Recovery of β-Carotene from Microalga Dunaliella sp. by HPCCC
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Production
2.2. Preparation of Extract of Dunaliella sp. Biomass
2.3. High-Performance Countercurrent Chromatography (HPCCC) Separation
2.3.1. HPCCC Equipment
2.3.2. Selection of the Suitable Biphasic Solvent System for HPCCC
2.3.3. HPCCC Separation Process
2.4. HPLC-DAD Analysis of Extract and Fractions
2.5. Confirmation of the Chemical Identity of the Purified Target Compound
2.6. Evaluation of HPCCC Process Efficiency
3. Results and Discussion
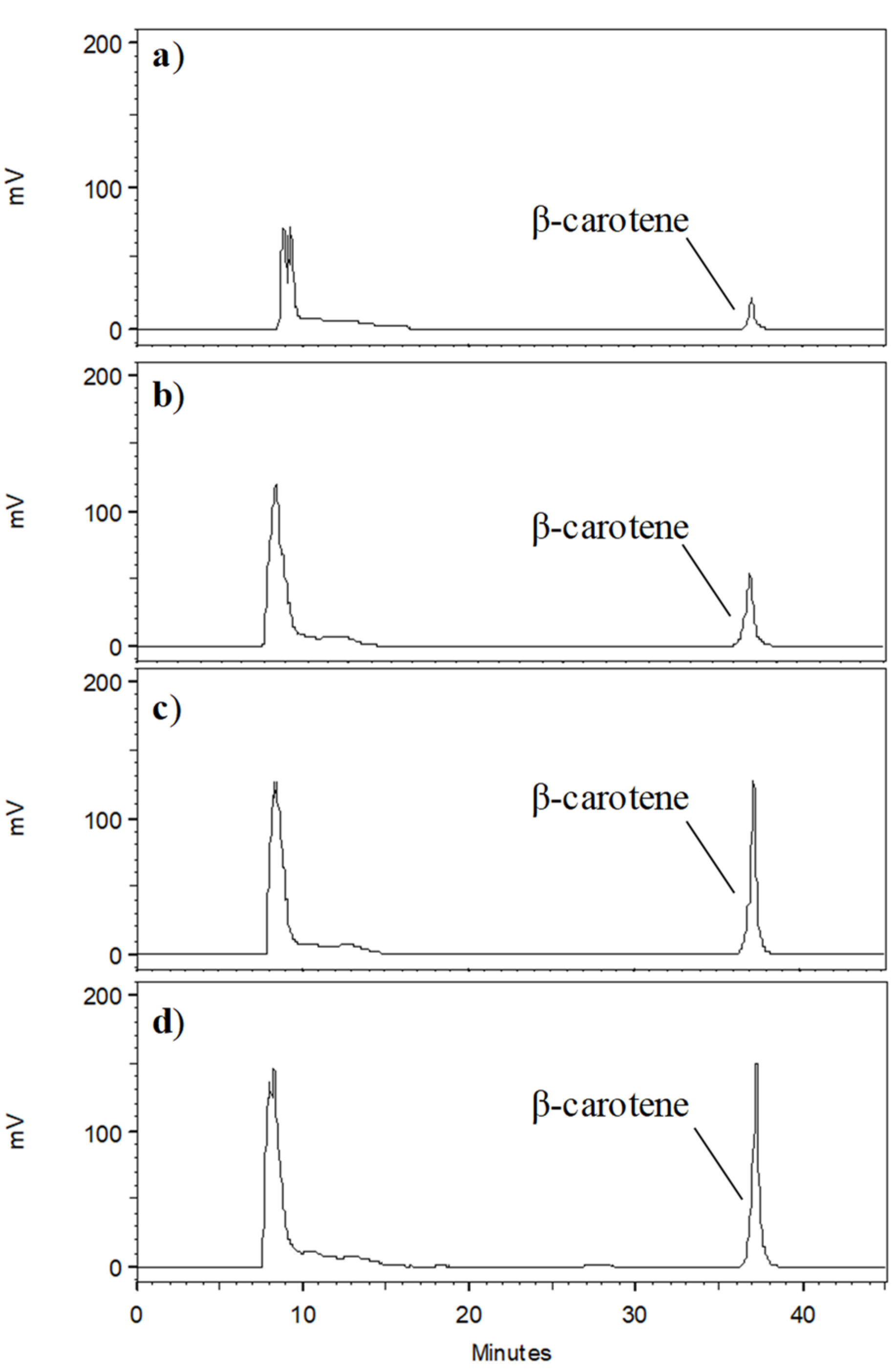
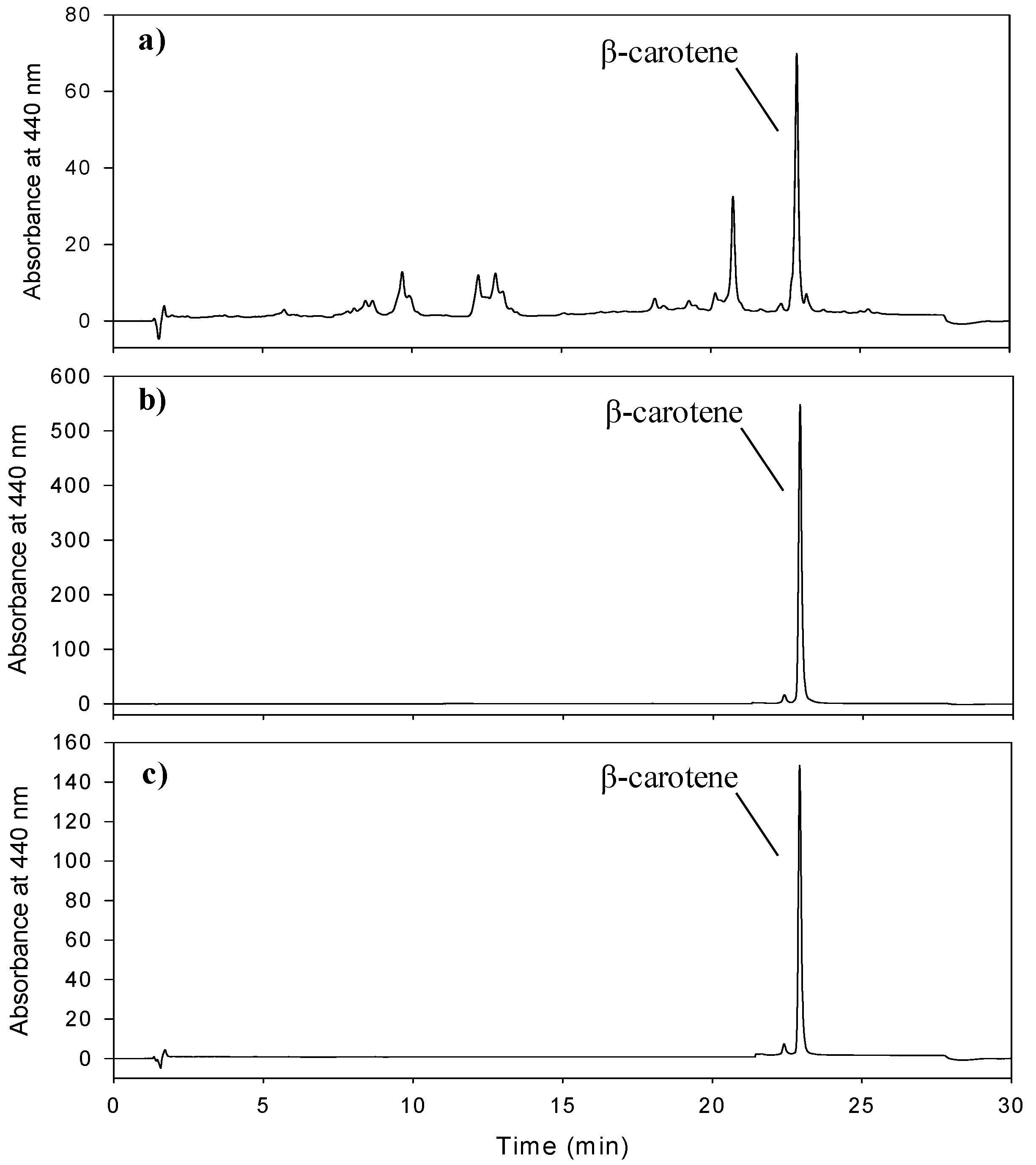
3.1. Development and Optimization of HPCCC Separation
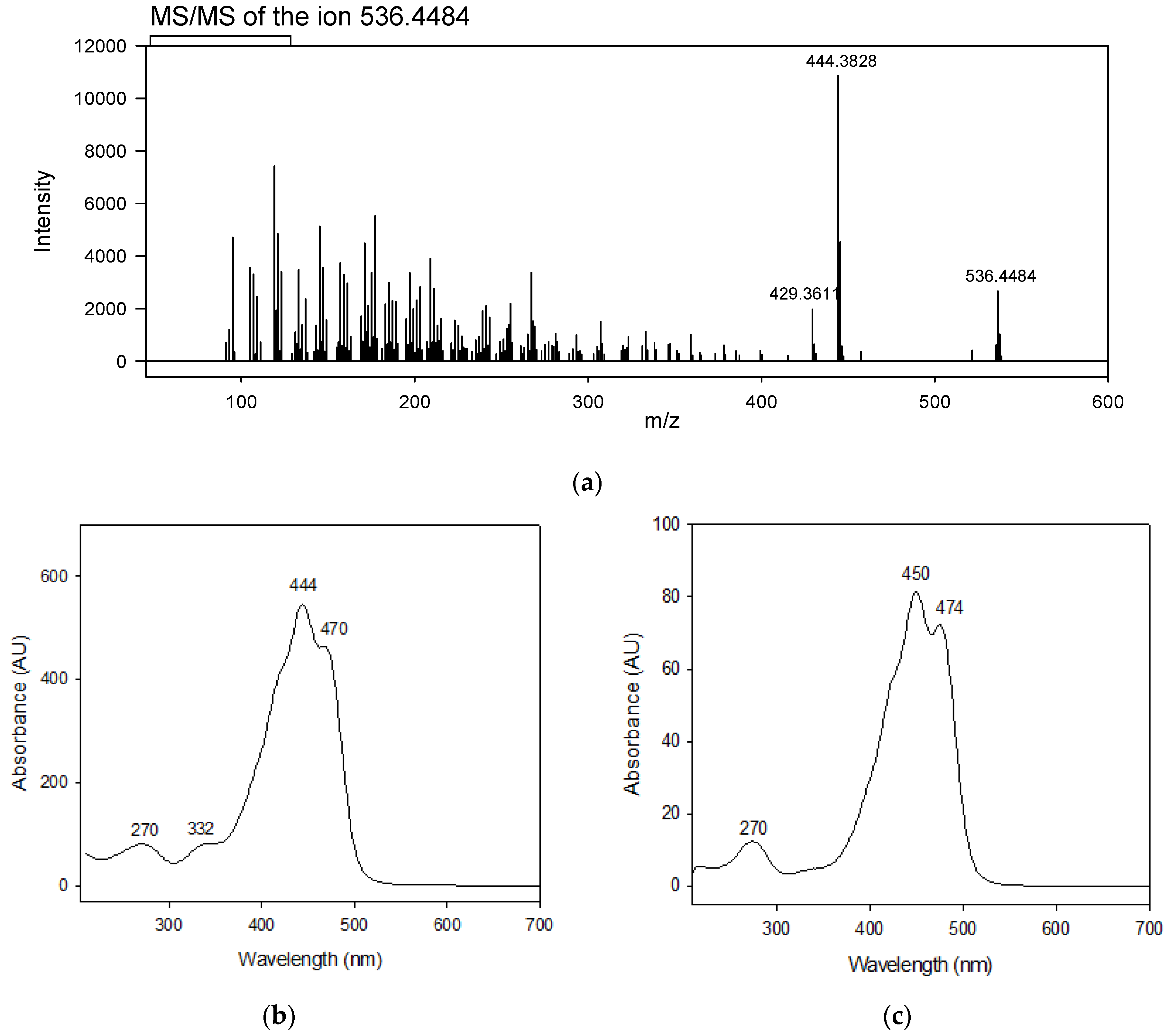
3.2. Confirmation of β-Carotene Identity Isolated by HPCCC
3.3. Process Performance
3.4. Theoretical Throughput Projections for β-Carotene Separation from Dunaliella sp. via HPCCC at Multiple Scales
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raja, R.; Hemaiswarya, S.; Rengasamy, R. Exploitation of Dunaliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007, 74, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Bogacz-Radomska, L.; Harasym, J. β-Carotene—Properties and production methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef]
- Jin, E.S.; Melis, A. Microalgal biotechnology: Carotenoid production by the green algae Dunaliella salina. Biotechnol. Bioprocess Eng. 2003, 8, 331–337. [Google Scholar] [CrossRef]
- Damergi, E.; Schwitzguébel, J.P.; Refardt, D.; Sharma, S.; Holliger, C.; Ludwig, C. Extraction of carotenoids from Chlorella vulgaris using green solvents and syngas production from residual biomass. Algal Res. 2017, 25, 488–495. [Google Scholar] [CrossRef]
- Di Caprio, F.; Altimari, P.; Pagnanelli, F. Sequential extraction of lutein and β-carotene from wet microalgal biomass. J. Chem. Technol. Biotechnol. 2020, 95, 3024–3033. [Google Scholar] [CrossRef]
- Soares, A.T.; Marques Júnior, J.G.; Lopes, R.G.; Derner, R.B.; Antoniosi Filho, N.R. Improvement of the extraction process for high commercial value pigments from Desmodesmus sp. microalgae. J. Braz. Chem. Soc. 2016, 27, 1083–1093. [Google Scholar] [CrossRef]
- Schüler, L.M.; Gangadhar, K.N.; Duarte, P.; Placines, C.; Molina-Márquez, A.M.; Léon-Bañares, R.; Sousa, V.S.; Varela, J.; Barreira, L. Improvement of carotenoid extraction from a recently isolated, robust microalga, Tetraselmis sp. CTP4 (chlorophyta). Bioprocess Biosyst. Eng. 2020, 43, 785–796. [Google Scholar] [CrossRef]
- Bachchhav, M.B.; Kulkarni, M.V.; Ingale, A.G. Process-intensified extraction of phycocyanin followed by β-carotene from Spirulina platensis using ultrasound-assisted extraction. Sep. Sci. Technol. 2019, 55, 932–944. [Google Scholar] [CrossRef]
- Rajput, A.; Singh, D.P.; Khattar, J.S.; Swatch, G.K.; Singh, Y. Evaluation of growth and carotenoid production by a green microalga Scenedesmus quadricauda PUMCC 4.1.40. under optimized culture conditions. J. Basic. Microbiol. 2022, 62, 1156–1166. [Google Scholar] [CrossRef]
- Gamlieli-Bonshtein, I.; Korin, E.; Cohen, S. Selective separation of cis-trans geometrical isomers of beta-carotene via CO2 supercritical fluid extraction. Biotechnol. Bioeng. 2002, 80, 169–174. [Google Scholar] [CrossRef]
- Jaime, L.; Mendiola, J.A.; Ibáñez, E.; Martin-Alvarez, P.J.; Cifuentes, A.; Reglero, G.; Señoráns, F.J. Beta-carotene isomer composition of sub- and supercritical carbon dioxide extracts. Antioxidant activity measurement. J. Agric. Food Chem. 2007, 55, 10585–10590. [Google Scholar] [CrossRef] [PubMed]
- Macias-Sanchez, M.D.; Mantell, C.; Rodriguez, M.; Martínez de la Ossa, E.; Lubian, L.M.; Montero, O. Comparison of Supercritical Fluid and Ultrasound-Assisted Extraction of Carotenoids and Chlorophyll a from Dunaliella salina. Talanta 2009, 77, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Marino, T.; Karatza, D.; Musmarra, D. Extraction of Bioactive Compounds Using Supercritical Carbon Dioxide. Molecules 2019, 24, 782. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.R.P.; Tavakoli, O.; Sarrafzadeh, M.H. Experimental Optimization of SC-CO2 Extraction of Carotenoids from Dunaliella salina. J. Supercrit. Fluids 2017, 121, 89–95. [Google Scholar] [CrossRef]
- Tirado, D.F.; Calvo, L. The Hansen theory to choose the best cosolvent for supercritical CO2 extraction of Β-carotene from Dunaliella salina. J. Supercrit. Fluids 2019, 145, 211–218. [Google Scholar] [CrossRef]
- Ludwig, K.; Rihko-Struckmann, L.; Brinitzer, G.; Kruse, A. β-Carotene Extraction from Dunaliella salina by Supercritical CO2. J. Appl. Phycol. 2021, 33, 1435–1445. [Google Scholar] [CrossRef]
- Berthod, A.; Maryutina, T.; Spivakov, B.; Shpigun, O.; Sutherland, I.A. Countercurrent chromatography in analytical chemistry (IUPAC Technical Report). Pure Appl. Chem. 2009, 81, 355–387. [Google Scholar] [CrossRef]
- Sutherland, I.A. Recent Progress on the Industrial Scale-Up of Counter-Current Chromatography. J. Chromatogr. A 2007, 1151, 6–13. [Google Scholar] [CrossRef]
- Englert, M.; Hammann, S.; Vetter, W. Isolation of β-carotene, α-carotene and lutein from carrots by countercurrent chromatography with the solvent system modifier benzotrifluoride. J. Chromatogr. A 2015, 1388, 119–125. [Google Scholar] [CrossRef]
- Marchal, L.; Mojaat-Guemir, M.; Foucault, A.; Pruvost, J. Centrifugal partition extraction of β-carotene from Dunaliella salina for efficient and biocompatible recovery of metabolites. Bioresour. Technol. 2013, 134, 396–400. [Google Scholar] [CrossRef]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Cheel, J.; Urajová, P.; Hájek, J.; Hrouzek, P.; Kuzma, M.; Bouju, E.; Faure, K.; Kopecký, J. Separation of cyclic lipopeptide puwainaphycins from cyanobacteria by countercurrent chromatography combined with polymeric resins and HPLC. Anal. Bioanal. Chem. 2017, 409, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Conway, W.D. Experimental Observations of the Hydrodynamic Behavior of Solvent Systems in High-Speed Countercurrent Chromatography. III. Effects of Physical Properties of the Solvent Systems and Operating Temperature on the Distribution of Two-Phase Solvent Systems. J. Chromatogr. A 1984, 301, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.A. Liquid stationary phase retention and resolution in hydrodynamic CCC. In Comprehensive Analytical Chemistry; Berthod, A., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2002; Volume 38, pp. 159–176. [Google Scholar]
- Bárcenas-Pérez, D.; Střížek, A.; Hrouzek, P.; Kopecký, J.; Barradas, M.; Sierra-Ramirez, A.; Fernandez-Marcos, P.J.; Cheel, J. Production of fucoxanthin from Phaeodactylum tricornutum using high performance countercurrent chromatography retaining its FOXO3 nuclear translocation-inducing effect. Mar. Drugs 2021, 19, 517. [Google Scholar] [CrossRef]
- Zhang, M.; Ignatova, S.; Liang, Q.; Wu, J.F.; Sutherland, I.; Wang, Y.; Luo, G. Rapid and High-Throughput Purification of Salvianolic Acid B from Salvia miltiorrhiza Bunge by High-Performance Counter-Current Chromatography. J. Chromatogr. A 2009, 1216, 3869–3873. [Google Scholar] [CrossRef]
- Renault, J.H.; Nuzillard, J.M.; Intes, O.; Maciuk, A. Solvent systems. In Comprehensive Analytical Chemistry; Berthod, A., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2002; Volume 38, pp. 49–83. [Google Scholar]
- Official Journal of the European Union; European Parliament and Council. Directive 2009/32/EC of 23 April 2009 on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients (Recast). Off. J. Eur. Union. 2009, L 140, 3–11. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32009L0032 (accessed on 10 January 2025).
- Berthod, A.; Faure, K. Separations with a liquid stationary phase: Countercurrent chromatography or centrifugal partition chromatography. In Analytical Separation Science, 1st ed.; Anderson, J.L., Berthod, A., Pino Estévez, V., Stalcup, A.M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1177–1206. [Google Scholar]
- Li, S.; He, S.; Zhong, S.; Duan, X.; Ye, H.; Shi, J.; Peng, A.; Chen, L. Elution-extrusion counter-current chromatography separation of five bioactive compounds from Dendrobium chrysototxum Lindl. J. Chromatogr. A 2011, 20, 3124–3128. [Google Scholar] [CrossRef]
- Stutz, H.; Bresgen, N.; Eckl, P.M. Analytical tools for the analysis of β-carotene and its degradation products. Free Radic. Res. 2015, 49, 650–680. [Google Scholar] [CrossRef]
- Zhang, Y.; Honda, M.; Wahyudiono; Kanda, H.; Goto, M. Enhanced production of β-carotene suspensions using supercritical CO2 via naturally occurring Z-isomerization-accelerating catalyst. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012008. [Google Scholar] [CrossRef]
- Xu, Y.; Harvey, P.J. Red light control of β-Carotene isomerisation to 9-cis β-carotene and carotenoid accumulation in Dunaliella salina. Antioxidants 2019, 27, 148. [Google Scholar] [CrossRef]



| Solvent Systems | Composition | Relative Proportions of Solvents (v/v/v) | Phase Volume Ratio (UP/LP) | Settling Time (s) | Density Difference (LP − UP, g/mL) | Partition Coefficient (K) of β-Carotene |
|---|---|---|---|---|---|---|
| 1 | n-Hep–EtOH–H2O | 6/5/1 | 1.00 | 10 | 0.1284 | 1180.08 |
| 2 | n-Hep–EtOH–H2O | 6/6/1 | 0.78 | 11 | 0.1125 | 1014.24 |
| 3 | n-Hep–EtOH–H2O | 6/7/1 | 0.65 | 13 | 0.0953 | 220.46 |
| 4 | n-Hep–EtOH–H2O | 6/8/1 | 0.50 | 14 | 0.0940 | 188.08 |
| 5 | n-Hep–EtOH–H2O | 6/9/1 | 0.39 | 27 | 0.0910 | 98.16 |
| 6 | n-Hep–EtOH–H2O | 6/10/1 | 0.27 | 20 | 0.0825 | 59.69 |
| 7 | n-Hep–EtOH–H2O | 6/11/1 | 0.22 | 29 | 0.0797 | 54.40 |
| 8 | n-Hep–EtOH–H2O | 6/12/1 | 0.13 | 29 | 0.0728 | 35.10 |
| 9 | n-Hep–EtOH–H2O | 6/13/1 | 0.02 | 29 | 0.0642 | 24.03 |
| 10 | n-Hep–EtOH–H2O | 6/14/1 | No phases | - | - | - |
| 11 | n-Hep–MeOH | 1/1 | 0.53 | 8 | 0.0700 | 9.00 |
| Optimization Experiments | Flow Rate (mL/min) | Sf at the Hydrodynamic Equilibrium in HPCCC (%) | Loading per Injection (mg) | Sf at the End of the HPCCC Separation Run (%) | β-Carotene HPLC Purity (%) | β-Carotene Yield (mg) |
|---|---|---|---|---|---|---|
| a | 10 | 50 | 100 | 47 | 98 | 13.5 |
| b | 10 | 50 | 200 | 36 | 98 | 28.1 |
| c | 10 | 50 | 400 | 29 | 97 | 55.6 |
| d | 10 | 50 | 800 | 21 | 97 | 112.7 |
| HPCCC Process | Purity (%) | Pt (g/h) | Pe (g/h) | Er (L/g) | Ge (g2 h−1 L−1) |
|---|---|---|---|---|---|
| Method in this paper | 97 | 0.6980 | 0.09836 | 6.1003 | 0.0161 |
| [19] | 95 | 0.0270 | 0.01348 | 14.1000 | 0.0009 |
| Column Volume (mL) | Throughput (g/h) | Throughput (g/week) |
|---|---|---|
| 134 | 0.698 | 27.92 a |
| 980 | 5.105 | 204.19 a |
| 4.6 | 23.962 | 575.09 b |
| 8.820 | 45.945 | 1102.67 b |
| 18 | 93.765 | 2250.37 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bárcenas-Pérez, D.; Gomes, D.; Parreira, C.; Costa, L.; Cheel, J. Recovery of β-Carotene from Microalga Dunaliella sp. by HPCCC. Processes 2025, 13, 1812. https://doi.org/10.3390/pr13061812
Bárcenas-Pérez D, Gomes D, Parreira C, Costa L, Cheel J. Recovery of β-Carotene from Microalga Dunaliella sp. by HPCCC. Processes. 2025; 13(6):1812. https://doi.org/10.3390/pr13061812
Chicago/Turabian StyleBárcenas-Pérez, Daniela, Diana Gomes, Celina Parreira, Luís Costa, and José Cheel. 2025. "Recovery of β-Carotene from Microalga Dunaliella sp. by HPCCC" Processes 13, no. 6: 1812. https://doi.org/10.3390/pr13061812
APA StyleBárcenas-Pérez, D., Gomes, D., Parreira, C., Costa, L., & Cheel, J. (2025). Recovery of β-Carotene from Microalga Dunaliella sp. by HPCCC. Processes, 13(6), 1812. https://doi.org/10.3390/pr13061812







