Electrocoagulation Coupled with TiO2 Photocatalysis: An Advanced Strategy for Treating Leachates from the Degradation of Green Waste and Domestic WWTP Biosolids in Biocells
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytical Methods for Biocell Leachate Characterization
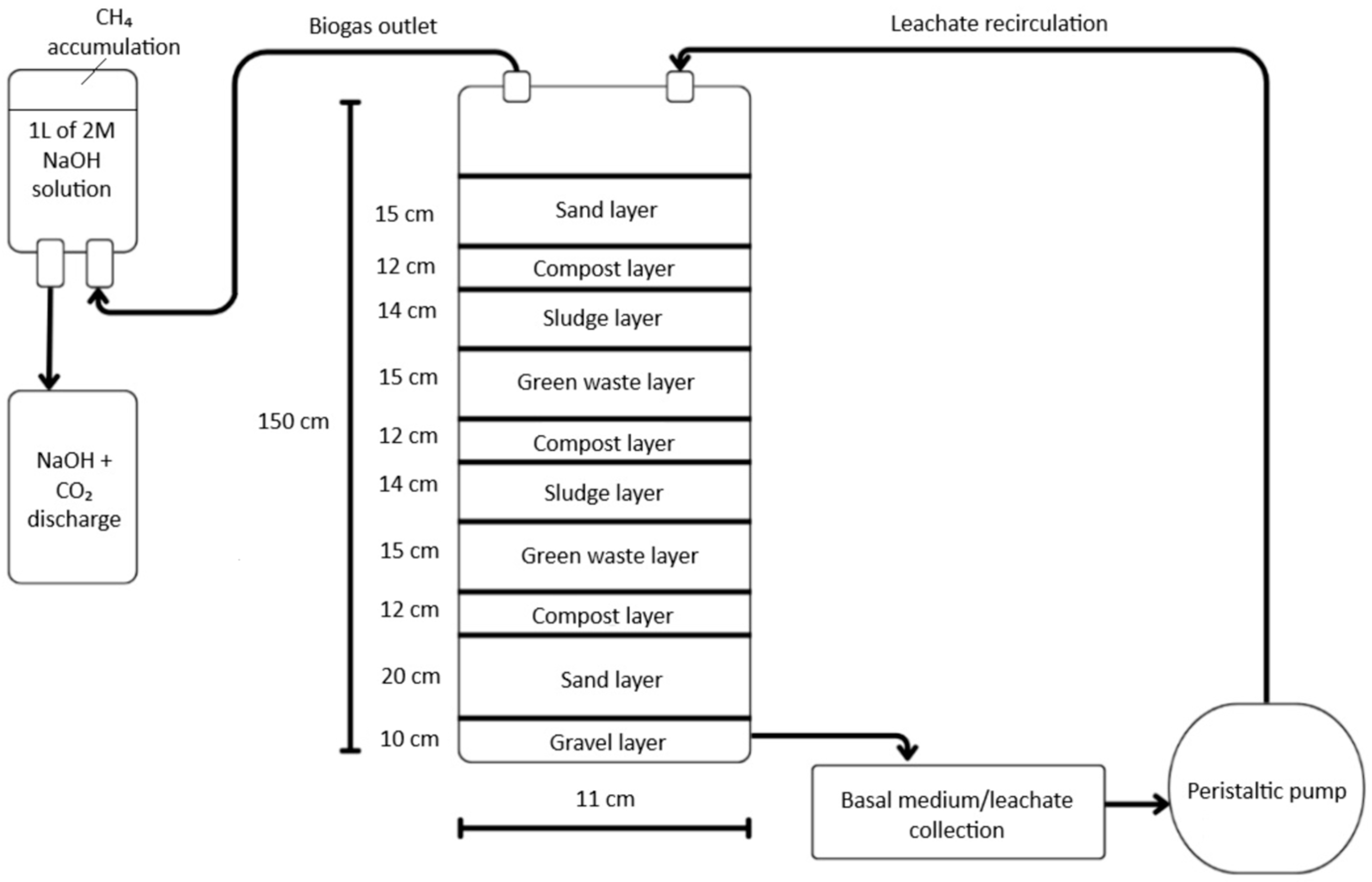
2.2. Implementation of Biocells for Green Waste and Biosolid Degradation
2.3. Biocell Leachate Pre-Treatment Using Electrocoagulation
2.4. Optimization of Photocatalytic Treatment for Electrocoagulation Pre-Treated Leachate
2.5. Photocatalytic Treatment Using Sand-Immobilized TiO2
2.6. Scanning Electron Microscopy (SEM) and Elemental Surface Analysis (EDS) of TiO2-Coated Sand
3. Results and Discussion
3.1. Biocell Leachate Characterization
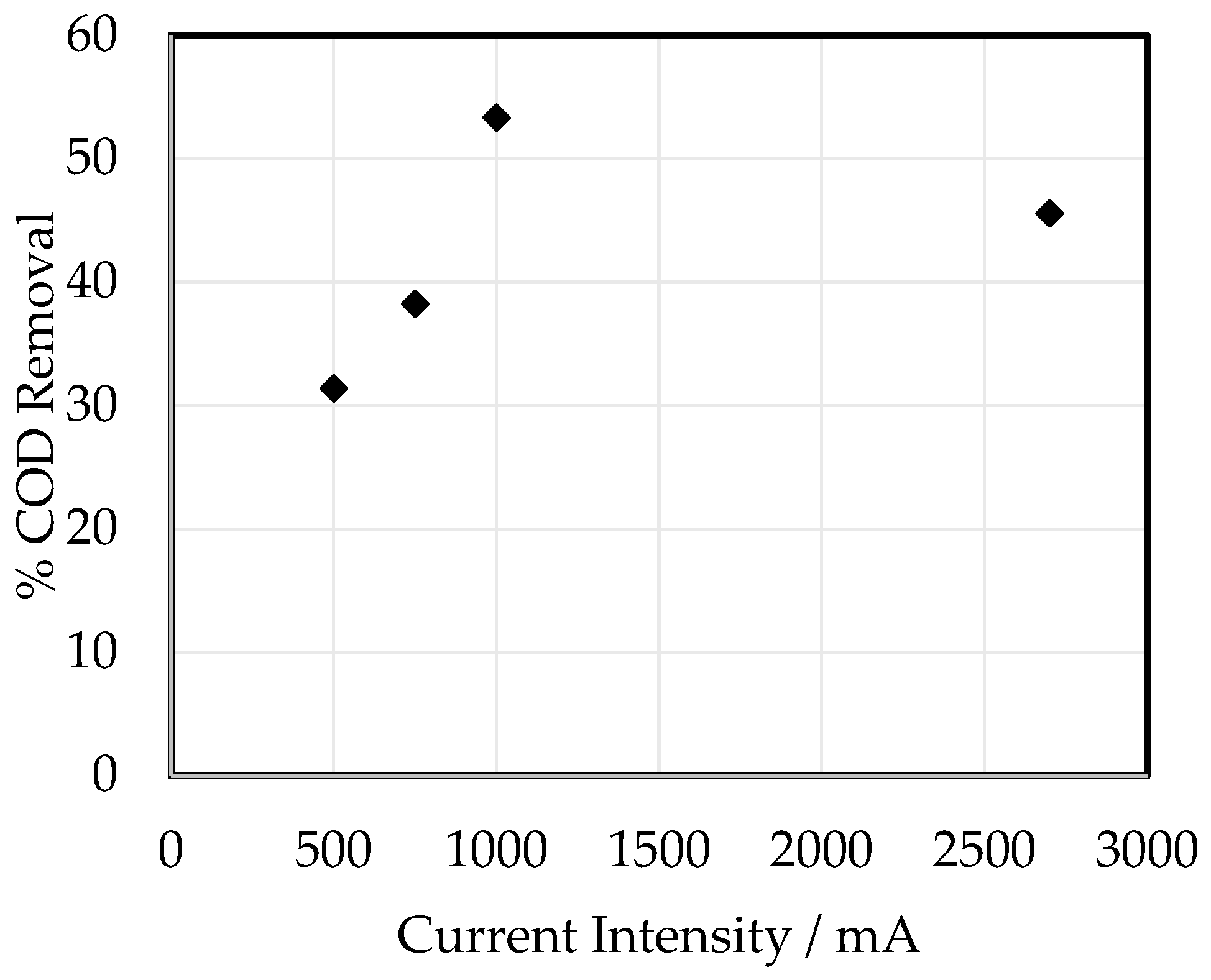
3.2. Biocell Leachate Decontamination Using Electrocoagulation
3.3. Optimization of Photocatalytic Decontamination for Electrocoagulation Pre-Treated Leachate
3.4. Continuous Photocatalytic Treatment Using Immobilized TiO2
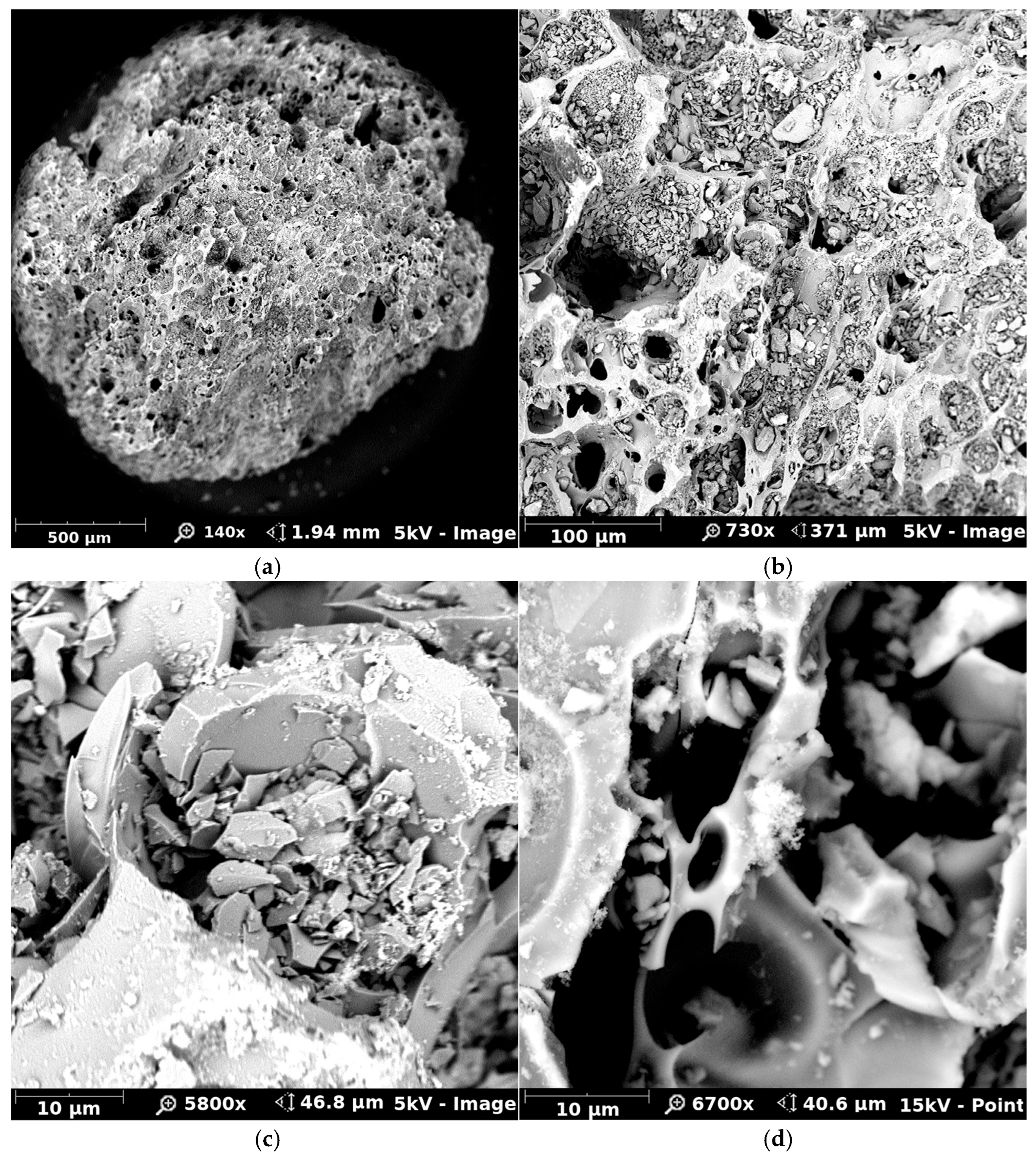
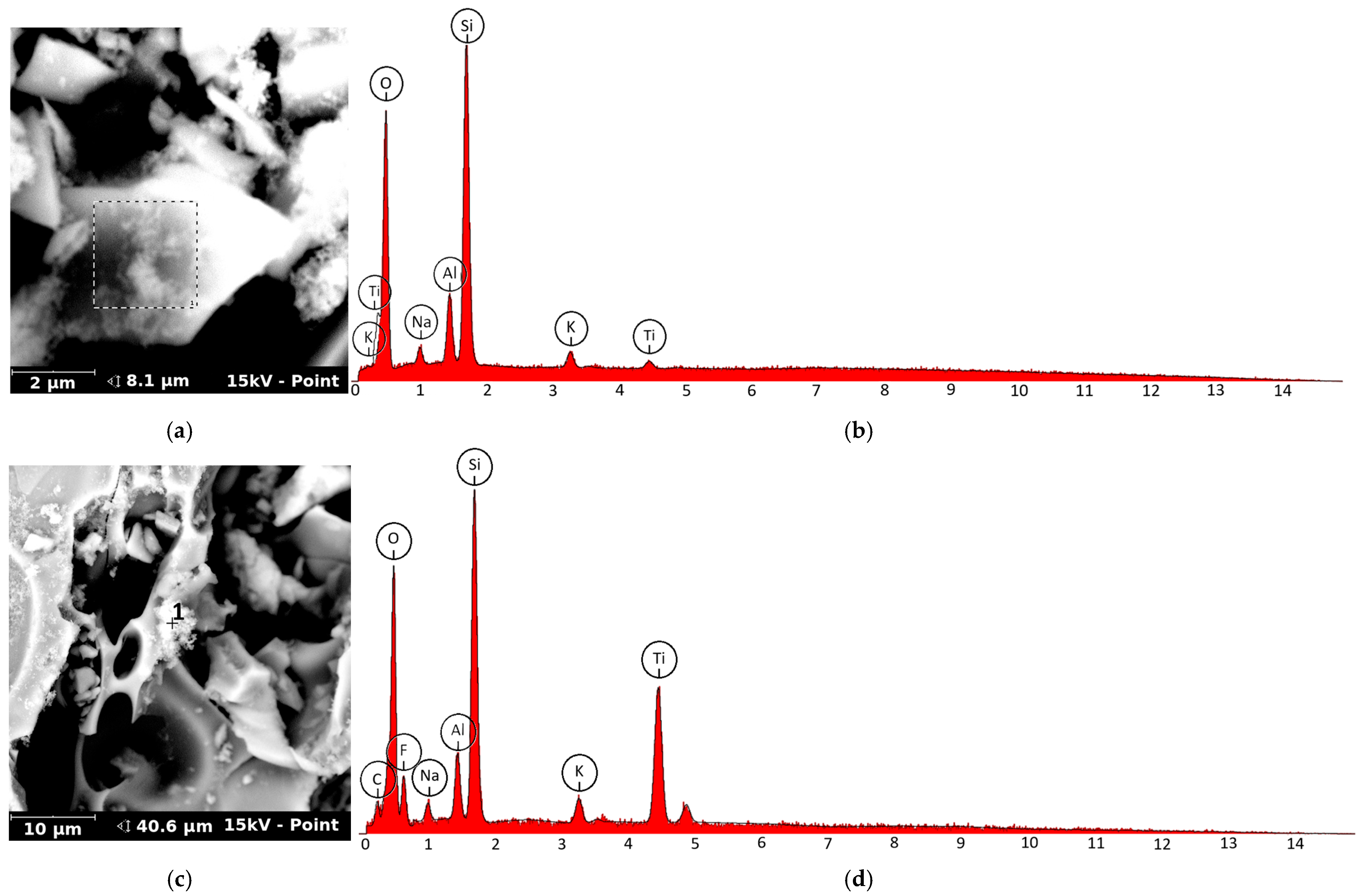
3.5. Surface Characterization of TiO2-Coated Sand by Scanning Electron Microscopy and EDS Mapping
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WWTP | Wastewater treatment plant |
| EC | Electrocoagulation |
| UVC | Ultraviolet C |
| COD | Chemical oxygen demand |
| BOD | Biochemical oxygen demand |
| POPs | Persistent organic pollutants |
| PC | Photocatalysis |
| SM | Standard Methods |
| APHA | American Public Health Association |
| TULSMA | Unified Text of Secondary Environmental Legislation (Ecuador) |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| BSD | Backscattered Electron Detector |
| TN | Total nitrogen |
| ANOVA | Analysis of Variance |
| %R | Percent Removal |
References
- Das, R.; Raj, D. Sources, distribution, and impacts of emerging contaminants—A critical review on contamination of landfill leachate. J. Hazard. Mater. Adv. 2025, 17, 100602. [Google Scholar] [CrossRef]
- Mor, S.; Ravindra, K.; Dahiya, R.P.; Chandra, A. Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ. Monit. Assess. 2006, 118, 435–456. [Google Scholar] [CrossRef]
- Ministerio del Ambiente y Agua del Ecuador. Manual de Aprovechamiento de Residuos Orgánicos Municipales. Quito; 2020. Available online: https://www.ambiente.gob.ec/wp-content/uploads/downloads/2020/07/MANUAL-DE-APROVECHAMIENTO-DE-RESIDUOS-ORGANICOS-MUNICIPAL.pdf (accessed on 3 February 2025).
- EPA. Bioreactor Landfills. 2023. Available online: https://www.epa.gov/landfills/bioreactor-landfills (accessed on 3 February 2025).
- Hettiaratchi, J.P.A. Landfill Bioreactors. In Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2012; pp. 5720–5732. [Google Scholar]
- Baun, A.; Ledin, A.; Reitzel, L.A.; Bjerg, P.L.; Christensen, T.H. Xenobiotic organic compounds in leachates from ten Danish MSW landfills—Chemical analysis and toxicity tests. Water Res. 2004, 38, 3845–3858. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and long-term composition of MSW landfill leachate: A review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- Jamrah, A.; AL-Zghoul, T.M.; Al-Qodah, Z. An Extensive Analysis of Combined Processes for Landfill Leachate Treatment. Water 2024, 16, 1640. [Google Scholar] [CrossRef]
- Mollah, Y.M.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)-science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Zailani, L.W.M.; Zin, N.S.M. Application of Electrocoagulation in Various Wastewater and Leachate Treatment-A Review. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2018. [Google Scholar]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Li, X.; Wei, H.; Song, T.; Lu, H.; Wang, X. A review of the photocatalytic degradation of organic pollutants in water by modified TiO2. Water Sci. Technol. 2023, 88, 1495–1507. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Yarbay, R.Z.; Şimşek, V.; Bogdan, L.; Tomašić, V. Photocatalytic Degradation of Neonicotinoids—A Comparative Study of the Efficacy of Hybrid Photocatalysts. Processes 2024, 12, 489. [Google Scholar] [CrossRef]
- Anucha, C.B.; Bacaksiz, E.; Stathopoulos, V.N.; Pandis, P.K.; Argirusis, C.; Andreouli, C.D.; Tatoudi, Z.; Altin, I. Molybdenum Modified Sol–Gel Synthesized TiO2 for the Photocatalytic Degradation of Carbamazepine under UV Irradiation. Processes 2022, 10, 1113. [Google Scholar] [CrossRef]
- Ates, H.; Dizge, N.; Yatmaz, H.C. Combined process of electrocoagulation and photocatalytic degradation for the treatment of olive washing wastewater. Water Sci. Technol. 2017, 75, 141–154. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Ministerio del Ambiente del Ecuador. Texto Unificado de Legislación Secundaria del Ministerio del Ambiente: Libro VI, Anexo 1—Norma de Calidad Ambiental y de Descarga de Efluentes al Recurso Agua. Registro Oficial Suplemento 387. 2015. Available online: https://www.gob.ec/sites/default/files/regulations/2018-09/Documento_Registro-Oficial-No-387-04-noviembre-2015_0.pdf (accessed on 9 February 2025).
- Hettiaratchi, J.P.A.; Jayasinghe, P.A.; Bartholameuz, E.M.; Kumar, S. Waste degradation and gas production with enzymatic enhancement in anaerobic and aerobic landfill bioreactors. Bioresour. Technol. 2014, 159, 433–436. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total Environ. 2020, 703, 135468. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiao, Z. A comprehensive review of landfill leachate treatment technologies. Front. Environ. Sci. 2024, 12, 1439128. [Google Scholar] [CrossRef]
- Lacerda, J.A.; Macedo, A.M.; Teixeira, R.I.; Simões, G.; Ribeiro, E.S.; Forero, J.S.; Corrêa, R.J. TiO2 Decorated Sand Grains for Photodegradation of Pollutants: Methylene Blue and Ciprofloxacin Study. J. Braz. Chem. Soc. 2020, 31, 201–210. [Google Scholar] [CrossRef]
- Sulaiman, F.; Alwared, A. Ability of Response Surface Methodology to Optimize Photocatalytic Degradation of Amoxicillin from Aqueous Solutions Using Immobilized TiO2/Sand. J. Ecol. Eng. 2022, 23, 293–304. [Google Scholar] [CrossRef]
- Sancha, M. Caracterización y Tratamiento de Lixiviados Generados en Vertederos de Residuos Urbanos. Available online: http://www.unioviedo.es/MBTA (accessed on 9 February 2025).
- Del Moro, G.; Mancini, A.; Mascolo, G.; Di Iaconi, C. Comparison of UV/H2O2 based AOP as an end treatment or integrated with biological degradation for treating landfill leachates. Chem. Eng. J. 2013, 218, 133–137. [Google Scholar] [CrossRef]
- Manahan, S.E. Environmental Chemistry, 11th ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Ali, Z.; Yousafzai, A.M.; Sher, N.; Muhammad, I.; Nayab, G.E.; Aqeel, S.A.M.; Shah, S.T.; Aschner, M.; Khan, I.; Khan, H. Toxicity and bioaccumulation of manganese and chromium in different organs of common carp (Cyprinus carpio) fish. Toxicol. Rep. 2021, 8, 343–348. [Google Scholar] [CrossRef]
- Ingelsson, M.; Yasri, N.; Roberts, E.P.L. Electrode passivation, faradaic efficiency, and performance enhancement strategies in electrocoagulation—A review. Water Res. 2020, 187, 116433. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, S.; Wali, A.; Buonerba, A.; Zarra, T.; Belgiorno, V.; Naddeo, V.; Ksibi, M. Efficient and sustainable treatment of tannery wastewater by a sequential electrocoagulation-UV photolytic process. J. Water Process Eng. 2020, 38, 101642. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Morkovsky, P.; Gomes, J.A.G.; Kesmez, M.; Parga, J.; Cocke, D.L. Fundamentals, present and future perspectives of electrocoagulation. J. Hazard. Mater. 2004, 114, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Rookesh, T.; Samaei, M.R.; Yousefinejad, S.; Hashemi, H.; Derakhshan, Z.; Abbasi, F.; Jalili, M.; Giannakis, S.; Bilal, M. Investigating the Electrocoagulation Treatment of Landfill Leachate by Iron/Graphite Electrodes: Process Parameters and Efficacy Assessment. Water 2022, 14, 205. [Google Scholar] [CrossRef]
- Khan, S.U.; Khalid, M.; Hashim, K.; Jamadi, M.H.; Mousazadeh, M.; Basheer, F.; Farooqi, I.H. Efficacy of Electrocoagulation Treatment for the Abatement of Heavy Metals: An Overview of Critical Processing Factors, Kinetic Models and Cost Analysis. Sustainability 2023, 15, 1708. [Google Scholar] [CrossRef]
- Valencia, S.H.; Marín, J.M.; Restrepo, G.M. Efecto del pH en la Degradación Fotocatalítica de Materia Orgánica Natural. Inf. Tecnol. 2011, 22, 57–66. [Google Scholar] [CrossRef]
- Harun, N.; Sheng, C.K.; Sabri, M.G.M.; Dagang, A.N.; Salleh, H. Impact of TiO2 and H2O2 on Photocatalytic Degradation of Rhodamine B under Ultraviolet C (UV-C) Radiation for Efficient Polluted Wastewater Treatment. J. Optoelectron. Biomed. Mater. 2020, 12, 9–15. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, T.G.; Hwangbo, S.A.; Jeong, J.R. Effect of the TiO2 Colloidal Size Distribution on the Degradation of Methylene Blue. Nanomaterials 2023, 13, 302. [Google Scholar] [CrossRef]
- Poulopoulos, S.G.; Yerkinova, A.; Ulykbanova, G.; Inglezakis, V.J. Photocatalytic treatment of organic pollutants in a synthetic wastewater using UV light and combinations of TiO2, H2O2 and Fe(III). PLoS ONE 2019, 14, e0216745. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Xu, Y.J. (Eds.) Heterogeneous Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Hänel, A.; Zaleska, A.; Hupka, J. Photocatalytic activity of TiO2 immobilized on glass beads. Physicochem. Probl. Miner. Process. 2010, 45, 49–56. Available online: https://www.researchgate.net/publication/279628443 (accessed on 9 February 2025).
- Umar, M.; Abdul, H. Photocatalytic Degradation of Organic Pollutants in Water. In Organic Pollutants—Monitoring, Risk and Treatment; IntechOpen Limited: London, UK, 2013. [Google Scholar]
- Chairungsri, W.; Pholchan, P.; Sumitsawan, S.; Chimupala, Y.; Kijjanapanich, P. Photocatalytic Degradation of Textile Dyeing Wastewater Using Titanium Dioxide on a Fixed Substrate: Optimization of Process Parameters and Continuous Reactor Tests. Sustainability 2023, 15, 12418. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Electro-Fenton degradation of synthetic dyes. Water Res. 2009, 43, 339–344. [Google Scholar] [CrossRef]
- Rahimi, S.; Poormohammadi, A.; Salmani, B.; Ahmadian, M.; Rezaei, M. Comparing the photocatalytic process efficiency using batch and tubular reactors in removal of methylene blue dye and COD from simulated textile wastewater. J. Water Reuse Desalination 2016, 6, 574–582. [Google Scholar] [CrossRef]
- Colombo, E.; Ashokkumar, M. Comparison of the photocatalytic efficiencies of continuous stirred tank reactor (CSTR) and batch systems using a dispersed micron sized photocatalyst. RSC Adv. 2017, 7, 48222–48229. [Google Scholar] [CrossRef]
- Jadaa, W.; Prakash, A.; Ray, A.K. Photocatalytic Degradation of Diazo Dye over Suspended and Immobilized TiO2 Catalyst in Swirl Flow Reactor: Kinetic Modeling. Processes 2021, 9, 1741. [Google Scholar] [CrossRef]
- Bertagna Silva, D.; Buttiglieri, G.; Babić, S. State-of-the-art and current challenges for TiO2/UV-LED photocatalytic degradation of emerging organic micropollutants. Environ. Sci. Pollut. Res. 2021, 28, 103–120. [Google Scholar] [CrossRef]
- Enesca, A. The Influence of Photocatalytic Reactors Design and Operating Parameters on the Wastewater Organic Pollutants Removal—A Mini-Review. Catalysts 2021, 11, 556. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Sand Supported Mixed-Phase TiO2 Photocatalysts for Water Decontamination Applications Decontamination Applications. Adv. Eng. Mater. 2014, 16, 248–254. [Google Scholar] [CrossRef]
- Kim, M.G.; Kang, J.M.; Lee, J.E.; Kim, K.S.; Kim, K.H.; Cho, M.; Lee, S.G. Effects of Calcination Temperature on the Phase Composition, Photocatalytic Degradation, and Virucidal Activities of TiO2 Nanoparticles. ACS Omega 2021, 6, 10668–10678. [Google Scholar] [CrossRef]





| Parameter | Units | Raw Biocell Leachate | Ecuadorian Freshwater Body Regulations | Typical Leachate Composition [22] |
|---|---|---|---|---|
| pH | 8.98 ± 0.01 | 6–9 | 4.5–9.0 | |
| Color | PCU | 7733 ± 289 | - | 2–25 |
| COD | mg/L | 1373 ± 55 | 200 | 500–30,000 |
| BOD5 | mg/L | 378 ± 44 | 100 | 100–5000 |
| Total Solids | mg/L | 12,835 ± 43 | 1600 | 1000–50,000 |
| Total Suspended Solids | mg/L | 208 ± 7 | 130 | 200–5000 |
| Total Nitrogen (TN) | mg/L | 393 ± 5 | - | 10–3000 |
| Ammonia (NH3-N) | mg/L | 201 ± 0.6 | 30 | 10–2000 |
| Nitrate (NO3−) | mg/L | n/d | - | 0.1–50 |
| Total Phosphorus (TP) | mg/L | 90 ± 1 | 10 | 0.1–50 |
| Parameter | Units | Raw Biocell Leachate | Ecuadorian Freshwater Body Regulations | Typical Leachate Composition [22] |
|---|---|---|---|---|
| Zn | mg/L | 2.6 ± 0.2 | 5 | 2.56 |
| Fe | mg/L | 1.7 ± 0.17 | 10 | 0.019–38.73 |
| Cd | mg/L | 0.043 ± 0.001 | 0.02 | <1 |
| Ni | mg/L | 7.063 ± 0.022 | 2 | <1 |
| Cr6+ | mg/L | 0.122 ± 0.002 | 0.5 | <1 |
| CrTOTAL | mg/L | 0.956 ± 0.020 | - | <1 |
| Pb | mg/L | 0.633 ± 0.012 | 0.2 | <1 |
| As | mg/L | 0.031 ± 0.001 | 0.1 | <1 |
| Mn | mg/L | 4.67 ± 0.58 | 2 | - |
| Cu | mg/L | 0.97 ± 0.12 | 1 | <1 |
| Parameter | Unit | Treated Leachate | % Removal | Freshwater Body Regulation |
|---|---|---|---|---|
| pH | 10.3 ± 0.04 | - | 6–9 | |
| Color | PCU | 1068 ± 6 | 86.2 | - |
| COD | mg/L | 513 ± 6 | 62.6 | 200 |
| BOD5 | mg/L | 210 ± 3 | 44.4 | 100 |
| Total Solids (TS) | mg/L | 11,953 ± 30 | 6.9 | 1600 |
| Total Nitrogen (TN) | mg/L | 240 ± 1 | 38.9 | - |
| Ammonia (N-NH3) | mg/L | 165 ± 1 | 17.9 | 30 |
| Nitrate (NO3−) | mg/L | n/d | - | - |
| Total Phosphorus (TP) | mg/L | 9.2 ± 0.1 | 89.8 | 10 |
| Parameter | Unit | Treated Leachate | % Removal | Freshwater Body Regulation |
|---|---|---|---|---|
| Zn | mg/L | 0.04 ± 0.01 | 98.5 | 5 |
| Fe | mg/L | 1.23 ± 0.02 | 27.7 | 10 |
| Cd | mg/L | 0.0121 ± 0.001 | 71.9 | 0.02 |
| Cr6+ | mg/L | 0.06 ± 0.001 | 50.8 | 0.5 |
| CrTOTAL | mg/L | 0.5707 ± 0.004 | 40.3 | - |
| Pb | mg/L | 0.0828 ± 0.004 | 86.9 | 0.2 |
| Mn | mg/L | 1.67 ± 0.06 | 64.2 | 2 |
| Cu | mg/L | 0.17 ± 0.01 | 82.5 | 1 |
| pH | 5.6 | 8 | 10 | Analysis of Variance, p-Value | ||
|---|---|---|---|---|---|---|
| %R (color) | 83.2 | 54.4 | 30.5 | <0.05 | ||
| H2O2 concentration, mg/L c(H2O2)/c(DQO) | 2 (0.016) | 20 (0.16) | 100 (0.08) | 200 (1.6) | 400 (3.2) | |
| %R (color) | 69.5 | 74.2 | 76.2 | 88.5 | 86.3 | <0.05 |
| TiO2 concentration, g/L | 2 | 4 | 8 | 12 | ||
| %R (color) | 62.7 | 77.2 | 88.5 | 87.7 | <0.05 | |
| Dilution | 1:4 | 1:3 | 1:2 | 1:1 | ||
| %R (color) | 88.5 | 77.3 | 61.3 | 48.1 | <0.05 | |
| UVC, μW/cm2 | 1554 | 2801 | ||||
| %R (color) | 88.5 | 88.7 | <0.05 |
| Parameter (%Removal) | Units | Leachate 130 mg H2O2/L (H2O2/COD Ratio of 0.27) (%Removal) | Leachate 800 mg H2O2/L (H2O2/COD Ratio of 1.6) (%Removal) | Ecuadorian Freshwater Body Regulations |
|---|---|---|---|---|
| pH | 8.04 | 8.20 | 6–9 | |
| Color | PCU | 114.7 (98.5%) | 90.0 (98.8%) | - |
| COD | mg/L | 310 (77.4%) | 230 (81.8%) | 200 |
| BOD5 | mg/L | 250 (33.9%) | 250 (33.9%) | 100 |
| Total Solids (TS) | mg/L | 20,321 (−58.3%) | 19,501 (−51.9%) | 1600 |
| Total Nitrogen (TN) | mg/L | 69.5 (82.3%) | 69.0 (82.4%) | - |
| Ammonia (NH3-N) | mg/L | 24.1 (88.0%) | 22.7 (88.7%) | 30 |
| Total Phosphorus (TP) | mg/L | 5.1 (94.3%) | 5.0 (94.4%) | 10 |
| CrTOTAL | mg/L | 0.0235 (97.5%) | 0.0519 (94.6%) | - |
| Cr6+ | mg/L | n/d (100%) | 0.006 (95%) | 0.5 |
| Zn | mg/L | 0.34 (86.9%) | 0.48 (81.5%) | 5 |
| Fe | mg/L | 0.21 (87.6%) | 0.07 (95.9%) | 10 |
| Pb | mg/L | 0.139 (78.0%) | 0.134 (78.8%) | 0.2 |
| Mn | mg/L | 0.40 (99.6%) | 0.40 (99.6%) | 2 |
| Cu | mg/L | 0.017 (98.3%) | 0.010 (99.0%) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñafiel, R.; Flores Tapia, N.E.; Mayacela Rojas, C.M.; Lema Chicaiza, F.R.; Pérez, L. Electrocoagulation Coupled with TiO2 Photocatalysis: An Advanced Strategy for Treating Leachates from the Degradation of Green Waste and Domestic WWTP Biosolids in Biocells. Processes 2025, 13, 1746. https://doi.org/10.3390/pr13061746
Peñafiel R, Flores Tapia NE, Mayacela Rojas CM, Lema Chicaiza FR, Pérez L. Electrocoagulation Coupled with TiO2 Photocatalysis: An Advanced Strategy for Treating Leachates from the Degradation of Green Waste and Domestic WWTP Biosolids in Biocells. Processes. 2025; 13(6):1746. https://doi.org/10.3390/pr13061746
Chicago/Turabian StylePeñafiel, Rodny, Nelly Esther Flores Tapia, Celia Margarita Mayacela Rojas, Freddy Roberto Lema Chicaiza, and Lander Pérez. 2025. "Electrocoagulation Coupled with TiO2 Photocatalysis: An Advanced Strategy for Treating Leachates from the Degradation of Green Waste and Domestic WWTP Biosolids in Biocells" Processes 13, no. 6: 1746. https://doi.org/10.3390/pr13061746
APA StylePeñafiel, R., Flores Tapia, N. E., Mayacela Rojas, C. M., Lema Chicaiza, F. R., & Pérez, L. (2025). Electrocoagulation Coupled with TiO2 Photocatalysis: An Advanced Strategy for Treating Leachates from the Degradation of Green Waste and Domestic WWTP Biosolids in Biocells. Processes, 13(6), 1746. https://doi.org/10.3390/pr13061746







