Bacillus Species: Evolving Roles in Bio-Based Detergents
Abstract
1. Introduction
2. Biosurfactants of Bacillus Species in Detergents
| Category | LAS | Rhamnolipids | Sophorolipids | Surfactin | Refs. |
|---|---|---|---|---|---|
| Typical Source | Chemical synthesis | Pseudomonas aeruginosa | Starmerella bombicola | Bacillus subtilis | [35,49] |
| CMC (ppm) | C10–14 433–650 C12LAS~360 C13LAS~150 | 10-200 | 40–100 | ~10–20 | [4,32,35,37] |
| Surface Tension (mN/m) | Commercial BIO-SOFT® S-101 C11.3 ~ 35 | ~30–35 | ~30–40 | ~27 | [35,50] |
| Biodegra-dability | 97–99% (Aerobic) | High | High | High | [28,51,52] |
| Eco-toxicity | EC50 = 3.5 ppm Dunaliella sp. | Low | Low | Low | [28,53] |
| Cost | USD ~2 highly scalable | USD ~223/100 g 90% pure | USD ~3/kg | USD ~22.3/mg ≥ 98% pure | [28,45] |
| Concentration (g/L) | Industrial scale | 39–112 | >200 | 0.1–1 WT Bacillus sp. | [43,49,54,55] |
| Scaling | Fully commercial | Limited commercial | Fully commercial | Pre-commercial | [55,56] |
| Use | Household and industrial detergents | Ecodetergents, bioremediation, cosmetics | Detergents, cosmetics, skincare | Pharma., cosmetics, skincare | [35] |
3. Enzymes from Bacillus Species in Detergents
3.1. Proteases from Bacillus Species
3.2. α-Amylases from Bacillus Species
3.3. Lipases from Bacillus Species
3.4. Cellulases from Bacillus Species
4. Recent Innovations in Harnessing Bacillus Species in Bio-Based Detergents
4.1. Affordable Green Detergents via Low-Cost Substrate Utilization
4.2. Energy-Saving Detergents with Cold-Adapted Microbes
| Bacillus Species | Culture Medium/Conditions | Growth Temp and Time | Enzyme/Biosurfactant | Enzyme Activity Characteristics | Scale | References |
|---|---|---|---|---|---|---|
| Bacillus sp. S1DI 10 (Himalayan Spring isolate) | Glucose–casein–peptone + salts; pH 7 | 20 °C; ~48 h | Cold-active metallo-protease | Optimum: 10 °C, pH 8; stable with 2% SDS and Tween-80; | Lab scale | [146] |
| Bacillus subtilis N8 (Turkey, alkaline soil) | Starch-based alkaline medium; 40 g/L glucose | 15–25 °C; ~48 h | Cold-active α-amylase | Optimum: 25 °C, pH 8; stable pH 8–12 and 10–40 °C; resists SDS, EDTA, Triton X-100, urea | Lab scale | [131] |
| Bacillus cereus GA6 (Himalayan glacier) | Glycerol + ammonium acetate; pH~10 | 20 °C; 96 h | Cold-active α-amylase | Optimum: 22 °C, pH 9; active 4–37 °C, pH 7–11; stable with SDS, EDTA, urea; active in detergents | Lab scale | [130] |
| Bacillus sp. SY-7 (oil-mill sewage) | Tributyrin and olive oil broth | 20 °C; 72 h | Cold-active lipase | Active 5–50 °C, pH 4–10; optimum at 20 °C, pH 8; stable in 5% SDS, detergents, metal ions | Lab scale | [133] |
| Bacillus subtilis SPB1 (Tunisian soil isolate) | Glucose, urea, NH4Cl, 2% kerosene; DO control | 30 °C; 48–72 h | Biosurfactant (surfactin) | Stable pH 2–9; 70 °C/1 h retention; improves detergent stain removal by 33–45% | Pilot (2.6 L bioreactor) | [34,147] |
4.3. Advanced Specialty Detergents Through Protein Engineering
| Strategies | Targeted Improvement | Results | References |
|---|---|---|---|
| Directed Evolution | Thermostability and substrate specificity |
| [150,152] |
| Post-translational Modification | pH-dependent activity enhancement |
| [153] |
| Recombination/Site-directed Mutagenesis | Oxidation stability |
| [154,155,156] |
| Semi-rational/Rational Design | Mentioned as complementary to directed evolution |
| [148] |
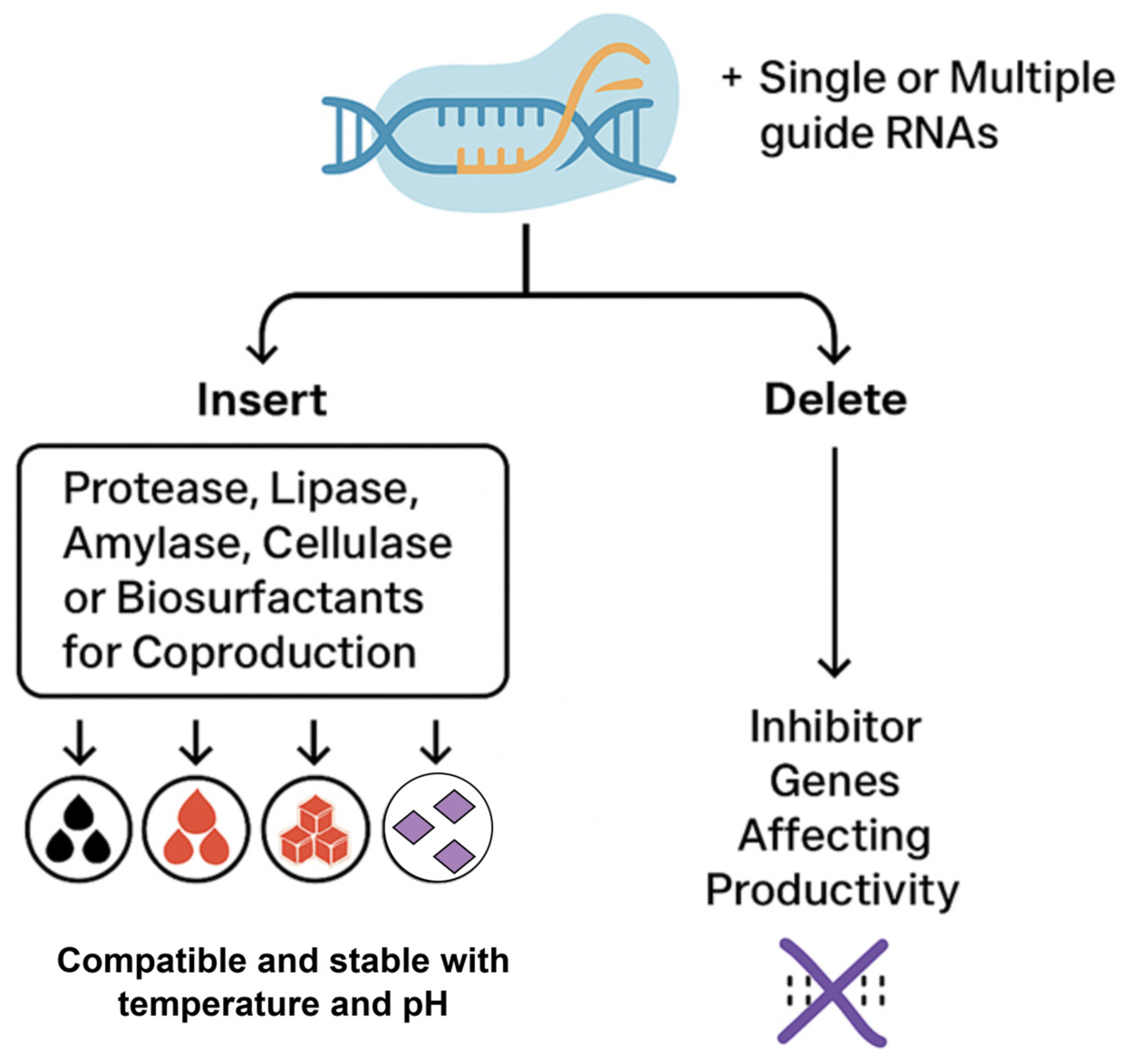
4.4. Smart Detergents for Precision Stain Removal with CRISPR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giagnorio, M.; Amelio, A.; Grüttner, H.; Tiraferri, A. Environmental impacts of detergents and benefits of their recovery in the laundering industry. J. Clean. Prod. 2017, 154, 593–601. [Google Scholar] [CrossRef]
- Cheng, K.C.; Khoo, Z.S.; Lo, N.W.; Tan, W.J.; Chemmangattuvalappil, N.G. Design and performance optimisation of detergent product containing binary mixture of anionic-nonionic surfactants. Heliyon 2020, 6, e03861. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology; Blackwell Scientific Publications Oxford: Oxford, UK, 1997. [Google Scholar]
- Yu, Y.; Zhao, J.; Bayly, A.E. Development of surfactants and builders in detergent formulations. Chin. J. Chem. Eng. 2008, 16, 517–527. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Khodadoost, F. Effects of detergents on natural ecosystems and wastewater treatment processes: A review. Environ. Sci. Pollut. Res. 2019, 26, 26439–26448. [Google Scholar] [CrossRef]
- Babajanzadeh, B.; Sherizadeh, S.; Ranji, H. Detergents and surfactants: A brief review. Open Access J. Sci. 2019, 3, 94–99. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Rita de Cássia, F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Warne, M.S.J.; Schifko, A. Toxicity of laundry detergent components to a freshwater cladoceran and their contribution to detergent toxicity. Ecotoxicol. Environ. Saf. 1999, 44, 196–206. [Google Scholar] [CrossRef]
- Household and Personal Care Today. Consumers Want Green and Clean Detergents. In Focus on Surfactants; Elsevier: Amsterdam, The Netherlands, 2009; p. 4.
- Scheibel, J.J. The evolution of anionic surfactant technology to meet the requirements of the laundry detergent industry. J. Surfactants Deterg. 2004, 7, 319–328. [Google Scholar] [CrossRef]
- Markets and Markets. Surfactants market worth $40.3 bn by 2019. In Focus on Surfactants; Elsevier: Amsterdam, The Netherlands, 2019; p. 4. [Google Scholar]
- Grbavčić, S.; Marković, D.; Rajilić-Stojanović, M.; Antov, M.; Šćiban, M.; Karadžić, I.; Knežević-Jugović, Z. Development of an environmentally acceptable detergent formulation for fatty soils based on the lipase from the indigenous extremophile Pseudomonas aeruginosa strain. J. Surfactants Deterg. 2015, 18, 383–395. [Google Scholar] [CrossRef]
- Benvegnu, T.; Plusquellec, D.; Lemiègre, L. Chapter 7: Surfactants from renewable sources: Synthesis and applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.-N., Gandini, A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2008; pp. 153–178. [Google Scholar]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef]
- Gurkok, S. Microbial Enzymes in Detergents: A Review. In Proceedings of the 4th International Conference on Advances in Natural & Applied Sciences Icanas, Agri, Turkey, 19–22 June 2019; pp. 19–22. [Google Scholar]
- Özbek Yazıcı, S.; Özmen, I. Optimization for coproduction of protease and cellulase from Bacillus subtilis M-11 by the Box–Behnken design and their detergent compatibility. Braz. J. Chem. Eng. 2020, 37, 49–59. [Google Scholar] [CrossRef]
- Nazareth, T.C.; Zanutto, C.P.; Maass, D.; de Souza, A.A.U.; Ulson, S.M.d.A.G. Bioconversion of low-cost brewery waste to biosurfactant: An improvement of surfactin production by culture medium optimization. Biochem. Eng. J. 2021, 172, 108058. [Google Scholar] [CrossRef]
- Singh, A.; Bajar, S.; Devi, A.; Pant, D. An overview on the recent developments in fungal cellulase production and their industrial applications. Bioresour. Technol. Rep. 2021, 14, 100652. [Google Scholar] [CrossRef]
- Song, Y.; He, S.; Jopkiewicz, A.; Setroikromo, R.; van Merkerk, R.; Quax, W.J. Development and application of CRISPR-based genetic tools in Bacillus species and Bacillus phages. J. Appl. Microbiol. 2022, 133, 2280–2298. [Google Scholar] [CrossRef]
- Niyonzima, F.N.; More, S.S. Coproduction of detergent compatible bacterial enzymes and stain removal evaluation. J. Basic Microbiol. 2015, 55, 1149–1158. [Google Scholar] [CrossRef]
- Vieira, I.M.M.; Santos, B.L.P.; Ruzene, D.S.; Silva, D.P. An overview of current research and developments in biosurfactants. J. Ind. Eng. Chem. 2021, 100, 1–18. [Google Scholar] [CrossRef]
- Berg, N.W.; Evans, M.R.; Sedivy, J.; Testman, R.; Acedo, K.; Paone, D.; Long, D.; Osimitz, T.G. Safety assessment of the use of Bacillus-based cleaning products. Food Chem. Toxicol. 2018, 116, 42–52. [Google Scholar] [CrossRef]
- OECD. Biosafety and the Environmental Uses of Micro-Organisms: Conference Proceedings; OECD Publishing: Paris, France, 2015. [Google Scholar]
- AB Enzymes GmbH. GRAS Notification for a Maltogenic Amylase from a Genetically Modified Bacillus subtilis; AB Enzymes Inc.: Plantation, FL, USA, 2020. [Google Scholar]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef]
- Li, J.; Deng, M.; Wang, Y.; Chen, W. Production and characteristics of biosurfactant produced by Bacillus pseudomycoides BS6 utilizing soybean oil waste. Int. Biodeterior. Biodegrad. 2016, 112, 72–79. [Google Scholar] [CrossRef]
- Karlapudi, A.P.; Venkateswarulu, T.; Tammineedi, J.; Kanumuri, L.; Ravuru, B.K.; Ramu Dirisala, V.; Kodali, V.P. Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Maria da Gloria, C.S.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ngueagni, P.T. A review on new aspects of lipopeptide biosurfactant: Types, production, properties and its application in the bioremediation process. J. Hazard. Mater. 2021, 407, 124827. [Google Scholar] [CrossRef]
- Smulders, E.; Sung, E. Laundry detergents, 2. Ingredients and products. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Hellmuth, H.; Dreja, M. Understanding interactions of surfactants and enzymes: Impact of individual surfactants on stability and wash performance of protease enzyme in detergents. Tenside Surfactants Deterg. 2016, 53, 502–508. [Google Scholar] [CrossRef]
- Jiang, X.; Yediler, A.; Yufang, S.; Sun, T.; Kettrup, A. Effect of linear alkylbenzene sulphonate (LAS) on the mineralization, metabolism and uptake of 14C-phenanthrene in a model ecosystem (water–lava–plant–air). Chemosphere 2005, 61, 741–751. [Google Scholar] [CrossRef]
- Domínguez Rivera, Á.; Martínez Urbina, M.Á.; López y López, V.E. Advances on research in the use of agro-industrial waste in biosurfactant production. World J. Microbiol. Biotechnol. 2019, 35, 155. [Google Scholar] [CrossRef]
- Bouassida, M.; Fourati, N.; Ghazala, I.; Ellouze-Chaabouni, S.; Ghribi, D. Potential application of Bacillus subtilis SPB1 biosurfactants in laundry detergent formulations: Compatibility study with detergent ingredients and washing performance. Eng. Life Sci. 2018, 18, 70–77. [Google Scholar] [CrossRef]
- Romero Vega, G.; Gallo Stampino, P. Bio-Based Surfactants and Biosurfactants: An Overview and Main Characteristics. Molecules 2025, 30, 863. [Google Scholar] [CrossRef]
- Sivapathasekaran, C.; Mukherjee, S.; Samanta, R.; Sen, R. High-performance liquid chromatography purification of biosurfactant isoforms produced by a marine bacterium. Anal. Bioanal. Chem. 2009, 395, 845–854. [Google Scholar] [CrossRef]
- Jauregi, P.; Coutte, F.; Catiau, L.; Lecouturier, D.; Jacques, P. Micelle size characterization of lipopeptides produced by B. subtilis and their recovery by the two-step ultrafiltration process. Sep. Purif. Technol. 2013, 104, 175–182. [Google Scholar] [CrossRef]
- Hamley, I.W. Lipopeptides: From self-assembly to bioactivity. Chem. Commun. 2015, 51, 8574–8583. [Google Scholar] [CrossRef]
- Janek, T.; Gudiña, E.J.; Połomska, X.; Biniarz, P.; Jama, D.; Rodrigues, L.R.; Rymowicz, W.; Lazar, Z. Sustainable surfactin production by Bacillus subtilis using crude glycerol from different wastes. Molecules 2021, 26, 3488. [Google Scholar] [CrossRef]
- Mukherjee, A. Potential application of cyclic lipopeptide biosurfactants produced by Bacillus subtilis strains in laundry detergent formulations. Lett. Appl. Microbiol. 2007, 45, 330–335. [Google Scholar] [CrossRef]
- Bhange, K.; Chaturvedi, V.; Bhatt, R. Simultaneous production of detergent stable keratinolytic protease, amylase and biosurfactant by Bacillus subtilis PF1 using agro industrial waste. Biotechnol. Rep. 2016, 10, 94–104. [Google Scholar] [CrossRef]
- Kavuthodi, B.; Sebastian, D. Biotechnological valorization of pineapple stem for pectinase production by Bacillus subtilis BKDS1: Media formulation and statistical optimization for submerged fermentation. Biocatal. Agric. Biotechnol. 2018, 16, 715–722. [Google Scholar] [CrossRef]
- Chong, H.; Li, Q. Microbial production of rhamnolipids: Opportunities, challenges and strategies. Microb. Cell Factories 2017, 16, 137. [Google Scholar] [CrossRef]
- Pardhi, D.S.; Panchal, R.R.; Raval, V.H.; Joshi, R.G.; Poczai, P.; Almalki, W.H.; Rajput, K.N. Microbial surfactants: A journey from fundamentals to recent advances. Front. Microbiol. 2022, 13, 982603. [Google Scholar] [CrossRef]
- Dhanarajan, G.; Sen, R. Cost analysis of biosurfactant production from a scientist’s perspective. Biosurfactants 2014, 159, 153. [Google Scholar]
- Wu, Q.; Zhi, Y.; Xu, Y. Systematically engineering the biosynthesis of a green biosurfactant surfactin by Bacillus subtilis 168. Metab. Eng. 2019, 52, 87–97. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Zhang, C.; Yao, Z.; Zhang, L.; Bie, X.; Lu, F.; Lu, Z. Genome shuffling of Bacillus amyloliquefaciens for improving antimicrobial lipopeptide production and an analysis of relative gene expression using FQ RT-PCR. J. Ind. Microbiol. Biotechnol. 2012, 39, 889–896. [Google Scholar] [CrossRef]
- Soberón-Chávez, G.; González-Valdez, A.; Soto-Aceves, M.P.; Cocotl-Yañez, M. Rhamnolipids produced by Pseudomonas: From molecular genetics to the market. Microb. Biotechnol. 2021, 14, 136–146. [Google Scholar] [CrossRef]
- Qiao, J.; Borriss, R.; Sun, K.; Zhang, R.; Chen, X.; Liu, Y.; Liu, Y. Research advances in the identification of regulatory mechanisms of surfactin production by Bacillus: A review. Microb. Cell Factories 2024, 23, 100. [Google Scholar] [CrossRef]
- Sałek, K.; Euston, S.R.; Janek, T. Phase behaviour, functionality, and physicochemical characteristics of glycolipid surfactants of microbial origin. Front. Bioeng. Biotechnol. 2022, 10, 816613. [Google Scholar] [CrossRef]
- Scott, M.J.; Jones, M.N. The biodegradation of surfactants in the environment. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1508, 235–251. [Google Scholar] [CrossRef]
- Ying, G.-G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 2006, 32, 417–431. [Google Scholar] [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. Int. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Zhen, C.; Ge, X.-F.; Lu, Y.-T.; Liu, W.-Z. Chemical structure, properties and potential applications of surfactin, as well as advanced strategies for improving its microbial production. AIMS Microbiol. 2023, 9, 195–217. [Google Scholar] [CrossRef]
- Henkel, M.; Geissler, M.; Weggenmann, F.; Hausmann, R. Production of microbial biosurfactants: Status quo of rhamnolipid and surfactin towards large-scale production. Biotechnol. J. 2017, 12, 1600561. [Google Scholar] [CrossRef]
- Dierickx, S.; Castelein, M.; Remmery, J.; De Clercq, V.; Lodens, S.; Baccile, N.; De Maeseneire, S.L.; Roelants, S.L.; Soetaert, W.K. From bumblebee to bioeconomy: Recent developments and perspectives for sophorolipid biosynthesis. Biotechnol. Adv. 2022, 54, 107788. [Google Scholar] [CrossRef]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial proteases applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef]
- Valls, C.; Pujadas, G.; Garcia-Vallve, S.; Mulero, M. Characterization of the protease activity of detergents laboratory practicals for studying the protease profile and activity of various commercial detergents. Biochem. Mol. Biol. Educ. 2011, 39, 280–290. [Google Scholar] [CrossRef]
- Jayasekara, S.; Ratnayake, R. Microbial cellulases: An overview and applications. In Cellulose; IntechOpen: Hamilton, NJ, USA, 2019; Volume 22, p. 130. [Google Scholar]
- American Cleaning Institue; AISE; AMFEP; Househood Commercial Products Association. The Role of Enzymes in Detergent Products. Available online: https://www.cleaninginstitute.org/sites/default/files/documents/Enzymes-factsheet.pdf (accessed on 12 March 2025).
- Gurkok, S.; Ozdal, M. Purification and characterization of a novel extracellular, alkaline, thermoactive, and detergent-compatible lipase from Aeromonas caviae LipT51 for application in detergent industry. Protein Expr. Purif. 2021, 180, 105819. [Google Scholar] [CrossRef]
- Ramnani, P.; Kumar, S.S.; Gupta, R. Concomitant production and downstream processing of alkaline protease and biosurfactant from Bacillus licheniformis RG1: Bioformulation as detergent additive. Process Biochem. 2005, 40, 3352–3359. [Google Scholar] [CrossRef]
- Enzymes Market Size, Share & Trends Analysis Report By Application (Industrial Enzymes, Specialty Enzymes), By Product (Carbohydrase, Proteases, Lipases), By Source, By Region, and Segment Forecasts, 2024–2030. Available online: https://www.grandviewresearch.com/industry-analysis/enzymes-industry/toc (accessed on 12 March 2025).
- Vojnovic, S.; Aleksic, I.; Ilic-Tomic, T.; Stevanovic, M.; Nikodinovic-Runic, J. Bacillus and Streptomyces spp. as hosts for production of industrially relevant enzymes. Appl. Microbiol. Biotechnol. 2024, 108, 185. [Google Scholar] [CrossRef]
- Herrmann, L.W.; Letti, L.A.J.; Penha, R.d.O.; Soccol, V.T.; Rodrigues, C.; Soccol, C.R. Bacillus genus industrial applications and innovation: First steps towards a circular bioeconomy. Biotechnol. Adv. 2024, 70, 108300. [Google Scholar] [CrossRef]
- Solanki, P.; Putatunda, C.; Kumar, A.; Bhatia, R.; Walia, A. Microbial proteases: Ubiquitous enzymes with innumerable uses. 3 Biotech 2021, 11, 428. [Google Scholar] [CrossRef]
- Vojcic, L.; Pitzler, C.; Körfer, G.; Jakob, F.; Martinez, R.; Maurer, K.-H.; Schwaneberg, U. Advances in protease engineering for laundry detergents. New Biotechnol. 2015, 32, 629–634. [Google Scholar] [CrossRef]
- Singh, S.; Bajaj, B.K. Potential application spectrum of microbial proteases for clean and green industrial production. Energy Ecol. Environ. 2017, 2, 370–386. [Google Scholar] [CrossRef]
- Klein, T.; Eckhard, U.; Dufour, A.; Solis, N.; Overall, C.M. Proteolytic Cleavage—Mechanisms, Function, and “Omic” Approaches for a Near-Ubiquitous Posttranslational Modification. Chem. Rev. 2018, 118, 1137–1168. [Google Scholar] [CrossRef]
- Saeki, K.; Ozaki, K.; Kobayashi, T.; Ito, S. Detergent alkaline proteases: Enzymatic properties, genes, and crystal structures. J. Biosci. Bioeng. 2007, 103, 501–508. [Google Scholar] [CrossRef]
- Nadeem, M.; Qazi, J.I.; Syed, Q.; Gulsher, M. Purification and characterization of an alkaline protease from Bacillus licheniformis UV-9 for detergent formulations. J. Sci. Technol. 2013, 35, 187–195. [Google Scholar]
- Hmidet, N.; Ali, N.E.-H.; Haddar, A.; Kanoun, S.; Alya, S.-K.; Nasri, M. Alkaline proteases and thermostable α-amylase co-produced by Bacillus licheniformis NH1: Characterization and potential application as detergent additive. Biochem. Eng. J. 2009, 47, 71–79. [Google Scholar] [CrossRef]
- El Hadj-Ali, N.; Agrebi, R.; Ghorbel-Frikha, B.; Sellami-Kamoun, A.; Kanoun, S.; Nasri, M. Biochemical and molecular characterization of a detergent stable alkaline serine-protease from a newly isolated Bacillus licheniformis NH1. Enzym. Microb. Technol. 2007, 40, 515–523. [Google Scholar] [CrossRef]
- Baweja, M.; Tiwari, R.; Singh, P.K.; Nain, L.; Shukla, P. An alkaline protease from Bacillus pumilus MP 27: Functional analysis of its binding model toward its applications as detergent additive. Front. Microbiol. 2016, 7, 1195. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Bhalla, T. Bacillus sp. APR-4 protease as a laundry additive. Indian J. Biotechnol. 2004, 3, 563–567. [Google Scholar]
- Mienda, B.S.; Huyop, F. Characterization of Bacillus cereus BM1 with Protease Activity. Res. Biotechnol. 2013, 4, 7–19. [Google Scholar]
- Emran, M.A.; Ismail, S.A.; Hashem, A.M. Production of detergent stable thermophilic alkaline protease by Bacillus licheniformis ALW1. Biocatal. Agric. Biotechnol. 2020, 26, 101631. [Google Scholar] [CrossRef]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and hot extremozymes: Industrial relevance and current trends. Front. Bioeng. Biotechnol. 2015, 3, 148. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Joseph, B. Current prospective in using cold-active enzymes as eco-friendly detergent additive. Appl. Microbiol. Biotechnol. 2020, 104, 2871–2882. [Google Scholar] [CrossRef]
- Souza, P.M.D. Application of microbial α-amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef]
- Saini, R.; Saini, H.S.; Dahiya, A. Amylases: Characteristics and industrial applications. J. Pharmacogn. Phytochem 2017, 6, 1865–1871. [Google Scholar]
- Azad, M.A.K.; Bae, J.-H.; Kim, J.-S.; Lim, J.-K.; Song, K.-S.; Shin, B.-S.; Kim, H.-R. Isolation and characterization of a novel thermostable α-amylase from Korean pine seeds. New Biotechnol. 2009, 26, 143–149. [Google Scholar] [CrossRef]
- Mitidieri, S.; Martinelli, A.H.S.; Schrank, A.; Vainstein, M.H. Enzymatic detergent formulation containing amylase from Aspergillus niger: A comparative study with commercial detergent formulations. Bioresour. Technol. 2006, 97, 1217–1224. [Google Scholar] [CrossRef]
- Lahmar, I.; El Abed, H.; Khemakhem, B.; Belghith, H.; Ben Abdallah, F.; Belghith, K. Optimization, purification, and starch stain wash application of two new α-amylases extracted from leaves and stems of Pergularia tomentosa. BioMed Res. Int. 2017, 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Mojsov, K. Aspergillus enzymes for food industries. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 215–222. [Google Scholar]
- Roy, J.K.; Rai, S.K.; Mukherjee, A.K. Characterization and application of a detergent-stable alkaline α-amylase from Bacillus subtilis strain AS-S01a. Int. J. Biol. Macromol. 2012, 50, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kanthi Kiran, K.; Chandra, T. Production of surfactant and detergent-stable, halophilic, and alkalitolerant alpha-amylase by a moderately halophilic Bacillus sp. strain TSCVKK. Appl. Microbiol. Biotechnol. 2008, 77, 1023–1031. [Google Scholar] [CrossRef]
- Kuddus, M.; Ahmad, I. Cold-active extracellular α-amylase production from novel bacteria Microbacterium foliorum GA2 and Bacillus cereus GA6 isolated from Gangotri glacier, Western Himalaya. J. Genet. Eng. Biotechnol. 2012, 10, 151–159. [Google Scholar] [CrossRef]
- Niyonzima, F.N.; More, S.S. Detergent-compatible bacterial amylases. Appl. Biochem. Biotechnol. 2014, 174, 1215–1232. [Google Scholar] [CrossRef]
- Offen, W.A.; Viksoe-Nielsen, A.; Borchert, T.V.; Wilson, K.S.; Davies, G.J. Three-dimensional structure of a variantTermamyl-like’Geobacillus stearothermophilus α-amylase at 1.9 Å resolution. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 66–70. [Google Scholar] [CrossRef]
- Rojo, R.; Mendoza, G.; Plata, F.; Lara, A.; Bárcena, R. Comparison of method of application on the effect of amylolytic enzymes on in vitro ruminal starch digestion. J. Appl. Anim. Res. 2007, 32, 81–84. [Google Scholar] [CrossRef]
- Guncheva, M.; Zhiryakova, D. Catalytic properties and potential applications of Bacillus lipases. J. Mol. Catal. B Enzym. 2011, 68, 1–21. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Javed, S.; Hameed, A. Enzymes used in detergents: Lipases. Afr. J. Biotechnol. 2010, 9, 4836–4844. [Google Scholar]
- Chandra, P.; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Factories 2020, 19, 1–42. [Google Scholar] [CrossRef]
- Verger, R. ‘Interfacial activation’of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzym. Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, N.; Rathi, P. Bacterial lipases: An overview of production, purification and biochemical properties. Appl. Microbiol. Biotechnol. 2004, 64, 763–781. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, N.; Pathania, S.; Handa, S. Purification and characterization of lipase by Bacillus methylotrophicus PS3 under submerged fermentation and its application in detergent industry. J. Genet. Eng. Biotechnol. 2017, 15, 369–377. [Google Scholar] [CrossRef]
- Verma, S.; Meghwanshi, G.K.; Kumar, R. Current perspectives for microbial lipases from extremophiles and metagenomics. Biochimie 2021, 182, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Ray, L. Purification and Immobilization of a Lipase from Bacillus cereus C7. Indian Chem. Eng. 2013, 55, 165–176. [Google Scholar] [CrossRef]
- Niyonzima, F.N. Detergent-compatible bacterial cellulases. J. Basic Microbiol. 2019, 59, 134–147. [Google Scholar] [CrossRef]
- Ito, S. Alkaline cellulases from alkaliphilic Bacillus: Enzymatic properties, genetics, and application to detergents. Extremophiles 1997, 1, 61–66. [Google Scholar] [CrossRef]
- Ladeira, S.A.; Cruz, E.; Delatorre, A.B.; Barbosa, J.B.; Leal Martins, M.L. Cellulase production by thermophilic Bacillus sp. SMIA-2 and its detergent compatibility. Electron. J. Biotechnol. 2015, 18, 110–115. [Google Scholar] [CrossRef]
- Jackson, A. Some problems of industrial scale-up. J. Biol. Educ. 1985, 19, 48–52. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Ramos, C.L.; Kuddus, M.; Rodriguez-Couto, S.; Srivastava, N.; Ramteke, P.W.; Mishra, P.K.; Molina, G. Enzymatic potential for the valorization of agro-industrial by-products. Biotechnol. Lett. 2020, 42, 1799–1827. [Google Scholar] [CrossRef]
- Chavan, S.; Yadav, B.; Atmakuri, A.; Tyagi, R.; Wong, J.W.; Drogui, P. Bioconversion of organic wastes into value-added products: A review. Bioresour. Technol. 2022, 344, 126398. [Google Scholar] [CrossRef]
- Moayedi, A.; Hashemi, M.; Safari, M. Valorization of tomato waste proteins through production of antioxidant and antibacterial hydrolysates by proteolytic Bacillus subtilis: Optimization of fermentation conditions. J. Food Sci. Technol. 2016, 53, 391–400. [Google Scholar] [CrossRef]
- Slivinski, C.T.; Mallmann, E.; de Araújo, J.M.; Mitchell, D.A.; Krieger, N. Production of surfactin by Bacillus pumilus UFPEDA 448 in solid-state fermentation using a medium based on okara with sugarcane bagasse as a bulking agent. Process Biochem. 2012, 47, 1848–1855. [Google Scholar] [CrossRef]
- Qureshi, A.S.; Khushk, I.; Ali, C.H.; Chisti, Y.; Ahmad, A.; Majeed, H. Coproduction of protease and amylase by thermophilic Bacillus sp. BBXS-2 using open solid-state fermentation of lignocellulosic biomass. Biocatal. Agric. Biotechnol. 2016, 8, 146–151. [Google Scholar] [CrossRef]
- Renge, V.; Khedkar, S.; Nandurkar, N.R. Enzyme synthesis by fermentation method: A review. Sci. Rev. Chem. Comm. 2012, 2, 585–590. [Google Scholar]
- Barros, F.F.C.; Simiqueli, A.P.R.; de Andrade, C.J.; Pastore, G.M. Production of Enzymes from Agroindustrial Wastes by Biosurfactant-Producing Strains of Bacillus subtilis. Biotechnol. Res. Int. 2013, 2013, 103960. [Google Scholar] [CrossRef]
- Gangadharan, D.; Nampoothiri, K.M.; Pandey, A. α-Amylase Production by Bacillus amyloliquefaciens Using Agro Wastes as Feed Stock. Food Technol. Biotechnol. 2011, 49, 336. [Google Scholar]
- Baysal, Z.; Uyar, F.; Aytekin, C. Solid state fermentation for production of α-amylase by a thermotolerant Bacillus subtilis from hot-spring water. Process Biochem. 2003, 38, 1665–1668. [Google Scholar] [CrossRef]
- Anto, H.; Trivedi, U.; Patel, K. α-Amylase production by Bacillus cereus MTCC 1305 using solid-state fermentation. Food Technol. Biotechnol. 2006, 44, 241–245. [Google Scholar]
- Abd-Elhalem, B.T.; El-Sawy, M.; Gamal, R.F.; Abou-Taleb, K.A. Production of amylases from Bacillus amyloliquefaciens under submerged fermentation using some agro-industrial by-products. Ann. Agric. Sci. 2015, 60, 193–202. [Google Scholar] [CrossRef]
- Annamalai, N.; Rajeswari, M.V.; Balasubramanian, T. Enzymatic saccharification of pretreated rice straw by cellulase produced from Bacillus carboniphilus CAS 3 utilizing lignocellulosic wastes through statistical optimization. Biomass Bioenergy 2014, 68, 151–160. [Google Scholar] [CrossRef]
- Annamalai, N.; Rajeswari, M.V.; Elayaraja, S.; Balasubramanian, T. Thermostable, haloalkaline cellulase from Bacillus halodurans CAS 1 by conversion of lignocellulosic wastes. Carbohydr. Polym. 2013, 94, 409–415. [Google Scholar] [CrossRef]
- Uyar, F.; Baysal, Z. Production and optimization of process parameters for alkaline protease production by a newly isolated Bacillus sp. under solid state fermentation. Process Biochem. 2004, 39, 1893–1898. [Google Scholar] [CrossRef]
- Bajaj, B.K.; Sharma, N.; Singh, S. Enhanced production of fibrinolytic protease from Bacillus cereus NS-2 using cotton seed cake as nitrogen source. Biocatal. Agric. Biotechnol. 2013, 2, 204–209. [Google Scholar] [CrossRef]
- Suci, M.; Arbianti, R.; Hermansyah, H. Lipase production from Bacillus subtilis with submerged fermentation using waste cooking oil. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012126. [Google Scholar] [CrossRef]
- Alkan, H.; Baysal, Z.; Uyar, F.; Dogru, M. Production of lipase by a newly isolated Bacillus coagulans under solid-state fermentation using melon wastes. Appl. Biochem. Biotechnol. 2007, 136, 183–192. [Google Scholar] [CrossRef]
- Baltaci, M.O.; Orak, T.; Taskin, M.; Adiguzel, A.; Ozkan, H. Enhancement of amylase and lipase production from Bacillus licheniformis 016 using waste chicken feathers as peptone source. Waste Biomass Valorization 2020, 11, 1809–1819. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Fernandes, E.C.; Rodrigues, A.I.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front. Microbiol. 2015, 6, 59. [Google Scholar] [CrossRef]
- Barros, F.F.C.; Ponezi, A.N.; Pastore, G.M. Production of biosurfactant by Bacillus subtilis LB5a on a pilot scale using cassava wastewater as substrate. J. Ind. Microbiol. Biotechnol. 2008, 35, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Fürst, C.; Frank, S.; Witt, A.; Koschke, L.; Makeschin, F. Assessment of the effects of forest land use strategies on the provision of ecosystem services at regional scale. J. Environ. Manag. 2013, 127, S96–S116. [Google Scholar] [CrossRef]
- Nandanwar, S.K.; Borkar, S.B.; Lee, J.H.; Kim, H.J. Taking Advantage of Promiscuity of Cold-Active Enzymes. Appl. Sci. 2020, 10, 8128. [Google Scholar] [CrossRef]
- Feller, G. Psychrophilic enzymes: From folding to function and biotechnology. Scientifica 2013, 2013, 512840. [Google Scholar] [CrossRef] [PubMed]
- Baghel, V.; Tripathi, R.; Ramteke, P.; Gopal, K.; Dwivedi, S.; Jain, R.; Rai, U.; Singh, S. Psychrotrophic proteolytic bacteria from cold environment of Gangotri glacier, Western Himalaya, India. Enzym. Microb. Technol. 2005, 36, 654–659. [Google Scholar] [CrossRef]
- Furhan, J.; Awasthi, P.; Sharma, S. Biochemical characterization and homology modelling of cold-active alkophilic protease from Northwestern Himalayas and its application in detergent industry. Biocatal. Agric. Biotechnol. 2019, 17, 726–735. [Google Scholar] [CrossRef]
- Kuddus, M. Cold-active detergent-stable extracellular α-amylase from Bacillus cereus GA6: Biochemical characteristics and its perspectives in laundry detergent formulation. J. Biochem. Technol. 2013, 4, 636–644. [Google Scholar]
- Arabacı, N.; Arıkan, B. Isolation and characterization of a cold-active, alkaline, detergent stable α-amylase from a novel bacterium Bacillus subtilis N8. Prep. Biochem. Biotechnol. 2018, 48, 419–426. [Google Scholar] [CrossRef]
- Joseph, B.; Ramteke, P.W. Extracellular solvent stable cold-active lipase from psychrotrophic Bacillus sphaericus MTCC 7526: Partial purification and characterization. Ann. Microbiol. 2013, 63, 363–370. [Google Scholar] [CrossRef]
- Yasemin, S.; ARABACI, N.; Güvenmez, H.K. Production of Cold Active Lipase from Bacillus sp. J. Appl. Biol. Sci. 2017, 11, 24–27. [Google Scholar]
- Perfumo, A.; Banat, I.M.; Marchant, R. Going green and cold: Biosurfactants from low-temperature environments to biotechnology applications. Trends Biotechnol. 2018, 36, 277–289. [Google Scholar] [CrossRef]
- Gu, T.; Sjöblom, J. Surfactant structure and its relation to the Krafft point, cloud point and micellization: Some empirical relationships. Colloids Surf. 1992, 64, 39–46. [Google Scholar] [CrossRef]
- Trudgeon, B.; Dieser, M.; Balasubramanian, N.; Messmer, M.; Foreman, C.M. Low-temperature biosurfactants from polar microbes. Microorganisms 2020, 8, 1183. [Google Scholar] [CrossRef]
- Vollú, R.E.; Jurelevicius, D.; Ramos, L.R.; Peixoto, R.S.; Rosado, A.S.; Seldin, L. Aerobic endospore-forming bacteria isolated from Antarctic soils as producers of bioactive compounds of industrial interest. Polar Biol. 2014, 37, 1121–1131. [Google Scholar] [CrossRef]
- Gesheva, V.; Stackebrandt, E.; Vasileva-Tonkova, E. Biosurfactant Production by Halotolerant Rhodococcusfascians from Casey Station, Wilkes Land, Antarctica. Curr. Microbiol. 2010, 61, 112–117. [Google Scholar] [CrossRef]
- Coronel-León, J.; de Grau, G.; Grau-Campistany, A.; Farfan, M.; Rabanal, F.; Manresa, A.; Marqués, A.M. Biosurfactant production by AL 1.1, a Bacillus licheniformis strain isolated from Antarctica: Production, chemical characterization and properties. Ann. Microbiol. 2015, 65, 2065–2078. [Google Scholar] [CrossRef]
- Vasileva-Tonkova, E.; Gesheva, V. Biosurfactant Production by Antarctic Facultative Anaerobe Pantoea sp. During Growth on Hydrocarbons. Curr. Microbiol. 2007, 54, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Zhang, B.; Chen, B.; Zhu, Z.; Lin, W.; Cao, T. Screening of biosurfactant producers from petroleum hydrocarbon contaminated sources in cold marine environments. Mar. Pollut. Bull. 2014, 86, 402–410. [Google Scholar] [CrossRef]
- Wu, S.; Liu, G.; Zhou, S.; Sha, Z.; Sun, C. Characterization of antifungal lipopeptide biosurfactants produced by marine bacterium Bacillus sp. CS30. Mar. Drugs 2019, 17, 199. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, L.; Li, K.; Chen, C.; Lin, X.; Zhang, C.; Xie, Q. Enhanced bioremediation of diesel oil-contaminated seawater by a biochar-immobilized biosurfactant-producing bacteria Vibrio sp. LQ2 isolated from cold seep sediment. Sci. Total Environ. 2021, 793, 148529. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, K.S. Bioactive compounds from polar regions: An account of chemical ecology and biotechnological applications. Curr. Org. Chem. 2022, 26, 1055–1087. [Google Scholar] [CrossRef]
- Janek, T.; Łukaszewicz, M.; Krasowska, A. Identification and characterization of biosurfactants produced by the Arctic bacterium Pseudomonas putida BD2. Colloids Surf. B Biointerfaces 2013, 110, 379–386. [Google Scholar] [CrossRef]
- Singh, D.; Thakur, S.; Thayil, S.M.; Kesavan, A.K. Characterization of a cold-active, detergent-stable metallopeptidase purified from Bacillus sp. S1DI 10 using Response Surface Methodology. PLoS ONE 2019, 14, e0216990. [Google Scholar] [CrossRef]
- Ghribi, D.; Ellouze-Chaabouni, S. Enhancement of Bacillus subtilis Lipopeptide Biosurfactants Production through Optimization of Medium Composition and Adequate Control of Aeration. Biotechnol. Res. Int. 2011, 2011, 653654. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Kundu, B. Protein engineering: Methods and applications. In Advances in Protein Molecular and Structural Biology Methods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 641–668. [Google Scholar]
- Engqvist, M.K.; Rabe, K.S. Applications of protein engineering and directed evolution in plant research. Plant Physiol. 2019, 179, 907–917. [Google Scholar] [CrossRef]
- Wintrode, P.L.; Miyazaki, K.; Arnold, F.H. Cold adaptation of a mesophilic subtilisin-like protease by laboratory evolution. J. Biol. Chem. 2000, 275, 31635–31640. [Google Scholar] [CrossRef] [PubMed]
- Tindbaek, N.; Svendsen, A.; Oestergaard, P.R.; Draborg, H. Engineering a substrate-specific cold-adapted subtilisin. Protein Eng. Des. Sel. 2004, 17, 149–156. [Google Scholar] [CrossRef]
- Martinez, R.; Jakob, F.; Tu, R.; Siegert, P.; Maurer, K.H.; Schwaneberg, U. Increasing activity and thermal resistance of Bacillus gibsonii alkaline protease (BgAP) by directed evolution. Biotechnol. Bioeng. 2013, 110, 711–720. [Google Scholar] [CrossRef]
- Jakob, F.; Martinez, R.; Mandawe, J.; Hellmuth, H.; Siegert, P.; Maurer, K.-H.; Schwaneberg, U. Surface charge engineering of a Bacillus gibsonii subtilisin protease. Appl. Microbiol. Biotechnol. 2013, 97, 6793–6802. [Google Scholar] [CrossRef]
- Despotovic, D.; Vojcic, L.; Blanusa, M.; Maurer, K.-H.; Zacharias, M.; Bocola, M.; Martinez, R.; Schwaneberg, U. Redirecting catalysis from proteolysis to perhydrolysis in subtilisin Carlsberg. J. Biotechnol. 2013, 167, 279–286. [Google Scholar] [CrossRef]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: A biotechnological perspective. Process Biochem. 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Nielsen, J.E.; Borchert, T.V. Protein engineering of bacterial α-amylases. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 2000, 1543, 253–274. [Google Scholar] [CrossRef]
- Chabhadiya, S.; Acharya, D.; Mangrola, A.; Shah, R.; Pithawala, E.A. Unlocking the potential of biosurfactants: Innovations in metabolic and genetic engineering for sustainable industrial and environmental solutions. Biotechnol. Notes 2024, 5, 111–119. [Google Scholar] [CrossRef]
- Karlson, C.K.S.; Mohd-Noor, S.N.; Nolte, N.; Tan, B.C. CRISPR/dCas9-Based Systems: Mechanisms and Applications in Plant Sciences. Plants 2021, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, E. CRISPR-Cas9: How research on a bacterial RNA-guided mechanism opened new perspectives in biotechnology and biomedicine. EMBO Mol. Med. 2015, 7, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, J.; Cheng, M.; Liao, X.; Peng, S. Review of CRISPR/Cas9 sgRNA design tools. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Liu, D.; Jia, X.; Zheng, Y.; Liu, W.; Xiao, Y. CRISPR-Cas9/Cas12a biotechnology and application in bacteria. Synth. Syst. Biotechnol. 2018, 3, 135–149. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y. CRISPR-based gene editing technology and its application in microbial engineering. Eng. Microbiol. 2023, 3, 100101. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Yang, S.; Zhu, M.; Wang, X. Technology prospecting on enzymes: Application, marketing and engineering. Comput. Struct. Biotechnol. J. 2012, 2, e201209017. [Google Scholar] [CrossRef]
- Fehler, A.O.; Kallehauge, T.B.; Geissler, A.S.; González-Tortuero, E.; Seemann, S.E.; Gorodkin, J.; Vinther, J. Flagella disruption in Bacillus subtilis increases amylase production yield. Microb. Cell Factories 2022, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, H.; Yuan, F.; Chai, H.; Wang, H.; Liu, F.; Li, Y.; Zhang, H.; Lu, F. Development and application of a CRISPR/Cas9 system for Bacillus licheniformis genome editing. Int. J. Biol. Macromol. 2019, 122, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hartz, P.; Gehl, M.; Koenig, L.; Bernhardt, R.; Hannemann, F. Development and application of a highly efficient CRISPR-Cas9 system for genome engineering in Bacillus megaterium. J. Biotechnol. 2021, 329, 170–179. [Google Scholar] [CrossRef]
- Price, M.A.; Cruz, R.; Baxter, S.; Escalettes, F.; Rosser, S.J. CRISPR-Cas9 In Situ engineering of subtilisin E in Bacillus subtilis. PLoS ONE 2019, 14, e0210121. [Google Scholar] [CrossRef] [PubMed]
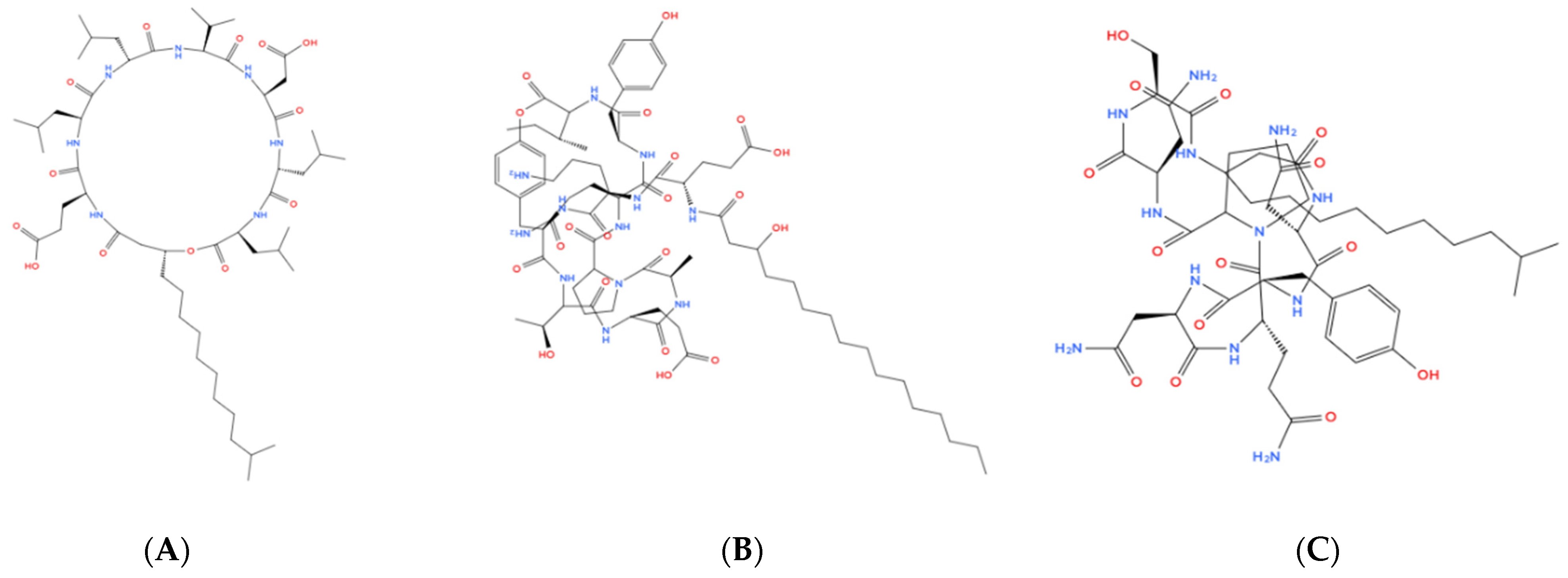

| Commercial Name | Specificity | Producer | Origin | Working Temperature |
|---|---|---|---|---|
| Alcalase® | Serine endopeptidase (Subtilisin A) | Novozymes | Bacillus licheniformis | Between 50 and 75 °C |
| Durazym® | Subtilisin | Novozymes | mutant Bacillus sp. | |
| Everlase™ | Subtilisin A | Novozymes | mutant Bacillus sp. | |
| Savinase® | Serine endopeptidase (Subtilisin A) | Novozymes | mutant Bacillus sp. | |
| Esperase® | Serine endopeptidase (Subtilisin A) | Novozymes | B. halodurans | |
| Neutrase® | Metalloprotease | Novozymes | B. amyloliquefaciens | |
| Protamex™ | Protease | Novozymes | Bacillus sp. | |
| Purafect® Prime | Subtilisin | Genencor Intl | Bacillus lentus | Between 20 and 40 °C |
| Properase® | Protease | Genencor Intl | Bacillus clausii |
| Types of Waste | Products | Strains | Types of Fermentation | Remarks | References |
|---|---|---|---|---|---|
| Wheat bran and rice husk as a carbon source | α-amylase | Bacillus subtilis | Solid-state fermentation | B. subtilis, isolated from hot springs. 7.3-fold higher enzyme production in wheat bran compared to rice husk | [113] |
| Wheat bran | α-amylase | Bacillus cereus MTCC 1305 | Solid-state fermentation | Highest enzyme production was observed with wheat bran (94 ± 2 U/g) after 72 h | [114] |
| Potato starch waste as the sole carbon source | α-, β-, γ-amylase | Bacillus amyloliquefaciens | Shaking flasks | Using the medium containing 2% potato starch waste in shaking flasks (150 rpm) at 50 °C produced the maximum α and β-amylase after 30 h, γ-amylase after 36 h | [115] |
| Rice bran as a carbon source | Cellulase | Bacillus carboniphilus CAS 3 | Shaking flasks | At initial pH 9.0, and temperature 50 °C, obtained 4040.4 U/mL of cellulose activity | [116] |
| Lignocellulosic wastes | Cellulase | Bacillus halodurans CAS 1 | Shaking flasks | With an optimum pH, temperature of 9.0 and 60 °C, an extracellular halotolerant, thermoalkaline cellulase was produced | [117] |
| Wheat bran and lentil husk as a carbon source | Alkaline protease | Bacillus sp. | Solid-state fermentation | Greatest yields of 429.04 and 168.64 U/g were achieved in 0.1 M carbonate/bicarbonate buffer at pH 10 | [118] |
| Cotton seed cake as a nitrogen source | Alkaline protease | B. cereus NS-2 | Shaking flasks | Wheat bran supported maximal fibrinolytic protease production (148 U/mL), cotton cake enhanced the fibrinolytic protease production to 315 U/mL, and Bacillus protease has the ability to remove blood stains. | [119] |
| Waste cooking oil | Lipase | Bacillus subtilis | Shaking flasks | The optimal lipolytic activity was 4.96 U/mL in 84 h of fermentation | [120] |
| Wheat bran, banana waste, melon waste, watermelon waste, lentil husk, and rice husk as carbon sources | Lipase | B. coagulans | Solid-state fermentation | Melon waste supplemented with 1% olive oil was found to be the best substrate for lipase production (78.069 U/g) | [121] |
| Chicken feather peptone (CFP) as a nitrogen source | Lipase and amylase | Bacillus licheniformis 016 | Shaking flasks | The optimum concentration of CFP for lipase and amylase production was determined as 5 and 6 g/L, respectively | [122] |
| Chicken feathers as a complex substrate of carbon and nitrogen source | Alkaline proteases and thermostable amylases | Bacillus licheniformis NH1 | Shaking flasks | Potential application as a detergent additive | [72] |
| Industrial waste (feather meal, potato peel and rape seed cake) | Keratinolytic protease, amylase, and biosurfactant | Bacillus subtilis PF1 | Shaking flasks | An overall 2.3% increase in proteases, 0.85% increase in amylase production, and 1.2% increase in biosurfactant production were achieved with optimized media. | [41] |
| Corn steep liquor | Biosurfactant | Bacillus subtilis | Shaking flasks | 10% (v/v) of Corn steep liquor, with a biosurfactant production of about 1.3 g/L | [123] |
| Soybean oil waste | Biosurfactant (lipoprotein) | Bacillus pseudomycoides BS6 | Liquid culture | 1.2 g crude biosurfactant was extracted from 1000 mL culture broth | [26] |
| Cassava wastewater as an unconventional carbon source | Biosurfactant | Bacillus subtilis LB5a | 40 L Bioreactor | An average of 25.7 g of surfactant was recovered per batch (0.68 g of surfactant/L of cassava wastewater | [124] |
| Wheat straw | Protease and amylase | Bacillus sp. BBXS-2 | Solid-state fermentation | 12,200 U/g and 6900 U/g dry matter for protease and amylase, respectively, after a 5-day fermentation at 45 °C, initial pH of 8.5, nonsterile open fermentation | [109] |
| Soybean flour and rice straw | Biosurfactant | Bacillus amyloliquefaciens XZ-173 | Solid-state fermentation | A surfactin yield of 15.03 mg/gram dry substrate was attained in a 1000-fold scale-up fermentation in a 50 L fermenter | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.-M.-L.; Ndao, A.; Peterson, E.C.; Blais, J.-F.; Adjallé, K. Bacillus Species: Evolving Roles in Bio-Based Detergents. Processes 2025, 13, 1885. https://doi.org/10.3390/pr13061885
Nguyen V-M-L, Ndao A, Peterson EC, Blais J-F, Adjallé K. Bacillus Species: Evolving Roles in Bio-Based Detergents. Processes. 2025; 13(6):1885. https://doi.org/10.3390/pr13061885
Chicago/Turabian StyleNguyen, Vu-Mai-Linh, Adama Ndao, Eric Charles Peterson, Jean-François Blais, and Kokou Adjallé. 2025. "Bacillus Species: Evolving Roles in Bio-Based Detergents" Processes 13, no. 6: 1885. https://doi.org/10.3390/pr13061885
APA StyleNguyen, V.-M.-L., Ndao, A., Peterson, E. C., Blais, J.-F., & Adjallé, K. (2025). Bacillus Species: Evolving Roles in Bio-Based Detergents. Processes, 13(6), 1885. https://doi.org/10.3390/pr13061885








