Abstract
Bioethanol is a promising alternative to fossil fuels. This study evaluated bioethanol production from orange peel (OP) and brewer’s spent grain (BSG) through acid hydrolysis, followed by fermentation with Saccharomyces cerevisiae. A factorial design was applied to assess the influence of temperature, acid concentration, and time on sugar release. Hydrolysis of OP at 125 °C with 0.5% H2SO4 yielded 52.14 g/L of glucose and 15.70 g/L of xylose. For BSG, the best results were obtained with 2.5% H2SO4 at 160 °C, producing 27.10 g/L of glucose and 14.22 g/L of xylose. Fermentation at 30 °C achieved 5.25% v/v bioethanol in OP and 1.5% v/v in BSG, representing 41.4 g/L and 11.8 g/L of bioethanol, respectively. The kinetic models showed high predictive accuracy (R2 > 0.98). These findings demonstrate the potential of OP and BSG as viable substrates for bioethanol production under mild processing conditions.
1. Introduction
In the context of climate change and the depletion of fossil resources, renewable energy sources such as biomass represent key alternatives for energy production. Bioethanol is produced through the fermentation of agricultural, forestry, and energy crop residues and it offers a way to diversify energy sources and reduce waste [1]. Its use in internal combustion engines can reduce greenhouse gas emissions by up to 52% compared with gasoline [2]. Furthermore, bioethanol is a chemical input in high demand within the public health sector, as demonstrated by the increased need during the COVID-19 pandemic [3]. With continued technological advancements and government support, bioethanol could play a critical role in the transition to clean energy.
In 2024, global orange production reached 48.8 million tons, with Brazil leading at 16.5 million tons. Mexico produced 4.8 million tons, positioning itself as one of the world’s top producers alongside countries including China, the United States of America, and Spain [4,5]. Orange juice production generates a significant amount of pomace, which consists of 50% peel, albedo, and seeds [6]. Orange peel (OP) is rich in bioactive compounds such as essential oils, polyphenols, fibers, and sugars, making it valuable for industrial and nutraceutical applications [7]. The sugars include glucose, fructose, and sucrose, as well as structural polysaccharides like cellulose, hemicellulose, and pectin.
Brewer’s spent grain (BSG) is a byproduct of beer production, which reached 1.88 billion hectoliters in 2023, generating 36.4 million tons of BSG [8]. Mexico is one of the world’s largest producers of BSG, with 124 million hectoliters of beer generating 2.48 million tons of BSG in 2019 [9]. This byproduct contains cellulose, hemicellulose, lignin, proteins, and phenolic compounds [10]. It is utilized in the production of organic fertilizers, bioethanol, xylitol, biogas, bioplastics, and enzymes through fermentation, supporting the circular economy and sustainability [11,12].
Improper management of OP and BSG can result in significant environmental impacts, such as methane emissions from landfills and water pollution due to leachates rich in organic matter, leading to eutrophication and negatively impacting biodiversity [13,14]. Furthermore, OP and BSG occupy space in landfills, increasing the demand for new disposal sites. Therefore, their reuse is essential for mitigating these effects and generating economic benefits [12].
In 2023, Mexico produced 157.4 million liters of ethanol, while 307 million liters were imported to cover the deficit [15]. This situation highlights the need to increase domestic ethanol production to reduce dependency on imports and strengthen the country’s energy self-sufficiency [16].
Bioethanol is obtained through a process that includes pretreatment, hydrolysis, and fermentation. Pretreatment involves physical, chemical, and biological methods that are essential for breaking down the complex structure of biomass. Physical methods normally consist of grinding and extrusion, chemical pretreatment uses acids or alkalis, and biological pretreatment uses enzymes or microorganisms capable of degrading lignin and cellulose [16].
The chemical hydrolysis of polysaccharides involves the breakdown of glycosidic bonds that link monosaccharides. Ayala et al. (2021) achieved glucose and xylose concentrations of 24.585 g/L and 9.015 g/L, respectively, through dilute acid hydrolysis. The cited authors used the 3,5-DNS acid method and UV spectroscopy for sugar quantification, demonstrating that reducing acidity enhances the production of fermentable sugars [17]. Xavier et al. (2022) investigated the pretreatment of BSG with subcritical water at 174 °C for 60 min. Simultaneous fermentation with a 25% load in a fed-batch system produced 32.5 g/L of bioethanol, surpassing the 21.8 g/L obtained from separate hydrolysis and fermentation processes [18].
Saccharomyces cerevisiae is highly efficient in fermenting glucose to ethanol, while Zymomonas mobilis tolerates ethanol well and ferments both glucose and fructose [19,20]. Modified strains of Escherichia coli can ferment xylose and arabinose, key components of hemicellulose [21]. Fermentation follows the glycolytic pathway, where glucose is converted into pyruvate, producing ethanol and CO2. Several factors, such as substrate concentration, temperature, pH, oxygen levels, and metabolic inhibitors, affect the efficiency of the process. The optimal temperature is critical for enzymatic activity, while an unsuitable pH can impair cell viability. In anaerobic conditions, oxygen reduces efficiency by promoting aerobic respiration. Additionally, compounds like acetic acid and furfural can inhibit microbial growth and negatively impact ethanol production [22].
Despite the potential of OP and BSG for bioethanol production, scalability faces both technical and economic challenges [23]. Pretreatment and hydrolysis processes require high temperatures, which increase energy consumption and raise the overall cost. Additionally, the use of acids accelerates the wear and tear on equipment and reactors, reducing their lifespan and increasing maintenance costs. On a large scale, reagents and enzymes lose stability more rapidly, affecting process efficiency and requiring greater expenditure on input [24]. As a result, the literature highlights a trend toward reducing chemical input consumption and operating temperatures to lower operational costs, extend equipment lifespan, and reduce energy consumption—factors that are crucial for the feasibility of large-scale processes.
Fermentation needs precise control of pH, temperature, and substrate concentration, requiring the use of advanced sensors and bioreactors equipped with continuous monitoring systems to prevent metabolic inhibition. Following fermentation, distillation and purification of bioethanol require separation systems that consume significant amounts of energy, which directly impacts the economic feasibility of the process [25]. Therefore, optimizing each stage is crucial in order to reduce operational costs and enhance the competitiveness of bioethanol.
In Mexico, the management of agro-industrial residues such as BSG and OP presents significant challenges. In the region of Mexicali, Baja California, BSG generated by microbreweries is primarily used as animal feed. However, the lack of adequate infrastructure for its storage and transportation limits its full utilization, often resulting in accumulation and potential environmental impacts due to its high organic matter and moisture content [9]. On the other hand, in Veracruz, orange juice producers generate large quantities of OP that sometimes exceed their handling capacity. As a result, surplus OP is disposed of in open dumps, posing risks of soil, water, and air contamination due to biomass decay and leachate generation [26]. These practices highlight the urgent need to develop sustainable strategies for the valorization of these residues, such as bioethanol conversion, which promotes a circular economy in the country.
The objective of this study was to develop a process for bioethanol production from OP and BSG while minimizing the use of chemical inputs and energy consumption. A factorial design was employed to evaluate different acid concentrations in the OP hydrolysis, including the absence of sulfuric acid, and various temperatures in the BSG hydrolysis. The sugars obtained were then fermented with Saccharomyces cerevisiae to produce bioethanol, validating the potential of these agroindustrial residues in a circular economy framework and highlighting their competitiveness compared with other reported processes.
2. Materials and Methods
2.1. Sample Collection and Preparation
The OP was collected from an external pilot-scale essential oil extraction process, using the operating conditions described by Armenta et al. (2023) [27]. The BSG was collected from a local brewery in Mexicali, Baja California, immediately after the lautering step, using clean and airtight containers to preserve its moisture and organic content. After collection, the BSG was stored for 48 h at 4 °C to prevent any uncontrolled fermentation process, while the OP was processed within the first 24 h. For experimental design purposes only, the OP samples were subjected to a thermal treatment process in a Thermolyne™ (Thermo Fisher Scientific Inc., Waltham, MA, USA) muffle furnace at 65 °C for 24 h.
2.2. Design of Experiments for Hydrolysis
Two factorial designs were conducted to determine the most effective conditions for glucose and xylose release, one for OP and another for BSG. The design of experiments for OP considered the following factors and levels: time at 1 and 2 h, H2SO4 concentration of 0, 0.5, and 1% v/v, and temperatures of 100 and 125 °C. The treatments were applied in duplicate and randomized. The factors and levels were selected based on the physicochemical properties of OP and the scientific literature [7]. OP samples were mixed using 10 g to 40 mL of H2SO4 at the corresponding concentration and underwent hydrolysis in a Thermolyne™ furnace at the time and temperature stated in the factorial design. The samples were then cooled, and the pH was adjusted to between 4.8 and 5.2 using 0.5 N NaOH.
The experimental design for BSG evaluated two factors: temperature at 130, 160, and 200 °C and H2SO4 concentration at 1%, 2.5%, and 5% v/v. The response variables were the concentration of glucose and xylose, with each treatment performed in duplicate and randomized. The factors and levels were selected based on the physicochemical characteristics of BSG and previous studies [8,10,11,13,19]. The BSG samples were mixed in a ratio of 10 g of dry BSG to 60 mL of H2SO4 at the corresponding concentration and underwent hydrolysis for 20 min in the Thermolyne™ furnace. Since the BSG had previously been fermented in local breweries, the residues required shorter hydrolysis times; longer processing times can lead to formation of fermentation inhibitors such as furfural [28].
The statistical significance of each factor and their interactions was determined through an analysis of variance (ANOVA) with a 95% confidence level (p < 0.05), using Minitab® software version 19.
The quantification of reducing sugars in the OP and BSG samples was performed using a UV-VIS-3100PC spectrophotometer and the DNS method. The DNS solution was prepared with 2.5 g of 3,5-dinitrosalicylic acid, 7.5 g of sodium potassium tartrate, and 4 g of NaOH. Reference curves for glucose and xylose were constructed using different concentrations in g/L. Glucose and xylose were chosen due to their higher concentrations in OP and BSG [18,22,29]. The standard points of both calibration curves were mixed with DNS solution in a 1:1 ratio, heated in a boiling bath for 5 min, and then cooled down to room temperature [18,29]. The samples were diluted 20 times for OP and 40 times for BSG. Then, an aliquot of each sample was mixed with DNS solution in a 1:1 ratio, heated in a boiling bath for 5 min and cooled down to room temperature. A 1 mL aliquot of each diluted hydrolysate was mixed with 1 mL of DNS solution. Each of the calibration curves for glucose and xylose were measured at 575 nm. All the samples from OP and BSG were compared to the calibration curves.
2.3. Response Surface Modeling
Based on the results from the experiments, regression and statistical modeling for curve and surface fitting were performed using MATLAB® R2025a. Second-order polynomial equations were implemented to describe the behavior of glucose and xylose as a function of the experimental factors. Model fitting was validated using the coefficients of determination (R2) and residual analysis.
The response surface plots were generated with an interpolated data approach using cubic functions in MATLAB®. These surfaces allowed the visualization of the combined effect of temperature and acid concentration on the release of glucose and xylose from both OP and BSG.
2.4. Orange Peel and Brewer’s Spent Grain Fermentation
After evaluating the factorial design for the hydrolysis of both residues, the optimal levels of the three studied factors were selected to proceed to the fermentation stage. For OP, the conditions were temperature 125 °C, acid concentration 0% v/v H2SO4, for 1 h; for BSG, the conditions were temperature 130 °C, acid concentration 2.5% v/v H2SO4, for 20 min, with an OP to solvent ratio of 1:1 and a BSG to solvent ratio of 0.7:1 [30,31]. These ratios were selected since they were not a significant factor affecting hydrolysis, according to the literature [32].
Taking advantage of its hydration, a mixture of 100 g of wet OP was combined with 100 mL of water and subjected to hydrolysis at laboratory scale. The resulting mixture was then filtered to obtain a concentrated syrup (Figure 1A). A total of 70 g of wet BSG was mixed with 100 mL of water and subjected to hydrolysis in the presence of 2.5% diluted sulfuric acid. The hydrolysate was then neutralized with sodium hydroxide and subsequently filtered to obtain a concentrated syrup (Figure 1B).

Figure 1.
Scheme of the bioethanol production process: hydrolysis, filtration, fermentation with Saccharomyces cerevisiae. (A) for OP, (B) for BSG.
Saccharomyces cerevisiae was selected as the yeast to ferment OP and BSG due to its high commercial availability, easy scalability of the process, reduced formation of undesired products in the fermentation stage, and its ability to ferment glucose, as this is the primary sugar present in OP and BSG [20]. The fermentation was conducted at 28, 30, and 32 °C, with 3 g of yeast added per 200 mL of filtrate. According to the literature for OP and BSG, based on the H2SO4 concentration of hydrolysis, the fermentation time was set to 5 days [33,34,35,36,37], 2 days more than the 3 days used as standard. The procedure was replicated in triplicate to ensure reproducibility of results. The process diagram for each residue is presented in Figure 1.
The bioethanol concentration in the fermented hydrolysates was determined using refractometry, a fast and reliable technique for alcoholic fermentation, achieving a precision of R2 = 0.975 [37]. The equipment used was a digital refractometer Abuycs ATC-MR0008 (Rockgood Technology Co., Zhongshan, China). Prior to each measurement, the device was calibrated with distilled water at 20 °C. Measurements were taken in triplicate and compared with values obtained from standardized alcohol and its respective dilutions.
2.5. Kinetic Modeling of Bioethanol Production and Parameter Estimation
In Equation (1), Cbioethanol(t) represents the ethanol concentration over time in g/L, Cmaxbioethanol is the maximum achievable ethanol concentration in g/L, k is the specific ethanol production rate in h−1, t represents time in h and t0 is the inflection time marking the end of the lag phase in h. This model is valid as it accurately reflects the ethanol production behavior during fermentation. Initially, the ethanol concentration is zero, and it follows a sigmoid curve, starting with a lag phase (t ≪ t0) where production remains low due to the slow growth of the yeast. Afterward, in the exponential phase (t ≈ t0), ethanol production increases rapidly due to cell proliferation. Finally, in the stationary phase (t ≫ t0), production stabilizes at Cmaxbioethanol due to nutrient depletion or inhibition caused by ethanol accumulation. Nonlinear regression was applied to adjust the parameters Cmaxbioethanol, k, and t0 into Equation (1), and Equations (2) and (3) were created [38].
2.6. Projection of the Potential Bioethanol Production
The annual bioethanol production potential from OP and BSG in Mexico was estimated using an extrapolation approach. This method was based on experimental yields obtained in the laboratory and national data on the generation and availability of these agro-industrial residues.
To determine biomass availability, national production reports related to the citrus and brewing industries were consulted [9,27,39]. For OP, only the recoverable fraction of the total generated from juice processing was considered. In the case of BSG, production was estimated based on the volume of beer produced nationally, applying an average technical ratio linking the amount of waste generated to the volume of product manufactured.
The experimental ethanol yields used for the projection were selected according to the highest results from the controlled fermentations under laboratory conditions, using Saccharomyces cerevisiae as the fermenting microorganism. The data were expressed in terms of the volume of ethanol produced per unit of treated wet biomass and were subsequently used as the basis for projecting a national-scale utilization scenario.
Finally, the estimated volume was used to make comparisons with other international benchmarks, such as bioenergy plants in Latin America, to analyze the potential impact of valorizing these residues.
3. Results and Discussion
3.1. Factorial Design Results for Glucose and Xylose by Hydrolysis
A total of 24 experimental hydrolysis treatments of OP were performed to assess the production of glucose and xylose. The detailed specifications are provided in Table 1. Hydrolysis of OP at 125 °C with 0.5% H2SO4 yielded 52.14 g/L of glucose and 15.70 g/L of xylose.

Table 1.
Glucose and xylose concentration results for OP (S.D. standard deviation).
Table 2 presents the ANOVA, which indicates that both the H2SO4 concentration (A) and temperature (B) had statistically significant effects, with p-values < 0.05, on glucose and xylose production. In contrast, time (C) and the interactions between factors did not show statistical significance (p > 0.05). Therefore, acid concentration and temperature were critical factors for the release of glucose and xylose, while extending the reaction time from 1 to 2 h did not lead to a significant increase. Although the concentration of H2SO4 was significant, the observed difference of approximately 3 g/L suggests that focusing on a primarily thermal treatment could be more advantageous in terms of saving sulfuric acid and sodium hydroxide for neutralization. The reduction in glucose and xylose production as sulfuric acid concentration increases was attributed to the conversion of sugars into furfural and 5-hydroxymethylfurfural [40], which negatively impacts the yield of glucose and xylose. Additionally, it is noted that temperature made a greater contribution to the response variables of glucose and xylose compared with acid concentration.

Table 2.
Analysis of variance for glucose and xylose concentration for OP.
Figure 2 presents the response surface plots illustrating the combined effects of H2SO4 concentration and temperature on sugar release after 1 and 2 h of treatment. The highest production point according to Figure 2 was 51.14 g/L for glucose and 15.42 g/L for xylose, at 125 °C, 0.25% v/v de H2SO4 and 2 h. The higher glucose release compared with xylose is attributed to the structural composition of the substrate, which contains a greater proportion of cellulose related to hemicellulose. These findings support the application of mild and controlled pretreatment conditions to optimize the recovery of glucose and xylose while minimizing thermal degradation byproducts.

Figure 2.
Response surfaces of sulfuric acid concentration (% v/v) and temperature (°C) on glucose (A,C) and xylose (B,D) release during OP hydrolysis at 1 h (A,B) and 2 h (C,D).
A residual analysis was conducted to assess the validity of the regression models applied to the glucose and xylose concentration data on Figure 3. The standardized residual plots versus the fitted values (Figure 3A) showed a random scatter around zero, indicating that the model errors were evenly distributed and did not follow a defined pattern. However, a slight clustering of residuals was observed at the lower fitted values for both glucose and xylose (Figure 3C). This may reflect some variability not fully explained by the model in that region, although it does not significantly affect the overall validity of the model.

Figure 3.
Residual analysis of the fitted models and normal probability plot for glucose and xylose concentration using OP hydrolysates. (A) Fitted values for glucose, (B) normal probability plot for glucose, (C) fitted values for xylose, (D) normal probability plot for xylose.
On the other hand, the normal probability plots (Figure 3B–D) show that most of the residuals closely followed the expected theoretical line, suggesting they were approximately normally distributed. Deviations at the extremes—particularly for glucose—may be attributed to experimental variation or the presence of mild outliers. Overall, the residual analysis supports the adequacy of the fitted models for describing the hydrolysis behavior under the studied conditions.
Based on the statistical analysis using multiple linear regression with interaction terms, predictive models were developed to estimate independently the concentrations of glucose and xylose released during the acid hydrolysis of biomass and whether those terms were significant or not, as described in Equations (2) and (3), respectively. These models considered sulfuric acid concentration (CH2SO4), temperature (T), and time (t) as independent variables, along with the bilinear interactions between these factors.
In a similar manner, Table 3 presents the average concentrations of glucose and xylose from two replicates and the standard deviation, based on the general factorial design with sulfuric acid percentage (%v/v) and temperature during the hydrolysis of BSG. For BSG, the best results were obtained with 2.5% H2SO4 at 160 °C, producing 27.10 g/L of glucose and 14.22 g/L of xylose.

Table 3.
Glucose and xylose concentration results for BSG (S.D., standard deviation).
The ANOVA in Table 4 indicated that both the H2SO4 concentration and temperature had a statistically significant effect on the release of glucose and xylose, whereas the interaction between these factors was not significant. Specifically, with a concentration of 2.5% H2SO4, glucose production was 22.0 g/L at 130 °C and 25.0 g/L at 160 °C, reflecting an increase of 3.0 g/L with the rise in temperature. Under the same temperature conditions, xylose production increased from 10.69 to 12.22 g/L, representing an improvement of approximately 1.53 g/L.

Table 4.
Analysis of variance for glucose and xylose concentration for BSG.
Energy consumption was calculated by determining the energy required to heat 1 L of water from 25 to 130 °C and 160 °C, using the specific heat capacity of water (4.18 kJ/kg·K). The energy needed to raise the temperature from 25 to 130 °C was 438.9 kJ, whereas to reach 160 °C, it was 564.3 kJ, representing a 28.6% increase in energy consumption. This increase in energy resulted in a 13.6% rise in glucose production and a 14.3% rise in xylose production. The yield of glucose was 0.0501 g/kJ at 130 °C and 0.0443 g/kJ at 160 °C. For xylose, the yield was 0.0244 g/kJ at 130 °C and 0.0217 g/kJ at 160 °C. These results suggest that while higher temperatures enhance the production of glucose and xylose, they also lead to higher energy costs, which should be considered in the overall process balance. Therefore, operating at 130 °C offers the best energy consumption rate per gram of sugar released.
Figure 4 shows the response surface graphs assessing the combined effect of sulfuric acid concentration and temperature on glucose and xylose release during the hydrolysis of BGS. The cubic interpolation of the experimental data generated a model that illustrates how sugar yields vary with these two variables. Intermediate conditions promote the release of both sugars, suggesting a balance between the catalytic action of the acid and the thermal input. The maximum concentration of glucose and xylose were reached at 160 °C and 2.5% v/v H2SO4 which were 25.00 g/L and 12.22 g/L. These values were identified through response surface analysis.
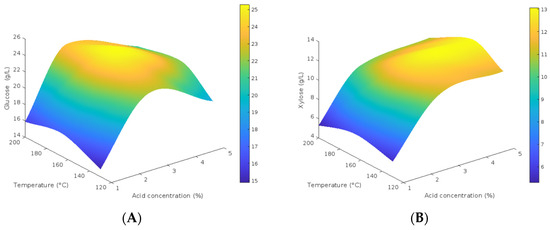
Figure 4.
Response surface graphs, OP, acid concentration %v/v and temperature °C, (A) glucose concentration (g/L), (B) xylose concentration (g/L).
A residual analysis was conducted to evaluate the regression models fitted for predicting glucose and xylose concentrations from brewer’s spent grain (BSG). In Figure 5A,C, which show standardized residuals versus fitted values, the residuals are generally scattered around zero, indicating that the model errors do not follow a systematic trend. However, a slight accumulation of positive residuals was observed in the mid-range of fitted values for both glucose and xylose, which may have reflected some variability associated with specific experimental conditions.

Figure 5.
Residual analysis of the fitted models and normal probability plot for glucose and xylose concentrations using BSG hydrolysates. (A) Fitted values for glucose, (B) normal probability plot for glucose, (C) fitted values for xylose, (D) normal probability plot for xylose.
In the normal probability plots (Figure 5B,D), the residuals align reasonably well with the theoretical reference line, suggesting that the assumption of normality is generally satisfied. Some deviations at the extremes may be attributable to outliers or non-linear behavior under certain combinations of temperature and acid concentration.
Based on the statistical analysis using multiple linear regression with interaction terms, predictive models were developed to estimate the concentrations of glucose and xylose released during the acid hydrolysis of BSG, as represented in Equations (4) and (5). These models consider sulfuric acid concentration (CH2SO4) and temperature (T) as independent variables, along with their combined effect through bilinear interaction.
Figure 6 compares the experimental and predicted concentrations of glucose and xylose obtained from the hydrolysates of OP and BSG. Graph A presents the results for OP, where the values predicted by the response surface regression model closely follow the experimental data, particularly in the case of xylose. The slight deviations observed for glucose may be attributable to experimental variability. Graph B shows the results for BSG, also demonstrating a consistent alignment between the measured and predicted values.

Figure 6.
Comparison between experimental and predicted values of glucose and xylose obtained from the hydrolysis of OP (A) and BSG (B), using response surface models.
Overall, the regression models accurately represented the trends for both residues, supporting their usefulness in estimating glucose and xylose under different hydrolysis conditions.
3.2. Glucose and Xylose Concentration Production Comparative
Table 5 presents a range of glucose and xylose production from OP and BSG. Each range was prepared after the highest concentrations of glucose and xylose reported. The studies described in Table 5 utilized different methods, including acid hydrolysis, enzymatic hydrolysis, and supercritical fluids. Table 5 also compares the highest result obtained in this study for glucose and xylose production.

Table 5.
Comparative analysis of the production of glucose and xylose from OP and BSG.
The present study achieved competitive concentrations of glucose and xylose through hydrolysis and its method offers significant advantages. For instance, although Córtes Ortiz et al. (2020) reported higher glucose concentrations, their method required delignification with NaOH and hydrolysis with 13.3% sulfuric acid, making it a more complex chemical process that involves additional reagents [49].
Similarly, Kuo et al. (2019) achieved high glucose and xylose concentrations, but their methodology involved specific enzymes and a reaction time of 5.5 h, which made the process more expensive [50]. Studies have reported that the cost of pretreatment and enzymatic hydrolysis for bioethanol production from lignocellulosic biomass is up to four times higher compared with acid hydrolysis. Additionally, the initial investment in enzymatic reactors can be up to five times higher for those of thermal or acid hydrolysis systems [41]. In contrast, the method used in this study is notable for its simplicity, as it does not require additional chemicals or long reaction times. Moreover, compared with studies such as that by Corona et al. (2020), which achieved lower sugar concentrations despite operating at high temperatures and controlled pressure, this study demonstrates that higher yields can be achieved with a less demanding process in terms of input materials and variable control [32]. Tsouko et al. (2020) reported up to 49 g/L of glucose and 16 g/L of xylose using 1.5% sulfuric acid at 121 °C, followed by enzymatic hydrolysis. Although these results are comparable to those achieved in the present study, their process involved more steps, higher energy consumption, and, as noted by Tsouko et al. (2020), issues related to the high viscosity of the hydrolysate, complicating subsequent operations such as fermentation. In contrast, the proposed method reached competitive sugar concentrations using 0% of H2SO4 and under similar temperature conditions [31].
Processes such as supercritical CO2 enable the highest yields of glucose and xylose production from OP, producing up to 79.29 g/L and 97.14 g/L of glucose and xylose, respectively [42]. When only acid hydrolysis at 130 °C is applied, it is possible to obtain up to 40.2 g/L of xylose from OP [43]. Nevertheless, the combination of high pressure and temperature negatively impacts xylose production. When the temperature exceeds 160 °C under high pressure, the yield drops significantly, with only 2.3 g/L of xylose produced [44,45]. Performing hydrolysis using bacteria such as Enterobacter cloacae, Pseudomonas aeruginosa, or Bacillus cereus results in glucose production of only up to 1.75 g/L [42].
Various studies on BSG hydrolysis reveal variations in the yields of glucose and xylose, primarily influenced by methodological conditions. Liguori et al. (2015) employed enzymatic hydrolysis, achieving a high conversion of cellulose to glucose, which underscores the effectiveness of enzymes in polysaccharide degradation. However, this method incurred high costs due to the need for specific enzymes and controlled reaction conditions [43].
López-Linares et al. (2020) and Mussatto and Roberto (2006) chose acid hydrolysis, applying different concentrations of sulfuric acid along with varying temperature and time conditions. This method has proven to be efficient for xylose release, although glucose production is more limited compared to enzymatic processes [46,47]. García et al. (2023) combined acid and enzymatic hydrolysis in a BSG fractionation process, which improved the utilization of biomass components, but at the expense of greater technical complexity and the need for multiple treatment stages [48].
In contrast, Treichel et al. (2023) investigated the use of biocatalysts, as well as homogeneous and heterogeneous catalysts, to enhance sugar conversion. This approach shows promise, though it faces challenges related to the stability and costs of the catalysts used [51].
The supercritical CO2 technique applied to BSG has been proven ineffective, as demonstrated by Lisci et al. (2024), where the maximum concentrations of glucose and xylose produced were 8.5 g/L and 9 g/L, respectively. This highlights a key advantage of hydrolysis over supercritical CO2 extraction, as the latter requires pressures above 73 atm, entailing high energy consumption, the need for highly specialized equipment, and costly operating conditions that hinder its large-scale implementation [44].
Similarly, Vičević et al. (2024) showed that the use of Thermomyces lanuginosus did not result in significant conversion of glucose and xylose from BSG [52]. However, operating at high temperatures above 180 °C can yield up to 18.3 g/L of glucose and 7.9 g/L of xylose. The enzyme Celluclast, when used on BSG, produced glucose concentrations comparable to those reported in the present study, ranging from 7.24 to 26.55 g/L. However, this was not the case for xylose, where the maximum yield was only 1.22 g/L.
In contrast to the studies described in this section, the present work relied solely on acid hydrolysis in the absence of enzymes, biocatalysts, or supercritical CO2. While the yields of glucose and xylose were lower compared with studies using more complex methodologies, the possibility of achieving significant sugar release from OP under hydrolysis at 0% of sulfuric acid, together with the overall simplicity of the diluted acid process, makes this strategy more attractive for industrial scalability.
3.3. Bioethanol Production
Figure 7 illustrates the increase in alcohol concentration (% v/v) produced during the fermentation at 28, 30, and 32 °C (Figure 7A, OP; Figure 7B, BSG, both for five days). Each data point represents the average of triplicate experiments. The results showed a general increase in alcohol production at all temperatures, with no significant changes after the third day of fermentation. Fermentation at 30 °C was the most efficient, reaching 5.25% and 1.5% alcohol by the end of the fifth day for OP and BSG, respectively. In contrast, the lowest yield (4.75%) was observed at 28 °C, while at 32 °C, the production reached 5%, suggesting that temperatures above 30 °C negatively impacted the fermentation process, which is likely to have been due to thermal stress on the yeast.

Figure 7.
Bioethanol production from OP (A) and BSG (B) at different temperatures.
These findings are consistent with prior studies that define an optimal temperature range for Saccharomyces cerevisiae growth, where deviations from this range affect both the fermentation rate and alcohol production [53]. Bioethanol production over time can be described by a modified logistic equation, which accounts for exponential and stationary phases, as expressed in Equation (1).
Equations (6) and (7) represent the fermentation kinetics for bioethanol production from OP and BSG, with correlation coefficients of 0.9877 and 0.9670, respectively, where Cbioethanol refers to bioethanol concentration depending on the biomass waste used in %v/v, and t is time in days.
Bioethanol production for OP yielded 41.4 g/L, which was comparable to the bioethanol yields reported in other studies. The range of bioethanol production from OP was 3.90–54.00 g/L [37,38,46,53,54].
In the method used by Corona et al. (2020), ethanol concentrations of approximately 35–38 g/L were achieved using OP hydrolysates fermented with a recombinant strain of Escherichia coli capable of fermenting both hexose and pentose sugars [32].
A separate study by Patsalou, M. (2020) reported a final ethanol yield of 30.3 g/L using hydrolysates obtained through sequential acid and enzymatic pretreatment. The fermentation was carried out using Saccharomyces cerevisiae and Mucor indicus [33].
Koutinas et al. (2015) conducted a study on high-temperature alcoholic fermentation of hydrolyzed OP, finding that the yeast maximized ethanol production at 42 °C, reaching 54 g/L of ethanol. Additionally, they explored the effects of OP oil on bioethanol formation and determined that the minimum inhibitory concentration of the essential oil was 0.01% (v/v) [55].
Oberoi et al. (2010) reported a yield of 50.55 g/L using a two-stage acid hydrolysis process [34]. In contrast, Santi et al. (2014) achieved ethanol production of 15 g/L by performing acid pretreatment on citrus waste in an innovative steam injection reactor at 180 °C for 150 s, followed by fermentation with an industrial strain of S. cerevisiae [56]. This analysis indicates a significantly higher bioethanol yield from OP compared with the previous studies [35,37,38].
The maximum amount of bioethanol produced was 54.00 g/L, using the yeast Pichia kudriavzevii [55]. Other studies have highlighted the amount of time used for the fermentation; with times varying from 24, 48, and 72 h, the amount of bioethanol produced increased from 3.90–12.80 [55].
Bioethanol production for BSG yielded 11.8 g/L which is compared with the bioethanol yields reported in other studies. The range of bioethanol production from BSG is 4–280 g/L [33,45,47,55,56,57].
The maximum amount of bioethanol obtained from BSG was 280.00 g/L from a combination of BSG with sugarcane [56,58], which can be explained by the content of sugars added via the sugarcane. BSG alone produced a maximum of 32.8 g/L using the enzyme ethanol red [52]. Supercritical CO2 under 24 h of operation produced a bioethanol concentration of 4 g/L [44].
In general, bioethanol production from BSG yielded less compared with OP. Since BSG had already undergone previous fermentation for beer production, it was expected that less bioethanol would be obtained.
3.4. Bioethanol from OP and BSG Projection
Mexican industry generates around 720,000 tons of OP annually, of which 80% is recoverable, equating to 576,000 tons available. With an experimental yield of 2.52 mL of ethanol per 100 g of OP, the estimated production potential is approximately 14.51 million liters of ethanol per year. Conversely, the Mexican brewing industry produces about 2.48 million tons of wet BSG annually. With a yield of 1.78 mL of ethanol per 100 g of wet BSG, the production potential is 44.14 million liters of ethanol per year. However, accounting for logistical and processing losses, it is estimated that only 80% of the BSG is usable, reducing the potential production to 35.31 million liters of ethanol annually [26,39].
Combining both residues, the total bioethanol production potential reaches 49.82 million liters annually. This volume accounted for approximately 31% of Mexico’s total ethanol production in 2023, as shown in Figure 8. Consequently, utilizing OP and BSG for bioethanol production could significantly reduce reliance on imports and enhance the country’s energy self-sufficiency.

Figure 8.
Production and demand for bioethanol in Mexico compared with the potential of agroindustrial waste.
Bioethanol represents a significant source of renewable energy that could contribute to Mexico’s sustainability objectives and reduce its dependence on fossil fuels.
This study highlights the potential of orange peel (OP) and brewer’s spent grain (BSG) as viable raw materials for bioethanol production through acid hydrolysis, followed by alcoholic fermentation with Saccharomyces cerevisiae. The process was optimized to enhance the yield of glucose and xylose sugars while reducing the consumption of chemicals and energy inputs.
In the case of OP, the results show that pretreatment at 125 °C for 1 h with 0.0% H2SO4 achieved concentrations of 39.64 g/L of glucose and 12.33 g/L of xylose. The ANOVA revealed that temperature was the most influential factor in hydrolysis, while the effect of sulfuric acid concentration, though statistically significant, did not lead to a substantial increase in glucose or xylose production. This suggests that reducing the acid concentration could be a viable strategy to reduce operational costs and environmental impact.
For BSG, the optimal conditions were 2.5% H2SO4 at 160 °C for 20 min, yielding concentrations of 25.08 g/L of glucose and 12.21 g/L of xylose. However, when the temperature was reduced to 130 °C, a 13.6% reduction in glucose yield and a 14.3% reduction in xylose yield were observed, but with a 28.6% decrease in energy consumption. This underscores the importance of assessing the balance between production efficiency and energy consumption when designing large-scale processes.
Fermentation with S. cerevisiae resulted in a maximum bioethanol concentration of 5.25% v/v for OP and 1.5% v/v for BSG at 30 °C. The kinetic modeling of fermentation using logistic equations revealed correlation coefficients above 0.98, indicating a high degree of predictability in ethanol production. The sugar-to-bioethanol conversion efficiency was higher for OP, suggesting that this substrate is more suitable for fermentation processes compared with BSG, probably due to its lower lignin content.
Based on the yields obtained in this study, Mexico’s potential bioethanol production is estimated at 49.82 million liters per year, equivalent to 31% of the country’s ethanol output in 2023. This amount also represents 20.8% of the installed capacity of FS Bioenergia—one of Brazil’s leading corn-based bioethanol plants, with an annual capacity of 240 million liters [58]. In Latin America, Colombia operates 14 bioethanol plants producing a total of 414.71 million liters annually, averaging 29.62 million liters per plant. Thus, the estimated output in this study exceeds the Colombian average by 68.2% [59], underscoring the high potential of converting agro-industrial waste into a sustainable energy source in the regional biofuel context.
4. Conclusions
This study demonstrates that OP and BSG are viable agro-industrial residues for bioethanol production through hydrolysis and alcoholic fermentation.
For OP, the highest sugar release was obtained at 125 °C with 0.5% H2SO4 for 1 h, reaching 52.14 g/L of glucose and 15.70 g/L of xylose. Significantly, from an operational standpoint, under the same temperature and time conditions but in the absence of H2SO4, the experimental design analysis showed production of 48.43 g/L of glucose and 13.79 g/L of xylose representing 92.9% and 87.8%, respectively, compared with the acid treatment. These results demonstrate a novel method for glucose and xylose production by hydrolysis, since the absence of H2SO4 is a relevant technical advantage, allowing for a reduction in the use of corrosive reagents and eliminating the neutralization stage.
In the case of BSG, the highest sugar release was achieved at 160 °C, with 27.10 g/L of glucose and 14.22 g/L of xylose. However, at 130 °C, 25.10 g/L of glucose and 11.00 g/L of xylose were obtained, corresponding to 92.6% and 77.4%, respectively, of the maximum yield. This reduction in temperature implies improved energy efficiency of the process, which is key for its industrial-scale feasibility. Regression models were developed to predict the release of glucose and xylose as a function of process parameters. The results demonstrate the feasibility of producing glucose and xylose from OP in the absence of sulfuric acid and from BSG at moderate temperatures. Compared with previous studies that use more complex or costly methods, such as enzymatic hydrolysis or temperatures above 160 °C, the yields obtained in this work are competitive and require lower energy input.
Fermentation with Saccharomyces cerevisiae yielded up to 5.25% v/v bioethanol from OP and 1.5% v/v from BSG at 30 °C. The fitted kinetic models showed correlation coefficients above 0.98. The bioethanol production from OP and BSG yielded 41.1 g/L and 11.8 g/L, respectively. These values are within the wide range of values reported in the literature, which makes OP and BSG suitable sources of glucose and xylose for bioethanol conversion.
Author Contributions
Conceptualization, J.R.A. and M.A.C.; methodology, J.M.A.; validation, E.E.A.; formal analysis, D.G.M.; investigation, J.M.A.; resources, E.E.A.; data curation, J.R.A.; writing—original draft preparation, J.M.A.; writing—review and editing, M.A.C. and L.Q.; visualization, D.G.M.; supervision, J.R.A.; project administration, M.A.C.; funding acquisition, L.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The authors would share any data regarding the findings upon request.
Acknowledgments
The authors thank the Secretary of Science, Humanities, Technology and Innovation (SECIHTI) and the Engineering Institute of the Universidad Autónoma de Baja California for their support in the development of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hoang, T.-D.; Nghiem, N. Recent Developments and Current Status of Commercial Production of Fuel Ethanol. Fermentation 2021, 7, 314. [Google Scholar] [CrossRef]
- Yelle, D.J.; Serwańska, K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Ulep, R.A.; Madigal, J.P.T.; Suarez, T.C.E.; Ramos, K.M.D.; Cariaga, J.F.; Agrupis, S.C. “Nipahol”: A Locally Formulated Sanitizer/Disinfectant from Nipa Bioethanol for Possible Use Against COVID-19. Agro Bali Agric. J. 2021, 5, 30–41. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture (USDA). Global Orange Production Will Total 48.8 Million Tons in the 2023–2024 Season. Citrus Committee. 2024. Available online: https://www.tridge.com/news/usda-world-orange-production-will-total-488-millio (accessed on 8 December 2024).
- Secretaría de Agricultura y Desarrollo Rural de México. Detrás de la naranja. Gobierno de México. 2024. Available online: https://www.gob.mx/agricultura/articulos/detras-de-la-naranja?idiom=es (accessed on 8 December 2024).
- Castro, L.A.d.; Lizi, J.M.; Chagas, E.G.L.d.; Carvalho, R.A.d.; Vanin, F.M. From Orange Juice By-Product in the Food Industry to a Functional Ingredient: Application in the Circular Economy. Foods 2020, 9, 593. [Google Scholar] [CrossRef]
- Munir, H.; Yaqoob, S.; Awan, K.A.; Imtiaz, A.; Naveed, H.; Ahmad, N.; Naeem, M.; Sultan, W.; Ma, Y. Unveiling the Chemistry of Citrus Peel: Insights into Nutraceutical Potential and Therapeutic Applications. Foods 2024, 13, 1681. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The Potential of Brewer’s Spent Grain in the Circular Bioeconomy: State of the Art and Future Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 870744. [Google Scholar] [CrossRef]
- Moreno Camarena, A. Caracterización Fisicoquímica e Hidrólisis del Bagazo Cervecero para la Obtención de Azúcares Reductores. Master’s Thesis, Universidad Autónoma de Baja California, Mexicali, México, 2017. Available online: https://repositorioinstitucional.uabc.mx/handle/20.500.12930/1891 (accessed on 8 December 2024).
- Devnani, B.; Moran, G.C.; Grossmann, L. Extraction, Composition, Functionality, and Utilization of Brewer’s Spent Grain Protein in Food Formulations. Foods 2023, 12, 1543. [Google Scholar] [CrossRef]
- Marcus, A.; Fox, G. Fungal Biovalorization of a Brewing Industry Byproduct, Brewer’s Spent Grain: A Review. Foods 2021, 10, 2159. [Google Scholar] [CrossRef]
- Assandri, D.; Bianco, A.; Pampuro, N.; Cavallo, E.; Zara, G.; Bardi, L.; Coronas, R.; Budroni, M. Enhancing Fertilizer Effect of Bioprocessed Brewers’ Spent Grain by Microbial Consortium Addition. Agronomy 2023, 13, 2654. [Google Scholar] [CrossRef]
- Jagiełło, K.; Uchańska, O.; Trusek, A. Brewer’s Spent Grains—Valuable Beer Industry By-Product. Biomolecules 2020, 10, 1669. [Google Scholar] [CrossRef]
- Tardiolo, G.; Nicolò, M.S.; Drago, C.; Genovese, C.; Fava, G.; Gugliandolo, C.; D’Antona, N. Orange Peel Waste as Feedstock for the Production of Glycerol-Free Biodiesel by the Microalgae Nannochloropsis oculata. Molecules 2023, 28, 6846. [Google Scholar] [CrossRef]
- Zurita Cabrales, T. Centro de Estudios para el Desarrollo Rural Sustentable y la Soberanía Alimentaria (CEDRSSA) Cámara de Diputados LXV Legislatura. Palacio Legislativo de San Lázaro. Consideraciones generales para la producción y utilización del bioetanol en México. SIIBA-CONADESUCA 2023. Available online: https://portales.diputados.gob.mx/CEDRSSA/publicaciones/detalles/7c9e4b14-60f9-4213-bdbc-13113cb0e1da (accessed on 3 April 2025).
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Ayala, J.R.; Montero, G.; Coronado, M.A.; García, C.; Curiel-Alvarez, M.A.; León, J.A.; Sagaste, C.A.; Montes, D.G. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules 2021, 26, 1348. [Google Scholar] [CrossRef]
- Xavier, A.M.R.B.; Beltrán, S.; Sanz, M.T. Subcritical Water as Pretreatment Technique for Bioethanol Production from Brewer’s Spent Grain Within a Biorefinery Concept. Polymers 2022, 14, 5218. [Google Scholar] [CrossRef]
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef]
- Rogers, P.L.; Jeon, Y.J.; Lee, K.J.; Lawford, H.G. Zymomonas mobilis for fuel ethanol and higher value products. Adv. Biochem. Eng. Biotechnol. 2007, 108, 263–288. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, X.; Yuan, Q.; Yan, Y. Metabolic Engineering Strategies for Co-Utilization of Carbon Sources in Microbes. Bioengineering 2016, 3, 10. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hsu, C.L.; Chang, T.C.; Jang, H.D. Enhancement of the Efficiency of Bioethanol Production by Saccharomyces cerevisiae via Gradually Batch-Wise and Fed-Batch Increasing the Glucose Concentration. Fermentation 2018, 4, 45. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Buller, L.S.; Mussatto, S.I.; Forster-Carneiro, T. Techno-economic assessment of bioenergy and fertilizer production by anaerobic digestion of brewer’s spent grains in a biorefinery concept. J. Clean. Prod. 2021, 297, 126600. [Google Scholar] [CrossRef]
- Paz, A.; Outeiriño, D.; Guerra, N.P.; Domínguez, J.M. Enzymatic hydrolysis of brewer’s spent grain to obtain fermentable sugars. Bioresour. Technol. 2019, 275, 402–409. [Google Scholar] [CrossRef]
- Gomes, J.; Batra, J.; Chopda, V.R.; Kathiresan, P.; Rathore, A.S. Monitoring and control of bioethanol production from lignocellulosic biomass. In Waste Biorefinery: Potential and Perspectives; Elsevier: Amsterdam, The Netherlands, 2018; pp. 727–749. [Google Scholar] [CrossRef]
- Galindo-Segura, L.A.; Pérez-Vázquez, A.; Ramírez-Martínez, A.; López-Romero, G.; Gómez-Merino, F.C. Managing Orange Bagasse in the Central Area of Veracruz State. Terra Latinoam. 2023, 41, e1673. [Google Scholar] [CrossRef]
- Armenta, E.E.; Coronado, M.A.; Ayala, J.R.; León, J.A.; Montes, D. Essential Oil Extraction for All: A Flexible and Modular System for Citrus Biomass Waste. BioResources 2023, 18, 4977–4993. [Google Scholar] [CrossRef]
- Mtashobya, L.A.; Mgeni, S.T.; Emmanuel, J.K. Bioethanol Production from Concentration Fruit Wastes Juice Using Bakery Yeast. Mater. Renew. Sustain. Energy 2025, 14, 6. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Mukherjee, G.; Goud, V.V.; Veeranki, V.D.; Sastri, C.V. Pitfalls in the 3,5-dinitrosalicylic acid (DNS) assay for the reducing sugars: Interference of furfural and 5-hydroxymethylfurfural. Int. J. Biol. Macromol. 2020, 156, 180–185. [Google Scholar] [CrossRef]
- Kaur, A.; Verma, A.; Prakash, R. Enhancing the Ethanol Fermentation Performance of Food and Fruit Waste Using Mild Acid Hydrolysis in Slurry Mode. Waste Biomass Valorization 2025, 16, 749–759. [Google Scholar] [CrossRef]
- Tsouko, E.; Maina, S.; Ladakis, D.; Kookos, I.K.; Koutinas, A. Integrated biorefinery development for the extraction of value-added components and bacterial cellulose production from orange peel waste streams. Renew. Energy 2020, 160, 944–954. [Google Scholar] [CrossRef]
- Corona Vázquez, B.; Roa-Morales, G.; Natividad, R.; Balderas-Hernández, P.; Saucedo-Luna, J. Thermal Hydrolysis of Orange Peel and its Fermentation with Alginate Beads to Produce Ethanol. BioResources 2017, 12, 2955–2964. [Google Scholar] [CrossRef][Green Version]
- Patsalou, M.; Menikea, K.K.; Nikolaou, C.; Tsoukos, P.; Koutinas, A.; Koutinas, M. A Citrus Peel Waste Biorefinery for Ethanol and Methane Production. Molecules 2019, 24, 2451. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Madl, R.L. Production of Ethanol from Orange Peels: Fermentation Studies and Two-Stage Hydrolysis Using Optimized Parameters through Experimental Design. J. Agric. Food Chem. 2010, 58, 3422–3429. [Google Scholar] [CrossRef] [PubMed]
- Kasaeian, A.; Fereidooni, L.; Ahmadbeigi, A.; Kahedi, S.; Shavali Koohshoori, M.; Ghafarian, S.; Tajmousavilangerudi, A.; Salaripoor, H. Review on Bioethanol Production from Fruit Peels. Waste Biomass Valorization 2025. [Google Scholar] [CrossRef]
- Kowsalya, R.; Prabhu, N.; Rajamehala, M.; Singh, M.V.P.; Karthikadevi, S. Statistical Optimization of Cellulase Production and Its Efficacy in Hesperidin Extraction from Orange Peel and Bioethanol Production from Rice Straw by Simultaneous Saccharification and Fermentation. Res. J. Biotechnol. 2022, 17, 20–31. [Google Scholar]
- Plugatar, Y.; Johnson, J.B.; Timofeev, R.; Korzin, V.; Kazak, A.; Nekhaychuk, D.; Borisova, E.; Rotanov, G. Prediction of Ethanol Content and Total Extract Using Densimetry and Refractometry. Beverages 2023, 9, 31. [Google Scholar] [CrossRef]
- Pavlečić, M.; Novak, M.; Trontel, A.; Marđetko, N.; Grubišić, M.; Didak Ljubas, B.; Petravić Tominac, V.; Čož Rakovac, R.; Šantek, B. Mathematical Modelling of Bioethanol Production from Raw Sugar Beet Cossettes in a Horizontal Rotating Tubular Bioreactor. Fermentation 2022, 8, 13. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Silva, S. Optimization of the Residual Biomass Supply Chain: Process Characterization and Cost Analysis. Logistics 2023, 7, 48. [Google Scholar] [CrossRef]
- Cui, J.; Tan, J.; Deng, T.; Cui, X.; Zhu, Y.; Li, Y. Conversion of carbohydrates to furfural via selective cleavage of the carbon–carbon bond: The cooperative effects of zeolite and solvent. Green Chem. 2016, 18, 1619–1624. [Google Scholar] [CrossRef]
- Verma, S.K.; Shastri, Y. Economic Optimization of Acid Pretreatment: Structural Changes and Impact on Enzymatic Hydrolysis. Ind. Crops Prod. 2020, 146, 112236. [Google Scholar] [CrossRef]
- Vinotha, T.; Umamaheswari, N.; Pandiyan, J.; Al-Ghanim, K.A.; Nicoletti, M.; Govindarajan, M. Biofuel Production from Mango and Orange Peel and Tapioca Shells by Fermentation Using Consortium of Bacteria: Agricultural and Food Waste Valorization. Fermentation 2023, 9, 678. [Google Scholar] [CrossRef]
- Liguori, R.; Soccol, C.R.; Vandenberghe, L.P.S.; Woiciechowski, A.L.; Faraco, V. Second Generation Ethanol Production from Brewers’ Spent Grain. Energies 2015, 8, 2575–2586. [Google Scholar] [CrossRef]
- Lisci, S.; Tronci, S.; Grosso, M.; Hajrizaj, R.; Sibono, L.; Karring, H.; Gerganov, A.; Maschietti, M.; Errico, M. Valorizing Brewer’s Spent Grain: A Sequential Pathway of Supercritical Extraction, Hydrolysis, and Fermentation. Chem. Eng. Sci. 2024, 285, 119620. [Google Scholar] [CrossRef]
- Pereira, B.S.; de Freitas, C.; Masarin, F.; Brienzo, M. Xylooligosaccharides from Industrial Fruit and Restaurant Waste Produced by Liquid Hot Water Treatment. BioEnergy Res. 2023, 16, 843–855. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Romero-García, J.M.; Robles, E.; Esteban, J.; Fermoso, J.; Castro, E. Brewer’s Spent Grain as a Source of Renewable Fuel through Optimized Dilute Acid Pretreatment. Renew. Energy 2020, 146, 1971–1983. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Chemical Characterization and Liberation of Pentose Sugars from Brewer’s Spent Grain. J. Chem. Technol. Biotechnol. 2006, 81, 268–274. [Google Scholar] [CrossRef]
- Bedő, S.; Rozbach, M.; Nagy, L.; Fehér, A.; Fehér, C. Optimised Fractionation of Brewer’s Spent Grain for a Biorefinery Producing Sugars, Oligosaccharides, and Bioethanol. Processes 2021, 9, 366. [Google Scholar] [CrossRef]
- Cortés Ortiz, W.G.; Ibla Gordillo, J.F.; Calderón Velásquez, L.M.; Herrera Bueno, A.F. Cuantificación de azúcares reductores en las cáscaras de naranja y banano. Rev. Tecnol. 2020, 12, 72–76. [Google Scholar] [CrossRef][Green Version]
- Kuo, C.-H.; Huang, C.-Y.; Shieh, C.-J.; Wang, H.-M.D.; Tseng, C.-Y. Hydrolysis of Orange Peel with Cellulase and Pectinase to Produce Bacterial Cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization 2019, 10, 85–93. [Google Scholar] [CrossRef]
- Treichel, H.; de Oliveira, D.; Mazutti, M.A.; Di Luccio, M.; de Oliveira, J.V. Applications of Brewer’s Spent Grain Hemicelluloses in Biorefineries: Extraction and Value-Added Product Obtention. Catalysts 2023, 13, 755. [Google Scholar] [CrossRef]
- Vičević, R.; Božinović, M.; Zekić, N.; Novak, M.; Grgić, D.K.; Šalić, A.; Zelić, B. Development of a Two-Stage Bioprocess for the Production of Bioethanol from the Acid Hydrolysate of Brewer’s Spent Grain. Energies 2024, 17, 3975. [Google Scholar] [CrossRef]
- Lewallen, L.; Loebeck, A.; Neighbors, D.; Wen, X.; Hoffman, R. The Effect Temperature Has on the Production of Carbon Dioxide in Saccharomyces cerevisiae. J. Introd. Biol. Investig. 2021, 14, 2. Available online: https://undergradsciencejournals.okstate.edu/index.php/jibi/article/view/13104 (accessed on 19 March 2024).
- Sarkar, R.; Nain, L.; Dutta, A.; Kundu, A.; Saha, S. Unraveling the Utilization Feasibility of Citrus Peel Solid Distillation Waste as Potential Source for Antioxidant as well as Bioethanol. Biomass Convers. Biorefin. 2024, 14, 27379–27391. [Google Scholar] [CrossRef]
- Koutinas, M.; Dourou, M.; Kookos, I.K.; Vlysidis, A.; Kopsahelis, N.; Papanikolaou, S. Life Cycle Assessment of a Novel Bioethanol Fermentation Process Using Pichia kudriavzevii. Lett. Appl. Microbiol. 2015, 62, 75–83. [Google Scholar] [CrossRef]
- Santi, G.; Crognale, S.; D’Annibale, A.; Petruccioli, M.; Ruzzi, M.; Valentini, R.; Moresi, M. Orange Peel Pretreatment in a Novel Lab-Scale Direct Steam-Injection Apparatus for Ethanol Production. Biomass Bioenergy 2014, 61, 146–156. [Google Scholar] [CrossRef]
- Tadesse, H.M.; Atnafu, T.; Kassahun, E.; Tessema, I.; Abewaa, M.; Tibebu, S. Optimization of Bioethanol Production from a Brewers’ Spent Grain and Sugarcane Molasses Mixture Utilizing Saccharomyces cerevisiae. Biomass Convers. Biorefin. 2025. [Google Scholar] [CrossRef]
- WEG. FS Bioenergia es Pionera en la Producción de Etanol de Maíz en Brasil. WEG. 2018. Available online: https://www.weg.net/institutional/NA/es/news/productos-y-soluciones/fs-bioenergia-es-pionera-en-la-produccion-de-etanol-de-maiz-en-brasil (accessed on 16 March 2024).
- Biofuels Working Group (GTB) of ARIAE. Statistical Benchmarking of Biofuels in Ibero-America 2023: Base Year 2022; Ibero-American Association of Energy Regulatory Entities (ARIAE): Madrid, Spain, 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).