Abstract
A green and mild chemical reaction of calcium citrate (CC) was successfully prepared from reactions between mussel shell waste and citric acid in the presence of acetone (AC), ethanol (Et), and isopropyl alcohol (IPA). All the synthesized CCs contained the same functional groups such as citrate (C6H5O73−), water (H2O), and calcium–oxygen (Ca–O). However, the differences in the spectra pointed out the differences in the crystal environment and structure of CCs. CC-AC and CC-IPA mainly crystallized in the monoclinic [Ca3(C6H5O7)2(H2O)2]·2H2O crystal system, whereas CC-Et mainly crystallized in the triclinic Ca3(C6H5O7)2∙(H2O)4 structure. The molecular alignments of triclinic CC-Et were different from monoclinic CC-AC and CC-IPA, resulting in differences in thermal behaviors. Two dehydration steps were observed for the monoclinic CC-AC and CC-IPA, whereas the triclinic CC-Et showed a single dehydration process. The TG mass losses further demonstrated that anhydrous Ca3(C6H5O7)2 phase, in addition to the Ca3(C6H5O7)2∙4H2O, was also observed for CC-AC and CC-IPA, whereas CC-Et contained a single Ca3(C6H5O7)2∙(H2O)4 phase. The morphologies of CC-AC and CC-IPA also differed from that of CC-Et. The differences in some properties of the synthesized CCs could be attributed to the change in the supersaturation state of the reaction solution. Due to the superior polarity, ethanol is more compatible with citric acid. The presence of ethanol could suppress the supersaturation rate of the reaction solution, causing the modulation of the precipitation mechanisms and reducing the particle growth rate of CC-Et, thereby explaining the difference in vibrational, structural, thermal, and morphological characteristics of CC-Et, compared to CC-AC and CC-IPA.
1. Introduction
Calcium citrate has been used as a calcium supplement [1] and an anti-inflammatory [2]. Compared to calcium carbonate (CaCO3), calcium citrate shows more potential in the medical field due to its enhanced bioavailability and absorption in patients with superior metabolism [3]. It has been utilized to provide sufficient calcium for the elderly for osteoporosis prevention [4,5]. Calcium citrate has been studied and employed as a bone repair material because of its biocompatibility and high concentrations of calcium ions, compared to CaCO3 [5,6]. Calcium citrate is combined with recombinant human bone morphogenetic-2 (rhBMP-2) to enhance bone regeneration effectively in bone defects [7]. Calcium citrate can positively stimulate fracture healing in white rabbits during the early time point when defects are not yet too large, because calcium citrate may resorb faster than calcium phosphate, such as hydroxyapatite (Ca10(PO4)6(OH)2) [8].
Calcium citrates with different physicochemical properties have been produced using different citrate-ion (C6H5O73−) sources, such as citric acid (C6H8O7) and sodium citrate (Na3C6H5O7), in the presence of various calcium sources, such as CaCO3, calcium nitrate tetrachloride (Ca(NO3)2·4H2O), and calcium chloride (CaCl2) [6]. However, CaCO3, derived from discarded materials such as oyster shells [2] and eggshells [9], has been employed because of its low cost and high availability. The differences in the physicochemical properties of calcium citrate were also observed from different synthetic conditions [1]. Needle-shaped crystals of Ca3(C6H5O7)2·4H2O or [Ca3(C6H5O7)2(H2O)2]·2H2O, which is the hydrated form of calcium citrate (Ca3(C6H5O7)2), were obtained through hydrothermal synthesis [10]. For the synthetic process, the components, including CaCO3, Ca(NO3)2·4H2O, anhydrous citric acid (C6H8O7 powder), and DI water, were placed in a Teflon and tightly sealed and then thermally treated at 160 °C for 72 h. Reactive crystallization was applied to prepare calcium citrate using citric acid (C6H8O7) and CaCO3 as reactants [1]. The supersaturation point occurred during the reaction, thereby forming hydrate ([Ca3(C6H5O7)2(H2O)2]·2H2O) and/or anhydrous (Ca3(C6H5O7)2) calcium citrates, depending on the reactants’ ratio (C6H8O7:CaCO3). [Ca3(C6H5O7)2(H2O)2]·2H2O (earlandite) was obtained through the dissolution of CaCO3 and subsequent crystallization by the fungus Trichoderma asperellum BDH65 [11].
The precursor precipitation method, followed by low-temperature treating, was used for the production of [Ca3(C6H5O7)2(H2O)2]·2H2O [5]. The salt solution, containing Na3C6H5O7 and CaCl2, was maintained at 25 °C. The reaction solution was allowed to achieve the supersaturation point, obtaining the precipitated precursor, which was then soaked with water or a mixture of ethanol (CH3CH2OH) and water. Fixing the CH3CH2OH/H2O volume ratio (2:1), the wire [Ca3(C6H5O7)2(H2O)2]·2H2O crystals were obtained when the mixture was treated at 90 °C with varying aging times of 0, 2, 4, 6, and 8 h. This synthesis technique was also reported by Li et al. [6]. The researchers observed that adding ethanol to the salt solution resulted in precursor precipitation. Three different volume ratios between CH3CH2OH and H2O of 1:2, 1:1, and 2:1 were used for the precipitation of [Ca3(C6H5O7)2(H2O)2]·2H2O. The results reported by Zhong et al. [5] and Li et al. [6] point out that the presence of ethanol, utilized for the precipitation step, could effectively influence the structural and physicochemical characteristics of the precipitated [Ca3(C6H5O7)2(H2O)2]·2H2O.
Molluscan aquaculture is a worldwide sustainable food resource that has increased global molluscan food production. Abundant shell wastes are generated after food production and consumption, which negatively impacts the land, air, and water environments [12]. Methods to transform shell waste into valuable products have been investigated to reduce their adverse environmental impact [11]. A major component of shell waste is CaCO3 [13]. Biominerals in shell wastes contain CaCO3 of around 95%, and the rest (around 5%) are proteins and polysaccharides [14]. CaCO3 can be transformed into calcium oxide (CaO) after the thermal treatment process, as expressed in the following calcination reaction: CaCO3(s) + heat → CaO(s) + CO2(g) [15]. Owing to the presence of CaO, calcined shell wastes can be used in many applications, such as the construction industry [16], plant-based energy [17], and agriculture [18]. Moreover, many studies revealed that CaO can be used as a bioactive material in food packaging [19] and has a high potential to kill bacteria [20] and fungi [21].
In Thailand, molluscan shells have been consumed increasingly, leading to an increase in shell waste from the seafood industry [14]. This waste can pollute the environment significantly due to the bad smell from the decomposition of residual attached meat on the waste. Some shell wastes are dumped in landfills or in the sea without appropriate control, increasing the risk of damaging the soil, water, and sea ecosystems [22]. This issue motivated scientists to research effective and green technology for utilizing shell waste. Due to the high amount of shell waste in Thailand, local people grind the waste into powder without applying heat and use the shell powder as feed or fertilizers for farming [14]. This has become a conventional technique for utilizing shell waste in Thailand. However, if the shell powder can be transformed to have more value, this resource will provide more benefits to people in communities.
The transformation of discarded shells into beneficial materials not only reduces the volume but also increases the value of shell waste [13]. Therefore, this research aims to synthesize calcium citrate through a mild, simple, green, and rapid process using citric acid (C6H8O7) and CaCO3. The CaCO3 was obtained from mussel shell waste as a sustainable calcium source. The CaCO3 was first dispersed in each organic solvent, namely, acetone (CH3COCH3), ethyl alcohol (ethanol, CH3CH2OH), and isopropyl alcohol (isopropanol, CH3CHOHCH3), which have been considered as recommended green solvents [23,24]. The suspension was then reacted with citric acid, forming calcium citrate. Therefore, the influence of the additive organic solvent on the physicochemical properties of synthesized calcium citrates was studied and compared with the effect of citric acid concentration with calcium carbonate derived from cockle shells in our previous report [25]. Waste management designed in this research highlighted an effective strategy to reduce negative environmental impact. Moreover, shell waste recycling is the most practical alternative for reducing environmental effects, while adding economic value to society [26]. These findings are consistent with the Sustainable Development Goals (SDGs) and the world’s carbon neutrality trend [27,28].
2. Materials and Methods
2.1. Preparation of CaCO3 from Mussel Shells
Mussel shell waste, collected from a local market in the Samut Prakan province, Thailand, was first washed to remove dust, tissue, and other impurities [2]. The cleaned waste was sun-dried and then finely ground. To control the particle size of the powdered shells, the ground powders were sieved using a 100-woven wire mesh sieve (Endecott™, Endercotts Limitted, Parsons Lane, UK) [26], thereby obtaining homogenized CaCO3 powder to employ as a green calcium source that is priced at 0.060 USD/kg for calcium citrate production.
2.2. Synthesis of Calcium Citrate
For the synthesis of calcium citrate, 10 g of CaCO3 powdered from shell waste was first mixed with 10 mL of each organic solvent, corresponding to the CaCO3/solvent ratio of 1 g/mL. The mixture was suspended using a stirrer (IKA®, C-MAG HS 7) at 150 rpm under ambient conditions. After stirring for 30 min, 25.62 mL of citric acid (50% C6H8O7 solution, Merck, Darmstadt, Germany, 0.92 USD/L) was added to the CaCO3 suspension under a stirring process, and the stirring rate was increased to 300 rpm to achieve a well-homogenized system. Using this stirring process, the citric acid reacted with CaCO3, initiating the calcium citrate production and releasing CO2, based on the following reaction (Equation (1)) [1].
3CaCO3(s) + 2C6H8O7(aq) → Ca3(C6H5O7)2(s) + 3H2O(l) + 3CO2(g)
The temperature and pH of the reaction were directly measured using a thermometer and a pH meter, respectively. The precipitation reaction was completed when gas (CO2) was not generated. At this step, the reaction time was recorded. The reaction product (calcium citrate) was corrected using a paper filter coupled with Buchner funnel vacuum filtration, washed with 20 mL of the used organic solvent, dried in an oven (60 °C), and finely ground with a mortar and pestle, obtaining calcium citrate powder. Based on the production weight, the production yield was calculated. The organic solvents used in this work were acetone (Merck, 99.5%, ACS grade; 2.29 USD/L), ethyl alcohol (ethanol, absolute anhydrous, Carlo Erba™, 99.9%, ACS grade; 1.01 USD/L), and isopropyl alcohol (isopropanol, Q RëC™, 99.7%, AR grade; 1.38 USD/L). Therefore, the calcium citrates synthesized from the reaction in the presence of acetone, ethanol, and isopropyl alcohol (IPA) were labeled as CC-AC, CC-Et, and CC-IPA, respectively. Additionally, the cost of the obtained CC products was estimated based solely on the cost of starting reagents according to Equation (1), without considering other parameters such as electricity, manpower, apparatus, and instrument costs.
2.3. Material Characterizations
The Spectrum GX Fourier-transform infrared (FTIR) spectrophotometer (Perkin Elmer) was used to investigate the functional groups that characterize the synthesized calcium citrate products. The changes in the transmittance percentages against the wavenumber, ranging between 4000 and 400 cm−1, were recorded using a resolution of 0.5 cm−1. The Rigaku MiniFlex benchtop X-ray diffractometer (XRD) was used to characterize the crystal structure of a sample. The 2-theta (2θ) angles were recorded in the range of 5–55° with a Cu-Kα radiation (λ = 0.15406 nm) at an angle interval of 0.01° and a scan speed of 1 s/step [28,29]. To examine the crystal structure property of a sample, the diffraction pattern was compared with the diffraction database of the Joint Committee on Powder Diffraction Standards (JCPDS) and Inorganic Crystal Structure Database (ICSD) [30,31]. The qualitative and quantitative analyses of a sample were conducted by using SRS 3400 X-ray fluorescence (XRF) spectroscopy (Bruker), hence revealing the elemental compositions and their corresponding percentages, which are available in each sample [32,33]. The Pyris Diamond thermogravimetric analyzer (TGA, Perkin Elmer (Waltham, MA, USA)) was used to analyze the thermal decomposition of a sample. Two alumina TG pans were first ultrasonically cleaned and heated at 900 °C to remove moisture and impurities from the pans. Around 10 mg of sample and calcined aluminum oxide (α-Al2O3, referent material) was placed separately in the TG pans, without a lid and sample pressing, and then heated from room temperature to 900 °C at a heating rate of 10 °C/min and N2 flow rate of 100 mL/min [34]. The morphological characteristics of the sample were examined using a LEO 1450VP scanning electron microscope (SEM, Zeiss (Jena, Germany)) [6]. Before the SEM observation, the sample was coated with gold nanoparticles to improve the conductivity and decrease the charging effect [35].
3. Results and Discussion
3.1. Production Results
Table 1 shows the reaction temperatures, reaction time, and reaction pH of the production of calcium citrates (CC-AC, CC-Et, and CC-IPA) produced from the reaction in the presence of different organic solvents: acetone (AC), ethanol (Et), and isopropanol (IPA). The reaction yield of the calcium citrate products was also evaluated. The reaction temperature, the highest temperature observed during the reaction between CaCO3 and citric acid in the presence of organic solvent, was measured and found to be 30.1, 28.4, and 27.3 °C when acetone, ethanol, and isopropanol were used in the reaction, respectively. The reaction temperatures observed in this work are lower than those observed in the previous report [25], which is caused by the use of differing medium agents in the synthesis route. The result shows that the temperature of the reaction is slightly different, indicating that all the utilized organic solvents showed an effective medium to produce calcium citrate because the low temperature was obtained during the reaction in the presence of acidic reagent, i.e., citric acid, pointing out the mild synthesis reaction. Generally, the temperature of the reaction between acid and calcium carbonate was observed to be higher than 50 °C because of a strong exothermic process [29].

Table 1.
Temperatures, time, pH, reaction yields, and costs of the production of calcium citrates (CCs) prepared from the reaction between CaCO3 and citric acid in the presence of organic solvents.
The reaction time was monitored when the citric acid was added into the suspension that contained CaCO3 and each organic solvent, and the final time was recorded when the CO2 gas was not observed as expressed in Equation (1). The reaction times for acetone, ethanol, and isopropanol were 37.69, 39.26, and 34.77 min, respectively. The time consumption for production in this work was shorter than that of the previous report, which was produced by the reaction between calcium carbonate derived from cockle shells with various citric acids without a medium agent in the process [25]. The highest reaction time was observed for the ethanol system, pointing out the lowest reaction rate between CaCO3 and citric acid in this system. This finding revealed that the presence of solvent can modulate the energies of reactants, changing the activation energy of the reaction, hence causing the reaction rate [34]. Moreover, the molecules of solvents, especially ethanol with the highest polarity, can participate in the reaction and compete with the reactants, change the properties of the reactants, intermediates, and the product, and change the solubility of components in the reaction [36,37]. These might affect the physicochemical properties of the product.
The pH of the reaction solution, containing CaCO3, organic solvent, and citric acid, was measured with a pH meter. Therefore, the exact pH was not observed, and an approximate value was reported. As presented in Table 1, the solution pHs were ~3.0–3.5 for the acetone and ethanol solvent systems, whereas a solution pH of ~2.5–3.0 was observed for the isopropanol system. The acidic pH is consistent with the presence of acidic reagents, e.g., citric acid, to form calcium citrate products; therefore, low pH values (<3.5) were observed for all synthetic systems. The slight difference in the solution pH also indicated that some citric acid content in the presence of isopropanol did not react with CaCO3 effectively, as expressed in Equation (1). In contrast, more citric acid molecules in the presence of acetone or ethanol could react with the CaCO3. Therefore, an ineffective reaction between the reactants caused higher citric acid in the reaction solution for the isopropanol system, resulting in a lower solution pH. For this reason, it could be assumed that higher reaction yields might be obtained in the acetone and ethanol systems due to the more complete reaction.
Reaction yield is one of the most important chemical parameters that should be investigated to achieve the optimal balance between productivity and cost-effectiveness. Economic considerations drive scientists to the chemical transformation of reactants into products with the highest yield possible [38]. Reaction yield is usually calculated and reported as a percentage of the desired product compared to the theoretical value. Therefore, the reaction yield (%) of calcium citrate was calculated using Equation (2) [29]:
where wexp is the weight of the calcium citrate powder in the preparation process obtained from each organic solvent, whereas wtheor is the weight of the theoretical calcium lactate product.
As demonstrated in Table 1, the reaction yields of all synthesized calcium citrates were more than 70%, indicating that the CaCO3 powdered from mussel shell waste could be effectively utilized as a green calcium source to prepare calcium citrate. The yields of production were found to be 77.30%, 77.87%, and 70.99% when acetone, ethanol, and isopropanol were used in the reaction, respectively. However, the yield results obtained in this work are lower than those obtained by the synthesis of cockle shell and various citric acids without a medium agent (>88%) [25]. The lowest reaction yield was obtained when isopropanol was employed as a co-solvent during the production of CC-IPA. In contrast, the higher reaction yield was achieved when acetone or ethanol was used for the reaction between CaCO3 and citric acid, thereby obtaining CC-AC or CC-Et, respectively. These findings are consistent with the solution pH as described above. The cost of the obtained CC products was estimated based on starting reagents in Equation (1), combined with the yields obtained, which were found to be in the price range of 1.8–2.5 USD/kg (Table 1). The cost of the CC products obtained in this work is higher than that obtained by the synthesis of cockle shell and various citric acids without a medium agent (0.20–0.30 USD/kg) [25], but these costs are lower than commercial calcium citrate in the general Thailand market (>5 USD/kg). Additionally, the results obtained clearly indicate that the different synthesis routes result in different preparation conditions, which may affect some physicochemical properties, which may result in applications such as fertilizer, food additives, calcium supplements, and bone repair.
3.2. Vibrational Spectroscopy
A Fourier-transform infrared (FTIR) spectrophotometer was utilized to investigate the functional groups present in the synthesized samples [11]. Figure 1 shows the FTIR spectra of calcium citrates (CC-AC, CC-Et, and CC-IPA) synthesized by CaCO3 derived from mussel shell waste and citric acid in the presence of different organic solvents, which are similar with the FTIR spectra of calcium citrate obtained from the reaction of calcium carbonate derived from cockle shells and various citric acids [25]. The functional groups of the compounds were analyzed through the vibrational patterns of molecular bonds.
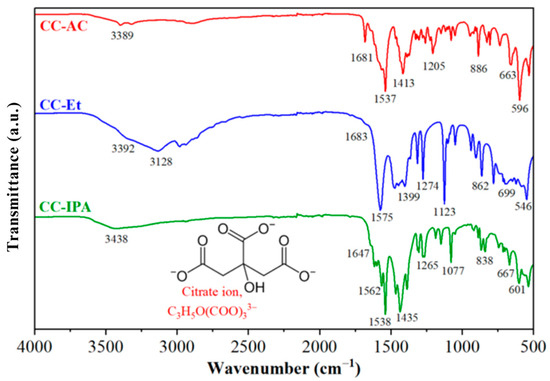
Figure 1.
FTIR spectra of calcium citrate products prepared in the presence of acetone (CC-AC), ethanol (CC-Et), and isopropanol (CC-IPA).
The chemical structure and atomic bonding interaction of citrate ions are presented in the inset of Figure 1. The absorbance band at the wavenumber range between 3650 and 3240 cm−1 is assigned as the vibrational mode of the O–H stretching of the hydroxyl functional (–OH) group of the citrate (C6H5O73− or C3H5O(COO)33−) ions [5]. It can be seen that among the synthesized samples, the calcium citrate prepared from the reaction in the presence of isopropanol (CC-IPA) showed the highest absorption energy (~3438 cm−1). This finding indicated the strongest chemical bonding between O and H atoms for the CC-IPA sample, whereas absorption bands at around 3392 and 3389 cm−1 were observed for CC-Et and CC-AC, respectively. Moreover, the stronger absorption band for the O–H stretching of the CC-Et sample, compared to other samples, pointed out the highest amount of hydroxyl groups. The vibrational band at a position of 3128 cm−1 also corresponded to a vibrational mode of the O-H bond [6,39].
The absorption energies at the positions between 1710 and 1480 cm−1 are assigned as the vibrational characteristics of C=O and C–O bonds, corresponding to the carboxylate group (RCOO−) in the C3H5O(COO)33− [11]. The vibrational stretching modes of the C–O bond, ranging from 1480 to 1160 cm−1 [5], were found to be close to each other. The vibrational bands, ranging from 1100 to 700 cm−1, corresponded to the bending mode of C–O–C bonds [11] and the rocking vibrations of the –CH2 bond [6] in the C3H5O(COO)33−. The vibrational bands observed at lower 700 cm−1 are attributed to the metal–oxygen (Ca–O) bond interaction [40]. The FTIR result could introduce the chemical formula of the synthesized sample. As presented in the results, similar spectra between CC-AC and CC-IPA were observed; however, they differed from the spectrum of CC-Et. These results indicated that the crystal structure of calcium citrate prepared in the presence of acetone or isopropanol differed from that of ethanol.
3.3. Structural and Phase Characteristics
The presence of organic solvents leads to a change in the solvation properties of Ca2+ ions that are solvated in aqueous-based citric acid [36,37,41]. The solvation property could then alter the nucleation and crystallization processes of the precipitated calcium citrate products. The crystal properties of calcium citrates prepared from the reaction in the presence of different organic solvents were characterized by the X-ray diffraction (XRD) technique, and the diffraction patterns are shown in Figure 2. As shown in the results, the diffraction patterns of calcium citrates were similar to each other; however, other different peaks were observed. These characteristics pointed out the obtained calcium citrate crystalized in both the same and different crystal systems [30,31].
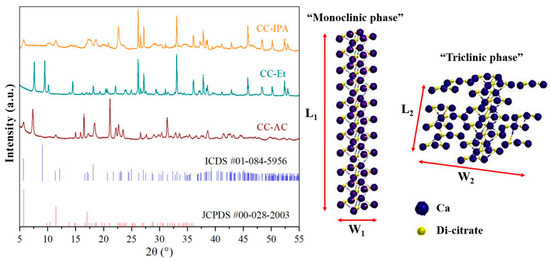
Figure 2.
X-ray diffraction (XRD) patterns of the synthesized calcium citrates prepared by the reaction between CaCO3 derived from mussel shell waste and citric acid in the presence of acetone (CC-AC), ethanol (CC-Et), and isopropanol (CC-IPA). Molecular alignments of monoclinic and triclinic crystal systems of calcium citrate [30].
The calcium citrates prepared from the reaction in the presence of acetone (CC-AC) and isopropanol (CC-IPA) mainly crystallized in the monoclinic crystal structure, corresponding to the chemical formula of [Ca3(C6H5O7)2(H2O)2]·2H2O [6,30] based on JCPDS #00-028-2003. However, the diffractions of both CC-AC and CC-IPA are also consistent with ICDS #01-084-5956, corresponding to the triclinic phase with the chemical formula of Ca3(C6H5O7)2∙(H2O)4 [30]. The calcium citrate prepared from the reaction in the presence of ethanol (CC-Et) mainly crystallized in the triclinic Ca3(C6H5O7)2∙(H2O)4 phase (ICDS #01-084-5956). At the same time, the monoclinic [Ca3(C6H5O7)2(H2O)2]·2H2O phase was also observed for CC-Et. These results demonstrate that all synthesized calcium citrates crystallized in both monoclinic and triclinic structures, with different phase quantities. These different crystal structures of calcium citrates also pointed out the difference in the chemical interaction of water (H2O), citrate (C6H5O73−), and metal–oxygen (Ca–O) in the crystal structures between monoclinic and triclinic calcium citrates, which is consistent with the difference in FTIR spectra between CC-Et and both CC-AC and CC-IPA. This difference is also demonstrated and discussed in the thermal properties of samples.
According to the XRD patterns, it can be concluded that all calcium citrates, synthesized by the reaction between the mussel shell powder and citric acid, were confirmed to be calcium citrate. However, the calcium citrates exhibited different crystal systems, in which CC-AC and CC-IPA were mainly crystallized in the monoclinic phase, whereas CC-Et mainly crystallized in the triclinic phase. The differences in the atomic alignment between monoclinic and triclinic crystal systems, proposed by Jang et al. [30], are presented in Figure 2. Two di-citrate (2C6H5O73−) molecules can bind with three calcium (3Ca2+) ions through chelation, forming the Ca–citrate complexes [Ca3(C6H5O7)2(H2O)2]·2H2O and Ca3(C6H5O7)2∙(H2O)4. The width of the atomic arrangement of the triclinic phase is longer than the monoclinic phase (W2 > W1), whereas the L1 of the monoclinic phase is longer than the L2 of the triclinic phase. There are three unequal axes for both crystal systems; however, each axis of the monoclinic phase is inclined to another one with a perpendicular form. Each axis is inclined to another one with oblique angles for the triclinic phase. For the monoclinic crystal system with the space group of C2/c, the lattice parameters (edge lengths) a, b, and c are 31.780, 10.255, and 5.984 Å, with a lattice (interaxial) angle β of 100.371° and a cell volume (V) of 1919.1 Å3, respectively. For the triclinic crystal system with the space group of , a, b, and c are 5.985, 10.257, and 16.668 Å, with lattice angles α, β, and γ of 72.380°, 80.085°, and 89.869°, respectively, and a V of 959.3 Å3 [10]. The difference in the atomic alignment between these crystal systems causes a difference in their symmetry. Compared to the monoclinic phase, the triclinic phase is a more irregular atomic arrangement. The XRD results in this work are significantly different from those obtained from different raw materials and synthetic routes reported in previous work [25]. The crystallite sizes (Sc) of each synthesized CC-AC, CC-Et, and CC-IPA powder were calculated using Scherrer’s equation (Sc = 0.094λ/βcosθ, where λ is the employed X-ray wavelength (0.154059 nm), β is the full width at half maximum (FWHM in radians) of each investigated diffraction peak, and θ is the diffraction peak angle). The calculated crystallite sizes of the synthesized CC-AC, CC-Et, and CC-IPA powders were 47 ± 5, 54 ± 6, and 45 ± 9 nm, respectively. These results indicate that the medium used in the preparation of the material affects the crystal size of the obtained product.
3.4. Chemical Composition
X-ray fluorescence (XRF) spectroscopy was used to determine the chemical composition and the purity of the synthesized calcium citrate samples [32]. Table 2 shows the chemical compositions of samples prepared using acetone (CC-AC), ethanol (CC-Et), and isopropanol (CC-IPA). The results revealed that all synthesized calcium citrates showed similar chemical composition. More than 97% of the major chemical composition of all samples was calcium (Ca), which corresponded to calcium oxide (CaO) [42]; however, this proportion was lower than those of the products (>98%) obtained from the reaction between cockle shells and various citric acids [25]. However, this CaO quantity is also related to sample purity; therefore, it could be interpreted that the high CaO percentage indicates the high purity of calcium citrate crystals synthesized from CaCO3 powdered from mussel shell waste. The results also demonstrated that the purity of the calcium citrates produced from mussel shell waste is consistent with the purity of the calcium citrate produced from oyster shell waste (98.48%) [2].

Table 2.
Chemical compositions of calcium citrate products prepared from the reaction in the presence of different organic solvents: acetone (CC-AC), ethanol (CC-Et), and isopropanol (CC-IPA).
Minor compositions such as silicon, iron, and strontium with quantities more than 0.5% were observed, which corresponded to silicon dioxide (SiO2), ferric oxide (Fe2O3), and strontium oxide (SrO), respectively. Other oxides were also observed in trace amounts (<0.2%), namely, aluminum oxide (Al2O3), sulfur trioxide (SO3), potassium oxide (K2O), phosphorus pentoxide (P2O5), chlorine dioxide (ClO2), and magnesium oxide (MgO). The similar chemical composition observed in CC-AC, CC-Et, and CC-IPA is due to the fact that all calcium citrates were prepared from the same precursor (mussel shell-derived CaCO3). Therefore, it could be concluded that the addition of organic solvents (acetone, ethanol, or isopropanol) could not influence the chemical composition of the obtained calcium citrates. Notably, the XRF results further suggested that the cleaned mussel shell waste is an effective source to prepare calcium citrate with high purity and without toxic elements, such as lead (Pb), mercury (Hg), arsenic (As), and cadmium (Cd). High-purity calcium citrate has attracted attention in both medical [41] and food [1] industries for applications such as calcium supplements, food additives, and bone repair.
3.5. Thermal Decomposition Behavior
The thermal analysis (TA) was used to investigate the thermal decomposition behavior of the synthesized calcium citrates [31] prepared from different organic solvents. The sample was thermally treated at a heating rate of 10 °C/min under a N2 gas flow rate of 100 mL/min. The thermogravimetric (TG) curves, as presented in Figure 3, show the percentage of weight loss (wt%) of the sample with increasing temperature. Figure 3 also demonstrates the derivative thermogravimetric (DTG) curves of the sample, which are the relative change in the weight loss percentage with respect to time (dW/dt). The resulting TG curve was employed to evaluate the thermal decomposition processes of calcium citrates, and the thermal decomposed products were then proposed in detail based on both the wt% and the remaining weight of the intermediates [10,11].
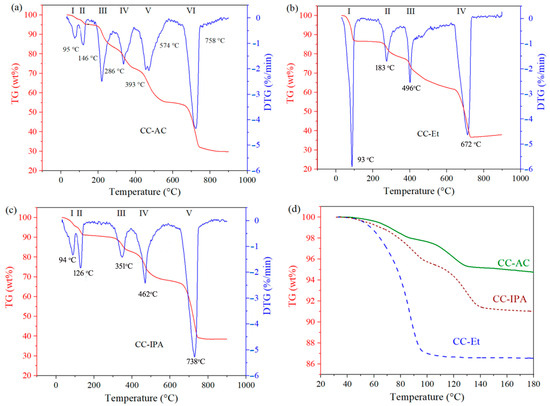
Figure 3.
Thermal decomposition behaviors (TG and DTG curves) of calcium citrates synthesized from different organic solvents: acetone ((a), CC-AC), ethanol ((b), CC-Et), and isopropanol ((c), CC-IPA). (d) Comparison of the TG curves of CC-AC, CC-Et, and CC-IPA in the dehydration step.
As shown in Figure 3a, six TG weight losses were observed, which are well consistent with six DTG peaks. Therefore, it could be mentioned that there were six thermal decomposition processes of the synthesized calcium citrate prepared from the reaction in the presence of acetone (CC-AC). The first thermal decomposition (Step I, Equation (3)) corresponded to the elimination of water (H2O) of [Ca3(C6H5O7)2(H2O)2]·2H2O. In this thermal step, two equivalent moles of H2O (2H2O) were eliminated from the tetra-hydrated form ([Ca3(C6H5O7)2(H2O)2]·2H2O), and the di-hydrated form (Ca3(C6H5O7)2(H2O)2) was obtained [6]. The second thermal decomposition (Step II, Equation (4)), 2H2O of the Ca3(C6H5O7)2(H2O)2 was eliminated, causing the formation of an anhydrous form (Ca3(C6H5O7)2) [31]. The obtained anhydrous Ca3(C6H5O7)2 crystalized in a monoclinic crystal system with a = 12.161, b = 10.251, c = 14.034 Å, β = 95.62°, and V = 1741.33 Å3 [43]. The third (Step III), fourth (Step IV), and fifth (Step V) thermal decompositions corresponded to the decomposition of the citrate section [5], which is the organic chelating of Ca [30]. These multi-step decompositions of citrate were also observed in the literature [10,31]. In these decomposition processes, H2O was eliminated with decarbonation (the elimination of carbon). Due to the presence of air during the thermal decomposition investigation, carbon could react with oxygen, causing the formation of carbon monoxide (CO) and/or carbon dioxide (CO2) [11]. Therefore, during the decomposition of citrate, H2O, CO, and/or CO2 were eliminated from Ca3(C6H5O7)2, resulting in the formation of CaCO3, as described in Equations (5)–(7). The final decomposition process (Step VI) is attributed to the decomposition of CaCO3. In this thermal decomposition, CO2 was eliminated from CaCO3 (decarbonization), resulting in the formation of CaO [10], as expressed in Equation (8). The starting and ending temperature points of each thermal decomposition process of all synthesized calcium citrates are summarized in Table 3.

Table 3.
Starting and ending temperature points of each thermal decomposition process obtained from the TG and DTG curves of CC-AC, CC-Et, and CC-IPA.
- CC-AC (Figure 3a)
Step I: First dehydration (30–95 °C)
[Ca3(C6H5O7)2(H2O)2]·2H2O(s) → Ca3(C6H5O7)2(H2O)2(s) + 2H2O(g)
Step II: Second dehydration (95–146 °C)
Ca3(C6H5O7)2(H2O)2(s) → Ca3(C6H5O7)2(s) + 2H2O(g)
Step III: First decomposition of citrate (146–286 °C)
Ca3(C6H5O7)2(s) → xCa3(C6H5O7)2(s) + a1CaCO3(s) + b1H2O(g) + c1C(g)
Step IV: Second decomposition of citrate (286–393 °C)
xCa3(C6H5O7)2(s) → yCa3(C6H5O7)2(s) + a2CaCO3(s) + b2H2O(g) + c2C(g)
Step V: Third decomposition of citrate (393–574 °C)
yCa3(C6H5O7)2(s) → a3CaCO3(s) + b3H2O(g) + c3C(g)
Step VI: Decarbonization (574–758 °C)
where a1 + a2 + a3 = 3; b1 + b2 + b3 = 5; c1 + c2 + c3 = 9; x + y = 1.
3CaCO3(s) → 3CaO(s) + 3CO2(g)
Figure 3b presents the thermal decomposition behavior (TG and DTG curves) of the synthesized calcium citrate prepared from the reaction in the presence of ethanol (CC-Et). It can be seen that four TG weight losses with four corresponding DTG peaks were observed. Therefore, the thermal decomposition of CC-Et occurred in four steps. The first thermal decomposition (Step I, Equation (9)) corresponded to the complete elimination of water (H2O) of hydrated Ca3(C6H5O7)2∙(H2O)4, and anhydrous Ca3(C6H5O7)2 was obtained. The second (Step II) and third (Step III) thermal decompositions corresponded to the decomposition of citrate. In these processes, H2O, CO, and/or CO2 were eliminated from Ca3(C6H5O7)2, hence forming CaCO3, (Equations (5) and (10)). The final decomposition process corresponded to the decomposition of CaCO3, resulting in the formation of CaO (Equation (8)).
- CC-Et (Figure 3b)
Step I: Dehydration (30–134 °C)
Ca3(C6H5O7)2∙(H2O)4(s) → Ca3(C6H5O7)2(s) + 4H2O(g)
Step II: First decomposition of citrate (134–343 °C) is illustrated in Equation (5).
Step III: Second decomposition of citrate (343–548 °C)
xCa3(C6H5O7)2(s) → a2CaCO3(s) + b2H2O(g) + c2C(g)
Step IV: Decarbonization (548–752 °C) is shown in Equation (8),
where a1 + a2 = 3; b1 + b2 = 5; c1 + c2 = 9.
Figure 3c presents five TG weight losses and five DTG peaks, corresponding to the five thermal decomposition processes of the synthesized calcium citrate prepared from the reaction in the presence of isopropanol (CC-IPA). In the first thermal decomposition (Step I, Equation (11)), 2H2O was eliminated from [Ca3(C6H5O7)2(H2O)2]·2H2O, forming Ca3(C6H5O7)2(H2O)2. In the second decomposition (Step II, Equation (12)), 2H2O of Ca3(C6H5O7)2(H2O)2 was eliminated, forming Ca3(C6H5O7)2. The third (Step III) and fourth (Step IV) decompositions are attributed to the decomposition of Ca3(C6H5O7)2, eliminating H2O, CO, and/or CO2 with the formation of CaCO3 (Equations (5) and (13)). The final decomposition (Step VI) corresponded to the decomposition of CaCO3, eliminating CO2 with the formation of CaO (Equation (8)).
- CC-IPA (Figure 3c)
Step I: First dehydration (30–105 °C)
[Ca3(C6H5O7)2(H2O)2]·2H2O(s) → Ca3(C6H5O7)2(H2O)2(s) + 2H2O(g)
Step II: Second dehydration (105–157 °C)
Ca3(C6H5O7)2(H2O)2(s) → Ca3(C6H5O7)2(s) + 2H2O(g)
Step III: First decomposition of citrate (157–395 °C) is tabulated in Equation (5).
Step IV: Second decomposition of citrate (395–592 °C)
xCa3(C6H5O7)2(s) → a2CaCO3(s) + b2H2O(g) + c2C(g)
Step V: Decarbonization (592–761 °C) is displayed in Equation (8),
where a1 + a2 = 3; b1 + b2 = 5; c1 + c2 = 9.
According to the thermal decomposition behaviors of samples, it could be observed that the calcium citrate can thermally decompose in three major processes [10,44] as follows (Equations (8), (14) and (15)):
Process I: Dehydration
Ca3(C6H5O7)2∙4H2O(s) → Ca3(C6H5O7)2(s) + 4H2O(g)
Process II: Decomposition of citrate
Ca3(C6H5O7)2(s) → 3CaCO3(s) + 5H2O(g) + 9C(g) (as CO and/or CO2)
Process III: Decarbonization is demonstrated in Equation (8).
The theoretical weight losses of the dehydration, decomposition of citrate, and decarbonization processes were 12.6 wt% (weight loss of 4H2O) [11], 52.3 wt% (the remaining weight of 3CaCO3), and 30.0 wt% (the remaining weight of 3CaO) [10]. The weight losses observed in this work are presented in Table 4.

Table 4.
TG weight losses derived from the TG curves of the synthesized CC-AC, CC-Et, and CC-IPA.
Compared to the theoretical value (12.6 wt%), there is a slight weight loss due to the dehydration for CC-Et due to the presence of adsorbed moisture on the particle surface, and the observed TG mass loss percentage (13.43 wt%) confirmed that CC-Et is Ca3(C6H5O7)2∙(H2O)4. In contrast, significant differences in dehydration for both CC-AC and CC-IPA are due to the presence of anhydrous calcium citrate (Ca3(C6H5O7)2) that is partially mixed with the hydrated form ([Ca3(C6H5O7)2(H2O)2]·2H2O). This result further confirmed that the utilization of different synthetic reactions resulted in differing forms of the calcium citrate product, thereby influencing the difference in weight loss [1]. For both as-prepared CC-AC and CC-IPA, they were [Ca3(C6H5O7)2(H2O)2]·2H2O and Ca3(C6H5O7)2, resulting in low weight loss in the dehydration process. CC-Et showed a strong DTG peak for the dehydration process (Figure 3b), indicating that bulk CC-Et contained more water constituent, compared to both CC-AC (Figure 3a) and CC-IPA (Figure 3c). This result is well consistent with the TG weight loss of the dehydration process as presented in Figure 3d, which shows a comparison of the TG weight losses obtained from CC-AC, CC-Et, and CC-IPA. Two dehydration processes were observed for both CC-AC and CC-IPA, whereas CC-Et showed a single dehydration process. Moreover, the highest dehydration rate (fastest weight loss) was observed for CC-Et, indicating that its structure was more easily decomposed. The differences in the weight losses observed from both the decomposition of citrate and decarbonization processes might be due to the complex decomposition processes of both decompositions that slightly shift the temperature, thereby shifting the weight losses. The traces of thermal decomposition of the products observed in this work are slightly different from those reported in previous works [25]; however, the thermal decomposition reaction occurs in a similar way.
3.6. Morphological Characteristics
The utilization of organic solvents is an important factor that influences the colloidal assembly mechanism and the particle growth process during material synthesis [45]. Interactions between the solvent molecules and ligand molecules or surface particles can affect the morphology and particle size [46]. In the present work, a scanning electron microscope (SEM) was utilized to observe the morphological characteristics of the synthesized calcium citrates. The particle sizes of the samples were directly measured through the SEM images. According to the SEM results (Figure 4), the particle morphologies of all CC-AC, CC-Et, and CC-IPA powders are in irregular forms of thin sheets (sheet-like structure); however, these irregular thin sheets are rather scattered with particle sizes from around 50 nm to 1.2 μm.

Figure 4.
SEM micrographs at a magnification of 50k× of calcium citrate powders synthesized from the reaction in the presence of acetone ((a), CC-AC), ethanol ((b), CC-Et), and isopropanol ((c), CC-IPA).
Although irregular thin sheets were observed for all synthesized samples, some significant differences in morphology were also observed among the samples. CC-Et (Figure 4b) showed lower particle agglomeration, compared to both CC-AC (Figure 4a) and CC-IPA (Figure 4c). During the synthesis of the CC-AC sample, acetone (CH3COCH3) was first mixed with mussel shell-derived CaCO3 before reacting with citric acid (C6H8O7). In this reaction, citric acid (in water) acted as both reagent and aqueous-based solvent, whereas acetone played the role of a co-solvent. However, due to the absence of a polar group such as a hydroxyl (–OH) group for acetone, compared to both ethanol (C2H5OH) and isopropanol ((CH3)2CHOH), the compatibility between acetone and water (that solvated citric acid) was lower than that between ethanol or isopropanol and water. The lowest compatibility resulted in the highest crystallization rate of calcium acetate, thereby making it difficult to control crystal growth.
As for the morphology of CC-Et, there are numerous gaps between crystals. The crystal structure is in the form of thin sheets (nanosheets) and is uniformly distributed. Furthermore, the CC-Et particles are arranged (tend to be of flower-like shape) and separated more than CC-AC and CC-IPA particles. Due to the presence of lower carbon atoms, the compatibility between ethanol and water is higher than that between isopropanol and water, and this phenomenon inhibited the supersaturation [5,6], resulting in controlled crystals without excessive crystal agglomeration. When isopropanol was utilized as a co-solvent, the CC-IPA particle shape was similar to the particle shape of CC-AC. More particle agglomeration was observed in comparison with CC-Et. Moreover, the crystal shape was denser and more disordered, with thicker plate-like crystals. Compared to higher polar solvents such as ethanol, isopropanol, with its lower polarity, could not inhibit the reaction between CaCO3 and citric acid effectively. Therefore, the crystallization rate of calcium citrate was higher than the utilization of ethanol. Moreover, the calcium citrate nuclei, formed in the presence of ethanol, absorbed the ethanol molecules with strong electric fields induced by the –OH group [41,45,46]. This electronic state could repulse and separate the formed CC-Et particles more than both CC-AC and CC-IPA. Additionally, the shapes and smaller particle sizes of the products obtained in this work are significantly different from the products obtained from different raw materials and synthetic methods reported in our previous work [25], which may result in some applications [46,47,48].
4. Conclusions
Mussel shell waste was successfully utilized as a green and sustainable calcium source to produce calcium citrate through a mild and simple chemical reaction. The mussel shell-derived CaCO3 precursor was dispersed in different organic solvents, namely, acetone (AC), ethanol (Et), and isopropyl alcohol (IPA), and then reacted with citric acid, obtaining CC-AC, CC-Et, and CC-IPA, respectively. The utilization of different organic solvents caused changes in the reaction temperature, time, and yields. The vibrational modes, revealed by the FTIR spectra, revealed the existence of water (H2O), citrate (C6H5O73−), and metal–oxygen (Ca–O), indicating the characteristics of the hydrated calcium citrate. The XRD patterns pointed out that the crystal structure of CC-AC and CC-IPA is different from that of CC-Et. CC-AC and CC-IPA are [Ca3(C6H5O7)2(H2O)2]·2H2O that mainly crystallizes in the monoclinic crystal system, whereas CC-Et is Ca3(C6H5O7)2∙(H2O)4 with a mainly triclinic structure. The differences between the triclinic CC-Et and monoclinic CC-AC or CC-IPA caused the differences in thermal decomposition behaviors between them. Two dehydration steps were observed for the monoclinic structure, whereas the triclinic phase showed a single dehydration process. The TG profiles of CC-AC and CC-IPA revealed that both samples mainly contained hydrated calcium citrate ([Ca3(C6H5O7)2(H2O)2]·2H2O) that was partially mixed with the anhydrous form (Ca3(C6H5O7)2). In contrast, a single Ca3(C6H5O7)2∙(H2O)4 phase was observed for CC-Et. A multi-step thermal decomposition of citrate was observed, obtaining CaCO3, followed by decarbonization, thereby obtaining CaO as the final decomposition product. The XRF result demonstrated the high purity of all calcium citrate products (>97%). Polarity and the existence of the –OH group of the additive organic solvent are important factors that influence the morphological characteristics of calcium citrates. Their different physicochemical properties could be ascribed to the change in the supersaturation state of the reaction solution. Compared to both acetone and isopropanol, ethanol, with the highest polarity and –OH group, is compatible with citric acid, thus suppressing the supersaturation rate of the reaction solution. This result caused the modulation of the precipitation mechanisms and the growth rate of the calcium citrate particles. Therefore, the vibrational, structural, thermal, and morphological characteristics of CC-Et are different from CC-AC and CC-IPA products. This work presents an easy, soft, and low-cost method with no environmental impact to recycle waste mussel shells into calcium citrate compounds via processing to obtain different medium agents, leading to specific properties for targeted uses, making it the best alternative for zero-waste management. The result of this research is very advantageous for specific applications such as fertilizers, food additives, calcium supplements, and bone repair.
Author Contributions
Conceptualization, B.B. and N.L.; methodology, P.C.; investigations and data curation, W.B. and N.L.; writing—original draft preparation, S.P., W.B. and S.S.; writing—review and editing, P.R. and B.B.; visualization, W.B.; supervision, S.S. and N.L.; project administration, B.B.; funding acquisition, S.P. and P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work is a result of the project entitled “Conversion of shell/eggshell biowastes for sustainable environmental remediation,” Grant No. RE-KRIS/FF67/030 and RE-KRIS/FF68/29 by King Mongkut’s Institute of Technology Ladkrabang (KMITL), which has received funding support from the NSRE.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank the Scientific Instruments Center KMITL for supporting TGA, FTIR, XRD, and SEM techniques.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Yan, H.; Liu, Y.; Peng, H.; Li, K.; Li, C.; Jiang, S.; Chen, M.; Han, D.; Gong, J. Improving calcium citrate food functions through spherulitic growth in reactive crystallization and a mechanism study. Food Chem. 2023, 404, 134550. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-Y.; Kim, H.-J.; Han, J.-S. Anti-inflammatory effects of calcium citrate in RAW 264.7 cells via suppression of NF-κB activation. Environ. Toxicol. Pharmacol. 2015, 39, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hany, M.; Wuyts, S.; Abouelnasr, A.A.; Zidan, A.; Demerdash, H.M.; Hussein, H.A.S.M.; Arida, R.E.; Elsharkawi, S.M.; Kramers, C.; Torensma, B. Comparison of calcium citrate and calcium carbonate absorption in patients with a Roux-en-Y gastric bypass, sleeve gastrectomy, and one-anastomosis gastric bypass: A double-blind, randomized cross-over trial. Surg. Obes. Relat. Dis. 2025, 21, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Boonen, S.; Vanderschueren, D.; Haentjens, P.; Lips, P. Calcium and vitamin D in the prevention and treatment of osteoporosis–a clinical update. J. Intern. Med. 2006, 259, 539–552. [Google Scholar] [CrossRef]
- Zhong, L.; Li, J.; Gao, Y.; Cao, W.; Zhang, P.; Lai, X. Preparation and characterisation of calcium citrate wires. Micro Nano Lett. 2015, 10, 419–421. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Gao, Y.; Zhong, L.; Zou, Q.; Lai, X. Preparation and properties of calcium citrate nanosheets for bone graft substitute. Bioengineered 2016, 7, 376–381. [Google Scholar] [CrossRef]
- Wang, L.-m.; Wang, W.; Li, X.-C.; Peng, L.; Lin, Z.-Q. Calcium citrate: A new biomaterial that can enhance bone formation in situ. Chin. J. Traumatol. 2012, 15, 291–296. [Google Scholar]
- Zhang, W.; Wang, W.; Chen, Q.-Y.; Lin, Z.-Q.; Cheng, S.-W.; Kou, D.-Q.; Ying, X.-Z.; Shen, Y.; Cheng, X.-J.; Nie, P.-F. Effect of calcium citrate on bone integration in a rabbit femur defect model. Asian Pac. J. Trop. Med. 2012, 5, 310–314. [Google Scholar] [CrossRef]
- Rovinaru, C.; Pasarin, D.; Matei, C. Optimization of conditions for production of calcium citrate from egg shells. Proceedings 2020, 57, 18. [Google Scholar] [CrossRef]
- Herdtweck, E.; Kornprobst, T.; Sieber, R.; Straver, L.; Plank, J. Crystal structure, synthesis, and properties of tri-calcium di-citrate tetra-hydrate [Ca3(C6H5O7)2(H2O)2]·2H2O. Z. Für Anorg. Und Allg. Chem. 2011, 637, 655–659. [Google Scholar] [CrossRef]
- Wei, S.; Sun, M. Transformation of calcite (CaCO3) into earlandite [Ca3(C6H5O7)2·4H2O] by the fungus Trichoderma asperellum BDH65. Int. Biodeterior. Biodegrad. 2021, 163, 105278. [Google Scholar] [CrossRef]
- Han, Y.; Yang, B.; Meng, L.-Y.; Cho, H.-K.; Lin, R.; Wang, X.-Y. Optimization of the life cycle environmental impact of shell powder and slag concrete using response surface methodology. Process Saf. Environ. Prot. 2025, 194, 272–288. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, J.-H.; Yoo, J.; Park, J.H.; Bae, J.-S.; Park, C.Y. Toward transformation of bivalve shell wastes into high value-added and sustainable products in South Korea: A review. J. Ind. Eng. Chem. 2024, 129, 38–52. [Google Scholar] [CrossRef]
- Srichanachaichok, W.; Pissuwan, D. Micro/nano structural investigation and characterization of mussel shell waste in Thailand as a feasible bioresource of CaO. Materials 2023, 16, 805. [Google Scholar] [CrossRef]
- Khosa, A.A.; Rehman, H.U.; Han, X.; Pan, J. In-depth review of CaCO3/CaO TCES system with the perspective of cyclic stability, reactors and its integration with CSPs. J. Energy Storage 2025, 106, 114820. [Google Scholar] [CrossRef]
- Huo, Y.; Lu, D.; Han, X.; Hu, S.; Sun, H.; Zhang, C.; Chen, Z.; Huang, J.; Yang, Y. The role of admixed CaO in a sulphoaluminate cement system under winter environments. J. Build. Eng. 2023, 78, 107638. [Google Scholar] [CrossRef]
- Amal, R.; Usman, M. A review of breakthroughs in biodiesel production with transition and non-transition metal-doped CaO nano-catalysts. Biomass Bioenergy 2024, 184, 107158. [Google Scholar] [CrossRef]
- Natsir, T.A.; Iknawati, A.M.; Wanadri, I.D.; Siswanta, D.; Lusiana, R.A.; Cahyaningrum, S.E. Environmentally friendly membrane based on chitosan, citric acid, and calcium for slow-release fertilizer. Heliyon 2025, 11, e41378. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Huang, Y.-C.; Chiu, S.-Y.; Kuo, K.-L.; Hwang, P.-A. Bacteriostatic effect of a calcined waste clamshell-activated plastic film for food packaging. Materials 2018, 11, 1370. [Google Scholar] [CrossRef]
- Watanabe, T.; Fujimoto, R.; Sawai, J.; Kikuchi, M.; Yahata, S.; Satoh, S. Antibacterial characteristics of heated scallop-shell nano-particles. Biocontrol Sci. 2014, 19, 93–97. [Google Scholar] [CrossRef]
- Xing, R.; Qin, Y.; Guan, X.; Liu, S.; Yu, H.; Li, P. Comparison of antifungal activities of scallop shell, oyster shell and their pyrolyzed products. Egypt. J. Aquat. Res. 2013, 39, 83–90. [Google Scholar] [CrossRef]
- Topić Popović, N.; Lorencin, V.; Strunjak-Perović, I.; Čož-Rakovac, R. Shell waste management and utilization: Mitigating organic pollution and enhancing sustainability. Appl. Sci. 2023, 13, 623. [Google Scholar] [CrossRef]
- Durak, H.; Aysu, T. Thermochemical liquefaction of algae for bio-oil production in supercritical acetone/ethanol/isopropanol. J. Supercrit. Fluids 2016, 111, 179–198. [Google Scholar] [CrossRef]
- Welton, T. Solvents and sustainable chemistry. Proc. R. Soc. A: Math. Phys. Eng. Sci. 2015, 471, 20150502. [Google Scholar] [CrossRef]
- Chanwetprasat, P.; Seangarun, C.; Seesanong, S.; Boonchom, B.; Laohavisuti, N.; Boonmee, W.; Rungrojchaipon, P. Effect of Citric Acid Concentration on the Transformation of Aragonite CaCO3 to Calcium Citrate Using Cockle Shells as a Green Calcium Source. Materials 2025, 18, 2003. [Google Scholar] [CrossRef]
- Prihanto, A.; Muryanto, S.; Ismail, R.; Jamari, J.; Bayuseno, A. Utilization of green mussel shell waste for calcium carbonate synthesis through the carbonation method with temperature variation. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Semarang, Indonesia, 1 September 2022; p. 012022. [Google Scholar]
- Boey, P.-l.; Maniam, G.P.; Abd Hamid, S.; Ali, D.M.H. Utilization of waste cockle shell (Anadara granosa) in biodiesel production from palm olein: Optimization using response surface methodology. Fuel 2011, 90, 2353–2358. [Google Scholar] [CrossRef]
- Ruslan, H.N.; Muthusamy, K.; Jaafar, M.; Zamri, N.A.; Jaya, R.P. Mechanical properties of mortar with Anadara granosa waste as partial sand replacement. Open Civ. Eng. J. 2023, 17, e187414952303280. [Google Scholar] [CrossRef]
- Seesanong, S.; Wongchompoo, Y.; Boonchom, B.; Sronsri, C.; Laohavisuti, N.; Chaiseeda, K.; Boonmee, W. Economical and environmentally friendly track of biowaste recycling of scallop shells to calcium lactate. ACS Omega 2022, 7, 14756–14764. [Google Scholar] [CrossRef]
- Jang, W.Y.; Pyun, J.C.; Chang, J.H. Comparative In Vitro dissolution assessment of calcined and uncalcined hydroxyapatite using differences in bioresorbability and biomineralization. Int. J. Mol. Sci. 2024, 25, 621. [Google Scholar] [CrossRef]
- Rimsueb, N.; Cherdchom, S.; Aksornkitti, V.; Khotavivattana, T.; Sereemaspun, A.; Rojanathanes, R. Feeding cells with a novel “trojan” carrier: Citrate nanoparticles. Acs Omega 2020, 5, 7418–7423. [Google Scholar] [CrossRef]
- Groarke, R.; Vijayaraghavan, R.K.; Powell, D.; Rennie, A.; Brabazon, D. Powder characterization—Methods, standards, and state of the art. Fundam. Laser Powder Bed Fusion. Met. 2021, 491–527. [Google Scholar] [CrossRef]
- Khan, M.M. X-ray Fluorescence Spectroscopy. In Photocatalysts: Synthesis and Characterization Methods; Elsevier: Amsterdam, The Netherlands, 2025; pp. 225–237. [Google Scholar]
- Sronsri, C.; Boonchom, B. Thermal kinetic analysis of a complex process from a solid-state reaction by deconvolution procedure from a new calculation method and related thermodynamic functions of Mn0.90Co0.05Mg0.05HPO4·3H2O. Trans. Nonferrous Met. Soc. China 2018, 28, 1887–1902. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, L.; Yu, P.; Tang, N.; Liu, L.; Wang, W.; Wang, P.; Yang, Q.; Guo, S.; Li, J. Comparison and development of scanning electron microscope techniques for delicate plant tissues. Plant Sci. 2024, 340, 111963. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Green, W.H. Computing kinetic solvent effects and liquid phase rate constants using quantum chemistry and COSMO-RS methods. J. Phys. Chem. A 2023, 127, 5637–5651. [Google Scholar] [CrossRef]
- Varghese, J.J.; Mushrif, S.H. Origins of complex solvent effects on chemical reactivity and computational tools to investigate them: A review. React. Chem. Eng. 2019, 4, 165–206. [Google Scholar] [CrossRef]
- Schwaller, P.; Vaucher, A.C.; Laino, T.; Reymond, J.-L. Prediction of chemical reaction yields using deep learning. Mach. Learn. Sci. Technol. 2021, 2, 015016. [Google Scholar] [CrossRef]
- Chandrajith, V.; Marapana, R. Physicochemical characters of bark exudates of Lannea coromandelica and its application as a natural fruit coating. J. Pharmacogn. Phytochem. 2018, 7, 1798–1802. [Google Scholar]
- Fakheri, H.; Tayyari, S.F.; Heravi, M.M.; Morsali, A. Low frequency vibrational spectra and the nature of metal-oxygen bond of alkaline earth metal acetylacetonates. J. Mol. Struct. 2017, 1150, 340–348. [Google Scholar] [CrossRef]
- Xiao, H.; Hu, C.; Chen, C.; Tao, C.; Wu, Y.; Jiang, J. The advantage of alcohol–calcium method on the formation and the stability of vaterite against ethanol–water binary solvent method. J. Mater. Res. 2020, 35, 289–298. [Google Scholar] [CrossRef]
- Mahmood, S.K.; Zakaria, M.Z.A.B.; Razak, I.S.B.A.; Yusof, L.M.; Jaji, A.Z.; Tijani, I.; Hammadi, N.I. Preparation and characterization of cockle shell aragonite nanocomposite porous 3D scaffolds for bone repair. Biochem. Biophys. Rep. 2017, 10, 237–251. [Google Scholar] [CrossRef]
- Kaduk, J.A. Crystal structures of tricalcium citrates. Powder Diffr. 2018, 33, 98–107. [Google Scholar] [CrossRef]
- Mansour, S.A. Thermal decomposition of calcium citrate tetrahydrate. Thermochim. Acta 1994, 233, 243–256. [Google Scholar] [CrossRef]
- Hussain, M.H.; Abu Bakar, N.F.; Mustapa, A.N.; Low, K.-F.; Othman, N.H.; Adam, F. Synthesis of various size gold nanoparticles by chemical reduction method with different solvent polarity. Nanoscale Res. Lett. 2020, 15, 140. [Google Scholar] [CrossRef] [PubMed]
- Zobel, M.; Neder, R.B.; Kimber, S.A. Universal solvent restructuring induced by colloidal nanoparticles. Science 2015, 347, 292–294. [Google Scholar] [CrossRef]
- Palermo, A.; Naciu, A.M.; Tabacco, G.; Manfrini, S.; Trimboli, P.; Vescini, F.; Falchett, A. Calcium citrate: From biochemistry and physiology to clinical applications. Rev. Endocr. Metab. Disord. 2019, 20, 353–364. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, C.; Su, M.; Zhou, D.; Tao, Z.; Wu, S.; Xiao, L.; Li, Y. Development of citric acid-based biomaterials for biomedical applications. J. Mater. Chem. B 2024, 12, 11611–11635. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).