Development of an Innovative and Sustainable Technological Process for Biogas Purification Through the Reuse of Autoclaved Aerated Concrete Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Experimental Setup
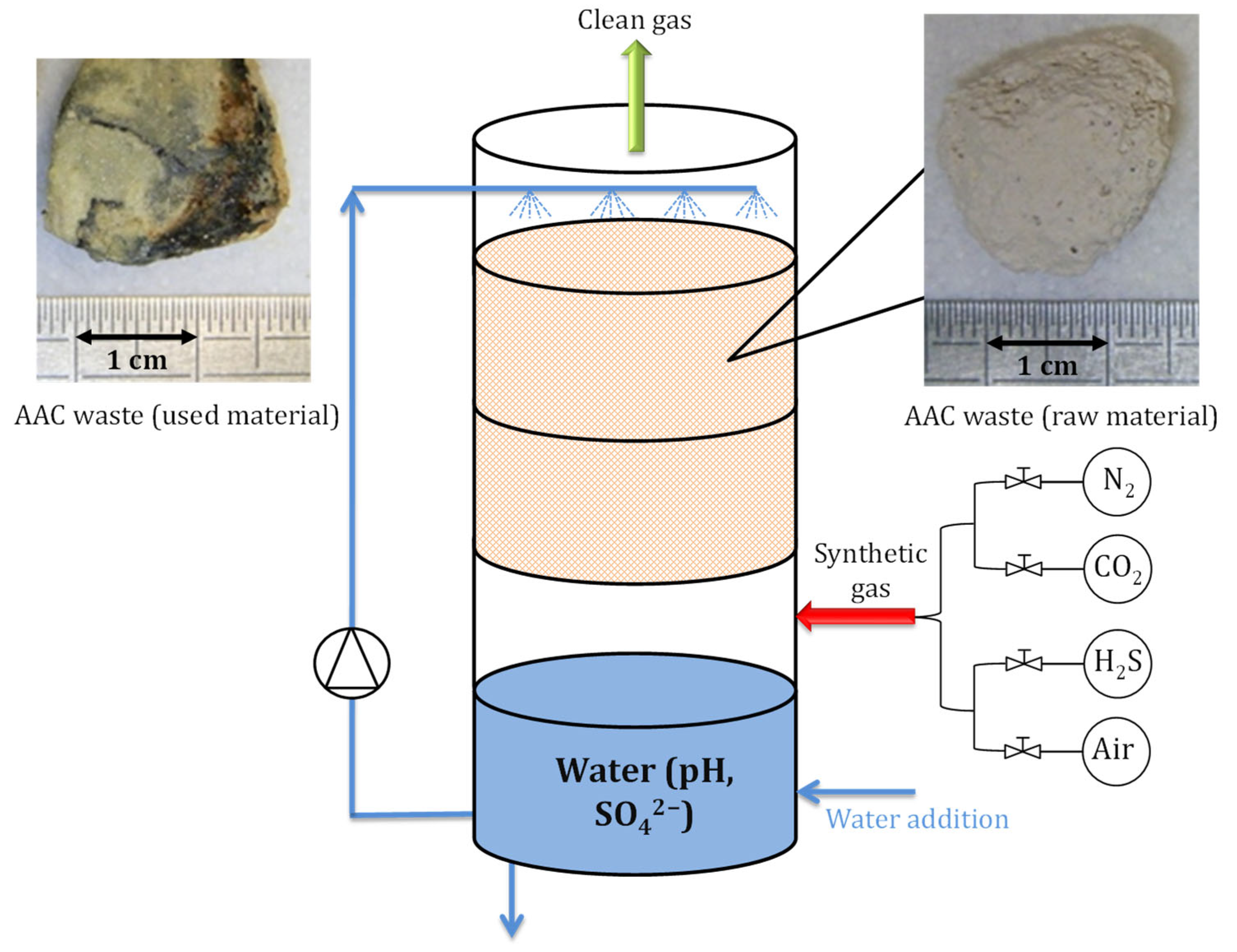
2.3. Operating Conditions
3. Results and Discussion
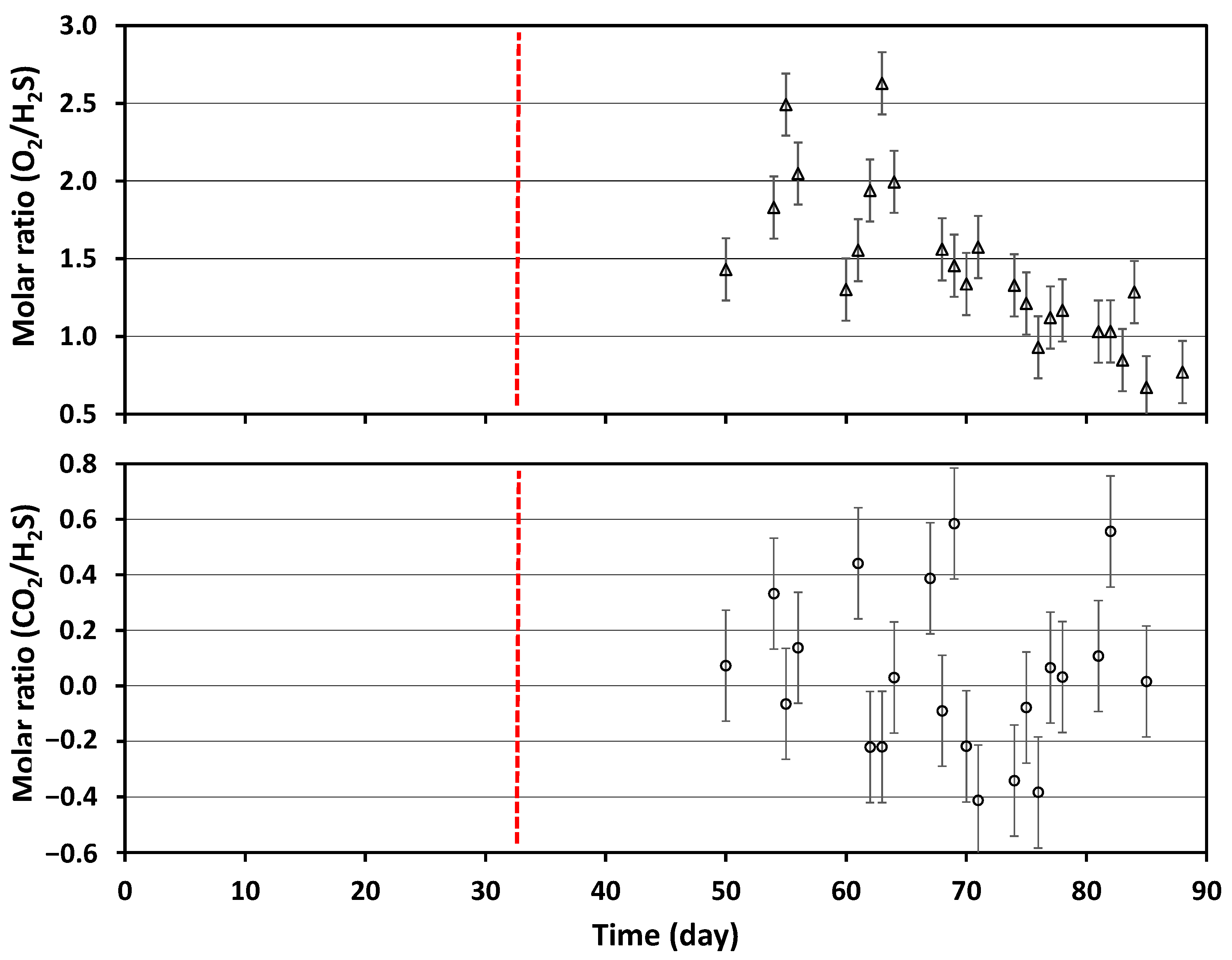
3.1. H2S Removal Efficiency
3.2. Pressure Drops
3.3. Change in Composition of AAC Waste over Time and Chemical Reactions
3.4. Lifespan of AAC Waste
3.5. Packed Bed Reactor Design and Operation Strategies for a Large Scale Biogas Purification
3.6. Advantages and Drawbacks of Using AAC Waste for H2S Removal in Biogas
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.; Wang, W.; Noguchi, T. Recyclable Calcium Carbonate-Based Concrete: Utilizing Calcium Carbonate to Bond Recycled Concrete Fines through an in-Situ Heterogeneous Dual-Precipitation Approach. Cem. Concr. Res. 2024, 186, 107679. [Google Scholar] [CrossRef]
- Olsson, J.A.; Miller, S.A.; Kneifel, J.D. A Review of Current Practice for Life Cycle Assessment of Cement and Concrete. Resour. Conserv. Recycl. 2024, 206, 107619. [Google Scholar] [CrossRef]
- Van Damme, H. Concrete Material Science: Past, Present, and Future Innovations. Cem. Concr. Res. 2018, 112, 5–24. [Google Scholar] [CrossRef]
- Volk, R.; Steins, J.J.; Kreft, O.; Schultmann, F. Life Cycle Assessment of Post-Demolition Autoclaved Aerated Concrete (AAC) Recycling Options. Resour. Conserv. Recycl. 2023, 188, 106716. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Zhang, X.; Liang, T. A New Insight: Investigating Calcium Silicate Hydrate as a Potential Adsorbent for Hydrogen Sulfide Removal. Surf. Interfaces 2025, 56, 105669. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.; Madi, H.; Rüsch-Pfund, F.; Gandiglio, M.; Biollaz, S. Biogas Composition from Agricultural Sources and Organic Fraction of Municipal Solid Waste. Renew. Energy 2022, 181, 1000–1007. [Google Scholar] [CrossRef]
- Thirumalaivasan, N.; Nangan, S.; Kanagaraj, K.; Rajendran, S. Assessment of Sustainability and Environmental Impacts of Renewable Energies: Focusing on Biogas and Biohydrogen (Biofuels) Production. Process Saf. Environ. Prot. 2024, 189, 467–485. [Google Scholar] [CrossRef]
- Pudi, A.; Rezaei, M.; Signorini, V.; Andersson, M.P.; Baschetti, M.G.; Mansouri, S.S. Hydrogen Sulfide Capture and Removal Technologies: A Comprehensive Review of Recent Developments and Emerging Trends. Sep. Purif. Technol. 2022, 298, 121448. [Google Scholar] [CrossRef]
- Paz, L.; Gentil, S.; Fierro, V.; Celzard, A. Activated Carbons Outperform Other Sorbents for Biogas Desulfurization. Chem. Eng. J. 2025, 506, 160304. [Google Scholar] [CrossRef]
- Jensen, H.S.; Lens, P.N.L.; Nielsen, J.L.; Bester, K.; Nielsen, A.H.; Hvitved-Jacobsen, T.; Vollertsen, J. Growth Kinetics of Hydrogen Sulfide Oxidizing Bacteria in Corroded Concrete from Sewers. J. Hazard. Mater. 2011, 189, 685–691. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Bhuiyan, M.; Robert, D.; Roychand, R.; Gao, L.; Cole, I.; Pramanik, B.K. Bio-Corrosion in Concrete Sewer Systems: Mechanisms and Mitigation Strategies. Sci. Total Environ. 2024, 921, 171231. [Google Scholar] [CrossRef] [PubMed]
- Rozière, E.; Loukili, A.; El Hachem, R.; Grondin, F. Durability of Concrete Exposed to Leaching and External Sulphate Attacks. Cem. Concr. Res. 2009, 39, 1188–1198. [Google Scholar] [CrossRef]
- Mohammadi, K.; Vaiskunaite, R.; Zagorskis, A. Innovative Biofiltration Materials for H2S Removal from Biogas. Environ. Health Eng. Manag. J. 2024, 11, 361–370. [Google Scholar] [CrossRef]
- Poser, M.; Duarte E Silva, L.R.; Peu, P.; Couvert, A.; Dumont, É. Cellular Concrete Waste: An Efficient New Way for H2S Removal. Sep. Purif. Technol. 2023, 309, 123014. [Google Scholar] [CrossRef]
- Germain, C.; Poser, M.; Peu, P.; Couvert, A.; Dumont, E. Hybrid Filtration Process for Gas Desulfurization. Processes 2023, 11, 3438. [Google Scholar] [CrossRef]
- Mohammadi, K.; Vaiškūnaitė, R.; Zigmontienė, A. Efficiency of Hydrogen Sulfide Removal from Biogas Using a Laboratory-Scale Biofilter Packed with Biochar, Cellular Concrete Waste, or Polyurethane Foam: A COMSOL Simulation Study. Processes 2025, 13, 329. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Jiao, F.; Qin, W.; Yang, C. Production and Resource Utilization of Flue Gas Desulfurized Gypsum in China—A Review. Environ. Pollut. 2021, 288, 117799. [Google Scholar] [CrossRef] [PubMed]
- Gasquet, V.; Kim, B.; Bonhomme, A.; Benbelkacem, H. Sewage Sludge Ash-Derived Materials for H2S Removal from a Landfill Biogas. Waste Manag. 2021, 136, 230–237. [Google Scholar] [CrossRef]
- Golmakani, A.; Ali Nabavi, S.; Wadi, B.; Manovic, V. Advances, Challenges, and Perspectives of Biogas Cleaning, Upgrading, and Utilisation. Fuel 2022, 317, 123085. [Google Scholar] [CrossRef]
- Pipatmanomai, S.; Kaewluan, S.; Vitidsant, T. Economic Assessment of Biogas-to-Electricity Generation System with H2S Removal by Activated Carbon in Small Pig Farm. Appl. Energy 2009, 86, 669–674. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, X.; Zhang, L. Biogenic Corrosion of Cementitious Composite in Wastewater Sewerage System–A Review. Process Saf. Environ. Prot. 2022, 165, 545–585. [Google Scholar] [CrossRef]
- Dumont, E. H2S Removal from Biogas Using Bioreactors: A Review. Int. J. Energy Environ. 2015, 6, 479–498. [Google Scholar]
- Wang, J.; Yang, P. Potential Flue Gas Desulfurization Gypsum Utilization in Agriculture: A Comprehensive Review. Renew. Sustain. Energy Rev. 2018, 82, 1969–1978. [Google Scholar] [CrossRef]
- Gao, X.; Xiao, Z.; Wu, P.; Liu, Y.; Xu, H.; Wang, Y. Recent Research Advances in Adsorption Removal of Gaseous Hydrogen Sulfide by Biochar. Fuel 2025, 388, 134390. [Google Scholar] [CrossRef]
- Steins, J.J.; Volk, R.; Schultmann, F. Modelling and Predicting the Generation of Post-Demolition Autoclaved Aerated Concrete (AAC) Volumes in Germany until 2050. Resour. Conserv. Recycl. 2021, 171, 105504. [Google Scholar] [CrossRef]
- Hassan, E.B.E.; Hoffmann, J. Review on Pressure Drop through a Randomly Packed Bed of Crushed Rocks. Discov. Appl. Sci. 2024, 6, 126. [Google Scholar] [CrossRef]
- Muller, C.; Guevarra, K.; Summers, A.; Pierce, L.; Shahbaz, P.; Zemke, P.E.; Woodland, K.; Hollingsworth, V.; Nakhla, G.; Bell, K.; et al. A Review of the Practical Application of Micro-Aeration and Oxygenation for Hydrogen Sulfide Management in Anaerobic Digesters. Process Saf. Environ. Prot. 2022, 165, 126–137. [Google Scholar] [CrossRef]
- Khadir, A.; Nakhla, G.; Karki, R.; Raskin, L.; Muller, C.; Guevarra, K.; Summers, A.; Pierce, L.; Shahbaz, P.; Bell, K.; et al. Micro-Aeration for Hydrogen Sulfide Reduction in Full-Scale Anaerobic Digesters with Limited Headspace: Performance and Sulfide Reduction Kinetics. Process Saf. Environ. Prot. 2025, 196, 106911. [Google Scholar] [CrossRef]
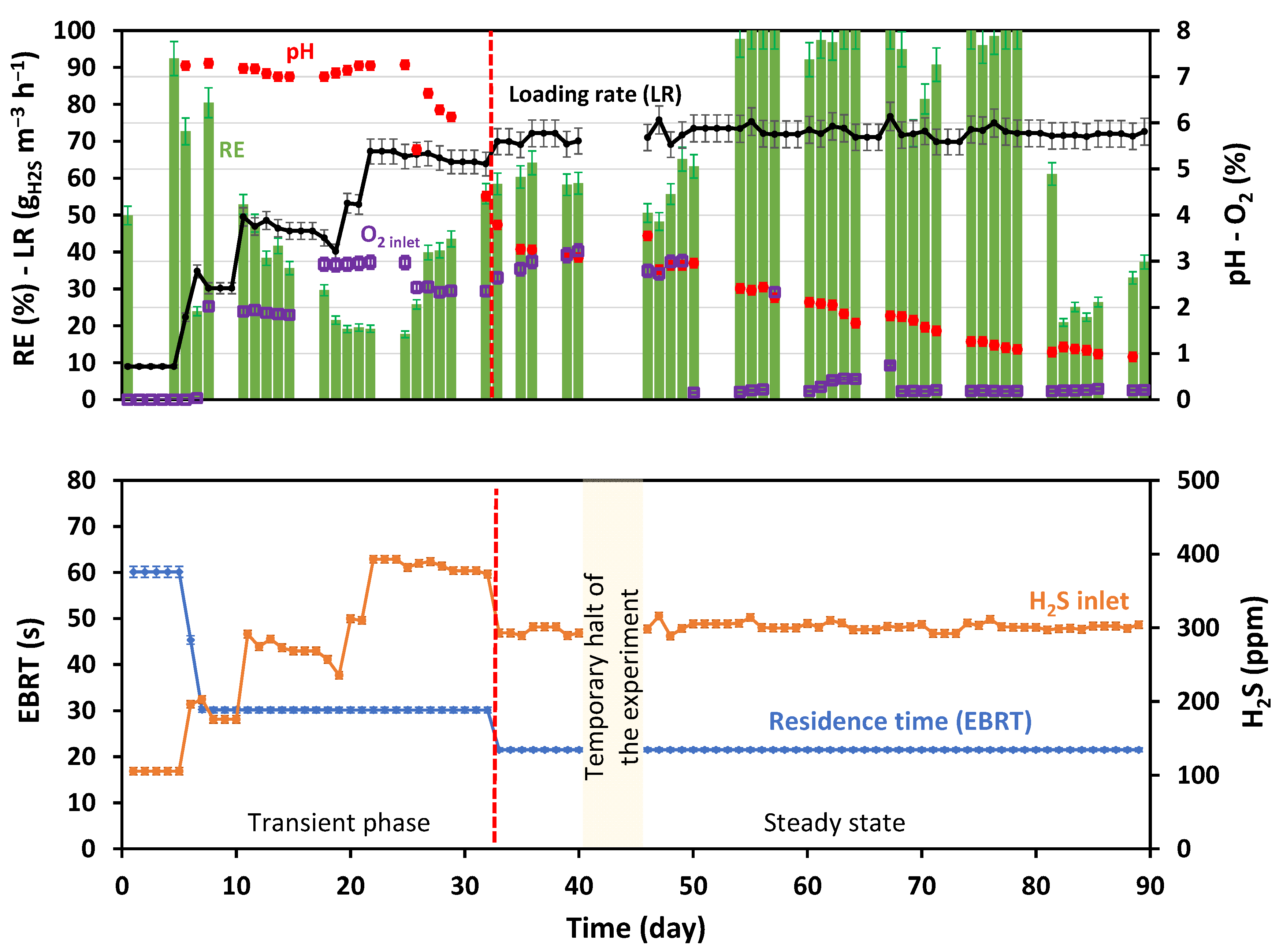



| Transient Phase | Steady State |
|---|---|
| From days 1 to 33 Residence time 1: 60 ± 1 ↘ 22 ± 1 s H2S concentration: 105 ↗ 400 ↘ 300 ± 6 ppm Loading Rate 2: 9.0 ± 0.5 ↗ 72 ± 4 gH2S m−3 h−1 O2 concentration: 2–3% (20,000–30,000 ± 600 ppm) | From days 34 to 89 Residence time = 22 ± 1 s H2S concentration = 300 ± 6 ppm Loading Rate = 72 ± 4 gH2S m−3 h−1 O2 concentration: 3 ↘ 0.3% (20,000–3000 ± 60 ppm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumont, E.; Kautzmann, N.; Couvert, A. Development of an Innovative and Sustainable Technological Process for Biogas Purification Through the Reuse of Autoclaved Aerated Concrete Waste. Processes 2025, 13, 1767. https://doi.org/10.3390/pr13061767
Dumont E, Kautzmann N, Couvert A. Development of an Innovative and Sustainable Technological Process for Biogas Purification Through the Reuse of Autoclaved Aerated Concrete Waste. Processes. 2025; 13(6):1767. https://doi.org/10.3390/pr13061767
Chicago/Turabian StyleDumont, Eric, Noé Kautzmann, and Annabelle Couvert. 2025. "Development of an Innovative and Sustainable Technological Process for Biogas Purification Through the Reuse of Autoclaved Aerated Concrete Waste" Processes 13, no. 6: 1767. https://doi.org/10.3390/pr13061767
APA StyleDumont, E., Kautzmann, N., & Couvert, A. (2025). Development of an Innovative and Sustainable Technological Process for Biogas Purification Through the Reuse of Autoclaved Aerated Concrete Waste. Processes, 13(6), 1767. https://doi.org/10.3390/pr13061767







