Abstract
This article discusses the development of a technology for producing nickel- and chromium-containing ferroalloy through the carbothermic reduction of low-grade ores from the Batamsha deposit. Thermodynamic modeling of phase formation was carried out using the Chemistry 10 software package, along with a series of pilot-scale smelting experiments in a submerged arc furnace. The influence of technological parameters, particularly slag basicity, on the distribution of nickel and chromium in the alloy was studied. The results of an X-ray phase analysis confirmed the modeling conclusions, revealing the presence of nickel and chromium silicide phases. The proposed technology demonstrated a high metal recovery rate and process stability, indicating its potential for industrial application in Kazakhstan.
1. Introduction
Kazakhstan possesses significant reserves of nickel ores. However, these ores are generally classified as low-grade and difficult to beneficiate, making their processing economically unfeasible using traditional hydrometallurgical methods. There is currently no established production method in the country for processing this type of raw material, resulting in valuable metals remaining unutilized and natural resources being used inefficiently [1,2,3].
Against this backdrop, pyrometallurgical processing methods, particularly carbothermic reduction, are gaining relevance. This approach is an economically viable method for processing low-grade and refractory nickel ores and is widely used in global practice. The thermal process, based on the reduction of ores with carbon-containing substances at high temperatures, offers lower capital and operating costs compared to hydrometallurgical processes. It enables the use of a broad range of thermal units, including induction, arc, and shaft furnaces. The technology has shown high efficiency when applied to laterites, silicate, and iron–nickel ores, achieving nickel recovery rates of up to 90% and above, along with the extraction of cobalt and chromium. A major advantage of carbothermic reduction is the ability to produce a marketable nickel–chromium ferroalloy without the need for complex refining processes. Additionally, the flexibility in choosing the reducing agent—including partial or complete substitution of coke with locally available coals—allows for cost reduction and adaptation to regional conditions. This makes the approach particularly promising for implementation in Kazakhstan’s metallurgical industry [4,5,6,7,8,9].
Against the background of the limited efficiency of traditional hydrometallurgical methods for processing low-grade and refractory nickel ores, there has been a growing interest in pyrometallurgical technologies particularly carbothermic reduction. A number of studies conducted at laboratory and semi-industrial scales have confirmed the high technological and economic efficiency of this approach. Recent works [10,11,12,13,14,15] demonstrate that carbothermic reduction of lateritic and iron–nickel ores can achieve nickel recovery rates of 90% and higher, while simultaneously producing marketable ferroalloys without the need for deep refining. The use of various reductants (anthracite, charcoal, petroleum coke, biomass) and additives (limestone, sulfates, slags) enables adaptation of the process to specific regional conditions. Thermodynamic and kinetic studies [16,17,18,19] further confirm the potential of the technology in terms of reduced energy consumption and environmental impact. The available data indicate the high adaptability of the method to ores with various mineral compositions and its suitability for industrial implementation in Kazakhstan’s metallurgical sector.
The work in [20] presents an improved approach to the pyrometallurgical processing of oxidized nickel ores for the production of a nickel–chromium ferroalloy. The technological process includes a stage of preliminary ore preheating in a rotary kiln at a temperature not exceeding 700 °C. Preheating is performed both with and without the addition of fluxing components, while it is crucial to avoid the formation of liquid melts at this stage. The ore then undergoes primary smelting with additives in a special smelting furnace, resulting in the formation of an ore–flux melt. This melt is then directed to an electric arc furnace for reductive smelting. The process gases from both the smelting and electric arc furnaces are efficiently utilized to preheat the raw material, thereby enhancing the overall energy efficiency of the process.
One of the widely adopted pyrometallurgical approaches to processing oxidized nickel ores is a two-stage scheme that includes a preliminary thermal stage followed by smelting. In the first stage, the raw material—nickel-bearing ore—undergoes drying to reduce its moisture content to 10–15%. The resulting mixture of ore and fluxing additives is then, if necessary, calcined in a rotary kiln at temperatures ranging from 500 to 1300 °C. The resulting calcine is fed in a hot state directly into the smelting unit without intermediate cooling. The main processing stage is carried out in a two-zone Vanyukov furnace. In the smelting zone of this furnace, thermal treatment of the calcine occurs through the combustion of carbonaceous fuel (natural gas, coal, etc.) in a mixture of process oxygen and air. In the subsequent reduction zone, iron, nickel, and other valuable metals are reduced. The smelting products—ferronickel or matte, as well as slag—are tapped from the furnace and directed to further processing according to the technological schemes of the specific enterprise [21].
The authors of [22] examine the process of the carbothermic reduction of nickel-bearing laterites from Eastern Anatolia using an induction furnace and graphite crucibles. Special attention is given to the influence of the amount of reductant, process duration, and flux addition on the efficiency of nickel, cobalt, and chromium recovery. The process optimization involved varying the proportion of reductant (from 5% to 30% metallurgical coke), the reduction time (from 15 to 35 min), and the amount of flux (CaO) in the charge (from 0% to 6%). Experiments showed that the best results were achieved with the addition of 30% coke and a 35-minute reduction time: the recovery rates reached 93.2% for Ni and 93.87% for Co. Additionally, the use of 6% CaO as a flux, with 10% reductant and 25 min of smelting, effectively reduced the slag formation temperature and increased the metal recovery rate.
The aim of this study is to develop and verify a technology for producing a nickel- and chromium-containing ferroalloy based on the carbothermic reduction of low-grade nickel ores from the Batamsha deposit, with an assessment of the phase composition of the products and the effect of slag basicity on the distribution of alloying elements.
2. Materials and Methods
Calculations of phase equilibrium and thermodynamic distribution of nickel among various chemical compounds during the carbothermic reduction of nickel ore were performed using the Chemistry 10 software package (Outotec Research Oy, Pori, Finland). Chemistry 10 is a software tool for thermodynamic modeling of chemical processes, based on the Gibbs free energy minimization method. An open system was used for the calculation of the reduction processes, with the gaseous phase (CO and CO2) being removed and a partial oxygen pressure of PO2 = 10−15. The program enables the calculation of equilibrium phase and chemical compositions of multicomponent systems under varying temperatures, pressures, and interaction conditions. It is widely used in research on reduction processes, smelting, oxidation, and the synthesis of alloys and compounds.
Phase equilibrium calculations and the thermodynamic distribution of nickel among various chemical compounds during the carbothermic reduction of nickel ore were carried out using the HSC Chemistry 10.0 software package. This software is designed for thermodynamic modeling of chemical processes and is based on the Gibbs free energy minimization method, which allows determination of the equilibrium state of a system under given conditions. Specifically, the Equilibrium Compositions (Gibbs Energy Minimization) module was used in the modeling. This module enables the calculation of phase and chemical compositions of multicomponent systems over a wide range of temperatures and pressures, including both closed and open systems.
The input data for the calculations included the initial composition of the nickel ore, the reductant (carbon), and temperature and pressure parameters corresponding to the intended technological process. The program takes into account the possible formation of various gas, solid, and liquid phases and determines their composition under thermodynamic equilibrium. This makes it particularly useful for analyzing processes such as reduction, smelting, oxidation, and synthesis, as well as the design of metallurgical operations.
The same charge materials used in the laboratory stage published in [23] were employed in the pilot-scale tests. The initial components included low-grade nickel ore from the Batamsha deposit, coke produced in the Republic of Kazakhstan, Shubarkol coal, and lime. The technical characteristics of the reductants, as well as the chemical composition of the ash, lime, and ore, are presented in Table 1.

Table 1.
Chemical and Technical Composition of Charge Materials.
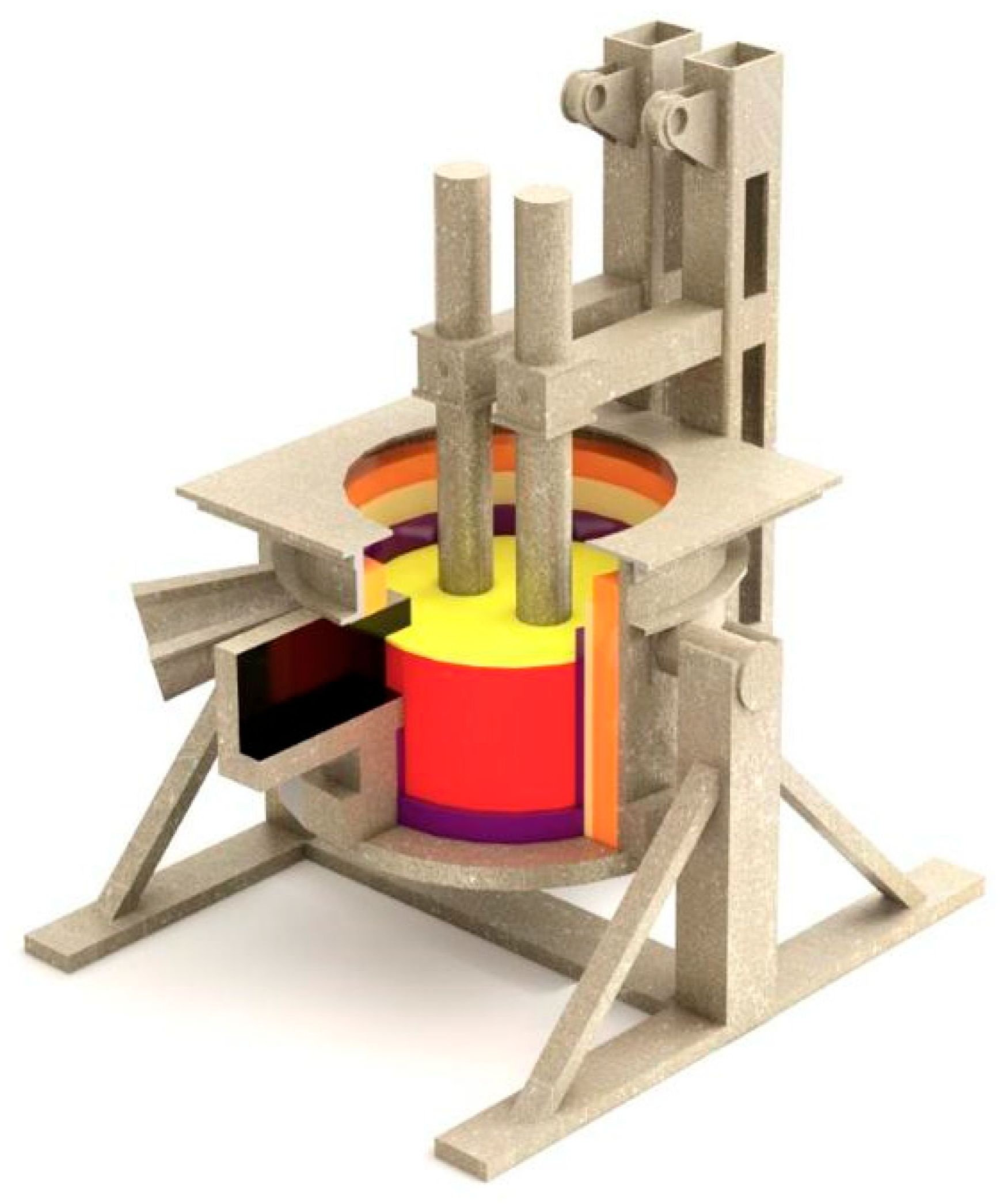
As part of the research, a series of pilot-scale smelting experiments were conducted to produce a nickel- and chromium-containing alloy using a refining-type submerged arc furnace equipped with a 100 kVA transformer and an inclined bath. The operating voltage of the transformer was 49 V (Figure 1). Reduction was carried out using a mixture of Kazakhstani coke and low-ash Shubarkol coal in a mass ratio of 75:25, selected based on positive results obtained during previous laboratory tests [4].
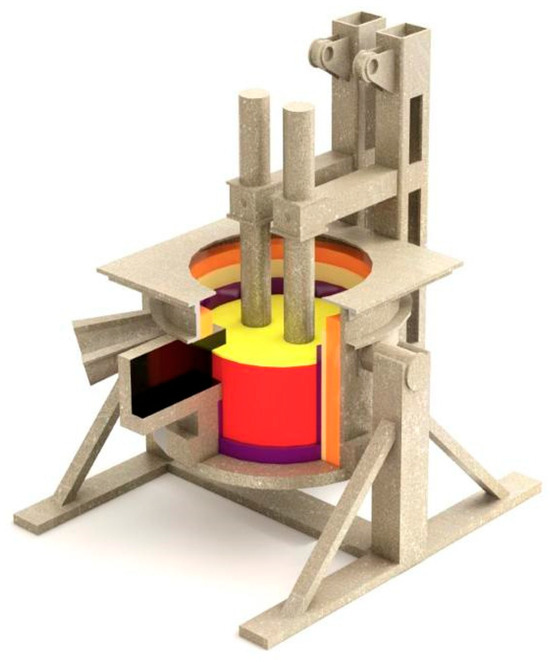
Figure 1.
3D Model of a 100 kVA Refining-Type Submerged Arc Furnace.
The required temperature in the reaction zone was achieved using an electric arc generated by a graphite electrode with a diameter of 150 mm. The electric furnace had four stages for regulating the secondary voltage within the range of 18.5 to 50 V (Figure 1). The furnace hearth was made of densely compacted conductive mass. To facilitate the outflow of the melt from the reduction zone, the hearth was inclined at an angle of 5–7° towards the tap hole. To ensure effective burn-through and opening of the tap hole, a system with a 30 mm graphite electrode was installed. The furnace’s interior lining consisted of fireclay bricks, and the tap hole was sealed using conical plugs made from a mixture of refractory clay and graphite fines.
The phase composition of the samples under study was determined using a D8 ADVANCE diffractometer (Bruker Elemental GmbH, Kalkar, Germany) equipped with a high-temperature chamber HTK 2000. Measurements were carried out in the 2θ angle range from 10° to 80°, with a scanning step of 0.02° and a data acquisition time of 1 s per step.
The chemical analysis of the samples was conducted in accordance with State Standard 22772.4-77, State Standard 22772.6-77, and State Standard 22772.7-96 [24,25,26].
3. Results and Discussion
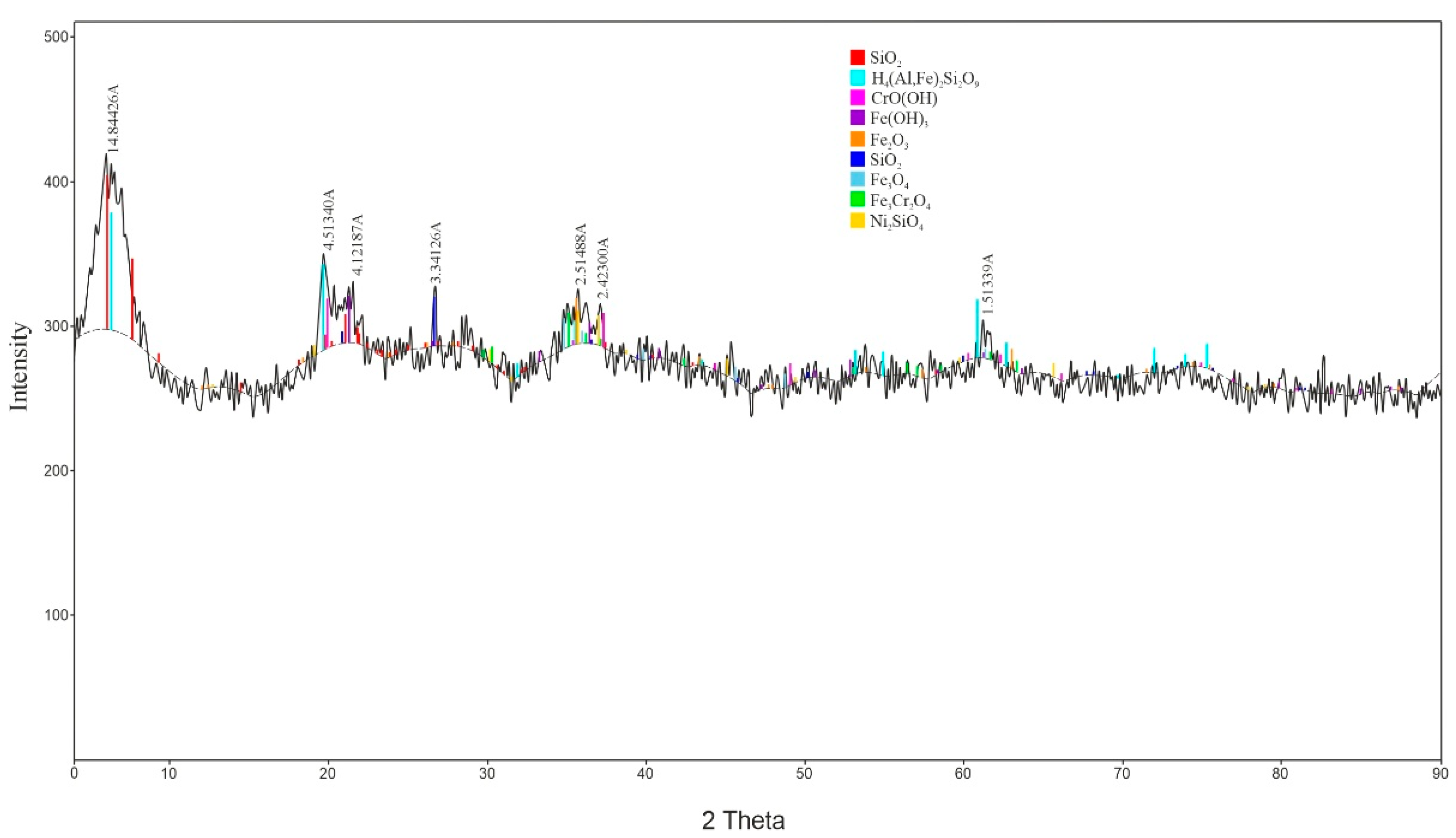
According to the results of the X-ray diffraction analysis conducted using a D8 ADVANCE X-ray diffractometer (BRUKER, Karlsruhe, Germany) (Figure 2), the mineral composition of the low-grade nickel ore is predominantly represented by quartz, nontronite, chromium metahydroxide, goethite, magnetite, and iron chromite. The nickel in the ore was detected in the form of nickel (II) silicate.
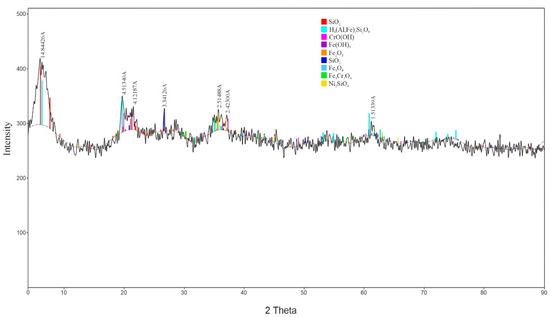
Figure 2.
X-ray Diffraction Pattern of the Low-Grade Nickel Ore Sample.
The thermodynamic modeling of the carbothermic reduction process of low-grade nickel ore from the Batamsha deposit was carried out using the Chemistry 10 software package. The initial data for the modeling included the mineral composition of the ore as determined by X-ray phase analysis. The modeling covered a temperature range from 100 to 1500 °C and allowed tracking of the phase distribution changes of nickel during reduction, including the formation of free metal, silicide, and intermetallic compounds. To simulate realistic process conditions, carbon (10 g) and lime (CaO, 39 g) were additionally included in the model to ensure reduction conditions and silica binding. This adjustment represented the excess presence of reductant and flux.
The results of the modeled phase distribution of nickel at various temperatures are presented in Table 2. As the temperature increases, nickel transitions from the oxide phase to the metallic phase and, subsequently, into more stable compounds with silicon.

Table 2.
Phase Distribution of Nickel Depending on Temperature.
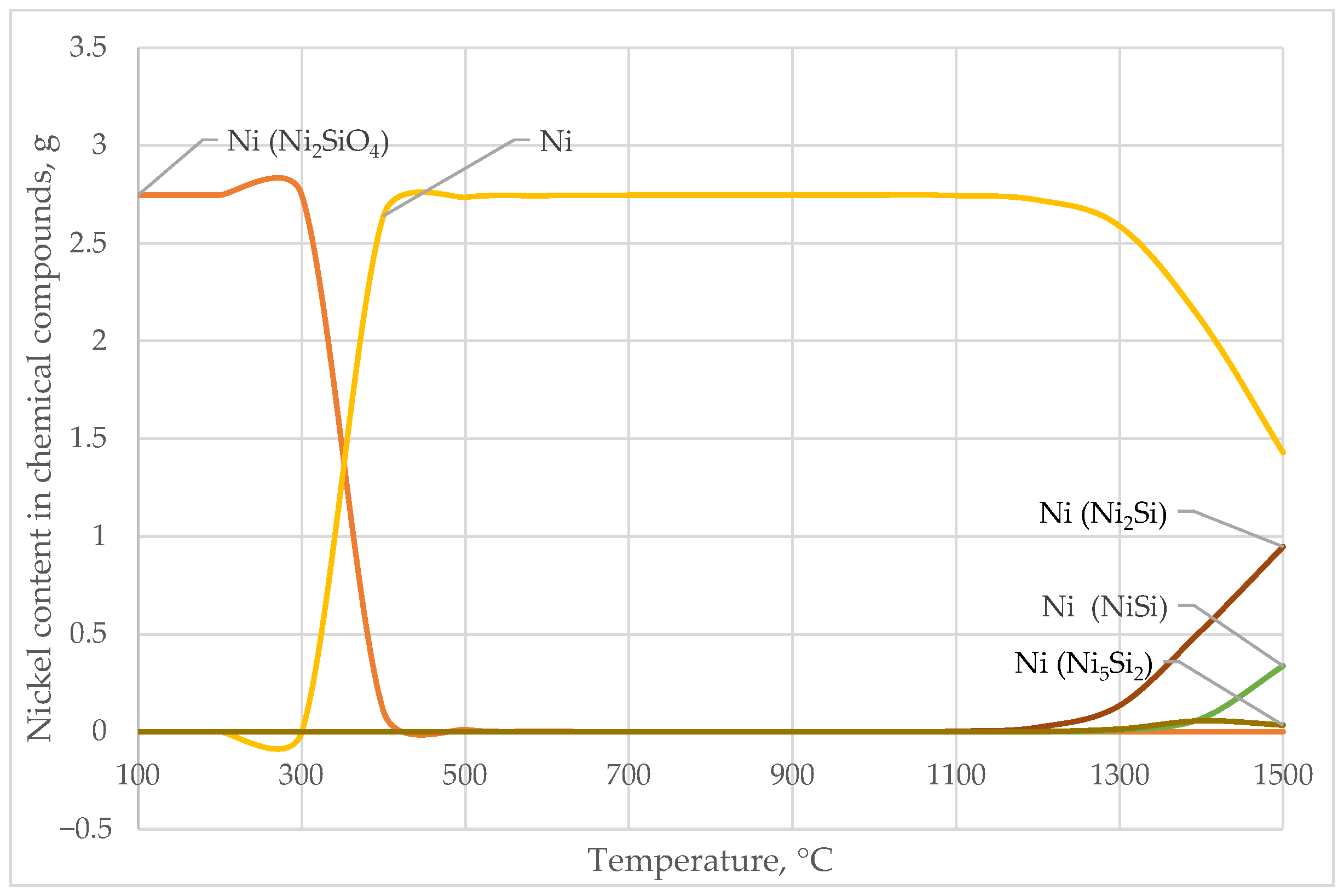
At low temperatures (up to ~300 °C), nickel is almost entirely present in the form of Ni2SiO4. As the temperature increases, the content of Ni2SiO4 sharply decreases, starting from 400 °C. Free nickel (Ni) begins to appear at 400 °C, reaching its maximum concentration between 700 and 900 °C, and then gradually decreases.
With further temperature increases, nickel silicides—Ni2Si, NiSi, Ni5Si2, and NiSi2—start to form gradually, initially in trace amounts (10−20–10−9 g), followed by a significant increase. Ni2Si becomes the dominant phase at T > 1000 °C (up to 0.95 g at 1500 °C), while NiSi and Ni5Si2 also increase substantially after 1100 °C.
Intermetallic compounds such as Cr-Ni, Cr-Fe-Ni, and Fe-Ni appear in very small quantities (10−50–10−20 g). These trends are clearly illustrated in the corresponding diagram (Figure 3). In Figure 3, the nickel (Ni) contents in the phase compounds Ni2SiO4, Ni2Si, and NiSi are presented, highlighting the redistribution of Ni among different silicide phases with increasing temperature.
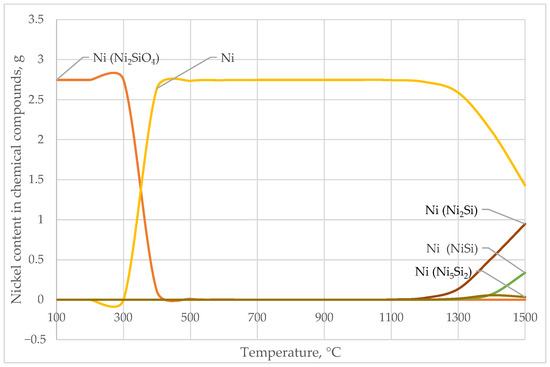
Figure 3.
Temperature Dependence of Nickel Content in Nickel-Bearing Phases.
During thermodynamic modeling of the reduction of chromium-containing components in the ore, a stepwise redistribution of chromium among various phases was observed, depending on the temperature (Table 3).

Table 3.
Phase Distribution of Chromium Depending on Temperature.
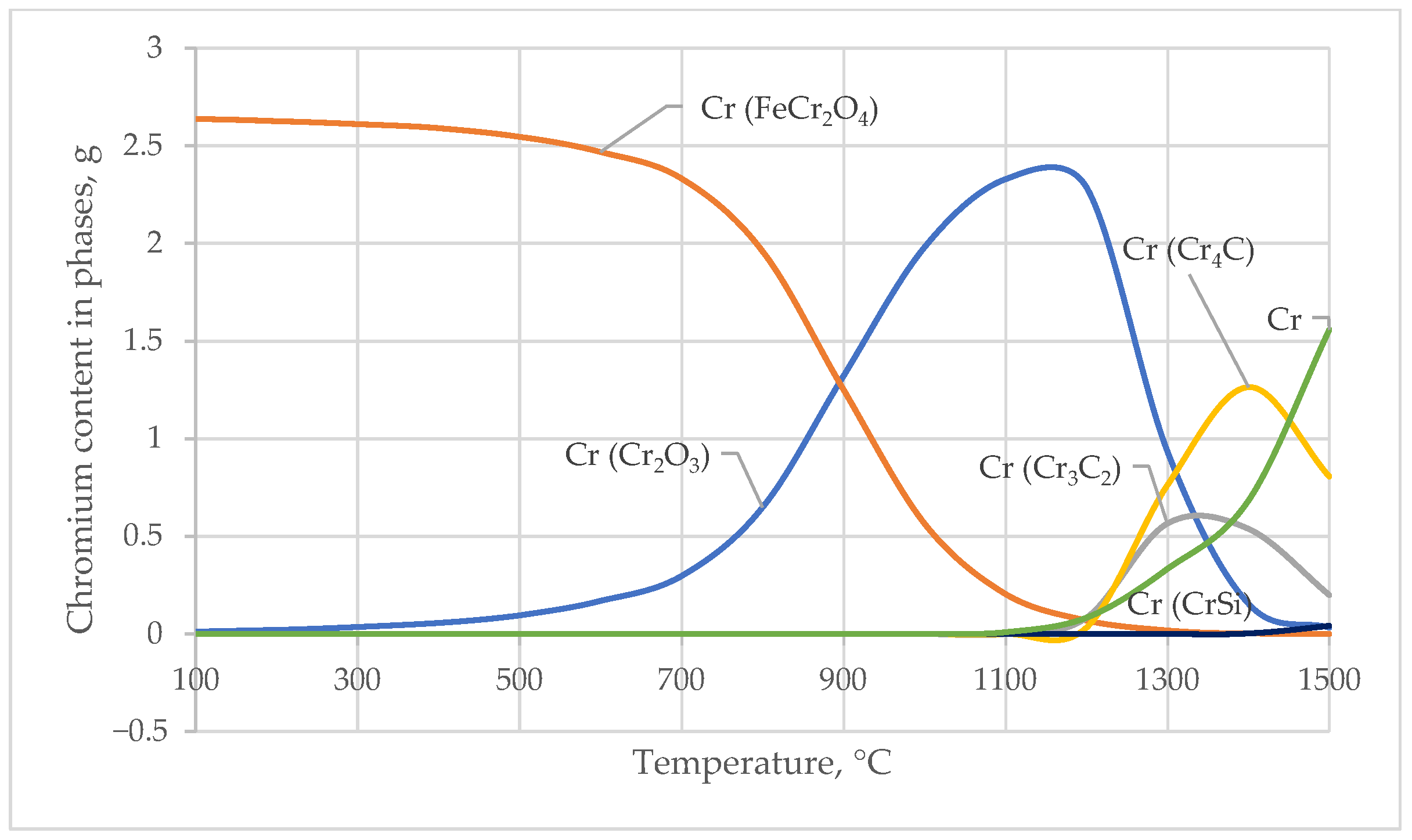
The main transformations involve the transition of Cr from ferrochromite and oxides into carbides and silicides. As the temperature increases, the content of ferrochromite gradually decreases, nearly disappearing at T > 1200 °C, indicating its decomposition and the redistribution of chromium into other phases.
The Cr2O3 phase increases as the ferrochromite decomposes and is present in significant amounts in the mid-temperature range. With further temperature increases, it is partially consumed in the formation of reduced compounds, such as carbides and silicides. Chromium carbides (Cr3C2, Cr4C) form at the high-temperature stage of reduction, with Cr3C2 forming most actively at 1300 °C. Cr4C also increases up to 1400 °C, indicating the deep reduction of chromium into carbide phases under conditions of carbon excess and elevated temperatures.
Iron–chromium phases appear in negligible quantities across the entire temperature range, suggesting weak formation of Fe-Cr intermetallics under the modeled conditions.
Chromium silicides (CrSi, CrSi2, Cr3Si, Cr5Si3) appear in extremely small quantities initially, but by 1500 °C, they become noticeable components of the phase composition, with CrSi and Cr3Si reaching the highest concentrations.
These trends are clearly illustrated in the corresponding diagram (Figure 4). Figure 4 shows the chromium (Cr) contents in the phase compounds Cr2O3, FeCr2O4, Cr4C, Cr3C2, and CrSi, reflecting the redistribution of chromium among oxide, carbide, and silicide phases as the temperature increases.
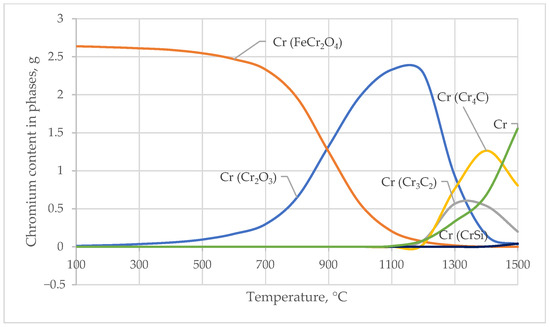
Figure 4.
Temperature Dependence of Chromium Content in Chromium-Bearing Phases.
The experiments were conducted using nickel ores from the Batamsha deposit, with a particle size range of 5–20 mm. The tests were carried out in a submerged arc furnace equipped with a 100 kVA transformer. The furnace was preheated over a period of 12 h using a coke bed, which served as both an electrical conductor and a base lining protector. Upon completion of the preheating phase, the furnace was completely cleared of residual coke bed material. The electrical parameters during the preheating period included a secondary voltage of 24.6 V and a current of 150–200 A on the high side. Smelting was carried out continuously, with the charge being fed in small portions as the charge level subsided, and metal tapped periodically every two hours into cast-iron molds. The tap hole was opened using either electric arc drilling or a steel rod. For each tapping, the metal and slag were weighed, and samples were taken for chemical analysis.
The charge materials were loaded around the electrode, maintaining a cone-shaped formation. During an 8-hour shift, up to 8 batches of charge were consumed, each containing 20 kg of nickel ore.
The charge cone around the electrode measured 0.3–0.45 m in height at an angle of 35–40°. The smelting electrical mode was selected to create the necessary thermal conditions in the shaft with deep electrode immersion. The secondary voltage ranged from 18 to 24 V, with an electrode current of 150–200 A (on the primary transformer winding). The charge descended into the reaction zone smoothly and evenly, without collapses. Current load fluctuations were observed 1–1.5 h after tapping, when metal accumulated in the furnace. After tapping (Figure 5), adjusting the burden, and loading a new portion of the charge, the current load stabilized. The charge was evenly heated by the outgoing reaction gases, creating favorable conditions for the progression of reduction processes.

Figure 5.
Tapping of Metal and Slag from the Furnace.
No significant deviations from the normal operation mode were observed during the first variant of smelting. The reaction zone was characterized by high temperatures, and both metal and slag were tapped actively. If necessary, the tap hole was poked using iron rods. Upon the completion of melt tapping, intense gas release from the tap hole was observed, indicating the complete drainage of metal from the reaction zone. Once these process indicators were confirmed, the tap hole was sealed, and a new batch of charge was loaded into the furnace, forming a cone around the electrode.
Overall, the process of producing nickel–chromium ferroalloy using coke and coal as reductants was characterized by deep electrode immersion and stable current load. In the first smelting variant, the ratio of charge materials (ore and reductants) was not altered. The process of producing nickel–chromium pig iron using coke and low-ash long-flame coal was characterized by smooth operation, stability, and consistent parameters. The technological parameters for producing nickel–chromium ferroalloy in a 100 kVA electric furnace are presented in Table 4. The chemical compositions of the final products are shown in Table 5.

Table 4.
Technological Parameters for Producing Nickel–Chromium Ferroalloy in a 100 kVA Electric Furnace.

Table 5.
Chemical Composition of Nickel–Chromium Ferroalloy and Slag.
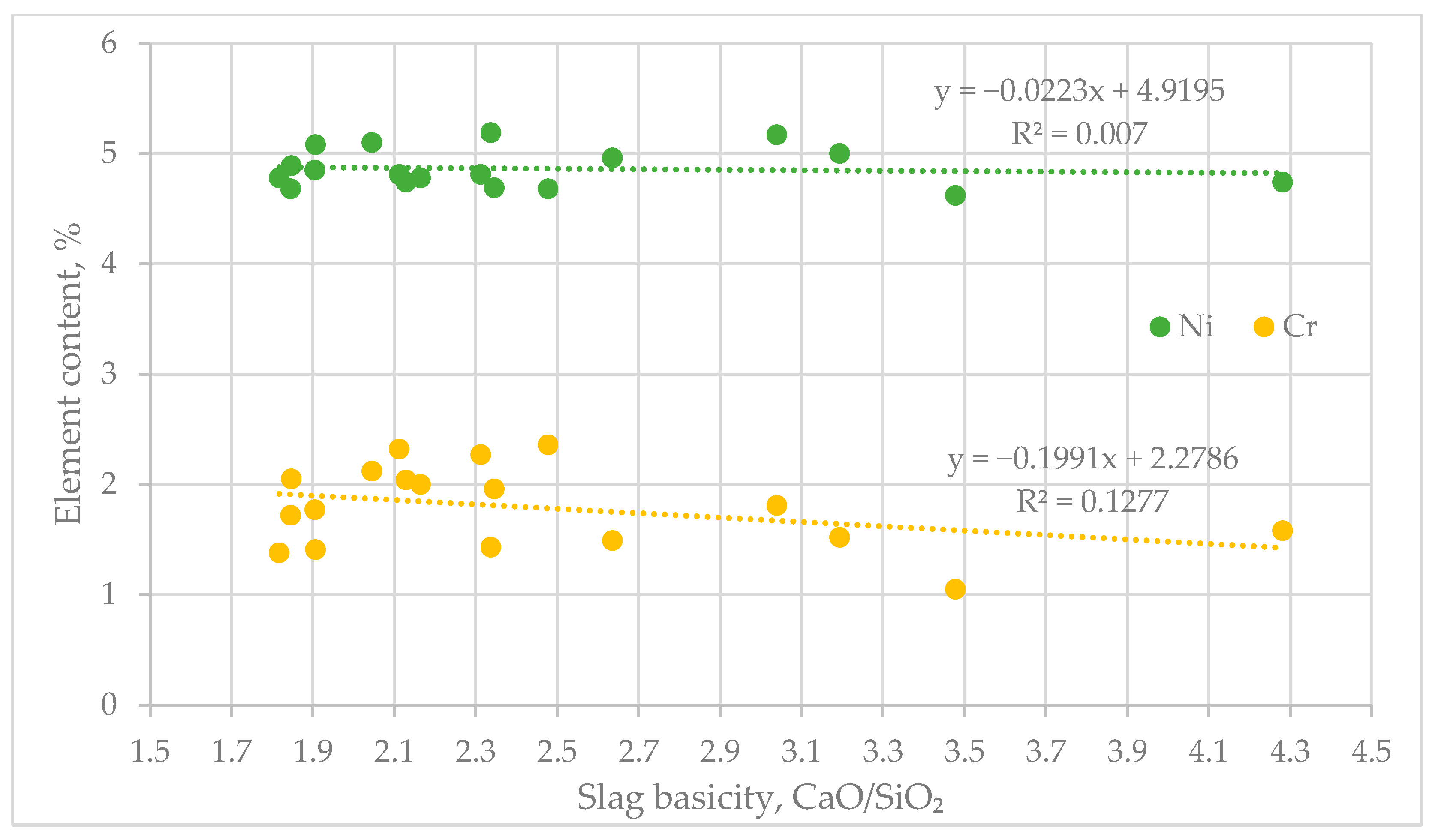
Based on the data presented in Table 4, an analysis was carried out on the effect of slag basicity on the nickel and chromium content in the alloy (Figure 6).
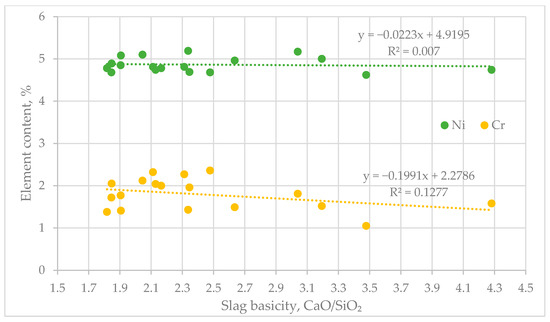
Figure 6.
Effect of Basicity on the Distribution of Nickel (Ni) and Chromium (Cr).
The diagram reflects the influence of the basicity of the medium (CaO/SiO2 ratio) on the distribution of nickel (Ni) and chromium (Cr). The analysis of the regression trends shows a slight negative correlation between increasing basicity and the content of the studied elements [27,28].
For nickel, the linear regression has a negligible slope (–0.0223) and a very low coefficient of determination (R2 = 0.007), indicating no significant influence of basicity changes on its content. In the case of chromium, the slope is more pronounced (–0.1991) and R2 = 0.1277, which indicates a moderate negative correlation. Optimal values for both elements were recorded at a CaO/SiO2 ratio within the range of 2.02–2.36. In this interval, the nickel content remains around 5%, and that of chromium ranges from 2% to 2.3%, suggesting favorable thermodynamic and kinetic conditions for the process.
Thus, the basicity range of 2.02–2.36 is considered optimal for the effective co-extraction of nickel and chromium during the processing of materials with the corresponding composition [29,30].
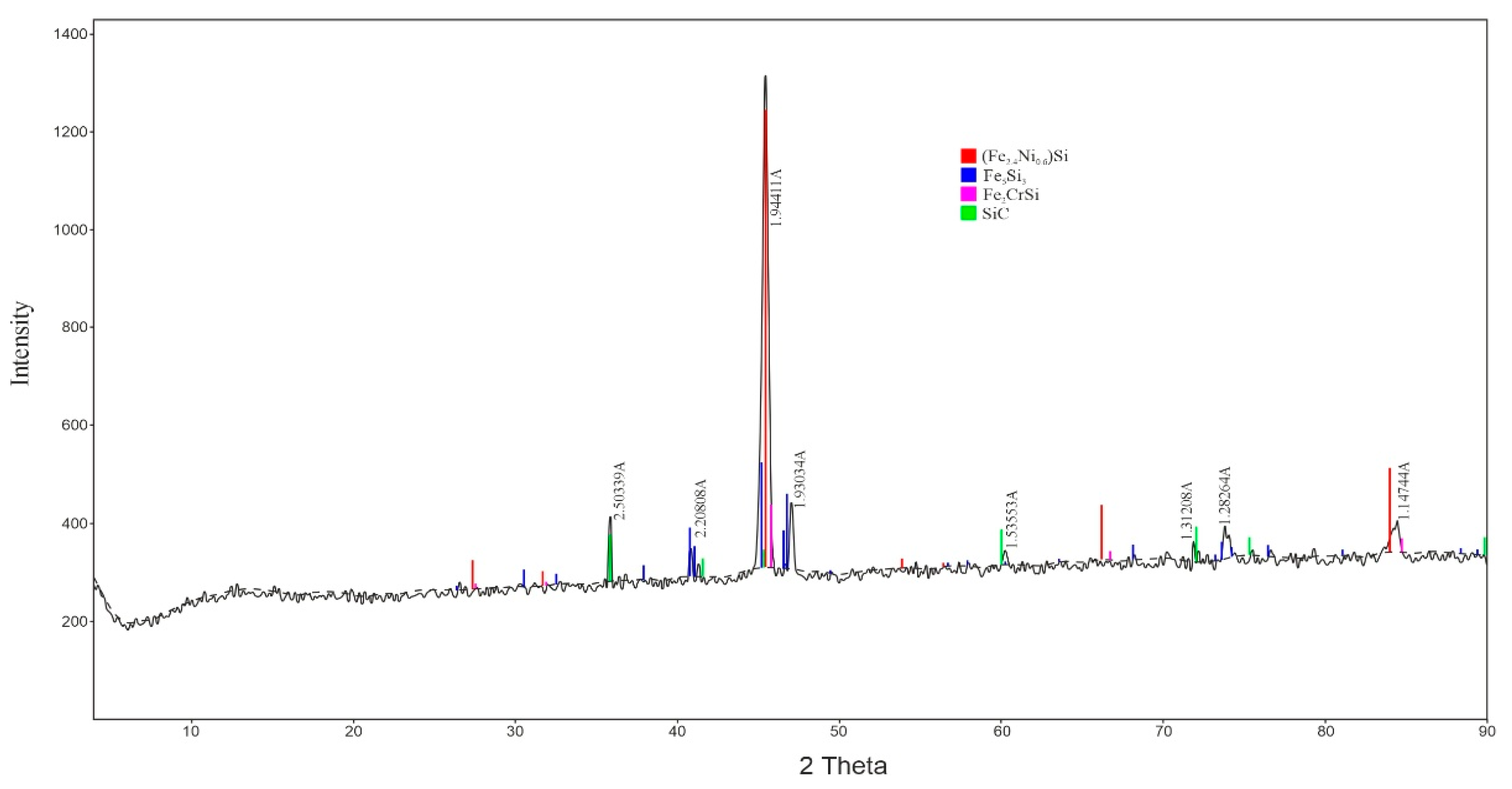
After smelting and tapping, samples of the obtained alloy were collected for subsequent laboratory analysis. To determine the phase composition of the resulting nickel–chromium ferroalloy, an X-ray phase analysis (XRD) was performed (Figure 7). The analysis revealed that the structure of the obtained nickel–chromium ferroalloy consists of a mixture of intermetallic and carbide phases. The main phase identified was the intermetallic compound (Fe2.4Ni0.6)Si, indicating a high degree of alloying with nickel and silicon.
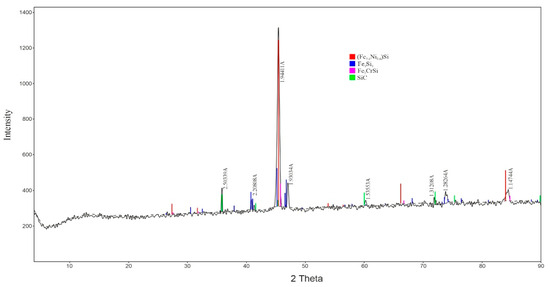
Figure 7.
X-ray Diffraction Pattern of the Nickel–Chromium Ferroalloy.
4. Conclusions
Successful thermodynamic modeling of nickel and chromium phase transformations during the reduction of ores from the Batamsha deposit was carried out, confirming the formation of silicide and intermetallic phases at high temperatures.
A series of pilot-scale smelting tests demonstrated the stability of the technological process when using coke and low-ash coal as reductants and confirmed the effectiveness of the selected charge composition.
The chemical analysis of the alloy and slag revealed a high nickel recovery rate (an average of 91%) and the formation of an alloy containing up to 5% Ni and 2.3% Cr. A comparative analysis of the modeling results obtained using HSC Chemistry 10 and the experimental data from the X-ray diffraction (XRD) and chemical analysis of the alloy showed that at a temperature of 1200 °C, the model predicts the formation of Ni (solid), Fe (solid), Ni3Si, and residual carbon phases, each of which are in good agreement with the phases identified experimentally.
The analysis of the slag basicity showed that optimal nickel and chromium contents in the alloy are achieved at CaO/SiO2 values in the range of 2.02–2.36.
The X-ray phase analysis of the resulting alloy confirmed the presence of silicide phases consistent with the results of the thermodynamic modeling, indicating the reliability of the proposed technological scheme.
Author Contributions
Conceptualization, B.K. and A.A. (Assylbek Abdirashit); methodology, B.K. and A.A. (Aigerim Abilberikova); software, O.S., T.Z. and Y.K.; validation, A.A. (Aigerim Abilberikova), O.S., T.Z. and B.U.; formal analysis, A.A. (Aigerim Abilberikova), B.U. and A.N.; investigation, O.S., D.Y. and B.U.; resources, A.A. (Assylbek Abdirashit), T.Z. and Y.K.; data curation, O.S. and A.N.; writing—original draft preparation, B.K., A.A. (Aigerim Abilberikova), T.Z., Y.K. and B.U.; writing—review and editing, B.K., A.A. (Aigerim Abilberikova), A.A. (Assylbek Abdirashit), O.S., D.Y. and A.N.; visualization, T.Z., Y.K. and A.A. (Assylbek Abdirashit); supervision, O.S. and D.Y.; project administration, B.K.; funding acquisition, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP 19679501).
Data Availability Statement
The data used to support the findings of this study are included within this article.
Acknowledgments
The authors express their gratitude for the administrative and technical support provided by K. Zhubanov Aktobe Regional University. The materials used in the experimental part of the study were kindly provided by the experimental base of the university.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tolymbekov, M.Z.; Kelamanov, B.S.; Baisanov, A.S.; Kaskin, K.K. Processing Kazakhstan’s chromonickel ore. Steel Transl. 2008, 38, 660–663. [Google Scholar] [CrossRef]
- Isatayev, K.K.; Seytimova, S.E.; Musayev, E.R. Prospects for the development of hard-to-enrich nickel ores in Kazakhstan. Min. J. Kazakhstan 2020, 2, 45–50. [Google Scholar]
- Bespalov, V.P.; Yermekbayev, K.T. Technological problems of processing low-grade nickel ores and ways to solve them. Bull. KASU 2021, 3, 32–39. [Google Scholar]
- Gurevich, L.I.; Udodov, V.G. Pyrometallurgy of Refractory Metals; Metallurgiya: Moscow, Russia, 1987; 320p. (In Russian) [Google Scholar]
- Derin, B.; Aydin, F.; Topkaya, Y.A. Production of ferro-nickel from nickel laterites in a DC arc furnace. Miner. Eng. 2008, 21, 683–689. [Google Scholar]
- Dalvi, A.D.; Bacon, W.G.; Osborne, R.C. The Past and the Future of Nickel Laterites. In Proceedings of the PDAC International Convention, Toronto, Canada, 26 January 2004; 21p. [Google Scholar]
- Kelamanov, B.; Yessengaliyev, D.; Sariev, O.; Akuov, A.; Samuratov, Y.; Zhuniskaliyev, T.; Kuatbay, Y.; Mukhambetgaliyev, Y.; Kolesnikova, O.; Zhumatova, A.; et al. Technological Analysis of the Production of Nickel-Containing Composite Materials. J. Compos. Sci. 2024, 8, 179. [Google Scholar] [CrossRef]
- Li, X.; Nie, J.; Wang, X.; Li, K.; Zhang, H. Effect of interface form on creep failure and life of dissimilar metal welds involving nickel-based weld metal and ferritic base metal. Chin. J. Mech. Eng. (Engl. Ed.) 2024, 37, 18. [Google Scholar] [CrossRef]
- Zhan, Z.; Shi, Z.; Wang, Z.; Lu, W.; Chen, Z.; Zhang, D.; Chai, F.; Luo, X. Effect of manganese on the strength–toughness relationship of low-carbon copper- and nickel-containing hull steel. Materials 2024, 17, 1012. [Google Scholar] [CrossRef]
- Abdul, F.; Firdausi, S.; Widyartha, A.B.; Setiyorini, Y.; Pintowantoro, S. The Role of Limestone in Enhancing Selective Reduction of Nickel in the Carbothermic Reduction of Laterite Nickel. Trans. Indian Inst. Met. 2023, 76, 2211–2219. [Google Scholar] [CrossRef]
- Prabowo, B.; Yuliah, Y.; Widodo, W. Effects of Reduction Time on Carbothermic Reduction of Lateritic Nickel Ore Using Palm Kernel Shell as Green Reducing Agent. IOP Conf. Ser. Earth Environ. Sci. 2018, 141, 012008. [Google Scholar] [CrossRef]
- Pintowantoro, S.; Abdul, F. Selective Reduction of Laterite Nickel Ore. Mater. Trans. 2019, 60, 2245–2255. [Google Scholar] [CrossRef]
- Ilyas, S.; Srivastava, R.R.; Kim, H.; Ilyas, N.; Sattar, R. Extraction of Nickel and Cobalt from a Laterite Ore Using the Carbothermic Reduction Roasting–Ammoniacal Leaching Process. Sep. Purif. Technol. 2020, 237, 115971. [Google Scholar] [CrossRef]
- Harjanto, S.; Rhamdhani, M.A. Sulfides Formation in Carbothermic Reduction of Saprolitic Nickel Laterite Ore with Addition of Sodium Thiosulfate and Soda Ash. Minerals 2019, 9, 631. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, C.; Xue, Z.; Li, F.; Li, S.; Duan, H.; Zhang, H. High-Grade Ferronickel Concentrates Prepared from Laterite Nickel Ore by a Carbothermal Reduction and Magnetic Separation Method. Minerals 2024, 14, 7132. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Z.; Wang, Z.; Sun, T. Effect of Co-Reduction Conditions of Nickel Laterite Ore and Red Mud on Ferronickel Particle Size Characteristics. Minerals 2022, 12, 357. [Google Scholar] [CrossRef]
- Setiawan, I.; Harjanto, S.; Subagja, R. Low-Temperature Carbothermic Reduction of Indonesia Nickel Lateritic Ore with Sub-Bituminous Coal. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012019. [Google Scholar] [CrossRef]
- Pickles, C.A.; Forster, J.; Elliott, R. Thermodynamic Analysis of the Carbothermic Reduction Roasting of a Nickeliferous Limonitic Laterite Ore. Miner. Eng. 2014, 61, 33–40. [Google Scholar] [CrossRef]
- Kelamanov, B.; Samuratov, Y.E.; Akuov, A.; Sariev, O.; Tastanova, L.; Abdirashit, A. Thermodynamic-diagram analysis of Fe–Ni–C–O system. Metalurgija 2022, 61, 261–264. [Google Scholar]
- Method for Processing Oxidized Nickel Ores to Produce a Nickel–Chromium-Containing Ferroalloy. RU Patent 2453617 C2 Russian Federation. No. 2010129269/02. 27 May 2012. Available online: https://patentimages.storage.googleapis.com/84/14/03/b822c146a5f8d1/RU2453617C2.pdf (accessed on 5 June 2025).
- Method for Processing Oxidized Nickel Ore. RU Patent 2624880 C2 Russian Federation. No. 2016133276. 6 July 2017. Available online: https://patenton.ru/patent/RU2624880C2 (accessed on 5 June 2025).
- Yildirim, H.; Turan, A.; Kahraman, N. Nickel Pig Iron Production from Lateritic Nickel Ores [Electronic Resource]//ResearchGate. 2018. Available online: https://www.researchgate.net/profile/Ahmet-Turan-3/publication/323177473_NICKEL_PIG_IRON_PRODUCTION_FROM_LATERITIC_NICKEL_ORES (accessed on 5 June 2025).
- Abdirashit, A.; Kelamanov, B.; Sariyev, O.; Yessengaliyev, D.; Abilberikova, A.; Zhuniskaliyev, T.; Kuatbay, Y.; Naurazbayev, M.; Nazargali, A. Study of Nickel–Chromium-Containing Ferroalloy Production. Processes 2025, 13, 1258. [Google Scholar] [CrossRef]
- GOST 22772.4-77; Manganese Ores, Concentrates and Agglomerates. Methods for Determination of Iron Content (Total). State Committee for Standards of the Council of Ministers of the USSR: Moscow, Russia, 1979.
- GOST 22772.6-77; Manganese Ores, Concentrates and Agglomerates. Methods for the Determination of Phosphorus. Committee for Standards of the Council of Ministers of the USSR: Moscow, Russia, 1979.
- GOST 22772.7-96; Manganese Ores, Concentrates and Agglomerates. Methods for Determination of Sulfur. Committee for Standards of the Council of Ministers of the USSR: Moscow, Russia, 1999.
- Mallieswaran, K.; Rajasekaran, S.; Kumar, M.V.; Rajendran, C. Steel shot peening effects on friction stir welded AA2014-T6 aluminum alloys. Mater. Test. 2022, 64, 1202–1213. [Google Scholar] [CrossRef]
- Mallieswaran, K.; Rajendran, C.; Padmanabhan, R.; Rajasekaran, S. Evaluation of nickel shot peening process on strength of friction stir welded AA2014-T6 aluminum alloy joints. Pract. Metallogr. 2023, 60, 442–460. [Google Scholar] [CrossRef]
- Lim, J.-D.; Kim, J.; Kim, D.; Park, Y. Effects of Oxygen Partial Pressure and Slag Basicity on the Behavioral Characteristics of Ni in Slag Drying Smelting. Arch. Metall. Mater. 2024, 69, 437–441. [Google Scholar] [CrossRef]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Chromium Partitioning and Speciation in Spinel-Bearing Slags: Implications for Chromium Leaching Behavior. J. Geochem. Explor. 2015, 154, 226–235. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).