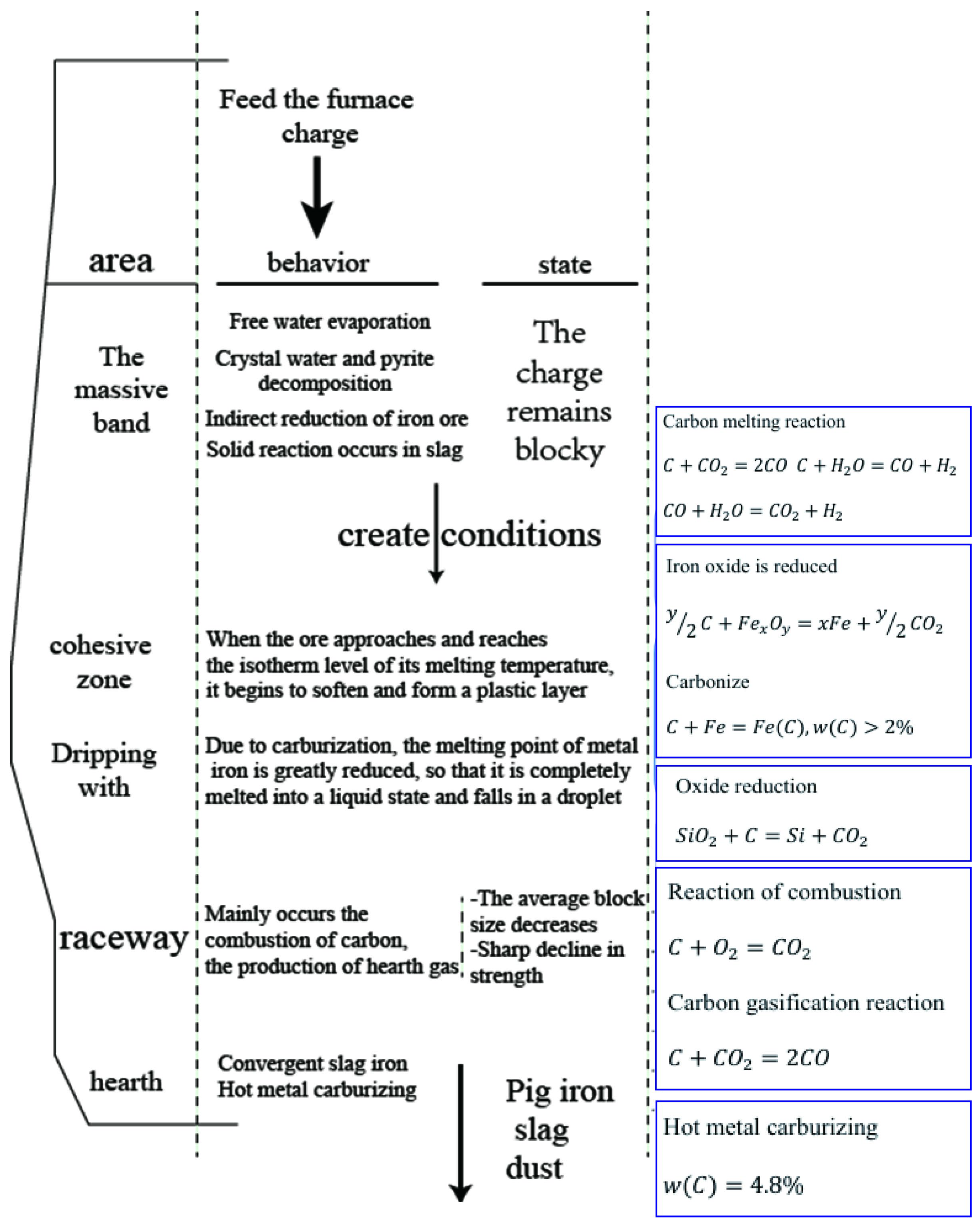
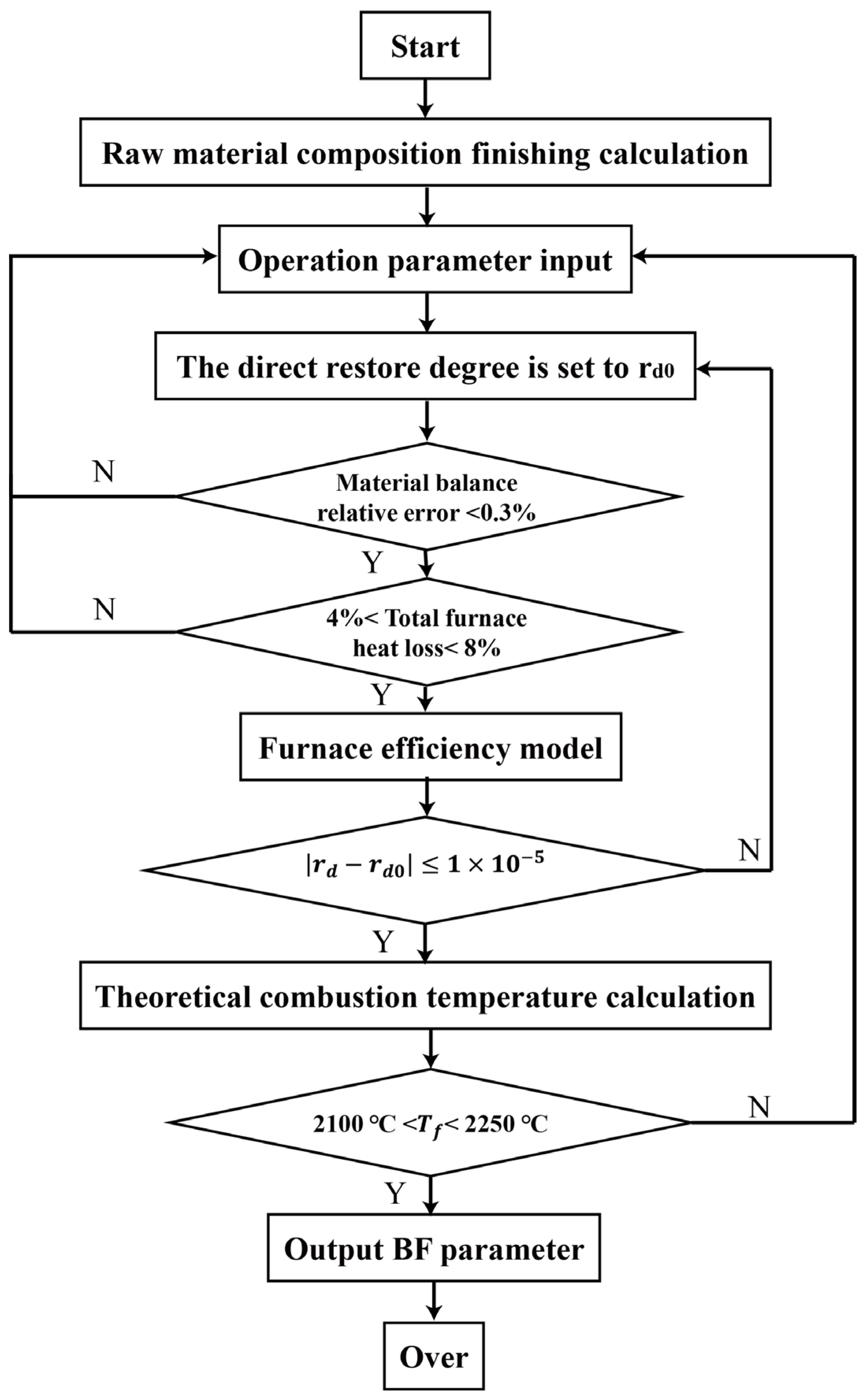
3.1. Model Validation
The accuracy of the hydrogen-rich blast furnace energy and mass balance model was validated using actual daily production data. The initial direct reduction degree was set to 0.45, and the key parameters calculated by the model are summarized in
Table 8. To ensure robust validation, operational data from nine randomly selected working days in March, July, and November were utilized. This selection accounts for potential fluctuations arising from variations in temperature, pressure, and furnace operating conditions.
In total, 10 sets of operational reports from the blast furnace of the steel plant in 2023 were randomly selected. Using the calculation method proposed by Professor A.H. Ram, the direct reduction degree values were derived, as shown in
Table 8. All calculated values fall within the range of 0.42 to 0.45, demonstrating consistency with actual operational conditions and further verifying the reliability of the model.
She Xue-feng et al. [
20] iteratively established the relationship between carbon consumption and the direct reduction degree in a blast furnace based on theoretical equations, as illustrated in
Figure 4. The curves labeled
and
represent carbon consumption as a function of the direct reduction degree during the indirect reduction in minerals, derived from carbon oxide equilibrium principles. These curves intersect the line representing the carbon required for heat supply at points A and B, corresponding to the minimum theoretical direct reduction degrees of 0.35 and 0.40, respectively.
In actual blast furnace operations, although carbon oxides undergo mutual conversion, reaction kinetics limitations prevent these reactions from reaching full equilibrium. As a result, the practical minimum direct reduction degree lies between 0.35 and 0.40, corresponding to a minimum carbon consumption range of 343 to 361 kg·t−1. Under the current mainstream production conditions in China, the direct reduction degree typically reaches about 0.45, which corresponds to a minimum carbon consumption of 399 kg·t−1. Therefore, China’s blast furnace ironmaking technology is considered mature, leaving limited room for further carbon reduction solely through process optimization within this framework.
To ensure robust validation, operational data from nine randomly selected working days in March, July, and November were used, accounting for potential fluctuations caused by variations in temperature, pressure, and furnace operating conditions.
As shown in
Table 9, the calculated values for B_(M-error), r_(Q-loss), and T_f all fall within normal operational ranges, confirming the model’s reliability under steady-state conditions. Furthermore,
Table 10 compares the calculated gas composition with on-site measured blast furnace gas data, demonstrating strong consistency and validating the model’s accuracy and applicability.
This comprehensive verification using field data confirms that the developed energy and mass balance model can accurately simulate the operation of hydrogen-enriched blast furnaces and is suitable for guiding process optimization in industrial applications.
Table 10 shows that the calculated CO
2, CO, and H
2 volume fractions closely match the field measurement values. However, a slight discrepancy is observed between the calculated and measured N
2 volume fractions. This difference is due to the inclusion of water content in the total gas calculation. After accounting for removing H
2O, the calculated N
2 volume fraction is 49.899%, with a relative error of 2.522%, compared to the field-measured value. The primary cause of this discrepancy is air infiltration caused by the imperfect sealing of the on-site measurement device during gas composition analysis, resulting in partial contamination by air. Therefore, the gas composition calculated by this model aligns well with the field measurement results, thus validating the accuracy and rationality of the model.
Furthermore, following the previously stated constraints, the heat loss in the high-temperature zone should account for 70% to 80% of the total heat loss in the entire furnace. The calculated result from the model is 5.958 GJ/t, which represents approximately 76.25% of the total heat loss in the furnace, falling within the blast furnace’s normal operating range.
These two verification procedures confirm the accuracy and rationality of the model.
3.2. Influence of Pulverized Coal Injection on Key Parameters in Blast Furnace Operation
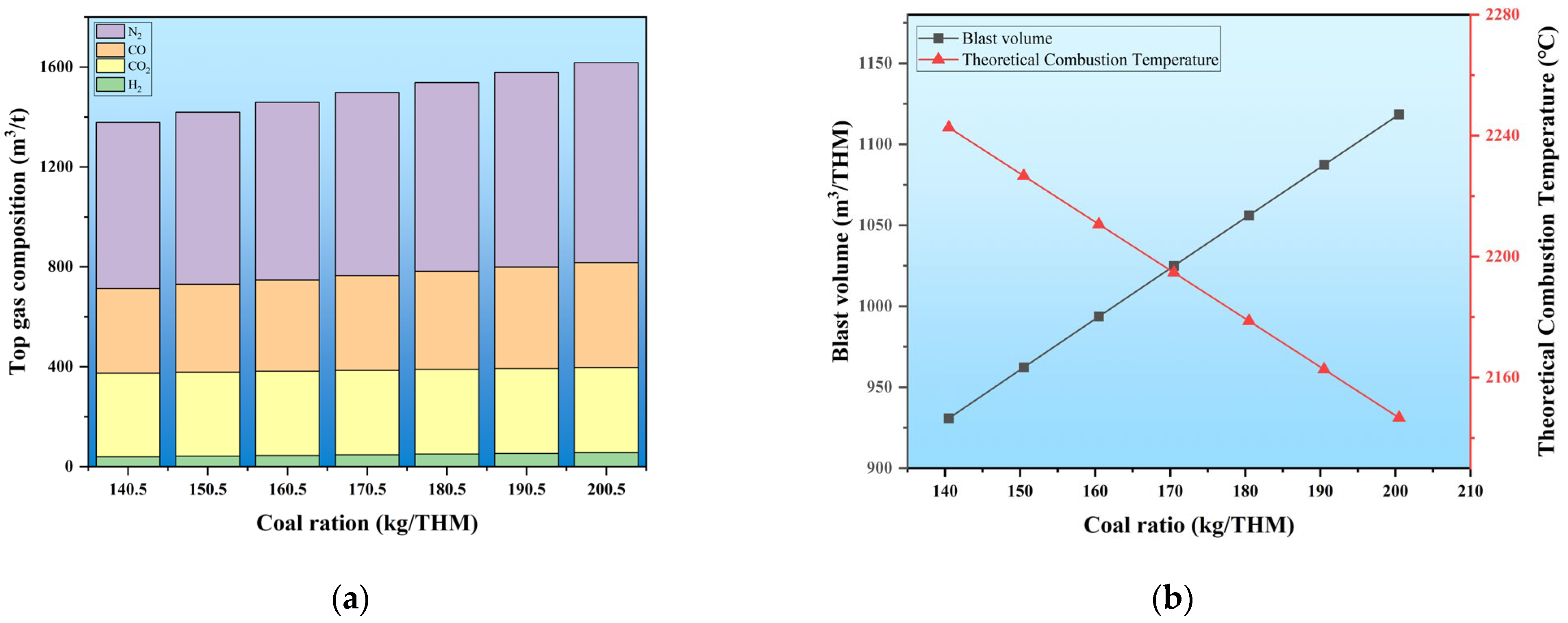
To understand the range of coal and CBM injection quantities after the blast furnace transformation under hydrogen-rich conditions, the coal injection quantity of the traditional blast furnace is defined using a model. The initial conditions are set: a coke ratio of 346.3 kg/THM, a coal ratio of 155.5 kg/THM, a step increase of 5 kg/THM for the coal ratio, and an oxygen enrichment rate of 6%. As the coal ratio increases, the changes in the gas composition in the furnace, the blast volume, and the theoretical combustion temperature are illustrated in
Figure 5.
With the increase in the amount of coal injected into the furnace,
Figure 5a shows the variation in the content of gas components within the furnace. Notably, CO, N
2, and H
2 levels exhibit a significant rise. Under the basic furnace conditions, for every 10 kg/THM increase in the coal injection quantity, the average increments of CO, N
2, and H
2 are 13.61 m
3/THM, 22.46 m
3/THM, and 2.84 m
3/THM, respectively, with increase rates of 3.73%, 3.15%, and 6.41%.
As the coal injection quantity increases, the demand for blast air also rises to maintain stable and smooth furnace operation, increasing N2 content within the furnace hearth. Additionally, due to the excess carbon, the combustion at the tuyere reaches its threshold, increasing the proportion of incomplete carbon combustion. The water–gas reaction intensifies, with reaction products including H2 and CO, as shown in Reactions (7) and (9), which increases the CO content in the furnace hearth. Simultaneously, as the amount of coal powder increases, the hydrogen and water introduced into the system also increase, leading to a rise in H2 content in the furnace hearth. As the pulverized coal injection rate increases within the blast furnace, the CO2 content remains relatively constant at 338.22 m3/THM. This stability is due to the constraints imposed by the input quantity of blended iron ore and the inherent limitations of the blast furnace itself. In summary, as the pulverized coal injection rate increases, the top gas volume rises correspondingly.
In conclusion, with the progressive increase in coal injection quantity, the proportion of reducing gases increases by as much as 17.28%. This change facilitates the smooth conduction of the reduction reaction. However, considering the limitations of theoretical combustion temperature and economic benefits, the optimal carbon consumption value must be calculated.
Figure 5b presents the variations in blast volume and theoretical combustion temperature. As coal injection increases, the oxygen input to the furnace must be enhanced, which raises the blast volume, while the theoretical combustion temperature shows a downward trend. For every 10 kg/THM increase in coal injection, the Tf decreases by approximately 16.28 °C. The boundary coal injection quantity must be calculated to keep the Tf within the normal range (2100 °C to 2250 °C). After computation, under these initial conditions, the coal injection quantity should fall within the 136.02 kg/THM to 228.14 kg/THM range.
3.3. Effect of CBM Injection on Pulverized Coal Injection and Oxygen Enrichment
The original working conditions are set as the initial baseline.
Figure 6 illustrates the impact of the CBM preheating temperature and injection volume on the coal ratio and the oxygen enrichment rate of the blast furnace air.
Firstly, as the injection volume of CBM increases, the coal ratio shows a linear downward trend. When the preheating temperature of CBM is 25 °C, for every increase of 10 m3/THM in CBM, the coal ratio decreases by approximately 8.08 kg/THM. Under these conditions, when the CBM injection volume reaches 120 m3/THM, the coal ratio decreases from 170.5 kg/THM to 73.27 kg/THM, while the oxygen enrichment rate of the blast increases from 4.28% to 10.30%. This phenomenon occurs because when room-temperature CBM is injected into the blast furnace, it must be heated to the combustion temperature at the tuyere–raceway zone, requiring additional heat from pulverized coal combustion, thereby increasing the oxygen enrichment rate of the blast. Additionally, injecting cold gas into the furnace reduces the coal ratio. It induces internal pressure changes in equipment like gas guns due to the alternation of hot and cold, significantly impacting the service life of the equipment.
In summary, to further enhance the reduction potential of CBM, the CBM was preheated to temperatures of 400 °C, 800 °C, and 1200 °C before injection into the blast furnace. Through model calculations, the effects of CBM injection volume at different preheating temperatures on the coal ratio and oxygen enrichment rate were obtained, as shown in
Figure 6. When the CBM injection rate is relatively low, the physical heat brought in by the CBM is minimal, and changing the preheating temperature does not significantly affect the reduction in the coal ratio. Compared to the room-temperature condition, when the preheating temperature is 1200 °C and the CBM injection volume is 10 m
3/THM, the coal ratio decreases by 0.421 kg/THM.
However, as the CBM injection volume increases, preheating the CBM significantly reduces the coal ratio in the blast furnace. When the CBM injection volume reaches 120 m3/THM, the coal ratio under room temperature decreases to 74.51 kg/THM. In contrast, at a preheating temperature of 1200 °C, it drops to 22.9 kg/THM, a reduction of approximately 69.26%.
As the preheating temperature of coalbed methane increases, the reduction in the coal ratio becomes more pronounced. Therefore, to maintain the energy and mass balance of the blast furnace system, it is necessary to supply additional physical heat through the blast, requiring an increase in blast volume. Since the blast temperature is fixed, the oxygen enrichment rate must be reduced to accommodate the increased blast volume. When 120 m3/THM of coalbed methane is injected into the blast furnace at room temperature and a preheating temperature of 1200 °C, the oxygen enrichment rate of the blast decreases from 10.30% to 6.81%. As the preheating temperature of coal mine gas inside the blast furnace increases, the pulverized coal injection rate decreases significantly. To maintain the energy and mass balance of the entire blast furnace system, it becomes necessary to increase the blast volume to supply additional thermal energy. Since the blast temperature is fixed, and elemental balance must be preserved during operation, the oxygen enrichment rate must be reduced to accommodate the higher blast volume. This explains the observed trend in the curve.
3.4. Effect of CBM Injection on the Raceway Adiabatic Flame Temperature
Previous studies have established that injecting CBM into the furnace to reduce the coal ratio is an effective carbon reduction technology. However, with the introduction of a significant amount of hydrogen elements, the temperature within the furnace may decrease [
10]. Reducing the coal ratio is crucial to ensuring the stable operation of the blast furnace.
A key parameter is required to assess the blast furnace’s thermal variations and operational performance. Since the majority of the heat needed in the metallurgical production of the blast furnace is supplied by the combustion of fuel at the tuyere–raceway zone, the variation in Raceway Adiabatic Flame Temperature (RAFT) serves as an important indicator of the thermal state of the furnace. The RAFT is a critical parameter for evaluating blast furnace operation. As blast furnace smelting calculations have evolved, numerous scholars have refined heat balance models, improving their accuracy. The adiabatic flame temperature calculation model is presented as Equation (17).
Furthermore, CBM is a biofuel with a relatively high hydrogen content. To maintain the thermal equilibrium of the blast furnace, the RAFT should be controlled to remain above 1900 °C [
21], and the T_top should be kept above 130 °C.
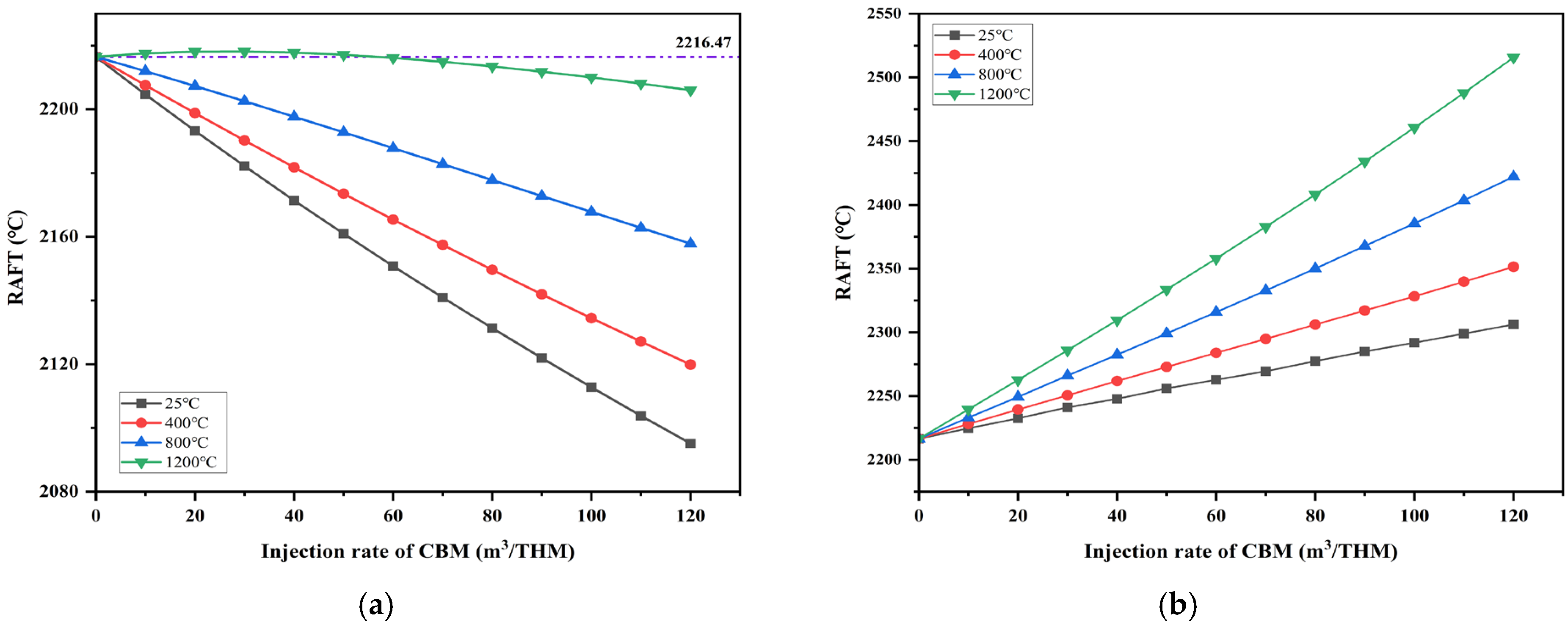
Figure 7a,b show the effects of injecting CBM at different preheating temperatures on the RAFT, with the original smelting parameters unchanged and the coal ratio varying, as shown in
Figure 6. The initial RAFT is set at 2216.47 °C, the initial T_top is set at 160 °C, the oxygen enrichment rate is 6%, and the injection temperatures of CBM are set at 25 °C, 400 °C, 800 °C, and 1200 °C, respectively.
When CBM is injected into the system, the RAFT exhibits a downward trend. This occurs because CBM, initially at room temperature, must absorb heat to reach the furnace belly temperature after entering the system. Additionally, methane pyrolysis at high temperatures is an endothermic reaction, as shown in Equation (16).
However, preheating the CBM can effectively alleviate this issue. Initially, when CBM is injected into the furnace at room temperature, and the injection volume reaches 120 m3/THM, the RAFT decreases from 2216.47 °C to 2095.11 °C, a reduction of 5.48%. According to the iterative model, when the CBM injection rate increases to 425 m3/THM, the RAFT further drops to 1902.74 °C. Due to the limitations mentioned earlier, the CBM injection volume is limited. Exceeding this volume could lead to cooling issues in the furnace and disrupt its operation. To further enhance the RAFT and ensure the furnace’s stability and efficiency, CBM was preheated to 400 °C, 800 °C, and 1200 °C.
When the CBM injection volume reaches 120 m3/THM after preheating to 400 °C, the RAFT decreases from 2216.47 °C to 2119.86 °C, a reduction of 4.36%. This is because, at the hot air temperature, CBM enters the furnace without needing to absorb heat from the environment to reach the reaction temperature. Additionally, preheating provides the necessary heat for the decomposition of CBM. This effect can be observed in the furnace’s top temperature variation curve.
However, the RAFT slightly increases when CBM is preheated to the hot air temperature (i.e., 1200 °C), and the injection rate is between 0 and 59.81 m3/THM. In this case, the physical heat from the preheated CBM and the heat released by combustion help compensate for the system’s heat deficit.
As shown in
Figure 7b, when the coal and CBM substitution ratio from
Figure 6 is applied to the model calculations, an increasing CBM injection rate leads to a corresponding increase in the oxygen enrichment of the blast air. Under these conditions, RAFT’s downward trend halts and rises with the increasing CBM injection rate. Similarly, the RAFT increases with the CBM injection rate, with preheating further amplifying this increase. Initially, when CBM enters the furnace at ambient temperature, an injection rate of 120 m
3/THM results in the RAFT rising to 2306.16 °C, a 4.05% increase compared to the previous year. When CBM is preheated to 400 °C and the injection rate reaches 120 m
3/THM, the RAFT increases to 2351.46 °C, marking a 6.09% year-on-year increase.
In conclusion, preheating CBM before its injection into the blast furnace significantly improves its stability and efficiency. However, preheating incurs certain economic costs, and from an economic standpoint, the optimal preheating temperature for CBM should be further investigated.
3.5. Effect of CBM Usage on Carbon Dioxide Emissions
According to previous research findings, substituting pulverized coal with CBM has become an effective process for carbon reduction in metallurgical operations. Throughout the smelting process, each parameter contributes to CO
2 emissions. Researchers proposed a CO
2 emission calculation method that aligns with modern production conditions, incorporating a set of measured emission factors to ensure calculation accuracy [
22].
The CO
2 emissions from the blast furnace process are calculated as the CO
2 equivalent at the input end minus the carbon emission allowance discount at the output end. The calculation results under baseline conditions are presented in
Table 11.
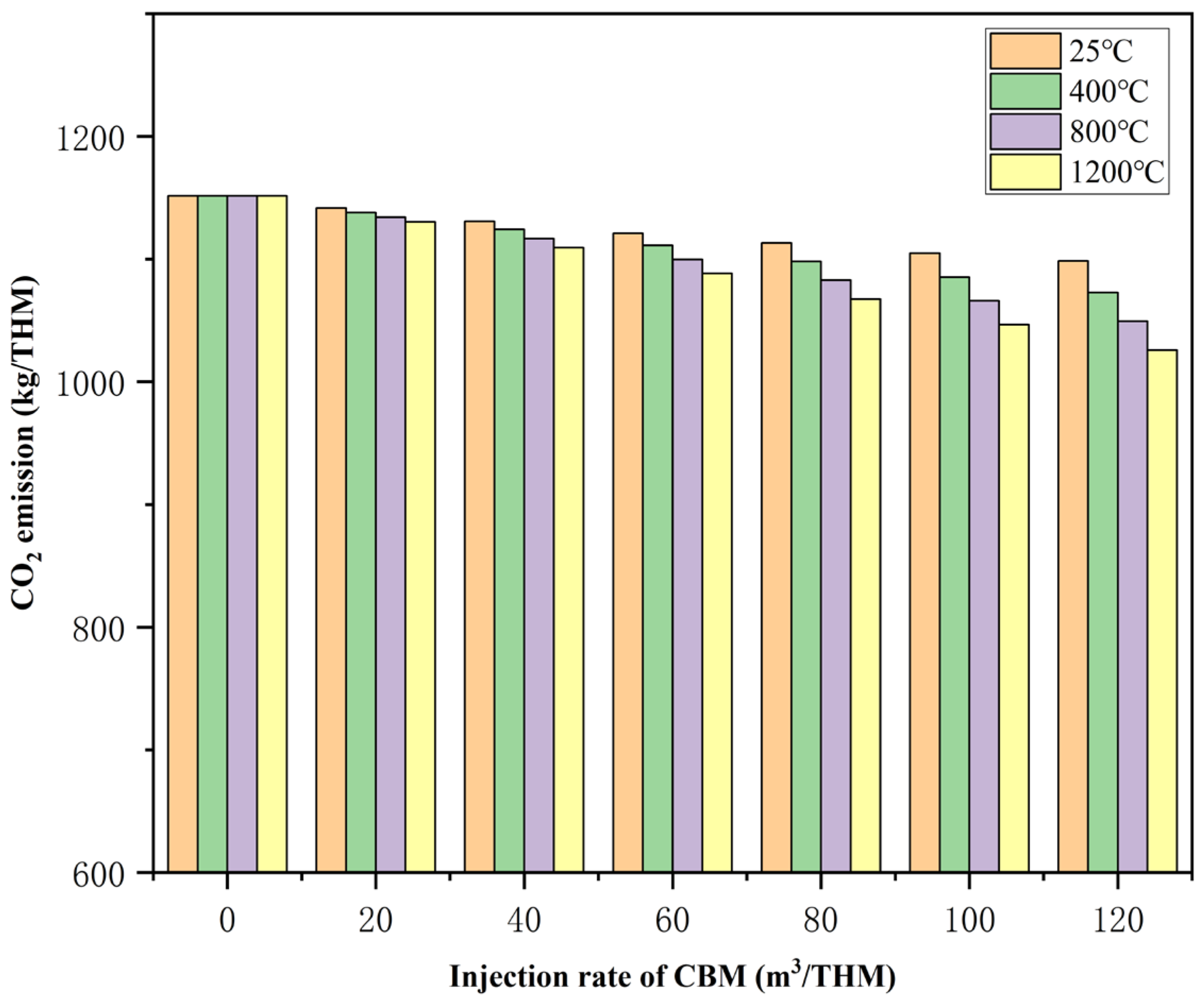
Through model calculations and analysis, we examined the impact of CBM injection volume and preheating temperature on smelting process CO
2 emissions, as shown in
Figure 8. As room-temperature CBM increases, CO
2 emissions decrease. For every 20 m
3/THM increase in the CBM injection volume, CO
2 emissions decrease by approximately 8.85 m
3/THM. As both the preheating temperature and injection volume of CBM increase, the carbon reduction potential of the process rises significantly. Compared to baseline conditions, when the CBM injection volume reaches 120 m
3/THM at room temperature and a preheating temperature of 1200 °C, CO
2 emissions are 1098.40 m
3/THM and 1025.91 m
3/THM, respectively, representing reductions of approximately 4.61% and 10.91%.
In conclusion, utilizing CBM to replace a portion of pulverized coal and preheating the injected CBM is a highly effective carbon reduction strategy. This approach offers valuable insights for introducing hydrogen-rich blast furnaces and developing hydrogen-to-coal replacement technologies.