Abstract
Biobased packaging solutions with active functions for different food categories are a very attractive topic nowadays. This packaging provides suitable preservation of the food quality and extends the shelf life of packed items. In addition, this is a promising pathway to overcome global pollution, to protect human health, as well as to provide a better planetary wellbeing. In this work, a packaging composition based on poly(lactic acid) (PLA) with the addition of Curcuma longa L. (C) extract prepared by the solution casting method is promoted as a potential packaging option for the active food packaging of cheese. The dopant levels of the extract were performed at 0.5%, 1%, 2%, 5%, and 10%, while the neat PLA film was used as a control. The obtained results are promising. By a thermal analysis, it is shown that C-extract has a plasticizing and nucleating effect on PLA molecules, as well as improving the barrier and other film properties. Moreover, this packaging was proven as a potential antimicrobial packaging for white cheese—it enables extending the shelf life by direct contact. This is a simple way of manufacturing biobased packaging doped with natural antimicrobials that could be used for other food categories that are prone to microbiological attack.
1. Introduction
Plastics have been used in a variety of sectors, from buildings, transportation, electrical and medical applications, as well as textiles, but the most is used in the packaging sector—more than 1/3 of the total produced amount goes to packaging purposes [1,2,3]. The attractive set of plastic properties, like being lightweight, durable, flexible, strong, user-friendly design, and fabrication capabilities is the main reason of plastic production that has increased a lot in the last century. Namely, plastic production reached approx. 368 million metric tons (Mt) in 2019, but it is estimated to double within the next 20 years. If it continues in this way, it is predicted that the annual global plastic production will exceed 1100 Mt by 2050. However, the practice of collecting plastic waste is not at a satisfactory level worldwide. It has been stated that only ~9% of plastic waste is recycled. It may take decades to transition toward effective plastic waste management and replace conventional plastics with bio-based and biodegradable alternatives. Plastic pollution poses a serious threat to global communities and planetary health, yet awareness and behavioral change remain slow and insufficient worldwide [4,5,6].
Many global initiatives for a better sustainable future and proper plastic treatment and management are found in the efforts proposed by the UN SDGs (2015) and the European Commission and their initiatives to mitigate the effects of climate change, such as the European Green Deal, the Circular Economy Action Plan, and the legislative initiatives that ban single-use plastics, which in general, promote finding new green methods and alternatives for petrochemical plastics and efficient life cycle management approaches. In the last century, plastics have drawn significant attention, not only in terms of production but also in exploring new sources, sustainable alternatives, and disposal or recycling options that offer potential benefits for the environment, economy, society, and overall planetary health [1]. The future scenario for eco-friendly options for plastics is a worthy challenge for many scientists nowadays [6,7]. In this context, bio-based plastics represent a promising part of the solution to this global threat. These are plastics derived from biomass, defined as any material of biological origin, excluding fossilized or geologically formed substances (European Standard EN 16575, Bio-based products. Vocabulary. 2014) [2,8].
One of the prominent representatives of biobased plastic that attracts a lot of attention is polylactic acid (PLA) or polylactide, which belongs to a group of thermoplastic polyesters. It can be made from either renewable or recycled resources. It has lactic acid as a monomer unit, which is usually obtained from the fermentation of sugar cane, potato, or maize. PLA is a safe plastic for use in contact with food items, so it is categorized as generally recognized as safe (GRAS) by the Food and Drug Administration (FDA) [9,10]. In general, PLA has similar characteristics to polypropylene (PP), polyethylene (PE), or polystyrene (PS) and can be processed even with the existing manufacturing equipment. More particularly, a variety of methods exist for PLA processing, like sheet extrusion, blow molding, and film forming [10,11]. PLA possesses great physical and mechanical properties, such as high transparency with excellent tensile strength and wear resistance, and is stiff, glossy, and clear, in addition to its biodegradability and biocompatibility. The properties of PLA can be modified in order to improve the inherent properties of the neat PLA film, like brittleness, poor thermal stability, or low elasticity [12]. The composite films based on PLA are investigated by many researchers as suitable functional/active packaging materials, such as, for example, the case of PLA combined with spent coffee [13], PLA films with algal cellulose nanocrystals and curcumin [14], PLA combined with essential oil and curcumin [15], etc. The degradation time or half-life of PLA ranges from approximately 6 to 24 months, depending on factors such as its stereochemistry and molecular weight [10]. This characteristic makes PLA particularly notable for its ability to degrade under environmental conditions.
Furthermore, PLA has a big potential to be used in antimicrobial packaging solutions [10]. Antimicrobial and antioxidant packaging materials based on PLA take an attractive place among active food packaging; natural additives have become a major focus of many scientists. Natural extracts, essential oils, and other bioactive compounds are promising alternatives to synthetic compounds due to their functionality and biodegradability [16,17,18,19,20]. Among these, curcumin extract stands out as a natural polyphenolic compound derived from turmeric roots/rhizomes (Curcuma longa L.), which belongs to the flavonoid compounds. In the food industry, it has been used for flavor and color due to its characteristic aroma and yellowish-orange appearance [21,22]. Traditionally, it has been used as a food coloring agent but also as a multifunctional additive in the production of dairy products, butter, and other foods. Curcumin utilized as a natural colorant is identified by the code E100, approved by the Codex Alimentarius Commission [23]. Its functional roles include extending shelf life, enhancing product quality, and providing antioxidant and antimicrobial effects [14,19,24,25,26]. In addition, it has a broad spectrum of beneficial health properties for humans, such as anti-inflammatory, anti-hypertensive, antioxidant, anti-cancer, antifungal, and other activities, but it is also being used in the treatment of cardiovascular diseases, metabolic syndrome, and arthritis [21,22,27,28,29,30,31,32].
Due to its unique properties, including its the two tautomeric forms, enol and keto (in acidic/neutral environments keto form is predominant, while the enol form prevails in alkaline conditions), curcumin is well-aligned with the concept of intelligent food packaging, e.g., for monitoring food freshness or other qualitative/quantitive parameters. This molecular versatility leads to a significant pH-responsive color change, transitioning from yellow in acidic/neutral environments to red in alkaline ones, which is visible with the naked eye [28,33,34]. For example, there are cases when the integration of curcumin is discussed for its ability to detect spoilage in meat, seafood, oils, fruits, and vegetables [14,15,19,34,35,36,37]. Moreover, curcumin can also play an active role by being released from contact packaging to the packed items, thereby prolonging their shelf life and improving the food quality. There is a study where a PBAT/curcumin film demonstrated antioxidant and antimicrobial activity, as well as an improved water vapor barrier [3]. In another study, curcumin incorporated into a PVA/SA matrix was found to delay lipid oxidation and provide antioxidant activity [19,36]. Additionally, ref. [37] reported on a curcumin-loaded TPCS/PBAT packaging composition, which contributed enhanced stability to chia oil, or a formulation based on gelatin/curcumin, which provided more than 99% UV-blocking effects (at only 1.5% added curcumin to a gelatin base), as well as significantly improved mechanical and barrier properties [24].
Furthermore, monitoring the quality of the packed food items aligns well with the global efforts to reduce food spoilage and wastage, which is a pressing issue worldwide. According to the Food and Agriculture Organization (FAO), food waste costs approx. USD 680 billion annually in industrialized countries and USD 310 billion in developing countries [25]. Dairy products, in particular, are highly perishable due to their susceptibility to microbial spoilage, which makes them an important target for improved packaging solutions.
This study focuses on the application of biopackaging for dairy products, specifically white cheese, aiming to extend shelf life while maintaining product quality. By incorporating natural antimicrobials found in curcumin extracts into a biobased PLA matrix, the authors present a suitable packaging approach that addresses consumer demands for eco-friendly production systems. White cheese is used as a model dairy product to evaluate the effectiveness of PLA/curcumin-based packaging material.
2. Materials and Methods
2.1. Materials
Poly(L-lactic acid) (PLA) was purchased from Biomer, Krailling, Germany (L9000, Mn = 101 kDa, Ð = 2.2, and 1.5% D-lactic acid). Curcuma longa L. powder (a commercial product) was used to obtain curcumin extract (C), properly characterized in a previous publication [38]. Chloroform (99.5%, product of Merck, Darmstadt, Germany) and ethanol (96%, product of Alkaloid, Skopje) solvents were used as received without previous purification. White cheese made from cow’s milk was used as a sample for packaging in the examined composite films based on PLA and curcumin extract.
2.2. Preparation of Polymer/C Films
Polymer solutions (5% w/v) were prepared by dissolving PLA pellets in chloroform by mechanical stirring for 5 h at room temperature. After complete dissolution of the polymer, the appropriate contents of dried Curcuma longa L. extract (0.5, 1, 2, 5, and 10 wt% related to the polymer) were added to the polymer solution and mixed together for an additional 2 h. The polymer solutions were cast onto Petri dishes (d = 9 cm) and left at room temperature to remove the solvent, and additionally vacuum dried for 24 h. The polymer films peeled from the glass molds were dried in a vacuum oven at 30 °C for 24 h. The sample films were denoted as PLA-C-x, where x is the percentage of the added C-extract.
2.3. Characterization of Polymer Films
2.3.1. Morphological Analysis
The surface morphology of the polymer films was observed by scanning electron microscopy (SEM, JSM-IT200 from JEOL, Peabody, MA, USA) using an accelerating voltage of 10 kV. Before the observations, the polymer films were coated with a thin layer of gold.
2.3.2. FTIR Spectroscopy Analysis
The ATR-FTIR spectra were analyzed using a Shimadzu IRAffinity 1S Fourier-transform infrared spectrometer (Shimadzu, Kyoto, Japan). All spectra were recorded in a range of 4000–400 cm−1, with 32 scans and a resolution of 4 cm−1. For each sample, the FTIR spectra were collected from five different positions on the film in order to evaluate the uniformity of extract distribution.
2.3.3. Thermal Analysis
The thermal properties of the films were analyzed with a TA Instruments DSC Q100 differential scanning calorimeter, equipped with a refrigerated cooling system. The specimens were heated from 20 °C to 200 °C at a heating rate of 10 °C min−1. The degree of crystallinity Xc was calculated according to Equation (1):
where Hm is the melting enthalpy (Jg−1) and is the melting of hypothetically 100% crystalline PLA, taken as 93.6 J g−1 [39], and is the PLA weight fraction in the corresponding film.
2.3.4. Surface Color and Film Transmittance
The surface color was determined from the captured images of the films, where the RGB coordinates were determined and converted to the L* a b parameters using software https://colormine.org/convert/rgb-to-lab (accessed on 21 May 2025).
The transmittance (T) of the films was analyzed by UV/VIS spectroscopy (Hewlett Packard 8452A Diode Array UV-Visible spectrometer, Santa Clara, CA, USA). The transmittance was determined at 660 nm (T660).
2.3.5. Water Contact Angle Measurements (WCA) and Water Vapor Permeability (WVP)
The contact angles were measured by the drop method using water. Approximately 5 μL of deionized water were dropped onto the surface of the polymer films. The images were captured within 5 s of water drop delivery and analyzed using ImageJ software, Ver. 2.0. Each water contact angle (WCA) was the mean value of five measurements taken from the different positions of the film.
The water vapor permeability (WVP) of the PLA/C films was determined gravimetrically at 20 °C, according to the ASTM E96/E96M-12 (1993) standard [40]. The polymer films (d = 5 cm) were cut and conditioned at 20 °C for 24 h. The films were sealed at the tops of the Petri dishes (d = 5 cm), which were filled with an equal quantity of distilled water. The Petri dishes were placed in a desiccator with a relative humidity of 54%. The mass change of the Petri dishes was monitored gravimetrically at 30 min intervals for 24 h. The water vapor permeability was determined according to Equation (2):
where N is the molar flux of water (mol/m2·s), L is polymer film thickness (m), and is the partial difference of the pressures from both sides of the film (Pa).
2.3.6. Photodegradation of Polymer Films
The photodegradation of the polymer films (1 cm × 1 cm) was followed under UV lamp exposure (1950 mW/cm2) with monochromatic light with a wavelength of 365 nm. The mass changes of the polymer films were followed over 14 days. The mass loss in percentage (Wloss) was determined using Equation (3):
where Wo is the initial mass of the polymer film, and Wt is the mass of the polymer film measured at time t of UV light exposure. The obtained values were the average of three measurements.
2.3.7. Curcumin Release Study
The release of the incorporated curcumin extract was followed by 3% (w/v) acetic acid in water, denoted as simulant B [3]. The polymer films were cut (1.5 cm × 1.5 cm) and placed in amber bottles filled with 5 mL of simulant. The samples were stored at room temperature (23 °C) for up to 24, 48, 72, and 144 h, and then, the simulant was appropriately spectrometrically analyzed in order to detect the released curcuminoids at λ = 422 nm.
2.4. Antimicrobial Activity Assessment of PLA/Curcumin Composite Films
The antimicrobial activity of the PLA/C films was assessed by the disk diffusion method, also known as the Kirby–Bauer method, which is a pivotal technique used for antimicrobial susceptibility testing in both clinical and food microbiology. The method was initially developed by Bauer et al. (1966) [41] and later standardized by the Clinical and Laboratory Standards Institute (CLSI, 2006) [42]. Its widespread adoption is attributed to its simplicity, cost-effectiveness, and reproducibility, making it a preferred method in many laboratories for assessing the effects of antimicrobial substances on bacteria and fungi. However, it is important to recognize that, while valuable, the disk diffusion method has certain limitations, such as subjective measurement of inhibition zones and the influence of agar thickness and inoculum density on diffusion rates [43,44].
The accelerated shelf-life testing method was also used to predict the shelf life of the white cheese layered with a PLA/curcumin film by storing it under elevated temperature (or other stress conditions such as humidity, light, or oxygen exposure) to accelerate spoilage or degradation.
2.4.1. Isolation and Identification of Spoilage Microorganisms from White Cheese
White cheese made from cow’s milk was purchased from a local producer, removed from its packaging, and stored at refrigerator temperature in a closed plastic container for 10 days. After this period, several fungal colonies appeared on the surface of the cheese. Each colony was aseptically transferred to sterile Sabouraud dextrose agar (Biolife, Milan, Italy) to obtain pure cultures. Three isolates were successfully obtained and purified. Based on microscopic examination and colony morphology, two isolates were tentatively identified as Penicillium spp. (characterized by the broom-like branching of conidiophores with metulae) and one isolate as Saccharomyces spp. (oval cells exhibiting unipolar budding) (Figure 1).
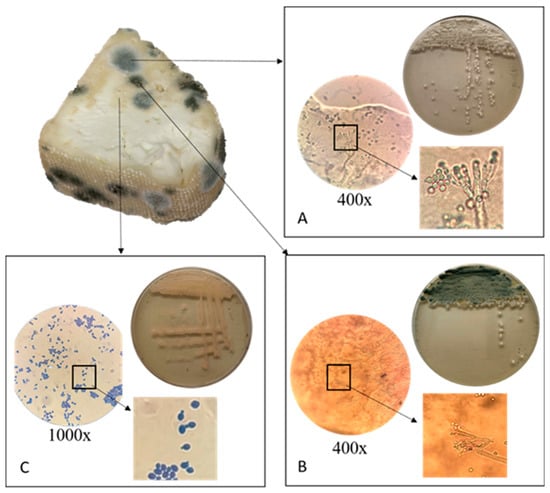
Figure 1.
Isolation and identification of spoilage microorganisms from white cheese: microscopic and cultural morphology of Penicillium spp. (A,B) and Saccharomyces spp. (C).
2.4.2. Antimicrobial Tests
The disk diffusion method was used to determine the antimicrobial properties of PLA films containing different concentrations of curcuma extract. In addition to the spoilage microorganisms isolated from the cheese, two pathogenic bacteria, Escherichia coli and Staphylococcus aureus, were tested. These bacteria, obtained from blood samples, were selected because they are capable of surviving in white cheese and are included in the microbiological criteria for milk and dairy products according to Commission Regulation (EC) No 2073/2005.
Pure cultures were inoculated into nutrient broth (Biolife, Milan, Italy) and incubated overnight (16–18 h) at 37 °C (E. coli and S. aureus) or at 28 °C (Penicillium spp. and Saccharomyces spp.). The overnight cultures were then diluted with peptone physiological saline as necessary to achieve an optical density of approximately 0.5 at 600 nm.
Approximately 20 mL of Mueller–Hinton agar (Biolife, Milan, Italy) (for bacteria) or Sabouraud dextrose agar (for fungi) were poured into Petri dishes to achieve a final depth of approximately 4 mm. The plates were inoculated by spreading 0.1 mL of the microbial suspension across the agar surface.
Within five minutes of inoculation, disks (6 mm diameter) cut from PLA films containing 0%, 0.5%, 1.0%, 2.0%, 5.0%, and 10.0% curcuma extract were placed onto the agar surface. Natamycin (500 μg/mL, corresponding to 5.0 μg per disk) applied onto sterile filter paper (6 mm diameter), was used as a positive control for antifungal activity. The plates were incubated at 37 °C (E. coli and S. aureus) or 28 °C (Penicillium spp. and Saccharomyces spp.). After 48 h of incubation, the plates were examined, and the diameters of the inhibition zones around each disk were recorded.
2.4.3. Accelerated Shelf-Life Testing of White Cheese Layered with PLA Films
The same type of white cheese used for isolating spoilage microorganisms was aseptically cut into cuboid pieces (3 cm × 3 cm × 1 cm, length × width × height). Each cheese piece was layered on the top surface with PLA films containing different concentrations of curcuma extract (direct contact analysis [15,44]). The samples were placed in sterilized glass Petri dishes and stored at a constant temperature of 21 °C. Visual observations of spoilage (e.g., mold growth, color changes) were recorded daily over a period of 16 days.
3. Results and Discussion
3.1. Morphology of Polymer Films
PLA and PLA-C prepared films appeared with almost identical thickness, around 160 ± 10 μm. The surface structure was observed by SEM microscopy, and some representative images are given in Figure 2. The presented images of the polymer film’s surface showed that the C-extract was well-distributed in the PLA, independently of the dry extract content. Some small imperfections (present in all films) are characteristic of the preparation of polymer films by the solvent casting method. These data, together with the almost constant thickness of all films, clearly show that the extract molecules were well-distributed and mixed together with the polymer matrix. Similar observations were reported by several authors [45,46], where curcumin was well-distributed throughout the PLA macromolecules.

Figure 2.
SEM images of polymer films surface (a) PLA, (b) PLA-C-2, (c) PLA-10 [bar scale: 10 μm].
3.2. FTIR Spectroscopy Analysis
FTIR spectroscopy was used to analyze the molecular structure of PLA films and C-extract molecules. The FTIR spectra of pure C dried extract, PLA film, and PLA-C films are shown in Figure 3A,B. Curcumin has a complex structure with interesting functionalities, such as hydroxyl and carbonyl groups. In a previous study, it was shown that the curcumin extract consisted of three curcuminoids: bisdemethoxycurcumin, demetoxycurcumin, and curcumin, with a total content of 1.12 w/w%, where curcumin was detected with the highest concentration of 0.78 w/w% [32]. Using FTIR spectroscopy, it is very difficult to distinguish all of the components present in the extract, since they have very similar (almost identical) chemical structures [47].
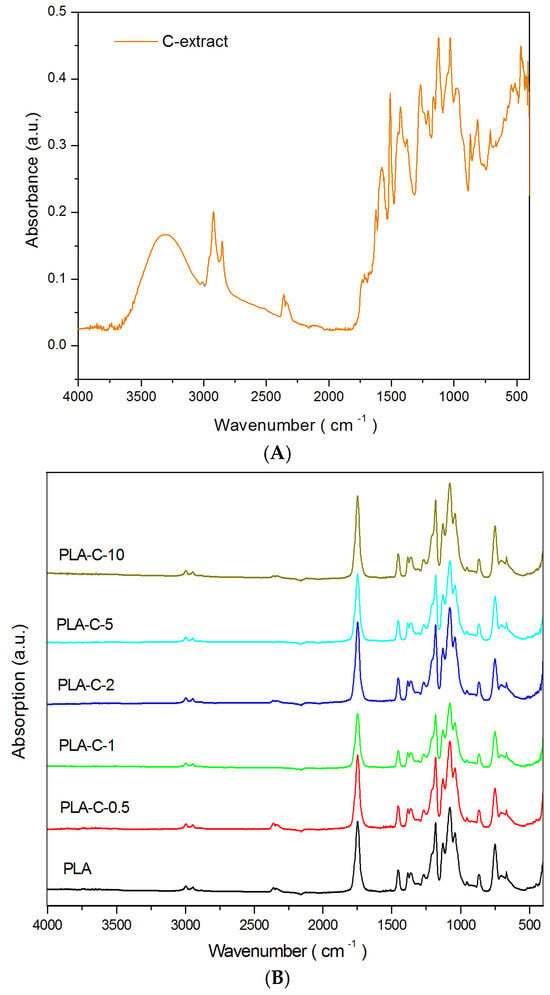
Figure 3.
(A). FTIR spectrum of dried C-extract. (B). FTIR spectra of PLA and PLA-C films.
The FTIR spectrum of dried C-extract (Figure 3A) showed an intensive absorption band around 3300 cm−1 due to the presence of OH groups. The two bands at 2916 cm−1 and 2846 cm−1 are related to the aliphatic stretching vibrations of C-H in methoxy O-CH3 groups. All other characteristic peaks related to the stretching vibrations of C=C that are relevant to the aromatic ring are positioned at 1618, 1570, 1502, and 1450 cm−1 [48,49].
Pure PLA film exhibited the main peak at 1752 cm−1, characteristic of the stretching vibration of the carbonyl group. The weak bands at 2947 and 2855 cm−1 are connected to the asymmetric and symmetric stretching vibrations of C-H in the methylene group [50], while the deformation vibrations of the same group are correlated with the peaks at 1454 and 1383 cm−1 [51]. The intensive band at 1180 cm−1 is indicative of the C-O-C stretching vibration [50,51]. FTIR spectra, relevant for PLA-C films, showed that the incorporation of C-extract did not cause any significant shift of the characteristic bands of PLA and C-extract, except for the intensity changes. This is probably due to the fact that the presence of carbonyls, as pendant chemical groups on the backbone chain, is usually inert to chemical modifications [52]. This apparent non-shift of the bands may further suggest that curcumin extract was well-distributed and mixed with PLA chains, as confirmed by SEM analysis. The slight intensity changes of the characteristic bands are in favor of the physical interactions between the PLA and the C-extract.
The bands positioned at 1180 cm−1 and 1383 cm−1 are usually indicative of the estimation of the crystallinity index of the PLA [53], defined as (Equation (4)):
where I1180 and I1383 are the intensities of the corresponding bands. The summarized CI indices (Table 1) showed that the highest CI value was obtained for the PLA-C-10 polymer film, while the increased content of the C-extract caused slightly increased CI values. This clearly supports the hypothesis that the presence of C extract molecules affects the crystallization behavior of PLA toward higher crystallinity.
CI = I1180/I1383

Table 1.
Crystallinity indices and DSC data.
3.3. Thermal Behavior of Polymer Films
The DSC curves are shown in Figure 4, and all relevant data derived from the measurements are summarized in Table 1. Only the first heating runs (without erasing the thermal history) were evaluated, in order to present the actual structural behavior of the obtained films and to link these results with the other measured properties. The DSC curve of the pure PLA film was characterized by a glass transition temperature at 47 °C, followed by cold crystallization exo peaks (at 76 °C and 94 °C) and a melting endotherm around 172 °C with a shoulder peak at 164 °C. The increased content of the C extract influenced the lowering of Tg (up to 35 °C for the PLA-C-10 film) and the decrement of cold crystallization peaks, confirming the plasticizing effect of the C extract. Similar data were reported in the literature, confirming the plasticizing effect and good distribution of curcumin within the polymer chains [45,54]. The melting peak in all investigated films was around 171 °C, accompanied by a shoulder peak showing slightly decreased values in the PLA-C-10 film. The estimated degrees of crystallinity increased with the increasing C-extract content. In addition to the plasticizing effect, C-extract molecules caused the arrangement of the PLA chains and acted as a nucleating agent [55].
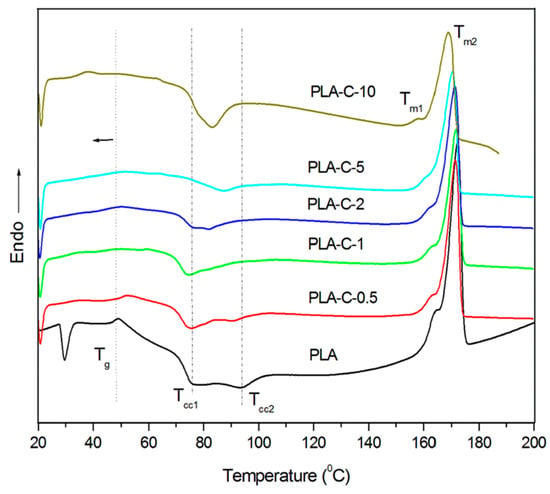
Figure 4.
DSC thermograms of PLA and PLA/C extract films.
3.4. Color Properties and Film Transmittance
The pure PLA film was visually the most transparent compared to all other investigated films (Figure 5). They are smooth and flexible, with a uniform film surface, indicating that the distribution of the filler in the matrix is uniform. This was additionally confirmed by the measurements of the UV/VIS spectra of the polymer films in the wavelength range between 190 and 800 nm. The highest transmittance at λ= 660 nm was determined for pure PLA film. The increased content of C-extract in the corresponding films caused the expected decrease in the transmittance of up to 25% for the PLA-C-10 film (Table 2). Similar results of the light transmittance reduction were obtained when incorporating curcumin into different polymer films [15,56,57].
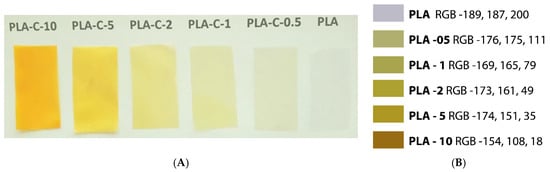
Figure 5.
(A). PLA films examined in this study. (B). RGB coordinates for each of the examined composite films (obtained by use of software: https://colormine.org).

Table 2.
Apparent color, light transmittance, and water vapor permeability data of PLA and PLA-C films.
Table 2 presents data for the color attributes of examined neat and composite PLA films. When the curcumin was added to the film, the lightness (L*) reduced drastically. As predicted, the a* (greenness, +redness) and b* (blueness, +yellowness) values of the PLA films were affected, too. Namely, the incorporation of curcumin into PLA film resulted in a decrease in the a* value, while there was a significant increase in the b*-value, owing to curcumin’s yellow color (Figure 5), which is confirmed by other researchers as well [15].
3.5. Water Contact Angles and Water Vapor Permeability
The contact angle measurements of all polymer films are presented in Figure 6. As it was found, PLA showed a low hydrophilic character, with a contact angle of 75.8 ± 1.2°. The WCA of the polymer films loaded with C extract showed an increase in the measured values (up to 88.9 ± 1.4°) due to the addition of C extract. These increased values were predominantly due to the hydrophobic nature of the curcuminoid molecules. The results demonstrate that the addition of the C extract would result in a decreased surface wettability of the polymer films. Similar observations were noted in the literature [58,59].
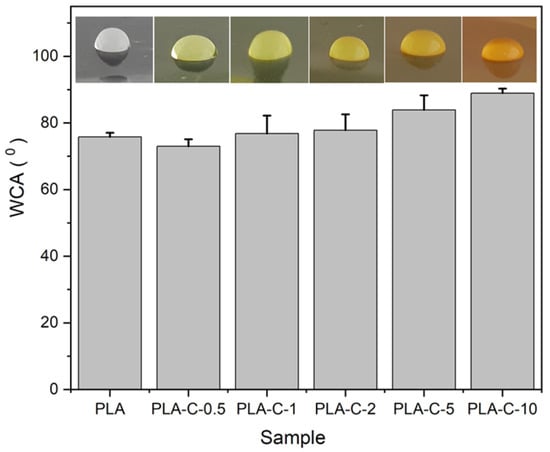
Figure 6.
WCA data of PLA and PLA-C films.
This increased hydrophobic character of the polymer film was expected to have a certain influence on the water vapor permeability, a property that is very important when evaluating polymer films used for food packaging. All results related to the WVP measurements are collected in Table 2. It could be detected that pure PLA film has the highest WVP compared to all other films with C extract. A significant reduction in WVP (for almost 43%) in the PLA-C-10 film was in perfect accordance with the data obtained from the contact angle measurements. The literature data also showed that the incorporation of curcumin improved the barrier properties of the polymer films due to their increased hydrophobicity [3,60,61]. In addition, the PLA loaded with other natural compounds, like thymol or cury, has shown a similar behavior decrease of WVP due to the addition of the active agent [44].
3.6. Photodegradation of Polymer Films
The materials used in food packaging are usually exposed to external influences, such as temperature and UV exposure. The photodegradation of the polymer film was followed within 14 days and is presented in Figure 7, as mass changes of the polymer film versus time of UV exposure. It could be noted that the pure PLA film degraded by 2.4% for the whole period of UV exposure, and these data are in accordance with the previously published results [62]. The addition of extract of various contents caused a decrease in PLA photodegradation (up to 1.25% for TPU-C-10). This behavior could be related to the phenolic structure of all curcuminoids present in the extract, which acts as a UV stabilizer of the PLA. This positive effect of the curcumin present in polymer films has already been noted in the scientific literature [63].
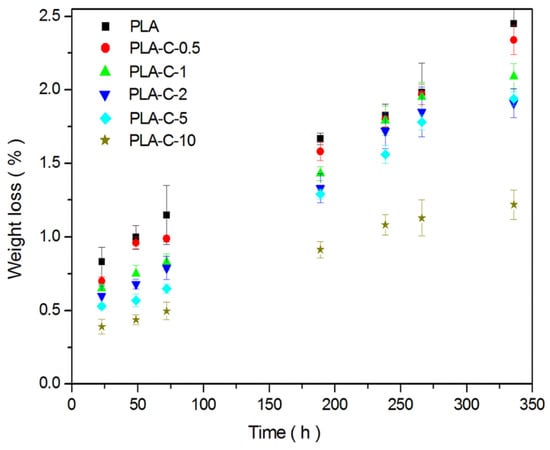
Figure 7.
Photodegradation behavior of PLA and PLA-C films.
3.7. Curcumin Release Profile into Food Simulant B
The release of curcumin from polymer films was monitored in 3% (w/v) acetic acid (simulant B), which ideally reflects the acidic environment of hard cheeses. The data were presented as C/C0 (%), where C is the released concentration of curcumin and C0 is the initial concentration of curcumin in the corresponding films (Figure 8). It is evident that, in films with low curcumin content (PLA-C-0.5, PLA-C-1, and PLA-C-2), no release can be observed in the first 48 h. Curcumin release was observed in these samples after 72 h, but the values were quite low, up to 0.89%. In the entire time interval, in the PLA-C-5 and PLA-C-10 samples, curcumin release of up to 1.36% was observed. The limited release of incorporated C extract is primarily due to the low degree of swelling of PLA films (between 0.08% and 1.32%) in the corresponding simulant. In addition to the degree of swelling, the hydrophobic nature of curcuminoids also has a significant impact on the release profile, resulting in their limited diffusion and dissolution in aqueous solutions. Similar results were observed in the literature, confirming the slow release of curcumin in the proposed food simulant [3]. Another study [44] proposed that active compounds are well-embedded in PLA film by the casting method, which causes an easier release of the active component. Namely, the release kinetics depend on many factors, like the polymer matrix and the filler nature, the interaction between them, the swelling of the polymers, the rate of dissolution and diffusion of the compound in the polymer film, and the solubility of the compound in the immersion solution [3].
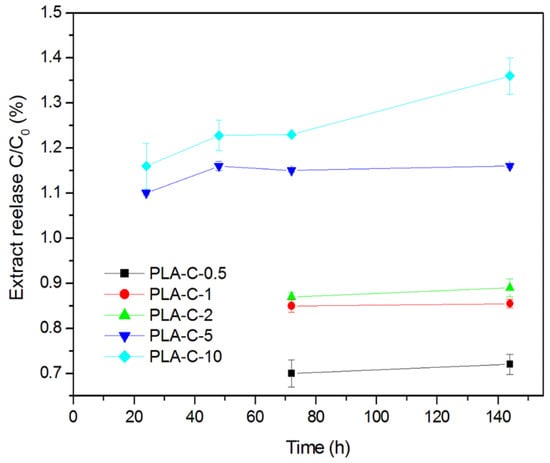
Figure 8.
Curcumin release in C/C0 (%) related to its initial concentration (C0) incorporated in polymer films.
3.8. Antimicrobial Properties of PLA Films
After the disk diffusion assay using PLA films, none of the tested PLA films produced a visible inhibition zone around the disks for any of the microorganisms. However, for the molds, no growth was observed directly beneath the disks, including the PLA film without curcuma extract. This suggests that, in addition to antimicrobial compounds originating from the curcuma extract, physical factors such as reduced oxygen availability beneath the films may have also contributed to the inhibition of mold growth.
In contrast, bacterial growth was observed beneath the PLA films without extract and those containing 0.5%, 1.0%, and 2.0% curcuma extract. PLA films with 5.0% and 10.0% curcuma extract prevented bacterial growth beneath the disks, indicating that the antimicrobial effect at higher extract concentrations is likely due to direct contact between the film and the microorganisms. These observations are consistent with the results of the migration tests, which showed that PLA films containing 5.0% and 10.0% curcuma extract exhibited the highest migration of active components into the simulant. Similar observations were presented by other authors [3], where a higher concentration of curcumin in the PBAT matrix showed better antibacterial activity.
Natamycin, used as a positive control, exhibited significant antifungal activity against molds, weaker activity against yeast, and no observable activity against bacteria. These results are in accordance with previous findings indicating that Natamycin, a secondary metabolite produced by certain Streptomyces species, is specifically effective against fungi in various food matrices [64]. Its specific antifungal mechanism involves binding to sterols in fungal cell membranes, thereby disrupting their integrity and function [65].
The absence of inhibition zones around the disks suggests that the antimicrobial compounds did not diffuse into the surrounding agar but acted through direct contact [44]. This finding aligns with previous reports that the antimicrobial activity of the PLA films incorporated with plant extracts is primarily contact-mediated. For instance, Sun et al. (2023) [66] demonstrated the antimicrobial activity of cinnamon oil-loaded PLA/chitosan films using immersion methods, highlighting the need for direct surface interaction rather than diffusion-based inhibition. Espinel-Ingroff et al. (2002) [67] also reported that certain antifungal agents, like caspofungin, showed no inhibition zones in standard diffusion assays, despite strong activity in broth cultures, indicating that the compound’s efficacy may depend on the direct interaction with fungal cells.
Similarly, the suppression of mold growth beneath all films, including those without curcuma extract, highlights the contribution of environmental factors, particularly reduced oxygen availability.
In the case of bacterial isolates, the complete absence of growth under PLA films with 5.0% and 10.0% curcuma extract confirms a dose-dependent antimicrobial effect. These results highlight the potential of curcuma-loaded PLA films as active packaging materials for controlling microbial contamination in dairy products, although the optimization of extract concentration and film properties would be necessary for broader applications. To complement these findings, the minimal inhibitory concentration (MIC) determination of turmeric released from the PLA matrix would be a valuable next step, providing quantitative insight into the bioavailable antimicrobial fraction and helping to distinguish the contact effects from release-based activity.
3.9. Accelerated Shelf-Life Testing of PLA Films on White Cheese
Visible spoilage of the cheese sample pieces was observed during storage. The most significant changes occurred in the cheese samples that were not layered with PLA films. By the sixth day, the cheese surface was largely covered with white colonies morphologically resembling yeast (Figure 9). Color changes to a yellowish hue were also evident, and by the end of the storage period (day 16), the texture of the cheese was completely disrupted (Figure 10).

Figure 9.
White cheese without PLA film after 6 days of storage at 21 °C.
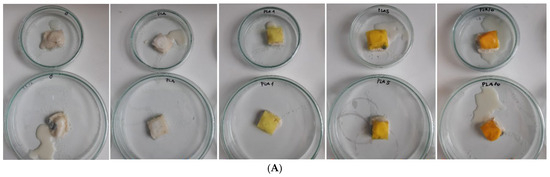
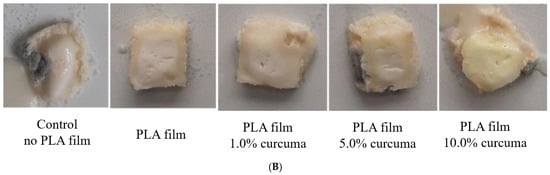
Figure 10.
White cheese samples layered with PLA films after 16 days of storage at 21 °C: view of the samples with the biofilms (A) and after removal of the films and zoomed for better visualization of the condition (B).
No noticeable difference in the spoilage rate (microbial growth, color, or texture changes) was observed between the PLA films with and without curcuma extracts (Figure 10). All cheese pieces covered with PLA films retained their white color, except at the edges where the film ended, and yellowing occurred. No visible microbial colonies (yeast or mold) appeared on the contact surfaces throughout the storage period. Texture degradation was minimal, except for the cheese pieces covered with PLA films containing 10.0% curcuma extract, where noticeable texture disruption was recorded. This could be attributed to the increased brittleness and decreased flexibility of the films with higher curcuma content, which may have reduced contact between the film and the cheese surface, allowing localized decomposition.
These results suggest that the mechanical and barrier properties of the PLA films played a crucial role in protecting the cheese from spoilage. Although the antimicrobial activity of curcuma extract has been extensively documented [23,68], in this case, its effects appear to have been overlapped by the strong physical protection provided by the films. On the other hand, a clear antimicrobial effect was observed against pathogenic bacteria such as S. aureus and E. coli, demonstrating that the combination of physical and antimicrobial protection was achieved. It can be concluded that PLA films containing 1.0–2.0% curcuma extract provided an optimal balance, with a great potential for significantly extending the shelf life of white cheese.
4. Conclusions
PLA–Curcuma lunga L. films with different content of C extract (0.5–10 wt%) were prepared by the solution casting method. C extract was well distributed within the polymer matrix. Thermal analysis showed that C extract has a plasticizing and nucleating effect on PLA molecules. Reduced moisture content, increased barrier properties, and limited photodegradation (up to 1.25%) were confirmed with an increasing C extract content in the polymer films. The release of the extract in a simulated acidic environment was limited and ranged up to 1.36% for PLA-C-10.
Although the films containing 5.0% and 10.0% curcumin extract exhibited the strongest antibacterial activity, better preservation of cheese quality was observed with films containing 1.0% to 2.0% extract. This discrepancy may be attributed to the increased rigidity and reduced flexibility of the films at higher extract concentrations, which likely compromised their contact with the cheese surface. Therefore, further optimization of the film composition is recommended to develop more elastic formulations that ensure improved adhesion to the product and enhanced preservation performance.
Author Contributions
Conceptualization, A.B. and A.T.P.; methodology, A.B. and D.D.; formal analysis, A.B. and D.D.; investigation, A.B.; resources, A.B., D.D. and A.T.P.; writing—original draft preparation, A.B.; writing—review and editing, A.B., D.D. and A.T.P.; visualization, A.B. and D.D.; supervision, A.B. and A.T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All experimental data are available upon request from the authors.
Acknowledgments
The authors would like to thank the Institute for Public Health of the Republic of North Macedonia for kindly providing the Escherichia coli and Staphylococcus aureus strains used in this study. Their support and contribution were essential to the successful completion of the microbiological analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hahladakis, J.N.; Iacovidou, E.; Gerassimidou, S. Plastic waste in a circular economy. In Plastic Waste and Recycling; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-817880-5. [Google Scholar] [CrossRef]
- Sinha, R.K.; Kumar, R.; Phartyal, S.S.; Sharma, P. Interventions of citizen science for mitigation and management of plastic pollution: Understanding sustainable development goals, policies, and regulations. Sci. Total Environ. 2024, 955, 176621. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Curcumin Incorporated Poly(Butylene Adipate-co-Terephthalate) Film with Improved Water Vapor Barrier and Antioxidant Properties. Materials 2020, 13, 4369. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, F.D.B. The role of plastic concerning the sustainable development goals: The literature point of view. Clean. Responsible Consum. 2021, 3, 100020. [Google Scholar] [CrossRef]
- Walker, T.R. (Micro)plastics and the UN Sustainable Development Goals. Curr. Opin. Green Sustain. Chem. 2021, 30, 100497. [Google Scholar] [CrossRef]
- Gonçalves, A.; Cardeal, G.; Henriques, E.; Ribeiro, I. Sustainable Value Roadmap for the Plastics Industry. In Proceedings of the 31st CIRP Conference on Life Cycle Engineering (LCE 2024), Turin, Italy, 9–21 June 2024. [Google Scholar]
- Kumar, N.; Pratibha Prasad, J.; Yadav, A.; Upadhyay, A.; Neeraj; Shukla, S.; Petkoska, A.T.; Heena; Suri, S.; Gniewosz, M.; et al. Recent Trends in Edible Packaging for Food Applications—Perspective for the Future. Food Eng. Rev. 2023, 15, 718–747. [Google Scholar] [CrossRef]
- DS/EN 16575:2014; Bio-Based Products—Vocabulary. ANSI: Washington, DC, USA, 2014.
- Jain, A.; Mehta, A.; Jain, S. Antimicrobial Property of Turmeric: A systemic Review. NMJ 2022, 5, 611–614. [Google Scholar] [CrossRef]
- Yusoff, N.H.; Pal, K.; Narayanan, T.; de Souza, F.G. Recent trends on bioplastics synthesis and characterizations: Polylactic acid (PLA) incorporated with tapioca starch for packaging applications. J. Mol. Struct. 2021, 1232, 129954. [Google Scholar] [CrossRef]
- Jacob, J.; Lawal, U.; Thomas, S.; Valapa, R.B. Biobased polymer composite from poly(lactic acid): Processing, fabrication, and characterization for food packaging. In Processing and Development of Polysaccharide-Based Biopolymers for Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Mohan, S.; Panneerselvam, K. A short review on mechanical and barrier properties of polylactic acid-based films. Mater. Today Proc. 2022, 56, 3241–3246. [Google Scholar] [CrossRef]
- Waisarikit, A.; Suadaung, N.; Khantho, B.; Hadad, B.; Ross, G.M.; Topham, P.D.; Ross, S.; Mahasaranon, S. Extracted Spent Coffee Grounds as a Performance-Enhancing Additive for Poly(Lactic Acid) Biodegradable Nursery Bags in Agriculture. Polymers 2025, 17, 561. [Google Scholar] [CrossRef]
- Mondal, K.; Soundararajan, N.; Goud, V.V.; Katiyar, V. Cellulose Nanocrystals Modulate Curcumin Migration in PLA-Based Active Films and Its Application as Secondary Packaging. ACS Sustain. Chem. Eng. 2024, 12, 9642–9657. [Google Scholar] [CrossRef]
- Subbuvel, M.; Kavan, P. Preparation and characterization of polylactic acid/fenugreek essential oil/curcumin composite films for food packaging applications. Int. J. Biol. Macromol. 2022, 194, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Daniloski, D.; D’cunha, N.M.; Naumovski, N.; Petkoska, A.T. Pomegranate peel extract—A natural bioactive addition to novel active edible packaging. Food Res. Int. 2022, 156, 111378. [Google Scholar] [CrossRef] [PubMed]
- Al-Hilifi, S.A.; Al-Ali, R.M.; Petkoska, A.T. Ginger Essential Oil as an Active Addition to Composite Chitosan Films: Development and Characterization. Gels 2022, 8, 327. [Google Scholar] [CrossRef]
- Al-Hilifi, S.A.; Al-Ali, R.M.; Al-Ibresam, O.T.; Kumar, N.; Paidari, S.; Trajkovska Petkoska, A.; Agarwal, V. Physicochemical, Morphological, and Functional Characterization of Edible Anthocyanin-Enriched Aloevera Coatings on Fresh Figs (Ficus carica L.). Gels 2022, 8, 645. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, S.; Li, N.; Lin, D. Curcumin: A Magical Small Molecule with a Large Role in Active-Intelligent Degradable Food Packaging. Int. J. Mol. Sci. 2025, 26, 3917. [Google Scholar] [CrossRef]
- Younis, M.; Alhamdan, A.; El-Abedein, A.I.Z.; Mohamed Ahmed, I.A.; Kamel, R.M.; Salama, M.A.; Abdelkarim, D.O.; Elsayed, M. Incorporation of safflower extract into sodium alginate and polyvinyl alcohol films: Impact on physicochemical properties and food packaging applications. Int. J. Food Sci. Technol. 2025, 60, vvaf072. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.; Egea, M.B. Edible Bioactive Film with Curcumin: A Potential “Functional” Packaging? Int. J. Mol. Sci. 2022, 23, 5638. [Google Scholar] [CrossRef]
- Bisht, S.; Gaikwad, K.K. Natural Pigments or Dyes for Sustainable Food Packaging Application. Food Bioprocess Technol. 2025, 18, 4301–4325. [Google Scholar] [CrossRef]
- Buniowska-Olejnik, M.; Mykhalevych, A.; Urbański, J.; Berthold-Pluta, A.; Michałowska, D.; Banach, M. The potential of using curcumin in dairy andmilk-based products—A review. J. Food Sci. 2024, 89, 5245–5254. [Google Scholar] [CrossRef]
- Roy, S.; Jong-Whan, R. Antioxidant and antimicrobial poly(vinyl alcohol)-based films incorporated with grapefruit seed extract and curcumin. J. Environ. Chem. Eng. 2021, 9, 104694. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Sar, T.; Kiraz, P.; Braho, V.; Harirchi, S.; Akbas, M.Y. Novel Perspectives on Food-Based Natural Antimicrobials: A Review of Recent Findings Published since 2020. Microorganisms 2023, 11, 2234. [Google Scholar] [CrossRef] [PubMed]
- Botalo, A.; Inprasit, T.; Ummartyotin, S.; Chainok, K.; Vatthanakul, S.; Pisitsak, P. Smart and UV-Resistant Edible Coating and Films Based on Alginate, Whey Protein, and Curcumin. Polymers 2024, 16, 447. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Jong-Whan, R. Curcumin and its uses in active and smart food packaging applications—A comprehensive review. Food Chem. 2022, 375, 131885. [Google Scholar] [CrossRef]
- Odo, E.O.; Ikwuegbu, J.A.; Obeagu, E.I.; Chibueze, S.A.; Ochiaka, R.E. Analysis of the antibacterial effects of turmeric on particular bacteria. Medicine 2023, 102, e36492. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective Properties of the Golden Spice Curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpinski, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Chiu, I.; Yang, T. Biopolymer-based intelligent packaging integrated with natural colourimetric sensors for food safety and sustainability. Anal. Sci. Adv. 2024, 5, e2300065. [Google Scholar] [CrossRef]
- Said, N.S.; Lee, W.Y. Pectin-Based Active and Smart Film Packaging: A Comprehensive Review of Recent Advancements in Antimicrobial, Antioxidant, and Smart Colorimetric Systems for Enhanced Food Preservation. Molecules 2025, 30, 1144. [Google Scholar] [CrossRef]
- Papadimitrioua, A.; Ketikidisa, I.; Stathopouloua, M.-E.K.; Bantia, C.N.; Papachristodoulou, C.; Zoumpoulakis, L.; Agathopoulos, S.; Vagenase, G.V.; Hadjikakou, S.K. Innovative material containing the natural product curcumin, with enhanced antimicrobial properties for active packaging. Mater. Sci. Eng. C 2018, 84, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Demircan, B.; McClements, D.J.; Velioglu, Y.S. Next-Generation Edible Packaging: Development of Water-Soluble, Oil-Resistant, and Antioxidant-Loaded Pouches for Use in Noodle Sauces. Foods 2025, 14, 1061. [Google Scholar] [CrossRef]
- De Campos, S.S.; de Oliveira, A.; Moreira, T.F.M.; da Silva, T.B.V.; da Silva, M.V.; Pinto, J.A.; Bilck, A.P.; Gonçalves, O.H.; Fernandes, I.P.; Barreiro, M.-F.; et al. TPCS/PBAT blown extruded films added with curcumin as a technological approach for active packaging materials. Food Pack. Shelf Life 2019, 22, 100424. [Google Scholar] [CrossRef]
- Bužarovska, A.; Stanoeva, J.P.; Karamanolevski, P.; Popa, A.D.; Dinescu, S.; Avérous, L. Thermoplastic Polyurethane/Polylactic Acid Blend Foams Loaded with Curcuma longa L. and Hypericum perforatum Extracts Towards Wound Dressing Applications. J. Appl. Polym. Sci. 2025, 142, e56708. [Google Scholar] [CrossRef]
- Garlotta, D.A. Literature review of poly (lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- ASTM E96/E96M-12; Standard Test Methods for Water Vapor Transmission of Materials. Annual Book of Standards. ASTM International: Brussels, Belgium, 1993; Volume 04.06.
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—9th ed.; Clinical and Laboratory Standards Institute Document M2-A9; CLSI (1)—Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006; ISBN 1-56238-586-0.
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Mohamad, N.; Mazlan, M.M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Fouad, H.; Jawaid, M. Development of active agents filled polylactic acid films for food packaging application. Int. J. Biol. Macrom. 2020, 163, 1451–1457. [Google Scholar] [CrossRef]
- Akshaykranth, A.; Jayarambabu, N.; Kumar, A.; Venkatappa Rao, T.; Rakesh Kumar, R.; Srinivasa Rao, L. Novel nanocomposite polylactic acid films with Curcumin-ZnO: Structural, thermal, optical and antibacterial properties. Curr. Res. Green Sustain. Chem. 2022, 5, 100332. [Google Scholar] [CrossRef]
- Roy, S.; Jong-Whan, R. Preparation of bioactive functional poly(lactic acid)/curcumin composite film for food packaging application. Int. J. Biol. Macrom. 2020, 162, 1780–1789. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Rakib, A.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Shahid-Ud-Daula, A.F.M.; Amin, M.N.; Simal-Gandara, J. The Genus Curcuma and Inflammation: Overview of the Pharmacological Perspectives. Plants 2021, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mittal, A.; Puri, V.; Kumar, P.; Singh, I. Curcumin-Loaded, Alginate–Gelatin Composite Fibers for Wound Healing Applications. 3 Biotech 2020, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H.; Illias, H.A.; Ching, K.Y.; Singh, R.; Nai-Shang, L. Influence of a Nonionic Surfactant on Curcumin Delivery of Nanocellulose Reinforced Chitosan Hydrogel. Int. J. Biol. Macrom. 2018, 118, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-X.; Jin, Y.-J.; Meng, Q.-Y.; Wang, L.; Zhang, M.; Wang, Y.-Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Gong, X.; Pan, L.; Tang, C.Y.; Chen, L.; Hao, Z.; Law, W.-C.; Wang, X.; Tsui, C.P.; Wu, C. Preparation, optical and thermal properties of CdSe–ZnS/poly(lactic acid) (PLA) nanocomposites. Compos. Part B Eng. 2014, 66, 494–499. [Google Scholar] [CrossRef]
- Baran, E.H.; Erbil, H.Y. Surface Modification of 3D Printed PLA Objects by Fused Deposition Modeling: A Review. Colloids Interfaces 2019, 3, 43. [Google Scholar] [CrossRef]
- Blomergen, S.; Holden, D.; Hamer, G.; Bluhm, T.; Marchessault, R. Studies of composition and crystallinity of bacterial poly(β-hydroxybutyrate-co-β-hydroxyvalerate. Macromolecules 1986, 19, 2865–2871. [Google Scholar] [CrossRef]
- Rojas, A.; Velásquez, E.; Patiño Vidal, C.; Guarda, A.; Galotto, M.J.; López de Dicastillo, C. Active PLA Packaging Films: Effect of Processing and the Addition of Natural Antimicrobials and Antioxidants on Physical Properties, Release Kinetics, and Compostability. Antioxidants 2021, 10, 1976. [Google Scholar] [CrossRef]
- Du, Y.; Wu, T.; Yan, N.; Kortschot, M.T.; Farnood, R. Fabrication and characterization of fully biodegradable natural fiber-reinforced poly(lactic acid) composites. Compos. Part B 2014, 56, 717–723. [Google Scholar] [CrossRef]
- Xie, Q.; Zheng, X.; Li, L.; Ma, L.; Zhao, Q.; Chang, S.; You, L. Effect of Curcumin Addition on the Properties of Biodegradable Pectin/Chitosan Films. Molecules 2021, 26, 2152. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Characterization of Chitosan Film Incorporated with Curcumin Extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Chiaoprakobkij, N.; Suwanmajo, T.; Sanchavanakit, N.; Phisalaphong, M. Curcumin-Loaded Bacterial Cellulose/Alginate/Gelatin as A Multifunctional Biopolymer Composite Film. Molecules 2020, 25, 3800. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Shen, C.; Deng, Z.; Wu, D.; Chen, K. Solution blow spinning of multilayer polycaprolactone/curcumin-loaded gelatin/polycaprolactone nanofilm for slow release and bacterial inhibition. Food Hydrocoll. Health 2022, 2, 100062. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Preparation of antibacterial poly(lactide)/poly(butylene adipate-co-terephthalate) composite films incorporated with grapefruit seed extract. Int. J. Biol. Macromol. 2018, 120, 846–852. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Imran, M.; Lian, Q.; Shehzad, F.K.; Athir, N.; Zhang, J.; Cheng, J. Curcumin incorporated polyurethane urea elastomers with tunable thermo-mechanical properties. React. Funct. Polym. 2018, 128, 97–103. [Google Scholar] [CrossRef]
- Buzarovska, A.; Grozdanov, A. Biodegradable poly(L-lactic acid)/TiO2 nanocomposites: Thermal properties and degradation. J. Appl. Polym. Sci. 2012, 123, 2187–2193. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, T.; Zhang, W.; Zhong, Y.; Fang, S.; Wang, G.; Li, Y.; Deng, Y.; Liu, X.; Li, H. Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability. e-Polymers 2023, 23, 20230009. [Google Scholar] [CrossRef]
- Serafini, K.F.C.; Alencar, E.R.; Ribeiro, J.L.; Ferreira, M.D.A. Influence of the salt concentration on action mechanisms of natamycin against microorganisms of importance in food manufacture. Food Sci. Technol. 2019, 40, 6–11. [Google Scholar] [CrossRef]
- Ciesielski, F.; Griffin, D.C.; Loraine, J.; Rittig, M.; Delves-Broughton, J.; Bonev, B.B. Recognition of Membrane Sterols by Polyene Antifungals Amphotericin B and Natamycin, A (13)C MAS NMR Study. Front. Cell. Dev. Biol. 2016, 4, 57. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Zhang, Y.; Yan, S.; Zhu, L.; Zhang, X. Development of active antibacterial CEO/CS@PLA nonwovens and the application on food preservation. ACS Omega 2023, 8, 42911–42920. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Canton, E.; Peman, J.; Pelaez, T. Activity of caspofungin against clinical isolates of Fusarium spp.: Lack of correlation between disk diffusion zones and MICs. Antimicrob. Agents Chemother. 2002, 46, 3084–3087. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Zhang, Y.; Gao, B.; Li, Y.; He, X.; Sun, J.; Choe, U.; Chen, P.; Blaustein, R.A.; et al. Chemical Composition of Turmeric (Curcuma longa L.) Ethanol Extract and Its Antimicrobial Activities and Free Radical Scavenging Capacities. Foods 2024, 13, 1550. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).