Microalgae Cultivation in Wastewater: How Realistic Is This Approach for Value-Added Product Production?
Abstract
1. Introduction
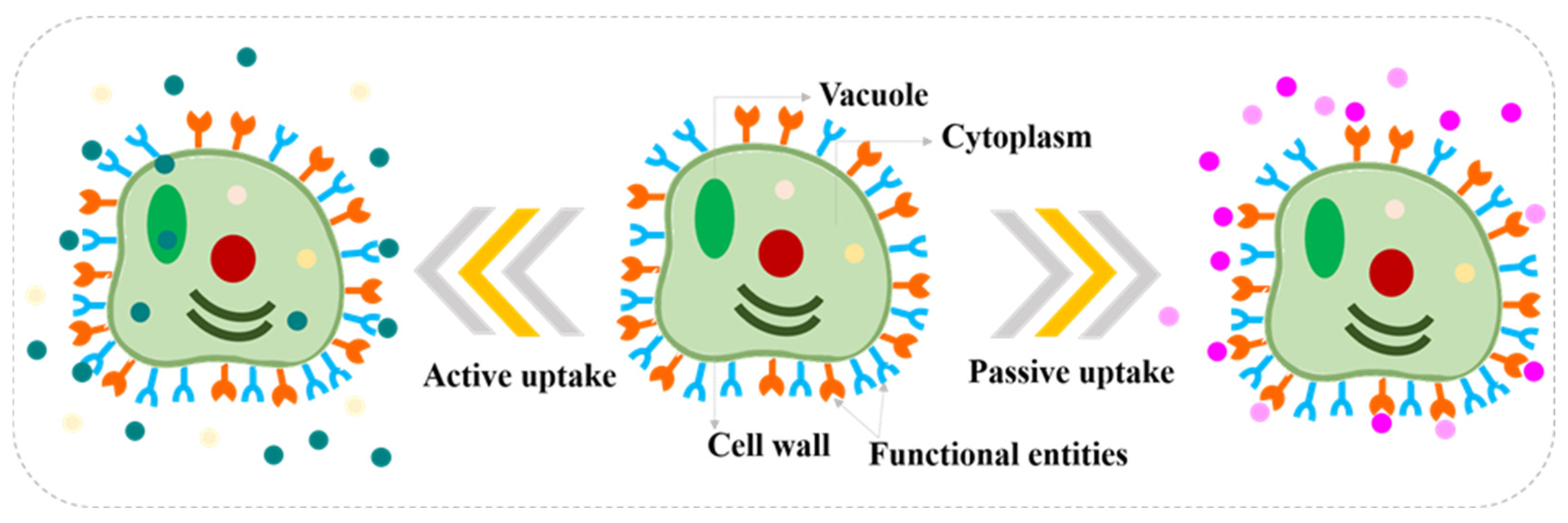
2. Microalgae-Based Phycoremediation
3. Technoeconomics of Microalgae Production Using Wastewater
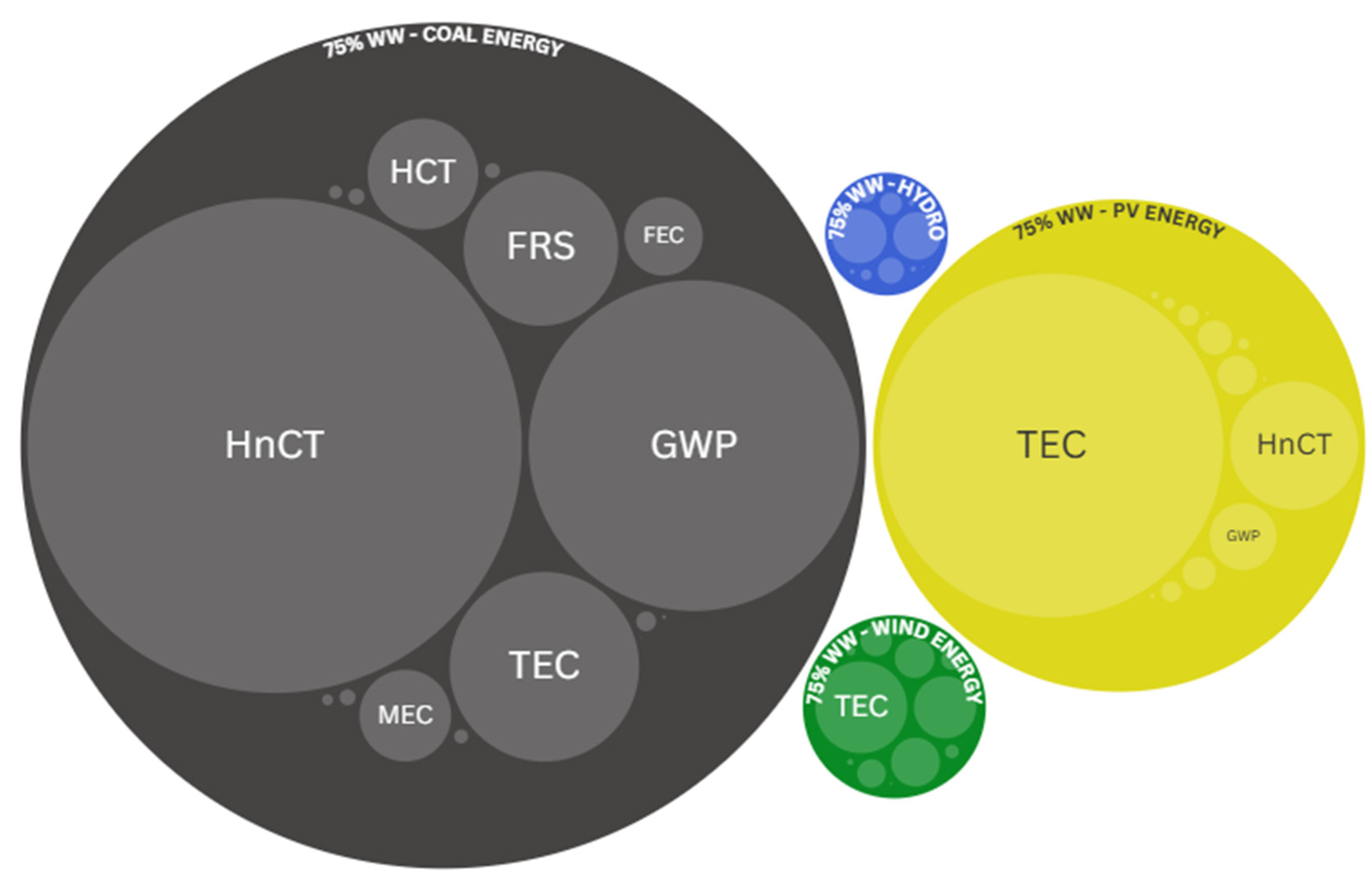
4. Environmental Impact of Microalgae Production Using Wastewater: Environmental Sustainability Metrics and Indicators
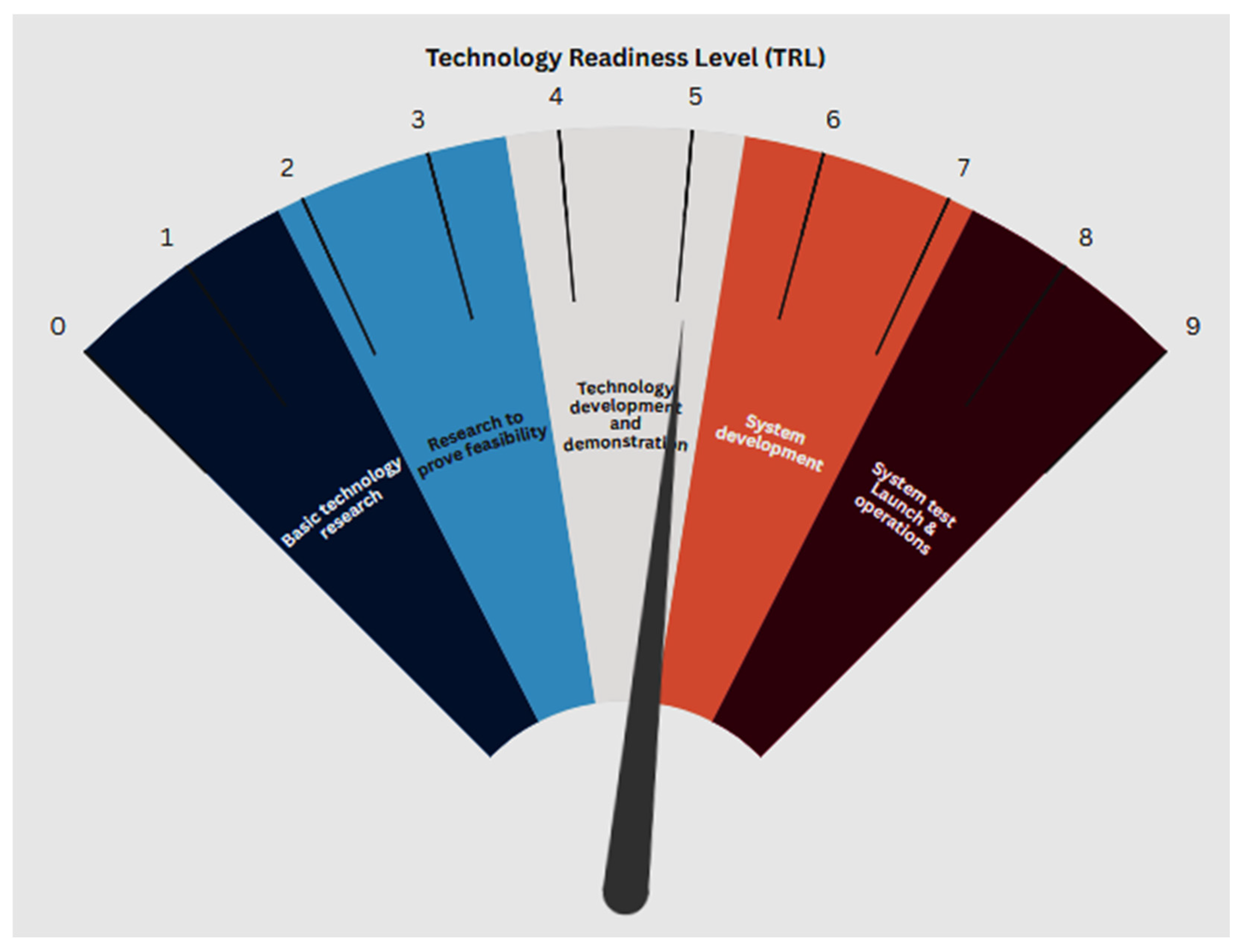
5. Technological Readiness Level of Microalgae Cultivation Using Wastewater
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TRL | Technology Readiness Level |
| WW | Wastewater |
| PV | Photovoltaic |
| FPMF | Fine particulate matter formation |
| FRS | Fossil resource scarcity |
| FEC | Freshwater ecotoxicity |
| FEU | Freshwater eutrophication |
| GWP | Global warming potential |
| HCT | Human carcinogenic toxicity |
| HnCT | Human non-carcinogenic toxicity |
| IR | Ionizing radiation |
| LU | Land use |
| MEC | Marine ecotoxicity |
| MRS | Mineral resource scarcity |
| OFTE | Ozone formation affecting terrestrial ecosystems |
| SOD | Stratospheric ozone depletion |
| TAC | Terrestrial acidification |
| TEC | Terrestrial eco-toxicity |
| TWC | Total water consumption |
Appendix A
| Base Case | ||||
| Coal | Photovoltaic | Onshore wind | Hydropower | |
| FPMF (kg PM2.5 eq.) | 3.00 × 10−1 | 2.64 × 10−2 | 7.41 × 10−3 | 2.51 × 10−3 |
| FRS (kg oil eq.) | 6.02 × 101 | 2.74 × 100 | 1.07 × 100 | 2.77 × 10−1 |
| FEC (kg 1,4-DCB) | 1.53 × 101 | 2.94 × 100 | 2.02 × 100 | 5.56 × 10−2 |
| FEU (kg P eq.) | 6.22 × 10−1 | 9.59 × 10−3 | 1.83 × 10−3 | 3.40 × 10−4 |
| GWP (kg CO2 eq.) | 2.74 × 102 | 1.12 × 101 | 3.93 × 100 | 1.66 × 100 |
| HCT (kg 1,4-DCB) | 3.07 × 101 | 1.06 × 100 | 5.94 × 100 | 8.07 × 10−1 |
| HnCT (kg 1,4-DCB) | 6.14 × 102 | 4.14 × 101 | 9.70 × 100 | 1.21 × 100 |
| IR (kBq Co-60 eq.) | 6.42 × 10−1 | 1.04 × 100 | 2.26 × 10−1 | 6.78 × 10−2 |
| LU (m2a crop eq.) | 4.85 × 10−1 | 3.12 × 10−1 | 4.59 × 10−1 | 5.36 × 10−2 |
| MEC (kg 1,4-DCB) | 2.11 × 101 | 3.88 × 100 | 2.49 × 100 | 7.77 × 10−2 |
| MRS (kg Cu eq.) | 3.15 × 10−2 | 1.41 × 10−1 | 9.32 × 10−2 | 1.83 × 10−2 |
| OFTE (kg NOx eq.) | 4.21 × 10−1 | 2.67 × 10−2 | 1.23 × 10−2 | 4.95 × 10−3 |
| SOD (kg CFC11 eq.) | 4.24 × 10−5 | 5.23 × 10−6 | 1.72 × 10−6 | 8.10 × 10−7 |
| TAC (kg SO2 eq.) | 9.75 × 10−1 | 5.61 × 10−2 | 1.35 × 10−2 | 4.11 × 10−3 |
| TEC (kg 1,4- DCB) | 9.01 × 101 | 2.97 × 102 | 2.11 × 101 | 5.66 × 100 |
| TWC (m3) | 5.51 × 10−1 | 3.43 × 10−1 | 4.87 × 10−2 | 7.44 × 100 |
| 10% WW | ||||
| Coal | Photovoltaic | Onshore wind | Hydropower | |
| FPMF (kg PM2.5 eq.) | 4.56 × 10−1 | 4.02 × 10−2 | 1.13 × 10−2 | 3.82 × 10−3 |
| FRS (kg oil eq.) | 9.16 × 101 | 4.17 × 100 | 1.62 × 100 | 4.21 × 10−1 |
| FEC (kg 1,4-DCB) | 2.33 × 101 | 4.48 × 100 | 3.08 × 100 | 8.47 × 10−2 |
| FEU (kg P eq.) | 9.47 × 10−1 | 1.46 × 10−2 | 2.78 × 10−3 | 5.18 × 10−4 |
| GWP (kg CO2 eq.) | 4.17 × 102 | 1.71 × 101 | 5.99 × 100 | 2.53 × 100 |
| HCT (kg 1,4-DCB) | 4.68 × 101 | 1.61 × 100 | 9.04 × 100 | 1.23 × 100 |
| HnCT (kg 1,4-DCB) | 9.35 × 102 | 6.30 × 101 | 1.48 × 101 | 1.84 × 100 |
| IR (kBq Co-60 eq.) | 9.78 × 10−1 | 1.58 × 100 | 3.44 × 10−1 | 1.03 × 10−1 |
| LU (m2a crop eq.) | 7.38 × 10−1 | 4.75 × 10−1 | 7.00 × 10−1 | 8.16 × 10−2 |
| MEC (kg 1,4-DCB) | 3.21 × 101 | 5.91 × 100 | 3.79 × 100 | 1.18 × 10−1 |
| MRS (kg Cu eq.) | 4.79 × 10−2 | 2.14 × 10−1 | 1.42 × 10−1 | 2.79 × 10−2 |
| OFTE (kg NOx eq.) | 6.42 × 10−1 | 4.06 × 10−2 | 1.87 × 10−2 | 7.54 × 10−3 |
| SOD (kg CFC11 eq.) | 6.46 × 10−5 | 7.96 × 10−6 | 2.61 × 10−6 | 1.23 × 10−6 |
| TAC (kg SO2 eq.) | 1.48 × 100 | 8.54 × 10−2 | 2.06 × 10−2 | 6.26 × 10−3 |
| TEC (kg 1,4- DCB) | 1.37 × 102 | 4.52 × 102 | 3.22 × 101 | 8.62 × 100 |
| TWC (m3) | 8.39 × 10−1 | 5.22 × 10−1 | 7.42 × 10−2 | 1.13 × 101 |
| 20% WW | ||||
| Coal | Photovoltaic | Onshore wind | Hydropower | |
| FPMF (kg PM2.5 eq.) | 6.40 × 10−1 | 5.64 × 10−2 | 1.58 × 10−2 | 5.36 × 10−3 |
| FRS (kg oil eq.) | 1.29 × 102 | 5.86 × 100 | 2.28 × 100 | 5.91 × 10−1 |
| FEC (kg 1,4-DCB) | 3.28 × 101 | 6.29 × 100 | 4.32 × 100 | 1.19 × 10−1 |
| FEU (kg P eq.) | 1.33 × 100 | 2.05 × 10−2 | 3.90 × 10−3 | 7.27 × 10−4 |
| GWP (kg CO2 eq.) | 5.86 × 102 | 2.40 × 101 | 8.41 × 100 | 3.55 × 100 |
| HCT (kg 1,4-DCB) | 6.56 × 101 | 2.26 × 100 | 1.27 × 101 | 1.72 × 100 |
| HnCT (kg 1,4-DCB) | 1.31 × 103 | 8.84 × 101 | 2.07 × 101 | 2.58 × 100 |
| IR (kBq Co-60 eq.) | 1.37 × 100 | 2.21 × 100 | 4.83 × 10−1 | 1.45 × 10−1 |
| LU (m2a crop eq.) | 1.04 × 100 | 6.67 × 10−1 | 9.82 × 10−1 | 1.14 × 10−1 |
| MEC (kg 1,4-DCB) | 4.50 × 101 | 8.30 × 100 | 5.31 × 100 | 1.66 × 10−1 |
| MRS (kg Cu eq.) | 6.72 × 10−2 | 3.00 × 10−1 | 1.99 × 10−1 | 3.92 × 10−2 |
| OFTE (kg NOx eq.) | 9.00 × 10−1 | 5.69 × 10−2 | 2.63 × 10−2 | 1.06 × 10−2 |
| SOD (kg CFC11 eq.) | 9.06 × 10−5 | 1.12 × 10−5 | 3.67 × 10−6 | 1.73 × 10−6 |
| TAC (kg SO2 eq.) | 2.08 × 100 | 1.20 × 10−1 | 2.89 × 10−2 | 8.79 × 10−3 |
| TEC (kg 1,4- DCB) | 1.93 × 102 | 6.34 × 102 | 4.52 × 101 | 1.21 × 101 |
| TWC (m3) | 1.18 × 100 | 7.32 × 10−1 | 1.04 × 10−1 | 1.59 × 101 |
| 75% WW | ||||
| Coal | Photovoltaic | Onshore wind | Hydropower | |
| FPMF (kg PM2.5 eq.) | 1.39 × 10−1 | 1.22 × 10−2 | 3.43 × 10−3 | 1.16 × 10−3 |
| FRS (kg oil eq.) | 2.78 × 101 | 1.27 × 100 | 4.93 × 10−1 | 1.28 × 10−1 |
| FEC (kg 1,4-DCB) | 7.09 × 100 | 1.36 × 100 | 9.35 × 10−1 | 2.57 × 10−2 |
| FEU (kg P eq.) | 2.88 × 10−1 | 4.44 × 10−3 | 8.46 × 10−4 | 1.57 × 10−4 |
| GWP (kg CO2 eq.) | 1.27 × 102 | 5.20 × 100 | 1.82 × 100 | 7.69 × 10−1 |
| HCT (kg 1,4-DCB) | 1.42 × 101 | 4.90 × 10−1 | 2.75 × 100 | 3.73 × 10−1 |
| HnCT (kg 1,4-DCB) | 2.84 × 102 | 1.91 × 101 | 4.49 × 100 | 5.59 × 10−1 |
| IR (kBq Co-60 eq.) | 2.97 × 10−1 | 4.79 × 10−1 | 1.05 × 10−1 | 3.14 × 10−2 |
| LU (m2a crop eq.) | 2.24 × 10−1 | 1.44 × 10−1 | 2.13 × 10−1 | 2.48 × 10−2 |
| MEC (kg 1,4-DCB) | 9.75 × 100 | 1.80 × 100 | 1.15 × 100 | 3.59 × 10−2 |
| MRS (kg Cu eq.) | 1.46 × 10−2 | 6.51 × 10−2 | 4.31 × 10−2 | 8.48 × 10−3 |
| OFTE (kg NOx eq.) | 1.95 × 10−1 | 1.23 × 10−2 | 5.70 × 10−3 | 2.29 × 10−3 |
| SOD (kg CFC11 eq.) | 1.96 × 10−5 | 2.42 × 10−6 | 7.94 × 10−7 | 3.75 × 10−7 |
| TAC (kg SO2 eq.) | 4.51 × 10−1 | 2.60 × 10−2 | 6.26 × 10−3 | 1.90 × 10−3 |
| TEC (kg 1,4- DCB) | 4.17 × 101 | 1.37 × 102 | 9.78 × 100 | 2.62 × 100 |
| TWC (m3) | 2.55 × 10−1 | 1.59 × 10−1 | 2.25 × 10−2 | 3.44 × 100 |
References
- Plöhn, M.; Spain, O.; Sirin, S.; Silva, M.; Escudero-Oñate, C.; Ferrando-Climent, L.; Funk, C. Wastewater treatment by microalgae. Physiol. Plant. 2021, 173, 568–578. [Google Scholar] [CrossRef]
- Tariq, A.; Mushtaq, A. Untreated wastewater reasons and causes: A review of most affected areas and cities. Int. J. Chem. Biochem. Sci 2023, 23, 121–143. [Google Scholar]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Ong, H.C. Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere 2022, 286, 131656. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Kumar, S.; Nand, S.; Azad, I.; Bharagava, R.N.; Ferreira, L.F.R.; Dutta, V. Wastewater generation and treatment by various eco-friendly technologies: Possible health hazards and further reuse for environmental safety. Chemosphere 2023, 313, 137547. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef] [PubMed]
- Kaloudas, D.; Pavlova, N.; Penchovsky, R. Phycoremediation of wastewater by microalgae: A review. Environ. Chem. Lett. 2021, 19, 2905–2920. [Google Scholar] [CrossRef]
- Singh, V.; Mishra, V. Evaluation of the effects of input variables on the growth of two microalgae classes during wastewater treatment. Water Res. 2022, 213, 118165. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Oulego, P.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Towards the implementation of circular economy in the wastewater sector: Challenges and opportunities. Water 2020, 12, 1431. [Google Scholar] [CrossRef]
- Göncü, S.; Şimşek Uygun, B.; Atakan, S. Nıtrogen and phosphorus removal from wastewater usıng Chlorella vulgarıs and Scenedesmus quadrıcauda mıcroalgae wıth a batch bıoreactor. Int. J. Environ. Sci. Technol. 2025, 1–16. [Google Scholar] [CrossRef]
- Prabhath, G.P.W.A.; Shukla, S.P.; Srivastava, P.P.; Kumar, K.; Sawant, P.B.; Verma, A.K.; Nuwansi, K.K.T. Downstream processing of biomass produced in aquaculture wastewater for valuable pigments from the cyanobacterium Spirulina (Arthrospira) platensis: A green and sustainable approach. Aquac. Int. 2022, 30, 3081–3106. [Google Scholar] [CrossRef]
- Mesa, A.P.; Grattz, P.A.C.; Vargas, J.J.V.; Ríos, L.A.; Echeverri, D.O.; Parra, A.M.M. Feasibility of nitrogen and phosphorus removal from treated wastewater using microalgae and potential microalgae use as biofertilizer. J. Water Process Eng. 2025, 70, 107023. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Markou, G.; Nicodemou, A.; Koutinas, M.; Tekerlekopoulou, A.G.; Vayenas, D.V. Cultivation of Arthrospira platensis in brewery wastewater. Water 2022, 14, 1547. [Google Scholar] [CrossRef]
- Tan, Y.H.; Chai, M.K.; Na, J.Y.; Wong, L.S. Microalgal growth and nutrient removal efficiency in non-sterilised primary domestic wastewater. Sustainability 2023, 15, 6601. [Google Scholar] [CrossRef]
- Amaro, H.M.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.; Esteves, A.F. Microalgae systems-environmental agents for wastewater treatment and further potential biomass valorisation. J. Environ. Manag. 2023, 337, 117678. [Google Scholar]
- Morais, E.G.; Cristofoli, N.L.; Maia, I.B.; Magina, T.; Cerqueira, P.R.; Teixeira, M.R.; Gouveia, L. Microalgal systems for wastewater treatment: Technological trends and challenges towards waste recovery. Energies 2021, 14, 8112. [Google Scholar] [CrossRef]
- Nobre, M.L.; Tavares, D.; Fraga, C.; Oliveira, B.; Dias, M.; Mesquita, S.; Pires, J.C. Techno-economic analysis of a circular microalgal approach for enhanced wastewater treatment and resource recovery in Northern Portugal. J. Clean. Prod. 2024, 434, 140389. [Google Scholar] [CrossRef]
- Álvarez-González, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Casas, M.E.; Matamoros, V.; Díez-Montero, R. The potential of wastewater grown microalgae for agricultural purposes: Contaminants of emerging concern, heavy metals and pathogens assessment. Environ. Pollut. 2023, 324, 121399. [Google Scholar] [CrossRef]
- Osman, M.E.; Abo-Shady, A.M.; Gheda, S.F.; Desoki, S.M.; Elshobary, M.E. Unlocking the potential of microalgae cultivated on wastewater combined with salinity stress to improve biodiesel production. Environ. Sci. Pollut. Res. 2023, 30, 114610–114624. [Google Scholar] [CrossRef]
- Ali, M.; Masood, A.; Saleem, M. Microalgae cultivation in wastewater for simultaneous nutrients removal and biomass production. Int. J. Energy Environ. Eng. 2021, 12, 475–485. [Google Scholar] [CrossRef]
- Bhuyar, P.; Trejo, M.; Dussadee, N.; Unpaprom, Y.; Ramaraj, R.; Whangchai, K. Microalgae cultivation in wastewater effluent from tilapia culture pond for enhanced bioethanol production. Water Sci. Technol. 2021, 84, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.A.H.; Yaakob, Z.; Abdullah, S.R.S.; Takriff, M.S. Assessing the feasibility of microalgae cultivation in agricultural wastewater: The nutrient characteristics. Environ. Technol. Innov. 2019, 15, 100402. [Google Scholar] [CrossRef]
- de Souza Leite, L.; Hoffmann, M.T.; Daniel, L.A. Coagulation and dissolved air flotation as a harvesting method for microalgae cultivated in wastewater. J. Water Process Eng. 2019, 32, 100947. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Koutra, E.; Kornaros, M.; Farhadian, O.; Bhatnagar, A. Sequential cultivation of microalgae in raw and recycled dairy wastewater: Microalgal growth, wastewater treatment and biochemical composition. Bioresour. Technol. 2019, 273, 556–564. [Google Scholar] [CrossRef]
- Chaudry, S. Integrating microalgae cultivation with wastewater treatment: A peek into economics. Appl. Biochem. Biotechnol. 2021, 193, 3395–3406. [Google Scholar] [CrossRef] [PubMed]
- Nur, M.M.A.; Buma, A.G. Opportunities and challenges of microalgal cultivation on wastewater, with special focus on palm oil mill effluent and the production of high value compounds. Waste Biomass Valorization 2019, 10, 2079–2097. [Google Scholar] [CrossRef]
- Aron, N.S.M.; Khoo, K.S.; Chew, K.W.; Veeramuthu, A.; Chang, J.S.; Show, P.L. Microalgae cultivation in wastewater and potential processing strategies using solvent and membrane separation technologies. J. Water Process Eng. 2021, 39, 101701. [Google Scholar] [CrossRef]
- Melo, J.M.; Ribeiro, M.R.; Telles, T.S.; Amaral, H.F.; Andrade, D.S. Microalgae cultivation in wastewater from agricultural industries to benefit next generation of bioremediation: A bibliometric analysis. Environ. Sci. Pollut. Res. 2022, 29, 22708–22720. [Google Scholar] [CrossRef]
- Purba, L.D.A.; Susanti, H.; Admirasari, R.; Praharyawan, S.; Iwamoto, K. Bibliometric insights into microalgae cultivation in wastewater: Trends and future prospects for biolipid production and environmental sustainability. J. Environ. Manag. 2024, 352, 120104. [Google Scholar] [CrossRef]
- Fal, S.; Smouni, A.; El Arroussi, H. Integrated microalgae-based biorefinery for wastewater treatment, industrial CO2 sequestration and microalgal biomass valorization: A circular bioeconomy approach. Environ. Adv. 2023, 12, 100365. [Google Scholar] [CrossRef]
- Zhuang, L.L.; Li, M.; Ngo, H.H. Non-suspended microalgae cultivation for wastewater refinery and biomass production. Bioresour. Technol. 2020, 308, 123320. [Google Scholar] [CrossRef] [PubMed]
- de Souza Leite, L.; Hoffmann, M.T.; Daniel, L.A. Microalgae cultivation for municipal and piggery wastewater treatment in Brazil. J. Water Process Eng. 2019, 31, 100821. [Google Scholar] [CrossRef]
- Garbowski, T.; Pietryka, M.; Pulikowski, K.; Richter, D. The use of a natural substrate for immobilization of microalgae cultivated in wastewater. Sci. Rep. 2020, 10, 7915. [Google Scholar] [CrossRef] [PubMed]
- Vaz, S.A.; Badenes, S.M.; Pinheiro, H.M.; Martins, R.C. Recent reports on domestic wastewater treatment using microalgae cultivation: Towards a circular economy. Environ. Technol. Innov. 2023, 30, 103107. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Hong, Y.; Liu, X.; Zhao, G.; Zhang, H.; Zhai, Q. Microalgae cultivation in domestic wastewater for wastewater treatment and high value-added production: Species selection and comparison. Biochem. Eng. J. 2022, 185, 108493. [Google Scholar] [CrossRef]
- Han, W.; Jin, W.; Li, Z.; Wei, Y.; He, Z.; Chen, C.; Zhou, X. Cultivation of microalgae for lipid production using municipal wastewater. Process Saf. Environ. Prot. 2021, 155, 155–165. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.H.; Sun, J. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef]
- Prosenc, F.; Piechocka, J.; Škufca, D.; Heath, E.; Bulc, T.G.; Istenič, D.; Buttiglieri, G. Microalgae-based removal of contaminants of emerging concern: Mechanisms in Chlorella vulgaris and mixed algal-bacterial cultures. J. Hazard. Mater. 2021, 418, 126284. [Google Scholar] [CrossRef]
- Matamoros, V.; Uggetti, E.; García, J.; Bayona, J.M. Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater: A laboratory scale study. J. Hazard. Mater. 2016, 301, 197–205. [Google Scholar] [CrossRef]
- Liu, R.; Li, S.; Tu, Y.; Hao, X. Capabilities and mechanisms of microalgae on removing micropollutants from wastewater: A review. J. Environ. Manag. 2021, 285, 112149. [Google Scholar] [CrossRef]
- Liu, L.; Lin, X.; Luo, L.; Yang, J.; Luo, J.; Liao, X.; Cheng, H. Biosorption of copper ions through microalgae from piggery digestate: Optimization, kinetic, isotherm and mechanism. J. Clean. Prod. 2021, 319, 128724. [Google Scholar] [CrossRef]
- Zhou, J.L.; Yang, L.; Huang, K.X.; Chen, D.Z.; Gao, F. Mechanisms and application of microalgae on removing emerging contaminants from wastewater: A review. Bioresour. Technol. 2022, 364, 128049. [Google Scholar] [CrossRef]
- Yadav, G.; Shanmugam, S.; Sivaramakrishnan, R.; Kumar, D.; Mathimani, T.; Brindhadevi, K.; Rajendran, K. Mechanism and challenges behind algae as a wastewater treatment choice for bioenergy production and beyond. Fuel 2021, 285, 119093. [Google Scholar] [CrossRef]
- Cinq-Mars, M.; Bourdeau, N.; Marchand, P.; Desgagné-Penix, I.; Barnabé, S. Characterization of two microalgae consortia grown in industrial wastewater for biomass valorization. Algal Res. 2022, 62, 102628. [Google Scholar] [CrossRef]
- de Morais, E.G.; da Silveira, J.T.; Schüler, L.M.; de Freitas, B.C.B.; Costa, J.A.V.; de Morais, M.G.; Barreira, L. Biomass valorization via pyrolysis in microalgae-based wastewater treatment: Challenges and opportunities for a circular bioeconomy. J. Appl. Phycol. 2023, 35, 2689–2708. [Google Scholar] [CrossRef]
- Morillas-España, A.; López-Serna, R.; Chikri, L.Y.R.; Jiménez, J.J.; Lafarga, T.; Uggetti, E.; González-López, C.V. Microalgae wastewater treatment: Pharmaceutical removal and biomass valorization. J. Environ. Manag. 2025, 380, 124942. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Bhattacharjya, R.; Saxena, A.; Mishra, B.; Tiwari, A. Utilization of wastewater as nutrient media and biomass valorization in marine Chrysophytes-Chaetoceros and Isochrysis. Energy Convers. Manag. X 2021, 10, 100062. [Google Scholar]
- Kumar, N.; Banerjee, C.; Chang, J.S.; Shukla, P. Valorization of wastewater through microalgae as a prospect for generation of biofuel and high-value products. J. Clean. Prod. 2022, 362, 132114. [Google Scholar] [CrossRef]
- Oswald, W.J.; Golueke, C.G. Harvesting and processing of waste-grown microalgae. Algae Man Environ. 1968, 1, 371–389. [Google Scholar]
- Oswald, W.J. Productivity of algae in sewage disposal. Sol. Energy 1973, 15, 107–117. [Google Scholar] [CrossRef]
- Oswald, W.J.; Benemann, J.R. A critical analysis of bioconversion with microalgae. In Biological Solar Energy Conversion; Academic Press: New York, NY, USA, 1977; pp. 379–398. [Google Scholar]
- Craggs, R. Energy from wastewater treatment. Water Atmos 2005, 13, 20. [Google Scholar]
- Benemann, J.R.; Van Olst, J.C.; Massingill, M.J.; Carlberg, J.A.; Weissman, J.C.; Brune, D.E. The controlled eutrophication process: Using microalgae for CO2 utilization and agricultural fertilizer recycling. In Greenhouse Gas Control Technologies—6th International Conference; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1433–1438. [Google Scholar]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Luong, T.T.; Lee, D.; Oh, Y.K.; Lee, T. Reuse of effluent water from a municipal wastewater treatment plant in microalgae cultivation for biofuel production. Bioresour. Technol. 2011, 102, 8639–8645. [Google Scholar] [CrossRef]
- Craggs, R.J.; Lundquist, T.J.; Benemann, J.R. Wastewater treatment and algal biofuel production. In Algae for Biofuels and Energy; Springer: Dordrecht, The Netherlands, 2012; pp. 153–163. [Google Scholar]
- Zhou, W.; Chen, P.; Min, M.; Ma, X.; Wang, J.; Griffith, R.; Ruan, R. Environment-enhancing algal biofuel production using wastewaters. Renew. Sustain. Energy Rev. 2014, 36, 256–269. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Howard-Williams, C.; Turnbull, M.H.; Broady, P.A.; Craggs, R.J. Enhancing microalgal photosynthesis and productivity in wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2015, 184, 222–229. [Google Scholar] [CrossRef]
- Singh, U.; Tiwari, G.L.; Tandon, R.; Tandon, R. Bioremediation of Heavy Metals Mediated by Cyanobacteria. In Biodegradation of Toxic and Hazardous Chemicals; CRC Press: Boca Raton, FL, USA, 2024; pp. 135–147. [Google Scholar]
- Goswami, R.K.; Agrawal, K.; Shah, M.P.; Verma, P. Bioremediation of heavy metals from wastewater: A current perspective on microalgae-based future. Lett. Appl. Microbiol. 2022, 75, 701–717. [Google Scholar] [CrossRef]
- Sarma, U.; Hoque, M.E.; Thekkangil, A.; Venkatarayappa, N.; Rajagopal, S. Microalgae in removing heavy metals from wastewater–An advanced green technology for urban wastewater treatment. J. Hazard. Mater. Adv. 2024, 15, 100444. [Google Scholar] [CrossRef]
- Surkatti, R.; Al-Zuhair, S. Microalgae cultivation for phenolic compounds removal. Environ. Sci. Pollut. Res. 2018, 25, 33936–33956. [Google Scholar] [CrossRef]
- Lindner, A.V.; Pleissner, D. Removal of phenolic compounds from olive mill wastewater by microalgae grown under dark and light conditions. Waste Biomass Valorization 2022, 13, 525–534. [Google Scholar] [CrossRef]
- Lindner, A.V.; Pleissner, D. Utilization of phenolic compounds by microalgae. Algal Res. 2019, 42, 101602. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Ismail, A.F.; Samawati, Z.; Liang, Y.Y.; Kanakaraju, D. Microalgae-enabled wastewater treatment: A sustainable strategy for bioremediation of pesticides. Water 2022, 15, 70. [Google Scholar] [CrossRef]
- Martínez-Burgos, W.J.; Pozzan, R.; da Silva Vale, A.; de Carvalho, J.C.; Iwamoto, H.; de Souza Vandenberghe, L.P.; Soccol, C.R. Bioremediation of Microalgae-Based Pesticides. In Algae as a Natural Solution for Challenges in Water-Food-Energy Nexus: Toward Carbon Neutrality; Springer Nature Singapore: Singapore, 2024; pp. 903–929. [Google Scholar]
- Castellanos-Estupiñan, M.A.; Carrillo-Botello, A.M.; Rozo-Granados, L.S.; Becerra-Moreno, D.; García-Martínez, J.B.; Urbina-Suarez, N.A.; Zuorro, A. Removal of nutrients and pesticides from agricultural runoff using microalgae and cyanobacteria. Water 2022, 14, 558. [Google Scholar] [CrossRef]
- Premaratne, M.; Nishshanka, G.K.S.H.; Liyanaarachchi, V.C.; Nimarshana, P.H.V.; Ariyadasa, T.U. Bioremediation of textile dye wastewater using microalgae: Current trends and future perspectives. J. Chem. Technol. Biotechnol. 2021, 96, 3249–3258. [Google Scholar] [CrossRef]
- Sudarshan, S.; Harikrishnan, S.; RathiBhuvaneswari, G.; Alamelu, V.; Aanand, S.; Rajasekar, A.; Govarthanan, M. Impact of textile dyes on human health and bioremediation of textile industry effluent using microorganisms: Current status and future prospects. J. Appl. Microbiol. 2023, 134, lxac064. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B. Removal of radon from radionuclide-contaminated water using microalgae. In The Role of Microalgae in Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 75–86. [Google Scholar]
- Kim, T.Y.; Hong, J.E.; Park, H.M.; Lee, U.J.; Lee, S.Y. Decontamination of low-level contaminated water from radioactive cesium and cobalt using microalgae. J. Radioanal. Nucl. Chem. 2020, 323, 903–908. [Google Scholar] [CrossRef]
- Ramirez, K.D.R.; Ñañez, K.B.; Gomez, C.L.G.; Moreira, Í.T.A. Efficient PAHs removal and CO2 fixation by marine microalgae in wastewater using an airlift photobioreactor for biofuel production. Environ. Res. 2024, 261, 119672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lu, B.; Wang, Z.; Wei, J.; Zhao, Y.; Wang, S. Enhanced antibiotics and antibiotics resistance genes removal from aquaculture wastewater by microalgae-based system induced with plant hormones. Int. Biodeterior. Biodegrad. 2025, 200, 106045. [Google Scholar] [CrossRef]
- Michelon, W.; Matthiensen, A.; Viancelli, A.; Fongaro, G.; Gressler, V.; Soares, H.M. Removal of veterinary antibiotics in swine wastewater using microalgae-based process. Environ. Res. 2022, 207, 112192. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, L.X.; Liu, Y.S.; Zhao, J.L.; He, L.Y.; Ying, G.G. Microalgae-based technology for antibiotics removal: From mechanisms to application of innovational hybrid systems. Environ. Int. 2021, 155, 106594. [Google Scholar] [CrossRef]
- Pereira, A.; de Morais, E.G.; Silva, L.; Pena, A.; Freitas, A.; Teixeira, M.R.; Barreira, L. Pharmaceuticals removal from wastewater with microalgae: A pilot study. Appl. Sci. 2023, 13, 6414. [Google Scholar] [CrossRef]
- Mokrani, S.; Houali, K.; Yadav, K.K.; Arabi, A.I.A.; Eltayeb, L.B.; AwjanAlreshidi, M.; Nabti, E.H. Bioremediation techniques for soil organic pollution: Mechanisms, microorganisms, and technologies-A comprehensive review. Ecol. Eng. 2024, 207, 107338. [Google Scholar] [CrossRef]
- Abuhasheesh, Y.; Ghazal, A.; Tang, D.Y.Y.; Banat, F.; Hasan, S.W.; Show, P.L. Advances in Chlorella Microalgae for Sustainable Wastewater Treatment and Bioproduction. Chem. Eng. J. Adv. 2025, 22, 100715. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of microalgae in global CO2 sequestration: Physiological mechanism, recent development, challenges, and future prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- Reisoglu, Ş.; Aydin, S. Microalgae as a promising candidate for fighting climate change and biodiversity loss. In Microalgae—Current and Potential Applications; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Aljabory, M.N.; Alhaboubi, N.A. Green Solutions for CO2 Mitigation: Exploring Microalgae-Based Carbon Capture and Utilization Technologies. J. Biotechnol. Res. Cent. 2025, 19, 52–64. [Google Scholar] [CrossRef]
- Santos, B.; Freitas, F.; Sobral, A.J.; Encarnação, T. Microalgae and circular economy: Unlocking waste to resource pathways for sustainable development. Int. J. Sustain. Eng. 2025, 18, 2501488. [Google Scholar] [CrossRef]
- Loaiza-González, J.M.; Rubio-Clemente, A.; Peñuela, G.A. Removal of contaminants through microalgae: Towards the development of a circular economy. Rev. UIS Ing. 2024, 23, 123–132. [Google Scholar] [CrossRef]
- Srivastava, N.; Maurya, V.K.; Bee, Z.; Singh, N.; Khare, S.; Singh, S.; Rai, P.K. Green Solutions for a blue planet: Harnessing bioremediation for sustainable development and circular economies. In Biotechnologies for Wastewater Treatment and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 283–296. [Google Scholar]
- Singh, S.K.; Shukla, L.; Singh, R.P.; Yadav, P.; Kumar, A. Microalgae mediated wastewater treatment and its production for biofuels and bioproducts. In Advances in Chemical Pollution, Environmental Management and Protection; Elsevier: Amsterdam, The Netherlands, 2023; Volume 9, pp. 153–165. [Google Scholar]
- Saldarriaga-Hernandez, S.; Hernandez-Vargas, G.; Iqbal, H.M.; Barceló, D.; Parra-Saldívar, R. Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. Sci. Total Environ. 2020, 715, 136978. [Google Scholar] [CrossRef]
- Dias, R.R.; Deprá, M.C.; Zepka, L.Q.; Jacob-Lopes, E. Microalgae-Based Bioremediation. Appl. Water Sci. Remediat. Technol. 2021, 2, 357–380. [Google Scholar]
- Mahlangu, D.; Mphahlele, K.; De Paola, F.; Mthombeni, N.H. Microalgae-mediated biosorption for effective heavy metals removal from wastewater: A review. Water 2024, 16, 718. [Google Scholar] [CrossRef]
- He, Z.; Fan, X.; Qu, L.; Zhou, X.; Jin, W.; Hatshan, M.R.; Wang, Q. Cultivation of Chlorella pyrenoidosa and Scenedesmus obliquus in swine wastewater: Nitrogen and phosphorus removal and microalgal growth. Process Saf. Environ. Prot. 2023, 179, 887–895. [Google Scholar] [CrossRef]
- Acebu, P.I.G.; de Luna, M.D.G.; Chen, C.Y.; Abarca, R.R.M.; Chen, J.H.; Chang, J.S. Bioethanol production from Chlorella vulgaris ESP-31 grown in unsterilized swine wastewater. Bioresour. Technol. 2022, 352, 127086. [Google Scholar] [CrossRef]
- Cheng, P.; Chu, R.; Zhang, X.; Song, L.; Chen, D.; Zhou, C.; Ruan, R. Screening of the dominant Chlorella pyrenoidosa for biofilm attached culture and feed production while treating swine wastewater. Bioresour. Technol. 2020, 318, 124054. [Google Scholar] [CrossRef]
- Carneiro, M.; Ranglová, K.; Lakatos, G.E.; Manoel, J.A.C.; Grivalský, T.; Kozhan, D.M.; Masojídek, J. Growth and bioactivity of two chlorophyte (Chlorella and Scenedesmus) strains co-cultured outdoors in two different thin-layer units using municipal wastewater as a nutrient source. Algal Res. 2021, 56, 102299. [Google Scholar] [CrossRef]
- Kisielewska, M.; Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Romanowska-Duda, Z.; Dudek, M. Effectiveness of Scenedesmus sp. biomass grow and nutrients removal from liquid phase of digestates. Energies 2020, 13, 1432. [Google Scholar] [CrossRef]
- Ansari, F.A.; Hassan, H.; Gani, K.M.; Rawat, I.; Gupta, S.K.; Bux, F. Influence of nitrogen sources on growth and biochemical composition of Chlorella sorokiniana. Biomass Bioenergy 2025, 197, 107832. [Google Scholar] [CrossRef]
- Guo, C.; Chen, J.; Ma, Y.; Lin, S.; Dong, R.; Ruan, R.; Liu, S. Enhanced Tolerance and Growth of Chlorella vulgaris and Scenedesmus quadricauda in Anaerobic Digestate Food Waste Effluent. Appl. Biochem. Biotechnol. 2025, 197, 4131–4156. [Google Scholar] [CrossRef]
- Singh, V.P.; Godara, P.; Srivastava, A. Sustainable Microalgal Bioremediation of Heavy Metals and Dyes from Synthetic Wastewater: Progressing Towards United Nations Sustainable Development Goals. Waste Manag. Bull. 2024, 2, 123–135. [Google Scholar] [CrossRef]
- Abd El-Reheem Mohammed, A.; Muhammad, S.S.; Mohamed, H.A.; El-Agamy, H.H. Potentiality of Spirulina platensis in zirconium ions biosorption and removal from aqueous solution as an eco-friendly process. Sep. Sci. Technol. 2025, 60, 1101–1112. [Google Scholar] [CrossRef]
- Osuna Barreto, M.P. Remediación de Suelos Contaminados con Cadmio a Partir de Mezclas de Enmiendas-Spirulina platensis. Diploma Thesis, Universidad de los Andes, Santiago, Chile, 2024. [Google Scholar]
- Nakarmi, K.J.; Daneshvar, E.; Mänttäri, M.; Bhatnagar, A. Removal and recovery of nutrients from septic tank wastewater using microalgae: Key factors and practical implications. J. Environ. Manag. 2023, 345, 118922. [Google Scholar] [CrossRef]
- López-Rosales, L.; López-García, P.; Benyachou, M.A.; Molina-Miras, A.; Gallardo-Rodríguez, J.J.; Cerón-García, M.C.; García-Camacho, F. Treatment of secondary urban wastewater with a low ammonium-tolerant marine microalga using zeolite-based adsorption. Bioresour. Technol. 2022, 359, 127490. [Google Scholar] [CrossRef]
- Aguiar Severo, I.; Azevedo, O.G.D.A.; da Silva, P.A.S.; Jacob-Furlan, B.; Mariano, A.B.; Ordonez, J.C.; Vargas, J.V.C. Wastewater treatment process using immobilized microalgae. Water Sci. Technol. 2024, 90, 1306–1320. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Z.; Lu, X. Advanced treatment of pharmaceutical wastewater with a combined Fe-C micro-electrolysis/EGSB system assisted by microalgae. Sep. Sci. Technol. 2021, 56, 2826–2837. [Google Scholar] [CrossRef]
- Nunes, N.S.P.; de Almeida, J.M.O.; Fonseca, G.G.; de Carvalho, E.M. Clarification of sugarcane (Saccharum officinarum) vinasse for microalgae cultivation. Bioresour. Technol. Rep. 2022, 19, 101125. [Google Scholar]
- Svierzoski, N.D.S.; Matheus, M.C.; Bassin, J.P.; Brito, Y.D.; Mahler, C.F.; Webler, A.D. Treatment of a slaughterhouse wastewater by anoxic–aerobic biological reactors followed by UV-C disinfection and microalgae bioremediation. Water Environ. Res. 2021, 93, 409–420. [Google Scholar] [CrossRef]
- Nagi, M.; He, M.; Li, D.; Gebreluel, T.; Cheng, B.; Wang, C. Utilization of tannery wastewater for biofuel production: New insights on microalgae growth and biomass production. Sci. Rep. 2020, 10, 1530. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Acién-Fernández, F.G.; Gómez-Serrano, C.; González-López, C.V. Annual production of microalgae in wastewater using pilot-scale thin-layer cascade photobioreactors. J. Appl. Phycol. 2021, 33, 3861–3871. [Google Scholar] [CrossRef]
- Abraham, J.; Abimbola, T.; Braida, W.J.; Terracciano, A.; Su, T.L.; Christodoulatos, C.; Lawal, A. On-site pilot-scale microalgae cultivation using industrial wastewater for bioenergy production: A case study towards circular bioeconomy. Bioengineering 2023, 10, 1339. [Google Scholar] [CrossRef]
- Fernández, F.A.; Sevilla, J.M.F.; Grima, E.M. Costs analysis of microalgae production. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 551–566. [Google Scholar]
- Rafa, N.; Ahmed, S.F.; Badruddin, I.A.; Mofijur, R.M.; Kamangar, S. Strategies to produce cost-effective third-generation biofuel from microalgae. Front. Energy Res. 2021, 9, 749968. [Google Scholar] [CrossRef]
- Matamoros, V.; Gutiérrez, R.; Ferrer, I.; García, J.; Bayona, J.M. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [CrossRef]
- Ovis-Sánchez, J.O.; Perera-Pérez, V.D.; Buitrón, G.; Quintela-Baluja, M.; Graham, D.W.; Morales-Espinosa, R.; Carrillo-Reyes, J. Exploring resistomes and microbiomes in pilot-scale microalgae-bacteria wastewater treatment systems for use in low-resource settings. Sci. Total Environ. 2023, 882, 163545. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P. Sub-pilot scale sequential microalgal consortium-based cultivation for treatment of municipal wastewater and biomass production. Environ. Pollut. 2024, 348, 123796. [Google Scholar] [CrossRef]
- Yu, Z.; Hou, Q.; Liu, M.; Xie, Z.; Ma, M.; Chen, H.; Pei, H. From lab to application: Cultivating limnetic microalgae in seawater coupled with wastewater for biodiesel production on a pilot scale. Water Res. 2023, 229, 119471. [Google Scholar] [CrossRef]
- Rossi, S.; Pizzera, A.; Bellucci, M.; Marazzi, F.; Mezzanotte, V.; Parati, K.; Ficara, E. Piggery wastewater treatment with algae-bacteria consortia: Pilot-scale validation and techno-economic evaluation at farm level. Bioresour. Technol. 2022, 351, 127051. [Google Scholar] [CrossRef]
- Mantovani, M.; Marazzi, F.; Fornaroli, R.; Bellucci, M.; Ficara, E.; Mezzanotte, V. Outdoor pilot-scale raceway as a microalgae-bacteria sidestream treatment in a WWTP. Sci. Total Environ. 2020, 710, 135583. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; Rodríguez-Miranda, E.; Gómez-Serrano, C.; González-López, C.V. Year-long evaluation of microalgae production in wastewater using pilot-scale raceway photobioreactors: Assessment of biomass productivity and nutrient recovery capacity. Algal Res. 2021, 60, 102500. [Google Scholar] [CrossRef]
- Dalvi, V.; Chawla, P.; Malik, A. Year-long performance assessment of an on-site pilot scale (100 L) photobioreactor on nutrient recovery and pathogen removal from urban wastewater using native microalgal consortium. Algal Res. 2021, 55, 102228. [Google Scholar] [CrossRef]
- Hussain, F.; Shah, S.Z.; Ahmad, H.; Abubshait, S.A.; Abubshait, H.A.; Laref, A.; Iqbal, M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review. Renew. Sustain. Energy Rev. 2021, 137, 110603. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Hernández, D.; Cruz García-González, M. Microalgae and wastewater treatment: Advantages and disadvantages. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 505–533. [Google Scholar]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Ruan, R. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Chai, W.S.; Tan, W.G.; Munawaroh, H.S.H.; Gupta, V.K.; Ho, S.H.; Show, P.L. Multifaceted roles of microalgae in the application of wastewater biotreatment: A review. Environ. Pollut. 2021, 269, 116236. [Google Scholar] [CrossRef]
- Do, C.V.T.; Pham, M.H.T.; Pham, T.Y.T.; Dinh, C.T.; Bui, T.U.T.; Tran, T.D.; Nguyen, V.T. Microalgae and bioremediation of domestic wastewater. Curr. Opin. Green Sustain. Chem. 2022, 34, 100595. [Google Scholar] [CrossRef]
- Li, L.; Gao, K.; Yang, M.; Zheng, Q.; Zhang, M.; Deng, X. Challenges and potential solutions of microalgae-based systems for wastewater treatment and resource recovery. Front. Bioeng. Biotechnol. 2023, 11, 1210228. [Google Scholar] [CrossRef]
- Priya, A.K.; Jalil, A.A.; Vadivel, S.; Dutta, K.; Rajendran, S.; Fujii, M.; Soto-Moscoso, M. Heavy metal remediation from wastewater using microalgae: Recent advances and future trends. Chemosphere 2022, 305, 135375. [Google Scholar] [CrossRef]
- All-Gas. A Major Step Forward Towards Sustainability. Available online: https://www.all-gas.eu/ (accessed on 14 April 2025).
- Valchev, D.; Ribarova, I. A review on the reliability and the readiness level of microalgae-based nutrient recovery technologies for secondary treated effluent in municipal wastewater treatment plants. Processes 2022, 10, 399. [Google Scholar] [CrossRef]
- Rahman, A.; Pan, S.; Houston, C.; Selvaratnam, T. Evaluation of Galdieria sulphuraria and Chlorella vulgaris for the Bioremediation of Produced Water. Water 2021, 13, 1183. [Google Scholar] [CrossRef]
- Sudhanthiran, M.C.; Perumalsamy, M. Bioremediation of dairy industry wastewater and assessment of nutrient removal potential of Chlorella vulgaris. Biomass Convers. Biorefinery 2024, 14, 10335–10346. [Google Scholar]
- Amaya-Santos, G.; Ruiz-Nieto, Á.; Sánchez-Zurano, A.; Ciardi, M.; Gómez-Serrano, C.; Acién, G.; Lafarga, T. Production of Chlorella vulgaris using urban wastewater: Assessment of the nutrient recovery capacity of the biomass and its plant biostimulant effects. J. Appl. Phycol. 2022, 34, 2971–2979. [Google Scholar] [CrossRef]
- Li, F.; Amenorfenyo, D.K.; Zhang, Y.; Zhang, N.; Li, C.; Huang, X. Cultivation of Chlorella vulgaris in membrane-treated industrial distillery wastewater: Growth and wastewater treatment. Front. Environ. Sci. 2021, 9, 770633. [Google Scholar] [CrossRef]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The use of microalgae for coupling wastewater treatment with CO2 biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef]
- You, X.; Yang, L.; Zhou, X.; Zhang, Y. Sustainability and carbon neutrality trends for microalgae-based wastewater treatment: A review. Environ. Res. 2022, 209, 112860. [Google Scholar] [CrossRef] [PubMed]
- Aravantinou, A.F.; Barkonikou, E.F.; Manariotis, I.D. Microalgae biomass and lipid production using primary treated wastewater. Desalination Water Treat. 2017, 91, 228–234. [Google Scholar] [CrossRef]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef]
- Norsker, N.H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Graça, S.; Sousa, C.; Ambrosano, L.; Ribeiro, B.; Botrel, E.P.; Silva, C.M. Microalgae biomass production using wastewater: Treatment and costs: Scale-up considerations. Algal Res. 2016, 16, 167–176. [Google Scholar] [CrossRef]
- Pajares, E.M.; Valero, L.G.; Sánchez, I.M.R. Cost of urban wastewater treatment and ecotaxes: Evidence from municipalities in southern Europe. Water 2019, 11, 423. [Google Scholar] [CrossRef]
- Suwanu Europe. Available online: https://suwanu-europe.eu/project/dam-recharge-irrigation-municipal/ (accessed on 14 April 2025).
- Liu, X.Y.; Hong, Y. Microalgae-based wastewater treatment and recovery with biomass and value-added products: A brief review. Curr. Pollut. Rep. 2021, 7, 227–245. [Google Scholar] [CrossRef]
- Bhatt, A.; Prajapati, S.K.; Arora, P. Assessing algae-based wastewater treatment—A life cycle assessment approach. In Clean Energy and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 137–154. [Google Scholar]
- Sangma, C.B.; Chalie-u, R. Life cycle assessment of wastewater treatment by microalgae. In Valorization of Microalgal Biomass and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 137–178. [Google Scholar]
- Jacob-Lopes, E.; Zepka, L.Q.; Deprá, M.C. Sustainability Metrics and Indicators of Environmental Impact: Industrial and Agricultural Life Cycle Assessmen; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Gurreri, L.; Rindina, M.C.; Luciano, A.; Lima, S.; Scargiali, F.; Fino, D.; Mancini, G. Environmental sustainability of microalgae-based production systems: Roadmap and challenges towards the industrial implementation. Sustain. Chem. Pharm. 2023, 35, 101191. [Google Scholar] [CrossRef]
- Togarcheti, S.C.; kumar Mediboyina, M.; Chauhan, V.S.; Mukherji, S.; Ravi, S.; Mudliar, S.N. Life cycle assessment of microalgae based biodiesel production to evaluate the impact of biomass productivity and energy source. Resour. Conserv. Recycl. 2017, 122, 286–294. [Google Scholar] [CrossRef]
- Porcelli, R.; Dotto, F.; Pezzolesi, L.; Marazza, D.; Greggio, N.; Righi, S. Comparative life cycle assessment of microalgae cultivation for non-energy purposes using different carbon dioxide sources. Sci. Total Environ. 2020, 721, 137714. [Google Scholar] [CrossRef]
- Ferreira, J.; de Assis, L.R.; de Sousa Oliveira, A.P.; de Siqueira Castro, J.; Calijuri, M.L. Innovative microalgae biomass harvesting methods: Technical feasibility and life cycle analysis. Sci. Total Environ. 2020, 746, 140939. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Devi, M.K.; Kumar, P.S.; Pandian, E. A review on biodiesel production by algal biomass: Outlook on lifecycle assessment and techno-economic analysis. Fuel 2022, 324, 124774. [Google Scholar] [CrossRef]
- Pechsiri, J.S.; Thomas, J.B.E.; El Bahraoui, N.; Fernandez, F.G.A.; Chaouki, J.; Chidami, S.; Gröndahl, F. Comparative life cycle assessment of conventional and novel microalgae production systems and environmental impact mitigation in urban-industrial symbiosis. Sci. Total Environ. 2023, 854, 158445. [Google Scholar] [CrossRef]
- Santurbano, V.; Marangon, B.; Castro, J.; Calijuri, M.L.; Leme, M.; Assemany, P. Enhancing environmental performance in biogas production from wastewater-grown microalgae: A life cycle assessment perspective. J. Environ. Manag. 2024, 362, 121251. [Google Scholar] [CrossRef]
- D’Imporzano, G.; Veronesi, D.; Salati, S.; Adani, F. Carbon and nutrient recovery in the cultivation of Chlorella vulgaris: A life cycle assessment approach to comparing environmental performance. J. Clean. Prod. 2018, 194, 685–694. [Google Scholar] [CrossRef]
- Magalhães, I.B.; Ferreira, J.; de Siqueira Castro, J.; de Assis, L.R.; Calijuri, M.L. Technologies for improving microalgae biomass production coupled to effluent treatment: A life cycle approach. Algal Res. 2021, 57, 102346. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Ma, Z.; Wang, G. The promising way to treat wastewater by microalgae: Approaches, mechanisms, applications and challenges. J. Water Process Eng. 2022, 49, 103012. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Lam, M.K.; Yusup, S.; Lim, J.W.; Lee, K.T. Life cycle evaluation of microalgae biofuels production: Effect of cultivation system on energy, carbon emission and cost balance analysis. Sci. Total Environ. 2019, 688, 112–128. [Google Scholar] [CrossRef]
- Gurreri, L.; Rindina, M.C.; Luciano, A.; Falqui, L.; Fino, D.; Mancini, G. Microalgae production in an industrial-scale photobioreactors plant: A comprehensive Life Cycle assessment. Sustain. Chem. Pharm. 2024, 39, 101598. [Google Scholar] [CrossRef]
- Zuorro, A.; García-Martínez, J.B.; Barajas-Solano, A.F.; Rodríguez-Lizcano, A.; Kafarov, V. Environmental Footprint of Inland Fisheries: Integrating LCA Analysis to Assess the Potential of Wastewater-Based Microalga Cultivation as a Promising Solution for Animal Feed Production. Processes 2023, 11, 3255. [Google Scholar] [CrossRef]
- Ferreira, J.; Braga, M.Q.; da Gama, R.C.N.; Magalhães, I.B.; Marangon, B.B.; de Siqueira Castro, J.; Calijuri, M.L. Carotenoids from wastewater-grown microalgae biomass: Life cycle assessment and techno-economical analysis. J. Clean. Prod. 2024, 434, 140526. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Montero, N.; Ferrer, I.; Acién, F.G.; Gómez, C.; Garfí, M. Life cycle assessment of high rate algal ponds for wastewater treatment and resource recovery. Sci. Total Environ. 2018, 622, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, I.B.; Ferreira, J.; de Siqueira Castro, J.; de Assis, L.R.; Calijuri, M.L. Agro-industrial wastewater-grown microalgae: A techno-environmental assessment of open and closed systems. Sci. Total Environ. 2022, 834, 155282. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Fixler, D.; Dubinsky, Z.; Iluz, D. Energy-input analysis of the life-cycle of microalgal cultivation systems and best scenario for oil-rich biomass production. Appl. Energy 2015, 154, 1082–1088. [Google Scholar] [CrossRef]
- Pérez-López, P.; De Vree, J.H.; Feijoo, G.; Bosma, R.; Barbosa, M.J.; Moreira, M.T.; Kleinegris, D.M. Comparative life cycle assessment of real pilot reactors for microalgae cultivation in different seasons. Appl. Energy 2017, 205, 1151–1164. [Google Scholar] [CrossRef]
- Lopes, A.C.; Valente, A.; Iribarren, D.; González-Fernández, C. Energy balance and life cycle assessment of a microalgae-based wastewater treatment plant: A focus on alternative biogas uses. Bioresour. Technol. 2018, 270, 138–146. [Google Scholar] [CrossRef]
- Arbour, A.J.; Bhatt, P.; Simsek, H.; Brown, P.B.; Huang, J.Y. Life cycle assessment on environmental feasibility of microalgae-based wastewater treatment for shrimp recirculating aquaculture systems. Bioresour. Technol. 2024, 399, 130578. [Google Scholar] [CrossRef]
- Dias, R.R.; Deprá, M.C.; Severo, I.A.; Zepka, L.Q.; Jacob-Lopes, E. Smart override of the energy matrix in commercial microalgae facilities: A transition path to a low-carbon bioeconomy. Sustain. Energy Technol. Assess. 2022, 52, 102073. [Google Scholar] [CrossRef]
- Morales, M.; Hélias, A.; Bernard, O. Optimal integration of microalgae production with photovoltaic panels: Environmental impacts and energy balance. Biotechnol. Biofuels 2019, 12, 1–17. [Google Scholar] [CrossRef]
- Sandoval, D.P. Environmental Impact Extrapolation of Environmental Processes at Low Technology Readiness Level (TRL): Application to Microalgae-Based Processes. Ph.D. Dissertation, Université Côte d’Azur, Nice, France, 2024. [Google Scholar]
- Lanjekar, P.R.; Panwar, N.L. A review on hydrogen production from biomass and commercialization assessment through technology readiness levels (TRLs). BioEnergy Res. 2024, 17, 912–931. [Google Scholar] [CrossRef]
- Petrini, S.; Foladori, P.; Andreottola, G. Laboratory-scale investigation on the role of microalgae towards a sustainable treatment of real municipal wastewater. Water Sci. Technol. 2018, 78, 1726–1732. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Manickam, P.; Muthukaruppan, V. Evaluation of distillery wastewater treatability in a customized photobioreactor using blue-green microalgae–Laboratory and outdoor study. J. Environ. Manag. 2019, 234, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Moondra, N.; Jariwala, N.D.; Christian, R.A. Microalgae based wastewater treatment: A shifting paradigm for the developing nations. Int. J. Phytoremediation 2021, 23, 765–771. [Google Scholar] [CrossRef]
- Xu, K.; Zou, X.; Xue, Y.; Qu, Y.; Li, Y. The impact of seasonal variations about temperature and photoperiod on the treatment of municipal wastewater by algae-bacteria system in lab-scale. Algal Res. 2021, 54, 102175. [Google Scholar] [CrossRef]
- Azam, R.; Kothari, R.; Singh, H.M.; Ahmad, S.; Sari, A.; Tyagi, V.V. Cultivation of two Chlorella species in open sewage contaminated channel wastewater for biomass and biochemical profiles: Comparative lab-scale approach. J. Biotechnol. 2022, 344, 24–31. [Google Scholar] [CrossRef]
- Noshadi, M.; Nouripour, R. Urban wastewater treatment by microalgae, bacteria and microalgae–bacteria system (Laboratory-scale study). Urban Water J. 2022, 19, 161–172. [Google Scholar] [CrossRef]
- Moondra, N.; Jariwala, N.; Christian, R. Impact of Microalgae in Domestic Wastewater Treatment: A Lab-Scale Experimental Study. Pollution 2023, 9, 211–221. [Google Scholar]
- Blanco-Vieites, M.; Álvarez-Gil, M.; Delgado, F.; García-Ruesgas, L.; Rodríguez, E. Livestock wastewater bioremediation through indigenous microalgae culturing as a circular bioeconomy approach as cattle feed. Algal Res. 2024, 78, 103424. [Google Scholar] [CrossRef]
- Deprá, M.C.; Dias, R.R.; Zepka, L.Q.; Jacob-Lopes, E. Tackling Old Challenges in Microalgal Biotechnology: The Role of Photobioreactors to Advance the Technology Readiness Level. Processes 2024, 13, 51. [Google Scholar] [CrossRef]
- Liberti, D.; Pinheiro, F.; Simões, B.; Varela, J.; Barreira, L. Beyond bioremediation: The untapped potential of microalgae in wastewater treatment. Water 2024, 16, 2710. [Google Scholar] [CrossRef]
- Bhujade, R.; Chidambaram, M.; Kumar, A.; Sapre, A. Algae to economically viable low-carbon-footprint oil. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 335–357. [Google Scholar] [CrossRef]
- Blanco-Vieites, M.; Casado, V.; Battez, A.H.; Rodríguez, E. Culturing Arthrospira maxima in mining wastewater: Pilot-scale culturing and biomass valorisation into C-phycocyanin and crude lipid extract. Environ. Technol. Innov. 2023, 29, 102978. [Google Scholar] [CrossRef]
- Mohit, A.; Mishel, V.K.; Remya, N. Life cycle assessment and technoeconomic analysis of biofuels produced from polyculture microalgae cultivated in greywater. J. Environ. Manag. 2024, 356, 120711. [Google Scholar] [CrossRef]
- Gondi, R.; Kavitha, S.; Kannah, R.Y.; Kumar, G.; Banu, J.R. Wastewater based microalgae valorization for biofuel and value-added products recovery. Sustain. Energy Technol. Assess. 2022, 53, 102443. [Google Scholar] [CrossRef]
- Al Ketife, A.M.; Almomani, F.; Muftah, E.N.; Judd, S. A technoeconomic assessment of microalgal culture technology implementation for combined wastewater treatment and CO2 mitigation in the Arabian Gulf. Process Saf. Environ. Prot. 2019, 127, 90–102. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Josa, I.; Ferrer, I.; Van Hulle, S.W.; Rousseau, D.P.; Garfí, M. Life cycle assessment of microalgae systems for wastewater treatment and bioproducts recovery: Natural pigments, biofertilizer and biogas. Sci. Total Environ. 2022, 847, 157615. [Google Scholar] [CrossRef]
- Verdelho Vieira, V.; Cadoret, J.P.; Acien, F.G.; Benemann, J. Clarification of most relevant concepts related to the microalgae production sector. Processes 2022, 10, 175. [Google Scholar] [CrossRef]
- De Francisci, D.; Su, Y.; Iital, A.; Angelidaki, I. Evaluation of microalgae production coupled with wastewater treatment. Environ. Technol. 2018, 39, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Ning, R.; Zhang, M.; Deng, X. Biofuel production as a promising way to utilize microalgae biomass derived from wastewater: Progress, technical barriers, and potential solutions. Front. Bioeng. Biotechnol. 2023, 11, 1250407. [Google Scholar] [CrossRef] [PubMed]


| Case Study | Base Case | 5% WW | 10% WW | 20% WW | |
|---|---|---|---|---|---|
| Biomass productivity | g/m2/day | 20 | 20 | 12.97 | 9.19 |
| CO2 usage | kg/kg biomass | 2 | 2 | 2 | 2 |
| Water evaporation | L/m2/day | 7.5 | 7.5 | 7.5 | 7.5 |
| Mixing power consumption | W/m3 | 10 | 10 | 10 | 10 |
| Labor | people/ha | 1 | 1 | 1 | 1 |
| Production days | days | 300 | 300 | 300 | 300 |
| Land area | ha | 5 | 5 | 5 | 5 |
| Ratio V/S | m3/m2 | 0.2 | 0.2 | 0.2 | 0.2 |
| CO2 fixation efficiency | 0.9 | 0.9 | 0.9 | 0.9 | |
| Dilution rate | 1/day | 0.2 | 0.2 | 0.2 | 0.2 |
| Total culture volume | m3 | 10,000 | 10,000 | 10,000 | 10,000 |
| Total biomass production | t/ha/year | 60 | 60 | 38.91 | 27.57 |
| Total CO2 consumption | t/ha/year | 120 | 120 | 77.82 | 55.14 |
| Total water evaporation | t/ha/year | 22,500 | 22,500 | 22,500 | 22,500 |
| Water and nutrients cost | EUR/kg | 1.1 | |||
| Power cost | EUR/kWh | 0.12 | 0.12 | 0.12 | 0.12 |
| Power for harvesting and others | kWh/m3 harvest | 0.1 | 0.1 | 0.1 | 0.1 |
| Bioreactor cost | EUR/m3 | 61.87 | 61.87 | 61.87 | 61.87 |
| Biomass sludge production cost | EUR/kg | 5.57 | 4.45 | 6.87 | 9.69 |
| Case Study | Base Case | 75% WW | |
|---|---|---|---|
| Biomass productivity | g/m2/day | 14.2 | 45 |
| CO2 usage | kg/kg algae biomass | 2 | 2 |
| Water evaporation | L/m2/day | 7.5 | 7.5 |
| Mixing power consumption | W/m3 | 10 | 10 |
| Labor | people/ha | 1 | 1 |
| Production days | days | 300 | 300 |
| Land area | ha | 5 | 5 |
| Ratio V/S | m3/m2 | 0.2 | 0.2 |
| CO2 fixation efficiency | 0.9 | 0.9 | |
| Dilution rate | 1/day | 0.2 | 0.2 |
| Total culture volume | m3 | 10,000 | 10,000 |
| Total biomass production | t/ha/year | 42.6 | 135 |
| Total CO2 consumption | t/ha/year | 85.2 | 270 |
| Total water evaporation | t/ha/year | 22,500 | 22,500 |
| Water and nutrients cost | EUR/kg | 1.1 | |
| Power cost | EUR/kWh | 0.12 | 0.12 |
| Power for harvesting and others | kWh/m3 harvest | 0.1 | 0.1 |
| Bioreactor cost | EUR/m3 | 61.87 | 61.87 |
| Biomass sludge production cost | EUR/kg | 7.84 | 1.98 |
| Base Case | 10% WW | 20% WW | 75% WW | ||
|---|---|---|---|---|---|
| Raceway pond | m3 | 10 | 15.41 | 21.76 | 4.44 |
| Electric energy for paddle wheel | kWh | 129.60 | 199.71 | 282.01 | 57.54 |
| Electric energy for water pumping | kWh | 24.00 | 36.98 | 52.22 | 10.66 |
| Electric energy for CO2 injection | kWh | 79.20 | 122.05 | 172.34 | 35.16 |
| CO2 consumption | kg/kg biomass | 2.00 | 2.00 | 2.00 | 2.00 |
| Biomass productivity | g/m2/day | 20.00 | 12.97 | 9.19 | 45 |
| Energy consumption centrifugation | kWh | 12.50 | 19.26 | 27.20 | 5.55 |
| Spray-dryer | kWh | 8.52 | 8.52 | 8.52 | 8.52 |
| Output | |||||
| Whole dried biomass | kg | 1 | 1 | 1 | 1 |
| Electricity | kWh | 253.82 | 386.53 | 542.30 | 117.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, R.R.; Deprá, M.C.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. Microalgae Cultivation in Wastewater: How Realistic Is This Approach for Value-Added Product Production? Processes 2025, 13, 2052. https://doi.org/10.3390/pr13072052
Dias RR, Deprá MC, de Menezes CR, Zepka LQ, Jacob-Lopes E. Microalgae Cultivation in Wastewater: How Realistic Is This Approach for Value-Added Product Production? Processes. 2025; 13(7):2052. https://doi.org/10.3390/pr13072052
Chicago/Turabian StyleDias, Rosangela Rodrigues, Mariany Costa Deprá, Cristiano Ragagnin de Menezes, Leila Queiroz Zepka, and Eduardo Jacob-Lopes. 2025. "Microalgae Cultivation in Wastewater: How Realistic Is This Approach for Value-Added Product Production?" Processes 13, no. 7: 2052. https://doi.org/10.3390/pr13072052
APA StyleDias, R. R., Deprá, M. C., de Menezes, C. R., Zepka, L. Q., & Jacob-Lopes, E. (2025). Microalgae Cultivation in Wastewater: How Realistic Is This Approach for Value-Added Product Production? Processes, 13(7), 2052. https://doi.org/10.3390/pr13072052









