Pyrometallurgical Recycling of Electric Motors for Sustainability in End-of-Life Vehicle Metal Separation Planning
Abstract
1. Introduction
2. Materials and Methods
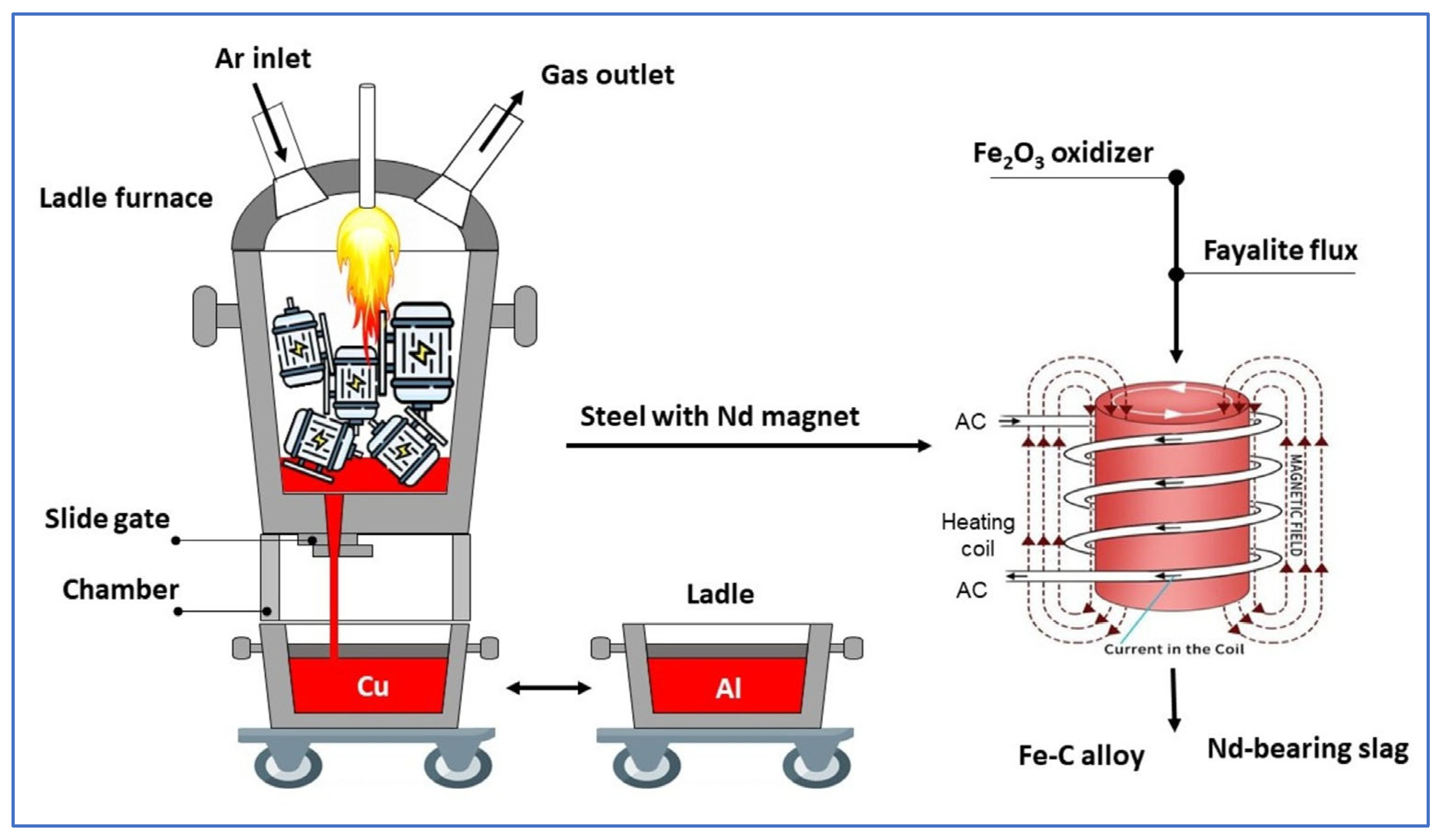
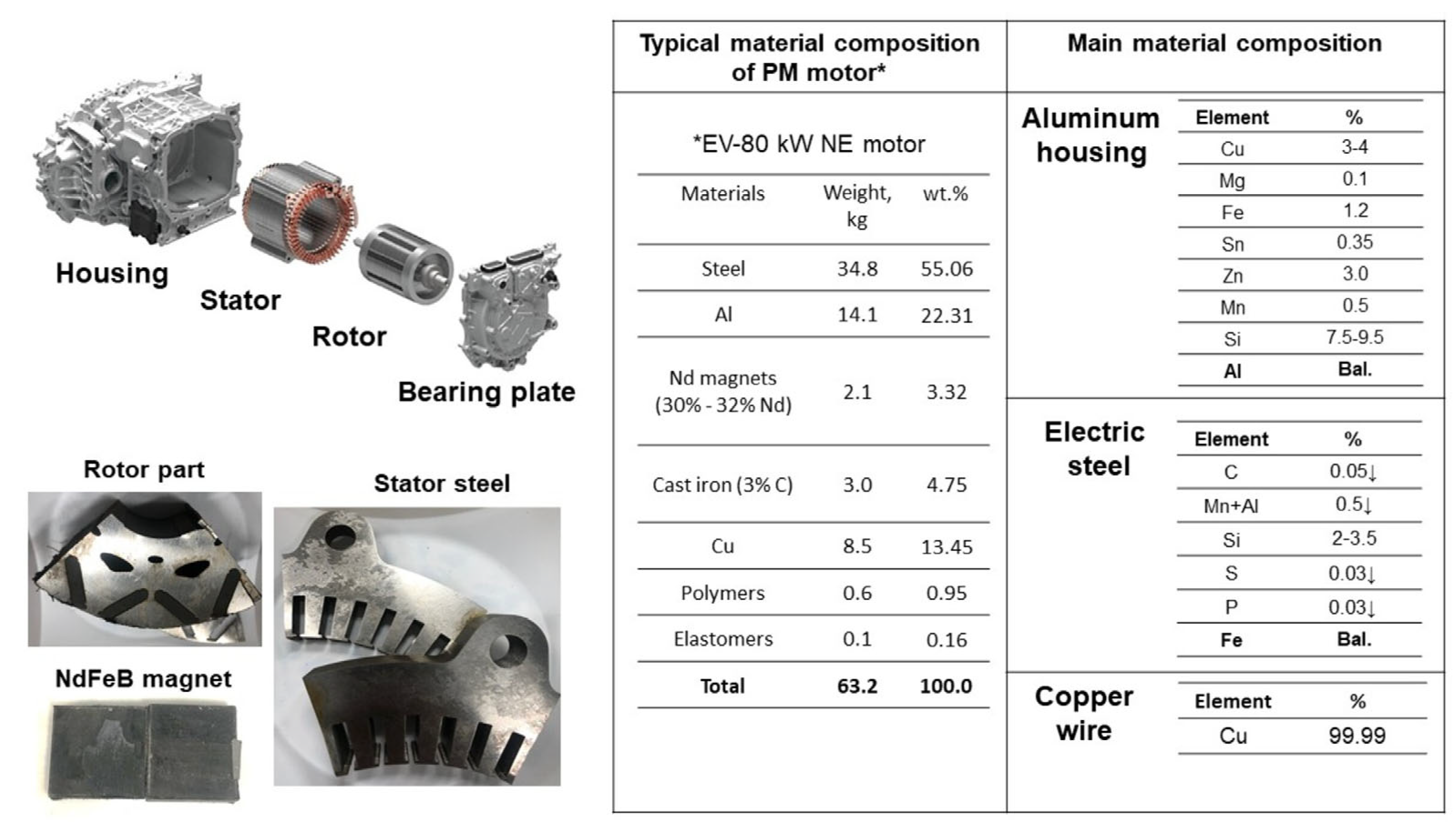
2.1. Experimental Materials
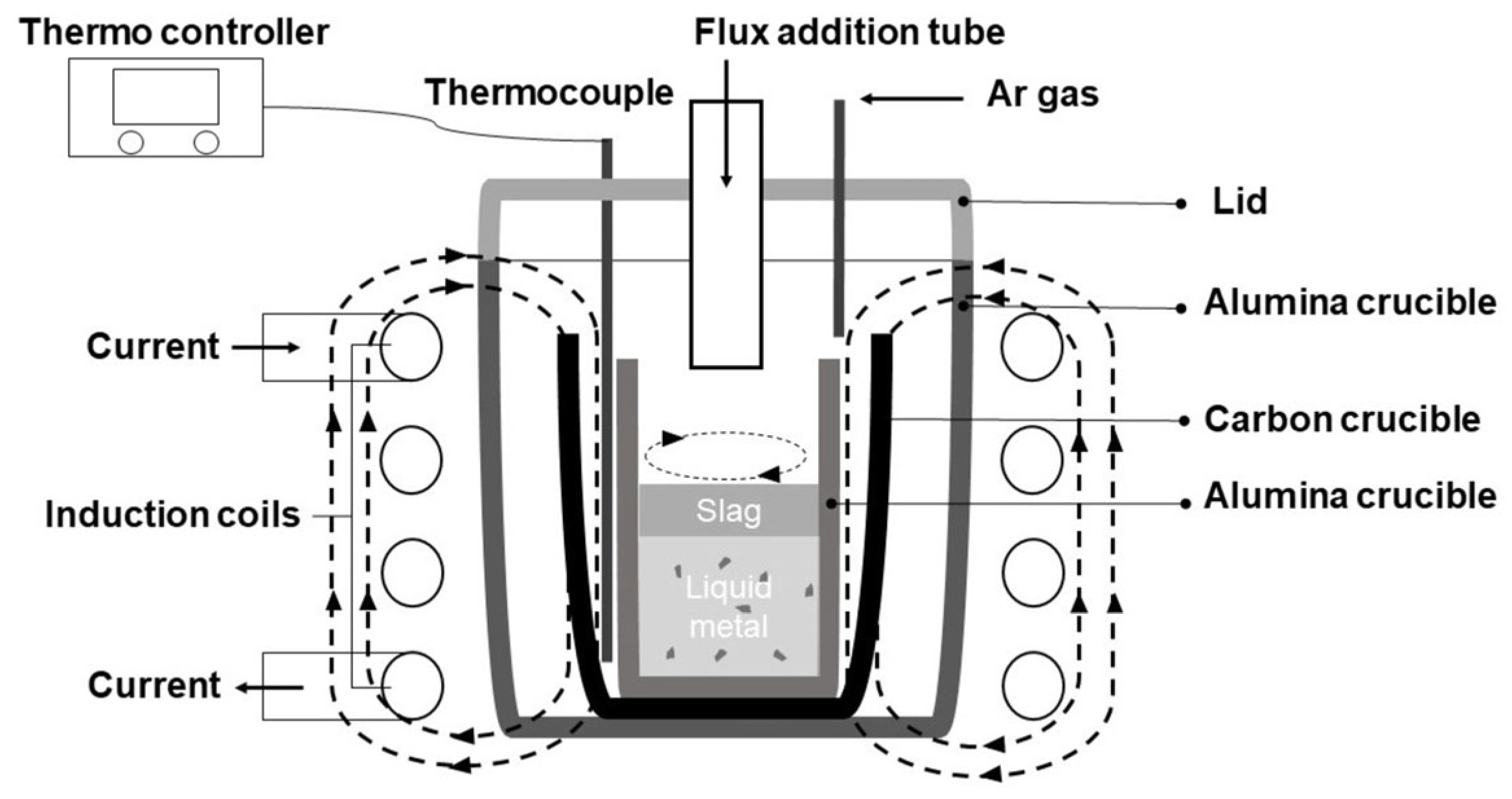
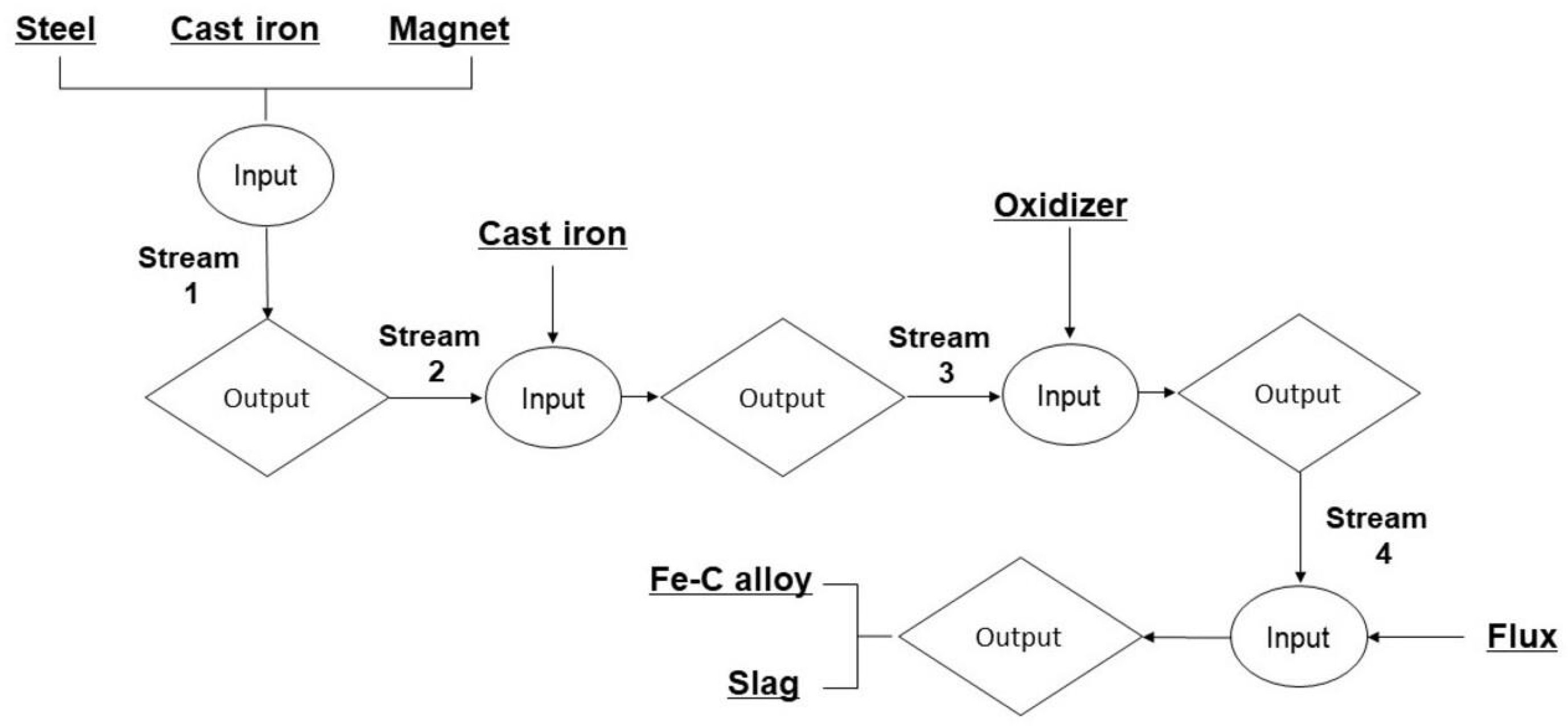
2.2. Experimental Procedure
2.3. Analytical Methods
2.3.1. Thermodynamic Modeling
2.3.2. Calculating Neodymium Recovery Degree
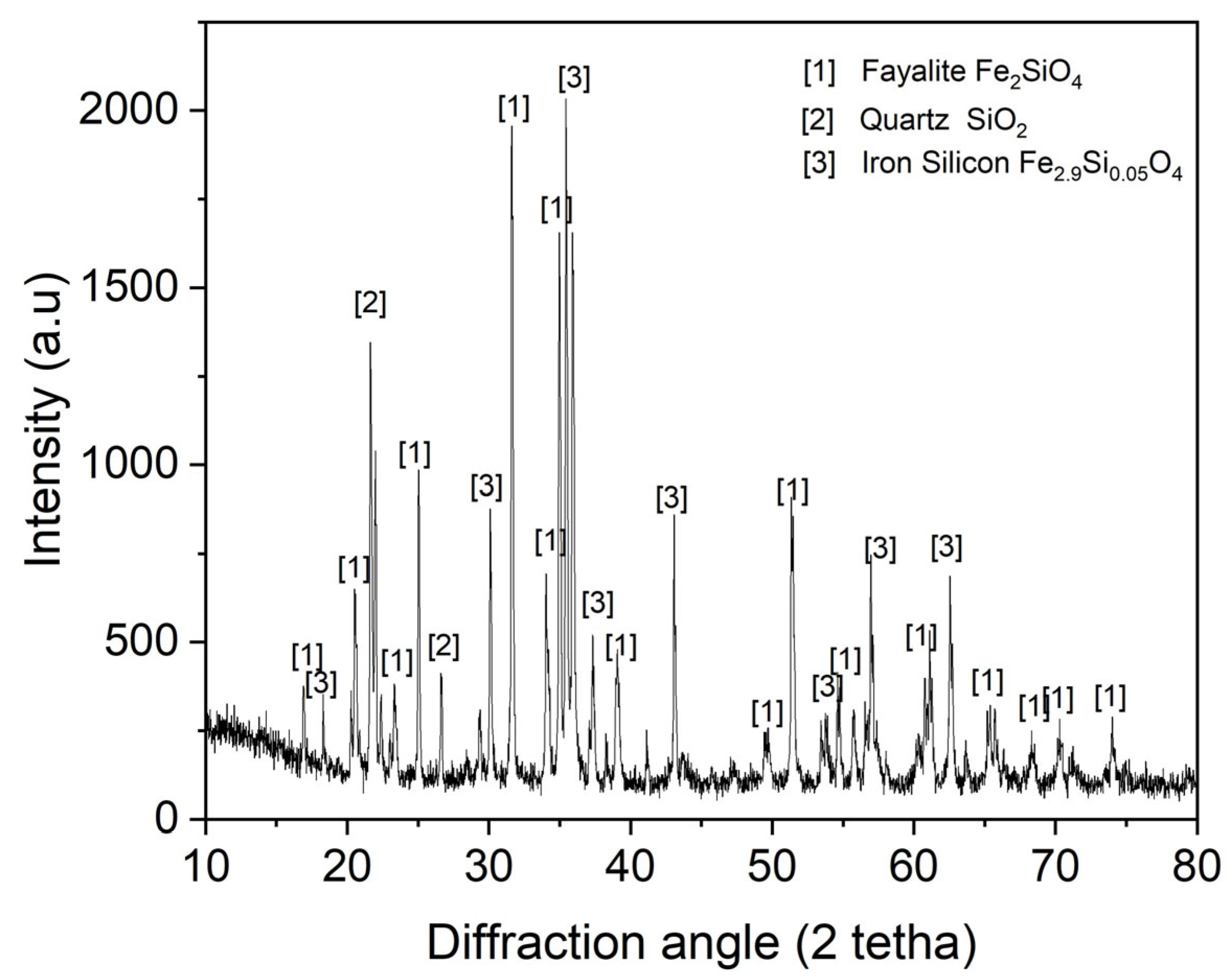
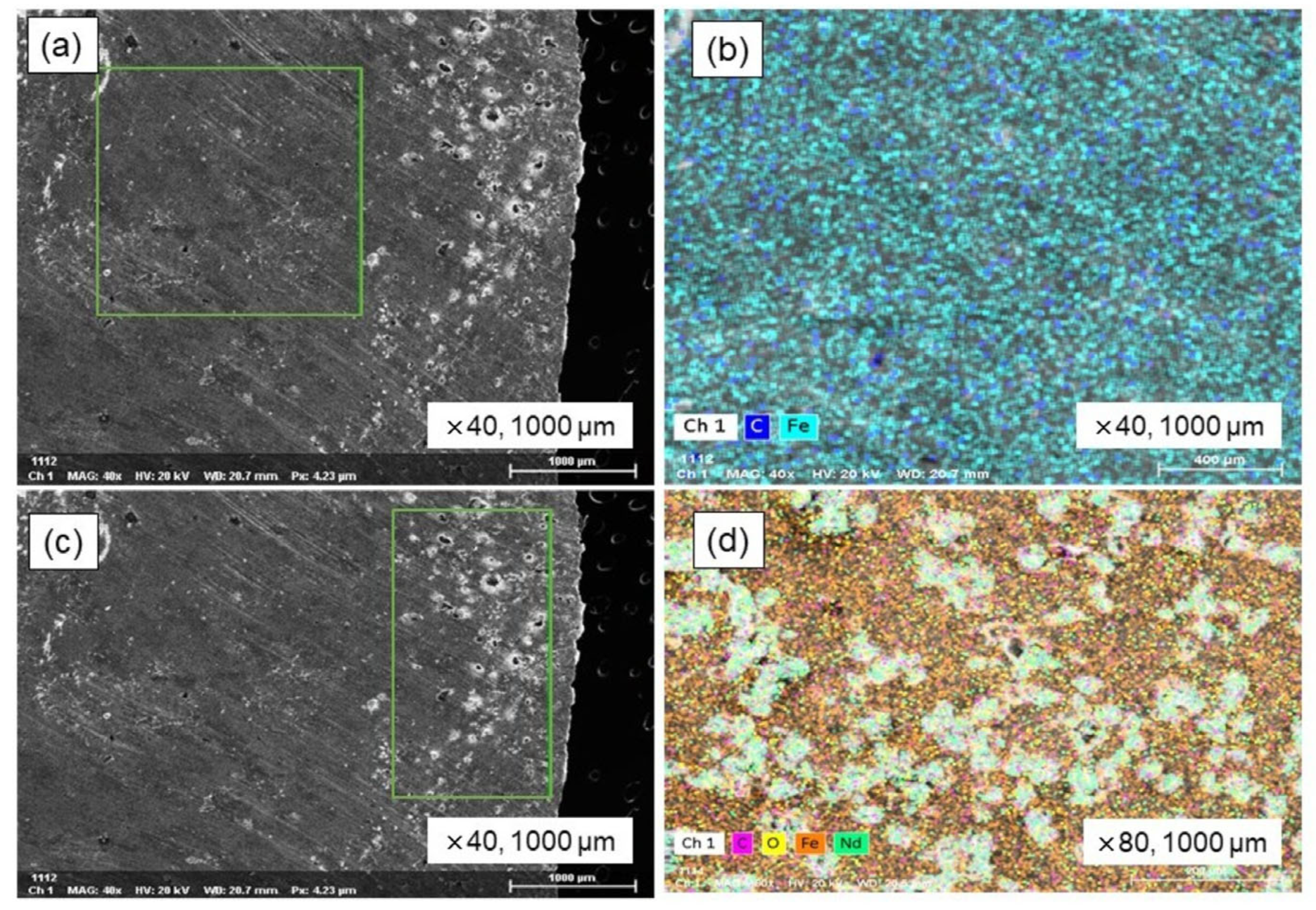
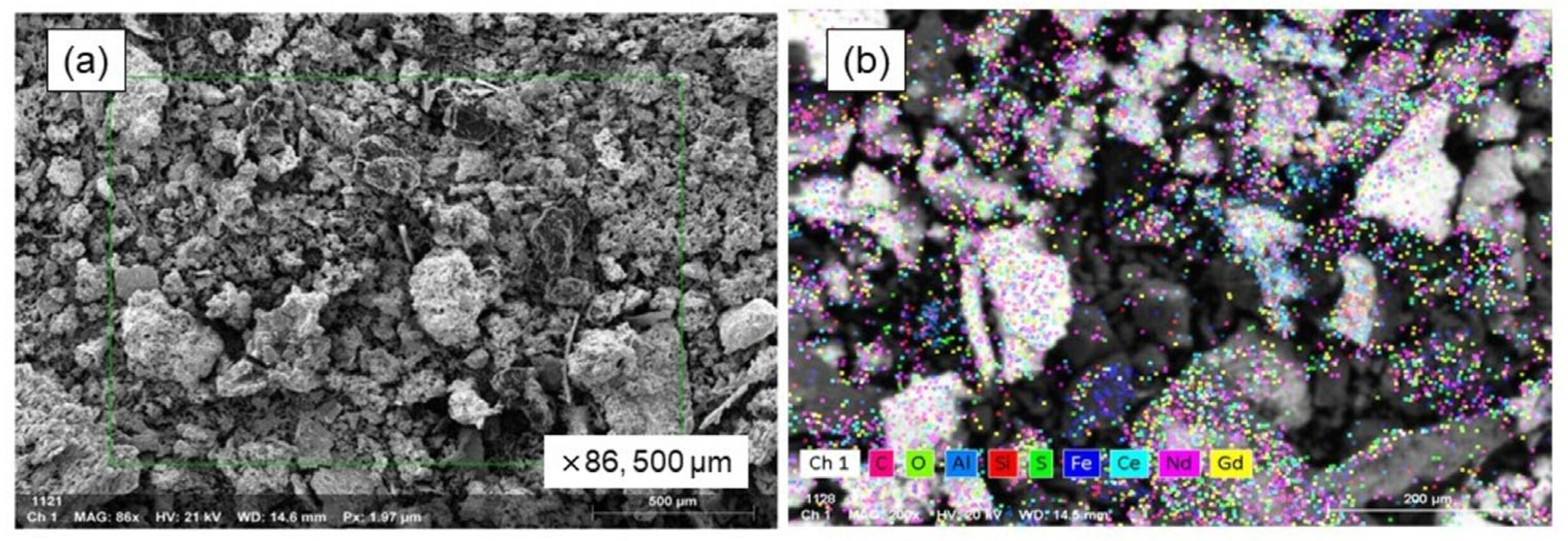
2.3.3. Chemical and Mineralogical Analysis
3. Results and Discussion
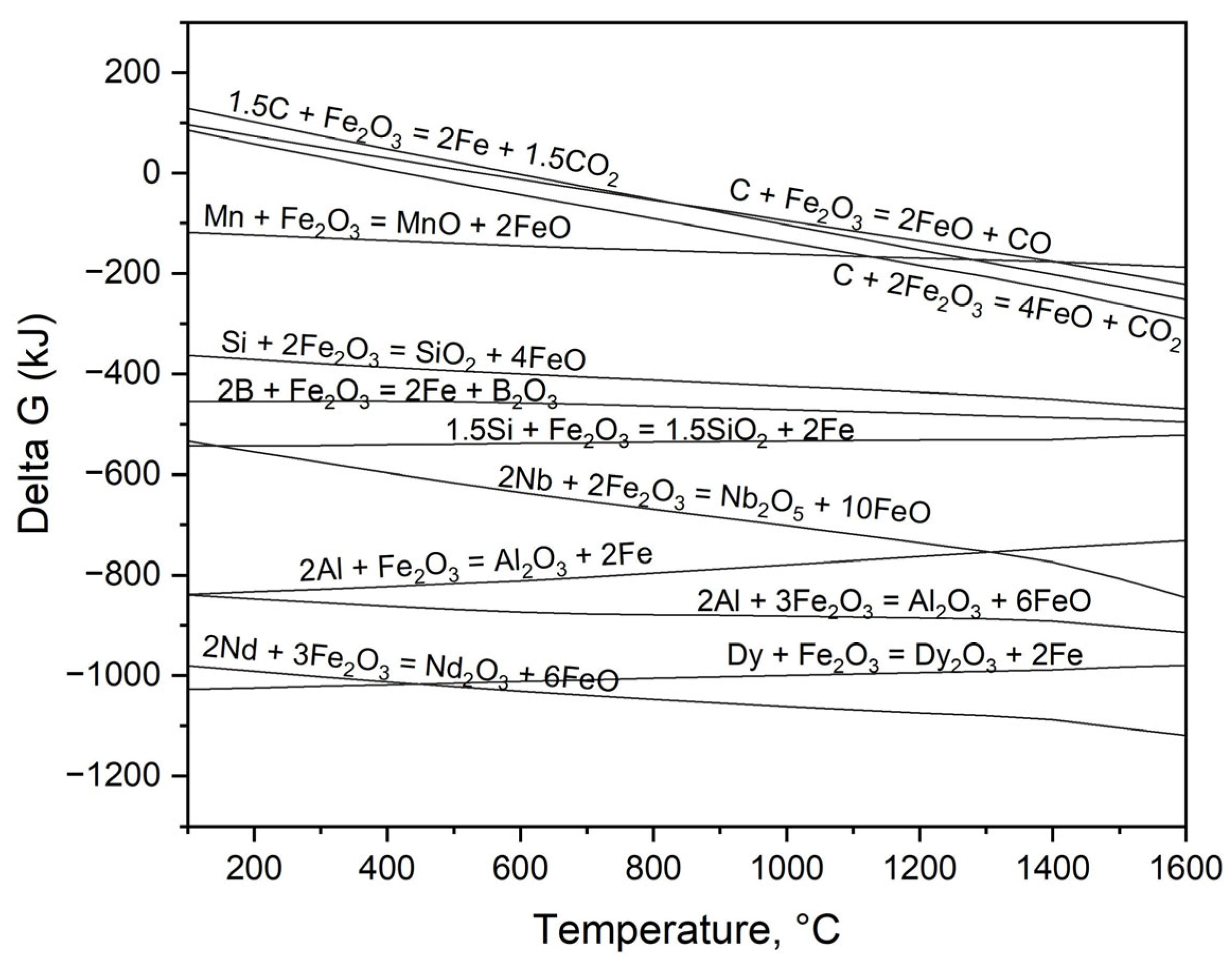
3.1. Thermodynamic Modeling of Steel and Magnet Smelting
| a[Re](l) + bFe2SiO4(s) = c(RexOy)(s) + d(SiO2)(l) + h[Fe](l) | at 1550 °C ΔG°[Nd] = −427 kJ ΔG°[Nb] = −186 kJ ΔG°[Dy] = −452 kJ | (7) |
| a[Me](l) + bFe2SiO4(s) = c(MexOy)(s) + d(SiO2) + h[Fe](l) | ΔG°[Si] = −219 kJ ΔG°[Al] = −311 kJ ΔG°[Mn] = −97 kJ | (8) |
| a[C](l) + bFe2SiO4(s) = c(CO)(s) + d(SiO2) + h[Fe](l) | ΔG°[C] = −75 kJ | (9) |
3.2. The Smelting Process of Fe-C Alloy and PM Based on Optimized Modeling
| [Fe](l) + Fe2O3(l) = 3FeO(l) | at 1550 °C ΔG°[FeO] = −86.9 kJ | (10) |
| 2[Nd](l) + 3(FeO)(l) = 3[Fe](l) + (Nd2O3)(s) | ΔG°[Nd2O3] = −816.7 kJ K ≈ 1022 | (11) |
| 2FeO(s) → 2Fe(l) + O2(g) at equal = | ΔG°[FeO] = 284.6 kJ | (12) |
| 2Nd2O3(s) → 4Nd(l) + 3O2(g) | ΔG°[FeO] = 2512.8 kJ | (13) |
| (14) |
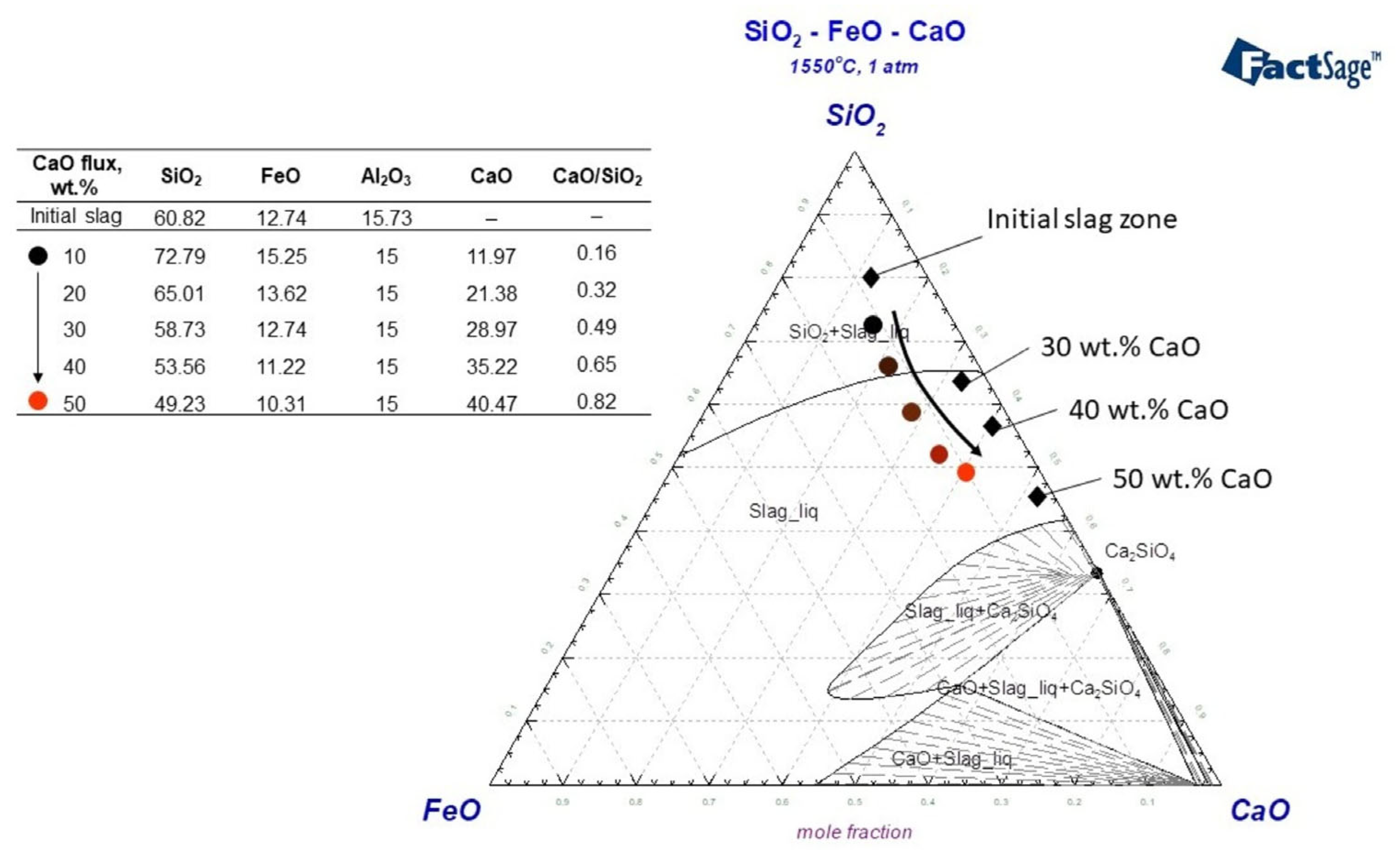
3.3. Influence of CaO Flux on the Slag Composition
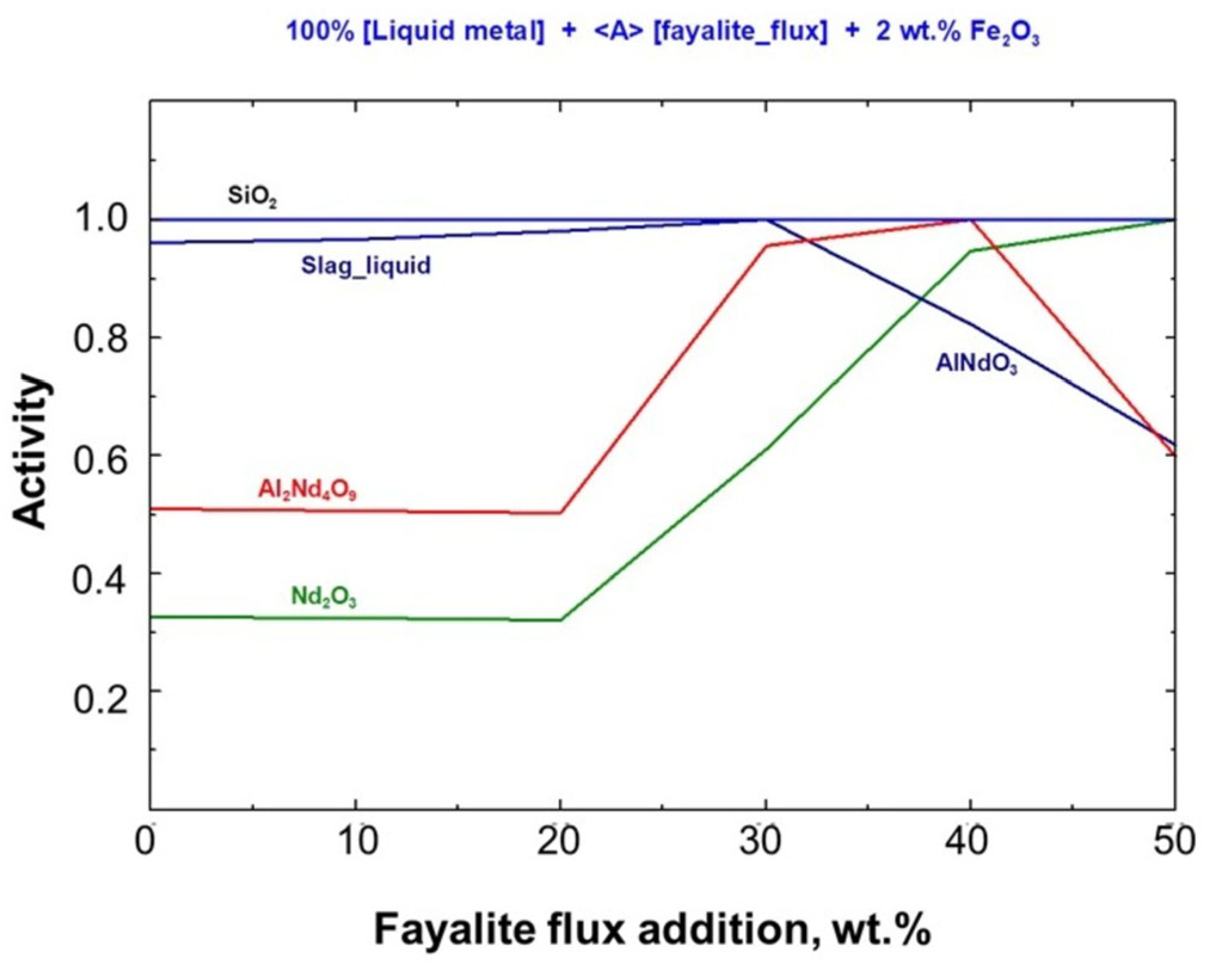
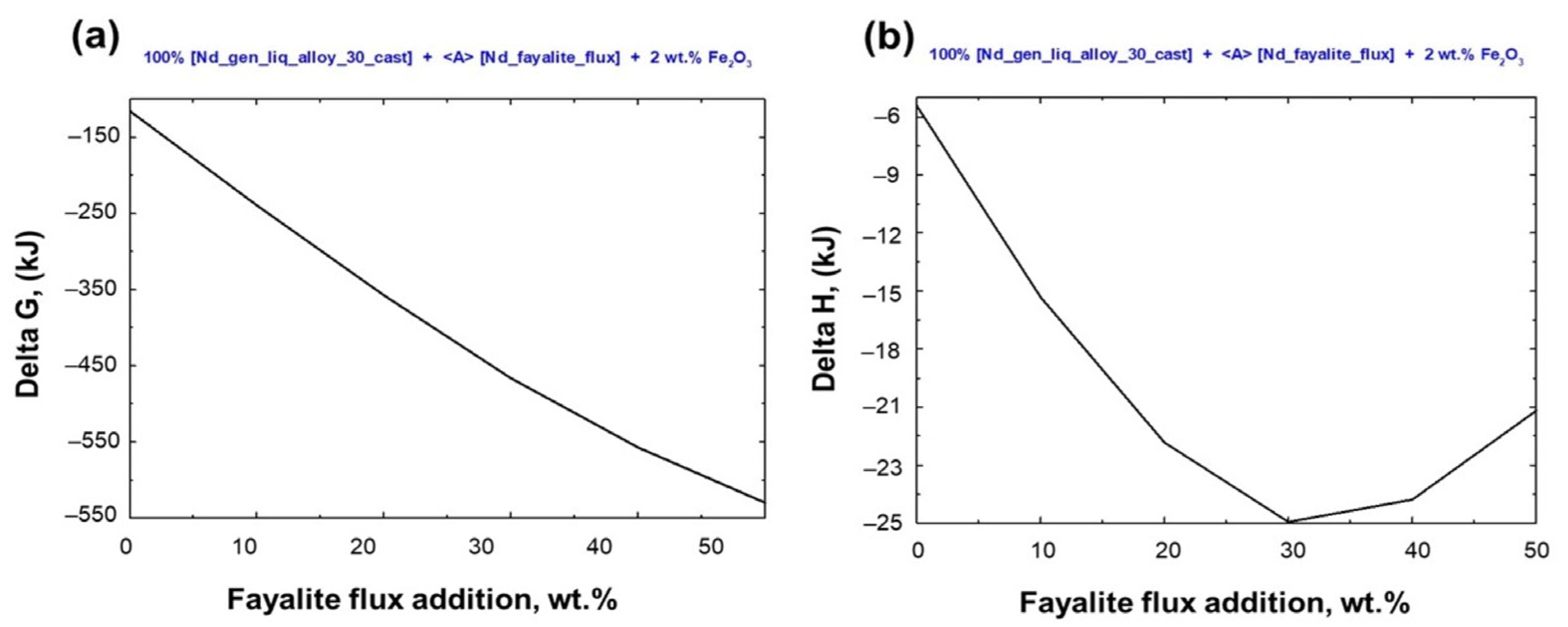
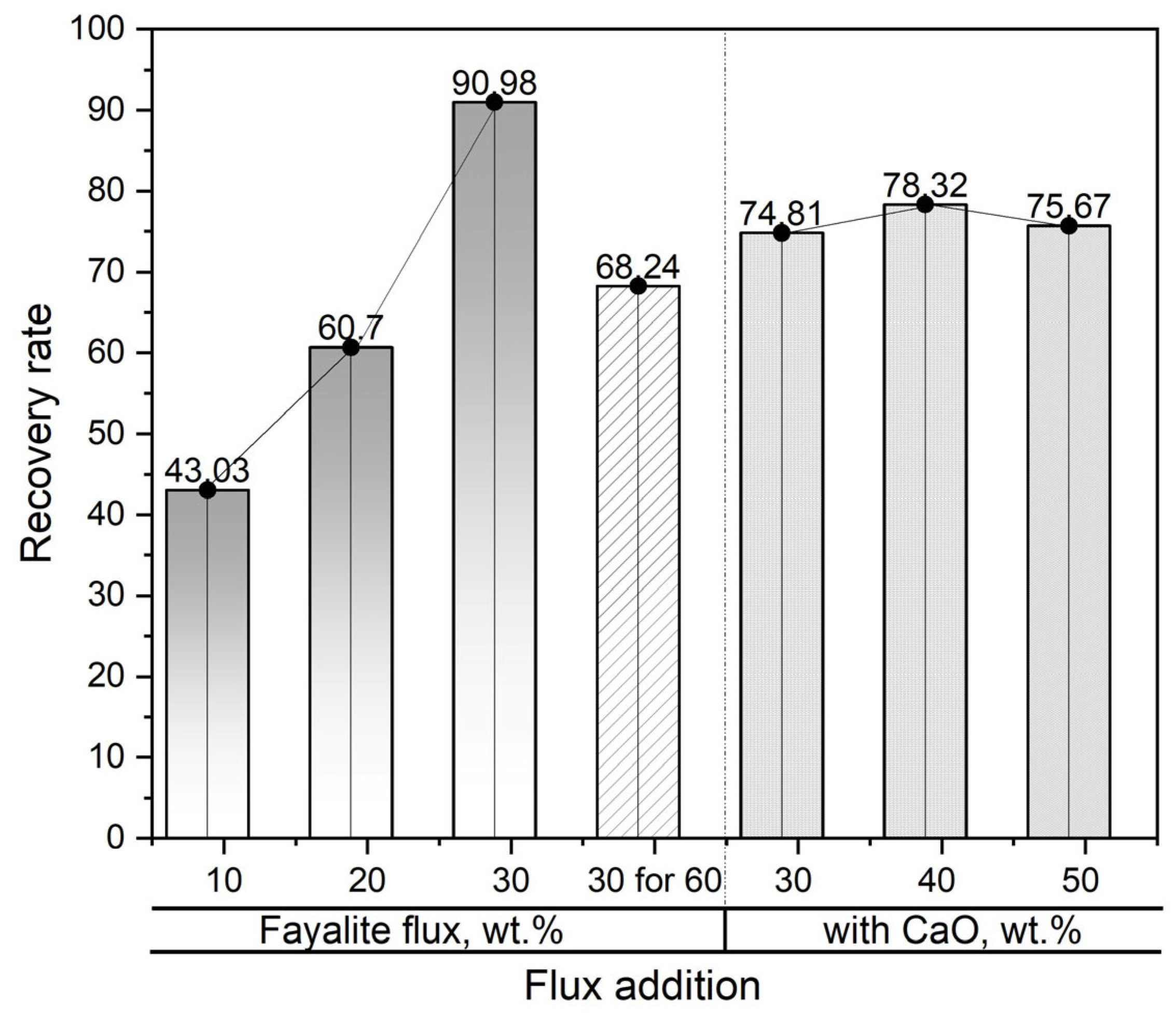
3.4. The Correlation Between the Flux Addition and the Neodymium Recovery Within the Slag
4. Conclusions
- REEs in liquid metal exhibit strong oxidation tendencies, with Gibbs free energy values for oxidation by fayalite of ΔG°[Nd] = −427 kJ, ΔG°[Dy] = −452 kJ, and ΔG°[Nb] = −186 kJ, respectively.
- At equilibrium smelting, electric steel and magnetic materials fully melted to form a bulk metal, but neodymium oxide inclusions heavily accumulated in the upper region, with REE oxide particles forming on the metal surface.
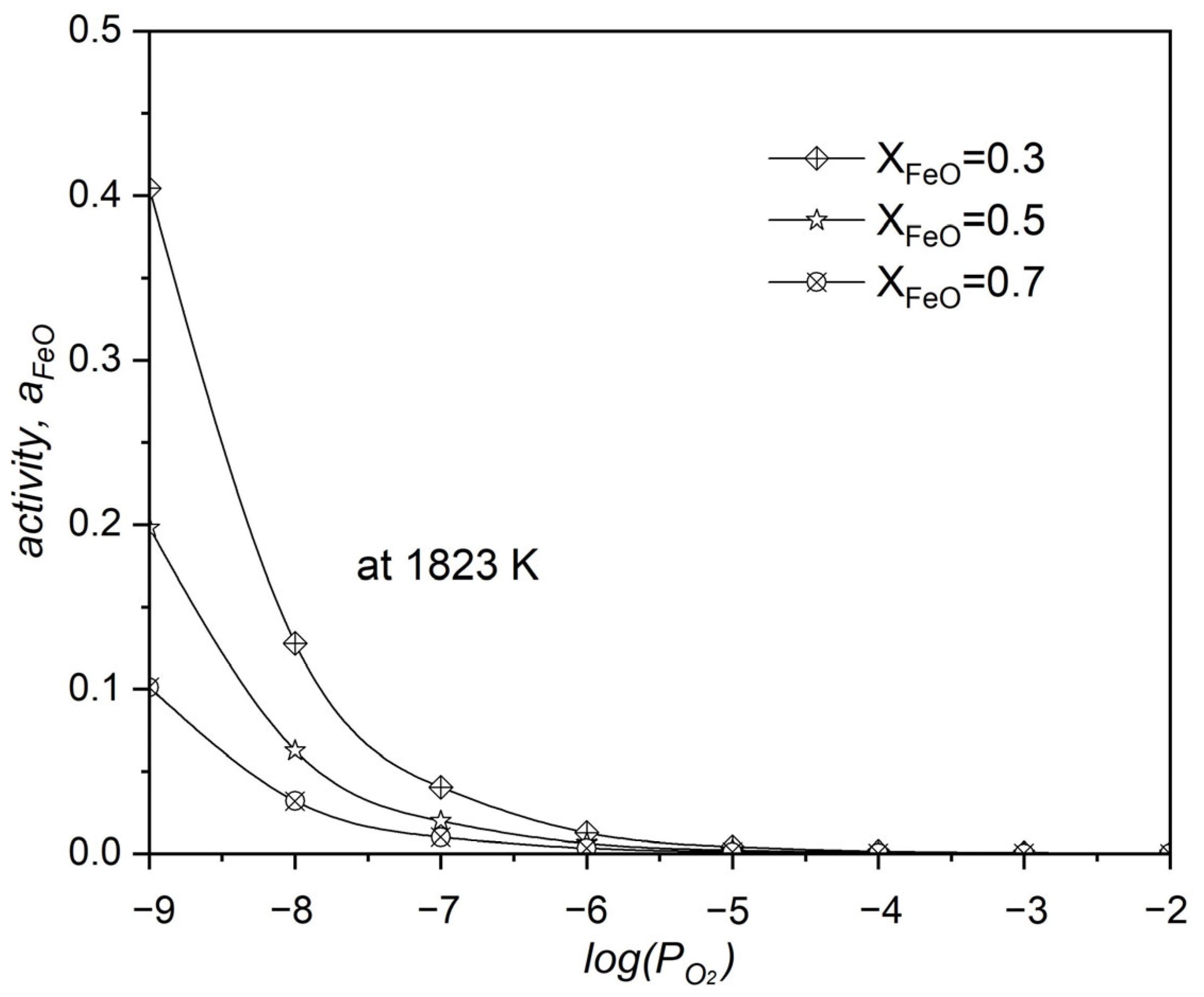
- For neodymium oxidation in liquid iron by FeO, the needed (1.8 × 10−9) is less than the FeO equilibrium partial pressure. Increased system correlated with increased and decreased .
- Nd3+ ions break down the base within the liquid slag, resulting in Nd2Si2O7 silicate formation, which is then broken down by Ca2+ cations.
- The 30 wt.% fayalite flux addition resulted in a maximum neodymium recovery efficiency of approximately 91% in the slag.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Racunaliske novice. Electric Cars with the Longest Range. Available online: https://www.racunalniske-novice.com/en/electric-cars-with-the-longest-range/ (accessed on 3 October 2024).
- ACKO. 15 Long-Range Electric Cars in India in 2025. Available online: https://www.acko.com/car-guide/long-range-electric-cars-in-india/ (accessed on 31 December 2024).
- Divyani, J.; Sunil, K.; Shally, V. Introduction to artificial intelligence-empowered electric vehicles in smart grids. In Artificial Intelligence-Empowered Modern Electric Vehicles in Smart Grid Systems; Aparna, K., Sudeep, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 3–31. [Google Scholar] [CrossRef]
- Arévalo, P.; Ochoa-Correa, D.; Villa-Ávila, E. A Systematic Review on the Integration of Artificial Intelligence into Energy Management Systems for Electric Vehicles: Recent Advances and Future Perspectives. World Electr. Veh. J. 2024, 15, 364. [Google Scholar] [CrossRef]
- Evs&Beyond. 2024 Breaks Global EV Sales Record with Over 17 Million Units Sold. Available online: https://evsandbeyond.co.nz/2024-breaks-global-ev-sales-record-with-over-17-million-units-sold/#:~:text=Electric%20vehicle%20(EV)%20sales%20reached,1.3%20million%20(+27%25) (accessed on 16 January 2025).
- RHO Motion. Over 17 Million EVs Sold in 2024—Record Year. Available online: https://rhomotion.com/news/over-17-million-evs-sold-in-2024-record-year/#:~:text=Over%2017%20million%20EVs%20sold%20in%202024,*%20Rest%20of%20World:%201.3%20million%2C%20+27% (accessed on 14 January 2025).
- Mohamed, K.; Nassar, Y.; El-Khozondar, H.J.; Monaem, E.R.Z.; Yaghoubi, E.; Yaghoubi, E. Electric Vehicles in China, Europe, and the United States: Current Trend and Market Comparison. Int. J. Electr. Eng. Sustain. 2024, 2, 1–20. Available online: https://ijees.org/index.php/ijees/article/view/70 (accessed on 9 January 2024).
- IEA.org. The Global Electric Vehicle Fleet is Set to Grow Twelve-Fold by 2035 Under Stated Policies. Available online: https://www.iea.org/reports/global-ev-outlook-2024/outlook-for-electric-mobility (accessed on 23 April 2024).
- Hou, L.; Guo, Y.; Ba, X.; Lei, G.; Zhu, J. Efficiency Improvement of Permanent Magnet Synchronous Motors Using Model Predictive Control Considering Core Loss. Energies 2024, 17, 773. [Google Scholar] [CrossRef]
- Özçiflikçi, O.E.; Koç, M.; Bahçeci, S.; Selcuk, E. Overview of PMSM control strategies in electric vehicles: A review. Int. J. Dynam. Control 2024, 12, 2093–2107. [Google Scholar] [CrossRef]
- Sergakis, A.; Salinas, M.; Gkiolekas, N.; Gyftakis, K.N. A Review of Condition Monitoring of Permanent Magnet Synchronous Machines: Techniques, Challenges and Future Directions. Energies 2025, 18, 1177. [Google Scholar] [CrossRef]
- Orlova, S.; Rassõlkin, A. Permanent Magnets in Sustainable Energy: Comparative Life Cycle Analysis. Energies 2024, 17, 6384. [Google Scholar] [CrossRef]
- Usman, A.; Saxena, A. Technical Roadmaps of Electric Motor Technology for Next Generation Electric Vehicles. Machines 2025, 13, 156. [Google Scholar] [CrossRef]
- Ranjan, P.; Kalla, U.K. Comprehensive Study on Various Types of Magnets Used in Permanent Magnet Electrical Motors. In Proceedings of the 2024 IEEE Region 10 Symposium (TENSYMP), New Delhi, India, 27–29 September 2024; pp. 1–7. [Google Scholar] [CrossRef]
- Karami, R.; Butler, D.; Tamimi, S. Manufacturing of non-grain-oriented electrical steels: Review. Int. J. Adv. Manuf. Technol. 2024, 133, 1083–1109. [Google Scholar] [CrossRef]
- Lukas, N.; Herbert, K.; Herbert, L.; Thomas, H.; Christof, S. Microstructural and textural evolution of double stage cold rolled non-grain oriented electrical steel. J. Magn. Magn. Mater. 2024, 597, 172032. [Google Scholar] [CrossRef]
- He, Y.; Kestens, L.A. The processing, microstructure, texture, and magnetic properties of electrical steels: A review. Int. Mater. Rev. 2025, 1, 1. [Google Scholar] [CrossRef]
- Ning, S.; Seangwong, P.; Fernando, N.; Siritaratiwat, A.; Khunkitti, P. A novel double stator hybrid-excited Halbach permanent magnet flux-switching machine for EV/HEV traction applications. Sci. Rep. 2024, 14, 18636. [Google Scholar] [CrossRef] [PubMed]
- Ma, J. Magnetic Field Analysis and Development of Disk Axial–Radial Hybrid Excitation Generator for Range Extenders in Extended-Range Electric Vehicles. World Electr. Veh. J. 2024, 15, 94. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Rahideh, A.; Abbas, A.; Iqbal, A.; El-Bayeh, C.Z.; Flah, A.; Ali, E.; Ramy, N.R.G. Calculating torque, back-EMF, inductance, and unbalanced magnetic force for a hybrid electrical vehicle by in-wheel drive application. Sci. Rep. 2024, 14, 12912. [Google Scholar] [CrossRef] [PubMed]
- Velikov, A.; Nachev, A.; Gueorguiev, N.; Bachvarov, R.; Stanev, S.; Nenova, L.; Maneva, A.; Nikolov, K.; Pavlova, D. Optimization the Phase Composition of Aluminum Alloys for Motor Parts Operating at Incresed Temperatures. In Proceedings of the 2024 6th International Conference on Control Systems, Mathematical Modeling, Automation and Energy Efficiency (SUMMA), Lipetsk, Russian, 13–15 November 2024; pp. 603–607. [Google Scholar] [CrossRef]
- Yavaş, A.; Cilingir, C.; Turk, A.; Celik, A. Production and Characterization of Highly Conductive Aluminum Metal for Electric Motor Applications. J. Mater. Eng. Perform. 2025, 34, 1705–1716. [Google Scholar] [CrossRef]
- Li, Z.; Ahmed, S.H.; Zhiming, Y.; Anwar, S.; Sumit, H.; Juliette, S.; Caroline, G.; Syed, H.A.; Friya, T. A circular economy approach for recycling Electric Motors in the end-of-life Vehicles: A literature review. Resour. Conserv. Recycl. 2024, 205, 107582. [Google Scholar] [CrossRef]
- Tiwari, D.; Miscandlon, J.; Tiwari, A.; Jewell, G.W. A Review of Circular Economy Research for Electric Motors and the Role of Industry 4.0 Technologies. Sustainability 2021, 13, 9668. [Google Scholar] [CrossRef]
- Chang, M.M.L.; Ong, S.K.; Nee, A.Y.C. Approaches and Challenges in Product Disassembly Planning for Sustainability. Procedia CIRP 2017, 60, 506–511. [Google Scholar] [CrossRef]
- Mitrouchev, P.; Wang, C.G.; Lu, L.X.; Li, G.O. Selective disassembly sequence generation based on lowest level disassembly graph method. Int. J. Adv. Manuf. Technol. 2015, 80, 141–159. [Google Scholar] [CrossRef]
- Cao, R.; Mi, C.; Cheng, M. Quantitative Comparison of Flux-Switching Permanent-Magnet Motors With Interior Permanent Magnet Motor for EV, HEV, and PHEV Applications. IEEE Trans. Magn. 2012, 48, 2374–2384. [Google Scholar] [CrossRef]
- James, D.W.; Richard, M.; Mohammed, K. Electric vehicle traction motors without rare earth magnets. Sustain. Mater. Technol. 2015, 3, 7–13. [Google Scholar] [CrossRef]
- Katsunori, Y.; Rare Earth Element Recovery Technology for Motor Magnets for Electric Vehicles that Aims to Reduce Costs in a Short Time. Science Technology Innovation Japan. Available online: https://sj.jst.go.jp/stories/2022/s0301-01a.html (accessed on 1 March 2022).
- Saito, T.; Sato, H.; Ozawa, S.; Yu, J.; Motegi, T. The extraction of Nd from waste Nd–Fe–B alloys by the glass slag method. J. Alloys Compd. 2003, 353, 189–193. [Google Scholar] [CrossRef]
- Yang, Y.; Abrahami, S.T.; Xiao, Y. Recovery of rare earth elements from EOL permanent magnets with molten slag extraction. In Proceedings of the 3rd International Slag Valorisation Symposium; ACCO: Leuven, Belgium, 2013; pp. 249–252. [Google Scholar]
- Zhang, Y.; Gu, F.; Su, Z.; Liu, S.; Anderson, C.; Jiang, T. Hydrometallurgical Recovery of Rare Earth Elements from NdFeB Permanent Magnet Scrap: A Review. Metals 2020, 10, 841. [Google Scholar] [CrossRef]
- Xiao, F.; Hu, W.; Zhao, J.; Zhu, H. Technologies of Recycling REEs and Iron from NdFeB Scrap. Metals 2023, 13, 779. [Google Scholar] [CrossRef]
- Oksana, D.; Natalia, K.; Vadim, K.; Gulaim, S. Recovery of rare earth elements from NdFeB magnet by mono- and bifunctional mesoporous silica: Waste recycling strategies and perspectives. Hydrometallurgy 2022, 210, 105855. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Cheng, G.; Xue, X.; Yang, H. Kinetics and mechanism of hydrochloric acid leaching of rare earths from Bayan Obo slag and recovery of rare earth oxalate and high purity oxides. Hydrometallurgy 2022, 208, 105782. [Google Scholar] [CrossRef]
- Blenau, L.W.; Vogt, D.; Lonski, O.; Abrar, A.; Fabrichnaya, O.; Charitos, A. Development of a Process to Recycle NdFeB Permanent Magnets Based on the CaO-Al2O3-Nd2O3 Slag System. Processes 2023, 11, 1783. [Google Scholar] [CrossRef]
- Yang, Z.; Xiao, F.; Sun, S.; Zhong, H.; Tu, G. REEs recovery from molten salt electrolytic slag: Challenges and opportunities for environmentally friendly techniques. J. Rare Earths 2024, 42, 1009–1019. [Google Scholar] [CrossRef]
- Elwert, T.; Goldmann, D.; Römer, F.; Buchert, M.; Merz, C.; Schueler, D.; Sutter, J. Current Developments and Challenges in the Recycling of Key Components of (Hybrid) Electric Vehicles. Recycling 2016, 1, 25–60. [Google Scholar] [CrossRef]
- Bast, U.; Recycling von Komponenten und Strategischen Metallen aus Elektrischen Fahrantrieben: MORE (Motor Recycling). Final Research Report. 2014. Available online: http://edok01.tib.uni-hannover.de/edoks/e01fb15/826920594.pdf (accessed on 16 September 2015).
- Li, Y.; Hu, A.; Fu, Y.; Liu, S.; Shen, W.; Hu, H.; Nie, X. Al Alloys and Casting Processes for Induction Motor Applications in Battery-Powered Electric Vehicles: A Review. Metals 2022, 12, 216. [Google Scholar] [CrossRef]
- Fonnov Aluminium. 6063 Aluminum Extrusion For Large Water-cooled Electric Motor Housing. Available online: https://www.fonnovaluminium.com/product/water-cooled-electric-motor-housing-aluminum-extrusions (accessed on 21 December 2023).
- Stornelli, G.; Faba, A.; Di Schino, A.; Folgarait, P.; Ridolfi, M.R.; Cardelli, E.; Montanari, R. Properties of Additively Manufactured Electric Steel Powder Cores with Increased Si Content. Materials 2021, 14, 1489. [Google Scholar] [CrossRef]
- Kim, K.O.; Jung, Y.H.; Park, J.C.; Lim, M.S. Comparative Study of Mechanical and Electrical Characteristics of High-Strength and Conventional Electrical Steel for EV Traction High-Speed Multilayer IPMSM Using Rare-Earth Free PM. IEEE Trans. Magn. 2023, 59, 8102205. [Google Scholar] [CrossRef]
- Lobo, J.A.; Geiger, G.H. Thermodynamics and solubility of carbon in ferrite and ferritic Fe-Mo alloys. Metall. Trans. A 1976, 7, 1347–1357. [Google Scholar] [CrossRef]
- Kiyoshi, K.; Yoshio, S. Rudimental research progress of rare-earth silicate oxyapatites: Their identification as a new compound until discovery of their oxygen ion conductivity. J. Ceram. Soc. Jpn. 2014, 122, 649–663. [Google Scholar] [CrossRef]
- Leszek, K.; Marek, W.; Marek, D. Interfacial reactions and silicate formation in highly dispersed Nd2O3–SiO2 system. Mater. Chem. Phys. 2006, 96, 353–360. [Google Scholar] [CrossRef]
- Yuji, M.; Mikio, H.; Kohei, K. Reinvestigation of phase relations around the oxyapatite phase in the Nd2O3–SiO2 system. J. Cryst. Growth 2003, 247, 207–212. [Google Scholar] [CrossRef]
- Thu, H.L.; Kai, T.; Sander, A.; Annelies, M.; Bart, B.; Muxing, G. Thermodynamic assessment of the Nd2O3-CaO-SiO2 ternary system. Calphad 2016, 55, 157–164. [Google Scholar] [CrossRef]
- Wei, W.; Li, S.; Zhang, B.; Cao, Z.M. Experimental study of the phase relations in Nd2O3-SiO2-FeOx system at 1773 K with p(O2) = 10−7 atm. Ceram. Int. 2025, 51, 18226–18235. [Google Scholar] [CrossRef]
- Le, T.H.; Malfliet, A.; Blanpain, B.; Muxing, G. Phase Relations of the CaO-SiO2-Nd2O3 System and the Implication for Rare Earths Recycling. Metall. Mater. Trans. B 2016, 47, 1736–1744. [Google Scholar] [CrossRef]
| Element | C | Si | Mn | P | S | Cr | Mo | Ni | Cu | Al | Fe | Nd * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 3.48 | 2.61 | 0.58 | 0.34 | 0.13 | 0.51 | 0.014 | 0.027 | 0.03 | <0.001 | Bal. | N.D. |
| Materials | Weight, kg | wt.% | Recalculated wt.% |
|---|---|---|---|
| Steel | 34.8 | 55.06 | 87.22 |
| Aluminum | 14.1 | 22.31 | - |
| NdFeB magnet (30% Nd) | 2.1 | 3.32 | 5.26 |
| Cast iron | 3.0 | 4.75 | 7.52 |
| Copper | 8.5 | 13.45 | - |
| Polymer | 0.6 | 0.95 | - |
| Elasromer | 0.1 | 0.16 | - |
| Total | 63.2 | 100.0 | 100.0 |
| Oxide | Fe2O3 | SiO2 | Al2O3 | MgO | Cr2O3 |
|---|---|---|---|---|---|
| wt.% | 55.45 | 41.49 | 0.8 | 1.5 | 0.65 |
| Materials | Element, wt.% | Weight.% | ||||||
|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | Al | Fe | ||
| Steel | 0.05 | 3.0 | 0.25 | 0.03 | 0.03 | 0.25 | 96.39 | 87.22 |
| Cast iron | 3.5 | 2.63 | 0.58 | 0.34 | 0.13 | – | 92.28 | 7.52 |
| Magnet | Nd | Si | B | Dy | Nb | Al | Fe | |
| 30.0 | – | 1.0 | 1.0 | 1.0 | 0.3 | 66.7 | 5.26 | |
| Materials | Element, wt.% | Weight, g | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Nd | Si | Al | B | Dy | Nb | P | S | Fe | ||
| Metal_liquid | 0.307 | 0.262 | 1.419 | 2.819 | 0.234 | 0.05 | 0.05 | 0.05 | 0.05 | 2 × 10−5 | 94.74 | 99.803 |
| NdS_solid | – | – | 81.82 | – | – | – | – | – | – | 18.18 | – | 0.197 |
| Cast Iron Addition, wt.% | Element of Metal, wt.% | Product Weight, g | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Al | Nd | Dy | B | Nb | Fe | Metal_liq | NdS_sol | |
| 10 | 0.59 | 2.80 | 0.29 | 3 × 10−5 | 0.07 | 0.21 | 1.237 | 0.047 | 0.047 | 0.047 | 94.63 | 109.73 | 0.071 |
| 20 | 0.84 | 2.79 | 0.31 | 3 × 10−5 | 0.10 | 0.19 | 1.086 | 0.043 | 0.043 | 0.043 | 94.53 | 119.66 | 0.142 |
| 30 | 1.04 | 2.78 | 0.33 | 4 × 10−5 | 0.11 | 0.18 | 0.95 | 0.04 | 0.04 | 0.04 | 94.45 | 129.59 | 0.214 |
| 40 | 1.22 | 2.77 | 0.35 | 5 × 10−5 | 0.13 | 0.16 | 0.84 | 0.037 | 0.037 | 0.037 | 94.38 | 139.52 | 0.285 |
| 50 | 1.37 | 2.76 | 0.36 | 6 × 10−5 | 0.14 | 0.15 | 0.75 | 0.035 | 0.035 | 0.035 | 94.32 | 149.45 | 0.357 |
| Fe2O3 Addition, wt.% | Element of Metal, wt.% | Product Weight, g | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Al | Nd | Dy | B | Nb | Fe | Metal_liq | AlNdO3 _solid | RexO3 _solid | SiO2_sol | |
| 2 | 1.0 | 2.71 | 0.33 | 4 × 10−5 | 0.11 | 1 × 10−4 | 1 × 10−7 | 1 × 10−7 | 1 × 10−7 | 0.04 | 95.73 | 129.31 | 1.813 | 0.136 | 0.199 |
| 4 | 0.95 | 2.34 | 0.33 | 4 × 10−5 | 0.11 | 9 × 10−5 | 1 × 10−7 | 1 × 10−7 | 1 × 10−7 | 0.04 | 96.16 | 130.20 | 1.813 | 0.136 | 1.176 |
| 6 | 0.89 | 1.99 | 0.33 | 4 × 10−5 | 0.11 | 8 × 10−5 | 1 × 10−8 | 9 × 10−8 | 9 × 10−8 | 0.04 | 96.58 | 131.08 | 1.813 | 0.136 | 1.813 |
| Flux Addition, wt.% | Element in Metal, wt.% | Product Weight, g | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Nd | Fe | Metal_liq | AlNdO3 _solid | RexO3 _solid | Slag_liq | SiO2_sol | |
| 10 | 0.84 | 1.75 | 0.32 | 4 × 10−5 | 0.11 | 6 × 10−8 | 96.87 | 133.42 | 1.81 | 0.136 | – | 5.69 |
| 20 | 0.62 | 0.93 | 0.31 | 4 × 10−5 | 0.11 | 2 × 10−8 | 97.92 | 137.54 | 1.81 | 0.136 | – | 10.95 |
| 30 | 0.34 | 0.29 | 0.3 | 4 × 10−5 | 0.11 | 1 × 10−8 | 98.89 | 141.69 | 1.77 | 0.163 | 2.159 | 13.75 |
| 40 | 0.05 | 0.09 | 0.02 | 3 × 10−5 | 0.1 | 9 × 10−10 | 99.73 | 144.8 | 1.54 | 0.164 | 4.696 | 14.59 |
| 50 | 0.04 | 0.004 | 0.004 | 3 × 10−5 | 0.11 | 6 × 10−10 | 99.76 | 145.17 | 1.35 | 0.167 | 18.40 | 13.39 |
| Flux Addition, wt.% | Oxide, % | ||||||
|---|---|---|---|---|---|---|---|
| SiO2 | FeO | MnO | Al2O3 | Fe2O3 | B2O3 | Etc. | |
| 30 | 95.84 | 0.35 | 0.54 | 0.46 | 9 × 10−5 | 2.79 | Bal. |
| 40 | 54.42 | 28.17 | 10.85 | 4.48 | 0.03 | 2.01 | Bal. |
| 50 | 50.50 | 43.18 | 3.04 | 2.34 | 0.08 | 0.90 | Bal. |
| Element | Fe | C | O | Nd | Sum. |
|---|---|---|---|---|---|
| wt.% | 73.56 | 12.04 | 7.21 | 7.19 | 100.0 |
| Element | Nd | O | Ce | C | Al | Fe | Gd | S | Si | Sum. |
|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 35.36 | 19.60 | 12.29 | 10.97 | 8.03 | 4.86 | 3.66 | 3.44 | 1.79 | 100.0 |
| Flux Addition, wt.% | Element of Metal, wt.% | Nd * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Cr | Ni | Cu | Al | Etc. | Fe | ||
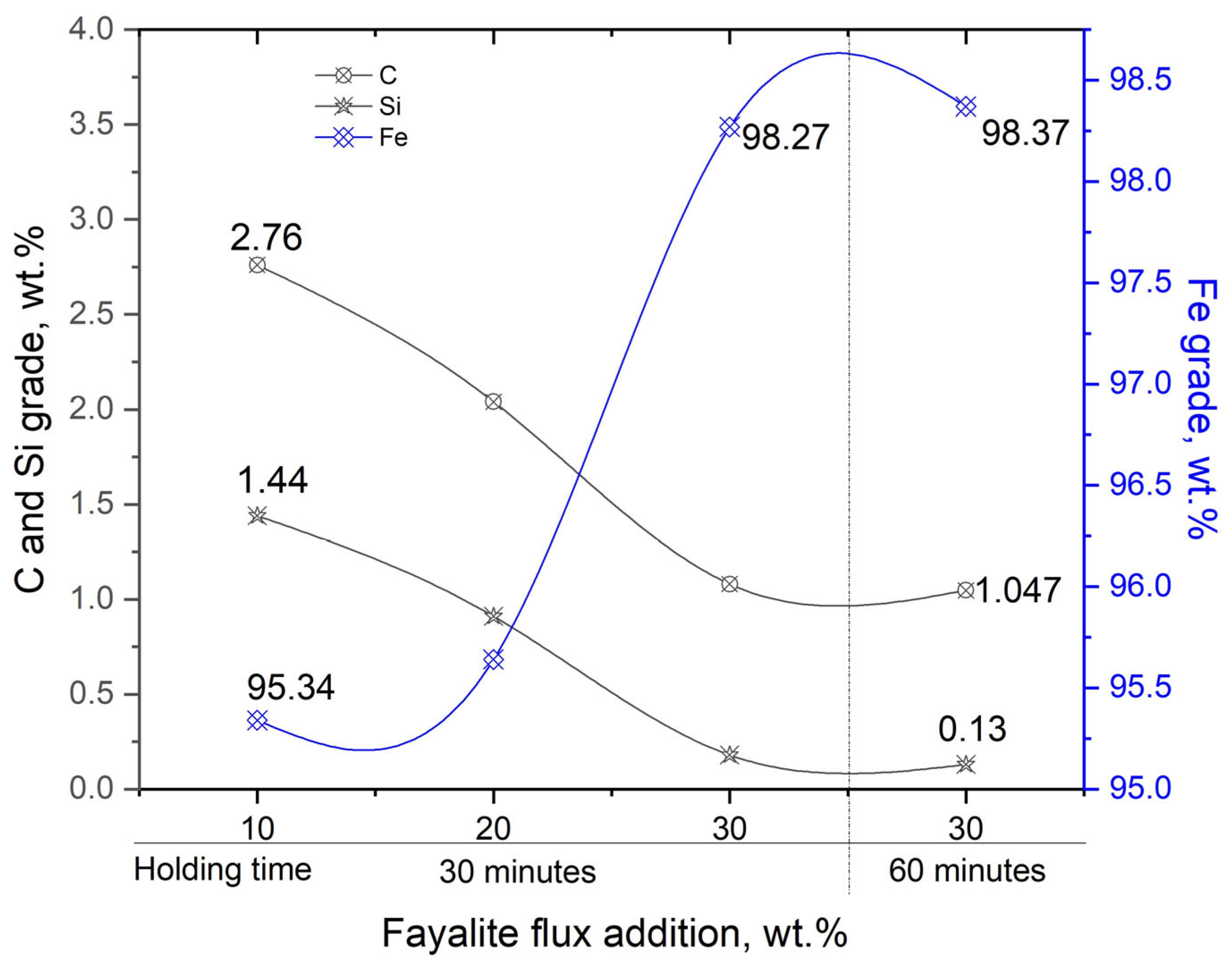
| 10 for 30 min | 2.76 | 1.44 | 0.15 | 0.008 | 0.05 | 0.053 | 0.035 | 0.045 | 0.022 | 0.094 | 95.34 | 0.018 |
| 20 for 30 min | 2.04 | 0.91 | 0.13 | 0.085 | 0.04 | 0.78 | 0.085 | 0.11 | 0.068 | 0.112 | 95.64 | 0.040 |
| 30 for 30 min | 1.08 | 0.18 | 0.05 | 0.015 | 0.05 | 0.02 | 0.01 | 0.10 | 0.01 | 0.215 | 98.27 | 0.0078 |
| 30 for 60 min | 1.047 | 0.13 | 0.034 | 0.01 | 0.055 | 0.026 | 0.014 | 0.15 | 0.001 | 0.154 | 98.38 | 0.006 |
| Flux Addition, wt.% | Oxide, wt.% | Nd * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Fe2O3 | Al2O3 | MnO | Cr2O3 | Gd2O3 | CaO | Pr6O11 | TiO2 | V2O5 | Nd2O3 | ||
| 10 for 30 min | 45.07 | 21.78 | 24.44 | 3.29 | 0.23 | 0.83 | – | 0.84 | 0.33 | 0.14 | 3.0 | 4.91 |
| 20 for 30 min | 47.66 | 20.76 | 23.69 | 2.91 | 0.27 | – | – | 0.80 | 0.33 | – | 3.58 | 5.83 |
| 30 for 30 min | 52.60 | 24.49 | 15.60 | 2.20 | 0.25 | – | 0.12 | – | 0.20 | 0.18 | 4.31 | 4.45 |
| 30 for 60 min | 46.17 | 26.67 | 19.59 | 2.07 | 0.27 | – | – | – | 0.17 | 0.20 | 4.81 | 3.32 |
| CaO Addition, wt.% | Oxide, wt.% | C/S | Nd * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Fe2O3 | Al2O3 | MnO | Nd2O3 | CaO | MgO | TiO2 | Cr2O3 | |||
| 30 | 39.78 | 5.22 | 28.67 | 1.23 | 4.01 | 20.23 | 0.62 | 0.23 | 0.011 | 0.50 | 2.80 |
| 40 | 34.83 | 4.73 | 30.23 | 1.38 | 3.20 | 24.81 | 0.50 | 0.18 | 0.09 | 0.71 | 2.51 |
| 50 | 24.65 | 0.99 | 40.89 | 0.76 | 2.83 | 28.89 | 0.54 | 0.17 | 0.13 | 1.17 | 2.01 |
| Flux Addition, wt.% | Nd in Magnet, g | Slag Weight, g | Nd in Slag, wt.% | Nd in Slag, g |
|---|---|---|---|---|
| Fayalite flux without CaO | ||||
| 10 | 6.32 | 55.51 | 4.91 | 2.72 |
| 20 | 6.32 | 65.82 | 5.83 | 3.83 |
| 30 | 4.17 | 85.26 | 4.45 | 3.79 |
| 30 for 60 min | 4.13 | 84.89 | 3.32 | 2.81 |
| 30 wt.% Fayalite flux with CaO | ||||
| 30 | 4.13 | 110.35 | 2.80 | 3.08 |
| 40 | 4.12 | 129.08 | 2.51 | 3.22 |
| 50 | 4.12 | 155.12 | 2.01 | 3.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urtnasan, E.; Park, J.-H.; Chung, Y.-J.; Wang, J.-P. Pyrometallurgical Recycling of Electric Motors for Sustainability in End-of-Life Vehicle Metal Separation Planning. Processes 2025, 13, 1729. https://doi.org/10.3390/pr13061729
Urtnasan E, Park J-H, Chung Y-J, Wang J-P. Pyrometallurgical Recycling of Electric Motors for Sustainability in End-of-Life Vehicle Metal Separation Planning. Processes. 2025; 13(6):1729. https://doi.org/10.3390/pr13061729
Chicago/Turabian StyleUrtnasan, Erdenebold, Jeong-Hoon Park, Yeon-Jun Chung, and Jei-Pil Wang. 2025. "Pyrometallurgical Recycling of Electric Motors for Sustainability in End-of-Life Vehicle Metal Separation Planning" Processes 13, no. 6: 1729. https://doi.org/10.3390/pr13061729
APA StyleUrtnasan, E., Park, J.-H., Chung, Y.-J., & Wang, J.-P. (2025). Pyrometallurgical Recycling of Electric Motors for Sustainability in End-of-Life Vehicle Metal Separation Planning. Processes, 13(6), 1729. https://doi.org/10.3390/pr13061729








