The Effect of Storage on the Absorption and Fluorescence Spectra of Petal Extracts of Selected Anthocyanin-Containing Flowers
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Material
2.2. Methods
3. Results
3.1. Absorption Spectra of Petal Extracts
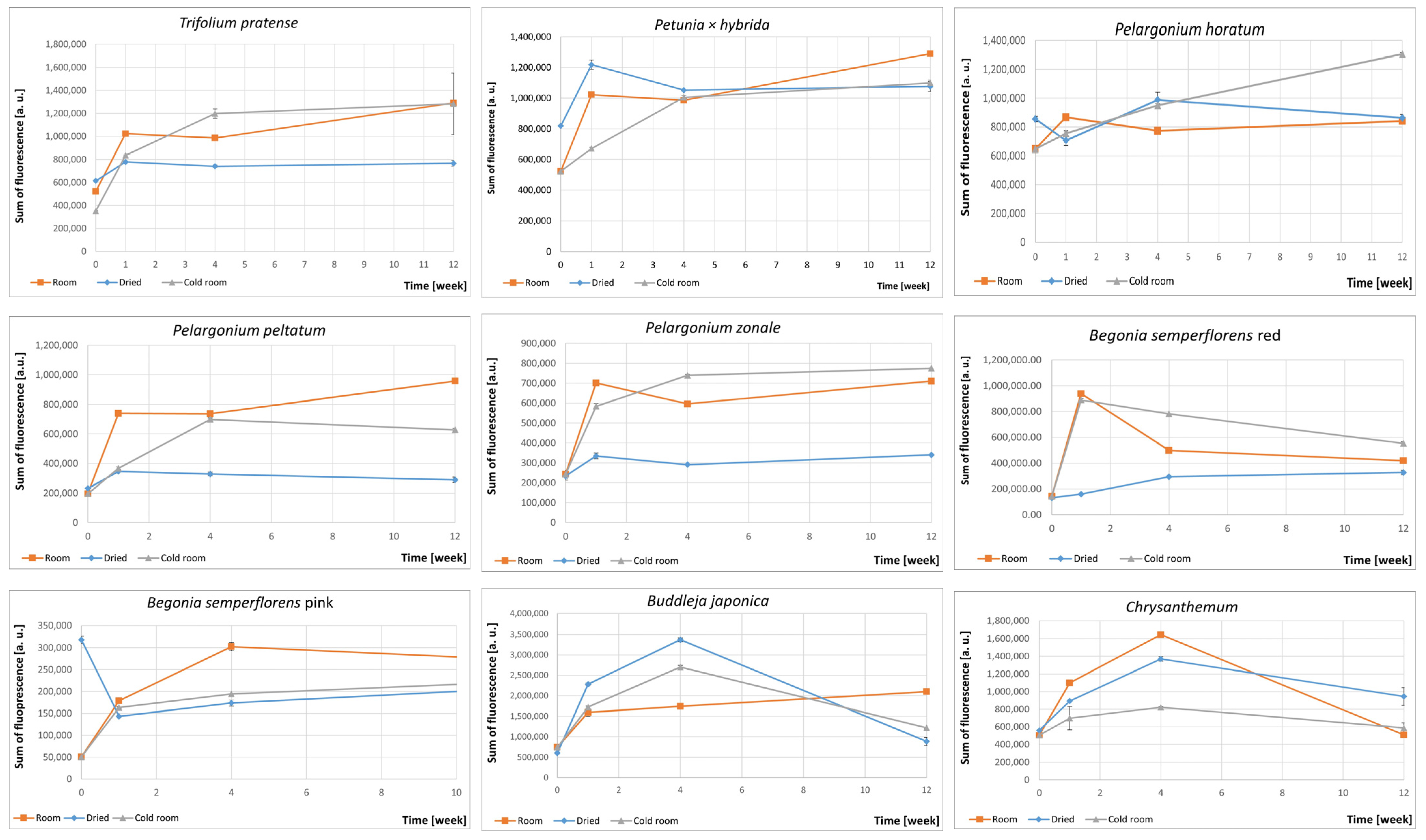
3.2. Fluorescence Spectra of Petals
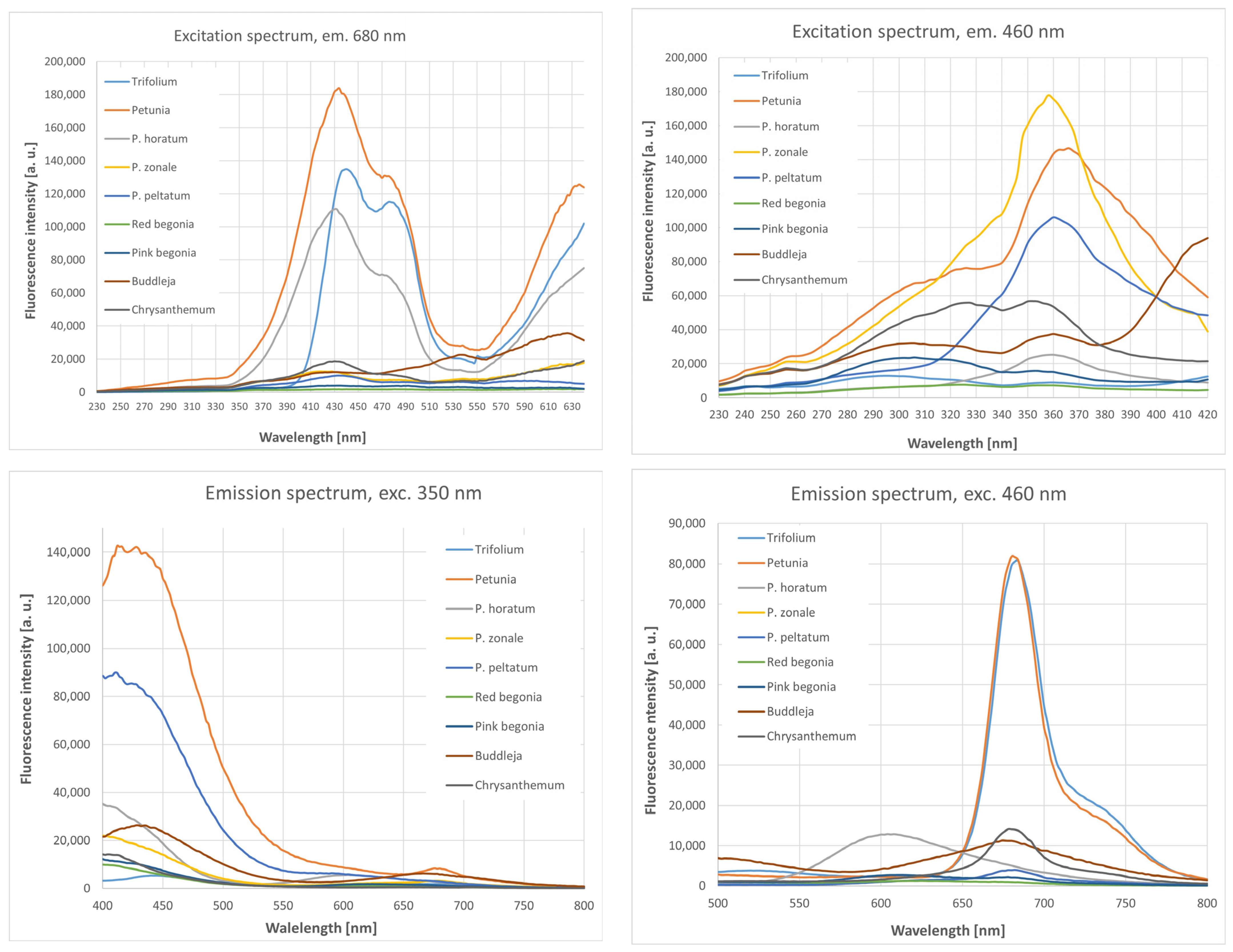
3.3. Fluorescence Spectra of Petal Extracts
3.3.1. Excitation Spectra Measured at the Emission Wavelength of 460 nm
3.3.2. Excitation Spectra Measured at the Emission Wavelength of 680 nm
3.3.3. Emission Spectra Measured at the Excitation Wavelength of 350 nm
3.3.4. Emission Spectra Measured at the Excitation Wavelength of 460 nm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quina, F.H.; Moreira, P.F., Jr.; Vautier-Giongo, C.; Rettori, D.; Rodrigues, R.F.; Freitas, A.A.; Silva, P.A.; Maçanita, A.L. Photochemistry of anthocyanins and their biological role in plant tissues. Pure Appl. Chem. 2009, 81, 1687–1694. [Google Scholar] [CrossRef]
- Nassour, R.; Ayash, A.; Al-Tameemi, K. Anthocyanin pigments: Structure and biological importance. J. Chem. Pharm. Sci. 2020, 13, 45–57. [Google Scholar]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant activity of anthocyanins and anthocyanidins: A critical review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef]
- Narbona, E.; del Valle, J.C.; Arista, M.; Buide, M.L.; Ortiz, P.L. Major flower pigments originate different colour signals to pollinators. Front. Ecol. Evol. 2021, 9, 743850. [Google Scholar] [CrossRef]
- Tenhumberg, B.; Dellinger, A.S.; Smith, S.D. Modelling pollinator and nonpollinator selection on flower colour variation. J. Ecol. 2023, 111, 746–760. [Google Scholar] [CrossRef]
- Iriel, A.; Lagorio, M.G. Implications of reflectance and fluorescence of Rhododendron indicum flowers in biosignaling. Photochem. Photobiol. Sci. 2010, 9, 342–348. [Google Scholar] [CrossRef]
- Arnold, K.E.; Owens, I.P.F.; Marshall, N.J. Fluorescent signaling in parrots. Science 2002, 295, 92. [Google Scholar] [CrossRef]
- Parker, A.R. Fluorescence of yellow budgerigars. Science 2002, 296, 655. [Google Scholar] [CrossRef][Green Version]
- Winter, Y.; Lopez, J.; von Helversen, O. Ultraviolet vision in a bat. Nature 2003, 425, 612–614. [Google Scholar] [CrossRef]
- De Ibarra, N.H.; Vorobyev, M.; Brandt, R.; Giurfa, M. Detection of bright and dim colours by honeybees. J. Exp. Biol. 2000, 203, 3289–3298. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta 2005, 222, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Kurup, R.; Johnson, A.J.; Sankar, S.; Hussain, A.A.; Kumar, C.S.; Sabulal, B. Fluorescent prey traps in carnivorous plants. Plant Biol. 2013, 15, 611–615. [Google Scholar] [CrossRef] [PubMed]
- van der Kooi, C.J.; Dyer, A.G.; Kevan, P.G.; Lunau, K. Functional significance of the optical properties of flowers for visual signalling. Ann. Bot. 2019, 123, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Iriel, A.; Lagorio, M.G. Is the flower fluorescence relevant in biocommunication? Naturwissenschaften 2010, 97, 915–924. [Google Scholar] [CrossRef]
- Figueiredo, P.; Pina, F. Fluorescence spectra and decays of malvidin 3, 5-diglucoside in aqueous solutions. J. Photochem. Photobiol. A Chem. 1990, 52, 411–424. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Santos, H.; Lima, J.C.; Abreu, I.; Ballardini, R.; Maestri, M. Excited state proton transfer in synthetic flavylium salts: 4-methyl-7-hydroxyflavylium and 4′, 7-dihydroxyflavylium. Example of a four-level molecular device to invert the population of the excited state. New J. Chem. 1998, 22, 1093–1098. [Google Scholar] [CrossRef]
- Lee, S.G.; Brownmiller, C.R.; Lee, S.O.; Kang, H.W. Anti-inflammatory and antioxidant effects of anthocyanins of Trifolium pratense (red clover) in lipopolysaccharide-stimulated RAW-267.4 macrophages. Nutrients 2020, 12, 1089. [Google Scholar] [CrossRef]
- Ando, T.; Tatsuzawa, F.; Saito, N.; Takahashi, M.; Tsunashima, Y.; Numajir, H.; Watanabe, H.; Kokubun, H.; Hara, R.; Seki, H.; et al. Differences in the floral anthocyanin content of red petunias and Petunia exserta. Phytochemistry 2000, 54, 495–501. [Google Scholar] [CrossRef]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Irving, L.J.; Jameson, P.E.; Davies, K.M. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef]
- Mitchell, K.A.; Markham, K.R.; Boase, M.R. Pigment chemistry and colour of Pelargonium flowers. Phytochemistry 1998, 47, 355–361. [Google Scholar] [CrossRef]
- Fujioka, M.; Kato, M.; Kakihara, F.; Tokumasu, S. Anthocyanidin composition of petals in Pelargonium× domesticum Bailey. J. Jpn. Soc. Hort. Sci. 1991, 59, 823–831. [Google Scholar] [CrossRef][Green Version]
- Zeid, M.H.; Mekky, T.M. Extraction of anthocyanins pigments from Pelargonium zonal petals as natural colorants in some foods. J. Food Dairy Sci. 2007, 32, 1159–1164. [Google Scholar] [CrossRef]
- Chirol, N.; Jay, M. Acylated anthocyanins from flowers of Begonia. Phytochemistry 1995, 40, 275–277. [Google Scholar] [CrossRef]
- Wu, Y.M.; Wu, Y.P.; Jin, X.H.; Meng, H. Effects of anthocyanin composition and distribution on flower color of Rieger begonia. CABI Abstr. 2020, 40, 58–68. [Google Scholar] [CrossRef]
- Madanakumar, A.J.; Kumaraswamy, M. Purified anthocyanin, its elicitation from cell cultures of Begonia malabarica and Begonia rex-cultorum ‘Baby Rainbow’ and it’s in vitro cytotoxicity Analysis by MTT Assay. Pharmacogn. J. 2018, 10, 553–558. [Google Scholar] [CrossRef]
- Dunn, B.L.; Lindstrom, J.T. Evaluation of a sib-mated population of Buddleja davidii ‘White Bouquet’× Buddleja indica hybrids. J. Environ. Hortic. 2009, 27, 67–69. [Google Scholar] [CrossRef]
- Chen, S.M.; Li, C.H.; Zhu, X.R.; Deng, Y.M.; Sun, W.; Wang, L.S.; Chen, F.D.; Zhang, Z. The identification of flavonoids and the expression of genes of anthocyanin biosynthesis in the chrysanthemum flowers. Biol. Plant. 2012, 56, 458–464. [Google Scholar] [CrossRef]
- Hong, Y.; Tang, X.; Huang, H.; Zhang, Y.; Dai, S. Transcriptomic analyses reveal species-specific light-induced anthocyanin biosynthesis in chrysanthemum. BMC Genom. 2015, 16, 202. [Google Scholar] [CrossRef]
- Mekapogu, M.; Vasamsetti, B.M.K.; Kwon, O.K.; Ahn, M.S.; Lim, S.H.; Jung, J.A. Anthocyanins in floral colors: Biosynthesis and regulation in chrysanthemum flowers. Int. J. Mol. Sci. 2020, 21, 6537. [Google Scholar] [CrossRef]
- Park, C.H.; Chae, S.C.; Park, S.Y.; Kim, J.K.; Kim, Y.J.; Chung, S.O.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Anthocyanin and carotenoid contents in different cultivars of chrysanthemum (Dendranthema grandiflorum Ramat.) flower. Molecules 2015, 20, 11090–11102. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G.; Grzesik-Pietrasiewicz, M.; Sadowska-Bartosz, I. Fluorescent products of anthocyanidin and anthocyanin oxidation. J. Agric. Food Chem. 2020, 68, 12019–12027. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; He, Y.; Liu, J.; Zhang, J.; Li, J.; Zhou, J.; Ye, Y.; Wang, J.; Wang, X. Kinetic investigation into pH-dependent color of anthocyanin and its sensing performance. Dye. Pigment. 2019, 170, 107643. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Leertouwer, H.L.; Dudek, B.; Van der Kooi, C.J. Coloration of flowers by flavonoids and consequences of pH dependent absorption. Front. Plant Sc. 2021, 11, 600124. [Google Scholar] [CrossRef]
- Rodríguez, N.; Real, B.D.; Ortiz, M.C.; Sarabia, L.A.; Herrero, A. Usefulness of parallel factor analysis to handle the matrix effect in the fluorescence determination of tetracycline in whey milk. Anal. Chim. Acta 2009, 632, 42–51. [Google Scholar] [CrossRef]
- Zhao, Y.W.; Wang, C.K.; Huang, X.Y.; Hu, D.G. Anthocyanin stability and degradation in plants. Plant Signal. Behav. 2021, 16, 1987767. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, H.; Shao, S.; Sun, S.; Yang, D.; Lv, S. Anthocyanin: A review of plant sources, extraction, stability, content determination and modifications. Int. J. Food Sci. Technol. 2022, 57, 7573–7591. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Osterc, G.; Stampar, F. Color and phenolic content changes during flower development in groundcover rose. J. Am. Soc. Hortic. Sci. 2010, 135, 195–202. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
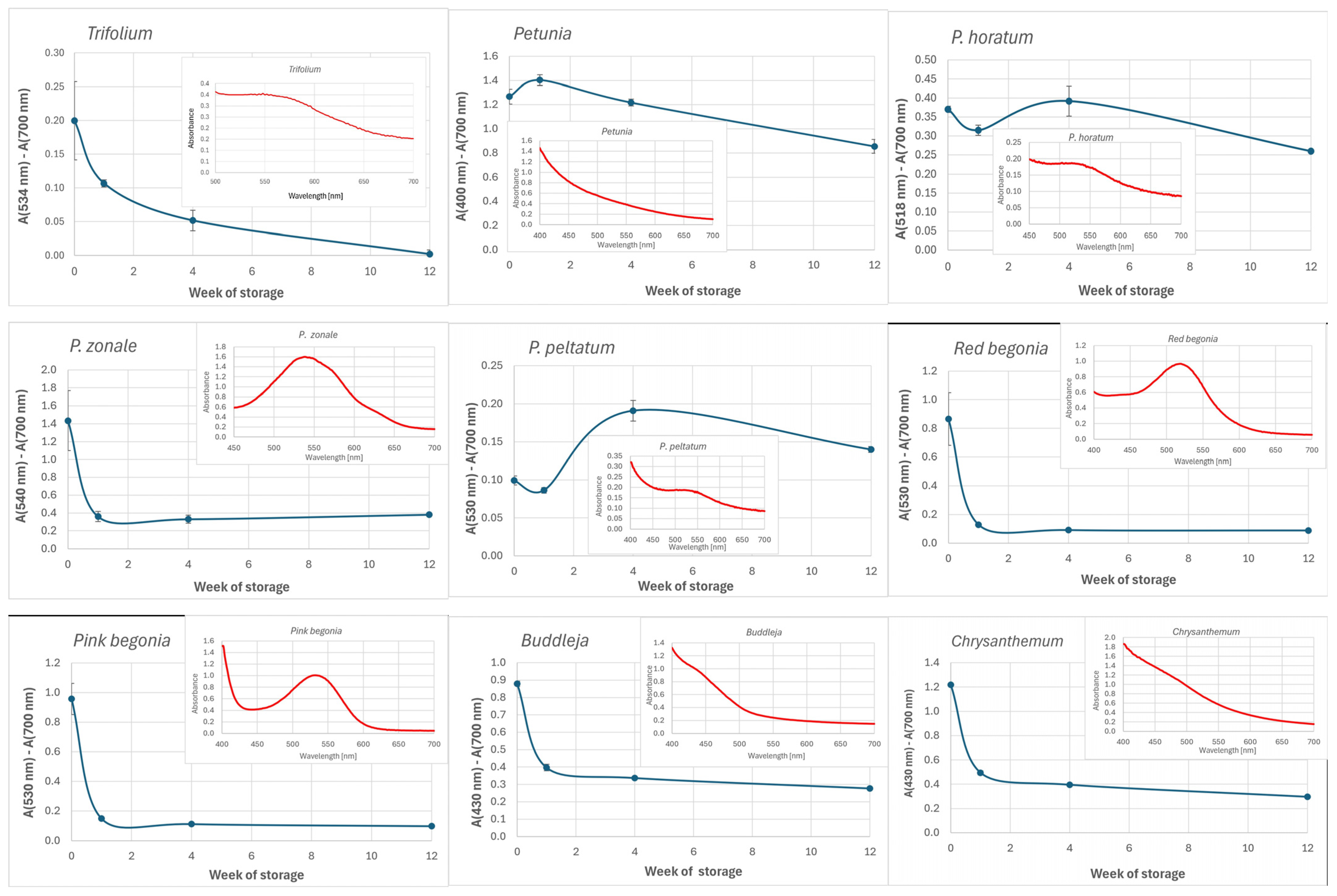
 extract of fresh petals,
extract of fresh petals,  extract of dried petals.
extract of dried petals.
 extract of fresh petals,
extract of fresh petals,  extract of dried petals.
extract of dried petals.
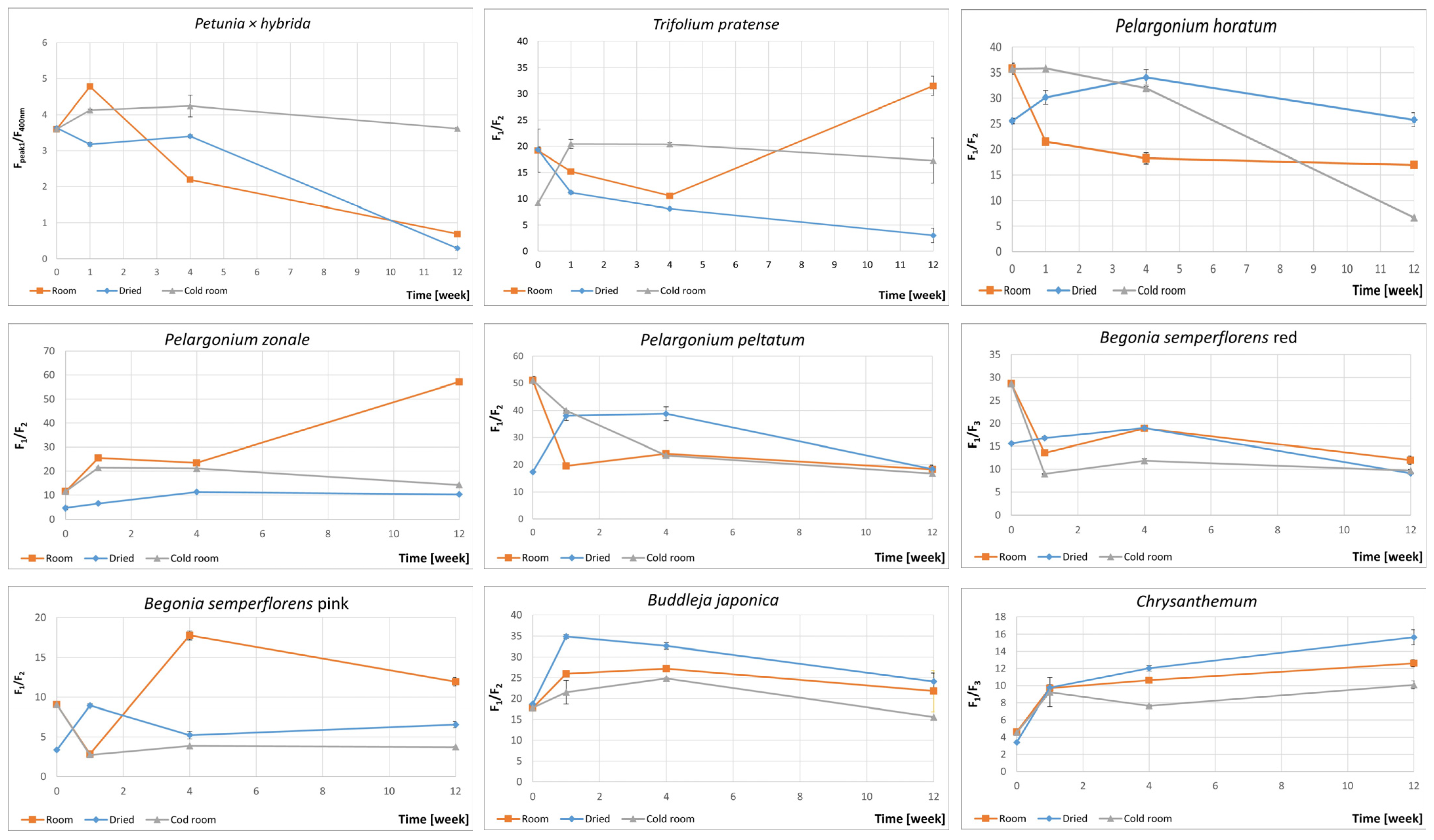
 extract of fresh petals,
extract of fresh petals,  extract of dried petals.
extract of dried petals.
 extract of fresh petals,
extract of fresh petals,  extract of dried petals.
extract of dried petals.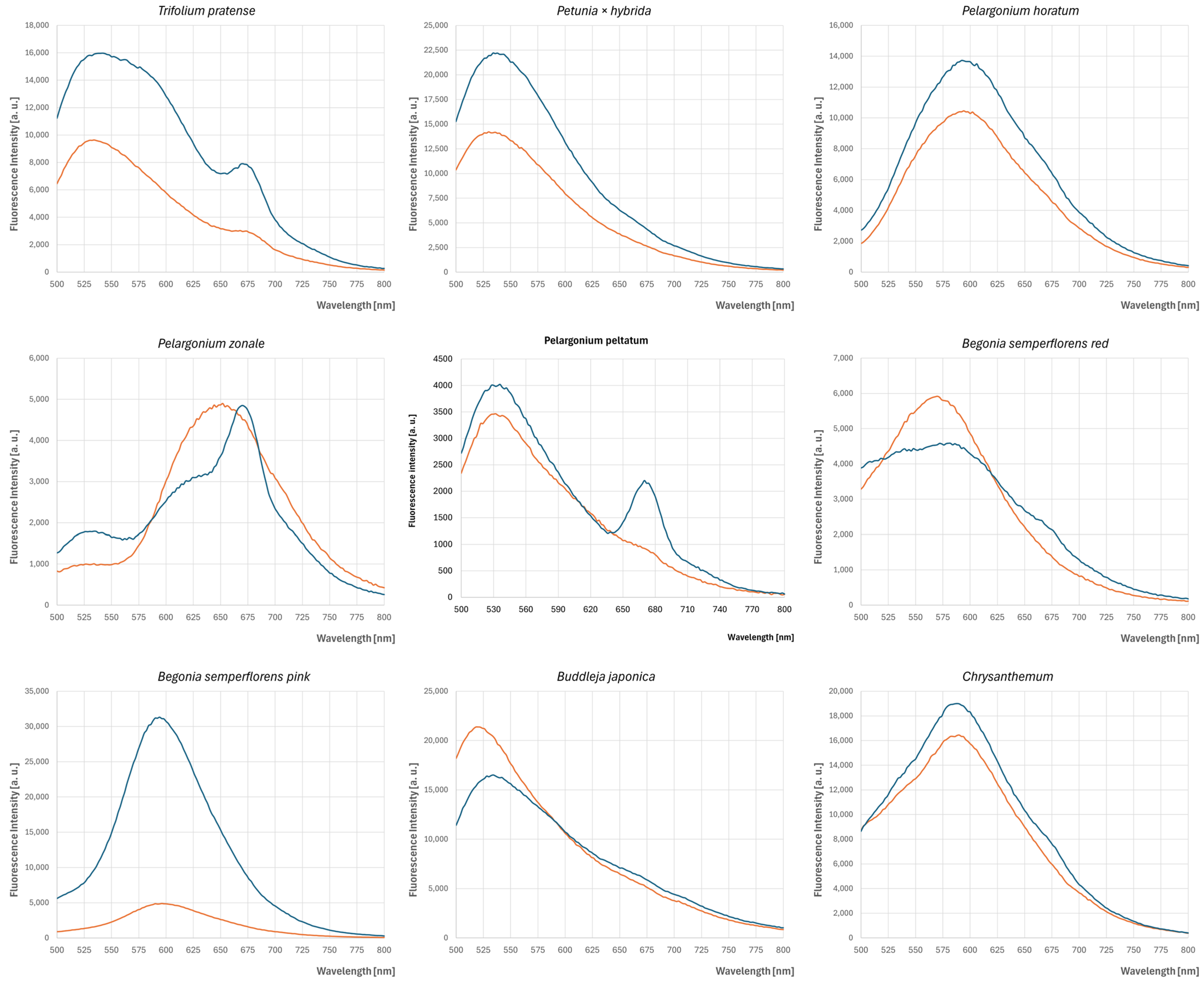
| Species | Common Name | Location | Date of Collection |
|---|---|---|---|
| Trifolium pratense | Red clover | Medow, Dobrzechów | 24 October 2024 |
| Petunia × hybrida | Pink petunia | Pot cultivation, home garden, Dobrzechów | 20 October 2024 |
| Pelargonium horatum | Red geranium | Pot cultivation, home garden Dobrzechów | 20 October 2024 |
| Pelargonium zonale | Pink-red geranium | Home pot cultivation, Dobrzechów | 20 October 2024 |
| Pelargonium peltatum | Pink ivy-leafed geranium | Pot cultivation, home garden, Dobrzechów | 20 October 2024 |
| Begonia semperflorens | Red begonia | Pot cultivation, home garden | 20 October 2024 |
| Begonia semperflorens | Pink begonia | Home garden, Święcany | 27 October 2024 |
| Buddleja japonica | Buddleia | Pot cultivation, home garden, Dobrzechów | 19 October 2024 |
| Chrysanthemum | Purple chrysanthemum | Home garden, Dobrzechów | 27 October 2024 |
| Species | Excitation Spectra λem 460 nm | Excitation Spectra λem 680 nm | Emission Spectra λex 350 nm | Emission Spectra λex 460 nm |
|---|---|---|---|---|
| Trifolium pratense | 242, 296 | 326, 440 | 444, 654 | 520, 684 |
| Petunia × hybrida | 366 | 434 | 412, 674 | 596, 680 |
| P. horatum | 360 | 430 | 604 | 604 |
| P. zonale | 358 | 420 | 678 | 682 |
| P. peltatum | 360 | 434, 590 | - | 682 |
| Red begonia | 324 | 450,480 | 610 | 618 |
| Pink begonia | 302 | 322, 432, 538, 620 | 624 | 612 |
| Buddleja japonica | 304, 360 | 432, 536, 626 | 668 | 674 |
| Chrysanthemum | 352 | 430, 538 | 646 | 514, 678 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kut, K.; Bartosz, G.; Sadowska-Bartosz, I. The Effect of Storage on the Absorption and Fluorescence Spectra of Petal Extracts of Selected Anthocyanin-Containing Flowers. Processes 2025, 13, 1826. https://doi.org/10.3390/pr13061826
Kut K, Bartosz G, Sadowska-Bartosz I. The Effect of Storage on the Absorption and Fluorescence Spectra of Petal Extracts of Selected Anthocyanin-Containing Flowers. Processes. 2025; 13(6):1826. https://doi.org/10.3390/pr13061826
Chicago/Turabian StyleKut, Kacper, Grzegorz Bartosz, and Izabela Sadowska-Bartosz. 2025. "The Effect of Storage on the Absorption and Fluorescence Spectra of Petal Extracts of Selected Anthocyanin-Containing Flowers" Processes 13, no. 6: 1826. https://doi.org/10.3390/pr13061826
APA StyleKut, K., Bartosz, G., & Sadowska-Bartosz, I. (2025). The Effect of Storage on the Absorption and Fluorescence Spectra of Petal Extracts of Selected Anthocyanin-Containing Flowers. Processes, 13(6), 1826. https://doi.org/10.3390/pr13061826









