential to enhance the microbial inhibitor resistance [43]. Processes like consolidated bioprocessing and simultaneous saccharification and fermentation can be employed so that the inhibitor’s concentration is less in the fermenter at any given time, as they are continuous processes [44,45].
2.3. Engineering Microbial Pathways to Enhance Tolerance Towards Biofuel
Microorganisms possess pathways that produce compounds that can be used for producing biofuels. As these compounds are anti-microbial in nature, microbes generally produce them in much lower amounts. So, there is a need to enhance the production of these compounds along with developing microbial tolerance towards them. This tolerance is often complex and mostly associated with stress response methods [46]. To induce biofuel tolerance in microbes, many strategies were employed. Some of them were modifying membrane proteins, expressing heat shock proteins (HSPs) or efflux pumps in host microbial cells, and activating the genes related to stress response [46,47]. When efflux pump-related operon is cloned into host cells, they enhance the tolerance by exporting the toxins out of the cells [46]. Different microbes have different efflux pumps, for instance E. coli has pumps which specifically pump out toluene, and others like acrAB-tolC pumps facilitate the pumping of toxins like octanes, heptanes, and hexane [47]. Generally, HSPs are produced to assist the proper folding of proteins along with their synthesis and transport. As a response to stress, their HSP production increases, as they help in the prevention of protein aggregate formation. In a study by Rutherford et al., it was reported that the HSP-related genes like htpG, rpoH, ibpAB, and dnaJ were observed to be affected when E. coli was exposed to butanol [48]. It was also reported that the plasma membrane composition alters when microbes are allowed to interact with solvents; this change is to prevent the entry of those solvents into the cell [46]. The membrane composition is altered by converting cis fatty acids in the membrane to trans with the help of the cis–trans isomerase enzyme. By converting cis-fatty acids to trans, the membrane fluidity changes, affecting its permeability, and in turn leading to the solvent tolerance [49].
2.4. Engineering the Plant Biomass for Biofuel Production
As discussed in the above Section 2.1, plant biomass is chiefly composed of lignin, hemicellulose, and cellulose. Cellulose fibrils are generally held together by lignin, thereby providing mechanical strength to the plant. The presence of lignin restricts the availability of cellulose for enzymatic hydrolysis. Hence, Wagner et al. engineered a plant Pinus radiata to produce less lignin by downregulating the expression of 4-coumarate-CoA ligase gene. This gene is important for synthesizing lignin precursors [50]. Similar work was also reported by Coleman et al., where p-coumaroyl-CoA 3′-hydroxylase enzyme was downregulated in a hybrid plant obtained by crossing Populus grandidentata with Populus alba with the help of the RNA interference (RNAi) approach. This downregulation resulted in a 60% drop in the lignin content of the plant [51].
3. Metabolic Engineering to Improve Bio-Butanol
3.1. n-Butanol
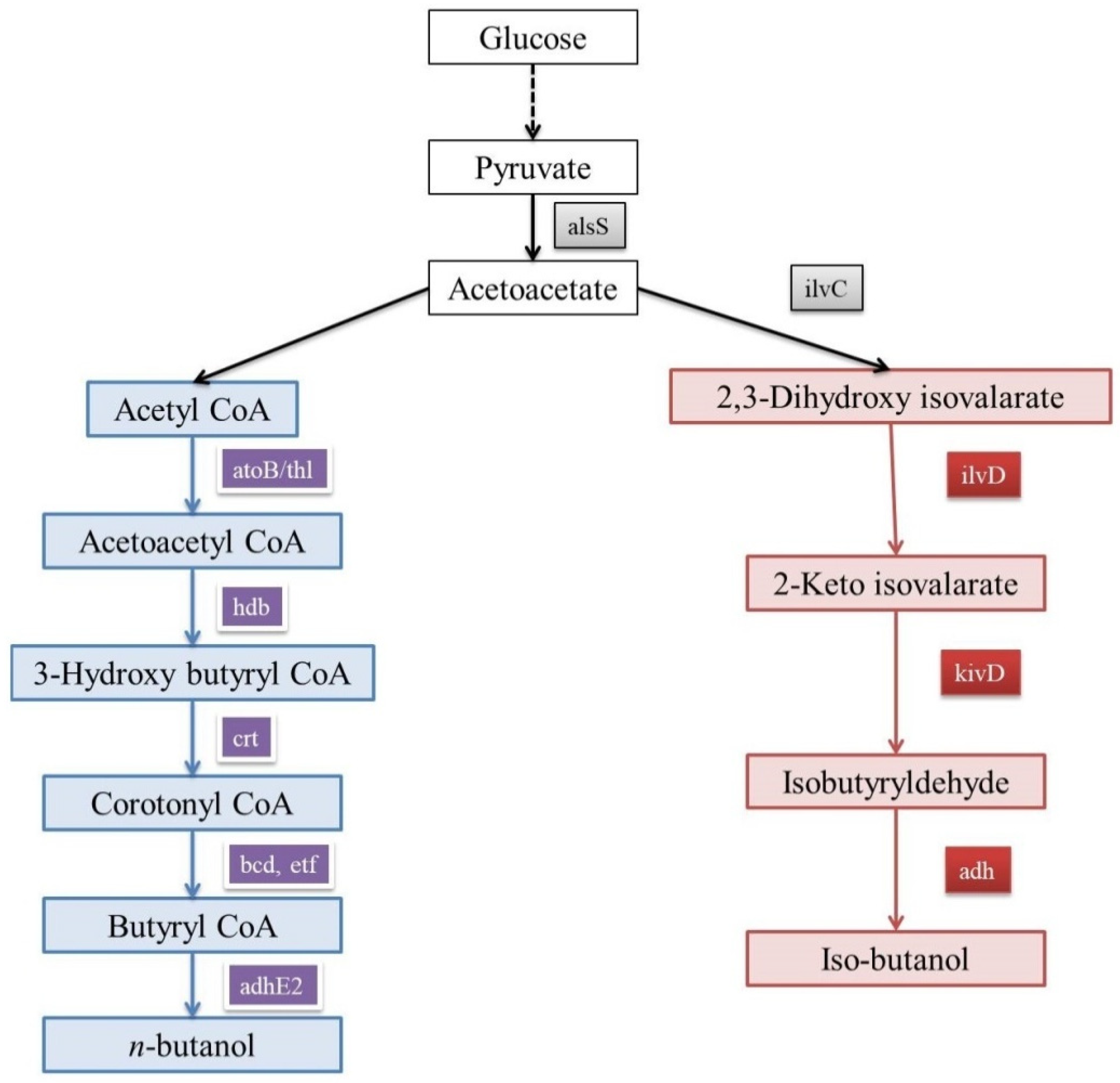
n-butanol can act as an alternative to gasoline, as the quantum of energy in them is almost same. n-butanol can also be blended with gasoline for usage, and its transportation is also easy [52]. Its precursor molecule is acetyl-coA; after a cascade of reactions, acetyl-coA is converted into butyryl-CoA before being converted to n-butanol (Figure 1). This pathway is known as the keto-acid pathway. Through acetone–butanol–ethanol fermentation, Clostridium acetobutylicum produces high amounts of n-butanol [53]. Yet the sluggish growth rate as well as sporulation-related issues make it unfit for producing n-butanol at an industrial scale [54]. Attempts were made to engineer E. coli so that it can produce n-butanol. One such attempt was reversing the β-oxidation pathway [53,55,56]. The acquired n-butanol-producing pathway in E. coli creates an inequity in NADH levels, which can be increased by introducing the trans-enoyl-CoA reductase enzyme, thereby increasing the flux for yielding high amounts of n-butanol [53,57,58]. For enhancing the n-butanol yield in E. coli, there is a need to increase NADP. By engineering the central metabolism of E. coli, flux towards the glycerol pathway was improved, and this in turn enhanced n-butanol production [57,59]. In a metabolomics study, it was reported that for enhancing n-butanol production handling, the CoA levels are vital, and it can be achieved by the overexpression go alcohol dehydrogenase (adhE2) in E. coli [60].
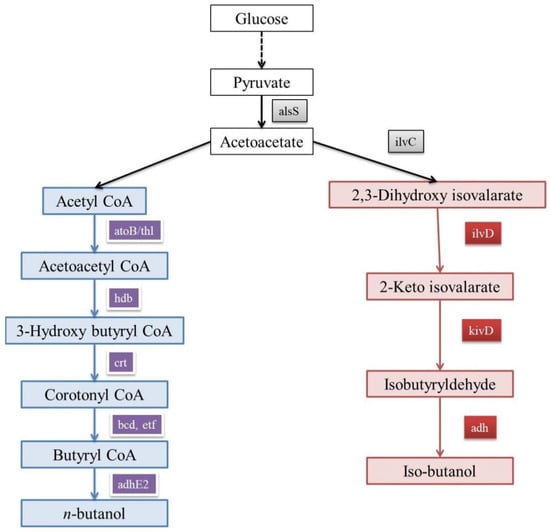
Figure 1.
Keto-acid pathway in E. coli for iso-butanol as well as n-butanol production.
There a few organisms (like S. cerevisiae) which produce less amounts of n-butanol through their innate keto-acid pathway [61]. Steen et al. have reported that the production of n-butanol in S. cerevisiae can be enhanced by introducing and overexpressing genes like crotonase, adhE2, and 3-hydroxybutyryl-CoA dehydrogenase from C. beijerinckii, along with the crotonase gene from Streptomyces collinus [52]. According to Krivoruchko et al., when the trans-enoyl-CoA reductase gene was expressed in S. cerevisiae, n-butanol production was improved [62]. In an another attempt by Schadeweg et al. to enhance n-butanol titer, alcohol dehydrogenase genes (adh1–5) were knocked out and the overexpression of the pantothenate kinase CoA gene (source—E. coli) gave encouraging results [61]. Moreover, when the NADH oxidase gene was expressed in the same strain under aerobic conditions, the n-butanol production was very much enhanced [63]. It was observed that co-culturing S. cerevisiae and C. acetobutylicum increased the n-butanol production [64]. At present, there a few companies like Green Biologics and Butamax that are producing commercial n-butanol [65]. Still, optimization for a better and more cost-effective n-butanol-producing host at the commercial level is necessary.
3.2. Iso-Butanol
Iso-butanol is one of the advanced biofuels which has gained importance recently. It is because unlike ethanol, iso-butanol is more aqueous, miscible, and less corrosive [66,67,68,69]. Like n-butanol, iso-butanol takes its origin from the keto-acid pathway (Figure 1) [70]. Enhanced iso-butanol production in E. coli was achieved when a set of new genes were overexpressed like kivD, alsS, and adh2 from Lactobacillus lactis, Bacillus subtilis, and S. cerevisiae, respectively [53]. Moreover, it was observed that high levels of iso-butanol were lethal for a host’s (E. coli) survival. So, a strain which can tolerate the elevated levels of iso-butanol was engineered by altering cAMP receptor protein. Even at high levels of iso-butanol, cells of this strain were mostly viable [71]. Recently, Noda et al. have engineered E. coli with deletions in the Embden–Meyerhof pathway, along with a few alterations in the Entner–Doudoroff pathway. These alterations have improved the production of iso-butanol in E. coli [72]. Klebsiella pneumoniae already has a pathway for iso-butanol production but in a quiescent state. Gu et al. have turned off a few genes related to the lactate pathway, leading to the activation of the iso-butanol pathway in K. pneumoniae. Apart from K. pneumoniae, Enterobacter aerogenes was also engineered for producing iso-butanol [73]. In E. aerogenes, nitrate and formate pathways (anoxic pathways) produced iso-butanol. In E. aerogenes, iso-butanol pathway-related genes (ilvD, kivD, budB, ilv C, and adhA) were expressed along with the knockout of a few genes (budA, pflB, and ldhA) to improve the yield of iso-butanol [74]. S. cerevisiae has the capability of producing lower amounts of iso-butanol as a side-effect of the Ehrlich pathway for amino acid synthesis [75,76]. S. cerevisiae was engineered by knocking out the threonine pathway [75] and overexpressing valine pathway-related genes [75,76]. Commercial iso-butanol is being produced by companies like Gevo, Butamax, and BASF SE. Iso-butanol is used only when blended with gasoline [65]. Though iso-butanol has good features, its blending with gasoline is costly. Production of iso-butanol needs to be optimized and has to be made cost-effective by advanced engineering to use it a substitute for fossil fuel.
4. Different Phases of Metabolic Engineering
There is no particular distinct classification as such, but various phases can be defined depending on altered character and the consequential biochemical result. Broadly it can be classified into five stages (Figure 2). The initial stage of metabolic engineering deals with the innate pathways in its regular host. The aim here is to enhance the production of the target metabolite only by overexpressing it and deleting the genes which hinder its production. The second phase deals with expressing a studied pathway into another relevant host strain. This has a very small effect on the total metabolic profile of the organism, but these changes will eventually enhance the targeted product by accelerating the kinetics or enhanced thermodynamic outline or usage of a highly viable co-factor, etc. For instance, in E. coli, acetoacetyl-CoA acetyltransferase (AtoB) was placed in the place of Clostridium acetoacetyl-CoA thiolase (Thl), and also a group of genes like ldhA, adhE, and frd were knocked out, thus creating a NADH flux and consequently enhancing the n-butanol production [58,77].

Figure 2.
The five stages of metabolic engineering (adapted from [77]).
These small changes, however, will not affect the main frame structure of the pathway, but will enhance the target metabolite production. The third approach is contrasting to the above approaches, where attempts are made to further construct the existing pathways. This strategy helps in creating new frontiers of metabolic engineering. With the help of this approach, a pathway is extended with a combination of enzymes which usually do not work together in nature. This integration is performed to achieve a specific metabolic work with an enhanced efficacy or sometimes a new functionality. Most of these approaches are not experimentally proven; they are merely software generated predictions. For instance, a pathway has been designed for total carbon conservation using non-oxidative glycolysis [78], the synthesis of propane from glucose [79,80], etc. A complete pathway analysis is very much required for identifying the most favourable pathways out of various routes. These comprehensive analyses efficiently relate the candidates characteristics like thermodynamic feasibility, toxicity, physiochemical properties, kinetic efficiency, hydrophobicity of the generated by-products, etc., with their metabolism [81]. The major advantage of this approach is that the pathways have novel extensions without evolutionary changes. To date, very few studies have applied these synthetic pathways with new catalytic changes. Two of these studies involve the artificial route for carbon dioxide fixation [82] and the synthetic pathway for the production of 1,4-butanediol [83]. The above-stated instances establish the potential of metabolic engineering to create new products and synthetic pathways. This strategy, if further applied, takes paces towards a fifth approach, a comprehensive “synthetic metabolism” that includes designed pathways with required products. Here, artificial pathways comprising designed enzymes are established to create a synthetic metabolism altogether.
5. Fuels from Isoprenoid and Fatty Acid Pathways
5.1. Biofuels from Fatty Acids
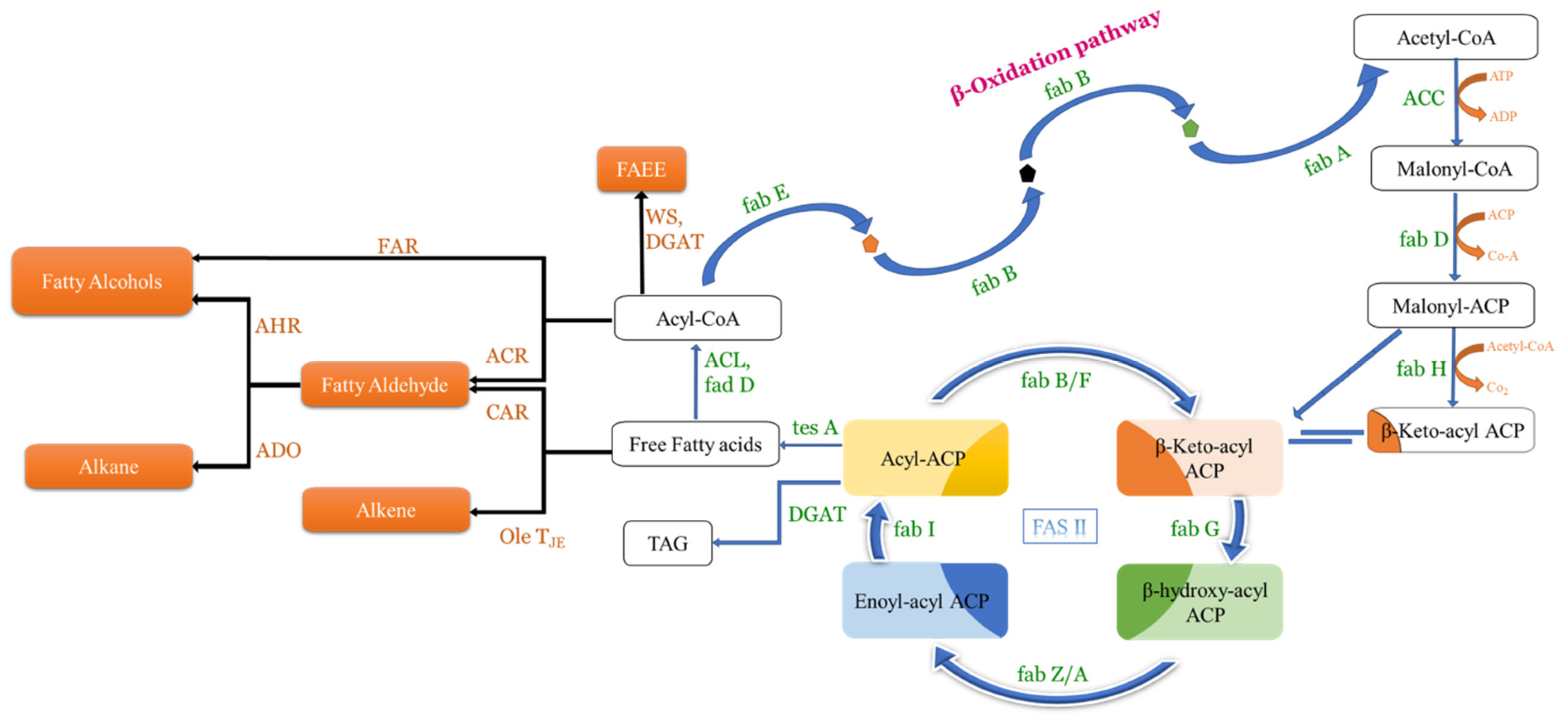
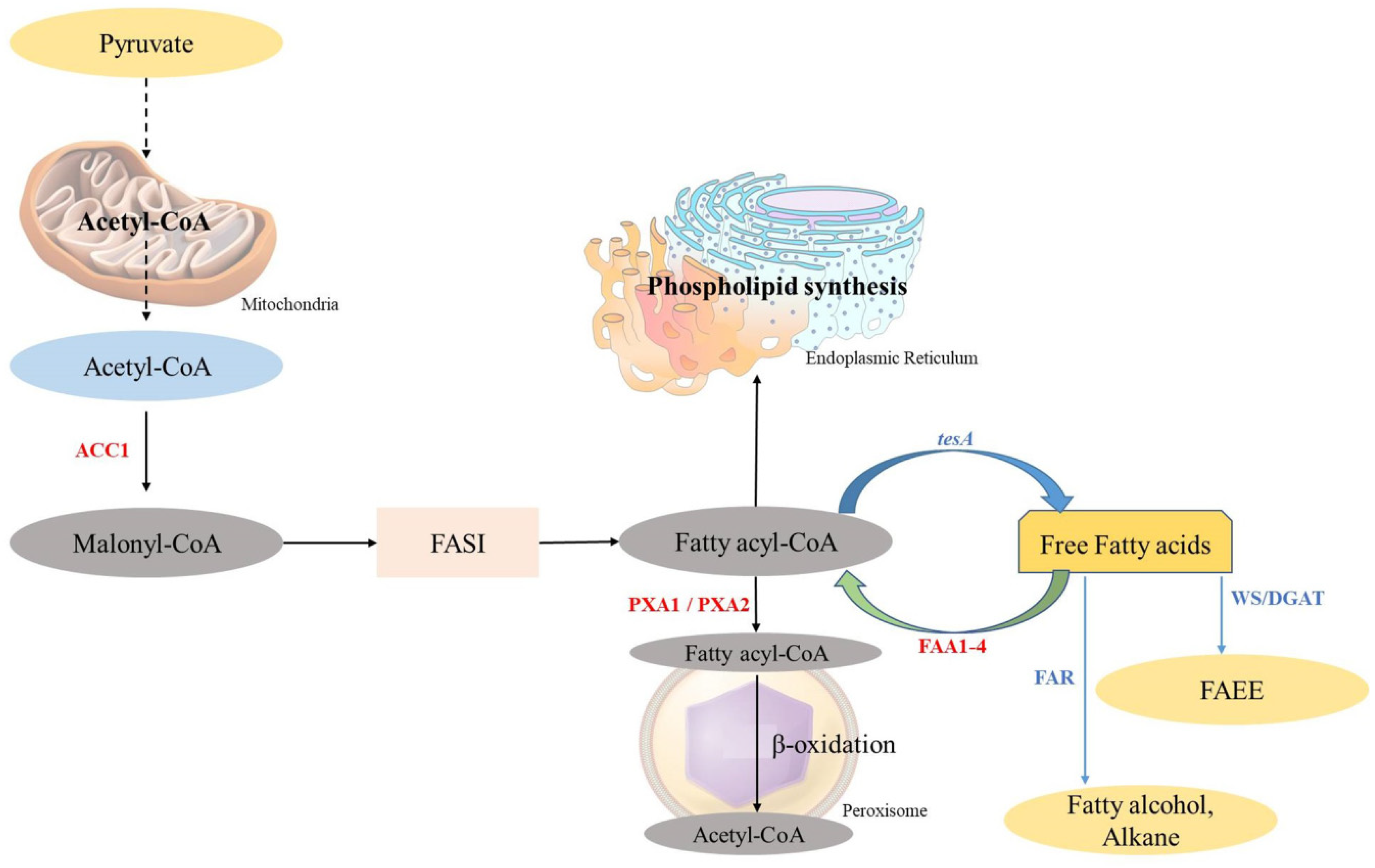
Fatty acids (FAs) are chains of hydrocarbons with a carboxylic group at one terminal and a methyl group at another terminal [65]. As the long chains of hydrocarbon are great sources of energy, they have become promising compounds for alternate fuel research [84]. Generally, two major steps take place in the biosynthesis of FAs: the first one is the ATP-dependent acetyl-CoA Carboxylase-driven conversion of acetyl-CoA to malonyl-CoA, and the second one is the integration of malonyl-CoA into the growing chain of FAs [85]. In bacteria, the same reaction is carried out through fatty acid synthase type II (FAS II) involving many enzymes like fabI, fabG, fabH, fabA/Z, and fabF/B (Figure 3). Whereas in yeast, it is carried out through fatty acid synthase type I (FAS I), a protein (2.6 MDa) which has two subunits, α-subunit and β-subunit, encoded by the genes FAS2 and FAS1, respectively (Figure 4).
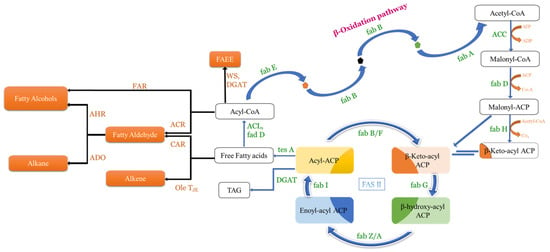
Figure 3.
Illustrative representation of β-oxidation pathways as well as fatty acid biosynthesis in E. coli. The native pathway is represented with blue arrows, whereas the heterologous pathway is represented with black arrows (adapted from [65]).
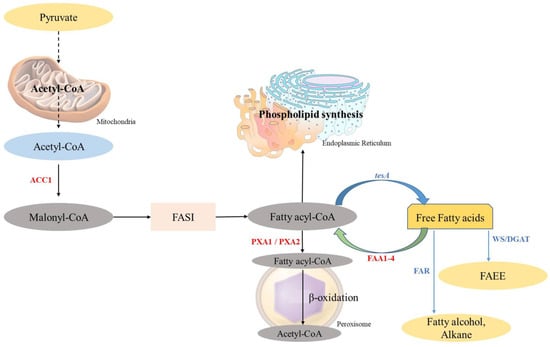
Figure 4.
Graphical representation of the fatty acid biosynthesis pathway for the production of biofuel and the β-oxidation pathway in S. cerevisiae. The native pathway is presented with black arrows and the heterologous pathway with blue arrows (adapted from [65]).
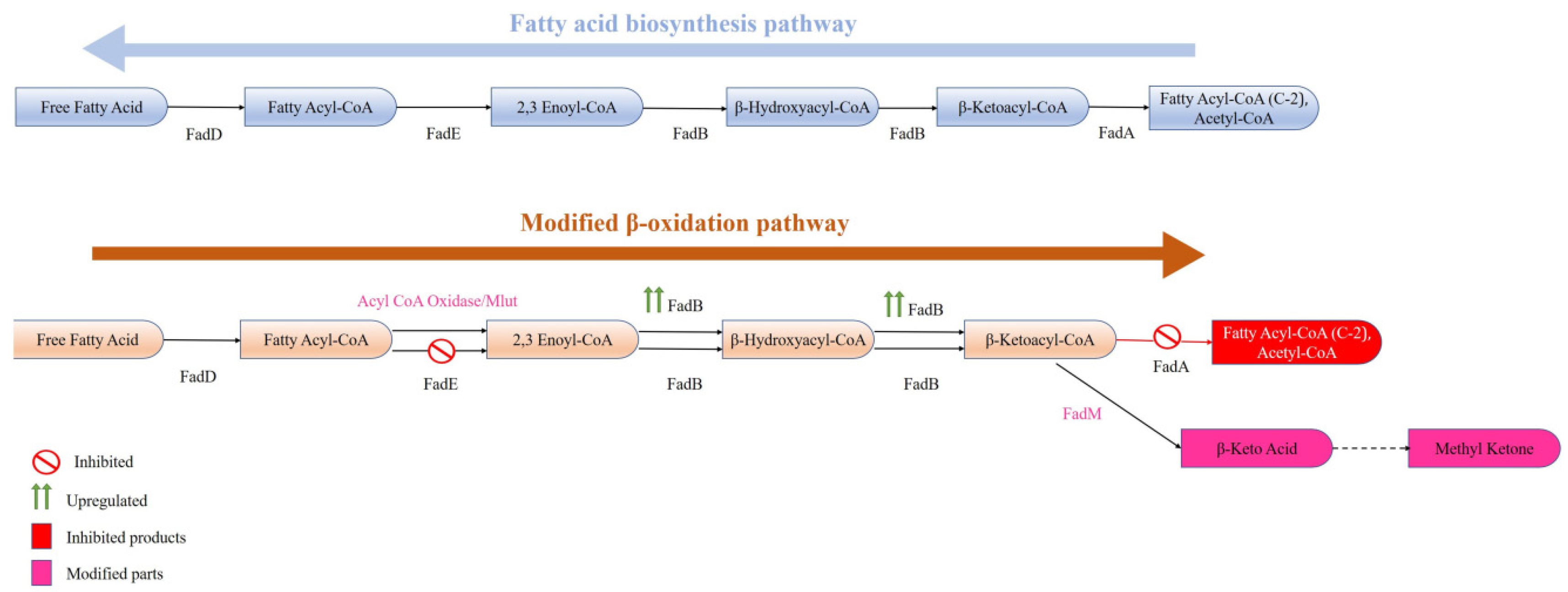
In general, FAs produced in any organism are required in low quantities and hence their enhanced production needs metabolic engineering. Furthermore, many commercially viable spinoffs (fatty alcohols-FOH, fatty acid ethyl esters-FAEE, etc.) can be produced in microbes from free fatty acids (FFAs) by introducing the required genes. By esterifying the fatty acyl product/FFA with ethanol in the presence of wax ester synthase, FAEE can be synthesized [86], and by reducing the fatty acyl product/FFA in the presence of various reductases, it can produce fatty aldehyde (Figure 3). However, by reducing FFA to fatty aldehydes and the thereafter decarbonylation of fatty aldehydes through aldehyde decarbonylase, enzyme alkenes as well as alkanes are synthesized. By modifying the β-oxidation pathway, even methyl ketones can be synthesized (Figure 5). Alkanes/alkenes as well as methyl ketones have applications in biofuel, perfume, and artificial flavour industries, whereas FOH, FFA, and FAEE are majorly involved in the detergent and biofuel industries.
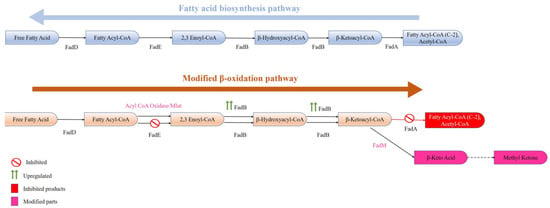
Figure 5.
An altered β-oxidation pathway in E. coli for the production of methyl ketones (adapted from [65]).
5.2. Bacterial Biofuels from Fatty Acids
Engineering bacteria to produce FFA has received attention lately due to its minimal metabolic technology and process economy. A few common approaches have been applied to enhance the fatty acid production in E. coli. For instance, malonyl-CoA and acetyl-CoA levels (precursors of fatty acids) in E. coli have been raised, and even the ACC enzyme is overexpressed to produce more FAs. Along with it, even the β-oxidation pathway was blocked by knocking out genes responsible for fadE and fadD enzymes. Steen et al. produced good amounts (1.2 g/L) of FFA in E. coli by knocking out fadE and fadD genes, along with increased expression of thiolase (‘tesA) [86]. In one study, depending on OptForce predictions, the fadD gene was knocked out and ‘tesA along with fabZ were overexpressed in E. coli, which resulted in increased (1.7 g/L) FFA production [84]. For eliminating the metabolic pathway issues, a fed-batch process was designed with an engineered E. coli strain. The strain overexpressed various genes associated with the fatty acid pathway (fabG, fabA, fabI, fabH, and ACC) and plant thioesterase enzymes (CnFatB2, BnFatA, and EgTE) along with the knockout of the fadD gene [87] (Table 1). While E. coli is one of the most ideal hosts for producing FA-related products, enhancing their production beyond a certain limit in E. coli is challenging due to the tough feedback inhibition, highly regulated transcription, and the post-transcriptional process [84]. Bacteria normally give out fatty acyl-ACP as an end product, and it has to be further hydrolysed to give FFA [86]. To overcome these drawbacks, a eukaryotic system has to be employed, and yeasts would be an interesting choice.

Table 1.
Metabolic engineering for biofuels from fatty acids (in host organisms E. coli and S. cerevisiae) (source: [65]).
5.3. Yeast Biofuels from Fatty Acids
In bacteria, FAS is encoded by different genes (approximately ten), whereas in yeasts, it is determined by only two genes which make the engineering process easy [96]. Though S. cerevisiae does not produce more FAs naturally, it has been metabolically altered to enhance the FAs production because of the ease of the engineering process. It was observed in S. cerevisiae that upregulating genes like DGTA, FAS2, ACC1, and FAS2 has enhanced the production of tri-acyl glycerol (TAG), which led to the accretion of lipid content by 17%. Further, the same strain was altered by knocking out genes like ROX, FAA1, and FAA4, and upregulating heterologous ‘tesA (E. coli) to produce higher amounts of FFA (400 mg/L) [96]. Leber et al. reported that knocking out genes FAA4, PXA1, FAA1, FAT1, and POX1, as well as upregulating lipases (that degrade TAG) and acyltransferases, produced enhanced levels of FFA (2.2 g/L) [90]. In a study, a fed-batch process was designed which employed an engineered S. cerevisiae. The engineering involved the overexpression of heterologous genes like ACP:citrate lyase (ACL), Malic enzymes (ME) from Rhodosporidium toruloides, and ‘tesA from E. coli along with knocking out HFD1, FAA4, POX1, and FAA1 genes. These alterations resulted in the enhanced production of FFa (10.4 g/L) [91]. A tabulation of a few modifications for fatty acid oleochemical production in S. cerevisiae and E. coli are presented in Table 1.
Apart from S. cerevisiae, researchers are on a quest to identify different yeasts with the capability to feed on various carbon sources (like lignocellulose), high fatty acid producing ability, and yeasts which are easy to handle at industrial scale. Yarrowia lipolytica, a yeast with enhanced flux of acetyl-CoA, is being considered as a better model for the biosynthesis of fatty acids and biofuels. In a study, genes like ACC1 and DGA1 were upregulated in Y. lipolytica, leading to enhanced lipid synthesis (−0.3 g/g) [100]. By knocking out the β-oxidation pathway as well as inserting lipoxygenase from soybean, Y. lipolytica was engineered to produce pentane (a short-chain alkane) [101]. Y. lipolytica was engineered for upregulating stearoyl-CoA desaturase, DGA1, and ACC1 genes to circumvent the highly regulated metabolism of FAs. This alteration resulted in the enhanced production of lipids (~55 g/L) [102]. A few strains of Y. lipolytica were also metabolically engineered to synthesize alkanes, FAEE, TAG, FOH, and FFA. In a study, directing different enzymes (like MmCAR, acyl-CoA/acyl-ACP-related enzymes, and AtfA) to different cellular compartments also enhanced the alkane (23.3 mg/L) and FAEE (142.5 mg/L) production. Moreover, by engineering substitute pathways for acetyl-CoA in cytosol, greater amounts of TAG (66.4 g/L) and FFA (9.67 g/L) were produced. Along with enhanced alkane, FAEE, TAG, and FFA production, the same study also reported the production of FOH (2.15 g/L). The FOH production was achieved by the expression of the heterologous genes FAR from Marinobacter aquaeolei and fadD from E. coli [103]. Consuming lignocellulosic biomass, engineered Y. lipolytica produced methyl ketones. This was achieved by localizing enzymes related to the methyl ketone pathway (bacterial) like FadM, FadB, ACO etc., in peroxisomes, and knocking out POT1 from its chromosomal DNA. This alteration has yielded about 315 mg/L of methyl ketone [104].
5.4. Biofuels from Isoprenoids
Isoprenoids are one of the most appropriate compounds naturally available for biofuel production as they have good traits that a fuel needs to have, like high energy, low freezing points, high cetane and octane numbers, etc. They are a set of various long-chained molecules and are also sometimes referred to as terpenoids [105,106]. Depending on the carbon atom number present, they are divided into sesquiterpenoids with 15 carbons (C15), monoterpenoids with 10 carbons (C10), and hemiterpenoids with 5 carbons C5. Terpenoids are synthesized by a five-carbon molecule condensation; the C5 precursor involved is isopentenyl diphosphate (IPP) along with dimethylallyl diphosphate (DMAPP; isomer of IPP). IPP and DMAPP can be synthesized through both the MEP (2-methyl-d-erythrito-4-phosphate) and MVA (mevalonate) pathways. Monoterpenes and sesquiterpenes are formed when two molecules of IPP are condensed with one molecule of DMAPP, whereas hemiterpenes are formed from DMAPP alone [105]. In general, the ratio of IPP and DMAPP is less than one, which can limit the synthesis of isoprenoids in the cells. This is because the primary reactant in the synthesis of isoprenoid is DMAPP alone [107]. For this reason, the target molecules for large-scale terpenoid microbial production are IPP and DMAPP [4].
A simple five-carbon hemiterpene that is used extensively in pesticide, rubber, and adhesive industries has been considered as an alternative fuel due to its enormous density of energy [1]. The global rubber production is about 0.012 billion tons per annum [108], and almost 95% of isoprene is used for its synthesis [109]. Though strains of S. cerevisiae, E. coli, and Synechocystis were engineered to synthesize isoprene, the insignificant production amount is insufficient for commercializing it. In Synechocystis, the underexpression of the enzyme isoprene synthase (IspS) [110] along with a reduced cellular IPP and DMAPP ratio [107] are the key limiting parameters for isoprene synthesis. Engineering Synechocystis by introducing the heterologous gene fni from Streptococcus pneumoniae and the ISPC-cpcB gene and expressing them has displayed a 62-fold enhancement in isoprene synthesis [111]. Apart from hemiterepenes, there are few monoterpenes (limonene and pinene) and sesquiterpenes (bisabolene and farnesene) that are being targeted for advanced biofuel production through the engineered microorganisms. However, monoterpenes have great industrial importance, as they are employed not only in the biofuel industry but also in the pharmaceutical, flavour, solvent and perfume industries [7,65]. In S. cerevisiae as well as E. coli, there exists a distinct monoterpene pathway, in which the substrate molecule geranyl pyrophosphate (GPP) is synthesized as the lone by-product for the sesquiterpene precursor molecule farnesyl pyrophosphate (FPP). So, there exists a competition for GPP between monoterpene synthases and sesquiterpene precursor FPP synthases (FPPS) [112].
Consequently, for enhancing the production of monoterpenes in S. cerevisiae as well as E. coli, novel approaches like downregulating FPPS and upregulating GPP synthases (GPPS) can be applied [113]. Furthermore, for addressing these issues, efforts were made to fuse a combination of three GPPS with three different pinene synthases. This resulted in an 6-fold-enhanced pinene production [114]. Terpenoids, which have wide applications in food industries, biofuels for jet planes, and personal care, are sesquiterpenes. These sesquiterpenes are formed when one molecule of DMAPP along with two IPP become condensed in the presence of farnesyl-diphosphate-isomerase to finally synthesize FPP [115,116]. Attempts were made to enhance the levels of cytosolic DMAPP along with upregulating bisabolene synthase or FPPS for the synthesis of sesquiterpenes [115,117]. Different metabolic engineering strategies performed on various microbial species for producing isoprene, pinene, limonene, farnesene, and bisabolene are listed in Table 2. In 2017, the market value for isoprenoids was about $0.51 billion, and it is predicted to escalate to $0.73 billion by 2025. The synthesis of isoprenoids has a great future, as they have the potential to produce bio-jet fuel, along with other applications in the pharmaceutical, solvent, and perfume industries. Hence, engineering the microbes metabolically to enhance the production of isoprenoids has gained importance. Already there are a few companies like Isobionics, Amyris, and Evolva that are commercially producing terpenoids from engineered yeasts and bacteria [118]. The major limiting factors for the large-scale production of terpenoids are the cytotoxicity of the terpenoids and inadequate precursor levels in the cells [114]. The challenges that the future research in this area should focus on are to enhance the metabolism of cells for enhanced terpenoid production, to balance the expression of the pathways, and to quest for an efficient promoter for enhanced terpenoid production.

Table 2.
Metabolic engineering for biofuels from isoprenoids through various microorganisms (source: [65]).
6. Metabolic Flux Analysis
To understand a system’s biology and metabolic engineering better, a complete understanding of the intricate metabolism of the cells is very much required. So, to understand the intricate cellular metabolism methods like complex metabolic prediction methods (in silico) [129,130,131,132], high-throughput omics methods [133,134] are necessary. To quantitatively estimate the intracellular metabolites involved in different pathways, methods like metabolic flux analysis (MFA) are being applied. This helps in understanding the key factors that regulate the processes like transcription as well as translation, and thereby the entire cellular metabolism [135,136].
6.1. Metabolic Pathways Analysis—A Quantitative Approach
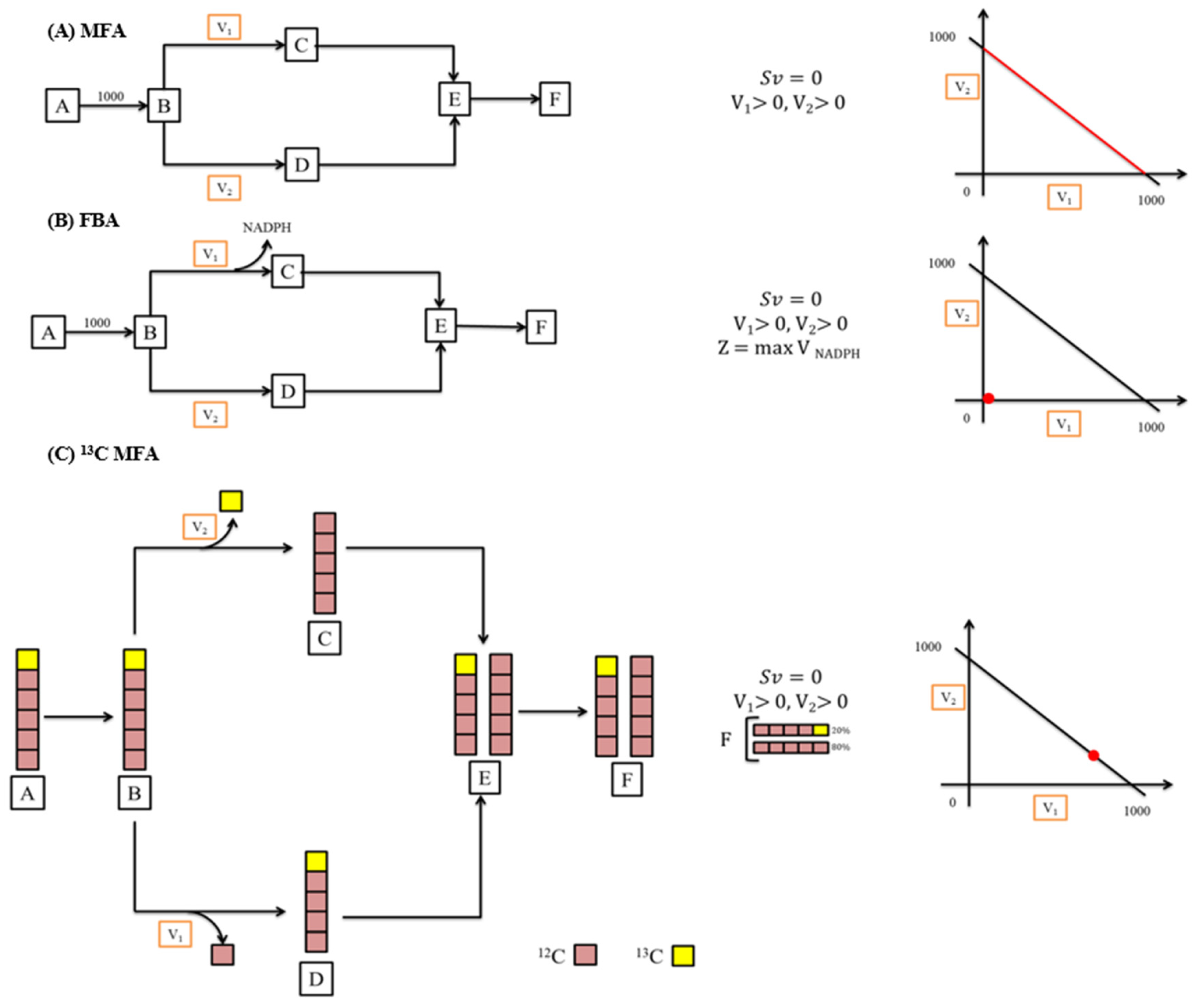
Using MFA, the intracellular metabolic fluxes of every reaction can be estimated. This is achieved with the help of elementary biochemical equations and their stoichiometric models. MFA also helps in understanding the regulatory mechanisms like the knockout or integration of genes in cells. For instance, in the MFA studies conducted on acetone/acid synthesizing and the wild type of Clostridium acetobutylicum strains by Desai et al., they revealed the quantities and the crucial roles played by pathways that form acid in their metabolisms [137]. Furthermore, MFA not only helps in understanding the regulatory mechanisms of a pathway and evidences regarding the crucial reactions, but it also suggests a possible way to enhance the yield with the help of approaches like metabolic engineering. MFA studies were more useful in understanding the roles of pivotal reactions involved in the metabolic pathways of C. acetobutylicum to identify targets that can produce butanol from sugars like xylose, glucose, etc., obtained from hydrolysed lignocellulosic biomass [138]. There are different kinds of MFA methods like (i) 13C MFA, (ii) flux balance analysis (FBA), and (iii) classical/standard stoichiometric MFA (Figure 6).
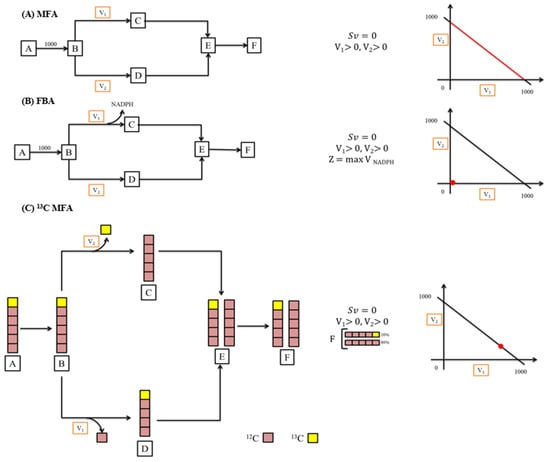
Figure 6.
Different MFA approaches. (A) Standard stoichiometric MFA, (B) flux balance analysis (FBA), (C) 13C-MFA.
In the case of regular parallel pathways, to estimate the branch fluxes (v2 and v1), regular MFA can be applied (Figure 6). Suppose v1 also produces NADPH, then the branch fluxes are estimated through FBA by the application of the objective function for maximizing the production of NADPH (Figure 6). Meanwhile, if v1 and v2 remove one C-atom from a particular position in B, then the branch fluxes can be calculated by 13C MFA. This can be performed with the help of the radiolabelling of substrate A and estimating the patterns of labelling in E or F.
6.2. Flux Balance Analysis
FBA is basically a constraint-based optimization method that is used for simulating different kinds of possible rete of reactions [139,140]. This approach initiates with the creation of a stoichiometric matrix S that has all the existing information related to the stoichiometry of the metabolic network. At first, the network is expected to be in a quasi-steady state, i.e., S.v = 0. Here v represents the fluxes over every reaction. By the application of lower and upper limits, the directionality and capacity necessities of the reactions are defined. Generally, in a constraint-based model with various resolutions regarding the distributions of flux, the system is underdetermined. The enhancement of biomass production is defined by an optimized objective function. A standard FBA design is categorized by various equivalents and provides solutions that are optimally non-unique to the problem. It also enhances particular objective functions related to its stoichiometric limitation with required lower and upper limits of the system fluxes. FBA attempts to maximize or minimize the objective function Z = cTv, which could be any linear mix of fluxes, where c denotes the vector of weights and recommends the contribution of each reaction to the total objective function. Hence, FBA is the method of elucidating the equation S.v = 0 with the help of linear programming. Here a linear combination of fluxes has been established as the objective function and the lower as well as upper limits of v have been provided. The output of FBA signifies a certain flux distribution (vj), which maximizes or minimizes the objective function [141].
The mass balance limitations as well as the objective function are applied in this approach, and here the biomass maximization or yield of ATP is primarily regarded as the objective function. Meanwhile, it is possible to estimate the interested compound’s maximum yield by considering the maximization of the target production rate as the objective function. The FBA method does not analyze any single tiny pathway; rather, it focuses on the total metabolic network across the genome, like central carbon metabolism. This approach has the capability of analyzing the pathway data even in the absence of enzyme kinetics information. FBA is one of the more powerful methods to solve the underdetermined systems, but still there are a few setbacks. One of the major setbacks is the identification of an appropriate objective function, and it is a cautious concern. In the prediction of one gene-deleted mutant, it is uncertain how to choose a single objective function for enhancing the biomass yield. To resolve these issues of designing gene-deletion mutants, advanced FBA methods like Regulatory on/off Minimization [142] as well as Minimization of Metabolic Adjustment [142] are being used. The predictions as well as flux determination are less accurate, as all of the approaches are dependent on assumptions only. Hence, to obtain highly reliable fluxes, 13C labelling approaches have been employed [143].
6.3. 13C-Metabolic Flux Analysis
13C-MFA manages the intricate metabolic networks involving reversible, parallel, and cyclic biochemical reactions. It is being used widely for understanding the metabolic traits of OptKnock, dependent knockout strains [83], confirming the activity for different pathways [144,145] and for identifying the metabolic blockages [146]. This approach uses 13C to label metabolites and thereby provides the essential information about the metabolism of the labelled source in any metabolic pathway [147,148,149]. This approach of labelling metabolites can even determine the distribution of flux across the different metabolic reactions. These experiments are performed under steady-state conditions and for continuous cultures only. In the experimental setup, only the medium is supplemented with a 13C labelled substrate at a particular position. Generally, for understanding the central carbon metabolism, a mix of [1–13C] and [U-13C] glucose is employed. As the proteins with 13C-labelled amino acids are used for estimating the flux in a particular biochemical reaction, the cellular metabolism needs to be carefully monitored till the protein acquires the 13C label. Usually 13C MFA is a four step process: (a) discerning the required analytical pathway and labelling the particular substrate, (b) performing a 13C-labelled experiment under steady-state circumstances and further label the pattern measurement of proteins with labelled amino acids using GC-MS/NMR [150], (c) formulating a stoichiometric model and, depending on the previously resolved network, preparing intracellular metabolite iso-topomer balance equations, and (d) determining the label patterns of proteins with labelled amino acids and computing them from the predicted fluxes for optimizing the distribution of flux.
In MFA, to analyze the isotope labelling pattern, the iso-topomer approach is mostly preferred [151]. In recent times, scientists have developed an open-source library, JBEI Quantitative Metabolic Modeling, to guide the design and execution of experiments related to metabolic modelling as well as flux analysis. It permits the users to determine the cellular fluxes by converting metabolomics information to isotope-labelled information from a 13C-labelled experiment. This data is very helpful to develop different genetic engineering approaches for metabolically engineering the cell [152]. Depending on various factors like metabolism and pace of growth., E. coli for prokaryotic studies and S. cerevisiae for eukaryotic studies are most preferred, and extensively engineered model organisms are preferred for the production of different biofuels. In an E. coli strain that has high fatty acid yields, 13C MFA was performed to analyze its metabolism, where the gene fadE was knocked out, and ‘tesA as well as fadR genes were highly upregulated. When the obtained results were compared with the wild-type strain of E. coli, it was observed that the metabolic flux was rerouted to the biosynthesis of the fatty acid pathway from the actual acetate pathway. These results recommended that the increase in the fatty acid precursor molecules, NADPH and acetyl-CoA, considerably diverted the fluxes towards the required pathway [153]. The fatty alcohol content and production were enhanced by employing innovative methods that involve the estimation of protein levels as well as metabolic flux quantitatively, engineering at the enzyme level, cofactor balancing, etc.; adapting these approaches yielded good amounts of fatty alcohols (1.2 g/L) in a shake flask as well as in fed batch (6.0 g/L) studies [11].
7. Synthetic Biology Methodologies to Enhance Biofuel Yields
For enhancing biofuel production, it is essential to have elevated levels of biofuel precursor molecules like malonyl-CoA, mevalonate, and acetyl-CoA. To reorganize the intricate metabolic reactions, there are genetic engineering tools like cloning new genes and pathways into different hosts, upregulating the required genes and downregulating the inhibiting gene products, or at times even knocking out the genes of competing pathways [8]. Few of many chief techniques and tools which are helpful in engineering the pathways for enhanced biofuel production are given below.
7.1. Multiplex Automated Genome Engineering
MAGE is an approach with which many loci in the same genome can be targeted at the same time to give out a combined genetic diversity by engineering a single cell or multiple cells. This approach is usually performed in prokaryotic cells. In a study by Wang et al., mutations were introduced into the binding sites of ribosomes for as many as 20 genes associated with the DXP pathway to enhance the lycopene production in E. coli [154]. Further, advanced MAGE was developed for introducing synthetic oligonucleotides to mutate the genome in S. cerevisiae. This advanced MAGE was termed eukaryotic MAGE. This technique has an edge over other gene editing tools, as it is independent of homologous recombination and does not even cleave the double stranded DNA. This approach employs oligonucleotides with synthetic mutations which bind near the DNA replication fork precisely at the lagging strand to create a gene manipulation with about 40% efficiency. This approaches was adapted by Barbieri et al. to alter the β-carotene levels in S. cerevisiae [155].
7.2. Clustered Regularly Interspaced Short Palindromic Repeats/Cas9
This is an advanced, adaptable molecular tool that has gained importance in recent times for its efficient and rapid engineering of genomes related to different organisms. This technique employs a Cas9 endonuclease to cleave the target site, and the target site is accurately reached with the help of a guide RNA that has −20 nucleotides attached to Cas9. Ever since the inception of this approach, it has been used in various organisms and for numerous applications [156,157]. Genome engineering of the host can be performed by the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9-mediated cleavage of the double strand, either with homology-directed repair or with non-homologous end joining. Abdelaal et al. and Li et al. have used the CRISPR/Cas9 method for engineering E. coli to adjust the production of n-butanol and β-carotene, respectively, in the fed-batch fermentation [158,159]. Zhang et al. have used the same approach but in a multiplexed fashion to engineer Clostridium tyrobutyricum in batch fermentation for regulating the n-butanol pathway, yielding huge n-butanol produce (26.2 g/L) [160]. Likewise in S. cerevisiae, this approach was used to knockout the contending pathway genes and introduce heterologous genes in required loci to enhance the production of fatty alcohols [11]. Not just a single locus, but multiple loci can be targeted with different genes to be inserted or deleted (multiplex CRISPR/Cas9) using this approach [161,162]. In a study by Jakocinas et al., CRISPR/Cas9 was multiplexed in S. cerevisiae with five targets, and it ended up with a 42-fold increase in its production of mevalonate. Similarly, Shi et al. have used CRISPR/Cas9 multiplexing in yeast genome to introduce 2,3-butandiol as well as xylose consumption pathways, resulting in the utilization of xylose and production of 2,3-butandiol [162]. 2-phenylethanol is an aromatic compound that is widely used in personal care products, foods, and beverages. It can be blended with gasoline and can be used as a biofuel additive. It was reported by Li et al. that engineering Ehrlich as well as Shikimate pathways using the CRISPR-mediated multigene integration system in Kluyveromyces marxianus improved stress tolerance and 2-phenylethanol production via the Shikimate pathway [163].
The rewiring leads to the creation of assorted pathways in the host that in turn create a new network of metabolites. This produces desired characters by streaming the cellular resources towards the target production rather than the cell’s growth. This approach has been effectively applied to produce many biofuel precursor compounds like isoprene [164], acetone [165], fatty acids [166], 1-butanol [167], ethylene [168], and 2-methyl-1-butanol [169]. All the above-mentioned studies depict the importance of artificial metabolic pathways for achieving the enhancement of biofuel production. Recently computational resources and algorithms have also been developed like the Pathway Prediction System [170], Retro-Biosynthesis Tool [171], and Biochemical Network Integrated Computational Explorer algorithm [172,173].
7.3. RNA Interference Approach for Controlling Transcription and CRISPRi/CRISPRa
Controlling transcription is also an alternative effective method involved in genetic engineering, which is most frequently applied in metabolic engineering as well. RNAi as well as CRISPR interference (CRISPRi) are the approaches that are employed for precise gene knockouts [174,175]. The RNAi approach is a naturally occurring transcription controlling mechanism observed in most of the eukaryotes. Recently, it has been used for the heterologous expression of argonaute as well as dicer in S. cerevisiae. This is further optimized to be used as an important tool for metabolically engineering the organisms. In S. cerevisiae, a test for putative gene targets was performed using the synthetic RNAi approach to enhance the production of itaconic acid [176], along with tolerance towards acetic acid [175]. CRISPRi, as the name suggests is a spin-off from the CRISPR/Cas9 approach; here, the nuclease coupled with the guiding RNA is absent (dCas). This dCas has the potential to physically obstruct the initiation and elongation steps of transcription [174]. In E. coli, this interference approach has also been applied to increase the production of flavonoids by adjusting the control networks related to crucial metabolic pathways like the TCA cycle and fatty acid biosynthesis [177]. Furthermore, CRISPR protein that is deficient of nuclease is coupled with an activator domain and can be used to activate the required gene (CRISPRa) [178]. By fusing dCas9 with RNA polymerase’s ω subunit as well as VP64 activator, the CRISPRa approach was successfully applied to activate the reported genes in the two hosts S. cerevisiae [178] and E. coli [179]. The above discussed approaches can upregulate the required genes and even downregulate the unnecessary genes for enhancing the production of biofuel and its related compounds.
7.4. Metabolic Reorganization at Genome Level
This is a bioinformatic approach that makes use of biochemical models to envisage the microbial response to abiotic as well as genetic stress. It is gaining importance, as it saves finances as well as time, and it is a substitute for in vitro lab tests. Metabolic engineering has shed light on the production of many novel molecules with immense commercial impact, and molecules that are custom designed also. Through genome-level reorganization, chemical conversions that take place in the cells and their associated pathways as well as genetic networks are precisely predicted [180,181,182]. The prominence of these in silico tools to predict the metabolic potential of different microalgae was brought into light by the genome-level metabolic reorganization of Nannochloropsis salina and Nannochloropsis gaditana [183,184]. Recent developments in bioinformatics and genetics have paved the way for the manipulation of microalgae-targeting enhancements in lipid engineering, photo utilization, and carbon flow management [185]. The available data about the biochemical pathways and the genome of the organisms helped in creating genome-scale metabolic models (GEMs). GEMs give a detailed insight into the pathways, associated metabolic reactions, and relations among genes and protein reactions. In the case of a novel species, GEMs need omics data as well as complete biochemical information. A GEM was constructed by Gomes de Oliveira Dal’Molin et al. depending on the genetic data of C. reinhardtii and it was entitled as AlgoGEM [186]. It is a comprehensive literature-dependent GEM which has the data pertaining to 1862 metabolites, 1725 exclusive reactions, 866 unique open reading frames, and about 2249 genes and protein reactions. Using the above discussed advanced information and tools, it is not impossible to reorganize/reconstruct the genomes for enhancing biofuel production [173].
8. Conclusive Remarks
Metabolic engineering has emerged as a transformative discipline in the sustainable production of biofuels, offering precise and efficient strategies to reprogram microbial and plant metabolic pathways. This review consolidates the significant advances made in utilizing metabolic engineering to overcome critical challenges in biofuel production, such as low yields, inhibitor toxicity, and substrate limitations. The integration of cutting-edge technologies—CRISPR/Cas9, multiplex genome engineering, synthetic biology, and metabolic flux analysis—has demonstrated the capacity to fine-tune cellular metabolism with unprecedented precision, thereby enhancing the production of advanced biofuels like n-butanol, iso-butanol, fatty acid derivatives, and isoprenoids.
Looking ahead, the convergence of systems biology, computational modelling, and omics data promises to accelerate the development of robust microbial platforms tailored for industrial-scale biofuel production. With increasing global emphasis on reducing carbon emissions and transitioning to renewable energy, the advancements in metabolic engineering not only pave the way for more sustainable fuel alternatives but also foster innovation in allied sectors such as pharmaceuticals, agriculture, and bioplastics. Continued interdisciplinary research and strategic policy support will be crucial in translating these scientific breakthroughs into commercially viable solutions that can redefine our energy future.
Author Contributions
Conceptualization, N.P.P.P.; visualization, N.P.P.P.; writing—original draft preparation, N.P.P.P. and S.P.; writing—review and editing, N.P.P.P., A.V. and P.K.G.; supervision, R.R.B.; project administration, R.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Authors thank the Department of Biotechnology, National Institute of Technology, Warangal (Telangana, India) for providing the support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rabinovitch-Deere, C.A.; Oliver, J.W.K.; Rodriguez, G.M.; Atsumi, S. Synthetic Biology and Metabolic Engineering Approaches To Produce Biofuels. Chem. Rev. 2013, 113, 4611–4632. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency. Energy Technology Perspectives 2020. Energy Technol. Perspect. 2020, 2020, 1–400. [Google Scholar] [CrossRef]
- Luque, R.; Herrero-Davila, L.; Campelo, J.M.; Clark, J.H.; Hidalgo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Biofuels: A Technological Perspective. Energy Environ. Sci. 2008, 1, 542–564. [Google Scholar] [CrossRef]
- Zhang, F.; Rodriguez, S.; Keasling, J.D. Metabolic Engineering of Microbial Pathways for Advanced Biofuels Production. Curr. Opin. Biotechnol. 2011, 22, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of Microbial Secondary Metabolites: Regulation by the Carbon Source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef]
- Subtil, T.; Boles, E. Competition between Pentoses and Glucose during Uptake and Catabolism in Recombinant Saccharomyces cerevisiae. Biotechnol. Biofuels 2012, 5, 14. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Zhang, F.; del Cardayre, S.B.; Keasling, J.D. Microbial Engineering for the Production of Advanced Biofuels. Nature 2012, 488, 320–328. [Google Scholar] [CrossRef]
- Lian, J.; Mishra, S.; Zhao, H. Recent Advances in Metabolic Engineering of Saccharomyces cerevisiae: New Tools and Their Applications. Metab. Eng. 2018, 50, 85–108. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, S.-J.; Lee, D.-W. Design and Development of Synthetic Microbial Platform Cells for Bioenergy. Front. Microbiol. 2013, 4, 92. [Google Scholar] [CrossRef]
- Chandrasegaran, S.; Carroll, D. Origins of Programmable Nucleases for Genome Engineering. J. Mol. Biol. 2016, 428, 963–989. [Google Scholar] [CrossRef]
- d’Espaux, L.; Ghosh, A.; Runguphan, W.; Wehrs, M.; Xu, F.; Konzock, O.; Dev, I.; Nhan, M.; Gin, J.; Reider Apel, A.; et al. Engineering High-Level Production of Fatty Alcohols by Saccharomyces cerevisiae from Lignocellulosic Feedstocks. Metab. Eng. 2017, 42, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.-X.; He, X.; Wu, Y.-Q.; Liu, J.-Z. Enhancing Production of Pinene in Escherichia coli by Using a Combination of Tolerance, Evolution, and Modular Co-Culture Engineering. Front. Microbiol. 2018, 9, 1623. [Google Scholar] [CrossRef] [PubMed]
- Esvelt, K.M.; Wang, H.H. Genome-Scale Engineering for Systems and Synthetic Biology. Mol. Syst. Biol. 2013, 9, 641. [Google Scholar] [CrossRef]
- Pabbathi, N.P.P.; Velidandi, A.; Tavarna, T.; Gupta, S.; Raj, R.S.; Gandam, P.K.; Baadhe, R.R. Role of Metagenomics in Prospecting Novel Endoglucanases, Accentuating Functional Metagenomics Approach in Second-Generation Biofuel Production: A Review. Biomass Convers. Biorefinery 2023, 13, 1371–1398. [Google Scholar] [CrossRef] [PubMed]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef]
- Collins, T.; Gerday, C.; Feller, G. Xylanases, Xylanase Families and Extremophilic Xylanases. FEMS Microbiol. Rev. 2005, 29, 3–23. [Google Scholar] [CrossRef]
- Kurosawa, K.; Wewetzer, S.J.; Sinskey, A.J. Triacylglycerol Production from Corn Stover Using a Xylose-Fermenting Rhodococcus opacus Strain for Lignocellulosic Biofuels. J. Microb. Biochem. Technol. 2014, 6, 254–259. [Google Scholar] [CrossRef]
- Mazzoli, R.; Lamberti, C.; Pessione, E. Engineering New Metabolic Capabilities in Bacteria: Lessons from Recombinant Cellulolytic Strategies. Trends Biotechnol. 2012, 30, 111–119. [Google Scholar] [CrossRef]
- Hu, M.-L.; Zha, J.; He, L.-W.; Lv, Y.-J.; Shen, M.-H.; Zhong, C.; Li, B.-Z.; Yuan, Y.-J. Enhanced Bioconversion of Cellobiose by Industrial Saccharomyces cerevisiae Used for Cellulose Utilization. Front. Microbiol. 2016, 7, 241. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.-H.; Lee, K.; Hahn, J.-S. Cellulosic Ethanol Production Using a Yeast Consortium Displaying a Minicellulosome and β-Glucosidase. Microb. Cell Fact. 2013, 12, 14. [Google Scholar] [CrossRef]
- Moraïs, S.; Stern, J.; Kahn, A.; Galanopoulou, A.P.; Yoav, S.; Shamshoum, M.; Smith, M.A.; Hatzinikolaou, D.G.; Arnold, F.H.; Bayer, E.A. Enhancement of Cellulosome-Mediated Deconstruction of Cellulose by Improving Enzyme Thermostability. Biotechnol. Biofuels 2016, 9, 164. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Lee, B.-R.; Sathiyanarayanan, G.; Song, H.-S.; Kim, J.; Jeon, J.-M.; Kim, J.-H.; Park, S.-H.; Yu, J.-H.; Park, K.; et al. Medium Engineering for Enhanced Production of Undecylprodigiosin Antibiotic in Streptomyces coelicolor Using Oil Palm Biomass Hydrolysate as a Carbon Source. Bioresour. Technol. 2016, 217, 141–149. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of Lignocellulose: Inhibitors and Detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-S.; Jeon, J.-M.; Kim, H.-J.; Bhatia, S.K.; Sathiyanarayanan, G.; Kim, J.; Won Hong, J.; Gi Hong, Y.; Young Choi, K.; Kim, Y.-G.; et al. Increase in Furfural Tolerance by Combinatorial Overexpression of NAD Salvage Pathway Enzymes in Engineered Isobutanol-Producing E. coli. Bioresour. Technol. 2017, 245, 1430–1435. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Lee, B.-R.; Sathiyanarayanan, G.; Song, H.S.; Kim, J.; Jeon, J.-M.; Yoon, J.-J.; Ahn, J.; Park, K.; Yang, Y.-H. Biomass-Derived Molecules Modulate the Behavior of Streptomyces Coelicolor for Antibiotic Production. 3 Biotech 2016, 6, 223. [Google Scholar] [CrossRef]
- Allen, S.A.; Clark, W.; McCaffery, J.M.; Cai, Z.; Lanctot, A.; Slininger, P.J.; Liu, Z.L.; Gorsich, S.W. Furfural Induces Reactive Oxygen Species Accumulation and Cellular Damage in Saccharomyces Cerevisiae. Biotechnol. Biofuels 2010, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.N.; Jarboe, L.R.; Turner, P.C.; Pharkya, P.; Yomano, L.P.; York, S.W.; Nunn, D.; Shanmugam, K.T.; Ingram, L.O. Furfural Inhibits Growth by Limiting Sulfur Assimilation in Ethanologenic Escherichia coli Strain LY180. Appl. Environ. Microbiol. 2009, 75, 6132–6141. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yomano, L.P.; Lee, J.Y.; York, S.W.; Zheng, H.; Mullinnix, M.T.; Shanmugam, K.T.; Ingram, L.O. Engineering Furfural Tolerance in Escherichia coli Improves the Fermentation of Lignocellulosic Sugars into Renewable Chemicals. Proc. Natl. Acad. Sci. USA 2013, 110, 4021–4026. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Yomano, L.P.; Geddes, R.D.; Shanmugam, K.T.; Ingram, L.O. Improving Escherichia coli FucO for Furfural Tolerance by Saturation Mutagenesis of Individual Amino Acid Positions. Appl. Environ. Microbiol. 2013, 79, 3202–3208. [Google Scholar] [CrossRef]
- Seo, H.-M.; Jeon, J.-M.; Lee, J.H.; Song, H.-S.; Joo, H.-B.; Park, S.-H.; Choi, K.-Y.; Kim, Y.H.; Park, K.; Ahn, J.; et al. Combinatorial Application of Two Aldehyde Oxidoreductases on Isobutanol Production in the Presence of Furfural. J. Ind. Microbiol. Biotechnol. 2016, 43, 37–44. [Google Scholar] [CrossRef]
- Gorsich, S.W.; Dien, B.S.; Nichols, N.N.; Slininger, P.J.; Liu, Z.L.; Skory, C.D. Tolerance to Furfural-Induced Stress Is Associated with Pentose Phosphate Pathway Genes ZWF1, GND1, RPE1, and TKL1 in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2006, 71, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Glebes, T.Y.; Sandoval, N.R.; Reeder, P.J.; Schilling, K.D.; Zhang, M.; Gill, R.T. Genome-Wide Mapping of Furfural Tolerance Genes in Escherichia coli. PLoS ONE 2014, 9, e87540. [Google Scholar] [CrossRef] [PubMed]
- Cavka, A.; Jönsson, L.J. Detoxification of Lignocellulosic Hydrolysates Using Sodium Borohydride. Bioresour. Technol. 2013, 136, 368–376. [Google Scholar] [CrossRef]
- Guo, X.; Cavka, A.; Jönsson, L.J.; Hong, F. Comparison of Methods for Detoxification of Spruce Hydrolysate for Bacterial Cellulose Production. Microb. Cell Fact. 2013, 12, 93. [Google Scholar] [CrossRef]
- Alriksson, B.; Cavka, A.; Jönsson, L.J. Improving the Fermentability of Enzymatic Hydrolysates of Lignocellulose through Chemical In-Situ Detoxification with Reducing Agents. Bioresour. Technol. 2011, 102, 1254–1263. [Google Scholar] [CrossRef]
- Persson, P.; Larsson, S.; Jönsson, L.J.; Nilvebrant, N.-O.; Sivik, B.; Munteanu, F.; Thörneby, L.; Gorton, L. Supercritical Fluid Extraction of a Lignocellulosic Hydrolysate of Spruce for Detoxification and to Facilitate Analysis of Inhibitors. Biotechnol. Bioeng. 2002, 79, 694–700. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. A Review of Biological Delignification and Detoxification Methods for Lignocellulosic Bioethanol Production. Crit. Rev. Biotechnol. 2015, 35, 342–354. [Google Scholar] [CrossRef]
- Okuda, N.; Soneura, M.; Ninomiya, K.; Katakura, Y.; Shioya, S. Biological Detoxification of Waste House Wood Hydrolysate Using Ureibacillus thermosphaericus for Bioethanol Production. J. Biosci. Bioeng. 2008, 106, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Nichols, N.N.; Sharma, L.N.; Mowery, R.A.; Chambliss, C.K.; van Walsum, G.P.; Dien, B.S.; Iten, L.B. Fungal Metabolism of Fermentation Inhibitors Present in Corn Stover Dilute Acid Hydrolysate. Enzym. Microb. Technol. 2008, 42, 624–630. [Google Scholar] [CrossRef]
- Parawira, W.; Tekere, M. Biotechnological Strategies to Overcome Inhibitors in Lignocellulose Hydrolysates for Ethanol Production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Kim, J.; Song, H.-S.; Kim, H.J.; Jeon, J.-M.; Sathiyanarayanan, G.; Yoon, J.-J.; Park, K.; Kim, Y.-G.; Yang, Y.-H. Microbial Biodiesel Production from Oil Palm Biomass Hydrolysate Using Marine rhodococcus sp. YHY01. Bioresour. Technol. 2017, 233, 99–109. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Trento, A.; Van Rensburg, E.; García-Aparicio, M.; Van Zyl, W.H.; Casella, S. Exploring Grape Marc as Trove for New Thermotolerant and Inhibitor-Tolerant Saccharomyces cerevisiae Strains for Second-Generation Bioethanol Production. Biotechnol. Biofuels 2013, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.O.A.; Church, G.M.; Dantas, G. A Functional Metagenomic Approach for Expanding the Synthetic Biology Toolbox for Biomass Conversion. Mol. Syst. Biol. 2010, 6, 360. [Google Scholar] [CrossRef]
- Olson, D.G.; McBride, J.E.; Joe Shaw, A.; Lynd, L.R. Recent Progress in Consolidated Bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, K.; Palmqvist, B.; Lidén, G. Improving Simultaneous Saccharification and Co-Fermentation of Pretreated Wheat Straw Using Both Enzyme and Substrate Feeding. Biotechnol. Biofuels 2010, 3, 17. [Google Scholar] [CrossRef]
- Dunlop, M.J. Engineering Microbes for Tolerance to Next-Generation Biofuels. Biotechnol. Biofuels 2011, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M.J.; Dossani, Z.Y.; Szmidt, H.L.; Chu, H.C.; Lee, T.S.; Keasling, J.D.; Hadi, M.Z.; Mukhopadhyay, A. Engineering Microbial Biofuel Tolerance and Export Using Efflux Pumps. Mol. Syst. Biol. 2011, 7, 487. [Google Scholar] [CrossRef]
- Rutherford, B.J.; Dahl, R.H.; Price, R.E.; Szmidt, H.L.; Benke, P.I.; Mukhopadhyay, A.; Keasling, J.D. Functional Genomic Study of Exogenous N-Butanol Stress in Escherichia coli. Appl. Environ. Microbiol. 2010, 76, 1935–1945. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Fischer, J. Bacterial Solvent Responses and Tolerance: Cis–Trans Isomerization. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 4203–4211. [Google Scholar] [CrossRef]
- Wagner, A.; Donaldson, L.; Kim, H.; Phillips, L.; Flint, H.; Steward, D.; Torr, K.; Koch, G.; Schmitt, U.; Ralph, J. Suppression of 4-Coumarate-CoA Ligase in the Coniferous Gymnosperm Pinus radiata. Plant Physiol. 2009, 149, 370–383. [Google Scholar] [CrossRef]
- Coleman, H.D.; Park, J.-Y.; Nair, R.; Chapple, C.; Mansfield, S.D. RNAi-Mediated Suppression of P-Coumaroyl-CoA 3′-Hydroxylase in Hybrid Poplar Impacts Lignin Deposition and Soluble Secondary Metabolism. Proc. Natl. Acad. Sci. USA 2008, 105, 4501–4506. [Google Scholar] [CrossRef]
- Steen, E.J.; Chan, R.; Prasad, N.; Myers, S.; Petzold, C.J.; Redding, A.; Ouellet, M.; Keasling, J.D. Metabolic Engineering of Saccharomyces cerevisiae for the Production of N-Butanol. Microb. Cell Fact. 2008, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Liao, J.C. Metabolic Engineering for Advanced Biofuels Production from Escherichia coli. Curr. Opin. Biotechnol. 2008, 19, 414–419. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Butanol Fermentation Research: Upstream and Downstream Manipulations. Chem. Rec. 2004, 4, 305–314. [Google Scholar] [CrossRef]
- Inui, M.; Suda, M.; Kimura, S.; Yasuda, K.; Suzuki, H.; Toda, H.; Yamamoto, S.; Okino, S.; Suzuki, N.; Yukawa, H. Expression of Clostridium acetobutylicum Butanol Synthetic Genes in Escherichia coli. Appl. Microbiol. Biotechnol. 2008, 77, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Dellomonaco, C.; Clomburg, J.M.; Miller, E.N.; Gonzalez, R. Engineered Reversal of the β-Oxidation Cycle for the Synthesis of Fuels and Chemicals. Nature 2011, 476, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Wang, Z.W.; Chiang, C.-J.; Chao, Y.-P. Metabolic Engineering of Escherichia coli for Production of N-Butanol from Crude Glycerol. Biotechnol. Biofuels 2017, 10, 173. [Google Scholar] [CrossRef]
- Shen, C.R.; Lan, E.I.; Dekishima, Y.; Baez, A.; Cho, K.M.; Liao, J.C. Driving Forces Enable High-Titer Anaerobic 1-Butanol Synthesis in Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 2905–2915. [Google Scholar] [CrossRef]
- Saini, M.; Li, S.-Y.; Wang, Z.W.; Chiang, C.-J.; Chao, Y.-P. Systematic Engineering of the Central Metabolism in Escherichia coli for Effective Production of N-Butanol. Biotechnol. Biofuels 2016, 9, 69. [Google Scholar] [CrossRef]
- Ohtake, T.; Pontrelli, S.; Laviña, W.A.; Liao, J.C.; Putri, S.P.; Fukusaki, E. Metabolomics-Driven Approach to Solving a CoA Imbalance for Improved 1-Butanol Production in Escherichia coli. Metab. Eng. 2017, 41, 135–143. [Google Scholar] [CrossRef]
- Schadeweg, V.; Boles, E. N-Butanol Production in Saccharomyces cerevisiae Is Limited by the Availability of Coenzyme A and Cytosolic Acetyl-CoA. Biotechnol. Biofuels 2016, 9, 44. [Google Scholar] [CrossRef]
- Krivoruchko, A.; Serrano-Amatriain, C.; Chen, Y.; Siewers, V.; Nielsen, J. Improving Biobutanol Production in Engineered Saccharomyces cerevisiae by Manipulation of Acetyl-CoA Metabolism. J. Ind. Microbiol. Biotechnol. 2013, 40, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Schadeweg, V.; Boles, E. Increasing N-Butanol Production with Saccharomyces cerevisiae by Optimizing Acetyl-CoA Synthesis, NADH Levels and Trans-2-Enoyl-CoA Reductase Expression. Biotechnol. Biofuels 2016, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zeng, Q.; Han, S.; Wang, Z.; Dong, Q.; Bi, Y.; Zhao, Y. High-Efficient n-Butanol Production by Co-Culturing Clostridium acetobutylicum and Saccharomyces cerevisiae Integrated with Butyrate Fermentative Supernatant Addition. World J. Microbiol. Biotechnol. 2017, 33, 76. [Google Scholar] [CrossRef]
- Das, M.; Patra, P.; Ghosh, A. Metabolic Engineering for Enhancing Microbial Biosynthesis of Advanced Biofuels. Renew. Sustain. Energy Rev. 2020, 119, 109562. [Google Scholar] [CrossRef]
- Buijs, N.A.; Siewers, V.; Nielsen, J. Advanced Biofuel Production by the Yeast Saccharomyces cerevisiae. Curr. Opin. Chem. Biol. 2013, 17, 480–488. [Google Scholar] [CrossRef]
- Lan, E.I.; Liao, J.C. Microbial Synthesis of N-Butanol, Isobutanol, and Other Higher Alcohols from Diverse Resources. Bioresour. Technol. 2013, 135, 339–349. [Google Scholar] [CrossRef]
- Fenkl, M.; Pechout, M.; Vojtisek, M. N-Butanol and Isobutanol as Alternatives to Gasoline: Comparison of Port Fuel Injector Characteristics. EPJ Web Conf. 2016, 114, 02021. [Google Scholar] [CrossRef]
- Generoso, W.C.; Brinek, M.; Dietz, H.; Oreb, M.; Boles, E. Secretion of 2,3-Dihydroxyisovalerate as a Limiting Factor for Isobutanol Production in Saccharomyces cerevisiae. FEMS Yeast Res. 2017, 17, fox029. [Google Scholar] [CrossRef]
- Koppolu, V.; Vasigala, V.K.R. Role of Escherichia coli in Biofuel Production. Microbiol. Insights 2016, 9, MBI.S10878. [Google Scholar] [CrossRef]
- Chong, H.; Geng, H.; Zhang, H.; Song, H.; Huang, L.; Jiang, R. Enhancing E. coli Isobutanol Tolerance through Engineering Its Global Transcription Factor CAMP Receptor Protein (CRP). Biotechnol. Bioeng. 2014, 111, 700–708. [Google Scholar] [CrossRef]
- Noda, S.; Mori, Y.; Oyama, S.; Kondo, A.; Araki, M.; Shirai, T. Reconstruction of Metabolic Pathway for Isobutanol Production in Escherichia coli. Microb. Cell Fact. 2019, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhou, J.; Zhang, Z.; Kim, C.H.; Jiang, B.; Shi, J.; Hao, J. Isobutanol and 2-Ketoisovalerate Production by Klebsiella pneumoniae via a Native Pathway. Metab. Eng. 2017, 43, 71–84. [Google Scholar] [CrossRef]
- Jung, H.-M.; Kim, Y.H.; Oh, M.-K. Formate and Nitrate Utilization in Enterobacter aerogenes for Semi-Anaerobic Production of Isobutanol. Biotechnol. J. 2017, 12, 1700121. [Google Scholar] [CrossRef]
- Ida, K.; Ishii, J.; Matsuda, F.; Kondo, T.; Kondo, A. Eliminating the Isoleucine Biosyntheticpathway to Reduce Competitive Carbon Outflow during Isobutanol Production by Saccharomyces cerevisiae. Microb. Cell Fact. 2015, 14, 62. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, S.; Hahn, J.-S. Metabolic Engineering of Saccharomyces Cerevisiae for the Production of Isobutanol and 3-Methyl-1-Butanol. Appl. Microbiol. Biotechnol. 2014, 98, 9139–9147. [Google Scholar] [CrossRef]
- Erb, T.J.; Jones, P.R.; Bar-Even, A. Synthetic Metabolism: Metabolic Engineering Meets Enzyme Design. Curr. Opin. Chem. Biol. 2017, 37, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Bar-Even, A.; Noor, E.; Lewis, N.E.; Milo, R. Design and Analysis of Synthetic Carbon Fixation Pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 8889–8894. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, I.W.; Lin, T.-S.; Liao, J.C. Synthetic Non-Oxidative Glycolysis Enables Complete Carbon Conservation. Nature 2013, 502, 693–697. [Google Scholar] [CrossRef]
- Kallio, P.; Pásztor, A.; Thiel, K.; Akhtar, M.K.; Jones, P.R. An Engineered Pathway for the Biosynthesis of Renewable Propane. Nat. Commun. 2014, 5, 4731. [Google Scholar] [CrossRef]
- Bar-Even, A.; Flamholz, A.; Noor, E.; Milo, R. Rethinking Glycolysis: On the Biochemical Logic of Metabolic Pathways. Nat. Chem. Biol. 2012, 8, 509–517. [Google Scholar] [CrossRef]
- Schwander, T.; Schada von Borzyskowski, L.; Burgener, S.; Cortina, N.S.; Erb, T.J. A Synthetic Pathway for the Fixation of Carbon Dioxide In Vitro. Science 2016, 354, 900–904. [Google Scholar] [CrossRef]
- Yim, H.; Haselbeck, R.; Niu, W.; Pujol-Baxley, C.; Burgard, A.; Boldt, J.; Khandurina, J.; Trawick, J.D.; Osterhout, R.E.; Stephen, R.; et al. Metabolic Engineering of Escherichia coli for Direct Production of 1,4-Butanediol. Nat. Chem. Biol. 2011, 7, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Tee, T.W.; Chowdhury, A.; Zomorrodi, A.R.; Yoon, J.M.; Fu, Y.; Shanks, J.V.; Maranas, C.D. An Integrated Computational and Experimental Study for Overproducing Fatty Acids in Escherichia coli. Metab. Eng. 2012, 14, 687–704. [Google Scholar] [CrossRef] [PubMed]
- d’Espaux, L.; Mendez-Perez, D.; Li, R.; Keasling, J.D. Synthetic Biology for Microbial Production of Lipid-Based Biofuels. Curr. Opin. Chem. Biol. 2015, 29, 58–65. [Google Scholar] [CrossRef]
- Steen, E.J.; Kang, Y.; Bokinsky, G.; Hu, Z.; Schirmer, A.; McClure, A.; del Cardayre, S.B.; Keasling, J.D. Microbial Production of Fatty-Acid-Derived Fuels and Chemicals from Plant Biomass. Nature 2010, 463, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Gu, Q.; Wang, W.; Wong, L.; Bower, A.G.W.; Collins, C.H.; Koffas, M.A.G. Modular Optimization of Multi-Gene Pathways for Fatty Acids Production in E. coli. Nat. Commun. 2013, 4, 1409. [Google Scholar] [CrossRef]
- Haushalter, R.W.; Groff, D.; Deutsch, S.; The, L.; Chavkin, T.A.; Brunner, S.F.; Katz, L.; Keasling, J.D. Development of an Orthogonal Fatty Acid Biosynthesis System in E. coli for Oleochemical Production. Metab. Eng. 2015, 30, 1–6. [Google Scholar] [CrossRef]
- Xiao, Y.; Bowen, C.H.; Liu, D.; Zhang, F. Exploiting Nongenetic Cell-to-Cell Variation for Enhanced Biosynthesis. Nat. Chem. Biol. 2016, 12, 339–344. [Google Scholar] [CrossRef]
- Leber, C.; Polson, B.; Fernandez-Moya, R.; Da Silva, N.A. Overproduction and Secretion of Free Fatty Acids through Disrupted Neutral Lipid Recycle in Saccharomyces cerevisiae. Metab. Eng. 2015, 28, 54–62. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Buijs, N.A.; Zhu, Z.; Qin, J.; Siewers, V.; Nielsen, J. Production of Fatty Acid-Derived Oleochemicals and Biofuels by Synthetic Yeast Cell Factories. Nat. Commun. 2016, 7, 11709. [Google Scholar] [CrossRef]
- Gajewski, J.; Pavlovic, R.; Fischer, M.; Boles, E.; Grininger, M. Engineering Fungal de Novo Fatty Acid Synthesis for Short Chain Fatty Acid Production. Nat. Commun. 2017, 8, 14650. [Google Scholar] [CrossRef]
- de Jong, B.W.; Shi, S.; Valle-Rodríguez, J.O.; Siewers, V.; Nielsen, J. Metabolic Pathway Engineering for Fatty Acid Ethyl Ester Production in Saccharomyces cerevisiae Using Stable Chromosomal Integration. J. Ind. Microbiol. Biotechnol. 2015, 42, 477–486. [Google Scholar] [CrossRef]
- Eriksen, D.T.; HamediRad, M.; Yuan, Y.; Zhao, H. Orthogonal Fatty Acid Biosynthetic Pathway Improves Fatty Acid Ethyl Ester Production in Saccharomyces cerevisiae. ACS Synth. Biol. 2015, 4, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Buijs, N.A.; Zhou, Y.J.; Siewers, V.; Nielsen, J. Long-Chain Alkane Production by the Yeast Saccharomyces cerevisiae. Biotechnol. Bioeng. 2015, 112, 1275–1279. [Google Scholar] [CrossRef]
- Runguphan, W.; Keasling, J.D. Metabolic Engineering of Saccharomyces Cerevisiae for Production of Fatty Acid-Derived Biofuels and Chemicals. Metab. Eng. 2014, 21, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-X.; Xiao, W.-H.; Liu, D.; Zhang, J.-L.; Ding, M.-Z.; Yuan, Y.-J. Biosynthesis of Odd-Chain Fatty Alcohols in Escherichia coli. Metab. Eng. 2015, 29, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Chen, J.; Zhou, J.; Wang, Y.; Yang, M.; Qi, X.; Xing, J.; Wang, Q.; Ma, Y. High Production of Fatty Alcohols in Escherichia coli with Fatty Acid Starvation. Microb. Cell Fact. 2016, 15, 129. [Google Scholar] [CrossRef]
- Teixeira, P.G.; Ferreira, R.; Zhou, Y.J.; Siewers, V.; Nielsen, J. Dynamic Regulation of Fatty Acid Pools for Improved Production of Fatty Alcohols in Saccharomyces cerevisiae. Microb. Cell Fact. 2017, 16, 45. [Google Scholar] [CrossRef]
- Tai, M.; Stephanopoulos, G. Engineering the Push and Pull of Lipid Biosynthesis in Oleaginous Yeast Yarrowia lipolytica for Biofuel Production. Metab. Eng. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Blazeck, J.; Hill, A.; Liu, L.; Knight, R.; Miller, J.; Pan, A.; Otoupal, P.; Alper, H.S. Harnessing Yarrowia lipolytica Lipogenesis to Create a Platform for Lipid and Biofuel Production. Nat. Commun. 2014, 5, 3131. [Google Scholar] [CrossRef]
- Qiao, K.; Imam Abidi, S.H.; Liu, H.; Zhang, H.; Chakraborty, S.; Watson, N.; Kumaran Ajikumar, P.; Stephanopoulos, G. Engineering Lipid Overproduction in the Oleaginous Yeast Yarrowia lipolytica. Metab. Eng. 2015, 29, 56–65. [Google Scholar] [CrossRef]
- Xu, P.; Qiao, K.; Ahn, W.S.; Stephanopoulos, G. Engineering Yarrowia lipolytica as a Platform for Synthesis of Drop-in Transportation Fuels and Oleochemicals. Proc. Natl. Acad. Sci. USA 2016, 113, 10848–10853. [Google Scholar] [CrossRef]
- Hanko, E.K.R.; Denby, C.M.; Sànchez i Nogué, V.; Lin, W.; Ramirez, K.J.; Singer, C.A.; Beckham, G.T.; Keasling, J.D. Engineering β-Oxidation in Yarrowia Lipolytica for Methyl Ketone Production. Metab. Eng. 2018, 48, 52–62. [Google Scholar] [CrossRef] [PubMed]
- George, K.W.; Alonso-Gutierrez, J.; Keasling, J.D.; Lee, T.S. Isoprenoid Drugs, Biofuels, and Chemicals—Artemisinin, Farnesene, and Beyond. Adv. Biochem. Eng. Biotechnol. 2015, 148, 355–389. [Google Scholar] [CrossRef]
- Li, M.; Hou, F.; Wu, T.; Jiang, X.; Li, F.; Liu, H.; Xian, M.; Zhang, H. Recent Advances of Metabolic Engineering Strategies in Natural Isoprenoid Production Using Cell Factories. Nat. Prod. Rep. 2020, 37, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.E.; Romero, P.R.; Kirst, H.; Melis, A. Role of Isopentenyl-Diphosphate Isomerase in Heterologous Cyanobacterial (Synechocystis) Isoprene Production. Photosynth. Res. 2016, 130, 517–527. [Google Scholar] [CrossRef]
- Association of Natural Rubber Producing Countries. News From Secretariat; The Association of Natural Rubber Producing Countries (ANRPC): Kuala Lumpur, Malaysia, 2021. [Google Scholar]
- Li, M.; Nian, R.; Xian, M.; Zhang, H. Metabolic Engineering for the Production of Isoprene and Isopentenol by Escherichia coli. Appl. Microbiol. Biotechnol. 2018, 102, 7725–7738. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.E.; Rueda-Romero, P.; Kirst, H.; Melis, A. Engineering Isoprene Synthase Expression and Activity in Cyanobacteria. ACS Synth. Biol. 2017, 6, 2281–2292. [Google Scholar] [CrossRef]
- Chaves, J.E.; Melis, A. Biotechnology of Cyanobacterial Isoprene Production. Appl. Microbiol. Biotechnol. 2018, 102, 6451–6458. [Google Scholar] [CrossRef]
- Tashiro, M.; Kiyota, H.; Kawai-Noma, S.; Saito, K.; Ikeuchi, M.; Iijima, Y.; Umeno, D. Bacterial Production of Pinene by a Laboratory-Evolved Pinene-Synthase. ACS Synth. Biol. 2016, 5, 1011–1020. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, C.; Yang, L.; Choi, E.-S.; Kim, S.-W. Geranyl Diphosphate Synthase: An Important Regulation Point in Balancing a Recombinant Monoterpene Pathway in Escherichia coli. Enzym. Microb. Technol. 2015, 68, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Sarria, S.; Wong, B.; Martín, H.G.; Keasling, J.D.; Peralta-Yahya, P. Microbial Synthesis of Pinene. ACS Synth. Biol. 2014, 3, 466–475. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and Microbial Production of a Terpene-Based Advanced Biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yoon, S.-H.; Jang, H.-J.; Chung, Y.-R.; Kim, J.-Y.; Choi, E.-S.; Kim, S.-W. Metabolic Engineering of Escherichia coli for α-Farnesene Production. Metab. Eng. 2011, 13, 648–655. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Yin, Q.; Zhang, J.; Zhang, C.; Qi, W.; Gao, L.; Tao, Z.; Su, R.; He, Z. Utilization of Biodiesel By-Product as Substrate for High-Production of β-Farnesene via Relatively Balanced Mevalonate Pathway in Escherichia coli. Bioresour. Technol. 2017, 243, 228–236. [Google Scholar] [CrossRef]
- Schempp, F.M.; Drummond, L.; Buchhaupt, M.; Schrader, J. Microbial Cell Factories for the Production of Terpenoid Flavor and Fragrance Compounds. J. Agric. Food Chem. 2018, 66, 2247–2258. [Google Scholar] [CrossRef]
- Ilmén, M.; Oja, M.; Huuskonen, A.; Lee, S.; Ruohonen, L.; Jung, S. Identification of Novel Isoprene Synthases through Genome Mining and Expression in Escherichia coli. Metab. Eng. 2015, 31, 153–162. [Google Scholar] [CrossRef]
- Yang, J.; Nie, Q.; Liu, H.; Xian, M.; Liu, H. A Novel MVA-Mediated Pathway for Isoprene Production in Engineered E. coli. BMC Biotechnol. 2016, 16, 5. [Google Scholar] [CrossRef]
- Pade, N.; Erdmann, S.; Enke, H.; Dethloff, F.; Dühring, U.; Georg, J.; Wambutt, J.; Kopka, J.; Hess, W.R.; Zimmermann, R.; et al. Insights into Isoprene Production Using the Cyanobacterium Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2016, 9, 89. [Google Scholar] [CrossRef]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting Yeast Central Carbon Metabolism for Industrial Isoprenoid Production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Yang, X.; Nambou, K.; Wei, L.; Hua, Q. Heterologous Production of α-Farnesene in Metabolically Engineered Strains of Yarrowia lipolytica. Bioresour. Technol. 2016, 216, 1040–1048. [Google Scholar] [CrossRef]
- Gomaa, L.; Loscar, M.E.; Zein, H.S.; Abdel-Ghaffar, N.; Abdelhadi, A.A.; Abdelaal, A.S.; Abdallah, N.A. Boosting Isoprene Production via Heterologous Expression of the Kudzu Isoprene Synthase Gene (KIspS) into Bacillus spp. Cell Factory. AMB Express 2017, 7, 161. [Google Scholar] [CrossRef]
- Lin, P.-C.; Saha, R.; Zhang, F.; Pakrasi, H.B. Metabolic Engineering of the Pentose Phosphate Pathway for Enhanced Limonene Production in the Cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2017, 7, 17503. [Google Scholar] [CrossRef] [PubMed]
- Tippmann, S.; Ferreira, R.; Siewers, V.; Nielsen, J.; Chen, Y. Effects of Acetoacetyl-CoA Synthase Expression on Production of Farnesene in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 911–922. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, S.; Cao, J.; Qiao, J.; Zhao, G.-R. Systematic Optimization of Limonene Production in Engineered Escherichia coli. J. Agric. Food Chem. 2019, 67, 7087–7097. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, X.; Jiang, G.; Wu, J.; Zhang, J.; Lei, D.; Yuan, Y.-J.; Qiao, J.; Zhao, G.-R. Orthogonal Engineering of Biosynthetic Pathway for Efficient Production of Limonene in Saccharomyces cerevisiae. ACS Synth. Biol. 2019, 8, 968–975. [Google Scholar] [CrossRef]
- Feist, A.M.; Herrgård, M.J.; Thiele, I.; Reed, J.L.; Palsson, B.Ø. Reconstruction of Biochemical Networks in Microorganisms. Nat. Rev. Microbiol. 2009, 7, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, K.; Yamada, Y.; Shinoda, K.; Nakayama, Y.; Tomita, M. GEM System: Automatic Prototyping of Cell-Wide Metabolic Pathway Models from Genomes. BMC Bioinform. 2006, 7, 168. [Google Scholar] [CrossRef]
- Durot, M.; Bourguignon, P.Y.; Schachter, V. Genome-Scale Models of Bacterial Metabolism: Reconstruction and Applications. FEMS Microbiol. Rev. 2009, 33, 164–190. [Google Scholar] [CrossRef]
- Reed, J.L.; Famili, I.; Thiele, I.; Palsson, B.O. Towards Multidimensional Genome Annotation. Nat. Rev. Genet. 2006, 7, 130–141. [Google Scholar] [CrossRef]
- Zhang, W.; Li, F.; Nie, L. Integrating Multiple “omics” Analysis for Microbial Biology: Application and Methodologies. Microbiology 2010, 156, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Nakahigashi, K.; Baba, T.; Robert, M.; Soga, T.; Kanai, A.; Hirasawa, T.; Naba, M.; Hirai, K.; Hoque, A.; et al. Multiple High-Throughput Analyses Monitor the Response of E. coli to Perturbations. Science 2007, 316, 593–597. [Google Scholar] [CrossRef]
- Trinh, C.T.; Wlaschin, A.; Srienc, F. Elementary Mode Analysis: A Useful Metabolic Pathway Analysis Tool for Characterizing Cellular Metabolism. Appl. Microbiol. Biotechnol. 2009, 81, 813–826. [Google Scholar] [CrossRef]
- Papin, J.A.; Stelling, J.; Price, N.D.; Klamt, S.; Schuster, S.; Palsson, B.O. Comparison of Network-Based Pathway Analysis Methods. Trends Biotechnol. 2004, 22, 400–405. [Google Scholar] [CrossRef]
- Desai, R.P.; Harris, L.M.; Welker, N.E.; Papoutsakis, E.T. Metabolic Flux Analysis Elucidates the Importance of the Acid-Formation Pathways in Regulating Solvent Production by Clostridium acetobutylicum. Metab. Eng. 1999, 1, 206–213. [Google Scholar] [CrossRef]
- Raganati, F.; Procentese, A.; Olivieri, G.; Russo, M.E.; Salatino, P.; Marzocchella, A. MFA of Clostridium acetobutylicum Pathway: The Role of Glucose and Xylose on the Acid Formation/Uptake. Chem. Eng. Trans. 2014, 38, 337–342. [Google Scholar] [CrossRef]
- Savinell, J.M.; Palsson, B.O. Optimal Selection of Metabolic Fluxes for in Vivo Measurement. I. Development of Mathematical Methods. J. Theor. Biol. 1992, 155, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Palsson, B.O. Metabolic Capabilities of Escherichia coli: I. Synthesis of Biosynthetic Precursors and Cofactors. J. Theor. Biol. 1993, 165, 477–502. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.D.; Thiele, I.; Palsson, B.Ø. What Is Flux Balance Analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef]
- Segrè, D.; Vitkup, D.; Church, G.M. Analysis of Optimality in Natural and Perturbed Metabolic Networks. Proc. Natl. Acad. Sci. USA 2002, 99, 15112–15117. [Google Scholar] [CrossRef]
- Simeonidis, E.; Price, N.D. Genome-Scale Modeling for Metabolic Engineering. J. Ind. Microbiol. Biotechnol. 2015, 42, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Young, J.D.; Shastri, A.A.; Stephanopoulos, G.; Morgan, J.A. Mapping Photoautotrophic Metabolism with Isotopically Nonstationary 13C Flux Analysis. Metab. Eng. 2011, 13, 656–665. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Berla, B.; He, L.; Pakrasi, H.B.; Tang, Y.J. 13C-MFA Delineates the Photomixotrophic Metabolism of Synechocystis sp. PCC 6803 under Light- and Carbon-Sufficient Conditions. Biotechnol. J. 2014, 9, 684–692. [Google Scholar] [CrossRef]
- Antoniewicz, M.R.; Kraynie, D.F.; Laffend, L.A.; González-Lergier, J.; Kelleher, J.K.; Stephanopoulos, G. Metabolic Flux Analysis in a Nonstationary System: Fed-Batch Fermentation of a High Yielding Strain of E. coli Producing 1,3-Propanediol. Metab. Eng. 2007, 9, 277–292. [Google Scholar] [CrossRef]
- Wiechert, W. 13C Metabolic Flux Analysis. Metab. Eng. 2001, 3, 195–206. [Google Scholar] [CrossRef]
- Sauer, U. Metabolic Networks in Motion: 13C-Based Flux Analysis. Mol. Syst. Biol. 2006, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, C. Fluxome Analysis Using GC-MS. Microb. Cell Fact. 2007, 6, 6. [Google Scholar] [CrossRef]
- Shimizu, K. Metabolic Flux Analysis Based on 13C-Labeling Experiments and Integration of the Information with Gene and Protein Expression Patterns. In Recent Progress of Biochemical and Biomedical Engineering in Japan II; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–49. ISBN 978-3-540-39876-9. [Google Scholar]
- Toya, Y.; Kono, N.; Arakawa, K.; Tomita, M. Metabolic Flux Analysis and Visualization. J. Proteome Res. 2011, 10, 3313–3323. [Google Scholar] [CrossRef]
- David, A.; Hector, G.M. Genome-Scale 13C Fluxomics Modeling for Metabolic Engineering of Saccharomyces cerevisiae. In The Elements of Law; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1859, pp. 99–106. ISBN 9781493987573. [Google Scholar]
- He, L.; Xiao, Y.; Gebreselassie, N.; Zhang, F.; Antoniewicz, M.R.; Tang, Y.J.; Peng, L. Central Metabolic Responses to the Overproduction of Fatty Acids in Escherichia coli Based on 13C-Metabolic Flux Analysis. Biotechnol. Bioeng. 2014, 111, 575–585. [Google Scholar] [CrossRef]
- Wang, H.H.; Isaacs, F.J.; Carr, P.A.; Sun, Z.Z.; Xu, G.; Forest, C.R.; Church, G.M. Programming Cells by Multiplex Genome Engineering and Accelerated Evolution. Nature 2009, 460, 894–898. [Google Scholar] [CrossRef]
- Barbieri, E.M.; Muir, P.; Akhuetie-Oni, B.O.; Yellman, C.M.; Isaacs, F.J. Precise Editing at DNA Replication Forks Enables Multiplex Genome Engineering in Eukaryotes. Cell 2017, 171, 1453–1467.e13. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Z.; Huang, C.; Zhang, Y.; Wang, Z.; Tang, Y.; Chen, T.; Zhao, X. Metabolic Engineering of Escherichia coli Using CRISPR–Cas9 Meditated Genome Editing. Metab. Eng. 2015, 31, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, A.S.; Jawed, K.; Yazdani, S.S. CRISPR/Cas9-Mediated Engineering of Escherichia coli for n-Butanol Production from Xylose in Defined Medium. J. Ind. Microbiol. Biotechnol. 2019, 46, 965–975. [Google Scholar] [CrossRef]
- Zhang, J.; Zong, W.; Hong, W.; Zhang, Z.-T.; Wang, Y. Exploiting Endogenous CRISPR-Cas System for Multiplex Genome Editing in Clostridium tyrobutyricum and Engineer the Strain for High-Level Butanol Production. Metab. Eng. 2018, 47, 49–59. [Google Scholar] [CrossRef]
- Lian, J.; Jin, R.; Zhao, H. Construction of Plasmids with Tunable Copy Numbers in Saccharomyces cerevisiae and Their Applications in Pathway Optimization and Multiplex Genome Integration. Biotechnol. Bioeng. 2016, 113, 2462–2473. [Google Scholar] [CrossRef]
- Shi, S.; Liang, Y.; Zhang, M.M.; Ang, E.L.; Zhao, H. A Highly Efficient Single-Step, Markerless Strategy for Multi-Copy Chromosomal Integration of Large Biochemical Pathways in Saccharomyces cerevisiae. Metab. Eng. 2016, 33, 19–27. [Google Scholar] [CrossRef]
- Li, M.; Lang, X.; Moran Cabrera, M.; De Keyser, S.; Sun, X.; Da Silva, N.; Wheeldon, I. CRISPR-Mediated Multigene Integration Enables Shikimate Pathway Refactoring for Enhanced 2-Phenylethanol Biosynthesis in Kluyveromyces marxianus. Biotechnol. Biofuels 2021, 14, 3. [Google Scholar] [CrossRef]
- Lindberg, P.; Park, S.; Melis, A. Engineering a Platform for Photosynthetic Isoprene Production in Cyanobacteria, Using Synechocystis as the Model Organism. Metab. Eng. 2010, 12, 70–79. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Zhang, Y.; Li, Y.; Ma, Y. Designing and Creating a Modularized Synthetic Pathway in Cyanobacterium Synechocystis Enables Production of Acetone from Carbon Dioxide. Metab. Eng. 2012, 14, 394–400. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, J.; Curtiss III, R. Fatty Acid Production in Genetically Modified Cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 6899–6904. [Google Scholar] [CrossRef]
- Lan, E.I.; Liao, J.C. ATP Drives Direct Photosynthetic Production of 1-Butanol in Cyanobacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 6018–6023. [Google Scholar] [CrossRef] [PubMed]
- Takahama, K.; Matsuoka, M.; Nagahama, K.; Ogawa, T. Construction and Analysis of a Recombinant Cyanobacterium Expressing a Chromosomally Inserted Gene for an Ethylene-Forming Enzyme at the PsbAI Locus. J. Biosci. Bioeng. 2003, 95, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.R.; Liao, J.C. Photosynthetic Production of 2-Methyl-1-Butanol from CO2 in Cyanobacterium Synechococcus elongatus PCC7942 and Characterization of the Native Acetohydroxyacid Synthase. Energy Environ. Sci. 2012, 5, 9574–9583. [Google Scholar] [CrossRef]
- Hou, B.K.; Ellis, L.B.M.; Wackett, L.P. Encoding Microbial Metabolic Logic: Predicting Biodegradation. J. Ind. Microbiol. Biotechnol. 2004, 31, 261–272. [Google Scholar] [CrossRef]
- Prather, K.L.J.; Martin, C.H. De Novo Biosynthetic Pathways: Rational Design of Microbial Chemical Factories. Curr. Opin. Biotechnol. 2008, 19, 468–474. [Google Scholar] [CrossRef]
- Hatzimanikatis, V.; Li, C.; Ionita, J.A.; Henry, C.S.; Jankowski, M.D.; Broadbelt, L.J. Exploring the Diversity of Complex Metabolic Networks. Bioinformatics 2005, 21, 1603–1609. [Google Scholar] [CrossRef]
- Jagadevan, S.; Banerjee, A.; Banerjee, C.; Guria, C.; Tiwari, R.; Baweja, M.; Shukla, P. Recent Developments in Synthetic Biology and Metabolic Engineering in Microalgae towards Biofuel Production. Biotechnol. Biofuels 2018, 11, 185. [Google Scholar] [CrossRef]
- Smith, J.D.; Suresh, S.; Schlecht, U.; Wu, M.; Wagih, O.; Peltz, G.; Davis, R.W.; Steinmetz, L.M.; Parts, L.; St. Onge, R.P. Quantitative CRISPR Interference Screens in Yeast Identify Chemical-Genetic Interactions and New Rules for Guide RNA Design. Genome Biol. 2016, 17, 45. [Google Scholar] [CrossRef]
- Si, T.; Luo, Y.; Bao, Z.; Zhao, H. RNAi-Assisted Genome Evolution in Saccharomyces Cerevisiae for Complex Phenotype Engineering. ACS Synth. Biol. 2015, 4, 283–291. [Google Scholar] [CrossRef]
- Crook, N.C.; Schmitz, A.C.; Alper, H.S. Optimization of a Yeast RNA Interference System for Controlling Gene Expression and Enabling Rapid Metabolic Engineering. ACS Synth. Biol. 2014, 3, 307–313. [Google Scholar] [CrossRef]
- Wu, J.; Du, G.; Chen, J.; Zhou, J. Enhancing Flavonoid Production by Systematically Tuning the Central Metabolic Pathways Based on a CRISPR Interference System in Escherichia coli. Sci. Rep. 2015, 5, 13477. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell 2013, 154, 442. [Google Scholar] [CrossRef]
- Bikard, D.; Jiang, W.; Samai, P.; Hochschild, A.; Zhang, F.; Marraffini, L.A. Programmable Repression and Activation of Bacterial Gene Expression Using an Engineered CRISPR-Cas System. Nucleic Acids Res. 2013, 41, 7429–7437. [Google Scholar] [CrossRef] [PubMed]
- Hlavova, M.; Turoczy, Z.; Bisova, K. Improving Microalgae for Biotechnology—From Genetics to Synthetic Biology. Biotechnol. Adv. 2015, 33, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- De Bhowmick, G.; Koduru, L.; Sen, R. Metabolic Pathway Engineering towards Enhancing Microalgal Lipid Biosynthesis for Biofuel Application—A Review. Renew. Sustain. Energy Rev. 2015, 50, 1239–1253. [Google Scholar] [CrossRef]
- Kumar, A.; Guria, C.; Chitres, G.; Chakraborty, A.; Pathak, A.K. Modelling of Microalgal Growth and Lipid Production in Dunaliella Tertiolecta Using Nitrogen-Phosphorus-Potassium Fertilizer Medium in Sintered Disk Chromatographic Glass Bubble Column. Bioresour. Technol. 2016, 218, 1021–1036. [Google Scholar] [CrossRef]
- Shah, A.R.; Ahmad, A.; Srivastava, S.; Jaffar Ali, B.M. Reconstruction and Analysis of a Genome-Scale Metabolic Model of Nannochloropsis gaditana. Algal Res. 2017, 26, 354–364. [Google Scholar] [CrossRef]
- Loira, N.; Mendoza, S.; Paz Cortés, M.; Rojas, N.; Travisany, D.; Di Genova, A.; Gajardo, N.; Ehrenfeld, N.; Maass, A. Reconstruction of the Microalga Nannochloropsis Salina Genome-Scale Metabolic Model with Applications to Lipid Production. BMC Syst. Biol. 2017, 11, 66. [Google Scholar] [CrossRef]
- Gimpel, J.A.; Mayfield, S.P. Analysis of Heterologous Regulatory and Coding Regions in Algal Chloroplasts. Appl. Microbiol. Biotechnol. 2013, 97, 4499–4510. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Oliveira Dal’Molin, C.; Quek, L.-E.; Palfreyman, R.W.; Nielsen, L.K. AlgaGEM—A Genome-Scale Metabolic Reconstruction of Algae Based on the Chlamydomonas reinhardtii Genome. BMC Genom. 2011, 12, S5. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).