Absorption-Based Optimization Technologies for Acid Gas Removal Units: A Review of Recent Trends and Challenges
Abstract
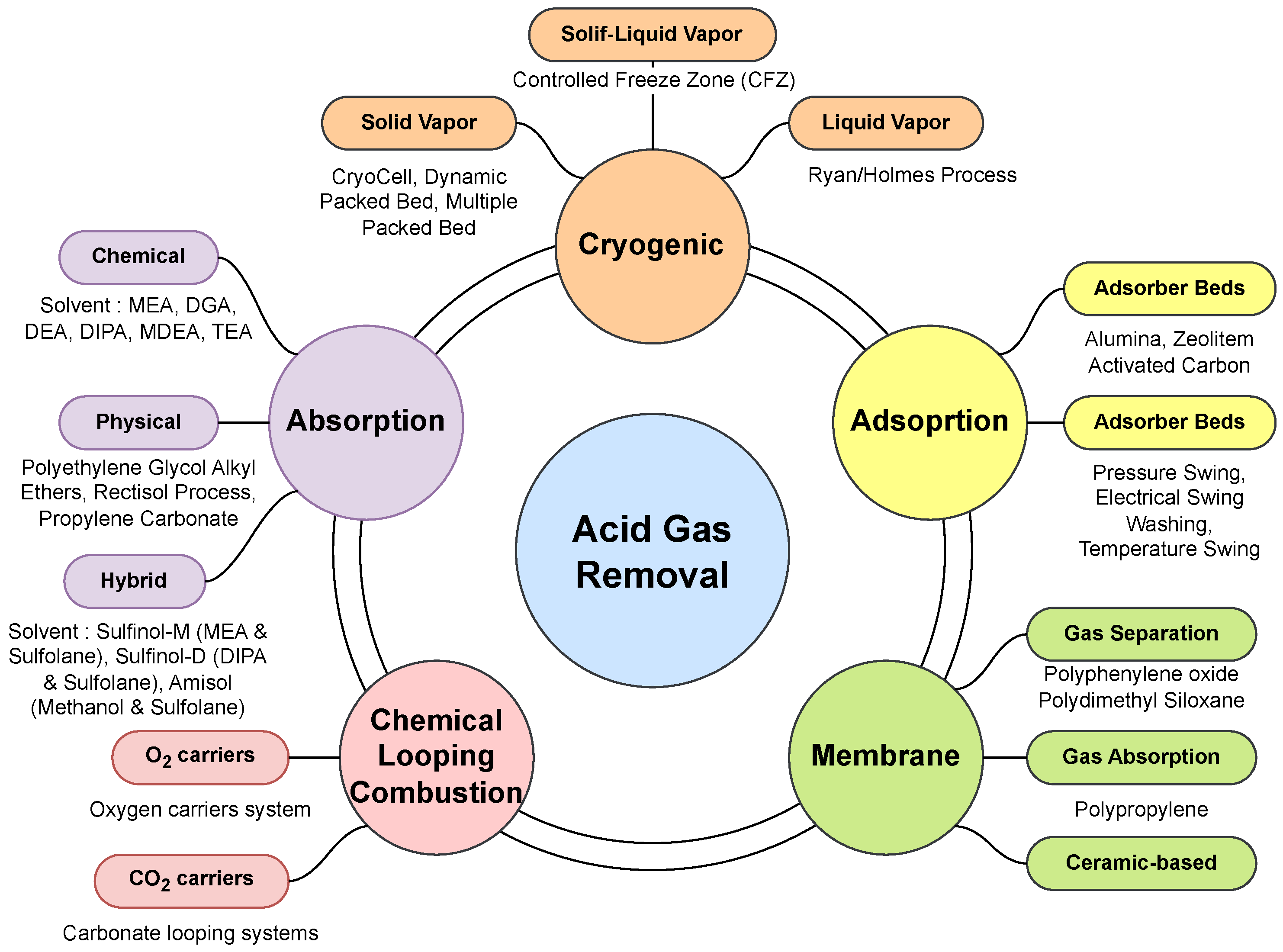
1. Introduction
2. Absorption-Based AGRU Optimization
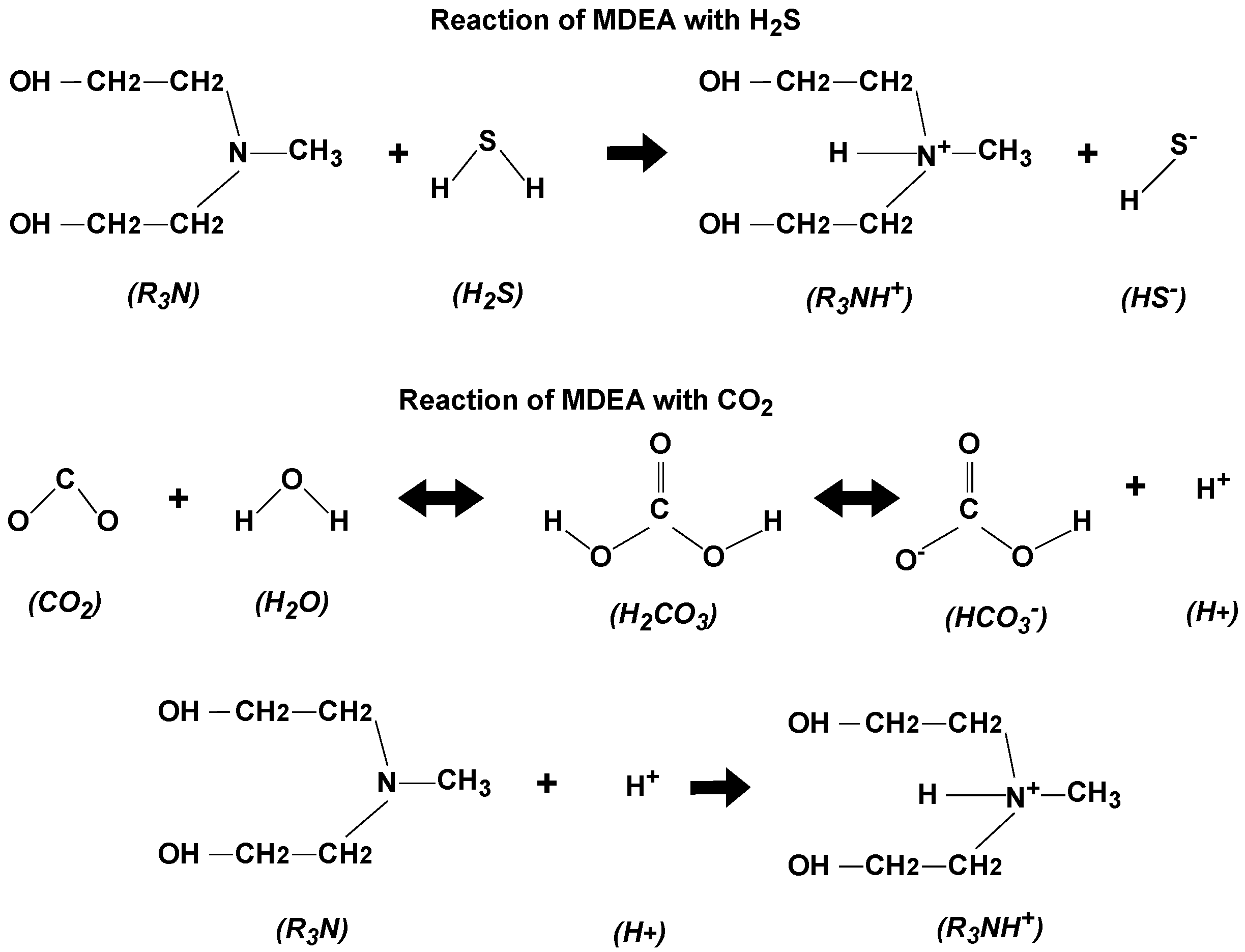
2.1. Solvent Optimization
2.1.1. Solvent Blending
| Solvent | Target Properties (Sweet Gas) | Results | Compatibility | References |
|---|---|---|---|---|
| Solvent Blending | ||||
| 42–50 wt% MDEA + 0–2.5 wt% PZ | – | Lower economic cost | MDEA (main amine), PZ (promoter) | [18] |
| 35–50 wt% TEA + 0–15 wt% DIPA | – | 18% increase in removal | TEA (main amine), DIPA (activator) | [19] |
| 40 wt% MEA + MDEA | ≤2 mol% , ≤4 ppm S | Reduce operation cost and energy saving | MEA (fast reaction), MDEA (high capacity) | [22] |
| MDEA + AEEA + NMP (Overall 50 wt%) | ≤1% , | Reduce energy consumption, improve S and capture | MDEA (main amine), AEEA (activator), NMP (enhances solubility at high pressure) | [23] |
| 50 wt% DGA + 0–15 wt% MDEA | – | Reduce energy consumption | DGA (low operating pressure), MDEA (high operating pressure) | [13] |
| 10–50 wt% DEA + 5 wt% 0.1 M Ca(OH)2 | ≤2 mol% , ≤4 ppm S | Increases S removal efficiency, reduces energy costs | DEA (main amine), Ca(OH)2 (improves cleaning process) | [24] |
| 40 wt% MDEA + 32 wt% DEA | ≤2 mol% , ≤4 ppm S | Increase S and removal | MDEA (main amine), DEA (improves absorption) | [25] |
| 17.5 wt% + DEA | Lower energy consumption | Reducing removal costs, reduce operating expenses | (main amine), DEA (improves absorption) | [26] |
| 44 wt% MDEA + 1.5 wt% Sulfolane | , | Reduces energy requirements, improves gas quality | MDEA (main amine), Sulfolane (S selective, low operating temperature) | [27] |
| [OHPy][TFA] + Methanol | ≤3 mass% , ≤S | Cost and energy saving | [OHPy][TFA] (S removal), Methanol (assist gas separation) | [28] |
| Composition Loading | ||||
| Solvent | Target Properties (Sweet Gas) | Approach | Results | References |
| 65 wt% MDEA | , | Trial and Error | Enhances gas purification and energy efficiency | [29] |
| MDEA | S content , content | Trial and error | Improve purified gas yield rate 0.5%, Reduce energy consumption 19.1% | [30] |
| 30 wt% DEA | 4–50 ppm v S | Trial and Error | Higher absorption rate | [31] |
| MDEA | <1– | Trial and Error | removal capacity | [32] |
2.1.2. Composition Loading
2.2. Process Optimization
2.2.1. Parameter Tuning
2.2.2. Process Control
2.2.3. System Integration
| Control Strategy/Unit | Controlled Variable (s) | Setpoint | Solvent | Target Properties (Sweet Gas) | Performance Metric | Reference |
|---|---|---|---|---|---|---|
| PI vs MMPC (feed pressure & makeup water loops) | Feed pressure Makeup water | psig USGPM | a-MDEA | < 2 mol % S < 4 ppm | Feed: ISE_SP 20.713.95 ISE_dist 453215 Water: ISE_SP 3.070.84 ISE_dist 166.8 | [42] |
| Feedforward MPC | S content Tray 1 temp | 52 °C | 45 wt % MDEA | < 1 mol % S < 4 ppm | Under % feed-flow: deviation mol % Temp deviation °C 0.5 h to recover | [43] |
| DMSND + PI network on 10 CVs | Loaded solvent T recovery P Stripper P Top T Semi-lean/lean cooler T Tray Water % at stripper HP/MP flash P | 65 °C 120 psi 90 psi 150 °C 50/45 °C 135 °C 2 wt % 300/100 psig | Selexol | < 2 mol % S < 4 ppm | IAE = 0.98 kmol /MWh (P) 25 % lower vs. SSND Recovers in 3 h vs. 4.5 h | [44] |
| APC on Amine Unit (multivariable) | Lean-amine/feed-gas ratio Lean-amine/feed T Overhead T Reflux ratio Inferred S loading Regenerator P Bottom temp Pressure minimization | < 2 mol % & S < 4 ppm Avoid condensation/flooding Prevent amine degradation | Aqueous amine blends (e.g., MDEA/MEA) | < 2 mol % S < 4 ppm | Aggregate APC (all units) saved ∼2000 × BTU/yr Lean-amine variability ↓ 50 % vs. DCS | [45] |
| Integration Type | Solvent | Target Properties (Sweet Gas) | Results | Reference |
|---|---|---|---|---|
| Direct Heat Integration (DHI) and Organic Rankine Cycle (ORC) Integration | MDEA, DEA | - | Significantly saves energy and generates electricity in natural gas processing | [50] |
| Novel Low Temperature Absorption Coupled with Cold Energy Recovery | MDEA (6.65%), H2O (93.25%) | - | New low-temperature absorption process with modified heat pump distillation, reducing energy consumption and operating costs | [51] |
| Integration of Novel Freezing-Based Acid Gas Removal Process with Cold Section | - | H2S (≤5 ppm), CO2 (9 ppm mmol) | Reduces energy requirements by 16.6% and increases production, leading to a 17.98% decrease in specific energy consumption | [52] |
2.3. Equipment Design Optimization
2.3.1. Absorber Design
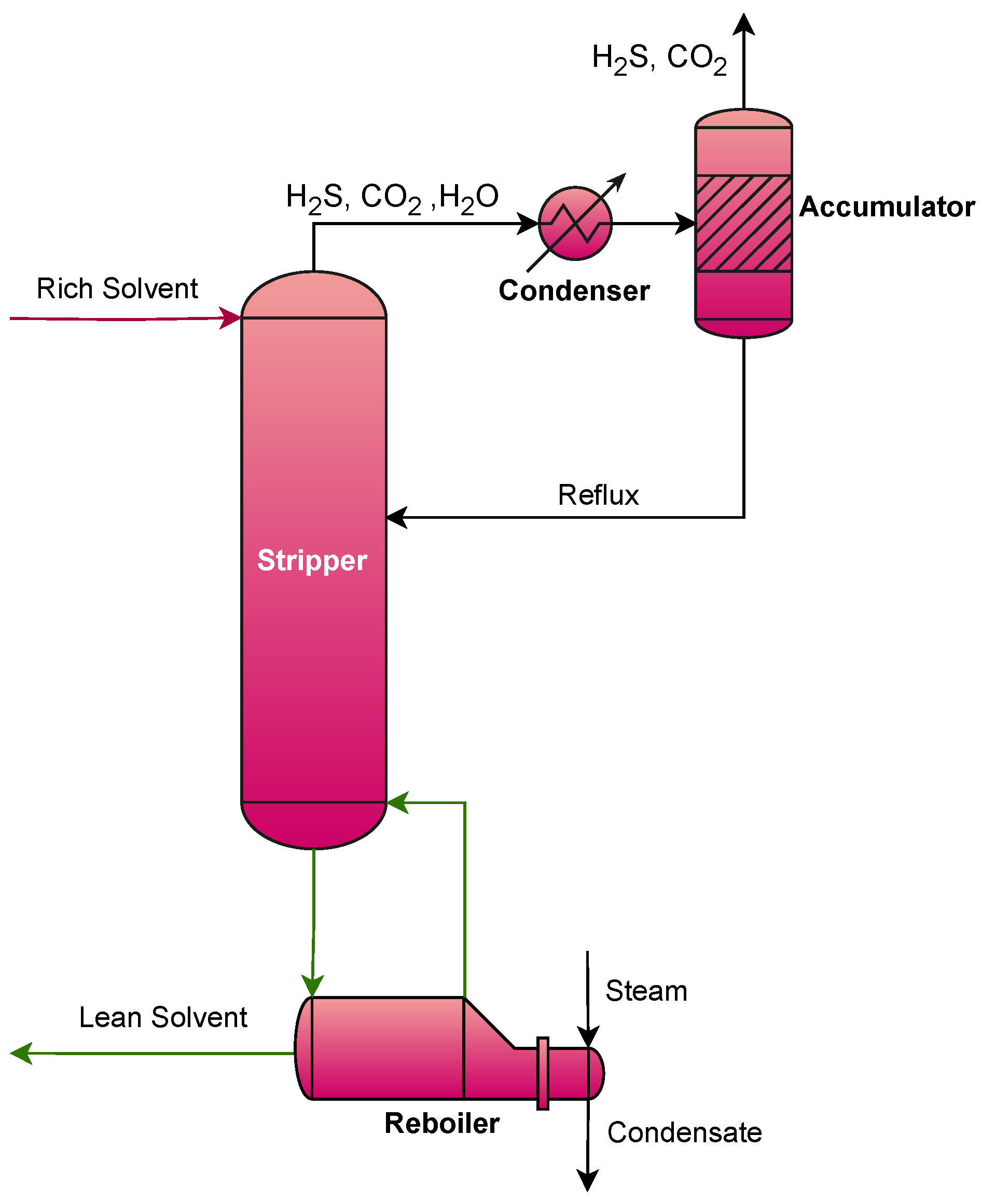
2.3.2. Stripper Design
| Equipment | Structure | Packing/Tray Type | Solvent | Software | Reference |
|---|---|---|---|---|---|
| Absorber | Tray | Sieve, Ballast | MEA 30 wt% | ASPEN Plus | [53,56] |
| Structured Packing | - | Piperazine (PZ) | ASPEN Plus | [58] | |
| Packed | Dixon ring, Sulzer DX, BX 500 | DETA, MEA, AMP | (Experiment) | [59,60,61] | |
| Structured and Random Packed Column | Pall Rings | MDEA: 15% MEA: 6% DEA: 6% | CFD | [62] | |
| Rotating Packed Bed | Stainless steel wire mesh | DETA: 10 wt%, 20 wt%, 25 wt%, 30 wt%, 40 wt% PZ, MDEA, AMP | (Experiment) | [63] | |
| Random Packing | IMTP #50 | MDEA 30 wt% | ProMax | [64] | |
| Packed | - | MDEA | Matlab | [65] | |
| Tray | - | Water | ASPEN Hysys v8.8 | [66] | |
| Packing | - | MEA, MDEA, PZ | ASPEN Plus | [69] | |
| Stripper | - | - | MDEA | - | [70] |
| Packed | SULZER Mellapak 350.Y | KOH, | CHEMCAD | [67] | |
| Packing | IMTP #40 (absorber) Flexipac 1Y (stripper) | MEA | Aspen Plus | [68] |
3. Data-Driven Application on an AGRU
3.1. Parameter Prediction
3.1.1. S and Parameters
| S and Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| Target Properties | Algorithm | Learning Type | Data Source | Data Set | MAE | RMSE | R² | Reference |
| and S removal efficiency | DT | Supervised | Simulation | - | 0.02 | - | - | [78] |
| S concentration in sweet gas | ANN, MLR | Supervised | Literature and simulation | 3015 | 0.066 | 0.122 | 0.966 | [73] |
| Rich amine loading and S concentration | SVM | Supervised | Industry | 145 | 0.034 | 0.262 | 0.99 | [75] |
| S concentration and reid vapor pressure | SVM | Supervised | Industry | 660 | 0.229 | 0.479 | 0.97 | [76] |
| S content | ENN | Supervised | Industry | 1600 | 5.3963 × | 3.872 × | - | [77] |
| Solubility of S | CNN, DBN, RNN, DJINN | Supervised | Experiment | 1516 | - | 0.0052 | 0.99 | [79] |
| S and concentration in rich amine | RF, SVM | Supervised | Industry | 550 | 0.003 | 0.004 | 0.992 | [80] |
| S Solubility | LM-ANN, BR-ANN, SCG-ANN | Supervised | Literature | 2526 | - | 0.0374 | 0.9817 | [81] |
| S and output concentration | BP-ANN, ICA-ANN | Supervised | Industry | 368 | 0.3706 × | 0.007 | 0.9307 | [82] |
| Operational Parameters | ||||||||
| Target Properties | Algorithm | Learning Type | Data Source | Data Set | MAE | RMSE | R² | Reference |
| Steam consumption | Density-Based Spatial Clustering, GB | Supervised | Industry | 4.8 × | 0.0014 | - | 0.98 | [85] |
| flow rate, emissions, and steam flow rate | RF, SVM, ANN | Supervised | Industry | 236,737 | 0.06 | 0.00206 | 0.98 | [86] |
| Power and water consumption | RBF-NN | Supervised | Experiment | - | - | 3.8 × | 0.99 | [87] |
| Mass transfer coefficient of absorption | BPNN | Supervised | Literature | 3935 | - | 0.0763 | 0.9905 | [88] |
| Mass transfer coefficient of absorption | RBFNN, RF | Supervised | Literature | 3935 | - | 0.1134 | 0.98106 | [89] |
| Vapor-liquid equilibrium ratio (KLV) | ANN | Supervised | Literature | - | - | - | 0.98 | [90] |
| Gas dew point temperature | PSO-ANN, ICA-ANN | Supervised | Industry | 1000 | - | 0.0721 | 0.9937 | [91] |
| Material Discovery | ||||||||
| Metal organic material | RF | Supervised | Experiment | 1600 | - | 0.3821 to 0.3206 | - | [92] |
| Metal organic frameworks | MLT, GBRT, XGBoost, SHAP | Supervised | Literature and simulation | 998 | 1.678 | 2.771 | 0.9 | [93] |
3.1.2. Operational Parameters
3.1.3. Material Discovery
3.2. Fault Detection
| Fault | Algorithm | Learning Type | Data Source | Data Set | Accuracy | Reference |
|---|---|---|---|---|---|---|
| Foaming, damaged trays, fouling | Shallow and Deep Sparse Autoencoders | Semi-Supervised | Simulation | - | 0.99 | [40] |
| Predicted process upsets and hazard events | DL, RF, GB | Supervised | Experiment | - | 0.78 | [97] |
| Natural gas composition, solvent contaminant | PLS | Supervised | Industry | 8580 | - | [98] |
| Absorber pressure drop fluctuation, Flash gas increment, Carryover amine from absorber or flash tank, Swinging liquid levels in any reservoir, increment with S decrease, Off-specification sweet gas | Gaussian Naïve Bayes | Supervised | Industry | - | 0.6291 | [99] |
| Hydrocarbon accumulation, Solid particles in amine, Contaminated amine | PCA-BN | Supervised | Industry | - | 0.94 | [100] |
| Liquid hydrocarbon in the lower part of absorber, Amine input valve failure, Temperature sensor failure | BN | Supervised | Industry | - | 0.961 | [101] |
| Foaming, damaged trays, fouling | ANN | Supervised | Simulation | - | 0.99 | [102] |
4. Future Scope and Conclusions
4.1. Future Scope
- The problem of high S selectivity in the MDEA as the most utilized solvent can be solved by mixing the amine solvent with the physical solvent, resulting in a hybrid amine. Furthermore, the research about hybrid amine needs to be explored.
- It is still challenging to determine the optimal solvent mix of certain AGRU systems with its operational parameters. Simulation and/or experiments are needed to find the optimized solvent with the input of certain solvent mix. There is an extensive amount of data that can be applied to develop a certain model to determine the optimum AGRU solvent.
- Currently, the optimization solvent composition mostly utilized trial and error by arbitrarily defining the concentration of solvent. However, it can be improved by applying the data-driven method.
- The utilization of packed column tower shows great performance in the absorber and stripper design in contrast with the tray column. For this reason, the packed column is recommended to be utilized in further research.
- Absorber and stripper design until now mostly still uses software simulation, and there is lack of experimentation since it is expensive. In order to reduce the cost, ANN has been utilized in this optimization technique; however, another technique could be applied in the equipment design optimization.
- Data collection in data-driven model development is essential. Current research collects data from simulation, but the variance of data will influence the model capability. Therefore, industrial data is needed to build a robust and reliable data-driven model.
- ANN is still the most utilized technique in the data-driven approach. However, since ANNs have a different layer of nodes, the training process will be time-consuming. Thus, the utilization ofa faster algorithm such as XG-Boost can be extensively applied.
- The semi-supervised learning technique still is not utilized extensively in AGRU optimization. Hence, the model development of the semi-supervised technique needs to be applied considering its high accuracy.
- Deep learning shows a great capability in the parameter prediction and fault detection task. Considering its performance, deep learning can be utilized in another task such as solvent or equipment design.
- Since the main purpose of AGRUs is to reduce the environmental impact with the removal of harmful sour gas, the optimization of AGRU with the consideration of its environmental impact is necessary.
4.2. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AARE | Average Absolute Relative Error | GNB | Gaussian Naïve Bayes |
| AAPRE | Average Absolute Percent Relative Error | S | Hydrogen Sulfide |
| AEEA | Aminoethylethanolamine | IAE | Integral Absolute Error |
| AGRU | Acid Gas Removal Unit | ICA | Imperialist Competitive Algorithm |
| AMP | Aminomethyl Propanol | IL | Ionic Liquid |
| ANN | Artificial Neural Network | Potassium Carbonate | |
| APC | Advanced Process Control | KLV | Vapor–Liquid Equilibrium Ratio |
| BFGS | Broyden–Fletcher–Goldfarb–Shanno | LMA | Levenberg-Marquadt Algorithm |
| BN | Bayesian Network | LNG | Liquefied Natural Gas |
| BP | Backpropagation | MEA | Monoethanolamine |
| BPNN | Back-Propagation Neural Network | MDEA | Methyldiethanolamine |
| BR | Bayesian Regularization | MEC | Modeling Error Compensation |
| Ca(OH)2 | Calcium Hydroxide | ML | Machine Learning |
| Ca()2 | Calcium Bicarbonate | MLP | Multi Layer Perceptron |
| Calcium Carbonate | MLR | Multiple Linear Regression | |
| CFD | Computer Fluid Dynamics | MPC | Model Predictive Control |
| CNN | Convolutional Neural Networks | MSE | Mean Square Error |
| Carbon Dioxide | NCL | Negative Correlation Learning | |
| DBN | Deep Belief Networks | NMP | N-Methylpyrrolidone |
| DBSCAN | Density-Based Spatial Clustering of Applications with Noise | NaOH | Sodium Hydroxide |
| DEA | Diethanolamine | PCA | Principal Component Analysis |
| DEPG | Dimethyl Ether of Polyethylene Glycol | PZ | Piperazine |
| DETA | Diethylenetriamine | PLS | Partial Least Squares |
| DGA | Diglycolamine | PSO | Particle Swarm Optimization |
| DIPA | Diisopropanolamine | RBF-NN | Radial Basis Function Neural Network |
| DJINN | Deep Jointly Informed Neural Network | RMSE | Root Mean Square Error |
| DMNSD | Dynamic Measurement Sensor and Network Design | RNN | Recurrent Neural Networks |
| ENN | Ensemble Neural Network | RPB | Rotating Packed Bed |
| FD | Fault Detection | SCG | Scaled Conjugate Gradient |
| GA | Genetic Algorithm | SFL | Sulfolane |
| GBM | Gradient Boosting Machine | SVM | Support Vector Machine |
| GBRT | Gradient Boosted Regression Trees | TEA | Triethanolamine |
| [OH]PyTFA | 1-methyl pyridinium trifluoroacetate |
References
- International Energy Agency. Growth in Global Energy Demand Surged in 2024 to Almost Twice Its Recent Average. 2025. Available online: https://www.iea.org/news/growth-in-global-energy-demand-surged-in-2024-to-almost-twice-its-recent-average (accessed on 3 June 2025).
- Holechek, J.L.; Geli, H.M.; Sawalhah, M.N.; Valdez, R. A global assessment: Can renewable energy replace fossil fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- BP. Energy Outlook—2024 Edition. 2024. Available online: https://www.bp.com/en/global/corporate/energy-economics/energy-outlook.html (accessed on 3 June 2025).
- Mohammad, N.; Mohamad Ishak, W.W.; Mustapa, S.I.; Ayodele, B.V. Natural gas as a key alternative energy source in sustainable renewable energy transition: A mini review. Front. Energy Res. 2021, 9, 625023. [Google Scholar] [CrossRef]
- Halliday, C.; Hatton, T.A. Sorbents for the Capture of CO2 and Other Acid Gases: A Review. Ind. Eng. Chem. Res. 2021, 60, 9313–9346. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lock, S.S.M.; Wong, M.K.; Yiin, C.L.; Loy, A.C.M.; Cheah, K.W.; Chai, S.Y.W.; Li, C.; How, B.S.; Chin, B.L.F.; et al. A State-of-the-Art Review on Capture and Separation of Hazardous Hydrogen Sulfide (H2S): Recent Advances, Challenges and Outlook. Environ. Pollut. 2022, 314, 120219. [Google Scholar] [CrossRef]
- Li, B.H.; Zhang, N.; Smith, R. Simulation and analysis of CO2 capture process with aqueous monoethanolamine solution. Appl. Energy 2016, 161, 707–717. [Google Scholar] [CrossRef]
- Sarker, A.I.; Aroonwilas, A.; Veawab, A. Equilibrium and kinetic behaviour of CO2 adsorption onto zeolites, carbon molecular sieve and activated carbons. Energy Procedia 2017, 114, 2450–2459. [Google Scholar] [CrossRef]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-combustion carbon capture by membrane separation, Review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Abuelgasim, S.; Wang, W.; Abdalazeez, A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress. Sci. Total Environ. 2021, 764, 142892. [Google Scholar] [CrossRef]
- Safdarnejad, S.M.; Hedengren, J.D.; Baxter, L.L. Plant-level dynamic optimization of Cryogenic Carbon Capture with conventional and renewable power sources. Appl. Energy 2015, 149, 354–366. [Google Scholar] [CrossRef]
- The Lens. The Lens—Free and Open Patent and Scholarly Search. 2025. Available online: https://www.lens.org/ (accessed on 2 June 2025).
- Zahid, U. Simulation of an Acid Gas Removal Unit Using a DGA and MDEA Blend Instead of a Single Amine. Chem. Prod. Process Model. 2020, 15, 1–17. [Google Scholar] [CrossRef]
- Kidnay, A.J.; Parrish, W.R.; McCartney, D.G. Fundamentals of Natural Gas Processing; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Haider, J.; Saeed, S.; Qyyum, M.A.; Kazmi, B.; Ahmad, R.; Muhammad, A.; Lee, M. Simultaneous Capture of Acid Gases from Natural Gas Adopting Ionic Liquids: Challenges, Recent Developments, and Prospects. Renew. Sustain. Energy Rev. 2020, 123, 109771. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Narayanan, V.A.; Yaashikaa, P.R.; Swetha, S.; Reshma, B. A Comprehensive Review on Different Approaches for CO2 Utilization and Conversion Pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Wong, S.; Keith, D.; Wichert, E.; Gunter, B.; McCann, T. Economics of Acid Gas Reinjection: An Innovative CO2 Storage Opportunity. In Proceedings of the 6th International Conference on Greenhouse Gas Control Technologies, Kyoto, Japan, 30 September–2 October 2003; pp. 1661–1664. [Google Scholar] [CrossRef]
- Patil, Y.; Chikorde, Y.S.; Omar, M.B.; Rosli, M.H.B.; Rosdiazli, B.I. Automated Determination of Optimal Component Design for a Binary Solvent for Absorption-Based Acid Gas Removal. Mater. Res. Proc. 2023, 29, 281–288. [Google Scholar] [CrossRef]
- Farooqi, A.S.; Ramli, R.M.; Lock, S.S.M.; Hussein, N.; Wajahat, S.M. Simulation of Acid Gas Removal Unit Using DIPA+TEA Amine Solvent. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1257, 012033. [Google Scholar] [CrossRef]
- Agarwal, N.; Nhien, L.C.; Lee, M. Rate-Based Modeling and Assessment of an Amine-Based Acid Gas Removal Process through a Comprehensive Solvent Selection Procedure. Energies 2022, 15, 6817. [Google Scholar] [CrossRef]
- Law, L.C.; Yusoff, N.; Syamsul, S.R. Optimization and Economic Analysis of Amine-Based Acid Gas Capture Unit Using Monoethanolamine/Methyl Diethanolamine. Clean Technol. Environ. Policy 2018, 20, 451–461. [Google Scholar] [CrossRef]
- Ulus, N.; Ali, S.A.S.; Khalifa, O.; Orhan, O.Y.; Elkamel, A. Optimization of Novel Nonaqueous Hexanol-Based Monoethanolamine/Methyl Diethanolamine Solvent for CO2 Absorption. Int. J. Energy Res. 2022, 46, 9000–9019. [Google Scholar] [CrossRef]
- Farooqi, A.S.; Ramli, R.M.; Lock, S.S.M.; Hussein, N.; Shahid, M.Z. Simulation of Natural Gas Treatment for Acid Gas Removal Using the Ternary Blend of MDEA, AEEA, and NMP. Sustainability 2022, 14, 10815. [Google Scholar] [CrossRef]
- Sanni, S.E.; Agboola, O.; Fagbiele, O.; Yusuf, E.O.; Emetere, M.E. Optimization of Natural Gas Treatment for the Removal of CO2 and H2S in a Novel Alkaline-DEA Hybrid Scrubber. Egypt. J. Pet. 2020, 29, 83–94. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Davani, Z.K.; Salehi, A.; Khosravi, A. Gas Sweetening Simulation and Its Optimization by Two Typical Amine Solutions: An Industrial Case Study in Persian Gulf Region. Nat. Gas Ind. B 2021, 8, 309–316. [Google Scholar] [CrossRef]
- Ngu, L.W.W.; How, B.S.; Mahmoud, A.; Rhamdhani, M.A.; Sunarso, J. Optimisation of K2CO3-Based Natural Gas Sweetening Process: A Hybrid Pareto and Fuzzy Optimisation Approach. J. Taiwan Inst. Chem. Eng. 2022, 132, 104128. [Google Scholar] [CrossRef]
- Esmaeili, A.; Yoon, T.; Atsbha, T.A.; Lee, C.J. Rate-Based Modeling and Energy Optimization of Acid Gas Removal from Natural Gas Stream Using Various Amine Solutions. Process Saf. Environ. Prot. 2023, 177, 643–663. [Google Scholar] [CrossRef]
- Lei, Y.; Du, L.; Liu, X.; Yu, H.; Liang, X.; Kontogeorgis, G.M.; Chen, Y. Natural Gas Sweetening Using Tailored Ionic Liquid-Methanol Mixed Solvent with Selective Removal of H2S and CO2. Chem. Eng. J. 2023, 476, 146424. [Google Scholar] [CrossRef]
- Adegunju, S.A.; Awad, M.R.; Berrouk, A.S.; Dara, S. Tackling Increased CO2 Concentration in Feeds for Existing Acid Gas Removal Units: A Simulation Study Based on a Customized CO2 Kinetics Model. Sep. Sci. Technol. 2018, 53, 2004–2015. [Google Scholar] [CrossRef]
- Shang, J.; Qiu, M.; Ji, Z. Efficiency Improvement, Consumption Reduction and Optimization of High-Sulfur Natural Gas Sweetening Units. Nat. Gas Ind. B 2019, 6, 472–480. [Google Scholar] [CrossRef]
- Darani, N.S.; Behbahani, R.M.; Shahebrahimi, Y.; Asadi, A.; Mohammadi, A.H. Simulation and Optimization of the Acid Gas Absorption Process by an Aqueous Diethanolamine Solution in a Natural Gas Sweetening Unit. ACS Omega 2021, 6, 12072–12080. [Google Scholar] [CrossRef]
- Otaraku, I.J. Simulation of Loading Capacity of MDEA and DEA for Amine-Based CO2 Removal Using HYSYS. Am. J. Chem. Eng. 2015, 3, 41. [Google Scholar] [CrossRef]
- Law, L.C.; Azudin, N.Y.; Shukor, S.R.A. Optimisation of Operational Parameter and Economic Analysis of Amine Based Acid Gas Capture Unit. Chem. Eng. Trans. 2017, 56, 73–78. [Google Scholar] [CrossRef]
- Tang, J.; Guo, Q.; Lin, R.; Zhang, C.; Li, Y.; Yu, X. Studies on Simulation and Optimization of Floating LNG Acid Gas Removal Process with Mixed Amine Solvent. In Proceedings of the 2011 International Conference on Electrical and Control Engineering (ICECE), Yichang, China, 16–18 September 2011; pp. 2573–2577. [Google Scholar] [CrossRef]
- Suebsook, J.; Eksaengsri, A.; Lertsakulsup, S.; Suwanvesh, K.; Iswigrai, J.; Sillapacharn, T. Process Optimisation in Acid Gas Removal System. In Proceedings of the International Petroleum Technology Conference (IPTC), Bangkok, Thailand, 15–17 November 2011; pp. 1–13. [Google Scholar]
- Gao, H.; Zhou, L.; Luo, X.; Liang, Z. Optimized Process Configuration for CO2 Recovery from Crude Synthesis Gas via a Rectisol Wash Process. Int. J. Greenh. Gas Control 2018, 79, 83–90. [Google Scholar] [CrossRef]
- Asani, R.R.; Mukherjee, R.; El-Halwagi, M.M. Optimal Selection of Shale Gas Processing and NGL Recovery Plant from Multiperiod Simulation. Process Integr. Optim. Sustain. 2021, 5, 123–138. [Google Scholar] [CrossRef]
- Godoy, J.L.; Gonzalez, A.H.; Ferramosca, A.; Bustos, G.; Normey-Rico, J.E. Tuning Methodology for Industrial Predictive Controllers Applied to Natural Gas Processing Unit. In Proceedings of the 2016 IEEE Conference on Control Applications (CCA), Buenos Aires, Argentina, 19–22 September 2016; pp. 1386–1391. [Google Scholar] [CrossRef]
- Jones, D.; Bhattacharyya, D.; Turton, R.; Zitney, S.E. Optimal Selection of Primary Controlled Variables for an Acid Gas Removal Unit as Part of an IGCC Plant with CO2 Capture. In Proceedings of the 2013 American Control Conference, Washington, DC, USA, 17–19 June 2013. [Google Scholar]
- Kathlyn, T.K.; Zabiri, H.; Aldrich, C.; Liu, X.; Mohd Amiruddin, A.A.A. Fault Detection and Identification in an Acid Gas Removal Unit Using Deep Autoencoders. ACS Omega 2023, 8, 19273–19286. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi-Baghmolaei, M.; Hajizadeh, A.; Zendehboudi, S.; Duan, X.; Shiri, H.; Saady, N.M.C. Exergy and Exergoeconomic Assessment of an Acid Gas Removal Unit in a Gas Refinery Plant. Ind. Eng. Chem. Res. 2021, 60, 14591–14612. [Google Scholar] [CrossRef]
- Wahid, A.; Meizvira, F.; Wiranoto, Y. Application of Multivariable Model Predictive Control to Overcome the Intervariable Interaction in CO2 Removal Process. E3S Web Conf. 2018, 67, 03049. [Google Scholar] [CrossRef]
- Mohajeri, M.; Panahi, M.; Shahsavand, A. Optimal Operation of a Natural Gas Sweetening Plant. Comput. Chem. Eng. 2024, 184, 108631. [Google Scholar] [CrossRef]
- Paul, P.; Bhattacharyya, D.; Turton, R.; Zitney, S.E. Dynamic model-based sensor network design algorithm for system efficiency maximization. Comput. Chem. Eng. 2016, 89, 27–40. [Google Scholar] [CrossRef]
- Kumar, M.S.; Ganapathy, K.; Al Yahyaee, N.; Ayoub, A. Enhance energy efficiency through advanced process control APC. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 9–12 November 2016; p. D031S071R002. [Google Scholar]
- Tikadar, D.; Gujarathi, A.M.; Guria, C. Multi-objective optimization of industrial gas-sweetening operations using economic and environmental criteria. Process Saf. Environ. Prot. 2020, 140, 283–298. [Google Scholar] [CrossRef]
- Thafseer, M.; Al Ani, Z.; Gujarathi, A.M.; Vakili-Nezhaad, G.R. Towards process, environment and economic based criteria for multi-objective optimization of industrial acid gas removal process. J. Nat. Gas Sci. Eng. 2021, 88, 103800. [Google Scholar] [CrossRef]
- Zhu, W.; Ye, H.; Zou, X.; Yang, Y.; Dong, H. Analysis and optimization for chemical absorption of H2S/CO2 system: Applied in a multiple gas feeds sweetening process. Sep. Purif. Technol. 2021, 276, 119301. [Google Scholar] [CrossRef]
- Chew, Y.E.; Putra, Z.A.; Foo, D.C.Y. Process simulation and optimisation for acid gas removal system in natural gas processing. J. Nat. Gas Sci. Eng. 2022, 107, 104764. [Google Scholar] [CrossRef]
- Berchiche, A.; Guenoune, M.; Belaadi, S.; Léonard, G. Optimal Energy Integration and Off-Design Analysis of an Amine-Based Natural Gas Sweetening Unit. Appl. Sci. 2023, 13, 6559. [Google Scholar] [CrossRef]
- Lian, L.; Liu, W.; Liu, S.; Wang, H.; Cheng, L.; Jiang, Y. Retrofit of the Acid Gas Sweetening Process for the Refinery Based on Exergy Analysis Method. Sep. Purif. Technol. 2023, 315, 123629. [Google Scholar] [CrossRef]
- Pal, A.; Almomani, F.; Al-Musleh, E.; Karimi, I. Freezing-Based Acid Gas Removal and Its Integration with the Cold Section in an LNG Plant. ACS Sustain. Chem. Eng. 2022, 10, 15148–15165. [Google Scholar] [CrossRef]
- Muhammad, A.; GadelHak, Y. Simulation Based Improvement Techniques for Acid Gases Sweetening by Chemical Absorption: A Review. Int. J. Greenh. Gas Control. 2015, 37, 481–491. [Google Scholar] [CrossRef]
- Hall, S.M. Absorbers. In Rules of Thumb for Chemical Engineers, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 293–303. [Google Scholar] [CrossRef]
- Sulzer, M.P.; Usa, C.; Holden, B.S. Back to Basics: Choosing Trays and Packings for Distillation. American Institute of Chemical Engineers. (CEP) Online. 2009. Available online: http://www.aiche.org/cep (accessed on 1 August 2024).
- Kasiri, N.; Ghayyem, M.A. Rate-Based Model in H2S and CO2 Absorption Column Using Alkanolamine Solutions. IUST Int. J. Eng. Sci. 2018, 19, 89–98. [Google Scholar]
- Moioli, S.; Pellegrini, L.A.; Picutti, B.; Vergani, P. Improved Rate-Based Modeling of H2S and CO2 Removal by Methyldiethanolamine Scrubbing. Ind. Eng. Chem. Res. 2013, 52, 2056–2065. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A. Improved Rate-Based Modeling of the Process of CO2 Capture with PZ Solution. Chem. Eng. Res. Des. 2015, 93, 611–620. [Google Scholar] [CrossRef]
- Fu, K.; Sema, T.; Liang, Z.; Liu, H.; Na, Y.; Shi, H.; Idem, R.; Tontiwachwuthikul, P. Investigation of Mass-Transfer Performance for CO2 Absorption into Diethylenetriamine (DETA) in a Randomly Packed Column. Ind. Eng. Chem. Res. 2012, 51, 12058–12064. [Google Scholar] [CrossRef]
- Fu, K.; Chen, G.; Sema, T.; Zhang, X.; Liang, Z.; Idem, R.; Tontiwachwuthikul, P. Experimental Study on Mass Transfer and Prediction Using Artificial Neural Network for CO2 Absorption into Aqueous DETA. Chem. Eng. Sci. 2013, 100, 195–202. [Google Scholar] [CrossRef]
- Gao, J.; Yin, J.; Zhu, F.; Chen, X.; Tong, M.; Kang, W.; Zhou, Y.; Lu, J. Postcombustion CO2 Capture Using Diethylenetriamine (DETA) Solvent in a Pilot-Plant Test Bed Compared to Monoethanolamine (MEA) Solvent. Environ. Prog. Sustain. Energy 2017, 36, 1131–1138. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Saidi, M.; Baniadam, M.; Parhoudeh, M. Investigation of Natural Gas Sweetening Process in Corrugated Packed Bed Column Using Computational Fluid Dynamics (CFD) Model. J. Nat. Gas Sci. Eng. 2013, 15, 127–137. [Google Scholar] [CrossRef]
- Sheng, M.; Xie, C.; Zeng, X.; Sun, B.; Zhang, L.; Chu, G.; Luo, Y.; Chen, J.; Zou, H. Intensification of CO2 Capture Using Aqueous Diethylenetriamine (DETA) Solution from Simulated Flue Gas in a Rotating Packed Bed. Fuel 2018, 234, 1518–1527. [Google Scholar] [CrossRef]
- Mahdipoor, H.R.; Mansoori, S.A.A.; Homayun, B.; Hosseinzadeh, M. Simulation and Optimization of Acid Gas Enrichment Column in an Industrial Sulfur Recovery Unit. Case Stud. Chem. Environ. Eng. 2024, 9, 100545. [Google Scholar] [CrossRef]
- Romero-Bustamante, J.A.; Zurita-Herrera, B.M.; Gutierrez-Limon, M.A.; Hernandez-Martinez, E. Robust Model-Based Control of a Packed Absorption Column for the Natural Gas Sweetening Process. Int. J. Chem. React. Eng. 2023, 21, 461–471. [Google Scholar] [CrossRef]
- Zahid, U.; Al-Qadri, A.; Al-Mousa, B.; Al-Nasser, A.; Ahmed, U. Design of a Novel Sour Water Stripping Unit. In Design of Novel Gas Processing Unit; Elsevier Masson SAS: Issy les Moulineaux, France, 2019. [Google Scholar] [CrossRef]
- Thiele, R.; Faber, R.; Repke, J.U.; Thielert, H.; Wozny, G. Design of Industrial Reactive Absorption Processes in Sour Gas Treatment Using Rigorous Modelling and Accurate Experimentation. Chem. Eng. Res. Des. 2007, 85, 74–87. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A. Regeneration Section of CO2 Capture Plant by MEA Scrubbing with a Rate-Based Model. Chem. Eng. Trans. 2013, 32, 1849–1854. [Google Scholar] [CrossRef]
- Oyenekan, B.A.; Rochelle, G.T. Alternative Stripper Configurations for CO2 Capture by Aqueous Amines. AIChE J. 2007, 53, 3144–3154. [Google Scholar] [CrossRef]
- Koolivand, M.; Abedini, R.; Adib, H.; Koolivand, H. Design of Neural Network for Manipulating Gas Refinery Sweetening Regenerator Column Outputs. Sep. Purif. Technol. 2011, 82, 1–9. [Google Scholar] [CrossRef]
- Jerng, S.E.; Park, Y.J.; Li, J. Machine Learning for CO2 Capture and Conversion: A Review. Energy AI 2024, 16, 100361. [Google Scholar] [CrossRef]
- Sarker, I.H. Machine Learning: Algorithms, Real-World Applications and Research Directions. SN Comput. Sci. 2021, 2, 160. [Google Scholar] [CrossRef]
- Hakimi, M.; Omar, M.B.; Ibrahim, R. Application of Neural Network in Predicting H2S from an Acid Gas Removal Unit (AGRU) with Different Compositions of Solvents. Sensors 2023, 23, 1020. [Google Scholar] [CrossRef]
- Ryan, P. Machine Learning for Design Principles for Single Atom Catalysts Towards Electrochemical Reactions. J. Mater. Chem. A 2022, 10, 15309–15331. [Google Scholar]
- Adib, H.; Sharifi, F.; Mehranbod, N.; Kazerooni, N.M.; Koolivand, M. Support Vector Machine Based Modeling of an Industrial Natural Gas Sweetening Plant. J. Nat. Gas Sci. Eng. 2013, 14, 121–131. [Google Scholar] [CrossRef]
- Adib, H.; Sabet, A.; Naderifar, A.; Adib, M.; Ebrahimzadeh, M. Evolving a Prediction Model Based on Machine Learning Approach for Hydrogen Sulfide Removal from Sour Condensate of South Pars Natural Gas Processing Plant. J. Nat. Gas Sci. Eng. 2015, 27, 74–81. [Google Scholar] [CrossRef]
- Azizkhani, J.S.; Jazayeri-Rad, H.; Nabhani, N. Design of an ensemble neural network to improve the identification performance of a gas sweetening plant using the negative correlation learning and genetic algorithm. J. Nat. Gas Sci. Eng. 2014, 21, 26–39. [Google Scholar] [CrossRef]
- Tikadar, D.; Gujarathi, A.M.; Guria, C. Towards retrofitting based multi-criteria analysis of an industrial gas sweetening process: Further insights of CO2 emissions. Process Saf. Environ. Prot. 2023, 175, 259–271. [Google Scholar] [CrossRef]
- Mousavi, S.P.; Atashrouz, S.; Nakhaei-Kohani, R.; Hadavimoghaddam, F.; Shawabkeh, A.; Hemmati-Sarapardeh, A.; Mohaddespour, A. Modeling of H2S solubility in ionic liquids using deep learning: A chemical structure-based approach. J. Mol. Liq. 2022, 351, 118418. [Google Scholar] [CrossRef]
- Rahaei, A.H.; Shokri, S.; Aroon, M.A.; Abolghasemi, H.; Zarrabi, S. Advancing Predictive Analytics for Gas Sweetening Plants Through Machine Learning and Feature Selection. J. Pet. Sci. Technol. 2024, 13, 12–19. [Google Scholar] [CrossRef]
- Nimmanterdwong, P.; Changpun, R.; Janthboon, P.; Nakrak, S.; Gao, H.; Liang, Z.; Tontiwachwuthikul, P.; Sema, T. Applied Artificial Neural Network for Hydrogen Sulfide Solubility in Natural Gas Purification. ACS Omega 2021, 6, 31321–31329. [Google Scholar] [CrossRef]
- Keshavarz, S.; Amin, J.S.; Mohsenipour, A.A.; Zendehboudi, S. Hybrid smart model to determine concentration of acidic gases in absorption tower of sweetening process. Can. J. Chem. Eng. 2022, 100, 2355–2373. [Google Scholar] [CrossRef]
- Memon, N.; Patel, S.B.; Patel, D.P. Comparative analysis of artificial neural network and XGBoost algorithm for PolSAR image classification. In Pattern Recognition and Machine Intelligence; Springer: Berlin/Heidelberg, Germany, 2019; pp. 452–460. [Google Scholar]
- Gajjar, A.; Kashyap, P.; Aysu, A.; Franzon, P.; Dey, S.; Cheng, C. FAXID: FPGA-accelerated XGBoost inference for data centers using HLS. In Proceedings of the 2022 IEEE 30th Annual International Symposium on Field-Programmable Custom Computing Machines (FCCM), New York City, NY, USA, 15–18 May 2022; pp. 1–9. [Google Scholar]
- Moghadasi, M.; Ozgoli, H.A.; Farhani, F. Steam consumption prediction of a gas sweetening process with methyldiethanolamine solvent using machine learning approaches. Int. J. Energy Res. 2021, 45, 879–893. [Google Scholar] [CrossRef]
- Grimaccia, F.; Montini, M.; Niccolai, A.; Taddei, S.; Trimarchi, S. A Machine Learning-Based Method for Modelling a Proprietary SO2 Removal System in the Oil and Gas Sector. Energies 2022, 15, 9138. [Google Scholar] [CrossRef]
- Khoshnevisan, L.; Hourfar, F.; Alhameli, F.; Elkamel, A. Combining design of experiments, machine learning, and principal component analysis for predicting energy consumption and product quality of a natural gas processing plant. Int. J. Energy Res. 2021, 45, 5974–5988. [Google Scholar] [CrossRef]
- Dong, S.; Quan, H.; Zhao, D.; Li, H.; Geng, J.; Liu, H. Generic AI models for mass transfer coefficient prediction in amine-based CO2 absorber, Part I: BPNN model. Chem. Eng. Sci. 2022, 264, 118165. [Google Scholar] [CrossRef]
- Quan, H.; Dong, S.; Zhao, D.; Li, H.; Geng, J.; Liu, H. Generic AI models for mass transfer coefficient prediction in amine-based CO2 absorber, Part II: RBFNN and RF model. AIChE J. 2023, 69, e17904. [Google Scholar] [CrossRef]
- Shams, A. Prediction of vapour-liquid equilibrium ratios for the CH4–CO2–H2S systems using artificial neural networks. Int. J. Oil Gas Coal Technol. 2022, 29, 226–240. [Google Scholar] [CrossRef]
- Rohani, M.; Jazayeri-Rad, H.; Behbahani, R.M. Continuous prediction of the gas dew point temperature for the prevention of the foaming phenomenon in acid gas removal units using artificial intelligence models. Int. J. Comput. Intell. Syst. 2017, 10, 165–175. [Google Scholar] [CrossRef]
- Cho, E.H.; Deng, X.; Zou, C.; Lin, L.C. Machine learning-aided computational study of metal-organic frameworks for sour gas sweetening. J. Phys. Chem. C 2020, 124, 27580–27591. [Google Scholar] [CrossRef]
- Gao, W.; Zheng, W.; Yan, K.; Sun, W.; Zhao, L. Accelerating the discovery of acid gas-selective MOFs for natural gas purification: A combination of machine learning and molecular fingerprint. Fuel 2023, 350, 128757. [Google Scholar] [CrossRef]
- Jia, B.; Mikalsen, R.; Smallbone, A.; Zuo, Z.; Feng, H.; Roskilly, A.P. Piston motion control of a free-piston engine generator: A new approach using cascade control. Appl. Energy 2016, 179, 1166–1175. [Google Scholar] [CrossRef]
- Wu, T.; Yin, H.; Yang, Z.; Yao, J.; Qin, Y.; Wu, P. Industrial process monitoring based on parallel global-local preserving projection with mutual information. Machines 2023, 11, 602. [Google Scholar] [CrossRef]
- Han, J.; Kamber, M.; Pei, J. Classification: Basic Concepts. In Data Mining: Concepts and Techniques, 3rd ed.; Morgan Kaufmann: San Francisco, CA, USA, 2012; pp. 327–391. [Google Scholar] [CrossRef]
- Cadei, L.; Camarda, G.; Montini, M.; Rossi, G.; Fier, P.; Bianco, A.; Lancia, L.; Loffreno, D.; Corneo, A.; Milana, D.; et al. Prediction and prescription of operation upset in H2S gas sweetening unit: Implementation of an innovative big data analytics procedure. In Proceedings of the Offshore Mediterranean Conference and Exhibition, Ravenna, Italy, 27–29 March 2019. [Google Scholar]
- Pradittiamphon, S.; Wongsa, S. Fault detection and isolation of acid gas removal units in a gas separation process using PLS. In Proceedings of the 2016 International Conference on Instrumentation, Control and Automation, Bandung, Indonesia, 29–31 August 2016; pp. 88–93. [Google Scholar] [CrossRef]
- Askarian, M.; Benítez, R.; Graells, M.; Zarghami, R. Data-based fault detection in chemical processes: Managing records with operator intervention and uncertain labels. Expert Syst. Appl. 2016, 63, 35–48. [Google Scholar] [CrossRef]
- Askarian, M.; Zarghami, R.; Jalali-Farahani, F.; Mostoufi, N. Fusion of Micro–Macro Data for Fault Diagnosis of a Sweetening Unit Using Bayesian Network. Chem. Eng. Res. Des. 2016, 115, 325–334. [Google Scholar] [CrossRef]
- Mohammadi, A.; Zarghami, R.; Lefebvre, D.; Golshan, S.; Mostoufi, N. Soft Sensor Design and Fault Detection Using Bayesian Network and Probabilistic Principal Component Analysis. J. Adv. Manuf. Process. 2019, 1, 1–14. [Google Scholar] [CrossRef]
- Rashidi, H.A.H. Fault Detection in Acid Gas Removal Unit (AGRU) System. Master’s Thesis, Universiti Teknologi Petronas, Seri Iskandar, Malaysia, 2021. [Google Scholar]
- Sun, W.; Zhou, Z.; Ma, F.; Wang, J.; Ji, C. Industrial Application of Data-Driven Process Monitoring with an Automatic Selection Strategy for Modeling Data. Processes 2023, 11, 402. [Google Scholar] [CrossRef]


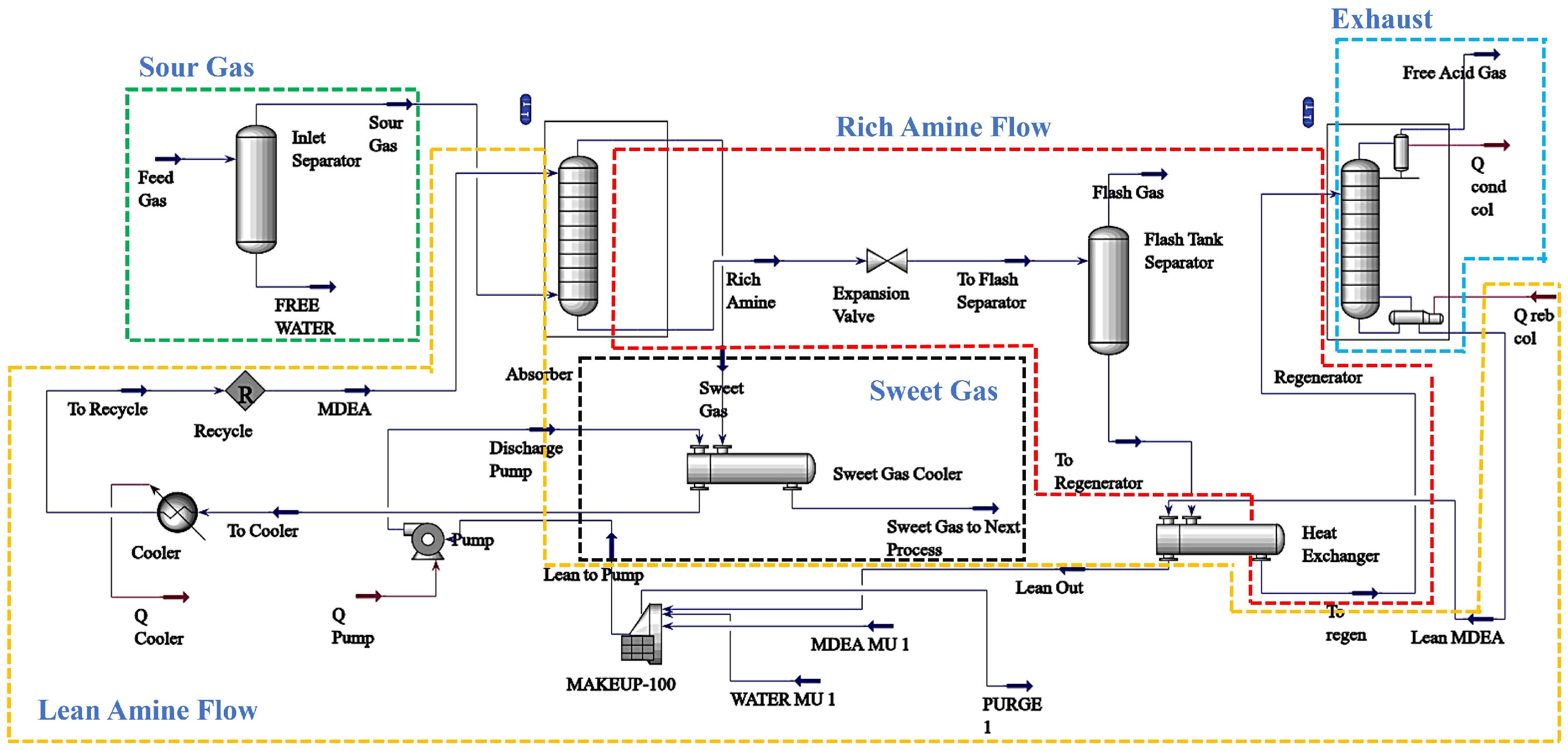

| Parameters | Solvent | Target Properties (Sweet Gas) | Results | Reference |
|---|---|---|---|---|
| Plant Capacity, NGL Recovery | 29 wt% DEA | Economic and Environmental | Offers a method for efficient shale gas processing, adaptable to changing gas flow rates for better economic returns | [33] |
| Feed Temperature, Feed Pressure, Permeate Pressure, Feed Flowrate | - | 23–40% CO2 | Optimizes CO2 removal, reduces operational losses | [34] |
| Lean Amine Temperature and Pressure, Feed Gas Temperature and Pressure, Regenerator Feed Temperature and Pressure, Feed Flow Rate | MDEA | H2S (≤0.001% mol), CO2 (≤2.0% mol) | Improves gas sweetening process, making it more cost-effective and environmentally friendly | [35] |
| H2S Purity, Energy Consumption, Exergy Loss | 30 wt% MDEA | H2S (≤20 ppmv) | Introduces an efficient method to purify gas and save energy using a new process that simplifies and speeds up the optimization | [36] |
| Solvent Flow Rate, Absorber Pressure | 38.97 wt% MDEA + 6.00 wt% PZ | CO2 (1%), H2S (≤4 ppmv) | Reduces energy use and costs in gas processing, increasing plant profit | [37] |
| Solvent Concentration, Absorption and Desorption Pressure | 40 wt% MDEA + 1 wt% PZ | CO2 (≤50 ppm), H2O (≤0.1 ppm) | Effectively reduces energy consumption in natural gas processing, significantly lowering CO2 and H2O levels | [38] |
| Sour Gas Split Ratio, Circulating Flowrate | 30 wt% MDEA | H2S (≤20 ppmv) | More efficient sweetening process for sour gases, improving gas purity and reducing energy consumption | [39] |
| Sour Gas Feed Temperature and Pressure, CO2 Volume Ratio, Solvent Temperature and Circulation Rate | 30% MDEA, 70% H2O | CO2 (10.35–1.5 vol%), H2S (25–0 ppm) | Optimized parameters for CO2 recovery, improving efficiency using actual industrial data | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wishnuwardana, R.J.; Omar, M.B.; Zabiri, H.B.; Faqih, M.; Ibrahim, R.; Bingi, K. Absorption-Based Optimization Technologies for Acid Gas Removal Units: A Review of Recent Trends and Challenges. Processes 2025, 13, 1909. https://doi.org/10.3390/pr13061909
Wishnuwardana RJ, Omar MB, Zabiri HB, Faqih M, Ibrahim R, Bingi K. Absorption-Based Optimization Technologies for Acid Gas Removal Units: A Review of Recent Trends and Challenges. Processes. 2025; 13(6):1909. https://doi.org/10.3390/pr13061909
Chicago/Turabian StyleWishnuwardana, Rafi Jusar, Madiah Binti Omar, Haslinda Binti Zabiri, Mochammad Faqih, Rosdiazli Ibrahim, and Kishore Bingi. 2025. "Absorption-Based Optimization Technologies for Acid Gas Removal Units: A Review of Recent Trends and Challenges" Processes 13, no. 6: 1909. https://doi.org/10.3390/pr13061909
APA StyleWishnuwardana, R. J., Omar, M. B., Zabiri, H. B., Faqih, M., Ibrahim, R., & Bingi, K. (2025). Absorption-Based Optimization Technologies for Acid Gas Removal Units: A Review of Recent Trends and Challenges. Processes, 13(6), 1909. https://doi.org/10.3390/pr13061909








