Experimental Adsorption Study of Pure CH4 and CO2 on Organic-Rich Shales from the Cesar-Ranchería Basin, Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Characterization
2.2. Mineralogical and Geochemical Characterization
2.3. Gas Adsorption Experiments
2.4. Surface Area and Pore Structure Characterization by N2 Adsorption–Desorption
3. Results
3.1. Mineralogical Composition and Its Role in Adsorption
3.2. Geochemical Properties and Thermal Maturity
3.3. Adsorption Behavior of CH4 and CO2
3.4. Textural Analysis by N2 Adsorption–Desorption
4. Discussion
4.1. Influence of Organic Matter and Thermal Maturity
4.2. Role of Mineralogy in Gas Storage
4.3. Textural Properties and Pore Structure
4.4. Performance and Langmuir Interpretation
4.5. Implications for Shale Gas Potential
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Energy Information Administration. International Energy Outlook 2023; U.S. EIA: Washington, DC, USA, 2023.
- Aguilera, R.F. Assessing shale gas potential globally: Energy return on investment (EROI) and other challenges. Energy Policy 2014, 64, 701–710. [Google Scholar]
- Agencia Nacional de Hidrocarburos (ANH). Potencial de Recursos en Yacimientos No Convencionales de Colombia; ANH: Bogotá, Colombia, 2020.
- Chalmers, G.R.L.; Bustin, R.M. The organic matter distribution and gas adsorption capacity of the Lower Cretaceous shales of northeastern British Columbia, Canada. Int. J. Coal Geol. 2007, 70, 223–239. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. Characterizing the shale gas resource potential of Devonian–Mississippian shales in western Canada. AAPG Bull. 2008, 92, 87–125. [Google Scholar] [CrossRef]
- Curtis, M.E.; Sondergeld, C.H.; Rai, C.S. Structural characterization of gas shales on the nanoscale. Microsc. Microanal. 2012, 18, 694–695. [Google Scholar]
- Wang, C.; Liu, H.H. A review on gas adsorption in shale nanopores: Modeling advances and challenges. J. Nat. Gas Sci. Eng. 2021, 94, 104127. [Google Scholar]
- Gasparik, M.; Ghanizadeh, A.; Gensterblum, Y.; Krooss, B.M.; Littke, R. CO2 and CH4 adsorption capacity of European shales. Energy Procedia 2013, 37, 5243–5252. [Google Scholar]
- Busch, A.; Alles, S.; Gensterblum, Y.; Prinz, D.; Dewhurst, D.N.; Raven, M.D.; Stanjek, H.; Krooss, B.M. Carbon dioxide storage potential of shales. Int. J. Greenh. Gas Control 2008, 2, 297–308. [Google Scholar] [CrossRef]
- Heller, R.; Vermylen, J.; Zoback, M. Experimental investigation of gas adsorption in shales. Fuel 2014, 115, 378–393. [Google Scholar]
- Rexer, T.F.T.; Benham, M.J.; Aplin, A.C.; Thomas, K.M. Methane adsorption on shale under simulated geological temperature and pressure conditions. Energy Fuels 2013, 27, 3099–3109. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.S.; Ruppel, S.C.; Milliken, K.; Yang, R. Effect of organic matter properties on methane sorption in shales. Int. J. Coal Geol. 2012, 103, 89–98. [Google Scholar]
- Pan, Z.; Connell, L.D. Comparative adsorption of CH4, CO2 and N2 on organic–inorganic shales. J. Nat. Gas Sci. Eng. 2015, 27, 82–95. [Google Scholar]
- Liu, X.; Zhou, Y.; Hu, Q.; Zhang, Y. Pore structure and CH4/CO2 adsorption capacity of high-TOC shales. Fuel 2018, 226, 125–135. [Google Scholar]
- Wang, S.; Liu, H.; Yang, R.; Zhang, L.; Li, Y. Adsorption of CO2 and CH4 in gas shale nanopores: Insights from molecular simulations. Langmuir 2014, 30, 13960–13968. [Google Scholar]
- Ríos-Reyes, C.A.; Buitrago, H.; Pulido, J.C. Thermal maturity and organic geochemistry of shales from the Cesar-Ranchería Basin. J. South Am. Earth Sci. 2017, 76, 283–297. [Google Scholar]
- Buitrago, H.; Arango, M.; De La Parra, M. Evaluation of unconventional resource potential in northern Colombia. In Proceedings of the AAPG International Conference and Exhibition, Cartagena, Colombia, 4–17 September 2014. [Google Scholar]
- Agencia Nacional de Hidrocarburos (ANH). Atlas Geológico de Colombia, Volumen IV: Cuenca Cesar-Ranchería; ANH: Bogotá, Colombia, 2015.
- Tang, X.; Ripepi, N.; Liu, B.; Liu, Z. Methane adsorption in shales: Influence of organic matter vs. clay content. Fuel 2016, 174, 560–570. [Google Scholar]
- Clarkson, C.R.; Bustin, R.M. Variation in permeability and storage capacity of shale gas reservoirs with mineralogy. Int. J. Coal Geol. 2000, 38, 33–45. [Google Scholar]
- Bustin, R.M.; Chalmers, G.R. Gas adsorption capacity and chemical composition of thermally mature shales. Int. J. Coal Geol. 2010, 82, 235–248. [Google Scholar]
- Guo, X.; Bustin, R.M.; Ning, Z. Impact of maturity and pore structure on methane adsorption in organic-rich shales. Mar. Pet. Geol. 2020, 117, 104399. [Google Scholar]
- Agencia Nacional de Hidrocarburos (ANH). Ronda Colombia 2021: Cuenca Cesar-Ranchería. 2021. Available online: https://www.anh.gov.co/es/ (accessed on 20 April 2025).
- Silva, C.; Toro, J.; Martínez, J.D. Integrated structural and basinal analysis of the Cesar–Ranchería Basin, Colombia: Implications for its tectonic history and petroleum systems. In Petroleum Basins and Hydrocarbon Potential of the Andes of Peru and Bolivia; AAPG Memoir 117; American Association of Petroleum Geologists: Tulsa, OK, USA, 2020; pp. 349–374. [Google Scholar]
- Busch, A.; Gensterblum, Y.; Krooss, B.M.; Littke, R. Methane and carbon dioxide adsorption–diffusion in organic-rich shales. Int. J. Coal Geol. 2008, 76, 89–101. [Google Scholar] [CrossRef]
- Pino, D.; Plantier, F.; Bessieres, D. Experimental determination of the adsorption isotherms in gas mixtures under extended pressure and temperature range. J. Therm. Anal. Calorim. 2014, 117, 1469–1477. [Google Scholar] [CrossRef]
- Gensterblum, Y.; Bode, M.; de Weireld, G.; Prinz, D.; Littke, R.; Busch, A.; Krooss, B.M. European inter-laboratory comparison of high-pressure CO2 sorption isotherms. I: Activated carbon. Carbon 2009, 47, 2958–2969. [Google Scholar] [CrossRef]
- Curtis, M.E.; Ambrose, R.J.; Sondergeld, C.H.; Rai, C.S. Structural characterization of gas shales on the micro- and nano-scales. J. Nat. Gas Sci. Eng. 2012, 8, 28–39. [Google Scholar] [CrossRef]
- Heller, R.; Zoback, M. Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. J. Unconv. Oil Gas Resour. 2014, 8, 14–24. [Google Scholar] [CrossRef]
- Guo, X.; Bustin, R.M. Pore structure evolution and fluid flow in thermally matured organic-rich shales. Mar. Pet. Geol. 2020, 117, 104399. [Google Scholar] [CrossRef]
- Tang, X.; Ripepi, N.; Liu, B.; Liu, Z. Quantitative evaluation of the relationship between surface area and methane adsorption in organic-rich shales. Fuel 2016, 182, 107–114. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Hu, Q.; Zhang, Y. Relationship between TOC and methane adsorption in shales: Experimental evidence from different formations. Fuel 2018, 226, 125–135. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide, 2nd ed.; Cambridge University Press: Cambridge, UK, 2005; Volume 1. [Google Scholar]
- Gasparik, M.; Ghanizadeh, A.; Gensterblum, Y.; Krooss, B.M.; Littke, R. Geological controls on the methane storage capacity of European shales. Energy Procedia 2013, 40, 511–520. [Google Scholar] [CrossRef]
- Gasparik, M.; Ghanizadeh, A.; Bertier, P.; Gensterblum, Y.; Bouw, S.; Krooss, B.M. High-pressure methane sorption isotherms of black shales from the Netherlands. Energy Fuels 2012, 26, 4995–5004. [Google Scholar] [CrossRef]
- Weniger, P.; Kalkreuth, W.; Busch, A.; Krooss, B.M. High pressure methane and carbon dioxide sorption on coal and shale samples from the Paraná Basin, Brazil. Int. J. Coal Geol. 2010, 84, 190–205. [Google Scholar] [CrossRef]
- Gensterblum, Y.; Merkel, A.; Busch, A.; Krooss, B.M. High-pressure CH4 and CO2 sorption isotherms as a function of coal maturity and the influence of moisture. Int. J. Coal Geol. 2013, 118, 45–57. [Google Scholar] [CrossRef]
- Pozo, M.; Pino, D.; Bessieres, D. Effect of thermal events on maturation and methane adsorption of Silurian black shales (Checa, Spain). Appl. Clay Sci. 2017, 136, 208–218. [Google Scholar] [CrossRef]
- Wei, L.; Liu, D.; Pan, Z.; Chen, J.; Liu, H. Pore structure evolution of organic matter in immature shales and its effect on methane adsorption. Fuel 2023, 344, 128180. [Google Scholar] [CrossRef]
- Zhang, H.; Song, Y.; Han, J.; Zhao, S. Thermal maturity controls on nanopore development and gas storage in lacustrine shales. Int. J. Coal Geol. 2022, 253, 104123. [Google Scholar]
- Wang, Y.; Tang, X.; Ripepi, N. Influence of kerogen type and morphology on methane adsorption in immature shales: An experimental and modeling approach. Energy Fuels 2023, 37, 10567–10580. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Zhou, Y.; Bai, Y.; He, H. Kerogen-hosted nanopores and their control on gas adsorption in early-mature shale: Insights from FIB-SEM and gas adsorption techniques. J. Nat. Gas Sci. Eng. 2024, 119, 105571. [Google Scholar]
- Zhou, W.; Hu, Q.; Liu, X.; Lu, S. Organic–inorganic interactions and their role in controlling pore accessibility and gas sorption in immature marine shales. Mar. Pet. Geol. 2022, 143, 105821. [Google Scholar] [CrossRef]



| Mineral | % in S1 | % in S2 | % in S3 |
|---|---|---|---|
| Quartz | 45.07 | 43.84 | 19.35 |
| Natrojarosite | 14.80 | ||
| Lizardite-1T | 13.89 | ||
| Pyrite | 11.39 | 6.34 | |
| Gypsum | 1.72 | ||
| Barite | 1.60 | ||
| Spinel | 1.38 | ||
| Kaolinite | 9.13 | 45.37 | |
| Chamosite-1MIIb | 1.02 | ||
| Jarosite | 2.77 | ||
| Anatase | 1.68 | ||
| Whewellite | 57.1 | ||
| Dickite | 23.55 |
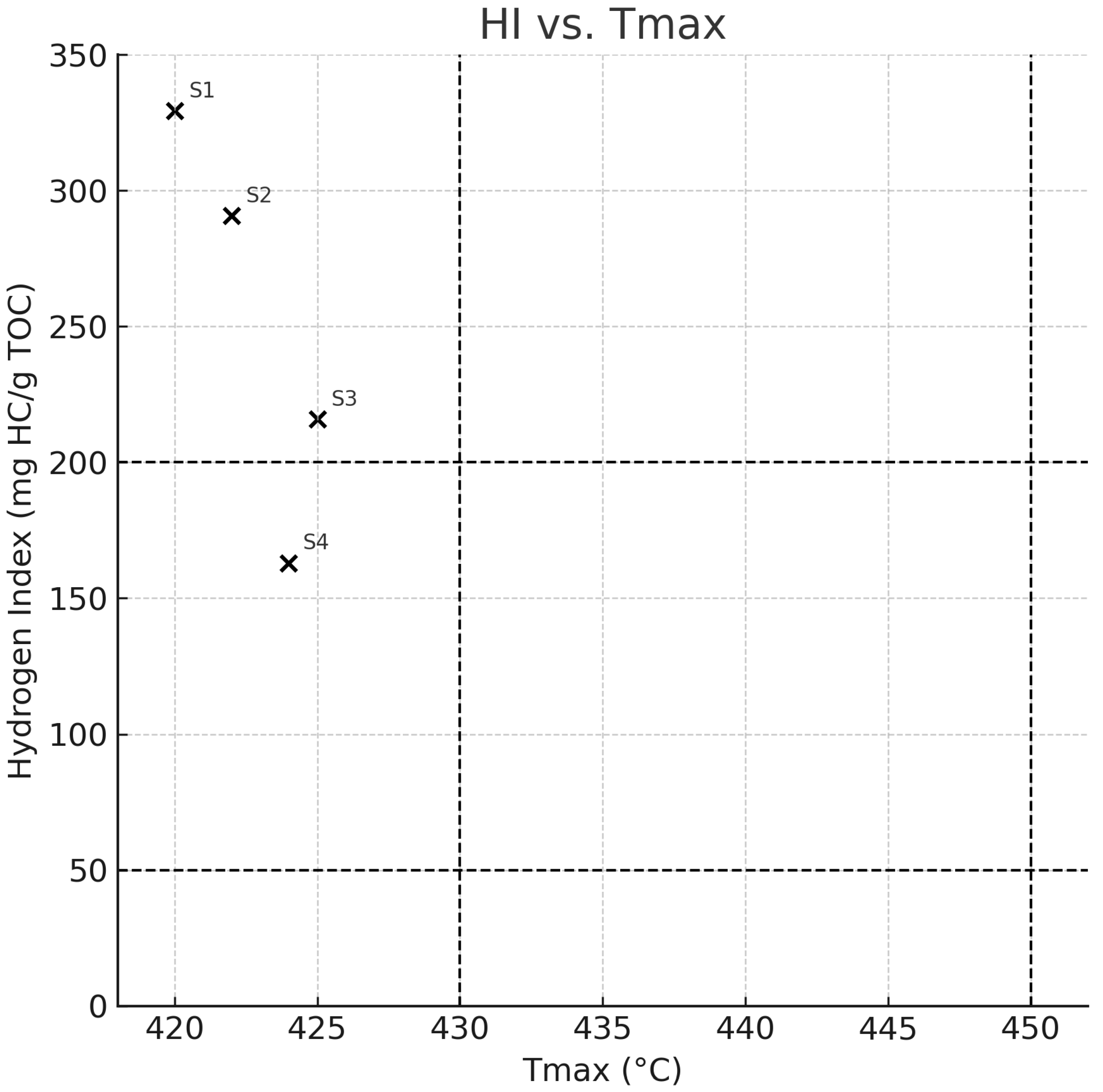
| Sample ID | TOC (wt%) | Tmax (°C) | S1 (mg HC/g Rock) | S2 (mg HC/g Rock) | S3 (mg CO2/g Rock) | HI (mg HC/g TOC) | OI (mg CO2/g TOC) | PI (S1/(S1 + S2)) | Ro (%) |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 33.44 | 420 | 2.45 | 110.10 | 1.70 | 329.22 | 5.08 | 0.02 | 0.36 |
| S2 | 53.67 | 422 | 2.11 | 155.98 | 2.65 | 290.62 | 4.94 | 0.01 | 0.39 |
| S3 | 66.58 | 425 | 1.45 | 143.70 | 10.78 | 215.84 | 16.19 | 0.01 | 0.51 |
| S4 | 69.63 | 424 | 1.05 | 113.49 | 10.44 | 162.99 | 14.99 | 0.01 | 0.53 |
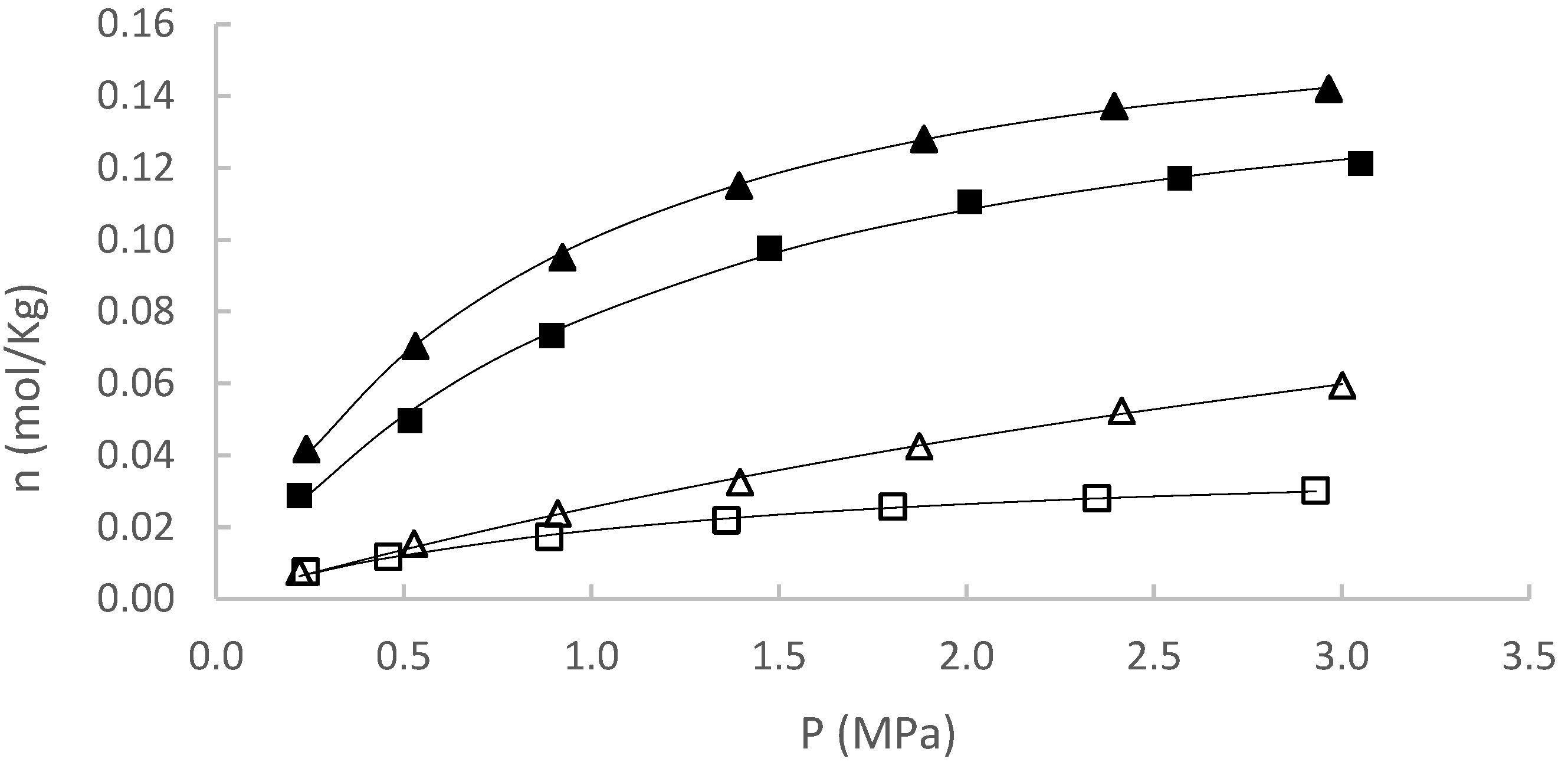
| Experiment | (mol/kg) [95% CI] | (MPa) [95% CI] | R2 | |
|---|---|---|---|---|
| CH4 at 50 °C | 0.2134 [0.2149–0.2118] | 7.2346 [7.3029–7.1672] | 0.0005 | 0.9970 |
| CH4 at 80 °C | 0.0456 [0.0454–0.0458] | 1.3625 [1.3492–1.3760] | 0.0002 | 0.9951 |
| CO2 at 50 °C | 0.1986 [0.1990–0.1982] | 0.9486 [0.9533–0.9438] | 0.0004 | 0.9928 |
| CO2 at 80 °C | 0.1837 [0.1845–0.1830] | 1.2885 [1.3015–1.2756] | 0.0007 | 0.9976 |
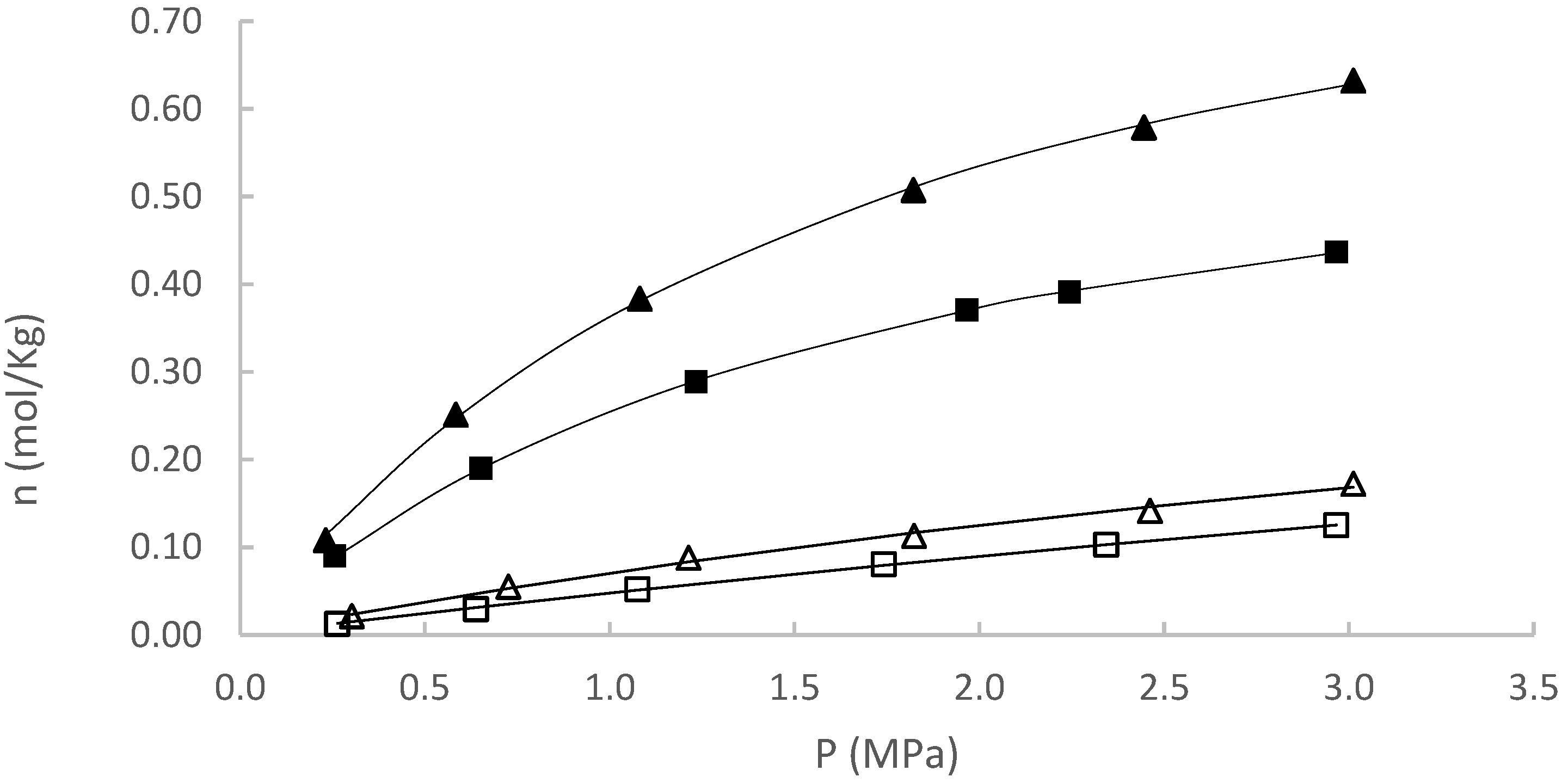
| Experiment | (mol/kg) [95% CI] | (MPa) [95% CI] | R2 | |
|---|---|---|---|---|
| CH4 at 50 °C | 0.6390 [0.6436–0.6345] | 7.9081 [7.9806–7.8366] | 0.0014 | 0.9945 |
| CH4 at 80 °C | 0.9517 [0.9491–0.9544] | 18.6652 [18.6195–18.7132] | 0.0004 | 0.9997 |
| CO2 at 50 °C | 1.1090 [1.1112–1.1068] | 2.0109 [2.0109–2.0030] | 0.0016 | 0.9974 |
| CO2 at 80 °C | 0.7515 [0.7521–0.7510] | 1.9051 [1.9077–1.9026] | 0.0004 | 0.9972 |
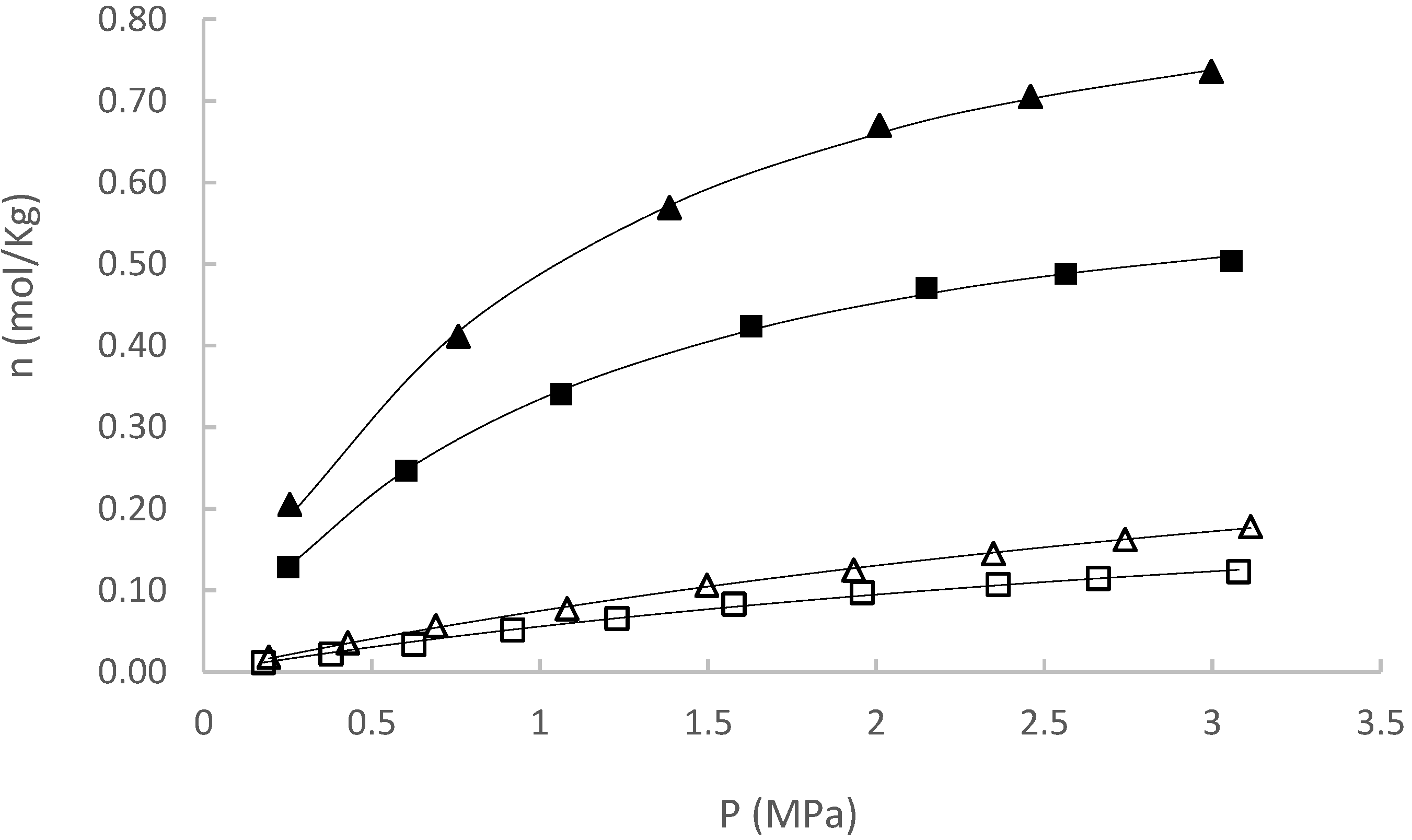
| Experiment | (mol/kg) [95% CI] | (MPa) [95% CI] | R2 | |
|---|---|---|---|---|
| CH4 at 50 °C | 0.5756 [0.5776–0.5736] | 6.5794 [6.6096–6.5493] | 0.0006 | 0.9991 |
| CH4 at 80 °C | 0.3553 [0.3575–0.3531] | 5.3011 [5.3473–5.2560] | 0.0007 | 0.9989 |
| CO2 at 50 °C | 1.0938 [1.0976–1.0899] | 1.2038 [1.2145–1.1932] | 0.0037 | 0.9970 |
| CO2 at 80 °C | 0.7467 [0.7486–0.7447] | 1.2004 [1.2083–1.1927] | 0.0017 | 0.9954 |
| Experiment | (mol/kg) [95% CI] | (MPa) [95% CI] | R2 | |
|---|---|---|---|---|
| CH4 at 50 °C | 0.8306 [0.8326–0.8287] | 2.0418 [2.0535–2.0349] | 0.0011 | 0.9962 |
| CH4 at 80 °C | 0.6608 [0.6619–0.6598] | 2.4018 [2.4087–2.3950] | 0.0005 | 0.9968 |
| CO2 at 50 °C | 1.9964 [1.9991–1.9937] | 1.4314 [1.4360–1.4267] | 0.0022 | 0.9929 |
| CO2 at 80 °C | 1.2662 [1.2718–1.2607] | 1.2371 [1.2508–1.2235] | 0.0040 | 0.9923 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz Cancino, O.P.; Santos Santos, N. Experimental Adsorption Study of Pure CH4 and CO2 on Organic-Rich Shales from the Cesar-Ranchería Basin, Colombia. Processes 2025, 13, 2108. https://doi.org/10.3390/pr13072108
Ortiz Cancino OP, Santos Santos N. Experimental Adsorption Study of Pure CH4 and CO2 on Organic-Rich Shales from the Cesar-Ranchería Basin, Colombia. Processes. 2025; 13(7):2108. https://doi.org/10.3390/pr13072108
Chicago/Turabian StyleOrtiz Cancino, Olga Patricia, and Nicolas Santos Santos. 2025. "Experimental Adsorption Study of Pure CH4 and CO2 on Organic-Rich Shales from the Cesar-Ranchería Basin, Colombia" Processes 13, no. 7: 2108. https://doi.org/10.3390/pr13072108
APA StyleOrtiz Cancino, O. P., & Santos Santos, N. (2025). Experimental Adsorption Study of Pure CH4 and CO2 on Organic-Rich Shales from the Cesar-Ranchería Basin, Colombia. Processes, 13(7), 2108. https://doi.org/10.3390/pr13072108








