Abstract
The yeast metabolic and evolutionary engineering, especially Saccharomyces cerevisiae, plays a significant role in the enhancement of its industrial applications in food, beverage, and biofuel production. This review integrates genetic engineering, systems biology, and evolutionary principles to optimize yeast performance, adaptability, and productivity. The key strategies which enable targeted genome modifications to improve substrate utilization, stress tolerance, and the biosynthesis of valuable metabolites such as flavor compounds, organic acids, vitamins, and antioxidants, including precise gene editing, notably CRISPR-Cas9. The metabolic pathway optimization through gene overexpression, deletion, and heterologous pathway integration, supported by multi-omics analyses and the Subcellular compartmentalization of metabolic pathways, which enhances biosynthetic efficiency. This review then discusses evolutionary engineering and global transcription machinery engineering by leveraging natural selection and global gene regulation to improve complex traits. The exploration of non-Saccharomyces species and genome shuffling expands the genetic toolkit for strain development. Emerging approaches, including machine learning and synthetic biology, are accelerating rational strain design. By critically synthesizing these diverse methodologies, this review highlights current advancements, identifies key challenges, and outlines future directions in engineering robust yeast strains for sustainable food biotechnology.
1. Introduction
Food yeasts, such as Saccharomyces cerevisiae, are widely used in industries for producing bread, beer, wine, biofuels, and other valuable compounds. These yeasts’ metabolic and evolutionary engineering aims to enhance their performance, adaptability, and productivity in industrial applications. Fermentation, a cornerstone of modern industrial biotechnology, plays a pivotal role in diverse sectors, including food, medicine, water, environment, energy, and construction, facilitating the chemical transformation of organic matter through microbial metabolism mediated by a myriad of enzymes [1].
Engineering microbial fermentation offers distinct advantages over multicellular tissue culture, including less fastidious growth requirements, significantly faster growth cycles, and reduced ethical and market resistance, particularly in biomedical and food applications. The capacity to harness microorganisms as production platforms has spurred the development of innovative tools aimed at enhancing and accelerating the creation of microbial-based foods, thereby surmounting existing limitations [2]. These advancements have led to the establishment of numerous startup companies focused on engineering microorganisms for the production of a wide array of food compounds, poised to exert an even greater impact on the food industry [3].
Recent advances in food yeast engineering involve the integration of advanced biotechnological tools, such as genome-scale metabolic modeling, systems biology, and synthetic biology [4,5,6]. These tools help in the design and optimization of yeast strains precisely for the production of a wide range of food ingredients, including flavors, fragrances, and nutraceuticals [1,7]. Additionally, the exploration of non-conventional yeast species, such as oleaginous red yeasts, offers new opportunities for the production of valuable compounds such as oleochemicals and specialty chemicals [8].
Despite significant developments, there is a wide range of limitations in the traditional yeast strains, including narrow substrate range, low tolerance to industrial stresses, and limited metabolic flexibility, which limits their applications in the industry. Meanwhile, the global demand for sustainable, efficient food and bio-based production systems is also growing. As a result, there is an increasing demand for the development of technological advancements that enable precise genetic modifications and adaptive improvement of microbial strains.
This review aims to provide a comprehensive and integrative overview of recent advancements in yeast strain development for industrial biotechnology. This review discusses the strategies to improve the performance, adaptability, and sustainability of food yeasts. This typically focuses on metabolic or evolutionary strategies in isolation, a unified framework combining both, while also incorporating cutting-edge tools such as CRISPR-Cas9, multi-omics, and machine learning. Notably, it expands the scope by discussing the underexplored potential of non-Saccharomyces yeasts and highlighting compartmentalized metabolic pathway engineering within organelles. The review addresses key gaps in the field, including the fragmented understanding of integrated engineering strategies, the need for predictive and AI-driven approaches, and challenges related to strain stability and regulatory hurdles. Through this synthesis, the review contributes novel insights and practical frameworks for advancing food yeast biotechnology.
2. Metabolic Engineering
Metabolic engineering stands as a pivotal discipline that leverages genetic engineering, systems biology, and evolutionary principles to optimize cellular processes for the enhanced production of desired compounds [9]. This interdisciplinary field empowers scientists to directly manipulate flavors in fermented foods, enabling the safe and economical production of flavor components [10]. Employing metabolic engineering strategies in food yeast, such as S. cerevisiae, allows for the augmentation of endogenous metabolic pathways to enhance the synthesis of targeted metabolites or the introduction of novel pathways to produce non-native compounds [11]. The application of metabolic engineering to food yeast involves several key strategies, including gene overexpression, gene deletion, and heterologous pathway introduction (Table 1). Overexpression of rate-limiting enzymes within a metabolic pathway can effectively increase the flux towards the desired product, while gene deletion can eliminate competing pathways or prevent the degradation of the target compound. The introduction of entirely new metabolic pathways from other organisms enables food yeast to produce novel compounds that it would not normally synthesize [12]. These genetic manipulations are often guided by a deep understanding of the yeast’s metabolic network and regulatory mechanisms, often obtained through transcriptomic and proteomic analyses [13]. Recent advancements in machine learning have further revolutionized metabolic engineering, allowing for the analysis of large datasets and the prediction of optimal genetic modifications to achieve desired metabolic outcomes [14]. While S. cerevisiae remains the primary model organism, the expanding use of non-Saccharomyces yeasts with distinctive metabolic capabilities has opened new avenues for flavor diversification and stress resilience, which are increasingly being integrated into metabolic engineering efforts to broaden the scope of fermented food products.
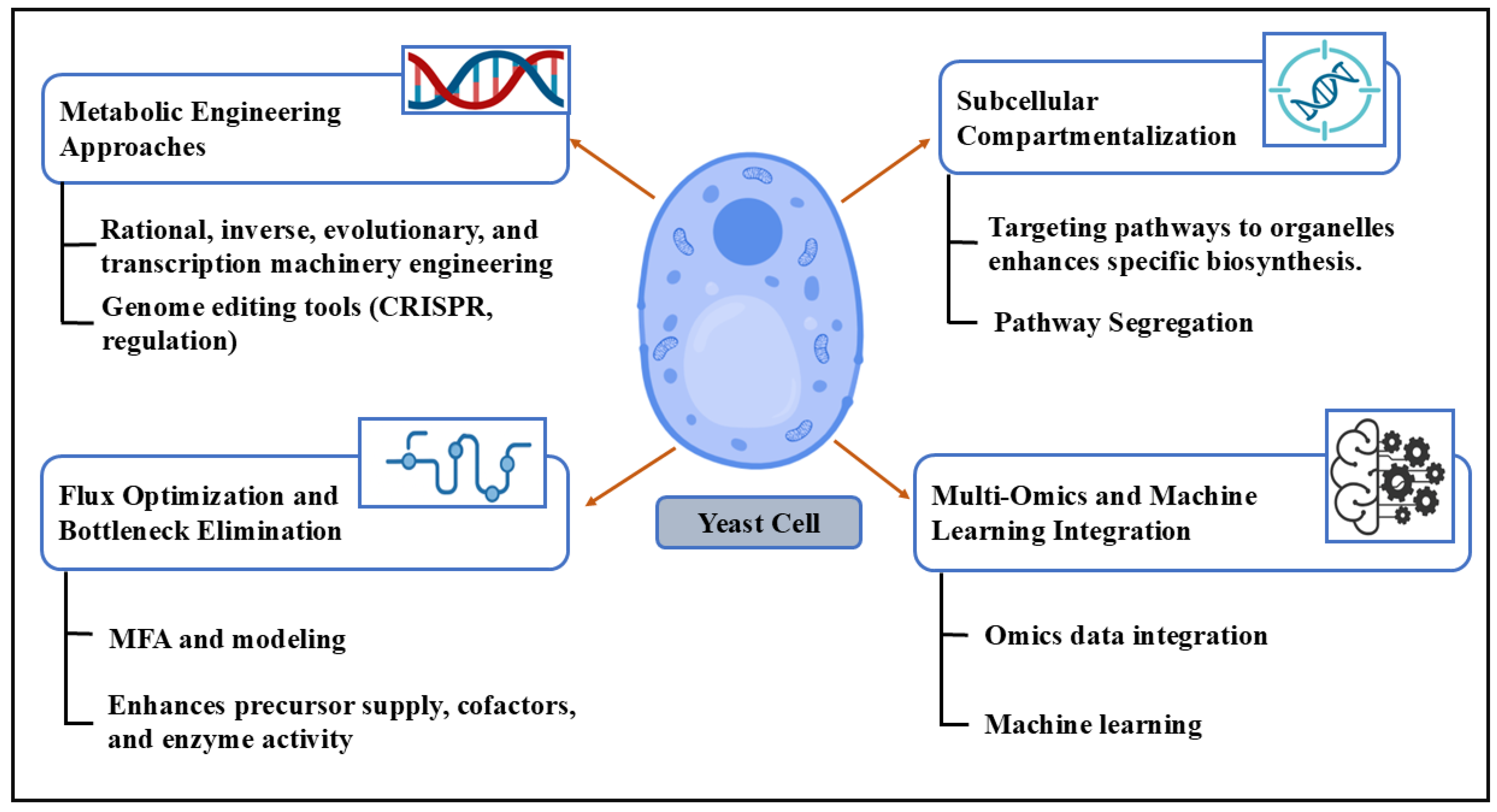
Metabolic engineering involves modifying the yeast’s metabolic pathways to optimize the production of desired compounds (Figure 1). This includes the following:
Figure 1.
Different strategies used in the metabolic engineering of yeast. This schematic illustrates key approaches in yeast metabolic engineering, including targeted genetic modifications, flux pathway optimization, subcellular pathway compartmentalization, and the integration of multi-omics and machine learning. These strategies collectively enhance biosynthetic efficiency, pathway regulation, and predictive capabilities in engineered yeast strains.
2.1. Gene Editing
The burgeoning global population and the imperative for sustainable food production have amplified the significance of food yeasts in diverse applications, ranging from baking and brewing to single-cell protein production and flavor enhancement. Traditional methods of strain improvement, such as random mutagenesis and selection, have been instrumental in shaping the characteristics of industrial yeast strains [11]. However, these approaches are often laborious, time-consuming, and lack the precision required to introduce specific, targeted modifications [15]. Gene editing technologies, particularly the CRISPR-Cas9 system, have emerged as powerful tools for precise and efficient manipulation of the yeast genome, offering unprecedented opportunities to tailor strains for enhanced performance in food-related processes [16]. The advent of CRISPR-Cas9 technology has revolutionized the landscape of yeast genetic engineering, enabling researchers to make targeted modifications with remarkable precision and efficiency [13].
One of the primary applications of gene editing in food yeasts lies in expanding their substrate utilization capabilities. Many wild-type yeast strains are limited in their ability to utilize complex carbohydrates, such as starch or cellulose, which are abundant in agricultural waste streams. Engineering yeasts to efficiently metabolize these substrates can not only reduce production costs but also contribute to sustainable waste management. For instance, researchers have employed CRISPR-Cas9 to introduce or enhance the expression of genes encoding amylolytic enzymes, enabling yeast strains to effectively break down starch into fermentable sugars [17]. Similarly, gene editing has been used to improve the ability of yeasts to utilize xylose, a major component of lignocellulosic biomass, for ethanol production.
Beyond substrate utilization, gene editing can be leveraged to enhance the production of desired metabolites in food yeasts. This is particularly relevant in the context of flavor compound production, where specific metabolites contribute to the unique sensory profiles of fermented foods and beverages. For instance, researchers have used CRISPR-Cas9 to optimize the production of esters, which impart fruity and floral notes to beer and wine. Furthermore, gene editing can be employed to increase the production of other valuable compounds, such as organic acids, vitamins, and antioxidants, in food yeasts, thereby enhancing their nutritional value and functional properties.
Stress tolerance is another critical trait that can be improved through gene editing. Industrial fermentation processes often subject yeast strains to various stresses, including high ethanol concentrations, osmotic pressure, and temperature fluctuations. By identifying and manipulating genes involved in stress response pathways, researchers can engineer more robust strains that can withstand harsh conditions and maintain high productivity. For example, CRISPR-Cas9 has been used to enhance the expression of heat shock proteins, which protect cells from thermal damage, and to improve tolerance to osmotic stress by modulating the expression of genes involved in glycerol synthesis. Additionally, some yeasts exhibit remarkable tolerance to multiple stressors, such as high temperatures and lignocellulosic inhibitors, making them ideal candidates for bioethanol production from lignocellulosic biomass [18].
The application of gene editing in food yeasts also extends to the modification of cell wall structure and composition. The cell wall plays a crucial role in protecting yeast cells from environmental stresses and maintaining their structural integrity. Modifying cell wall components can have a significant impact on yeast cell properties, such as flocculation, adhesion, and enzyme accessibility. For instance, researchers have used CRISPR-Cas9 to alter the glycosylation patterns of cell wall proteins, leading to improved flocculation properties in brewing yeasts. Flocculation is the phenomenon by which yeast cells aggregate and settle out of the fermentation broth, facilitating their removal after fermentation.
Gene editing offers a promising avenue for tailoring food yeast strains with enhanced performance characteristics, but it is essential to address safety concerns and regulatory considerations associated with genetically modified organisms. While gene-edited organisms do not necessarily contain foreign DNA, they may still be subject to regulatory scrutiny depending on the specific modifications made and the intended application. As the understanding of yeast genetics and the capabilities of gene-editing technologies continue to advance, the potential for improving food yeasts through targeted genome manipulation is immense [1]. The development of a yeast strain with a genome containing over 50% synthetic DNA exemplifies the possibilities of genetic engineering [19]. The ongoing research and development efforts in this field hold great promise for enhancing the efficiency, sustainability, and nutritional value of food production processes.
The development of novel methods for the genetic analysis and modification of yeasts, along with genomic and post-genomic analysis, provides a platform for understanding the molecular mechanisms underlying simple and complex biological features, which are useful for developing novel and eco-compatible applications. In industrial settings, Saccharomyces cerevisiae has faced challenges in expanding its carbon source utilization, product synthesis, and tolerance to harsh conditions, prompting the exploration of alternative yeast species [20]. Importantly, the exploration of non-Saccharomyces yeasts, which exhibit unique metabolic pathways and enhanced stress tolerance, has broadened the scope for metabolic engineering and strain improvement. Non-Saccharomyces yeasts, with their unique metabolic pathways and stress tolerance mechanisms, offer a valuable resource for strain improvement. By employing gene editing tools, researchers can harness the desirable traits of non-Saccharomyces yeasts and transfer them to S. cerevisiae or other industrial strains, creating hybrid strains with superior performance characteristics. Intergeneric genome shuffling, combined with adaptive evolution, has proven to be an effective strategy for breeding strains with improved traits, such as glucose-xylose co-fermentation [21].
There exists strategies for metabolic engineering in food yeasts (Table 1). However, several factors contribute to the successful application of metabolic engineering in food yeasts. These factors include the availability of efficient transformation methods, the development of precise and reliable gene-editing tools, and a comprehensive understanding of yeast genetics and physiology. Delivery of CRISPR-Cas9 components into yeast cells can be achieved through various methods, including plasmid-based transformation, ribonucleoprotein delivery, and viral transduction. The choice of delivery method depends on the specific application and the characteristics of the target strain. Furthermore, the design of guide RNAs (gRNAs) is crucial for ensuring the specificity and efficiency of gene editing.

Table 1.
Strategies in metabolic engineering of food yeasts.
Table 1.
Strategies in metabolic engineering of food yeasts.
| Strategy | Description | Applications | References |
|---|---|---|---|
| Gene Overexpression | Amplifying rate-limiting enzymes to increase metabolite flux | Enhanced ester or flavor compound production in S. cerevisiae | [10,12] |
| Gene Deletion/Knockout | Removing genes that divert or degrade target compounds | Deleting competitive pathways to improve ethanol yield | [11] |
| Heterologous Pathway Introduction | Incorporating metabolic pathways from other organisms | Producing novel flavors or bioactive compounds | [12] |
| CRISPR-Cas9 Gene Editing | Using RNA-guided endonucleases for precise genome modifications | Enhancing stress tolerance, flavor production, and substrate utilization | [13,16] |
| Pathway Compartmentalization | Targeting pathways to specific organelles to boost efficiency | Directing ester synthesis to mitochondria or peroxisomes | [22] |
| Adaptive Laboratory Evolution | Evolving strains under selective pressures to improve traits | Developing yeast strains tolerating high ethanol or temperature | [21] |
2.2. Pathway Optimization
Food yeasts, distinguished by their capacity to enhance flavor, nutritional value, and overall quality, represent a cornerstone in food biotechnology. Metabolic engineering has emerged as a pivotal tool, enabling scientists to directly manipulate flavors in fermented foods, offering a safe and economical route to produce specific flavor components [10]. The inherent robustness, rapid growth rates, and well-characterized genetics of food yeasts render them amenable to genetic modification and metabolic pathway optimization [1,2]. The strategic reconstruction and expression of heterologous pathways, coupled with spatial optimization within the yeast cell, allows for enhanced production of target compounds [22]. By targeting metabolic pathway proteins to specific subcellular organelles, such as mitochondria, peroxisomes, the endoplasmic reticulum, and vacuoles, researchers can leverage the unique environments and distinct physicochemical properties of each organelle to optimize biosynthesis [22]. These approaches hold immense potential for tailoring food yeast strains to specific applications, enhancing the quality and diversity of food products.
Metabolic engineering strategies encompass a diverse array of approaches, including rational metabolic engineering, inverse metabolic engineering, evolutionary engineering, and global transcription machinery engineering [23]. Rational metabolic engineering involves the targeted manipulation of specific genes and pathways based on a deep understanding of cellular metabolism, enzyme kinetics, and regulatory mechanisms [24]. Inverse metabolic engineering, conversely, focuses on identifying the genetic basis of desired phenotypes through the analysis of mutant strains or naturally occurring variants with superior traits. Evolutionary engineering, another powerful approach, harnesses the principles of natural selection to improve desired traits in microorganisms [22]. Global transcription machinery engineering targets the modification of global transcriptional regulators to fine-tune the expression of multiple genes involved in complex metabolic pathways [25]. These approaches collectively underscore the versatility and power of metabolic engineering in optimizing food yeast strains for enhanced performance and desired characteristics [14]. Furthermore, by employing advanced techniques such as genome editing and transcriptional regulation, scientists can achieve precise control over metabolic fluxes, rerouting carbon flow towards desired compounds [26]. Combining these approaches promises to create new yeast strains that can synthesize valuable molecules [27].
Optimizing metabolic pathways in food yeasts involves a multifaceted approach that integrates genetic engineering, enzyme kinetics, and systems biology. Firstly, enhancing precursor supply, such as pyruvate, is crucial for augmenting the production of target metabolites [28]. Secondly, optimizing cofactor availability is essential, as many metabolic enzymes rely on specific cofactors, such as NADH or NADPH, for their activity. Thirdly, relieving feedback inhibition, a common regulatory mechanism in metabolic pathways, can unlock increased flux towards desired products. Fourthly, improving enzyme activity through protein engineering can also enhance metabolic flux. These engineering strategies are underpinned by the integration of multi-omics data, including genomics, transcriptomics, proteomics, and metabolomics, which provide a holistic understanding of cellular metabolism and regulatory networks. Ultimately, the study’s purpose determines the different downstream studies that are performed, such as enrichment analysis or PCA-based phylogenetic analysis [29]. In addition, the use of compartmentalization strategies, like segregating pathways, helps reduce metabolic cross-talk [30].
Enhancing glycolytic flux represents a pivotal strategy for improving the performance of food yeasts, and is achieved through augmenting the rate of sugar uptake, which directly boosts the productivity of microbial catalysts [24]. Overexpressing homologous or heterologous genes encoding glycolytic enzymes, however, has often proven unsuccessful in enhancing glycolytic flux [24]. In contrast, significant enhancement in glycolytic flux has been observed in studies with bacteria, specifically, Corynebacterium glutamicum [24]. The underlying reason could be the deregulation of transcription factors in C. glutamicum. Genome-scale modeling and constraint-based flux analysis have become a valuable tool for calculating intracellular fluxes based on the complex stoichiometric relationships of metabolites within the metabolic network [31]. Analyzing these flux distributions reveals critical information about the rate-limiting steps in metabolic pathways, the degree of pathway activation, and the overall efficiency of carbon utilization [32]. By systematically analyzing and optimizing the expression of key enzymes, scientists can fine-tune the metabolic network to achieve maximal flux toward desired products. Furthermore, advancements in analytical techniques, such as high-resolution mass spectrometry and liquid chromatography, enable the precise quantification of intracellular metabolite concentrations, providing valuable insights into pathway dynamics and regulatory mechanisms [29].
Metabolic flux analysis, combined with gene expression analysis, offers a powerful approach to elucidate the underlying mechanisms responsible for enhanced performance in engineered cells [33]. By simultaneously measuring gene expression levels and metabolic fluxes, researchers can identify key regulatory genes and metabolic bottlenecks that limit pathway productivity [34]. Subsequently, pathway enrichment analysis can be used to classify the enriched pathways within the cell [29]. This information can then be used to inform further engineering strategies. For instance, in silico genome-scale models are promising tools that accelerate the design of cells with improved and desired properties [35]. However, these models need to be validated in vivo to ensure their reliability and accuracy [35]. Such models predict cell phenotypes through flux balance analysis, where a cellular objective is used to achieve realistic flux distributions [36].
Multi-omics data integration and machine learning offer powerful tools for analyzing and optimizing complex metabolic networks [29]. The integration of transcriptomics data with flux balance analysis can help decipher cell metabolism [37]. Correlation networks visualize disruptions to community structure and can examine the interactions between environmental factors, metabolites, and clinical features [29]. Moreover, machine learning algorithms can be used to predict the effects of genetic perturbations on metabolic phenotypes, facilitating the design of strains with enhanced capabilities [38].
3. Evolutionary Engineering of Food Yeast
Evolutionary engineering, also known as adaptive laboratory evolution, is a complementary approach to metabolic engineering that harnesses the power of natural selection to improve desired traits in microorganisms. This method involves subjecting microbial populations to selective pressures that favor the evolution of specific characteristics, such as increased tolerance to stress conditions, enhanced substrate utilization, or improved product yield. Unlike directed genetic modifications, evolutionary engineering relies on spontaneous mutations and recombination events to drive adaptation. Typically, microbial populations undergo long-term cultivation under controlled laboratory conditions, designed to select for the desired phenotype. The process typically involves long-term cultivation of microbial populations under controlled laboratory conditions, where the environment is designed to select for the desired phenotype. Over time, beneficial mutations accumulate within the population, leading to gradual improvement in the targeted trait. The evolved strains can then be analyzed to identify the genetic changes responsible for the observed phenotypic improvements, providing valuable insights into the underlying mechanisms of adaptation. By applying evolutionary engineering to food yeast, researchers can enhance its performance in industrial fermentation processes, improve its ability to utilize low-cost substrates, and increase its tolerance to inhibitory compounds. Moreover, evolutionary engineering can be used to diversify the metabolic capabilities of food yeast, enabling it to produce a wider range of flavor compounds and other valuable metabolites [27]. Integration of multi-omics data with machine learning techniques enhances our ability to understand host-microbe interactions, providing a comprehensive view of the gut microbiome’s functional characteristics. This integration facilitates the discovery of novel prebiotics and probiotics and the development of personalized dietary interventions to modulate the gut microbiome for improved health outcomes [39,40].
Evolutionary engineering uses natural selection principles to improve yeast strains. This approach involves:
3.1. Adaptive Evolution
Adaptive evolution plays a pivotal role in the domestication and improvement of food yeasts, enabling these microorganisms to thrive in specific industrial environments and enhance desired traits for food production. Yeasts, particularly Saccharomyces cerevisiae, have been integral to human society for millennia, underpinning the production of bread, beer, wine, and other fermented foods [41]. The ability of yeasts to efficiently convert sugars into ethanol and carbon dioxide during fermentation, a process elucidated by Louis Pasteur in the 19th century, has been a cornerstone of the food and beverage industry [42]. The selection and propagation of yeast strains with superior fermentation capabilities, stress tolerance, and flavor profiles have been crucial for optimizing food production processes [43]. This process can occur naturally or through human intervention. Microbes’ ability to transform organic matter through enzymatic reactions is also important [1].
The exploitation of natural yeast diversity represents a powerful strategy for obtaining strains with enhanced industrial performance [15]. Wild yeast strains often exhibit unique characteristics, such as tolerance to high sugar concentrations, elevated temperatures, or inhibitory compounds present in industrial substrates. Adaptive laboratory evolution is a technique used to mimic natural selection in a controlled environment [10]. By subjecting yeast populations to prolonged exposure to specific stress conditions, such as high ethanol concentrations or nutrient limitation, researchers can drive the selection of mutants with improved fitness [44]. These adaptive mutations can arise through various mechanisms, including single-nucleotide polymorphisms, copy number variations, and chromosomal rearrangements. Genome resequencing analysis can unravel genomic changes, such as the loss of a 146 kb fragment in a hybrid strain, which was regained after being evolved for a while, and the one-copy loss of an 11 kb fragment only found after being evolved for a longer time [21]. In addition, the overexpression of the *GPD1* gene encoding glycerol-3-phosphate dehydrogenase has been shown to enhance osmotolerance in S. cerevisiae. Adaptive evolution strategies have successfully improved various industrially relevant traits in yeasts, including ethanol production, xylose utilization, and thermotolerance.
The genetic manipulation of yeasts has emerged as a complementary approach to adaptive evolution, enabling the introduction of targeted modifications to enhance specific traits. Metabolic engineering involves the deliberate modification of metabolic pathways to optimize the production of desired compounds or improve substrate utilization. Through the incorporation of entire biosynthetic pathways into S. cerevisiae, the fitness and variety of biochemicals that can be synthesized from this microorganism have expanded [45]. For instance, researchers have engineered S. cerevisiae to produce valuable metabolites, such as biofuels, pharmaceuticals, and flavor compounds. Combining adaptive evolution with genetic engineering can lead to synergistic improvements in yeast performance. Through metabolic pathway optimization, spatial optimization, and protein localization, the production of target compounds can be enhanced [22].
The use of genome-scale metabolic models has significantly accelerated the rational design of cells with improved and desired properties [35]. The compartmentalization of metabolic pathways offers advantages such as using each organelle’s environment for compound biosynthesis and distinct physiochemical properties [22]. For example, engineering organelles in yeast has been shown to enhance the production of protopanaxadiol through the manipulation of peroxisome proliferation in Saccharomyces cerevisiae [22]. By integrating experimental data with computational models, researchers can predict the effects of genetic modifications on yeast metabolism and identify optimal engineering strategies. Furthermore, global transcription machinery engineering, inverse metabolic engineering, and evolutionary engineering are important strategies in yeast strain improvement [23]. However, the introduction of synthetic pathways may lead to several problems that compromise the fitness and the ecological and evolutionary stability of the yeasts [46]. These can include, for example, competition for resources, accumulation of toxic intermediates, or instability of the synthetic pathway [47]. Synthetic biology approaches enable the construction of novel metabolic pathways and regulatory circuits, expanding the functional capabilities of food yeasts. For example, biologists have produced a strain of yeast whose genome is more than 50% synthetic DNA [19].
The application of systems biology approaches, including genomics, transcriptomics, proteomics, and metabolomics, provides a comprehensive understanding of yeast physiology and metabolism [22]. By analyzing the global changes in gene expression, protein abundance, and metabolite profiles in response to different environmental conditions or genetic modifications, researchers can gain insights into the complex regulatory networks that govern yeast behavior [9].
3.2. Genome-Wide Mutations
Introducing random mutations creates genetic diversity and allows the selection of strains with improved traits. The domestication and directed evolution of food yeasts, particularly Saccharomyces cerevisiae, have been instrumental in shaping various industries, including baking, brewing, and winemaking [15]. The inherent genetic plasticity of yeasts, coupled with human intervention, has led to the selection and propagation of strains exhibiting desirable traits such as enhanced fermentation efficiency, improved flavor profiles, and tolerance to specific environmental conditions [43]. Genome-wide mutations, encompassing single-nucleotide polymorphisms, insertions, deletions, and copy number variations, constitute the raw material upon which natural selection and artificial selection act [21]. Over time, the accumulation of these mutations has driven the divergence of domesticated yeast strains from their wild progenitors, resulting in specialized lineages optimized for particular industrial applications. In a study, a strain of wine yeast, S. cerevisiae has been evolved for the enhanced production of glutathione (GSH) by selecting for molybdate resistance. The resulting strain carried an extra copy of the chromosome and showed altered gene expression, contributing to its increased glutathione production. This non-GMO evolutionary strategy demonstrates how chromosomal alterations and spontaneous mutations can be made to improve antioxidant production in food yeasts, thereby improving the quality of the product and reducing the need for additives like sulfur dioxide [48]. Understanding the nature and impact of these mutations is crucial for further enhancing the performance of food yeasts and developing novel strains with tailored functionalities.
The application of CRISPR-Cas9 technology has revolutionized the precision and efficiency with which the S. cerevisiae genome can be modified, allowing for targeted editing of native genetic elements and the inclusion of heterologous pathways [16]. This approach overcomes the limitations of standard cloning and integrating methods [49]. The use of synthetic biology to create new yeast platforms can solve previous genetic manipulation issues [20]. This technology facilitates gene knock-out and knock-in, and transcriptional activation and interference [50]. Omics technologies, including genomics, transcriptomics, and proteomics, provide invaluable insights into the functional consequences of genome-wide mutations in food yeasts [13]. These approaches enable researchers to correlate specific genetic changes with phenotypic variations, unraveling the complex interplay between genotype and phenotype [1]. The USDA has declared that macrofungi that are genetically altered via CRISPR-Cas9 are exempt from regulatory oversight, due to the high amount of protein, fiber, vitamins, and antioxidants that they contain [1]. These technologies are revolutionizing the creation of new functional food products. A strain of yeast with more than 50% synthetic DNA has been produced [19]. Standard brewer’s yeast stores its genetic code across 16 chromosomes, and in the new strain, 6.5 of those chromosomes were edited and synthesized in the laboratory [19].
The ability to introduce targeted genomic sequence changes into living cells and organisms provides a powerful tool for biological research as well as a potential avenue for the therapy of genetic diseases [51].
4. Different Approaches
By visualizing evolution in real time, researchers can gain a deeper understanding of the adaptive landscape and identify the key genetic changes that drive microbial evolution [52]. The integration of theoretical microbial ecology with engineering principles promotes the development of predictive frameworks, facilitating the design and optimization of engineered water systems for sustainable bioprocesses [53]. The continuous development of novel technologies, such as advanced NGS platforms and sophisticated MS techniques, are refining the instruments used in multi-omics research, enabling the capture of a more nuanced and comprehensive view of microbial communities [29].
Several strategies are employed in yeast engineering:
4.1. Targeted Genetic Modifications
Targeted genetic modifications involve precise changes to the yeast genome to obtain the desired traits. These alterations are often directed by metabolic engineering and synthetic biology tools. For instance, CRISPR/Cas9 systems have been widely accepted for single-nucleotide resolution editing, multiple-gene editing, and transcriptional regulation in yeast. Such tools have enhanced the development of yeast cell factories for the sustainable production of biofuels, chemicals, and pharmaceuticals [54,55]. Metabolic engineering strategies aim to redirect metabolic fluxes to obtain desired products, improve substrate utilization, and enhance physiological properties. For example, yeast has been genetically engineered to produce free fatty acids and their derivatives, such as fatty alcohols and esters, by modifying fatty acid biosynthesis and lipid accumulation pathways [56,57]. These strategies involve heterologous pathway optimization, such as introducing plant-derived enzymes to produce polyphenols, which are significant for their antioxidant and therapeutic properties [58].
Multiplex genome engineering methods, including delta integration and rDNA cluster integration, have evolved to enable simultaneous modifications at multiple genomic loci. These techniques, along with CRISPR/Cas tools, have significantly accelerated the efficiency and developed accurate strains. For instance, the integration of multiple copies of a gene is facilitated by pre-placed gate systems, which streamlines the development of intricate heterologous pathways [59].
4.2. Directed Evolution
Directed evolution is an influential strategy that refers to mimicking the process of natural selection in a controlled laboratory environment to develop yeast strains and obtain the desired traits. It helps to enhance the adaptation of the yeast to specific selective pressures in the environment. This strategy involves exposing the populations of the yeast to the conditions that create the situation of emergence and selection of variants with desired traits, without necessarily requiring in-depth knowledge of the principles of genetic engineering [60]. This strategy combines evolutionary principles with advanced genome editing technologies to generate desired properties in the yeast strains. For example, genome-scale modifications have been used to study stress resistance, metabolic pathway optimization, and organismal adaptation [5].
Evolutionary engineering techniques, such as continuous evolution and adaptive laboratory evolution, have been utilized to enhance the phenotypic traits of the yeast. These methods involve selecting desired phenotypes under specific conditions, such as high ethanol tolerance or thermotolerance. For instance, non-conventional yeasts like Yarrowia lipolytica and Kluyveromyces marxianus have been engineered for enhanced biofuel production using evolutionary approaches [61,62]. The integration of evolutionary engineering with synthetic biology has enabled the development of robust production hosts. For example, yeast strains have evolved to utilize alternative carbon sources, such as CO2, methanol, and acetate, for the sustainable production of lipids and biofuels [56,63].
4.3. Synthetic Biology
The integration of biology, mathematics, chemistry, biophysics, and automation to construct synthetic enzymes, circuits, pathways, chromosomes, and organisms in a systematic, modular, and standardized fashion is synthetic biology [64]. It has advanced efforts to introduce modular cloning (MoClo) systems, synthetic promoters, and terminators, create dynamic regulatory circuits, and enable the synthesis of efficient metabolic pathways. These advances, along with the systems biology strategy, have altered yeasts to become a potent and manageable set of host organisms [65]. This synthetic genome has allowed unprecedented control over cellular function, opening the door to tailor-made biological solutions [66,67]. Synthetic biology has focused on the design of new building blocks, such as promoters, terminators, and enzymes, to engineer yeast metabolism. For example, synthetic signal peptides and codon-optimized genes have been developed to enhance protein secretion and folding in yeast cell factories [68,69].
Pathway engineering involves the construction of heterologous pathways to produce specific compounds. For instance, yeast has been engineered to produce polypeptides, terpenes, and commodity chemicals by harnessing endogenous pathways and introducing novel enzymes [70,71]. Gene circuits, such as feedback regulation systems, have been designed to optimize pathway performance and ensure predictable behaviors in engineered strains [55,62].
5. Current State-of-the-Art Technologies and Advancements
There are advanced genome editing technologies which enable precise and effective modification of the yeast genome and the integration of novel metabolic pathways, which transform yeast into a powerful cell factory for the synthesis of valuable industrial products [65]. There exists several strategies for gene editing in food yeasts (Table 2).
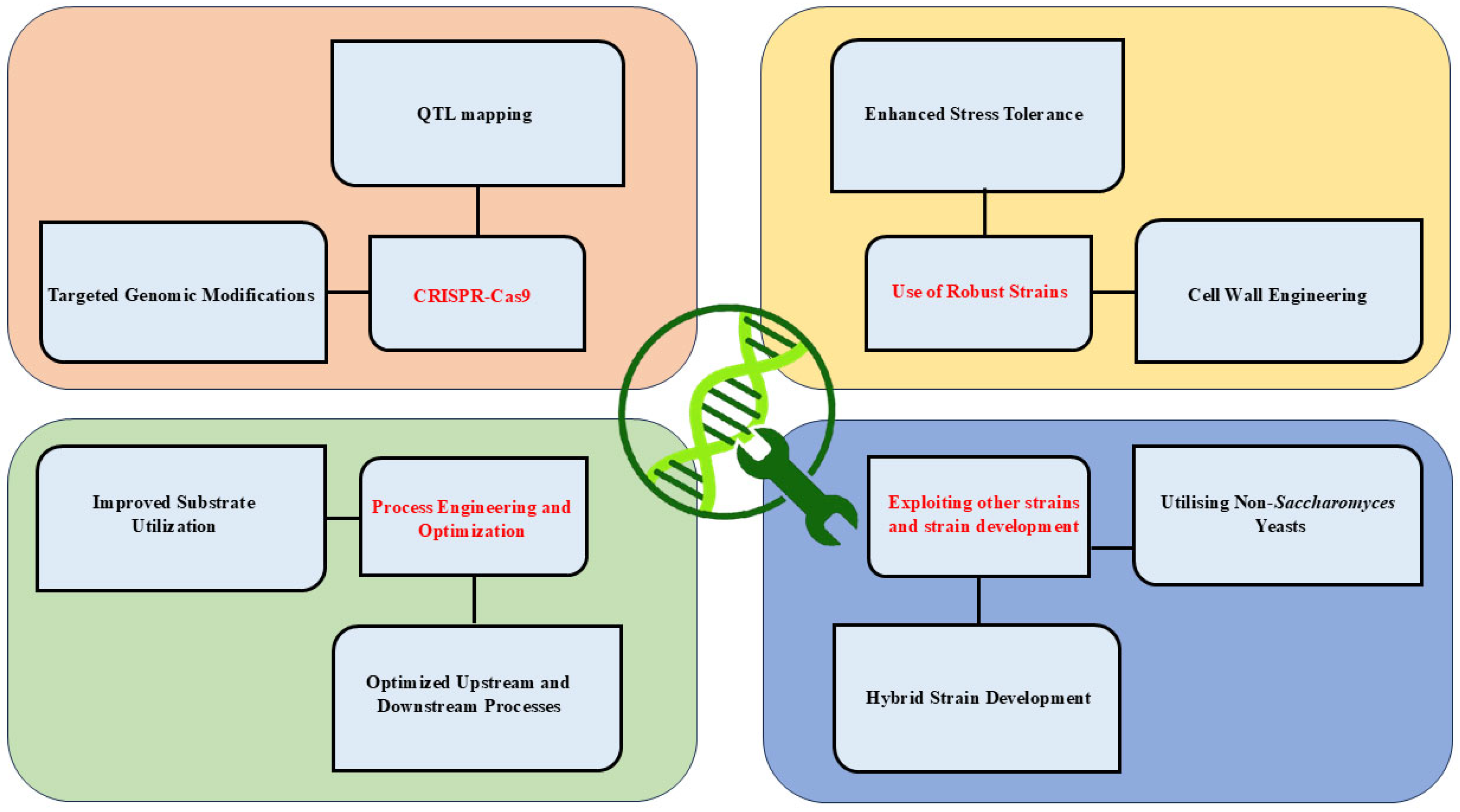
There are several strategies for strain improvement and others (Figure 2). Recent advancements in yeast engineering include:
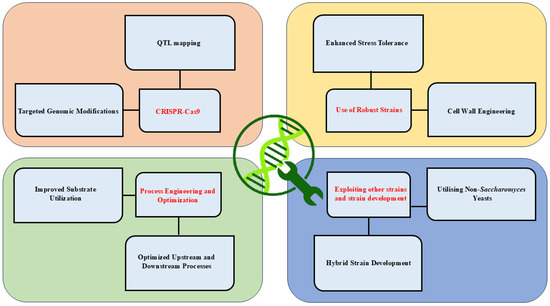
Figure 2.
Strategies for yeast strain improvement in industrial biotechnology. This figure highlights diverse strategies for enhancing yeast strains, including genetic modifications, process engineering, stress tolerance, and exploitation of non-conventional yeast species. These approaches aim to improve substrate utilization, stress resilience, and production efficiency, enabling robust industrial applications.
5.1. CRISPR-Cas9 Technology
The development of the CRISPR-Cas system has revolutionized genome editing by enabling unprecedented precision and efficiency [65]. The CRISPR–Cas9 system is the most widely used gene editing tool among the various CRISPR systems. It consists of the Cas9 protein, CRISPR RNA (crRNA), and trans-activating RNA, and enables gene deletion and the integration of expression cassettes [72,73]. This technique has been utilized to edit multiple genes simultaneously, enhance metabolic pathways, and establish yeast libraries for high-throughput screening. Previously, several challenges related to gene editing were faced in yeast metabolic engineering. However, with the development of high-efficiency gene editing techniques, genetic manipulation has been explored in both conventional yeasts, such as S. cerevisiae and nonconventional yeast, including Schizosaccharomyces pombe, Pichia pastoris, Yarrowia lipolytica, Candida tropicalis, Starmerella bombicola, and Methylotrophic yeasts, among others. The CRISPR-associated protein Cas9 enables precise and simultaneous editing of multiple gene loci or genes, offering a significant advantage over conventional genome editing methods [74].
The CRISPR-Cas9 system, derived from bacterial adaptive immune systems, relies on a customizable RNA guide molecule (sgRNA) to direct the Cas9 endonuclease to a specific DNA sequence in the genome, where it induces a double-stranded break [75]. This break triggers the cell’s natural DNA repair mechanisms, which can be harnessed to introduce desired changes, such as gene deletions, insertions, or point mutations. The simplicity, modularity, and scalability of the CRISPR-Cas9 system have made it widely accessible to researchers, facilitating rapid advancements in yeast strain engineering [50]. The efficiency and precision of CRISPR-Cas9-mediated gene editing have opened new avenues for improving key traits in food yeasts, including enhanced substrate utilization, increased product yield, improved stress tolerance, and novel flavor compound production. The basic components of the CRISPR system are highly similar in practice across different organisms; many findings are directly applicable to the entire field and are not restricted to only Saccharomyces or fungi [49].
In agriculture, CRISPR-Cas9 offers a promising approach for crop improvement by modifying genes related to disease resistance, yield, and nutritional content [17]. The CRISPR/Cas9 system has revolutionized the field of genome engineering through the efficient creation of targeted breaks in the DNA of almost any organism and cell type, opening an avenue for a wide range of applications in biomedical research and medicine [76]. This genome editing and transcriptional regulation system has rapidly gained popularity, being applied to many yeast species [75]. Combining high-throughput genotyping and phenotyping with diverse developed CRISPR toolboxes will greatly facilitate the application of genome editing to wheat improvement [77]. CRISPR-Cas9 is an efficient, cost-effective, easier, and highly precise genome editing tool as compared to other genome editing tools [78]. In its simplest form, genome editing can improve crops by knocking out genes conferring undesirable traits [79]. More complex applications involve rewriting or inserting new genes to introduce desirable traits.
The rapid advances in directed genome evolution are overcoming the limits of evolutionary engineering by simplifying mutation generation, screening, and pathway identification. Combining lab evolution with omics and CRISPR will greatly accelerate research in the field of yeast engineering [80].
Despite CRISPR/Cas-based genome engineering being a promising technique for yeast metabolic engineering, it contains several risks, such as off-target effects, which means unwanted genetic modifications that lead to compromised strain stability, product yield, or safety, especially in food applications. These unwanted modifications may disrupt essential genes or regulatory elements, leading to unpredictable phenotypes or metabolic imbalances. While CRISPR has advanced the field, careful validation and monitoring are required to reduce these risks and ensure the reliability of engineered strains [81].
5.2. Machine Learning
Advancements in machine learning (ML) have made significant progress in yeast metabolic engineering by enhancing the efficiency and precision of strain development. One of the key applications of ML is its integration into the Design–Build–Test–Learn (DBTL) cycle, where it aids in predicting optimal gene combinations, regulatory elements, and metabolic pathway designs, thereby minimizing the reliance on trial-and-error methods. Machine learning models trained on large-scale multi-omics data, such as genomics, transcriptomics, and metabolomics, can identify beneficial genetic modifications and forecast strain performance. This data-driven approach enables researchers to pre-select high-performing constructs before physical testing, saving both time and resources [60].
Moreover, ML plays a vital role in automated high-throughput screening platforms, where it helps analyze vast experimental datasets and uncover complex, non-intuitive relationships between genetic changes and phenotypic outputs. Another noteworthy advancement is the use of ML for designing synthetic promoters and ribosome binding sites (RBS), allowing precise control over gene expression levels. Additionally, the implementation of active learning strategies enables iterative improvement by selecting the most informative experiments to perform next, thereby speeding up the convergence toward optimal strain designs. Overall, machine learning is transforming yeast metabolic engineering into a more predictive and intelligent process, enabling the discovery of novel metabolic solutions with greater speed and accuracy [60].
Machine learning has emerged as a powerful tool in metabolic engineering, enabling the optimization of biosynthetic pathways and the prediction of optimal production conditions. By analyzing large datasets from omics studies and fermentation experiments, machine learning algorithms can identify patterns and correlations that inform pathway design and optimization [82]. Machine learning algorithms have become indispensable across industries, with specific algorithms and case studies showcasing their real-world effectiveness. Several key algorithms stand out for their versatility and performance. The most common machine learning algorithms are Neural Networks (NNs), which are used for complex pattern recognition, including image and speech analysis, and medical diagnostics. Deep learning, a subset of NNs, enables large-scale data analysis in fields like healthcare and smart cities [83]. Random Forests, an ensemble method based on decision trees, prove effective for classification and regression tasks, often used in business, finance, and geosciences [83]. Support Vector Machines (SVMs) are popular for classification problems, including medical diagnosis and accidental prediction [83]. Genetic Algorithms (GAs) serve as metaheuristic optimization techniques, often combined with ML for tasks like image reconstruction and parameter tuning [84]. Bayesian Optimization is used for hyperparameter tuning and model selection, particularly in large-scale or complex ML tasks [85]. These algorithms have demonstrated their value in various applications, from healthcare and finance to smart cities and beyond.
Machine learning techniques have been applied to pathway engineering to overcome the limitations of traditional trial-and-error methods such as optimization, modifications in metabolism, and pathway remodeling, to name a few (Figure 3). For instance, multiplex experimentation and machine learning have been used to explore the enzyme expression landscape and identify optimal pathway configurations for enhanced molecule production [86]. Additionally, dynamic pathway engineering has leveraged machine learning to design metabolic production systems with intracellular control mechanisms, enabling self-regulation of pathway activity in response to environmental perturbations [87]. The integration of machine learning with genome-scale metabolic network models (GSMMs) has further enhanced the design of microbial cell factories. Machine learning algorithms have been used to analyze GSMMs and identify optimal metabolic engineering strategies for increasing the yield of desired products [88]. This approach has been particularly useful in the engineering of yeasts for the production of fatty acid-derived hydrocarbons and other biofuels [89].
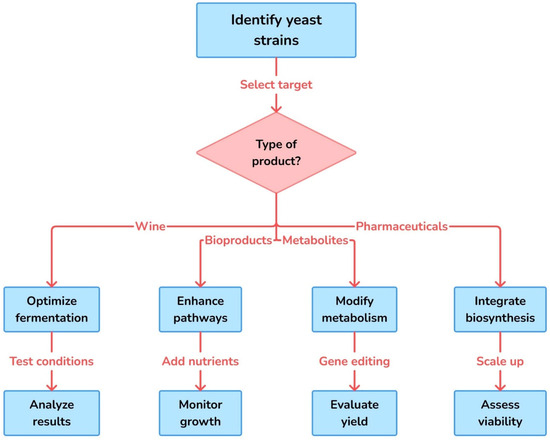
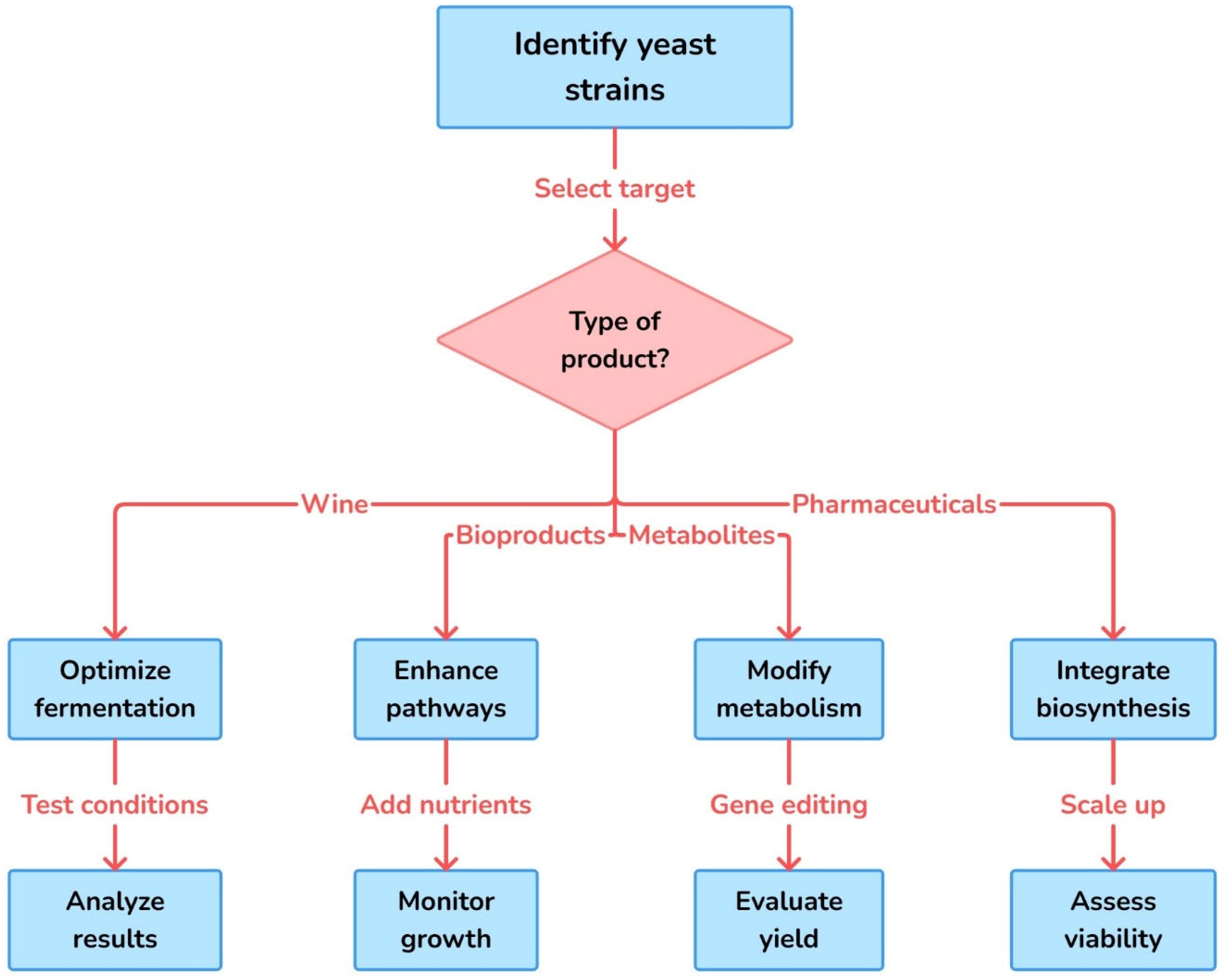
Figure 3.
Workflow for targeted engineering of yeast strains based on product type. This flowchart outlines a strategic approach to yeast strain development tailored to specific end products, such as wine, bioproducts, metabolites, and pharmaceuticals. The process begins with strain identification and target selection, followed by product-specific interventions like fermentation optimization, pathway enhancement, metabolic modification, and biosynthesis integration. Each branch includes critical experimental steps for improving productivity, functionality, and industrial feasibility.
The increasing use of genome-scale metabolic models (GEMs) and machine learning strategies to predict and optimize yeast metabolism. However, their precision and accuracy are highly reliant on the quality and comprehension of input data, such as enzyme kinetics, proteomics, and transcriptomics. Incomplete or low-quality datasets can lead to inaccurate phenotype predictions, misidentification of metabolic bottlenecks, or suboptimal strain designs. For example, models that do not account for enzyme abundances or kinetic constraints may fail to predict real-world metabolic behavior, especially under stress or non-standard growth conditions [90,91].
5.3. Metabolic Modeling
Metabolic modeling is a powerful tool that utilizes genome-scale metabolic models (GEMs) and constraint-based methods such as flux balance analysis (FBA) for understanding and engineering cellular metabolism. It increases the flow of metabolites in metabolic networks under specific constraints to predict cellular behavior and guide metabolic engineering strategies. In Saccharomyces cerevisiae, constraint-based modeling such as FBA and GEMs have been extensively used in yeast for fundamental research and industrial applications. Yeast GEMs enable predictions of metabolic fluxes, optimization of production processes, and rational design of yeast strains for industrial applications [90].
Traditional GEMs often rely on nutrient uptake rates as constraints. Enzyme-constrained models (ecModels), however, incorporate enzymatic limitations, ensuring metabolic fluxes align with available enzyme capacity. Recent advancements have focused on integrating additional biological constraints to enhance model accuracy and predictive power [90].
Recent advancements include the GECKO toolbox and Compartment-Specific Constraints. The GECKO toolbox is a prominent method for enhancing GEMs with enzyme constraints, enabling the integration of proteomics data and improving phenotype predictions. It facilitates the integration of enzyme constraints and proteomics data into GEMs, supporting model-based design in metabolic engineering and synthetic biology [90]. Whereas Constraint-based modeling has been successfully applied to yeast metabolic engineering [92]. For instance, researchers used FBA and ecModels to design yeast strains that secrete formic acid under aerobic conditions. The engineered strain performed as predicted, demonstrating the effectiveness of these models in guiding systems-level engineering strategies. Enzyme-constrained models have also been used to optimize bioprocesses, accurately capturing key metabolic phenomena. Advanced models now consider compartment-specific protein pools, revealing how cellular constraints drive metabolic strategies and adaptation in yeast [90].
5.4. Non-Conventional Yeasts
The unique properties and diverse applications of non-conventional yeasts, including Yarrowia lipolytica, Rhodotorula toruloides, and Kluyveromyces marxianus, play a significant role in biotechnological applications. These yeasts exhibit a wide range of substrate utilization, tolerance to environmental stressors, and the ability to produce high-value compounds such as lipids, hydrocarbons, and nutraceuticals [93,94,95].
They have been extensively used in food and additives production, leveraging their metabolic diversity and niche adaptations. For example, Zygosaccharomyces rouxii has been employed in the production of balsamic vinegar and soy sauce, while Yarrowia lipolytica has been used for the production of nutraceuticals and biofuels [95,96,97]. The use of non-conventional yeasts in bioprocesses contributes to a circular economy by enabling the conversion of waste and by-products into valuable compounds. For instance, Candida famata has been used to produce riboflavin from cheese whey, while Ogataea polymorpha has been employed for the production of biofuels from crude glycerol [98,99]. Despite their potential, non-conventional yeasts face challenges such as limited genetic information and the need for tailored engineering tools. However, recent advancements in CRISPR-Cas9 systems and synthetic biology have expanded the genetic toolboxes for these yeasts, enabling their further development as microbial cell factories [100,101].
They offer unique metabolic capabilities but present additional challenges. The non-conventional yeasts do not have well-developed genetic tools and possess complex or poorly characterized genomes, which makes precise engineering difficult, leading to lower efficiency, unpredictable outcomes, or limited scalability of the engineered yeast. They do not have the complete molecular data, which further complicates developing and validating metabolic models for these species [91,102].

Table 2.
Applications and traits improved via gene editing in food yeasts.
Table 2.
Applications and traits improved via gene editing in food yeasts.
| Application Area | Specific Traits Enhanced | Approach | References |
|---|---|---|---|
| Flavor Compound Production | Increased ester, acid, and antioxidant production | Overexpressing flavor biosynthesis genes, CRISPR-Cas9 modifications | [1,19] |
| Substrate Utilization | Ability to ferment complex carbohydrates like starch and xylose | Introducing amylolytic or xylose metabolism genes | [17] |
| Stress Tolerance | Tolerance to heat, ethanol, osmotic, and lignocellulosic inhibitors | Enhancing heat shock protein genes, modulating glycerol synthesis | [18] |
| Cell Wall Engineering | Improved flocculation, stress resistance, and enzyme accessibility | Modifying glycosylation patterns via CRISPR | [13] |
| Strain Hybridization | Combining desirable traits of S. cerevisiae and non-Saccharomyces yeasts | Intergeneric genome shuffling and adaptive evolution | [20,21] |
| Synthetic Genome Construction | Creating yeast strains with synthetic DNA for tailored traits | Developing strains with >50% synthetic genome | [19] |
6. Technological Challenges
Although there are significant developments in the yeast metabolic and evolutionary engineering technologies, several challenges remain:
6.1. Genetic Complexity
Genetic complexity is a key challenge in yeast engineering, particularly in industrial strains like Saccharomyces pastorianus (lager brewer’s yeast), an allopolyploid hybrid [103]. The hybrid nature of these strains complicates genetic modification due to the presence of multiple copies of chromosomes, which makes it difficult to predict and control the impacts of genetic alterations. Additionally, the genetic redundancy in polyploid yeasts results in unintended interactions between homologous genes, further complicating engineering efforts to obtain the desired traits. Even in haploid or diploid yeast strains, metabolic engineering often leads to multiple gene responses due to the intricate interconnected behavior of cellular regulatory systems [104]. For instance, introducing a single genetic modification can activate or repress multiple downstream genes, leading to unpredictable metabolic fluxes. This complexity is exacerbated by the redundancy in regulatory pathways, where multiple genes may control the same metabolic function, making it challenging to achieve precise control over engineered traits. Non-conventional yeasts, such as those with high stress tolerance or unique metabolic pathways, offer promising alternatives for industrial applications. However, their genetic complexity poses additional challenges. These yeasts often have distinct regulatory mechanisms and metabolic pathways that are not yet fully understood, making it difficult to apply conventional engineering strategies [105,106].
6.2. Strain Stability
Ensuring the long-term strain stability of engineered strains is one of the primary challenges in yeast engineering. Genetic instability can arise from homologous recombination events, particularly when multiple genetic modifications are introduced [107]. For example, the use of identical promoter sequences in metabolic engineering can lead to recombination, resulting in genetic rearrangements that compromise strain performance. This instability is particularly problematic in large-scale industrial fermentations, where consistent productivity is critical.
Additionally, engineered strains of yeast often experience a fitness burden due to the metabolic costs associated with producing non-native compounds or maintaining synthetic pathways [108]. This burden can lead to selective pressure against engineered cells in competitive environments, causing the loss of high-performing strains over time. For instance, strains engineered for vanillin-β-glucoside production exhibit reduced growth rates compared to their parental strains, highlighting the need for strategies to mitigate fitness deficits.
To address these challenges, researchers have developed synthetic control circuits that link pathway flux to essential cellular functions [108]. These circuits use biosensors to monitor intermediate metabolites and regulate gene expression dynamically, ensuring stable production over multiple generations. Additionally, genome replication engineering-assisted continuous evolution (GREACE) has been employed to accumulate beneficial genetic modifications iteratively, improving strain stability and inheritance of desired traits [109].
6.3. Regulatory Hurdles
The regulation of genetically modified organisms (GMOs) has evolved significantly since the Asilomar Conference in 1975, which first emphasized biosafety in recombinant DNA research [110]. While these regulations aim to protect human health and the environment, they often present considerable challenges that can hinder innovation and delay the application of engineered yeasts [110]. The genetically modified organisms (GMOs) are reviewed, analyzed, and approved through the critical processes governed by regulatory frameworks that differ by country yet share common principles [111].
In many regions, such as the European Union, genetically modified yeasts are subject to the same rigorous regulatory frameworks as traditional GMOs. This includes lengthy risk assessments, extensive documentation, and mandatory public consultations, regardless of whether the modification introduces foreign DNA [112]. The approval process typically involves comprehensive evaluations of the engineered yeast’s safety, including molecular characterization, environmental impact assessments, and toxicological data. These requirements demand substantial time, expertise, and financial resources, making it particularly difficult for smaller research groups and startups to navigate the system [110].
Biosafety concerns are central to the responsible development of engineered yeasts. These include containment strategies, prevention of horizontal gene transfer, and assessments of environmental and health risks [113]. Strategies like using well-characterized promoters and regulatory systems reduce genetic instability risks and unintended gene expression. Adaptive laboratory evolution (ALE) and metabolic engineering approaches improve strain robustness and stability, essential for biosafety, but introduce new genetic variations requiring safety evaluation [80]. Compartmentalizing metabolic pathways within organelles reduces metabolic crosstalk and unintended interactions, improving biosafety [114].
Moreover, public concerns regarding the safety of GMOs, including potential health risks, ecological consequences, and ethical considerations, further complicate regulatory pathways. These concerns often influence policy decisions and contribute to prolonged approval timelines [115]. While current regulatory frameworks prioritize safety, advocacy is growing for a more balanced and risk-proportionate approach. A regulatory system that distinguishes between low-risk and high-risk modifications, while maintaining rigorous safety standards, could accelerate the responsible development and commercialization of engineered yeasts [112]. Such reforms would help to introduce innovations and unlock the full potential of genetically modified organisms. Consumer acceptance of genetically modified organism-derived products is also controlled by technological perceptions of safety, transparency, and benefits. Food and pharmaceutical applications are more sensitive to public concerns and have many acceptance barriers, while yeast-based biofuels and industrial chemicals have fewer [80]. As a result, effective science communication and transparent engagement about the safety, benefits, and regulatory oversight of the engineered yeast with the public are essential to fostering acceptance and trust in genetically modified yeast applications [115]. In some cases, the regulatory burden can result in delays of several years before market authorization is granted. Regulatory frameworks often do not align with the actual risk posed by the organisms, leading to calls for a more proportional regulatory approach [116].
The responsible application of CRISPR-Cas9 raises significant ethical and regulatory hurdles that must be addressed. CRISPR-Cas9 use in gene drives can lead to the rapid and irreversible dissemination of genetic changes in populations, raising concerns about imprudent ecological consequences and biodiversity loss. There is ongoing discussion about the acceptability of modifying organisms at the genetic level, particularly regarding the potential for misuse, unpredicted long-term effects, and the ethical boundaries of genetic alterations. Ethical use requires transparent engagement about the rationales, risks, and benefits of CRISPR use to develop public trust and ensure informed societal consent [117].
The diverse regulatory approaches for CRISPR-modified organisms vary by country, leading to inconsistencies in overseen, approval processes, and definitions of what constitutes a genetically modified organism. Regulatory bodies require comprehensive risk assessments to address potential off-target effects, genetic instability, and the risk of horizontal gene transfer. Containment strategies are especially important for gene drive applications in yeast to minimize the risk of unintended environmental release. The emergence of CRISPR technologies has led to complex patent landscapes, complicating access, commercialization, and collaboration in research and industry [117].
Non-conventional yeasts produce unpredictable responses to interventions to CRISPR-Cas9, which requires effective regulatory and safety evaluations for each strain and application. Ongoing technical challenges include improving the specificity, efficiency, and predictability of CRISPR edits, which are important for meeting regulatory safety standards [105].
7. Future Prospects
The metabolic and evolutionary engineering of yeast is a promising technique that holds immense potential in various industries, which is driven by recent advancements in tools such as CRISPR-Cas systems, synthetic biology, and machine learning. These advancements in yeast engineering technologies are creating new opportunities for the production of biofuels, chemicals, bioplastics, innovative flavors, ingredients, and drug and vaccine development [65].
7.1. Sustainable Production
The technique of yeast engineering plays a significant role in the sustainable production of biofuels and biodegradable plastics. Yeast engineering addresses the concerns related to the environment, such as fossil fuel depletion and environmental degradation, and helps to develop greener alternatives. For instance, Saccharomyces cerevisiae and other yeast strains have been engineered to produce ethanol, which is a key biofuel characterized by low carbon emissions and yields high energy [80]. Advanced metabolic engineering strategies have not only enabled the production of ethanol but also enabled the biosynthesis of free fatty acids and their derivatives, serving as a precursor for biodiesel and other chemicals. Moreover, it also supports the utilization of alternative carbon sources such as CO2 and methanol to enhance sustainable production and resource efficiency [56,62]. Furthermore, yeasts are increasingly being engineered to synthesize non-native chemicals, which expands their utility beyond traditional fermentation processes [118].
7.2. Food Innovation
The applications of yeast engineering in food innovation are rapidly accelerating beyond the process of traditional fermentation. Genetic engineering has facilitated the production of novel and innovative food ingredients, flavors, and functional compounds, thereby enriching the sensory and nutritional profiles of food products [119]. Yeast has been engineered with enhanced stress tolerance and the ability to metabolize a wide range of substrates, which allows them to become suitable for diverse food processing conditions [60]. One of the recent advancements is the biosynthesis of terpenes using yeast as a sustainable alternative to traditional plant extraction methods [120].
7.3. Pharmaceuticals
The metabolic engineering of yeast has also transformed manufacturing in the pharmaceutical industry by enabling the efficient, expandable, and cost-effective production of complex therapeutics. Yeast engineering has shown immense potential in the production of pharmaceutical compounds such as vaccines, hormones, and antimicrobial agents [119]. The integration of synthetic biology, genome-scale modeling, and high-throughput screening has enhanced yeast strain development with complex phenotypes, which are tailored for specific pharmaceutical applications [60]. Moreover, the eukaryotic nature of yeast supports post-translational modifications similar to those in higher organisms, improving the functionality, efficacy, and biosafety of drugs and vaccines [74].
7.4. Emerging Directions and Technologies
Yeast engineering is rapidly evolving with the development of new scientific tools and advances in synthetic biology, artificial intelligence (AI), and genome editing. The convergence of AI-designed synthetic genomes and other emerging technologies is opening new horizons for both basic scientific research and industrial applications, with significant implications for the industry. The intersection of life sciences and information sciences enables the use of AI and computer-assisted design tools to create synthetic genomes, minimal genomes, pan-genomes, neochromosomes, and metagenomes in yeast, especially Saccharomyces cerevisiae, offering precise control over yeast cellular systems [121]. The Synthetic Yeast Genome (Sc2.0) Project has demonstrated the feasibility of fully synthetic, designer eukaryote genomes with the significant achievement of synthesizing all 16 yeast chromosomes and a de novo neochromosome [122,123]. AI-driven modeling and data-centric engineering, which enables more precise and efficient engineering, are poised to further accelerate the design, optimization, and functional prediction of synthetic yeast genome systems [121,124]. The advanced genome editing techniques, such as CRISPR/Cas systems, delta integration, and multiplex genome engineering, allow rapid, simultaneous, and multiloci modifications, accelerating the development of yeast cell factories. Moreover, the development of standardized DNA parts and modular assembly methods promotes the rapid construction of synthetic yeast strains, supporting both S. cerevisiae and non-conventional yeast species like Yarrowia lipolytica and Pichia pastoris [59,125].
8. Conclusions
Food yeast’s metabolic and evolutionary engineering, particularly Saccharomyces cerevisiae, has revolutionized the food biotechnology industry by making precise and efficient advancements in the performance, adaptability, and productivity of the yeast. Through the integration of advanced genetic engineering tools, such as CRISPR-Cas9, systems biology, and evolutionary strategies, researchers have attained targeted modifications that enhance substrate utilization, stress tolerance, and the biosynthesis of valuable metabolites, including flavors, organic acids, vitamins, and antioxidants. Metabolic engineering strategies, including gene overexpression, gene deletion, and the introduction of heterologous pathways, have allowed the optimization of metabolic fluxes for enhanced production and efficiency. The compartmentalization of metabolic pathways within specific organelles has further improved biosynthetic capabilities, while multi-omics analyses and machine learning have accelerated the identification and implementation of optimal genetic modifications. Evolutionary engineering and global transcription machinery engineering complement these efforts by harnessing natural selection and global gene regulation, enabling the development of yeast strains with complex, desirable traits. Moreover, exploring non-Saccharomyces yeasts and employing intergeneric genome shuffling have expanded the genetic diversity and robustness of industrial strains, offering new avenues for strain improvement. Collectively, these advancements are driving the development of sustainable, efficient, and high-quality food and beverage production systems. The continued evolution of metabolic and evolutionary engineering strategies promises to further enhance the nutritional value, sensory qualities, and overall performance of food yeasts, positioning them as essential platforms for the future of industrial biotechnology.
Author Contributions
Conceptualization, P.G., T.C.D., and B.R.G.; methodology, S.D., J.B., T.C.D., B.R.G., and P.G.; software, S.D., J.B., T.C.D., B.R.G., and P.G.; validation, S.D., J.B., T.C.D., B.R.G., and P.G.; formal analysis, S.D., J.B., T.C.D., B.R.G., and P.G.; investigation, S.D., J.B., T.C.D., B.R.G., and P.G.; resources, S.D., J.B., T.C.D., B.R.G., and P.G.; data curation, S.D., J.B., T.C.D., B.R.G., and P.G.; writing—original draft preparation, S.D., J.B., T.C.D., B.R.G., and P.G.; writing—review and editing, S.D., J.B., T.C.D., B.R.G., and P.G.; visualization, S.D., J.B., T.C.D., B.R.G., and P.G.; supervision, S.D., J.B., T.C.D., B.R.G., and P.G.; project administration, S.D., J.B., T.C.D., B.R.G., and P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data was created.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chai, K.F.; Ng, K.R.; Samarasiri, M.; Chen, W.N. Precision fermentation to advance fungal food fermentations. Curr. Opin. Food Sci. 2022, 47, 100881. [Google Scholar] [CrossRef]
- Graham, A.E.; Ledesma-Amaro, R. The microbial food revolution. Nat. Commun. 2023, 14, 2231. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for future food systems: Precision fermentation can complement the scope and applications of traditional fermentation. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef] [PubMed]
- Tekarslan-Sahin, S.H. Metabolic Engineering of Saccharomyces cerevisiae for Industrial Biotechnology; IntechOpen: London, UK, 2021. [Google Scholar]
- Yao, Z.; Wang, Q.; Dai, Z.J. Recent advances in directed yeast genome evolution. J. Fungi 2022, 8, 635. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Nielsen, J. Genome-scale modeling of yeast metabolism: Retrospectives and perspectives. FEMS Yeast Res. 2022, 22, foac003. [Google Scholar] [CrossRef]
- Cao, C.; Gao, J.; Zhu, B.; Zhou, Y.J. Engineering yeast for bio-production of food ingredients. Syst. Microbiol. Biomanuf. 2023, 3, 2–11. [Google Scholar] [CrossRef]
- Gong, G.; Wu, B.; Liu, L.; Li, J.; He, M. Engineering oleaginous red yeasts as versatile chassis for the production of olechemicals and valuable compounds: Current advances and perspectives. Biotechnol. Adv. 2024, 76, 108432. [Google Scholar] [CrossRef]
- Patané, A.; Jansen, G.; Conca, P.; Carapezza, G.; Costanza, J.; Nicosia, G. Multi-objective optimization of genome-scale metabolic models: The case of ethanol production. Ann. Oper. Res. 2019, 276, 211–227. [Google Scholar] [CrossRef]
- Hilgendorf, K.; Wang, Y.; Miller, M.J.; Jin, Y.-S. Precision fermentation for improving the quality, flavor, safety, and sustainability of foods. Curr. Opin. Biotechnol. 2024, 86, 103084. [Google Scholar] [CrossRef]
- Seo, S.-O.; Jin, Y.-S. Next-generation genetic and fermentation technologies for safe and sustainable production of food ingredients: Colors and flavorings. Annu. Rev. Food Sci. Technol. 2022, 13, 463–488. [Google Scholar] [CrossRef]
- Kallscheuer, N. Engineered microorganisms for the production of food additives approved by the European Union—A systematic analysis. Front. Microbiol. 2018, 9, 1746. [Google Scholar] [CrossRef]
- Pan, M.; Barrangou, R.J. Combining omics technologies with CRISPR-based genome editing to study food microbes. Curr. Opin. Biotechnol. 2020, 61, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Patra, P.; Disha, B.; Kundu, P.; Das, M.; Ghosh, A. Recent advances in machine learning applications in metabolic engineering. Biotechnol. Adv. 2023, 62, 108069. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Snoek, T.; Meersman, E.; Nicolino, M.P.; Voordeckers, K.; Verstrepen, K.J. Improving industrial yeast strains: Exploiting natural and artificial diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef]
- Costa, J.M.R.; Rodrigues, J.L.; Rodrigues, L.R. CRISPR-Cas9: A powerful tool to efficiently engineer Saccharomyces cerevisiae. Life 2020, 11, 13. [Google Scholar] [CrossRef]
- Ansori, A.N.; Antonius, Y.; Susilo, R.J.; Hayaza, S.; Kharisma, V.D.; Parikesit, A.A.; Zainul, R.; Jakhmola, V.; Saklani, T.; Rebezov, M.J.N.J. Application of CRISPR-Cas9 genome editing technology in various fields: A review. Narra J. 2023, 3, e184. [Google Scholar] [CrossRef] [PubMed]
- Pilap, W.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. The potential of multistress tolerant yeast, Saccharomycodes ludwigii, for second-generation bioethanol production. Sci. Rep. 2022, 12, 22062. [Google Scholar] [CrossRef]
- Bourzac, K. Engineered yeast breaks new record: A genome with over 50% synthetic DNA. Nature, 2023; online ahead of print. [Google Scholar]
- Cernak, P.; Estrela, R.; Poddar, S.; Skerker, J.M.; Cheng, Y.-F.; Carlson, A.K.; Chen, B.; Glynn, V.M.; Furlan, M.; Ryan, O.W. Engineering Kluyveromyces marxianus as a robust synthetic biology platform host. mBio 2018, 9, e01410-18. [Google Scholar] [CrossRef]
- Lin, Y.; Cai, Y.; Guo, Y.; Li, X.; Qi, X.; Qi, Q.; Wang, Q. Development and genomic elucidation of hybrid yeast with improved glucose-xylose co-fermentation at high temperature. FEMS Yeast Res. 2019, 19, foz015. [Google Scholar] [CrossRef]
- Choi, B.H.; Kang, H.J.; Kim, S.C.; Lee, P.C. Organelle engineering in yeast: Enhanced production of protopanaxadiol through manipulation of peroxisome proliferation in Saccharomyces cerevisiae. Microorganisms 2022, 10, 650. [Google Scholar] [CrossRef]
- Yu, A.-Q.; Juwono, N.K.P.; Foo, J.L.; Leong, S.S.J.; Chang, M.W. Metabolic engineering of Saccharomyces cerevisiae for the overproduction of short branched-chain fatty acids. Metab. Eng. 2016, 34, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Jojima, T.; Inui, M. Engineering the glycolytic pathway: A potential approach for improvement of biocatalyst performance. Bioengineered 2015, 6, 328–334. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, X.; Li, M.; Ma, M.; Zheng, Q.; Li, D.; An, T.; Wang, G. Advances in the optimization of central carbon metabolism in metabolic engineering. Microb. Cell Factories 2023, 22, 76. [Google Scholar] [CrossRef]
- Selma, S.; Ntelkis, N.; Nguyen, T.H.; Goossens, A. Engineering the plant metabolic system by exploiting metabolic regulation. Plant J. 2023, 114, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Radivojević, T.; Costello, Z.; Workman, K.; Garcia Martin, H. A machine learning Automated Recommendation Tool for synthetic biology. Nat. Commun. 2020, 11, 4879. [Google Scholar] [CrossRef]
- Yuan, W.; Du, Y.; Yu, K.; Xu, S.; Liu, M.; Wang, S.; Yang, Y.; Zhang, Y.; Sun, J. The production of pyruvate in biological technology: A critical review. Microorganisms 2022, 10, 2454. [Google Scholar] [CrossRef]
- Dakal, T.C.; Xu, C.; Kumar, A. Advanced Computational Tools, Artificial Intelligence and Machine-learning Approaches in Gut Microbiota and Biomarker Identification. Front. Med. Technol. 2025, 6, 1434799. [Google Scholar] [CrossRef]
- Kang, W.; Ma, T.; Liu, M.; Qu, J.; Liu, Z.; Zhang, H.; Shi, B.; Fu, S.; Ma, J.; Lai, L.T.F. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux. Nat. Commun. 2019, 10, 4248. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Park, J.M.; Kim, T.Y.; Yun, H.; Lee, S.Y. The genome-scale metabolic network analysis of Zymomonas mobilis ZM4 explains physiological features and suggests ethanol and succinic acid production strategies. Microb. Cell Factories 2010, 9, 94. [Google Scholar] [CrossRef]
- Shi, H.; Shimizu, K. On-line metabolic pathway analysis based on metabolic signal flow diagram. Biotechnol. Bioeng. 1998, 58, 139–148. [Google Scholar] [CrossRef]
- Chandrawanshi, V.; Kulkarni, R.; Prabhu, A.; Mehra, S.J. Enhancing titers and productivity of rCHO clones with a combination of an optimized fed-batch process and ER-stress adaptation. J. Biotechnol. 2020, 311, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Metallo, C.M.; Vander Heiden, M.G. Understanding metabolic regulation and its influence on cell physiology. Mol. Cell 2013, 49, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Bro, C.; Regenberg, B.; Förster, J.; Nielsen, J. In silico aided metabolic engineering of Saccharomyces cerevisiae for improved bioethanol production. Metab. Eng. 2006, 8, 102–111. [Google Scholar] [CrossRef]
- Malina, C.; Di Bartolomeo, F.; Kerkhoven, E.J.; Nielsen, J. Constraint-based modeling of yeast mitochondria reveals the dynamics of protein import and iron-sulfur cluster biogenesis. iScience 2021, 24, 103294. [Google Scholar] [CrossRef]
- Guo, W.; Feng, X. OM-FBA: Integrate transcriptomics data with flux balance analysis to decipher the cell metabolism. PLoS ONE 2016, 11, e0154188. [Google Scholar] [CrossRef]
- Milne, C.B.; Kim, P.J.; Eddy, J.A.; Price, N.D. Accomplishments in genome-scale in silico modeling for industrial and medical biotechnology. Biotechnol. J. 2009, 4, 1653–1670. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Nguyen, T.; Arbter, P.; Utesch, T.; Zeng, A.P. Phenotype analysis of cultivation processes via unsupervised machine learning: Demonstration for Clostridium pasteurianum. Eng. Life Sci. 2022, 22, 85–99. [Google Scholar] [CrossRef]
- Lawson, C.E.; Martí, J.M.; Radivojevic, T.; Jonnalagadda, S.V.R.; Gentz, R.; Hillson, N.J.; Peisert, S.; Kim, J.; Simmons, B.A.; Petzold, C.J. Machine learning for metabolic engineering: A review. Metab. Eng. 2021, 63, 34–60. [Google Scholar] [CrossRef]
- Yildiran, H.; Başyiğit Kiliç, G.; Karahan Çakmakçi, A.G. Characterization and comparison of yeasts from different sources for some probiotic properties and exopolysaccharide production. Food Sci. Technol. 2019, 39, 646–653. [Google Scholar] [CrossRef]
- Vanderwaeren, L.; Dok, R.; Voordeckers, K.; Nuyts, S.; Verstrepen, K.J. Saccharomyces cerevisiae as a model system for eukaryotic cell biology, from cell cycle control to DNA damage response. Int. J. Mol. Sci. 2022, 23, 11665. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.; Sewalt, V. GEMs: Genetically engineered microorganisms and the regulatory oversight of their uses in modern food production. Crit. Rev. Food Sci. Nutr. 2021, 61, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Chemler, J.A.; Yan, Y.; Koffas, M.A. Biosynthesis of isoprenoids, polyunsaturated fatty acids and flavonoids in Saccharomyces cerevisiae. Microb. Cell Factories 2006, 5, 20. [Google Scholar] [CrossRef]
- Belda, I.; Williams, T.C.; de Celis, M.; Paulsen, I.T.; Pretorius, I.S. Seeding the idea of encapsulating a representative synthetic metagenome in a single yeast cell. Nat. Commun. 2021, 12, 1599. [Google Scholar] [CrossRef]
- Shomar, H.; Bokinsky, G. Towards a synthetic biology toolset for metallocluster enzymes in biosynthetic pathways: What we know and what we need. Molecules 2021, 26, 6930. [Google Scholar] [CrossRef]
- Mezzetti, F.; Fay, J.C.; Giudici, P.; De Vero, L. Genetic variation and expression changes associated with molybdate resistance from a glutathione producing wine strain of Saccharomyces cerevisiae. PLoS ONE 2017, 12, e0180814. [Google Scholar] [CrossRef]
- Giersch, R.M.; Finnigan, G.C. Yeast still a beast: Diverse applications of CRISPR/Cas editing technology in S. cerevisiae. Yale J. Biol. Med. 2017, 90, 643. [Google Scholar]
- Lian, J.; HamediRad, M.; Zhao, H. Advancing metabolic engineering of Saccharomyces cerevisiae using the CRISPR/Cas system. Biotechnol. J. 2018, 13, 1700601. [Google Scholar] [CrossRef]
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef]
- Zhang, W.; Nielsen, D.R. Synthetic biology applications in industrial microbiology. Front. Microbiol. 2014, 5, 451. [Google Scholar] [CrossRef]
- McDaniel, E.A.; Wahl, S.A.; Ishii, S.i.; Pinto, A.; Ziels, R.; Nielsen, P.H.; McMahon, K.D.; Williams, R.B. Prospects for multi-omics in the microbial ecology of water engineering. Water Res. 2021, 205, 117608. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Qiu, Y.; Shi, S. CRISPR/Cas9 systems for the development of Saccharomyces cerevisiae cell factories. Front. Bioeng. Biotechnol. 2020, 8, 594347. [Google Scholar] [CrossRef]
- Jensen, M.K.; Keasling, J.D. Recent applications of synthetic biology tools for yeast metabolic engineering. FEMS Yeast Res. 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Saha, T.R.; Kang, N.K.; Lee, E.Y. Advanced metabolic Engineering strategies for the sustainable production of free fatty acids and their derivatives using yeast. J. Biol. Eng. 2024, 18, 73. [Google Scholar] [CrossRef]
- Jiang, W.; Li, C.; Li, Y.; Peng, H. Metabolic engineering strategies for improved lipid production and cellular physiological responses in yeast Saccharomyces cerevisiae. J. Fungi 2022, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Rainha, J.; Gomes, D.; Rodrigues, L.R.; Rodrigues, J.L. Synthetic biology approaches to engineer Saccharomyces cerevisiae towards the industrial production of valuable polyphenolic compounds. Life 2020, 10, 56. [Google Scholar] [CrossRef]
- Malcı, K.; Walls, L.E.; Rios-Solis, L. Multiplex genome engineering methods for yeast cell factory development. Front. Bioeng. Biotechnol. 2020, 8, 589468. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Chen, Y.; Nielsen, J. Metabolic Engineering of Yeast. Annu. Rev. Biophys. 2025, 54, 101–120. [Google Scholar] [CrossRef]
- Lacerda, M.P.; Oh, E.J.; Eckert, C. The model system Saccharomyces cerevisiae versus emerging non-model yeasts for the production of biofuels. Life 2020, 10, 299. [Google Scholar] [CrossRef]
- Liu, Z.; Moradi, H.; Shi, S.; Darvishi, F. Yeasts as microbial cell factories for sustainable production of biofuels. Renew. Sustain. Energy Rev. 2021, 143, 110907. [Google Scholar] [CrossRef]
- Sahoo, A.; Das, P.K.; Patra, S.; Veeranki, V.D. Engineered yeasts for the production of biofuel and platform chemicals. In Advances in Yeast Biotechnology for Biofuels and Sustainability; Elsevier: Amsterdam, The Netherlands, 2023; pp. 21–46. [Google Scholar]
- Liu, Z.; Wang, J.; Nielsen, J. Yeast synthetic biology advances biofuel production. Curr. Opin. Microbiol. 2022, 65, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.; Alper, H.S. Recent advances in genetic engineering and chemical production in yeast species. FEMS Yeast Res. 2025, 25, foaf009. [Google Scholar] [CrossRef] [PubMed]
- Taghon, G.J.; Strychalski, E.A. Rise of synthetic yeast: Charting courses to new applications. Cell Genom. 2023, 3, 100438. [Google Scholar] [CrossRef]
- Schindler, D. Genetic engineering and synthetic genomics in yeast to understand life and boost biotechnology. Bioengineering 2020, 7, 137. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Nielsen, J. Synthetic biology of yeast. Biochemistry 2019, 58, 1511–1520. [Google Scholar] [CrossRef]
- Thak, E.J.; Yoo, S.J.; Moon, H.Y.; Kang, H.A. Yeast synthetic biology for designed cell factories producing secretory recombinant proteins. FEMS Yeast Res. 2020, 20, foaa009. [Google Scholar] [CrossRef]
- Ma, J.; Gu, Y.; Marsafari, M.; Xu, P. Synthetic biology, systems biology, and metabolic engineering of Yarrowia lipolytica toward a sustainable biorefinery platform. J. Ind. Microbiol. Biotechnol. 2020, 47, 845–862. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, H.J. Metabolic engineering of oleaginous yeasts for production of fuels and chemicals. Front. Microbiol. 2017, 8, 2185. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wu, L.; Guo, S.; Cao, W.; Ma, W.; Wang, X.; Shen, J.; Wang, M.; Zhang, Q.; Huang, M. Metabolic engineering of yeast for the production of carbohydrate-derived foods and chemicals from C1–3 molecules. Nat. Catal. 2024, 7, 21–34. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.-F.; Yu, T.; et al. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef]
- Chettri, D.; Verma, A.K.; Verma, A.K. Past, present and future of yeast engineering. In Advances in Yeast Biotechnology for Biofuels and Sustainability; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–20. [Google Scholar]
- Stovicek, V.; Holkenbrink, C.; Borodina, I. CRISPR/Cas system for yeast genome engineering: Advances and applications. FEMS Yeast Res. 2017, 17, fox030. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lage, M.; Torres-Ruiz, R.; Rodriguez-Perales, S. CRISPR/Cas9 technology: Applications and human disease modeling. Prog. Mol. Biol. Transl. Sci. 2017, 152, 23–48. [Google Scholar]
- Li, S.; Zhang, C.; Li, J.; Yan, L.; Wang, N.; Xia, L. Present and future prospects for wheat improvement through genome editing and advanced technologies. Plant Commun. 2021, 2, 100211. [Google Scholar] [CrossRef]
- Das, J.; Kumar, S.; Mishra, D.C.; Chaturvedi, K.K.; Paul, R.K.; Kairi, A. Machine learning in the estimation of CRISPR-Cas9 cleavage sites for plant system. Front. Genet. 2023, 13, 1085332. [Google Scholar] [CrossRef]
- Scheben, A.; Wolter, F.; Batley, J.; Puchta, H.; Edwards, D. Towards CRISPR/Cas crops–bringing together genomics and genome editing. New Phytol. 2017, 216, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Topaloğlu, A.; Esen, Ö.; Turanlı-Yıldız, B.; Arslan, M.; Çakar, Z.P. From Saccharomyces cerevisiae to ethanol: Unlocking the power of evolutionary engineering in metabolic engineering applications. J. Fungi 2023, 9, 984. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Mishra, S.; Zhao, H.J. Recent advances in metabolic engineering of Saccharomyces cerevisiae: New tools and their applications. Metab. Eng. 2018, 50, 85–108. [Google Scholar] [CrossRef]
- Song, Y.; Prather, K.L. Strategies in engineering sustainable biochemical synthesis through microbial systems. Curr. Opin. Chem. Biol. 2024, 81, 102493. [Google Scholar] [CrossRef]
- Sarker, I.H. Machine learning: Algorithms, real-world applications and research directions. SN Comput. Sci. 2021, 2, 160. [Google Scholar] [CrossRef]
- Cavallaro, C.; Cutello, V.; Pavone, M.; Zito, F. Machine Learning and Genetic Algorithms: A case study on image reconstruction. Knowl.-Based Syst. 2024, 284, 111194. [Google Scholar] [CrossRef]
- Bottou, L.; Curtis, F.E.; Nocedal, J. Optimization methods for large-scale machine learning. SIAM Rev. 2018, 60, 223–311. [Google Scholar] [CrossRef]
- Boob, A.G.; Chen, J.; Zhao, H. Enabling pathway design by multiplex experimentation and machine learning. Metab. Eng. 2024, 81, 70–87. [Google Scholar] [CrossRef]
- Merzbacher, C.; Oyarzún, D.A. Applications of artificial intelligence and machine learning in dynamic pathway engineering. Biochem. Soc. Trans. 2023, 51, 1871–1879. [Google Scholar] [CrossRef]
- Bai, L.; You, Q.; Zhang, C.; Sun, J.; Liu, L.; Lu, H.; Chen, Q. Advances and applications of machine learning and intelligent optimization algorithms in genome-scale metabolic network models. Syst. Microbiol. Biomanuf. 2023, 3, 193–206. [Google Scholar] [CrossRef]
- Lu, R.; Shi, T.-Q.; Lin, L.; Ledesma-Amaro, R.; Ji, X.-J.; Huang, H. Advances in metabolic engineering of yeasts for the production of fatty acid-derived hydrocarbon fuels. Green Chem. Eng. 2022, 3, 289–303. [Google Scholar] [CrossRef]
- Sánchez, B.J.; Zhang, C.; Nilsson, A.; Lahtvee, P.J.; Kerkhoven, E.J.; Nielsen, J. Improving the phenotype predictions of a yeast genome-scale metabolic model by incorporating enzymatic constraints. Mol. Syst. Biol. 2017, 13, 935. [Google Scholar] [CrossRef] [PubMed]
- Timouma, S.; Balarezo-Cisneros, L.N.; Schwartz, J.-M.; Delneri, D. Development of a genome-scale metabolic model for the lager hybrid yeast S. pastorianus to understand the evolution of metabolic pathways in industrial settings. Msystems 2024, 9, e00424–e00429. [Google Scholar] [CrossRef]
- Elsemman, I.E.; Rodriguez Prado, A.; Grigaitis, P.; Garcia Albornoz, M.; Harman, V.; Holman, S.W.; van Heerden, J.; Bruggeman, F.J.; Bisschops, M.M.; Sonnenschein, N. Whole-cell modeling in yeast predicts compartment-specific proteome constraints that drive metabolic strategies. Nat. Commun. 2022, 13, 801. [Google Scholar] [CrossRef]
- Qian, Z.; Song, L.; Liu, Q.; Gong, X.; Kang, Y.; He, Z.; Long, H.; Cai, M. Biosynthesis of natural products by non-conventional yeasts. Sheng Wu Gong Cheng Xue Bao 2023, 39, 2284–2312. [Google Scholar]
- Rodriguez-Ocasio, E.; Khalid, A.; Truka, C.J.; Blenner, M.A.; Jarboe, L.R. Survey of nonconventional yeasts for lipid and hydrocarbon biotechnology. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac010. [Google Scholar] [CrossRef]
- Gu, Y.; Lu, X.; Liu, T.; Song, Y.; Sang, E.; Ding, S.; Liu, L.; Xue, F.; Xu, P. Engineering the oleaginous yeast Yarrowia lipolytica to produce nutraceuticals: From metabolic design to industrial applications. Food Bioeng. 2023, 2, 187–199. [Google Scholar] [CrossRef]
- Solieri, L. The revenge of Zygosaccharomyces yeasts in food biotechnology and applied microbiology. World J. Microbiol. Biotechnol. 2021, 37, 96. [Google Scholar] [CrossRef]
- Binati, R.L.; Salvetti, E.; Bzducha-Wróbel, A.; Bašinskienė, L.; Čižeikienė, D.; Bolzonella, D.; Felis, G.E. Non-conventional yeasts for food and additives production in a circular economy perspective. FEMS Yeast Res. 2021, 21, foab052. [Google Scholar] [CrossRef] [PubMed]
- Sibirny, A.A. Metabolic engineering of non-conventional yeasts for construction of the advanced producers of biofuels and high-value chemicals. BBA Adv. 2023, 3, 100071. [Google Scholar] [CrossRef] [PubMed]
- Geijer, C.; Ledesma-Amaro, R.; Tomás-Pejó, E. Unraveling the potential of non-conventional yeasts in biotechnology. FEMS Yeast Res. 2022, 22, foab071. [Google Scholar] [CrossRef]
- Navarrete, C.; Martínez, J.L. Non-conventional yeasts as superior production platforms for sustainable fermentation based bio-manufacturing processes. AIMS Bioeng. 2020, 7, 289–305. [Google Scholar] [CrossRef]
- Park, J.; Kim, I.J.; Kim, S.R. Nonconventional yeasts engineered using the CRISPR-cas system as emerging microbial cell factories. Fermentation 2022, 8, 656. [Google Scholar] [CrossRef]
- Löbs, A.-K.; Schwartz, C.; Wheeldon, I. Genome and metabolic engineering in non-conventional yeasts: Current advances and applications. Synth. Syst. Biotechnol. 2017, 2, 198–207. [Google Scholar] [CrossRef]
- Saerens, S.M.; Duong, C.T.; Nevoigt, E. Genetic improvement of brewer’s yeast: Current state, perspectives and limits. Appl. Microbiol. Biotechnol. 2010, 86, 1195–1212. [Google Scholar] [CrossRef]
- Bro, C.; Regenberg, B.; Nielsen, J. Yeast functional genomics and metabolic engineering: Past, present and future. In Functional Genetics of Industrial Yeasts; Springer: Berlin/Heidelberg, Germany, 2003; pp. 331–360. [Google Scholar]
- Xia, Y.; Li, Y.; Shen, W.; Yang, H.; Chen, X. CRISPR-Cas technology for bioengineering conventional and non-conventional yeasts: Progress and new challenges. Int. J. Mol. Sci. 2023, 24, 15310. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, W.-B.; Wang, Y.-T.; Zhao, X.-Q. Regulatory mechanisms underlying yeast chemical stress response and development of robust strains for bioproduction. Curr. Opin. Biotechnol. 2024, 86, 103072. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Wood, R.J.; Nielsen, L.K.; Vickers, C.E. An expanded heterologous GAL promoter collection for diauxie-inducible expression in Saccharomyces cerevisiae. ACS Synth. Biol. 2018, 7, 748–751. [Google Scholar] [CrossRef]
- D’ambrosio, V.; Dore, E.; Di Blasi, R.; van den Broek, M.; Sudarsan, S.; Ter Horst, J.; Ambri, F.; Sommer, M.O.; Rugbjerg, P.; Keasling, J.D. Regulatory control circuits for stabilizing long-term anabolic product formation in yeast. Metab. Eng. 2020, 61, 369–380. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Niu, C.; Wang, J.; Zheng, F.; Li, Y.; Li, Q. Rationally designed perturbation factor drives evolution in Saccharomyces cerevisiae for industrial application. J. Ind. Microbiol. Biotechnol. 2018, 45, 869–880. [Google Scholar] [CrossRef]
- Gowrishankar, J. Regulation of genetically modified organisms: Has the time come to amend the law? Curr. Sci. 2009, 96, 1574. [Google Scholar]
- Mirsalami, S.M.; Mirsalami, M. Advances in genetically engineered microorganisms: Transforming food production through precision fermentation and synthetic biology. Futur. Foods 2025, 11, 100601. [Google Scholar] [CrossRef]
- Bratlie, S.; Halvorsen, K.; Myskja, B.K.; Mellegård, H.; Bjorvatn, C.; Frost, P.; Heiene, G.; Hofmann, B.; Holst-Jensen, A.; Holst-Larsen, T. A novel governance framework for GMO: A tiered, more flexible regulation for GMO s would help to stimulate innovation and public debate. EMBO Rep. 2019, 20, e47812. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Plan, M.R.; Carpenter, A.; Nielsen, L.K.; Vickers, C.E. Coupling gene regulatory patterns to bioprocess conditions to optimize synthetic metabolic modules for improved sesquiterpene production in yeast. Biotechnol. Biofuels 2017, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.K.; Avalos, J.L. Harnessing yeast organelles for metabolic engineering. Nat. Chem. Biol. 2017, 13, 823–832. [Google Scholar] [CrossRef]
- Mamta, S.; Supriya, A.; Devi, L.; Nagma, K.; Chandana, B.; Verma, S.K. Biosafety Concerns and Regulatory Framework for Transgenics. Res. J. Agric. For. Sci. 2014, 2, 7–13. [Google Scholar]
- Lee, D.; Chen, A.; Nair, R. Genetically engineered crops for biofuel production: Regulatory perspectives. Biotechnol. Genet. Eng. Rev. 2008, 25, 331–362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, D.; Hussain, A.; Manghwar, H.; Xie, K.; Xie, S.; Zhao, S.; Larkin, R.M.; Qing, P.; Jin, S.; Ding, F. Genome editing with the CRISPR-Cas system: An art, ethics and global regulatory perspective. Plant Biotechnol. J. 2020, 18, 1651–1669. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hoang Nguyen Tran, P.; Lee, S.-M. Current challenges and opportunities in non-native chemical production by engineered yeasts. Front. Bioeng. Biotechnol. 2020, 8, 594061. [Google Scholar] [CrossRef]
- Turanlı-Yıldız, B.; Hacısalihoğlu, B.; Çakar, Z.P. Advances in Metabolic Engineering of Saccharomyces cerevisiae for the Production of Industrially and Clinically Important Chemicals; IntechOpen: London, UK, 2017. [Google Scholar]
- Yang, L.; Liu, H.; Jin, Y.; Liu, J.; Deng, L.; Wang, F. Recent advances in multiple strategies for the synthesis of terpenes by engineered yeast. Fermentation 2022, 8, 615. [Google Scholar] [CrossRef]
- Dixon, T.A.; Walker, R.S.; Pretorius, I.S. Visioning synthetic futures for yeast research within the context of current global techno-political trends. Yeast 2023, 40, 443–456. [Google Scholar] [CrossRef]
- Pretorius, I.S. Synthetic genome engineering forging new frontiers for wine yeast. Crit. Rev. Biotechnol. 2017, 37, 112–136. [Google Scholar] [CrossRef]
- Schindler, D.; Walker, R.S.; Cai, Y. Methodological advances enabled by the construction of a synthetic yeast genome. Cell Rep. Methods 2024, 4, 100761. [Google Scholar] [CrossRef]
- Pretorius, I.S. Visualizing the next frontiers in wine yeast research. FEMS Yeast Res. 2022, 22, foac010. [Google Scholar] [CrossRef]
- Patra, P.; Das, M.; Kundu, P.; Ghosh, A. Recent advances in systems and synthetic biology approaches for developing novel cell-factories in non-conventional yeasts. Biotechnol. Adv. 2021, 47, 107695. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).