Valorization of Grape Seed By-Products Using Subcritical Water Extraction: A Sustainable Approach for Bioactive Compound Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Preparation
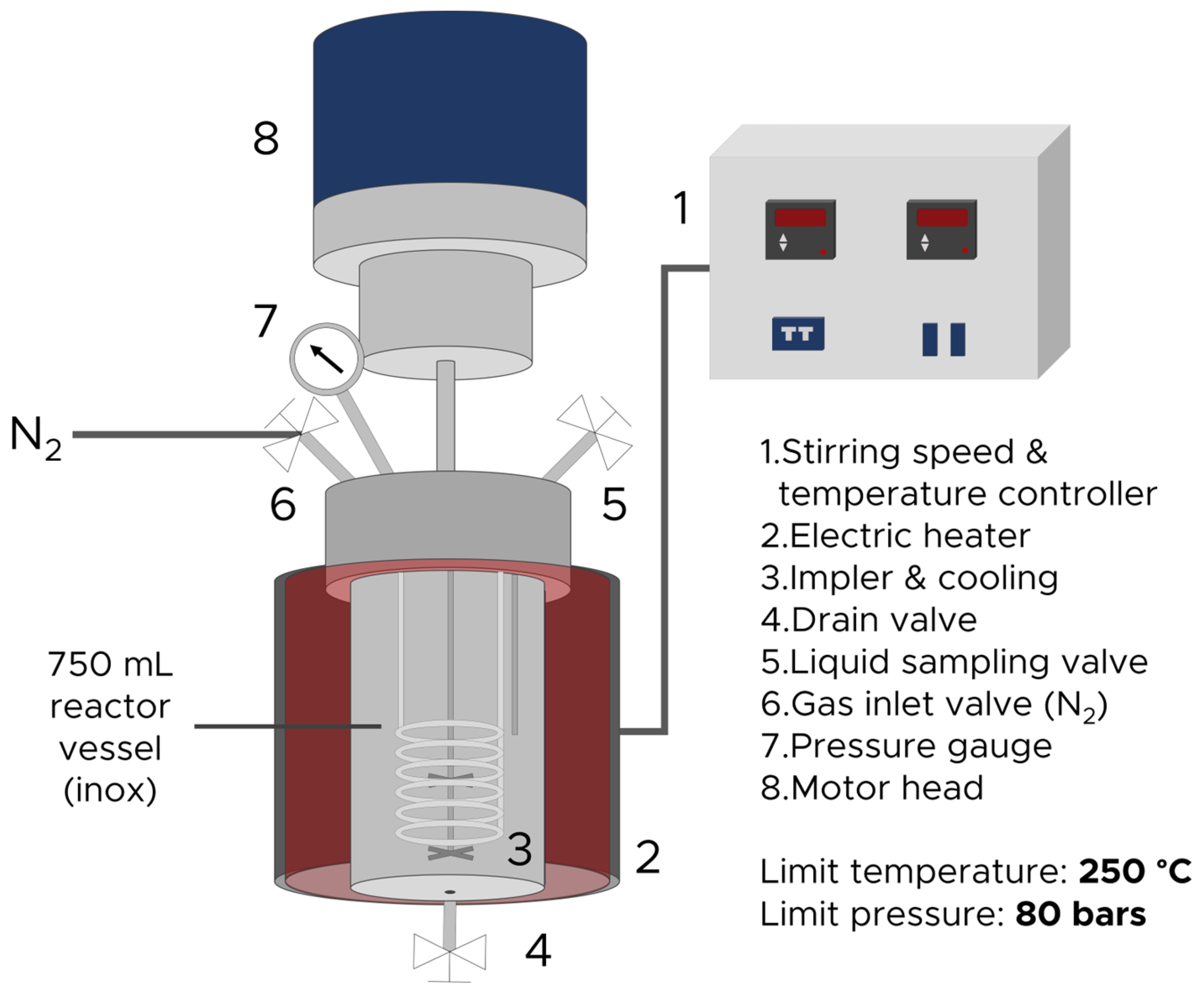
2.2. Subcritical Water Extraction (SWE)
2.3. Conventional Solvent Extraction
2.4. Experimental Design of SWE by RSM
2.5. Validation of the Model
2.6. Characterization of SWE Grape Seed Extracts
2.6.1. Dry Matter Determination
2.6.2. Total Phenolic Content (TPC) Determination
2.6.3. Protein Determination
2.6.4. DPPH Radical Scavenging Activity Determination
2.7. Quantification of Phenolic Compounds Through HPLC-DAD
2.8. Identification of Phenolic Compounds Through HPLC-PDA-MSMS
2.9. Maillard Reaction Products Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Experimental Design
3.2. Response Surface Analysis of AA
3.3. Response Surface Analysis of TPC
3.4. Response Surface Analysis of Tprot
3.5. Phenolic Compounds Identification and Quantification
3.6. Formation of Maillard Reaction Products During SWE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape Seed Extract: Having a Potential Health Benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- García-Marino, M.; Rivas-Gonzalo, J.C.; Ibáñez, E.; García-Moreno, C. Recovery of Catechins and Proanthocyanidins from Winery By-Products Using Subcritical Water Extraction. Anal. Chim. Acta 2006, 563, 44–50. [Google Scholar] [CrossRef]
- Cherubim, D.J.; Martins, C.V.; Fariña, L.; Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; la Paz, S.M. Grape (Vitis vinifera L.) Seed Oil: A Functional Food from the Winemaking Industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T.; Liu, W.; Zhao, G. Physicochemical Characteristics and Functional Properties of Grape (Vitis vinifera L.) Seeds Protein. Int. J. Food Sci. Technol. 2011, 46, 635–641. [Google Scholar] [CrossRef]
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine Fining with Plant Proteins. Molecules 2019, 24, 2186. [Google Scholar] [CrossRef]
- Gazzola, D.; Vincenzi, S.; Marangon, M.; Pasini, G.; Curioni, A. Grape Seed Extract: The First Protein-based Fining Agent Endogenous to Grapes. Aust. J. Grape Wine Res. 2017, 23, 215–225. [Google Scholar] [CrossRef]
- Rajbhar, K.; Dawda, H.; Mukundan, U. Polyphenols: Methods of Extraction. Sci. Rev. Chem. Commun. 2015, 5, 1–6. [Google Scholar]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Supriatno; Seow, E.M. Effect of Solvents in Extracting Polyphenols and Antioxidants of Selected Raw Vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of Polyphenols from Grape Skins and Defatted Grape Seeds Using Subcritical Water: Experiments and Modeling. Food Bioprod. Process. 2015, 94, 29–38. [Google Scholar] [CrossRef]
- Yammine, S.; Delsart, C.; Vitrac, X.; Peuchot, M.M.; Ghidossi, R. Characterisation of Polyphenols and Antioxidant Potential of Red and White Pomace By-Product Extracts Using Subcritical Water Extraction. OENO One 2020, 54, 263–278. [Google Scholar] [CrossRef]
- Ferreira, C.; Moreira, M.M.; Delerue-Matos, C.; Sarraguça, M. Subcritical Water Extraction to Valorize Grape Biomass—A Step Closer to Circular Economy. Molecules 2023, 28, 7538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent Advances in the Extraction of Bioactive Compounds with Subcritical Water: A Review. Trends Food Sci. Technol. 2019, 95, 183–195. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical Water Extraction of Phenolic Compounds from Onion Skin Wastes (Allium cepa Cv. Horcal): Effect of Temperature and Solvent Properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef]
- Giombelli, C.; Iwassa, I.J.; da Silva, C.; Barros, B.C.B. Valorization of Peach Palm By-Product through Subcritical Water Extraction of Soluble Sugars and Phenolic Compounds. J. Supercrit. Fluids 2020, 165, 104985. [Google Scholar] [CrossRef]
- Rodrigues, L.G.G.; Mazzutti, S.; Vitali, L.; Micke, G.A.; Ferreira, S.R.S. Recovery of Bioactive Phenolic Compounds from Papaya Seeds Agroindustrial Residue Using Subcritical Water Extraction. Biocatal. Agric. Biotechnol. 2019, 22, 101367. [Google Scholar] [CrossRef]
- Ibañez, E.; Kubátová, A.; Señoráns, F.J.; Cavero, S.; Reglero, G.; Hawthorne, S.B. Subcritical Water Extraction of Antioxidant Compounds from Rosemary Plants. J. Agric. Food Chem. 2003, 51, 375–382. [Google Scholar] [CrossRef]
- Singh, P.P.; Saldaña, M.D.A. Subcritical Water Extraction of Phenolic Compounds from Potato Peel. Food Res. Int. 2011, 44, 2452–2458. [Google Scholar] [CrossRef]
- Carr, A.G.; Mammucari, R.; Foster, N.R. A Review of Subcritical Water as a Solvent and Its Utilisation for the Processing of Hydrophobic Organic Compounds. Chem. Eng. J. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Möller, M.; Nilges, P.; Harnisch, F.; Schröder, U. Subcritical Water as Reaction Environment: Fundamentals of Hydrothermal Biomass Transformation. ChemSusChem 2011, 4, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, Y.; Ma, Y.; Zhu, P.; He, J.; Lei, J. Optimization of Subcritical Water Extraction of Resveratrol from Grape Seeds by Response Surface Methodology. Appl. Sci. 2017, 7, 321. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nioi, C.; Lisanti, M.T.; Meunier, F.; Redon, P.; Massot, A.; Moine, V. Antioxidant Activity of Yeast Derivatives: Evaluation of Their Application to Enhance the Oxidative Stability of White Wine. LWT 2022, 171, 114116. [Google Scholar] [CrossRef]
- Mateus, A.R.S.; Barros, S.C.; Cortegoso, S.M.; Sendón, R.; Barbosa-Pereira, L.; Khwaldia, K.; Pataro, G.; Ferrari, G.; Breniaux, M.; Ghidossi, R.; et al. Potential of Fruit Seeds: Exploring Bioactives and Ensuring Food Safety for Sustainable Management of Food Waste. Food Chem. X 2024, 23, 101718. [Google Scholar] [CrossRef]
- Salagoity-Auguste, M.-H.; Tricard, C.; Sudraud, P. Dosage Simultané Des Aldéhydes Aromatiques et Des Coumarines Par Chromatographie Liquide Haute Performance Application Aux Vins et Eaux-de-Vie Vieillis En Fŭt de Chĕne. J. Chromatogr. A 1987, 392, 379–387. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of Antioxidants from Winery Wastes Using Subcritical Water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- Kronholm, J.; Hartonen, K.; Riekkola, M.-L. Analytical Extractions with Water at Elevated Temperatures and Pressures. TrAC Trends Anal. Chem. 2007, 26, 396–412. [Google Scholar] [CrossRef]
- Ju, Z.; Howard, L.R. Subcritical Water and Sulfured Water Extraction of Anthocyanins and Other Phenolics from Dried Red Grape Skin. J. Food Sci. 2005, 70, S270–S276. [Google Scholar] [CrossRef]
- Gabaston, J.; Leborgne, C.; Valls, J.; Renouf, E.; Richard, T.; Waffo-Teguo, P.; Mérillon, J.-M. Subcritical Water Extraction of Stilbenes from Grapevine By-Products: A New Green Chemistry Approach. Ind. Crops Prod. 2018, 126, 272–279. [Google Scholar] [CrossRef]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on Phenolic Compounds Stability during Microwave-Assisted Extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B. Virgin Grape Seed Oil: Is It Really a Nutritional Highlight? Eur. J. Lipid Sci. Technol. 2008, 110, 645–650. [Google Scholar] [CrossRef]
- Pesavento, I.C.; Bertazzo, A.; Flamini, R.; Vedova, A.D.; Rosso, M.D.; Seraglia, R.; Traldi, P. Differentiation of Vitis vinifera Varieties by MALDI-MS Analysis of the Grape Seed Proteins. J. Mass Spectrom. 2008, 43, 234–241. [Google Scholar] [CrossRef]
- Elkatry, H.O.; Ahmed, A.R.; El-Beltagi, H.S.; Mohamed, H.I.; Eshak, N.S. Biological Activities of Grape Seed By-Products and Their Potential Use as Natural Sources of Food Additives in the Production of Balady Bread. Foods 2022, 11, 1948. [Google Scholar] [CrossRef]
- Oprea, O.B.; Popa, M.E.; Apostol, L.; Gaceu, L. Research on the Potential Use of Grape Seed Flour in the Bakery Industry. Foods 2022, 11, 1589. [Google Scholar] [CrossRef]
- Hata, S.; Wiboonsirikul, J.; Maeda, A.; Kimura, Y.; Adachi, S. Extraction of Defatted Rice Bran by Subcritical Water Treatment. Biochem. Eng. J. 2008, 40, 44–53. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Li, C.; Duan, Y.; Zhang, H.; Ma, H. Antioxidant Peptide Fractions Isolated from Wheat Germ Protein with Subcritical Water Extraction and Its Transport Across Caco-2 Cells. J. Food Sci. 2019, 84, 2139–2146. [Google Scholar] [CrossRef]
- Baca-Bocanegra, B.; Nogales-Bueno, J.; Hernández-Hierro, J.M.; Heredia, F.J. Optimization of Protein Extraction of Oenological Interest from Grape Seed Meal Using Design of Experiments and Response Surface Methodology. Foods 2021, 10, 79. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized Hot Water Extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.d.S.S.; Genovese, M.I.; Fett, R. Phenolic Compounds and Antioxidant Activity of Seed and Skin Extracts of Red Grape (Vitis vinifera and Vitis labrusca) Pomace from Brazilian Winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Sochorova, L.; Prusova, B.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Baron, M.; Sochor, J. The Study of Antioxidant Components in Grape Seeds. Molecules 2020, 25, 3736. [Google Scholar] [CrossRef] [PubMed]
- Guendez, R.; Kallithraka, S.; Makris, D.P.; Kefalas, P. Determination of Low Molecular Weight Polyphenolic Constituents in Grape (Vitis vinifera Sp.) Seed Extracts: Correlation with Antiradical Activity. Food Chem. 2005, 89, 1–9. [Google Scholar] [CrossRef]
- Tița, O.; Lengyel, E.; Stegăruș, D.I.; Săvescu, P.; Ciubara, A.B.; Constantinescu, M.A.; Tița, M.A.; Rață, D.; Ciubara, A. Identification and Quantification of Valuable Compounds in Red Grape Seeds. Appl. Sci. 2021, 11, 5124. [Google Scholar] [CrossRef]
- López-Gutiérrez, N.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Determination of Polyphenols in Grape-Based Nutraceutical Products Using High Resolution Mass Spectrometry. LWT Food Sci. Technol. 2016, 71, 249–259. [Google Scholar] [CrossRef]
- Galkin, A.A.; Lunin, V.V. Subcritical and Supercritical Water: A Universal Medium for Chemical Reactions. Russ. Chem. Rev. 2005, 74, 21–35. [Google Scholar] [CrossRef]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of Heat Treatment on the Phenolic Compounds and Antioxidant Capacity of Citrus Peel Extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef]
- Alibekov, R.S.; Kamal, S.M.M.; Taip, F.S.; Sulaiman, A.; Azimov, A.M.; Urazbayeva, K.A. Recovery of Phenolic Compounds from Jackfruit Seeds Using Subcritical Water Extraction. Foods 2023, 12, 3296. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Facts about the Formation of New Antioxidants in Natural Samples after Subcritical Water Extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Neoformation of Antioxidants in Glycation Model Systems Treated under Subcritical Water Extraction Conditions. Food Res. Int. 2010, 43, 1123–1129. [Google Scholar] [CrossRef]
- Ellis, G.P. The Maillard Reaction. Adv. Carbohydr. Chem. 1959, 14, 63–134. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Luo, W. Maillard Reaction and Processed Foods—Main Chemical Products. In Chemistry of Maillard Reactions in Processed Foods; SpringerBriefs in Molecular Science; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Kheirkhah, H.; Baroutian, S.; Quek, S.Y. Evaluation of Bioactive Compounds Extracted from Hayward Kiwifruit Pomace by Subcritical Water Extraction. Food Bioprod. Process. 2019, 115, 143–153. [Google Scholar] [CrossRef]
- Fan, R.; Gao, Y. Maillard and Hydrolytic Reactions in Subcritical Water Extraction of Bioactive Compounds from Licorice. Molecules 2022, 27, 6851. [Google Scholar] [CrossRef]
- He, L.; Zhang, X.; Xu, H.; Xu, C.; Yuan, F.; Knez, Ž.; Novak, Z.; Gao, Y. Subcritical Water Extraction of Phenolic Compounds from Pomegranate (Punica granatum L.) Seed Residues and Investigation into Their Antioxidant Activities with HPLC–ABTS+ Assay. Food Bioprod. Process. 2012, 90, 215–223. [Google Scholar] [CrossRef]
- Plaza, M.; Abrahamsson, V.; Turner, C. Extraction and Neoformation of Antioxidant Compounds by Pressurized Hot Water Extraction from Apple Byproducts. J. Agric. Food Chem. 2013, 61, 5500–5510. [Google Scholar] [CrossRef]
- Liu, S.-C.; Yang, D.-J.; Jin, S.-Y.; Hsu, C.-H.; Chen, S.-L. Kinetics of Color Development, PH Decreasing, and Anti-Oxidative Activity Reduction of Maillard Reaction in Galactose/Glycine Model Systems. Food Chem. 2008, 108, 533–541. [Google Scholar] [CrossRef]
- Kanmaz, E.Ö. 5-Hydroxymethylfurfural (HMF) Formation during Subcritical Water Extraction. Food Sci. Biotechnol. 2018, 27, 981–986. [Google Scholar] [CrossRef]
- Asghari, F.S.; Yoshida, H. Acid-Catalyzed Production of 5-Hydroxymethyl Furfural from d-Fructose in Subcritical Water. Ind. Eng. Chem. Res. 2006, 45, 2163–2173. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Vergara, M.; Altamirano, C.; Gonzalez, Á.; Pérez-Correa, J.R. Characterization of Pressurized Hot Water Extracts of Grape Pomace: Chemical and Biological Antioxidant Activity. Food Chem. 2014, 171, 62–69. [Google Scholar] [CrossRef]
- Usui, T.; Shizuuchi, S.; Watanabe, H.; Hayase, F. Cytotoxicity and Oxidative Stress Induced by the Glyceraldehyde-Related Maillard Reaction Products for HL-60 Cells. Biosci. Biotechnol. Biochem. 2004, 68, 333–340. [Google Scholar] [CrossRef]
- Kitts, D.D.; Chen, X.-M.; Jing, H. Demonstration of Antioxidant and Anti-Inflammatory Bioactivities from Sugar–Amino Acid Maillard Reaction Products. J. Agric. Food Chem. 2012, 60, 6718–6727. [Google Scholar] [CrossRef]


| Variable | F1 Temperature (°C) | F2 Time (min) | F3 Pressure (Bar) |
|---|---|---|---|
| Low level (−1) | 100 | 10 | 20 |
| Middle level (0) | 150 | 35 | 45 |
| High level (+1) | 200 | 60 | 70 |
| Extraction Procedure | Responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Repetition | F1 (°C) | F2 (h) | F3 (bar) | AA (mg TROLOXeq/g DM) | TPC (mg GAE/g DM) | Tprot (mg BSAeq/g DM) | |
| Subcritical water | 1 | 1 | 100 | 0.17 | 45 | 235.2 ± 1.9 | 120.2 ± 1.6 | 77.2 ± 3.4 |
| 2 | 1 | 200 | 0.17 | 45 | 300.5 ± 2.0 | 240.0 ± 0.7 | 29.5 ± 3.7 | |
| 3 | 1 | 100 | 1 | 45 | 275.2 ± 2.2 | 150.9 ± 1.7 | 66.8 ± 4.2 | |
| 4 | 1 | 200 | 1 | 45 | 328.2 ± 2.0 | 293.0 ± 0.7 | 19.6 ± 1.7 | |
| 5 | 1 | 100 | 0.58 | 20 | 251.1 ± 2.8 | 151.7 ± 2.9 | 75.6 ± 4.8 | |
| 6 | 1 | 200 | 0.58 | 20 | 302.7 ± 2.5 | 255.2 ± 0.8 | 24.8 ± 2.7 | |
| 7 | 1 | 100 | 0.58 | 70 | 270.5 ± 2.2 | 152.5 ± 0.8 | 70.5 ± 2.4 | |
| 8 | 1 | 200 | 0.58 | 70 | 327.9 ± 2.7 | 224.1 ± 0.9 | 20.1 ± 1.5 | |
| 9 | 1 | 150 | 0.17 | 20 | 368.4 ± 2.9 | 213.0 ± 1.2 | 78.9 ± 4.6 | |
| 10 | 1 | 150 | 1 | 20 | 337.0 ± 1.4 | 208.8 ± 2.0 | 66.0 ± 3.3 | |
| 11 | 1 | 150 | 0.17 | 70 | 373.2 ± 1.9 | 228.5 ± 3.9 | 79.2 ± 4.8 | |
| 12 | 1 | 150 | 1 | 70 | 331.2 ± 3.5 | 239.1 ± 2.1 | 59.1 ± 2.1 | |
| 13 | 1 | 150 | 0.58 | 45 | 334.1 ± 1.1 | 331.2 ± 0.7 | 73.7 ± 3.5 | |
| 14 | 1 | 150 | 0.58 | 45 | 341.3 ± 2.9 | 305.7 ± 0.7 | 72.2 ± 3.0 | |
| 15 | 1 | 150 | 0.58 | 45 | 317.8 ± 3.1 | 287.3 ± 1.1 | 72.5 ± 3.23.2 | |
| Water/ethanol extraction | 16 | 1 | 25 | 24 | 1 | 177.0 ± 10.1 | 150.0 ± 5.8 | 64.4 ± 1.9 |
| 17 | 1 | 25 | 24 | 1 | 192.5 ± 8.5 | 166.0 ± 5.3 | 73.2 ± 3.2 | |
| 18 | 1 | 25 | 24 | 1 | 193.8 ± 8.6 | 166.7 ± 7.4 | 72.7 ± 2.7 | |
| Source | Degree of Freedom | Sum of Square | Mean Square | F Value | p Value | R2 | Adjusted R2 | Signification Code | |
|---|---|---|---|---|---|---|---|---|---|
| AA (mg TROLOXeq/g DM) | Model | 9 | 19,659.0 | 2184.3 | 3.584 | 0.087 | 0.866 | 0.801 | . |
| Residual | 5 | 3047.6 | 609.5 | ||||||
| Total corrected | 14 | 22,706.7 | |||||||
| TPC (mg GAE/g DM) | Model | 9 | 51,725.1 | 5747.2 | 8.494 | 0.015 | 0.939 | 0.898 | * |
| Residual | 5 | 3383.1 | 676.6 | ||||||
| Total corrected | 14 | 55,108.2 | |||||||
| Tprot (mg BSAeq/g DM) | Model | 9 | 7305.9 | 811.7 | 145.405 | <0.0001 | 0.996 | 0.989 | *** |
| Residual | 5 | 27.9 | 5.5 | ||||||
| Total corrected | 14 | 7333.9 |
| Conditions (°C; min, bar) | Values | Desirability | Relative Standard Error | |
|---|---|---|---|---|
| Optimal AA 165 °C, 51 min, 20 bar | Predicted value | 351.6 mg TROLOXeq/gDM | 0.949 | 5.6% |
| Experimental value | 332.7 ± 1.4 mg TROLOXeq/gDM | |||
| Optimal TPC | Predicted value | 276.0 mg GAE/g DM | 0.969 | 4.1% |
| 170 °C, 39 min, 20 bar | Experimental value | 288.3 ± 2.1 mg GAE/g DM | ||
| Optimal Tprot | Predicted value | 84.2 mg BSA/g DM | 1.000 | 6.6% |
| 105 °C, 10 min, 20 bar | Experimental value | 78.0 ± 2.0 mg BSA/g DM |
| N | Compound | Rt (min) | λmax (nm) | Concentration Range (µg/mL) | Calibration Curve Equation | R2 | LOD (µg/mL) | LOQ (µg/mL) | Grape Seed * |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | 2.59 | 278 | 0.1–50 | y = 75.71089x − 65.71346 | 0.9978 | 0.05 | 0.10 | 6.59 ± 0.05 |
| 2 | Protocatechuic acid | 4.37 | 300 | 0.1–50 | y = 48.46254x − 33.52003 | 0.9957 | 0.05 | 0.10 | 7.83 ± 0.11 |
| 3 | p-hydroxybenzoic acid | 6.72 | 278 | 0.05–5 | y = 55.64851x − 0.88561 | 0.9999 | 0.025 | 0.05 | <LOQ |
| 4 | Delphinidin chloride | 7.62 | 325 | 2.5–50 | y = 2.19073x − 2.43698 | 0.9978 | 1 | 2.50 | 1.92 ± 0.67 |
| 5 | Catechin | 8.69 | 278 | 0.25–5 | y = 18.27697x − 1.51585 | 0.9993 | 0.1 | 0.25 | 0.44 ± 0.12 |
| 6 | Gentisic acid | 9.40 | FLD | 0.25–5 | y = 138.89148x − 48.88005 | 0.9922 | 0.1 | 0.25 | 0.20 ± 0.00 |
| 7 | Chlorogenic acid | 12.33 | 325 | 0.25–10 | y = 75.63289x − 33.69450 | 0.9930 | 0.1 | 0.25 | <LOQ |
| 8 | Epicatechin | 12.48 | 278 | 0.25–5 | y = 19.67890x − 0.36473 | 0.9996 | 0.1 | 0.25 | 0.07 ± 0.00 |
| 9 | Isoquercitrin | 22.76 | 360 | 0.25–5 | y = 56.13872x − 11.33972 | 0.9984 | 0.1 | 0.25 | <LOQ |
| 10 | Rutin | 22.80 | 360 | 0.1–5 | y = 35.67480x − 3.80415 | 0.9991 | 0.05 | 0.10 | <LOQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breniaux, M.; Poulain, B.; Mariño-Cortegoso, S.; Barbosa-Pereira, L.; Nioi, C.; Ghidossi, R. Valorization of Grape Seed By-Products Using Subcritical Water Extraction: A Sustainable Approach for Bioactive Compound Recovery. Processes 2025, 13, 1788. https://doi.org/10.3390/pr13061788
Breniaux M, Poulain B, Mariño-Cortegoso S, Barbosa-Pereira L, Nioi C, Ghidossi R. Valorization of Grape Seed By-Products Using Subcritical Water Extraction: A Sustainable Approach for Bioactive Compound Recovery. Processes. 2025; 13(6):1788. https://doi.org/10.3390/pr13061788
Chicago/Turabian StyleBreniaux, Marion, Benjamin Poulain, Sandra Mariño-Cortegoso, Letricia Barbosa-Pereira, Claudia Nioi, and Rémy Ghidossi. 2025. "Valorization of Grape Seed By-Products Using Subcritical Water Extraction: A Sustainable Approach for Bioactive Compound Recovery" Processes 13, no. 6: 1788. https://doi.org/10.3390/pr13061788
APA StyleBreniaux, M., Poulain, B., Mariño-Cortegoso, S., Barbosa-Pereira, L., Nioi, C., & Ghidossi, R. (2025). Valorization of Grape Seed By-Products Using Subcritical Water Extraction: A Sustainable Approach for Bioactive Compound Recovery. Processes, 13(6), 1788. https://doi.org/10.3390/pr13061788








