Sustainable Production of 2,5-Furandicarboxylic Acid via Nickel-Based Heterogeneous Catalysis from 5-Hydroxymethylfurfural
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Nickel-Based Catalysts
2.3. Catalyst Characterization
2.4. Catalytic Oxidation of HMF to FDCA
2.5. Kinetic Studies
2.6. Experimental Design for FDCA Production
2.7. Catalyst Recycling Tests
2.8. Oxidation of Crude HMF
2.9. Analytical Methods
3. Results and Discussion
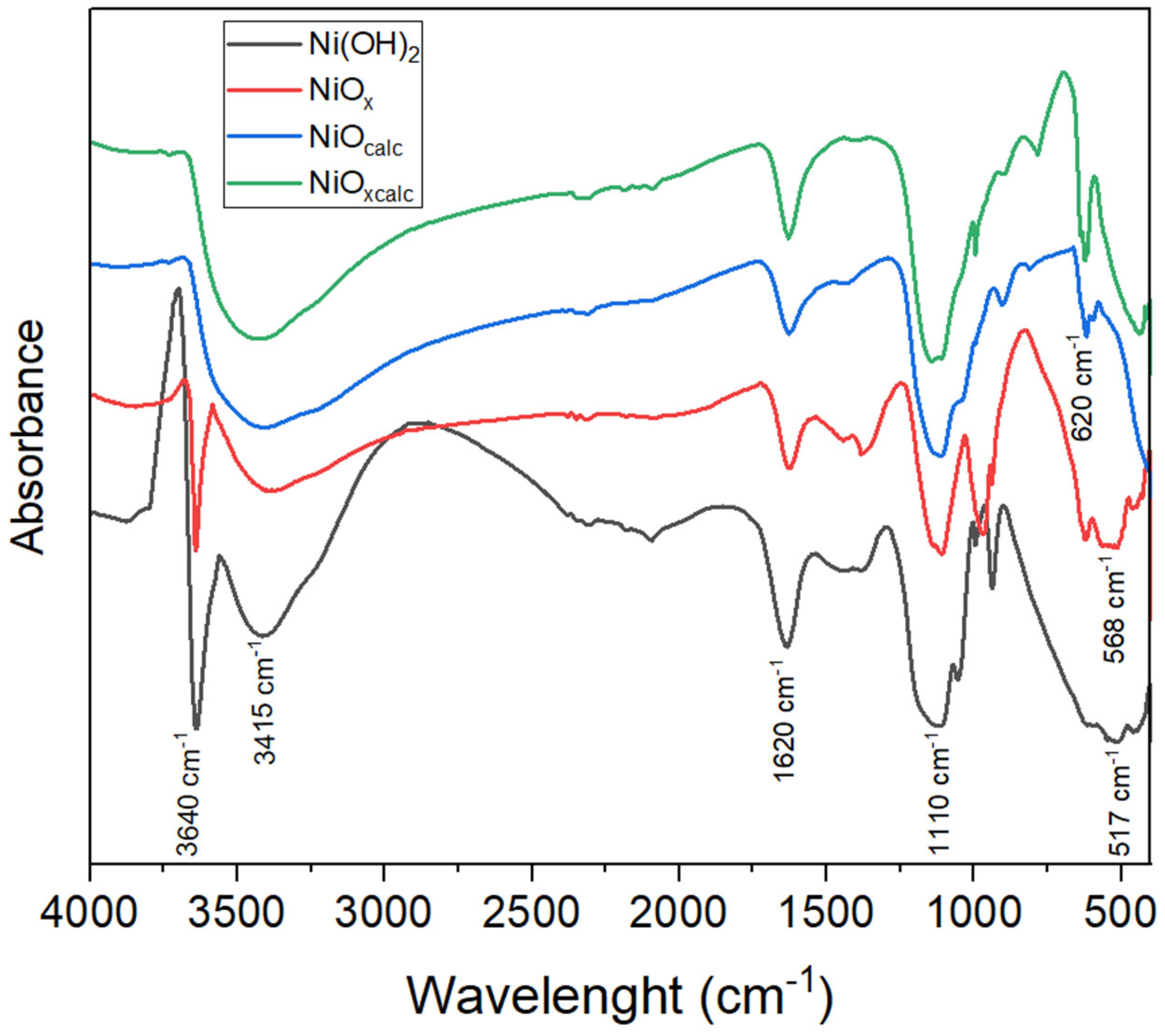
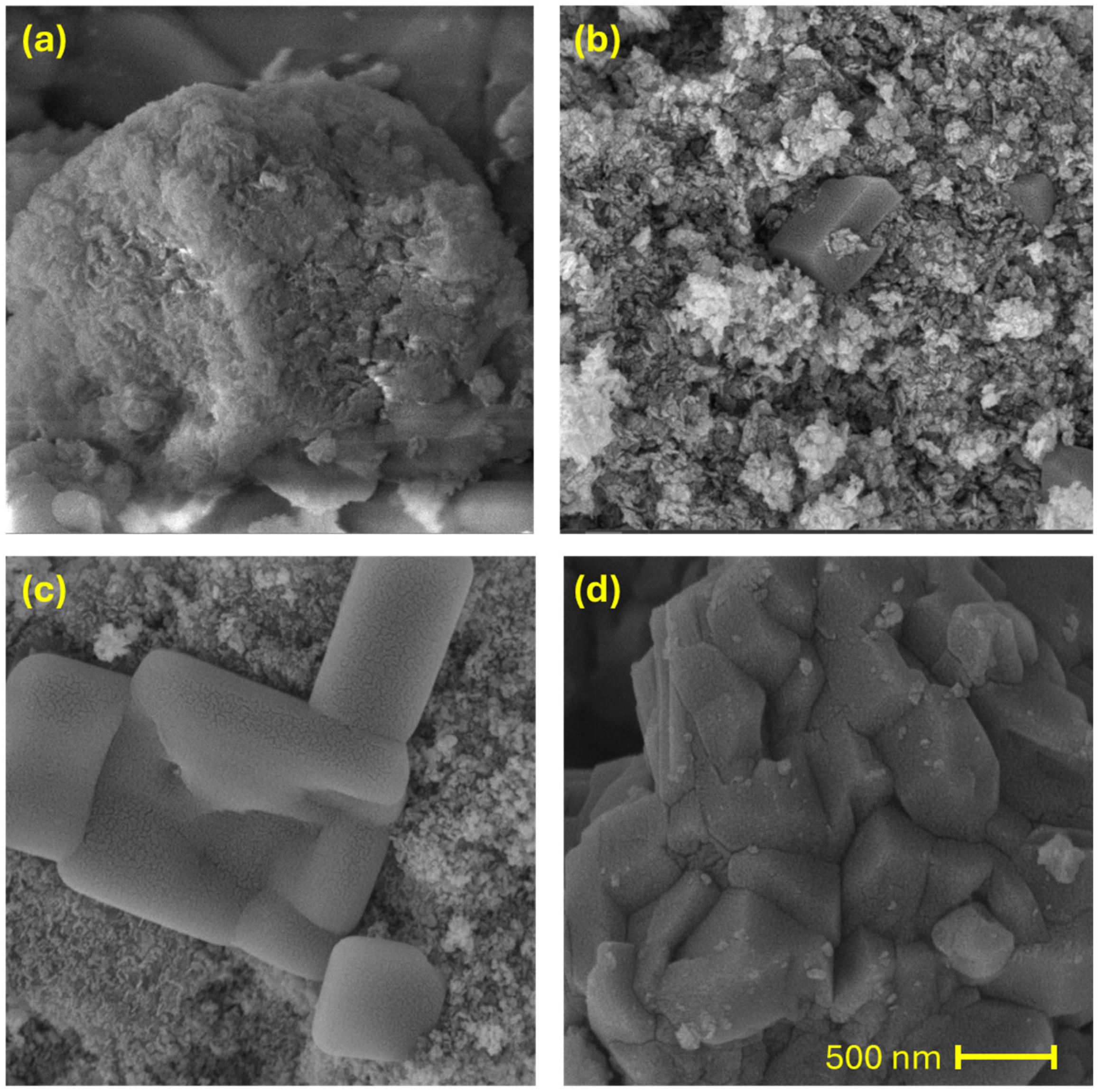
3.1. Catalyst Characterization
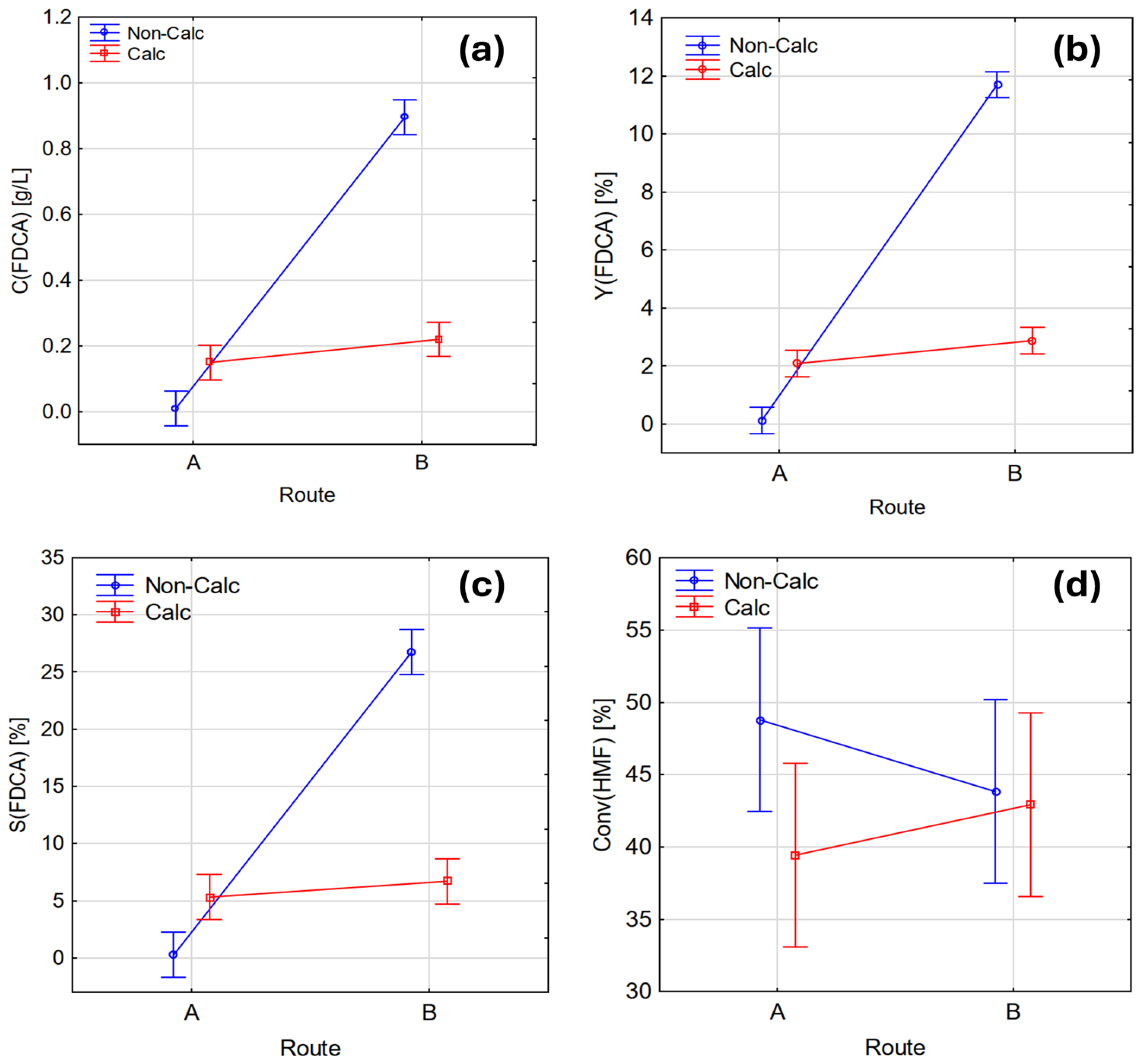
3.2. Influence of Synthesis Route and Calcination Conditions on Catalyst Properties
3.3. Reaction Kinetics and Oxidant Addition Strategies
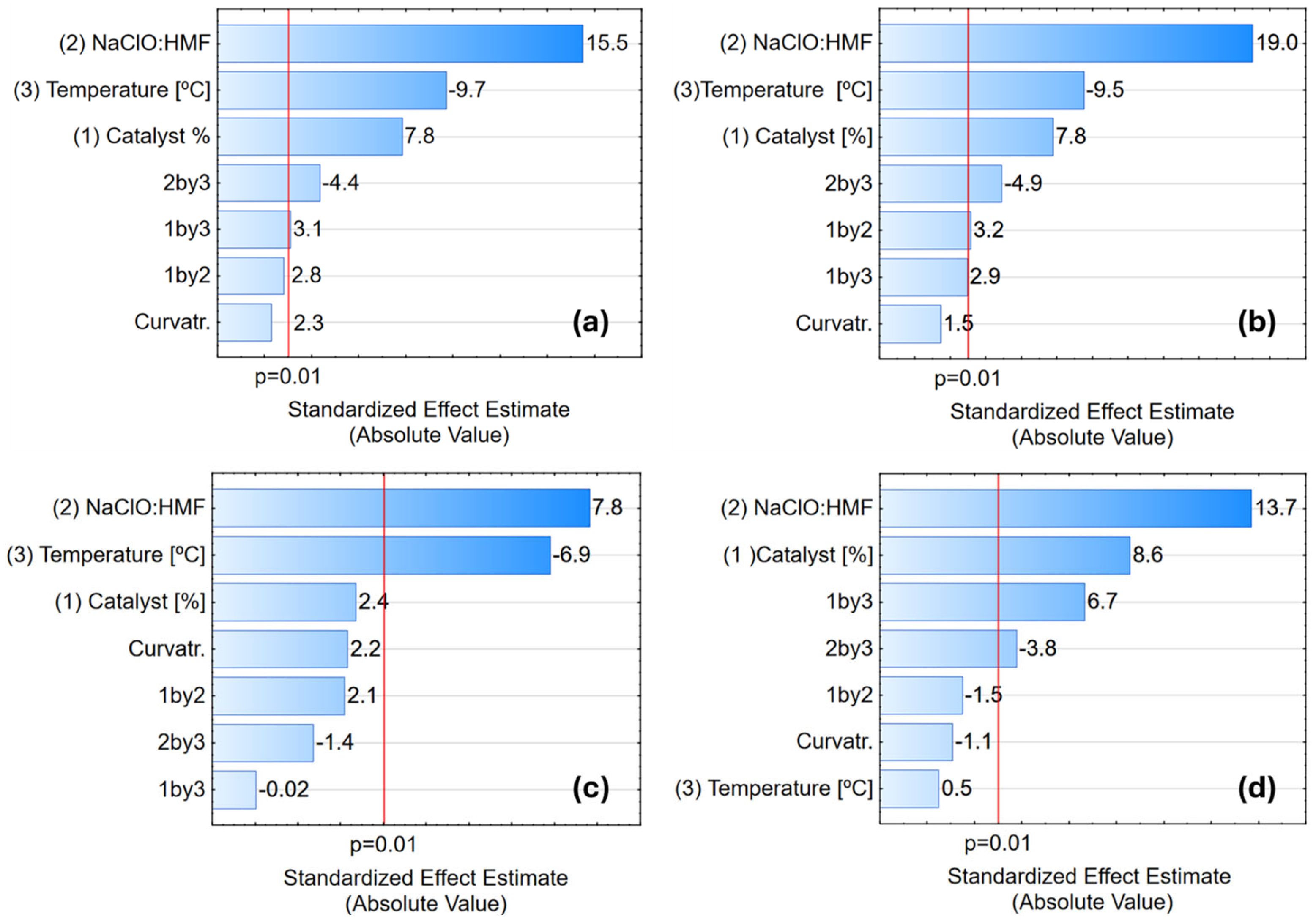
3.4. Reaction Optimization Using Factorial Design
3.5. Reaction Kinetics Under Optimized Conditions
3.6. Catalyst Reusability and Stability
3.7. FDCA Production from Crude HMF
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lecona-Vargas, C.S.; Orsat, V.; Dumont, M.-J. Carbohydrate-Based Biorefineries for the Production of 5-Hydroxymethylfurfural and 2,5-Furandicarboxylic Acid and Their Separation and Purification Methods. Biomass Convers. Biorefin 2022, 12, 26575–26595. [Google Scholar] [CrossRef]
- Dessbesell, L.; Souzanchi, S.; Venkateswara Rao, K.T.; Carrillo, A.A.; Bekker, D.; Hall, K.A.; Lawrence, K.M.; Tait, C.L.J.; Xu, C. Production of 2,5-Furandicarboxylic Acid (FDCA) from Starch, Glucose, or High-Fructose Corn Syrup: Techno-Economic Analysis. Biofuels Bioprod. Biorefining 2019, 13, 1234–1245. [Google Scholar] [CrossRef]
- Gao, R.; Wang, J.; Liu, F.; Dai, H.; Zhang, X.; Wang, X.; Li, Y.; Zhu, J. Synthesis of Bio-Based Poly(Ester-Ether) Elastomers from 2,5-Furandicarboxylic Acid (FDCA) with Excellent Thermo-Mechanical Properties. Polym. Degrad. Stab. 2023, 210, 110292–110304. [Google Scholar] [CrossRef]
- Hayashi, E.; Komanoya, T.; Kamata, K.; Hara, M. Heterogeneously-Catalyzed Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid with MnO2. ChemSusChem 2017, 10, 654–658. [Google Scholar] [CrossRef]
- Jiang, M.; Hu, F.; Feng, G.; Zhang, H.; Hu, H.; Gan, T.; Huang, Z.; Zhang, Y. In-Situ Growth of MnO2 on Hierarchical Porous Carbon Foam with Enhanced Oxygen Vacancy Concentration and Charge Transfer for Efficient Catalytic Oxidation of 5-Hydroxymethylfurfural. Appl. Surf. Sci. 2022, 598, 153849–153859. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Dumesic, J.A. Production and Upgrading of 5-Hydroxymethylfurfural Using Heterogeneous Catalysts and Biomass-Derived Solvents. Green. Chem. 2013, 15, 85–90. [Google Scholar] [CrossRef]
- Biradar Tamboli, A.T.; Kirdant, S.P.; Jadhav, V.H. Metal-Free Approach towards Efficient Synthesis of FDCA Using a p-Toluene Sulfonic Acid (p-TSA)-Derived Heterogeneous Solid Acid Catalyst and Oxone over Two Steps from HMF, Fructose and Glucose. New J. Chem. 2022, 46, 10272–10279. [Google Scholar] [CrossRef]
- Bonincontro, D.; Lolli, A.; Villa, A.; Prati, L.; Dimitratos, N.; Veith, G.M.; Chinchilla, L.E.; Botton, G.A.; Cavani, F.; Albonetti, S. AuPd-nNiO as an Effective Catalyst for the Base-Free Oxidation of HMF under Mild Reaction Conditions. Green. Chem. 2019, 21, 4090–4099. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Zuo, M.; Tang, X.; Zeng, X.; Sun, Y.; Lei, T.; Fang, H.; Li, T.; Lin, L. Facile and Efficient Two-Step Formation of a Renewable Monomer 2,5-Furandicarboxylic Acid from Carbohydrates over the NiOx Catalyst. Ind. Eng. Chem. Res. 2020, 59, 4895–4904. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.; Xia, H. Photocatalytic Oxidation of 5-Hydroxymethylfurfural to Furandicarboxylic Acid over the Au-Ag/TiO2 Catalysts under Visible Light Irradiation. Appl. Surf. Sci. 2023, 613, 156036–156047. [Google Scholar] [CrossRef]
- Wang, S.; Feng, H.; Liu, T.; Deng, Y.; Zhang, M.; Zhao, S.; Han, J.; Zhang, X. Theoretical Exploration of the Origin of Alkaline Dependence in the Oxidation of 5-Hydroxymethylfurfural Catalyzed by NiO2Hx. ACS Catal. 2024, 14, 9860–9869. [Google Scholar] [CrossRef]
- Zhu, Y.; Wei, J.; Wu, J.; Chen, R.; Tsiakaras, P.; Yin, S. Built-in Electric Field in NiO-CuO Heterostructures to Regulate the Hydroxide Adsorption Sites for 5-Hydroxymethylfurfural Electrooxidation Assisted Hydrogen Production. J. Colloid. Interface Sci. 2024, 673, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Li, Y.; Xing, L.; Wang, N.; Zhong, R.; Qian, Z.; Du, C.; Yin, G.; Wang, Y.; Du, L. A Dynamic Ni(OH)2-NiOOH/NiFeP Heterojunction Enabling High-Performance E-Upgrading of Hydroxymethylfurfural. Appl. Catal. B 2022, 311, 121357–121368. [Google Scholar] [CrossRef]
- Herlina, I.; Krisnandi, Y.K.; Ridwan, M. Oxidation of 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic Acid over CuO and NiO Modified Natural Sourced Hierarchical ZSM-5. S. Afr. J. Chem. Eng. 2024, 47, 75–82. [Google Scholar] [CrossRef]
- Holzhäuser, F.J.; Janke, T.; Öztas, F.; Broicher, C.; Palkovits, R. Electrocatalytic Oxidation of 5-Hydroxymethylfurfural into the Monomer 2,5-Furandicarboxylic Acid Using Mesostructured Nickel Oxide. Adv. Sustain. Syst. 2020, 4, 1900151–1900156. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Cheng, H.; Zhou, H. Integrated Chemo- and Biocatalytic Processes: A New Fashion toward Renewable Chemicals Production from Lignocellulosic Biomass. J. Chem. Technol. Biotechnol. 2023, 98, 331–345. [Google Scholar] [CrossRef]
- Singh, D.; Batoo, K.M.; Hussain, S.; Kumar, A.; Aziz, Q.H.; Sheri, F.S.; Tariq, H.; Singh, P. Enhancement of the Photocatalytic Activity of RGO/NiO/Ag Nanocomposite for Degradation of Methylene Blue Dye. RSC Adv. 2024, 14, 2429–2438. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Li, C.; Gong, M.; Wang, X.; Yang, X.; Wang, H.; Li, Y.-F.; Liu, Z.-P. The Identity of Nickel Peroxide as a Nickel Superoxyhydroxide for Enhanced Electrocatalysis. JACS Au 2023, 3, 2964–2972. [Google Scholar] [CrossRef]
- Kumar, A.; Mustafa, M.A.; Fouly, A.; Bains, P.S.; Sharma, R.; Bisht, Y.S.; Awwad, E.M.; Singh, P. NiOx/PANI Nanocomposite Doped Carbon Paste as Electrode for Long-Term Stable and Highly Efficient Perovskite Solar Cells. RSC Adv. 2024, 14, 13374–13383. [Google Scholar] [CrossRef]
- Gao, W.; Guan, X.; Wang, J.; Heinig, N.F.; Thomas, J.P.; Zhang, L.; Ding, K.; Leung, K.T. Supported NiOx Nanocatalysts on Graphene for Nonenzymatic Lactate Sensing. ACS Appl. Nano Mater. 2024, 7, 7162–7171. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, T.; Cao, Q.; Fang, W. Tailoring the Reactive Oxygen Species in Mesoporous NiO for Selectivity-Controlled Aerobic Oxidation of 5-Hydroxymethylfurfural on a Loaded Pt Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 6056–6067. [Google Scholar] [CrossRef]
- Christoskova, S.G.; Stoyanova, M.; Danova, N.; Argirov, O. Low-Temperature Methanol Oxidation on a Higher Nickel Oxide in Gaseous and Aqueous Phase. Part II. Appl. Catal. A Gen. 1998, 173, 101–105. [Google Scholar] [CrossRef]
- He, H.; Yang, S.; Yu, K.; Ju, Y.; Sun, C.; Wang, L. Microwave Induced Catalytic Degradation of Crystal Violet in Nano-Nickel Dioxide Suspensions. J. Hazard. Mater. 2010, 173, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wang, T.; Zhang, M.; She, Y.; Wang, L. Simple Fabrication of Nano-Sized NiO2 Powder and Its Application to Oxidation Reactions. Appl. Catal. A Gen. 2005, 282, 25–30. [Google Scholar] [CrossRef]
- Lai, T.-L.; Lee, C.-C.; Wu, K.-S.; Shu, Y.-Y.; Wang, C.-B. Microwave-Enhanced Catalytic Degradation of Phenol over Nickel Oxide. Appl. Catal. B 2006, 68, 147–153. [Google Scholar] [CrossRef]
- Lai, T.-L.; Liu, J.-Y.; Yong, K.-F.; Shu, Y.-Y.; Wang, C.-B. Microwave-Enhanced Catalytic Degradation of 4-Chlorophenol over Nickel Oxides under Low Temperature. J. Hazard. Mater. 2008, 157, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fan, B.; Bian, L.; Tang, Q.; Cao, Q.; Fang, W. Influence of NiO-Calcination on Pt-Supported Catalyst for Selective Oxidation of 5-Hydroxymethylfurfural. Catal. Commun. 2023, 184, 106791–106798. [Google Scholar] [CrossRef]
- Rathod, P.V.; Jadhav, V.H. Efficient Method for Synthesis of 2,5-Furandicarboxylic Acid from 5-Hydroxymethylfurfural and Fructose Using Pd/CC Catalyst under Aqueous Conditions. ACS Sustain. Chem. Eng. 2018, 6, 5766–5771. [Google Scholar] [CrossRef]
- Vuyyuru, K.R.; Strasser, P. Oxidation of Biomass Derived 5-Hydroxymethylfurfural Using Heterogeneous and Electrochemical Catalysis. Catal. Today 2012, 195, 144–154. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Won, W.; Sener, C.; Alonso, D.M.; Maravelias, C.T.; Dumesic, J.A. Toward Biomass-Derived Renewable Plastics: Production of 2,5-Furandicarboxylic Acid from Fructose. Sci. Adv. 2018, 4, 9722–9729. [Google Scholar] [CrossRef]
- Rao, K.T.V.; Rogers, J.L.; Souzanchi, S.; Dessbesell, L.; Ray, M.B.; Xu, C.C. Inexpensive but Highly Efficient Co–Mn Mixed-Oxide Catalysts for Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. ChemSusChem 2018, 11, 3323–3334. [Google Scholar] [CrossRef]
- Reports and Data 2,5-Furandicarboxylic Acid (FDCA) Market. Available online: https://www.reportsanddata.com/report-detail/2-5-furandicarboxylic-acid-fdca-market (accessed on 7 April 2023).
- KBV Research POLYETHYLENE Terephthalate Market Size, Share & Industry Forecast Report. Available online: https://www.kbvresearch.com/polyethylene-terephthalate-market (accessed on 27 September 2024).
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |










| Assay | Oxidant (NaClO) Addition Method |
|---|---|
| C1 | 100% of the volume added at t = 0 min |
| C2 | 50% of the volume added at t = 0 min and 50% at t = 30 min |
| C3 | 25% of the volume added at each of t = 0, 10, 20, and 30 min |
| C4 | 100% of the volume added continuously from t = 0 min to t = 30 min at a constant flow rate using a peristaltic pump |
| Catalyst | Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| NiOx | 59.1 | 0.1 | 6.7 |
| Catalyst | Route | Calcination (400 °C; 4 h) | FDCA Concentration (g/L) | FDCA Yield (%) | FDCA Selectivity (%) | HMF Conversion (%) |
|---|---|---|---|---|---|---|
| Ni(OH)2 | A | NO | 0.01 | 0.13 | 0.28 | 47.15 |
| 0.01 | 0.13 | 0.26 | 50.41 | |||
| NiOcalc | A | YES | 0.17 | 2.22 | 5.82 | 38.21 |
| 0.13 | 1.96 | 4.82 | 40.65 | |||
| NiOx | B | NO | 0.90 | 11.77 | 27.41 | 42.93 |
| 0.89 | 11.64 | 26.02 | 44.72 | |||
| NiOx-calc | B | YES | 0.21 | 2.75 | 6.65 | 41.30 |
| 0.23 | 3.01 | 6.75 | 44.55 |
| CFDCA | Sum of Squares | Degrees of Freedom | Mean Squares | F Value | p-Value |
|---|---|---|---|---|---|
| Route (1) | 0.456013 | 1 | 0.456013 | 1737.190 | 0.000002 * |
| Calcination (2) | 0.143113 | 1 | 0.143113 | 545.190 | 0.000020 * |
| 1 × 2 | 0.332113 | 1 | 0.332113 | 1265.190 | 0.000004 * |
| Error | 0.001050 | 4 | 0.000263 | ||
| Total SS | 0.932287 | 7 | R2 = 0.99887 | ||
| YFDCA | Sum of Squares | Degrees of Freedom | Mean Squares | F Value | p-Value |
|---|---|---|---|---|---|
| Route (1) | 76.4466 | 1 | 76.44661 | 4020.861 | 0.000000 * |
| Calcination (2) | 23.5641 | 1 | 23.56411 | 1239.401 | 0.000004 * |
| 1 × 2 | 58.1581 | 1 | 58.15811 | 3058.941 | 0.000001 * |
| Error | 0.0760 | 4 | 0.01901 | ||
| Total SS | 158.2449 | 7 | R2 = 0.99952 | ||
| SFDCA | Sum of Squares | Degrees of Freedom | Mean Squares | F Value | p-Value |
|---|---|---|---|---|---|
| Route (1) | 387.1153 | 1 | 387.1153 | 1052.480 | 0.000005 * |
| Calcination (2) | 111.9756 | 1 | 111.9756 | 304.437 | 0.000063 * |
| 1 × 2 | 314.1271 | 1 | 314.1271 | 854.041 | 0.000008 * |
| Error | 1.4713 | 4 | 0.3678 | ||
| Total SS | 814.6893 | 7 | R2 = 0.99819 | ||
| XHMF | Sum of Squares | Degrees of Freedom | Mean Squares | F Value | p-Value |
|---|---|---|---|---|---|
| Route (1) | 1.0658 | 1 | 1.06580 | 0.28096 | 0.624133 |
| Calcination (2) | 52.5313 | 1 | 52.53125 | 13.84779 | 0.020452 * |
| 1 × 2 | 35.70125 | 1 | 35.70125 | 9.41123 | 0.037374 * |
| Error | 15.1739 | 4 | 3.79347 | ||
| Total SS | 104.4722 | 7 | R2 = 0.85476 | ||
| Assay | Experimental Conditions | Response Variables | |||||
|---|---|---|---|---|---|---|---|
| Catalyst% (w/v) | Temperature (°C) | NaClO: HMF | [FDCA] (g L−1) | FDCA Yield (%) | FDCA Selectivity (%) | HMF Conversion (%) | |
| 1 | 2 | 25 | 6:1 | 1.10 | 14.38 | 25.94 | 55.45 |
| 1.02 | 13.34 | 24.19 | 55.12 | ||||
| 2 | 6 | 25 | 6:1 | 1.21 | 15.82 | 27.10 | 58.37 |
| 1.17 | 15.30 | 26.73 | 57.24 | ||||
| 3 | 2 | 75 | 6:1 | 0.66 | 8.63 | 15.52 | 55.61 |
| 0.80 | 10.46 | 23.48 | 44.55 | ||||
| 4 | 6 | 75 | 6:1 | 1.06 | 13.86 | 18.73 | 73.98 |
| 1.03 | 13.47 | 17.97 | 74.96 | ||||
| 5 | 2 | 25 | 12:1 | 1.79 | 26.92 | 34.30 | 78.49 |
| 1.93 | 29.03 | 36.89 | 78.68 | ||||
| 6 | 6 | 25 | 12:1 | 2.27 | 34.14 | 42.57 | 80.19 |
| 2.03 | 30.53 | 37.11 | 82.26 | ||||
| 7 | 2 | 75 | 12:1 | 1.02 | 15.34 | 22.46 | 68.30 |
| 1.09 | 16.39 | 24.75 | 66.23 | ||||
| 8 | 6 | 75 | 12:1 | 1.81 | 27.22 | 32.49 | 83.77 |
| 1.61 | 24.21 | 28.97 | 83.58 | ||||
| 9 | 4 | 50 | 9:1 | 1.36 | 18.98 | 26.97 | 70.38 |
| 1.50 | 20.94 | 31.13 | 67.25 | ||||
| 10 | 4 | 50 | 9:1 | 1.38 | 19.26 | 29.10 | 66.20 |
| 1.47 | 20.52 | 32.09 | 63.94 | ||||
| 11 | 4 | 50 | 9:1 | 1.42 | 19.82 | 28.66 | 69.16 |
| 1.55 | 21.63 | 32.59 | 66.38 | ||||
| Results | |||
|---|---|---|---|
| CFDCA (gL−1) | YFDCA (%) | SFDCA (%) | XHMF (%) |
| 0.39 | 5.43 | 23.47 | 23.14 |
| Catalyst | HMF Origin | Solvent, Additive and/or Oxidant | Reaction Conditions | FDCA Yield (%) | References |
|---|---|---|---|---|---|
| % Pt/C | Fructose | Gama-valerolactone/H2O (50:50) | 110°C, PO2 = 40 bar, 20 h, cat 7.5% (w/v), no HMF purification | 0 | [30] |
| Co-Mn | Cellulosic biomass | H2O/NaHCO3 | 120 °C, PO2 = 10 bar, 5 h, cat 10% (w/v), non-optimized stage, no HMF purification | 4.6 | [31] |
| NiOx | Glucose | H2O and NaClO:HMF 12:1 | 25 °C, 50 min, cat 6% (w/v), non-optimized stage, no HMF purification | 5.4 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Aquino Santos, C.L.; Silva, J.P.A.; Mussatto, S.I.; Carneiro, L.M. Sustainable Production of 2,5-Furandicarboxylic Acid via Nickel-Based Heterogeneous Catalysis from 5-Hydroxymethylfurfural. Processes 2025, 13, 2026. https://doi.org/10.3390/pr13072026
de Aquino Santos CL, Silva JPA, Mussatto SI, Carneiro LM. Sustainable Production of 2,5-Furandicarboxylic Acid via Nickel-Based Heterogeneous Catalysis from 5-Hydroxymethylfurfural. Processes. 2025; 13(7):2026. https://doi.org/10.3390/pr13072026
Chicago/Turabian Stylede Aquino Santos, Celso Luiz, João Paulo Alves Silva, Solange I. Mussatto, and Livia Melo Carneiro. 2025. "Sustainable Production of 2,5-Furandicarboxylic Acid via Nickel-Based Heterogeneous Catalysis from 5-Hydroxymethylfurfural" Processes 13, no. 7: 2026. https://doi.org/10.3390/pr13072026
APA Stylede Aquino Santos, C. L., Silva, J. P. A., Mussatto, S. I., & Carneiro, L. M. (2025). Sustainable Production of 2,5-Furandicarboxylic Acid via Nickel-Based Heterogeneous Catalysis from 5-Hydroxymethylfurfural. Processes, 13(7), 2026. https://doi.org/10.3390/pr13072026








