Integrated In Silico and In Vitro Assessment of the Anticancer Potential of Origanum vulgare L. Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. OEO Extraction and GC–MS Analysis
2.2. Determination of OEO DPPH Scavenging Activity
2.3. ABTS Radical Scavenging Assay
2.4. Determination of the Total Phenolic Content
2.5. Cell Culture
2.6. Cell Viability Assessment
2.7. Molecular Docking
2.8. In Silico ADMET Predictions for the OEO Components
| Protein (PDB ID) | Grid Box Center Coordinates | Grid Box Size | Chain/ Domain Type | Residue Range (Sequence Length) | Native Ligand Name/Description (PDB ID) | Ref. |
|---|---|---|---|---|---|---|
| PDPK1 (2PE1) | x = −7.0111 y = 44.0378 z = 43.8068 | x = 14.4075 y = 14.4075 z = 14.4075 | A/Kinase domain | 82–360 (279 aa) | BX-517/Indolinone-based ATP-competitive inhibitor (517) | [43] |
| mTOR (4JSX) | x = 51.6285 y = −2.0404 z = −48.8188 | x = 18.7676 y = 16.3518 z = 18.7676 | A/Kinase domain (ΔN-mTOR) | 2015–2549 (535 aa) | Torin2/ATP-site inhibitor (17G) | [44] |
| MEK1 (3DV3) | x = 37.7734 y = −13.7113 z = −1.1927 | x = 17.4625 y = 14.0291 z = 12.8563 | A/Kinase domain | 59–364 (306 aa) | PF-04622664/MEK inhibitor (MEK) | [45] |
| AKT/PKB (4GV1) | x = −20.8519 y = 3.6032 z = 10.7428 | x = 15.2397 y = 11.6572 z = 14.4921 | A/Kinase domain | 144–480 (337 aa) | AZD5363/ATP-competitive inhibitor (0XZ) | [46] |
| PI3Kα (6GVF) | x = −16.9238 y = 148.1857 z = 29.3169 | x = 14.5772 y = 21.3565 z = 12.8758 | A/Catalytic subunit (p110α) | 107–1051 (945 aa) | 3-(2-Amino-benzooxazol-5-yl)-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamine (FE5) | [47] |
| PI3Kγ (4FA6) | x = 45.4506 y = 14.5595 z = 30.3998 | x = 20.2093 y = 12.4970 z = 16.1645 | A/Catalytic subunit | 144–1102 (959 aa) | PI3Kalpha/mTOR-IN1/Pyrrolidinyl pyrido pyrimidinone derivative (0TA) | [48] |
| BCL-2 (4LVT) | x = 6.3606 y = −2.7407 z = −7.7449 | x = 18.7676 y = 27.4958 z = 15.6622 | A/BH3-binding domain | 1–34, 92–207 (150 aa) | Navitoclax/ABT-263, BH3-mimetic inhibitor (1XJ) | [49] |
| BCL-XL (2YXJ) | x = −7.9224 y = −16.8631 z = 10.5365 | x = 17.4625 y = 28.5719 z = 12.8563 | A/BH3-binding domain | 1–209 (209 aa) | ABT-737/BH3-mimetic inhibitor (N3C) | [50] |
| EGFR1 (1XKK) | x = 18.7130 y = 33.5455 z = 36.7358 | x = 19.8569 y = 19.8569 z = 19.8569 | A/Kinase domain | 695–1022 (328 aa) | Lapatinib/GW572016, dual EGFR/ErbB2 inhibitor (FMM) | [51] |
| VEGFR2 (4ASD) | x = −25.0080 y = −0.1288 z = −10.9439 | x = 20.2093 y = 14.4098 z = 16.1645 | A/Kinase domain | 787–1171 (385 aa) | Sorafenib/BAY 43-9006, ATP-competitive inhibitor (BAX) | [52] |
2.9. Lipid Peroxidation Assay
2.10. Caspase-3/7 Assay
2.11. High-Resolution Respirometry
2.12. Statistical Analysis
3. Results
3.1. Chemical Composition of OEO
3.2. Determination of the Antioxidant Activity of OEO
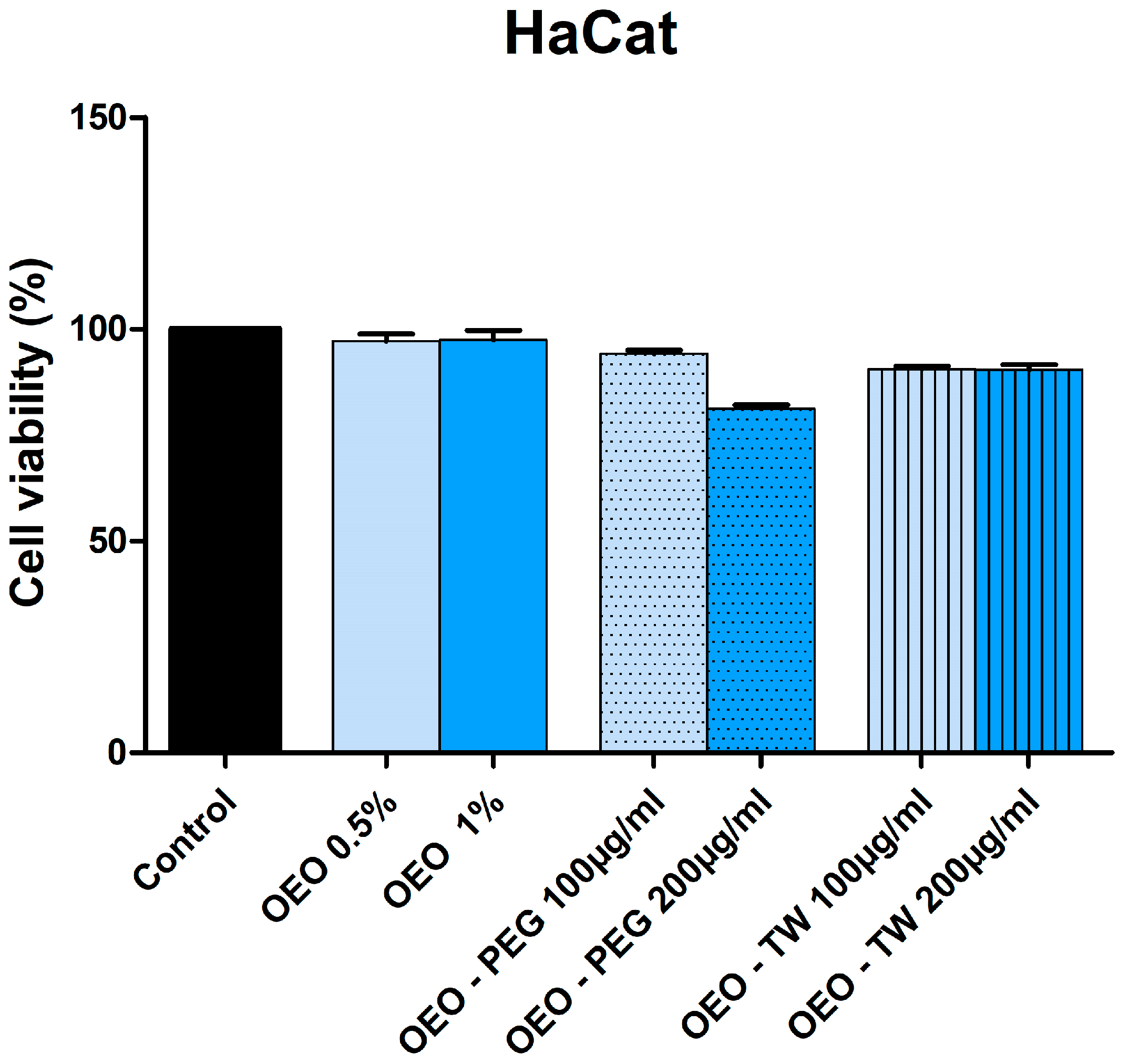
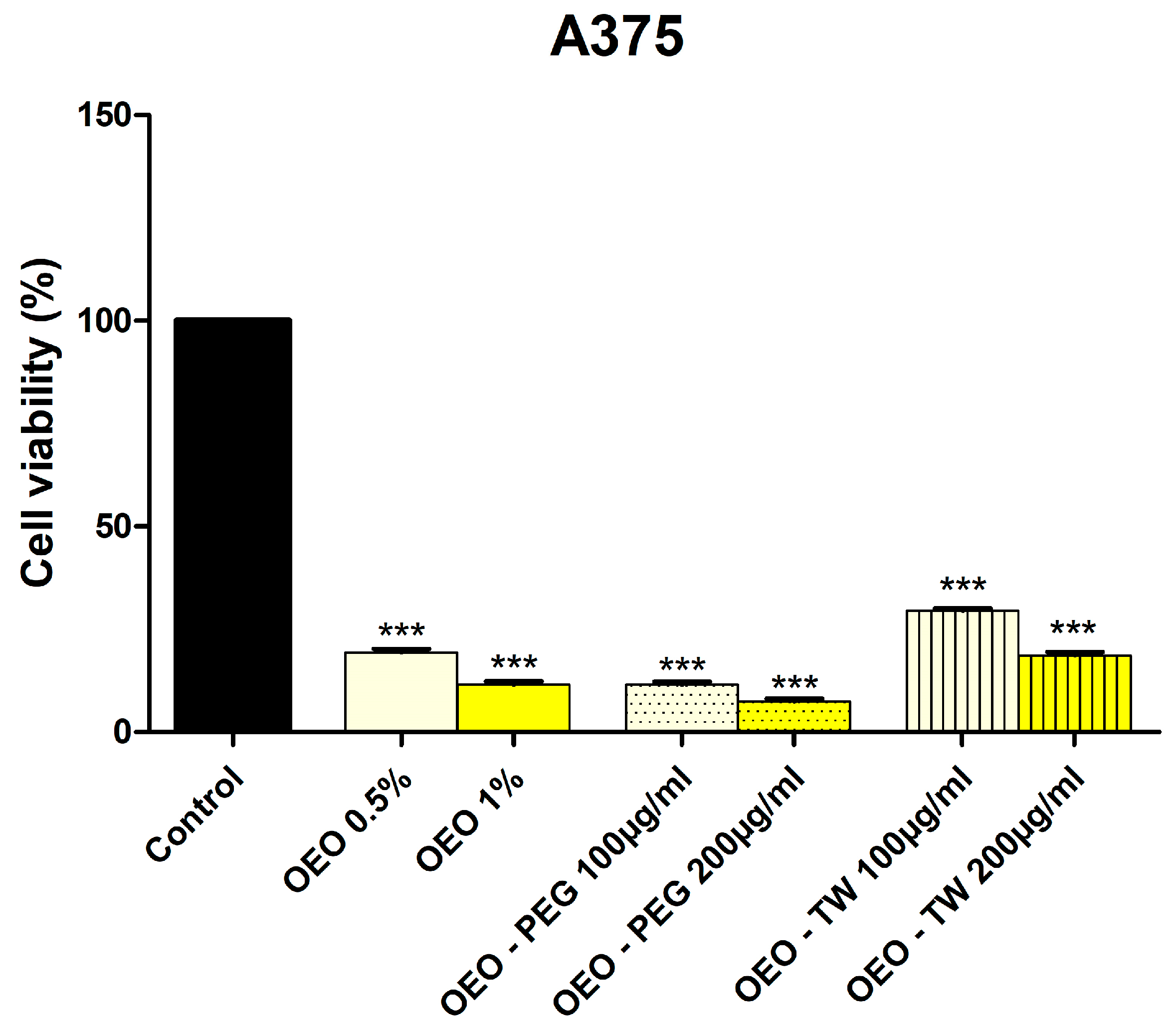
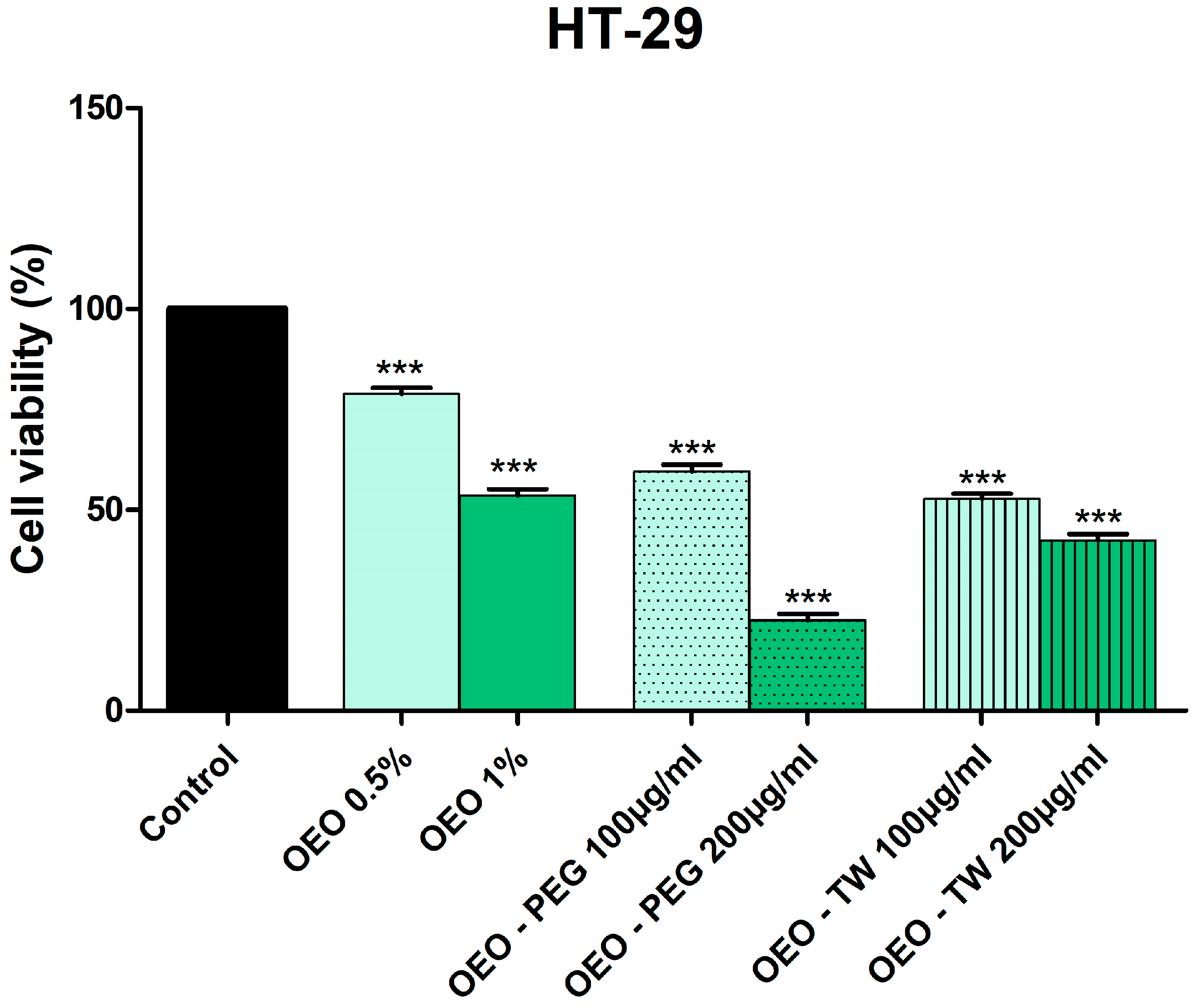
3.3. Evaluation of the Cytotoxic Effect of OEO
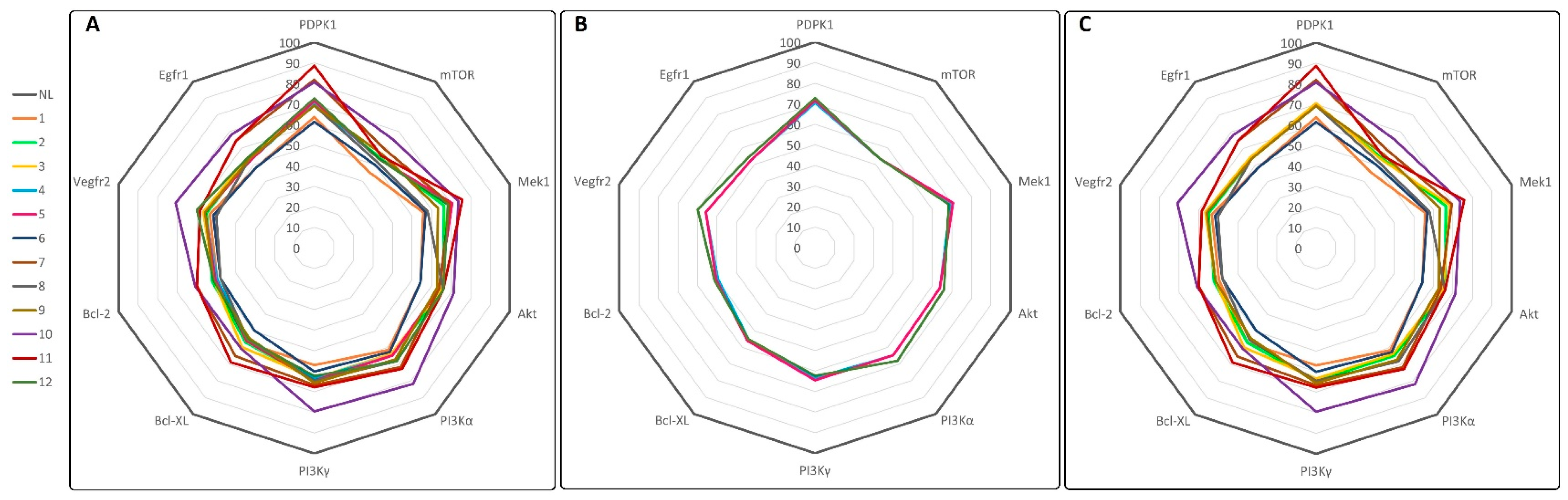
3.4. Molecular Docking and ADMET Prediction
3.5. OEO Decreases Lipid Peroxidation
3.6. OEO Increases Caspase 3/7 Activation
3.7. Evaluation of the Effect of OEO on Mitochondrial Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Global Cancer Facts & Figures, 5th ed.; American Cancer Society: Atlanta, GA, USA, 2024. [Google Scholar]
- Gogulescu, A.; Blidisel, A.; Soica, C.; Mioc, A.; Voicu, A.; Jojic, A.; Voicu, M.; Banciu, C. Neurological Side Effects of TNF-α Inhibitors Revisited: A Review of Case Reports. Medicina 2024, 60, 1409. [Google Scholar] [CrossRef]
- Chizenga, E.P.; Abrahamse, H. Biological Therapy with Complementary and Alternative Medicine in Innocuous Integrative Oncology: A Case of Cervical Cancer. Pharmaceutics 2021, 13, 626. [Google Scholar] [CrossRef]
- Jojic, A.A. Juniperi galbulus: Screening of phytochemicals and bioactivity investigation of the aqueous extract. Farmacia 2024, 72, 934–945. [Google Scholar] [CrossRef]
- Shaik, B.B.; Katari, N.K.; Jonnalagadda, S.B. Role of Natural Products in Developing Novel Anticancer Agents: A Perspective. Chem. Biodivers. 2022, 19, e202200535. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Samani, M.; Kaffash Farkhad, N.; Reza Mahmoudian-Sani, M.; Shirzad, H. Antioxidants as a Double-Edged Sword in the Treatment of Cancer. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar]
- Tietbohl, L.A.C.; Oliveira, A.P.; Esteves, R.S.; Albuquerque, R.D.D.G.; Folly, D.; Machado, F.P.; Correa, A.L.; Santos, M.G.; Ruiz, A.L.G.; Rocha, L. Antiproliferative activity in tumor cell lines, antioxidant capacity and total phenolic, flavonoid and tannin contents of Myrciaria floribunda. An. Acad. Bras. Cienc. 2017, 89, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Meireles, D.R.; Fernandes, H.M.; Rolim, T.L.; Batista, T.M.; Mangueira, V.M.; de Sousa, T.K.; Pita, J.C.; Xavier, A.L.; Beltrão, D.M.; Tavares, J.F.; et al. Toxicity and antitumor efficacy of Croton polyandrus oil against Ehrlich ascites carcinoma cells. Rev. Bras. Farmacogn. 2016, 26, 751–758. [Google Scholar] [CrossRef]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Mohamed, J.; Ainane, T. Exploring the Potent Anticancer Activity of Essential Oils and Their Bioactive Compounds: Mechanisms and Prospects for Future Cancer Therapy. Pharmaceuticals 2023, 16, 1086. [Google Scholar] [CrossRef]
- Pfeffer, C.; Singh, A. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Yu, J.; Zhong, B.; Xiao, Q.; Du, L.; Hou, Y.; Sun, H.-S.; Lu, J.-J.; Chen, X. Induction of programmed necrosis: A novel anti-cancer strategy for natural compounds. Pharmacol. Ther. 2020, 214, 107593. [Google Scholar] [CrossRef]
- Fu, W.; Chen, J.; Cai, Y.; Lei, Y.; Chen, L.; Pei, L.; Zhou, D.; Liang, X.; Ruan, J. Antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective potential of the extract from Parathelypteris nipponica (Franch. et Sav.) Ching. J. Ethnopharmacol. 2010, 130, 521–528. [Google Scholar] [CrossRef]
- Luk, S.C.-W.; Siu, S.W.-F.; Lai, C.-K.; Wu, Y.-J.; Pang, S.-F. Cell Cycle Arrest by a Natural Product via G2/M Checkpoint. Int. J. Med. Sci. 2005, 2, 64–69. [Google Scholar] [CrossRef]
- Mohamed, F.; Essam, A. Regulation of Apoptosis, Invasion and Angiogenesis of Tumor Cells by Caffeic Acid Phenethyl Ester. In Carcinogenesis; InTech: Nappanee, IN, USA, 2013. [Google Scholar]
- Albonici, L.; Giganti, M.G.; Modesti, A.; Manzari, V.; Bei, R. Multifaceted Role of the Placental Growth Factor (PlGF) in the Antitumor Immune Response and Cancer Progression. Int. J. Mol. Sci. 2019, 20, 2970. [Google Scholar] [CrossRef] [PubMed]
- Morshedloo, M.R.; Craker, L.E.; Salami, A.; Nazeri, V.; Sang, H.; Maggi, F. Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono- and sesquiterpene synthesis in two oregano (Origanum vulgare L.) subspecies. Plant Physiol. Biochem. 2017, 111, 119–128. [Google Scholar] [CrossRef]
- Guo, X.; Hao, Y.; Zhang, W.; Xia, F.; Bai, H.; Li, H.; Shi, L. Comparison of Origanum Essential Oil Chemical Compounds and Their Antibacterial Activity against Cronobacter sakazakii. Molecules 2022, 27, 6702. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutiérrez-Grijalva, E.; Vazquez-Olivo, G.; Heredia, J. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Lupea-Chilom, D.-S.; Solovan, C.S.; Farcas, S.S.; Gogulescu, A.; Andreescu, N.I. Latent Tuberculosis in Psoriasis Patients on Biologic Therapies: Real-World Data from a Care Center in Romania. Medicina 2023, 59, 1015. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Mitić, K.V.; Nešić, M.; Stanković, M.; Petrović, V.; Baralić, M.; Randjelović, P.J.; Sokolović, D.; Radulović, N. Oregano (Origanum vulgare) Essential Oil and Its Constituents Prevent Rat Kidney Tissue Injury and Inflammation Induced by a High Dose of L-Arginine. Int. J. Mol. Sci. 2024, 25, 941. [Google Scholar] [CrossRef] [PubMed]
- Kosakowska, O.; Węglarz, Z.; Pióro-Jabrucka, E.; Przybył, J.L.; Kraśniewska, K.; Gniewosz, M.; Bączek, K. Antioxidant and Antibacterial Activity of Essential Oils and Hydroethanolic Extracts of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare). Molecules 2021, 26, 988. [Google Scholar] [CrossRef]
- Erenler, R. Antiproliferative activity and cytotoxic effect of essential oil and water extract from origanum Vulgare L. Sigma J. Eng. Nat. Sci.–Sigma Mühendislik Ve Fen Bilim. Derg. 2023, 41, 202–206. [Google Scholar] [CrossRef]
- Elshafie, H.; Armentano, M.; Carmosino, M.; Bufo, S.; De Feo, V.; Camele, I. Cytotoxic Activity of Origanum Vulgare L. on Hepatocellular Carcinoma cell Line HepG2 and Evaluation of its Biological Activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Li, L.; Lu, J.X.; Ma, S.X. Anti-tumor activity of Origanum oil in vitro. Chin. J. Microecol. 2010, 22, 1101–1102. [Google Scholar]
- Spyridopoulou, K.; Fitsiou, E.; Bouloukosta, E.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Oreopoulou, A.; Papavassilopoulou, E.; Pappa, A.; Chlichlia, K. Extraction, Chemical Composition, and Anticancer Potential of Origanum onites L. Essential Oil. Molecules 2019, 24, 2612. [Google Scholar] [CrossRef] [PubMed]
- Kebriti, I.; Solgi, M.; Velashjerdi, M. Improving quality of strawberry by novel essential oil nanoemulsions of Echinophora platyloba combined with Aloe vera gel and gum arabic. Sci. Rep. 2025, 15, 1731. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Laigle, M.; Michel, T.; Palmeri, V. Essential oil-based nano-emulsions: Effect of different surfactants, sonication and plant species on physicochemical characteristics. Ind. Crops Prod. 2020, 157, 112935. [Google Scholar] [CrossRef]
- Khairan, K.; Marfu’ah, M.; Idroes, R.; Sriwati, R.; Diah, M. An overview of essential oil-based nanoemulsion and their biological activities against some microbial pathogenic. IOP Conf. Ser. Earth Environ. Sci. 2024, 1297, 012083. [Google Scholar] [CrossRef]
- Craveiro, A.A. Oleos essenciais de plantas do Nordeste. In Coleção Ciência; Edição UFC: Fortaleza, Brazil, 1981; p. 209. [Google Scholar]
- Munteanu, A.; Gogulescu, A.; Șoica, C.; Mioc, A.; Mioc, M.; Milan, A.; Lukinich-Gruia, A.T.; Pricop, M.-A.; Jianu, C.; Banciu, C.; et al. In Vitro and In Silico Evaluation of Syzygium aromaticum Essential Oil: Effects on Mitochondrial Function and Cytotoxic Potential Against Cancer Cells. Plants 2024, 13, 3443. [Google Scholar] [CrossRef]
- Jianu, C.; Rusu, L.-C.; Muntean, I.; Cocan, I.; Lukinich-Gruia, A.T.; Goleț, I.; Horhat, D.; Mioc, M.; Mioc, A.; Șoica, C.; et al. In Vitro and In Silico Evaluation of the Antimicrobial and Antioxidant Potential of Thymus pulegioides Essential Oil. Antioxidants 2022, 11, 2472. [Google Scholar] [CrossRef]
- Pricop, M.-A.; Lukinich-Gruia, A.T.; Cristea, I.-M.; Păunescu, V.; Tatu, C.A. Aristolochia clematitis L. Ethanolic Extracts: In Vitro Evaluation of Antioxidant Activity and Cytotoxicity on Caco-2 Cell Line. Plants 2024, 13, 2987. [Google Scholar] [CrossRef]
- Jianu, C.; Goleț, I.; Stoin, D.; Cocan, I.; Lukinich-Gruia, A. Antioxidant Activity of Pastinaca sativa L. ssp. sylvestris [Mill.] Rouy and Camus Essential Oil. Molecules 2020, 25, 869. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.; Mioc, M.; Mioc, A.; Gogulescu, A.; Mardale, G.; Avram, Ș.; Maksimović, T.; Mara, B.; Șoica, C. Cytotoxic Potential of Betulinic Acid Fatty Esters and Their Liposomal Formulations: Targeting Breast, Colon, and Lung Cancer Cell Lines. Molecules 2024, 29, 3399. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef]
- Eskandani, M.; Hamishehkar, H.; Ezzati Nazhad Dolatabadi, J. Cyto/Genotoxicity study of polyoxyethylene (20) sorbitan monolaurate (tween 20). DNA Cell Biol. 2013, 32, 498–503. [Google Scholar] [CrossRef]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar]
- Toxicity Prediction—PreADMET. Available online: https://preadmet.webservice.bmdrc.org/toxicity-prediction (accessed on 16 May 2025).
- Islam, I.; Bryant, J.; Chou, Y.-L.; Kochanny, M.J.; Lee, W.; Phillips, G.B.; Yu, H.; Adler, M.; Whitlow, M.; Ho, E.; et al. Indolinone based phosphoinositide-dependent kinase-1 (PDK1) inhibitors. Part 1: Design, synthesis and biological activity. Bioorg. Med. Chem. Lett. 2007, 17, 3814–3818. [Google Scholar] [CrossRef]
- Yang, H.; Rudge, D.G.; Koos, J.D.; Vaidialingam, B.; Yang, H.J.; Pavletich, N.P. mTOR kinase structure, mechanism and regulation. Nature 2013, 497, 217–223. [Google Scholar] [CrossRef]
- Tecle, H.; Shao, J.; Li, Y.; Kothe, M.; Kazmirski, S.; Penzotti, J.; Ding, Y.-H.; Ohren, J.; Moshinsky, D.; Coli, R.; et al. Beyond the MEK-pocket: Can current MEK kinase inhibitors be utilized to synthesize novel type III NCKIs? Does the MEK-pocket exist in kinases other than MEK? Bioorg. Med. Chem. Lett. 2009, 19, 226–229. [Google Scholar] [CrossRef]
- Addie, M.; Ballard, P.; Buttar, D.; Crafter, C.; Currie, G.; Davies, B.R.; Debreczeni, J.; Dry, H.; Dudley, P.; Greenwood, R.; et al. Discovery of 4-Amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide (AZD5363), an Orally Bioavailable, Potent Inhibitor of Akt Kinases. J. Med. Chem. 2013, 56, 2059–2073. [Google Scholar] [CrossRef] [PubMed]
- Ouvry, G.; Clary, L.; Tomas, L.; Aurelly, M.; Bonnary, L.; Borde, E.; Bouix-Peter, C.; Chantalat, L.; Defoin-Platel, C.; Deret, S.; et al. Impact of Minor Structural Modifications on Properties of a Series of mTOR Inhibitors. ACS Med. Chem. Lett. 2019, 10, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Le, P.T.; Cheng, H.; Ninkovic, S.; Plewe, M.; Huang, X.; Wang, H.; Bagrodia, S.; Sun, S.; Knighton, D.R.; LaFleur Rogers, C.M.; et al. Design and synthesis of a novel pyrrolidinyl pyrido pyrimidinone derivative as a potent inhibitor of PI3Kα and mTOR. Bioorg. Med. Chem. Lett. 2012, 22, 5098–5103. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Lee, E.F.; Czabotar, P.E.; Smith, B.J.; Deshayes, K.; Zobel, K.; Colman, P.M.; Fairlie, W.D. Crystal structure of ABT-737 complexed with Bcl-xL: Implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 2007, 14, 1711–1713. [Google Scholar] [CrossRef]
- Wood, E.R.; Truesdale, A.T.; McDonald, O.B.; Yuan, D.; Hassell, A.; Dickerson, S.H.; Ellis, B.; Pennisi, C.; Horne, E.; Lackey, K.; et al. A Unique Structure for Epidermal Growth Factor Receptor Bound to GW572016 (Lapatinib). Cancer Res. 2004, 64, 6652–6659. [Google Scholar] [CrossRef]
- McTigue, M.; Murray, B.W.; Chen, J.H.; Deng, Y.-L.; Solowiej, J.; Kania, R.S. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 18281–18289. [Google Scholar] [CrossRef]
- Abcam, C. (UK): Lipid Peroxidation (MDA) Assay Kit-ab118970. Available online: https://www.abcam.com/en-us/products/assay-kits/lipid-peroxidation-mda-assay-kit-colorimetric-fluorometric-ab118970?srsltid=AfmBOooSt5XYd52jEjk-dFQUE8Quw7ATT6_poQMSo-KMw40gzgHQJKne (accessed on 13 January 2025).
- Scientific, T. CellEventTM Caspase-3/7 Detection Reagents. Available online: https://www.thermofisher.com/order/catalog/product/C10423 (accessed on 27 January 2025).
- Petrus, A.; Ratiu, C.; Noveanu, L.; Lighezan, R.; Rosca, M.; Muntean, D.; Duicu, O. Assessment of Mitochondrial Respiration in Human Platelets. Rev. Chim. 2017, 68, 768–771. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer activity of essential oils: A review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef] [PubMed]
- Cinbilgel, I.; Kurt, Y. Oregano and/or marjoram: Traditional oil production and ethnomedical utilization of Origanum species in southern Turkey. J. Herb. Med. 2019, 16, 100257. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Bora, L.; Burkard, T.; Juan, M.H.S.; Radeke, H.H.; Muț, A.M.; Vlaia, L.L.; Magyari-Pavel, I.Z.; Diaconeasa, Z.; Socaci, S.; Borcan, F.; et al. Phytochemical Characterization and Biological Evaluation of Origanum vulgare L. Essential Oil Formulated as Polymeric Micelles Drug Delivery Systems. Pharmaceutics 2022, 14, 2413. [Google Scholar] [CrossRef]
- Bora, L.; Avram, S.; Pavel, I.; Muntean, D.; Liga, S.; Buda, V.; Gurgus, D.; Danciu, C. An up-to-date review regarding cutaneous benefits of Origanum vulgare L. essential oil. Antibiotics 2022, 11, 549. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 1, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Mutu, A.; Budeanu, O.; Martea, R.; Clapco, S.; Gille, E. RAPD molecular marker study of the intraspecific variability of Origanum vulgare subsp. vulgare naturally occurring in Moldova. Rev. Bot. 2014, 1, 23–28. [Google Scholar]
- Nurzyńska-Wierdak, R.E.; Bogucka-Kocka, A.; Sowa, I.; Szymczak, G. The composition of essential oil from three ecotypes of Origanum vulgare L. ssp. vulgare cultivated in Poland. Farmacia 2012, 1, 571–577. [Google Scholar]
- Port, A.; Mutu, A.; Duca, M.; Gille, E. Analiza biochimică a plantelor de Origanum vulgare ssp. vulgare din flora spontană a Republicii Moldova. Bul. AŞM. Ştiinţele Vieţii. 2020, 340, 95–105. [Google Scholar]
- Gales, R.; Preotu, A.; Necula, R.; Gille, E.; Toma, C. Altitudinal variations of morphology, distribution and secretion of glandular hairs in Origanum vulgare L. leaves. Stud. Univ. Vasile Goldis. Ser. Stiint. Vietii. 2010, 1, 59–62. [Google Scholar]
- Tămaș, M.; Balica, G.; Ştefănescu, C. Research on some plant species contining essential oils performed at University of Medicine and Pharmacy „Iuliu Hațieganu” Cluj-Napoca. Contrib. Bot. 2020, 55, 141–151. [Google Scholar] [CrossRef]
- Tămaș, M.; Oprean, I. Precizări asupra compoziției chimice a uleiului volatil de Origanum vulgare L. Rev. Med. Chir. Soc. Med. Nat. 2003, 107, 31–34. [Google Scholar]
- Shafiee-Hajiabad, M.; Hardt, M.; Honermeier, B. Comparative investigation about the trichome morphology of Common oregano (Origanum vulgare L. subsp. vulgare) and Greek oregano (Origanum vulgare L. subsp. hirtum). J. Appl. Res. Med. Aromat. Plants 2014, 1, 50–58. [Google Scholar] [CrossRef]
- Raclariu-Manolică, A.C.; Anmarkrud, J.; Kierczak, M.; Rafati, N.; Thorbek, B.; Schrøder-Nielsen, A.; de Boer, H. DNA metabarcoding for quality control of basil, oregano, and paprika. Front. Plant Sci. 2021, 12, 665618. [Google Scholar] [CrossRef]
- Ke, W.-T.; Lin, S.-Y.; Ho, H.-O.; Sheu, M.-T. Physical characterizations of microemulsion systems using tocopheryl polyethylene glycol 1000 succinate (TPGS) as a surfactant for the oral delivery of protein drugs. J. Control. Release 2005, 102, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Taher, S.S.; Al-Kinani, K.K.; Hammoudi, Z.M.; Ghareeb, M. mohammed Co-surfactant effect of polyethylene glycol 400 on microemulsion using BCS class II model drug. J. Adv. Pharm. Educ. Res. 2022, 12, 63–69. [Google Scholar] [CrossRef]
- Krishna, G.; Thrimothi, D. Nano Emulsions: A Novel Targeted Delivery of Cancer Therapeutics. In Design and Applications of Self-Assembly Aggregates—From Micelles to Nanoemulsions; IntechOpen: London, UK, 2024. [Google Scholar]
- da Silva, C.C.; Giordani, C.; Picoli, T.; Guterres, K.A.; Perera, S.C.; Capella, G.A.; Waller, S.B.; Corcini, C.D.; Junior, A.S.V.; Freitag, R.A.; et al. Potencial antiproliferativo do óleo essencial de Origanum majorana Linn. em células de melanoma e seus efeitos intracelulares em células neoplásicas e não-neoplásicas. Res. Soc. Dev. 2021, 10, e7110917788. [Google Scholar] [CrossRef]
- Begnini, K.R.; Nedel, F.; Lund, R.G.; Carvalho, P.H.d.A.; Rodrigues, M.R.A.; Beira, F.T.A.; Del-Pino, F.A.B. Composition and Antiproliferative Effect of Essential Oil of Origanum vulgare Against Tumor Cell Lines. J. Med. Food 2014, 17, 1129–1133. [Google Scholar] [CrossRef]
- Kozics, K.; Mesárošová, M.; Šramková, M.; Bučková, M.; Puškárová, A.; Galová, D.; Pangallo, D. Evaluation of Bioactivity of Essential Oils: Cytotoxic/Genotoxic Effects on Colorectal Cancer Cell Lines, Antibacterial Activity, and Survival of Lactic Acid Bacteria. Molecules 2025, 30, 890. [Google Scholar] [CrossRef]
- Savini, I.; Arnone, R.; Catani, M.V.; Avigliano, L. Origanum Vulgare Induces Apoptosis in Human Colon Cancer Caco 2 Cells. Nutr. Cancer 2009, 61, 381–389. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Wang, Y.; Leng, Q.; Sun, Y.; Hoffman, R.M.; Jin, H. The Anti-oxidant Monoterpene p -Cymene Reduced the Occurrence of Colorectal Cancer in a Hyperlipidemia Rat Model by Reducing Oxidative Stress and Expression of Inflammatory Cytokines. Anticancer Res. 2021, 41, 1213–1218. [Google Scholar] [CrossRef]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- Acikgul, F.C.; Duran, N.; Kutlu, T.; Ay, E.; Tek, E.; Bayraktar, S. The therapeutic potential and molecular mechanism of Alpha-pinene, Gamma-terpinene, and P-cymene against melanoma cells. Heliyon 2024, 10, e36223. [Google Scholar] [CrossRef]
- Sampaio, L.A.; Pina, L.T.S.; Serafini, M.R.; Tavares, D.d.S.; Guimarães, A.G. Antitumor Effects of Carvacrol and Thymol: A Systematic Review. Front. Pharmacol. 2021, 12, 702487. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Bahuguna, A.; Paul, S.; Chul, S. Thymol Elicits HCT-116 Colorectal Carcinoma Cell Death Through Induction of Oxidative Stress. Anticancer. Agents Med. Chem. 2017, 17, 1942–1950. [Google Scholar] [CrossRef]
- Blažíčková, M.; Blaško, J.; Kubinec, R.; Kozics, K. Newly Synthesized Thymol Derivative and Its Effect on Colorectal Cancer Cells. Molecules 2022, 27, 2622. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Kubiliene, A.; Munius, E.; Songailaite, G.; Kokyte, I.; Baranauskaite, J.; Liekis, A.; Sadauskiene, I. A Comparative Evaluation of Antioxidant Activity of Extract and Essential Oil of Origanum onites L. In Vivo. Molecules 2023, 28, 5302. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Walasek-Janusz, M. Chemical Composition, Biological Activity, and Potential Uses of Oregano (Origanum vulgare L.) and Oregano Essential Oil. Pharmaceuticals 2025, 18, 267. [Google Scholar] [CrossRef]
- Shim, M.K.; Yoon, H.Y.; Lee, S.; Jo, M.K.; Park, J.; Kim, J.-H.; Jeong, S.Y.; Kwon, I.C.; Kim, K. Caspase-3/-7-Specific Metabolic Precursor for Bioorthogonal Tracking of Tumor Apoptosis. Sci. Rep. 2017, 7, 16635. [Google Scholar] [CrossRef] [PubMed]
- Gökhan, A. Evaluation of Cytotoxic, Membrane Damaging and Apoptotic Effects of Origanum majorana Essential Oil on Lung Cancer and Epidermoid Carcinoma Cells. Cyprus J. Med. Sci. 2022, 7, 201–206. [Google Scholar] [CrossRef]
- Arafat, K.; Sulaiman, S.; Al-Azawi, A.M.; Yasin, J.; Sugathan, S.; Nemmar, A.; Karam, S.; Attoub, S. Origanum majorana essential oil decreases lung tumor growth and metastasis in vitro and in vivo. Biomed. Pharmacother. 2022, 155, 113762. [Google Scholar] [CrossRef] [PubMed]
- Terada, H. The interaction of highly active uncouplers with mitochondria. Biochim. Biophys. Acta-Rev. Bioenerg. 1981, 639, 225–242. [Google Scholar] [CrossRef]
- Custodio, J.; Ribeiro, M.V.; Silva, F.; Rodrigues Machado, S.M.; Sousa, M. The essential oils component p-cymene induces proton leak through Fo-ATP synthase and uncoupling of mitochondrial respiration. J. Exp. Pharmacol. 2011, 3, 69–76. [Google Scholar] [CrossRef]
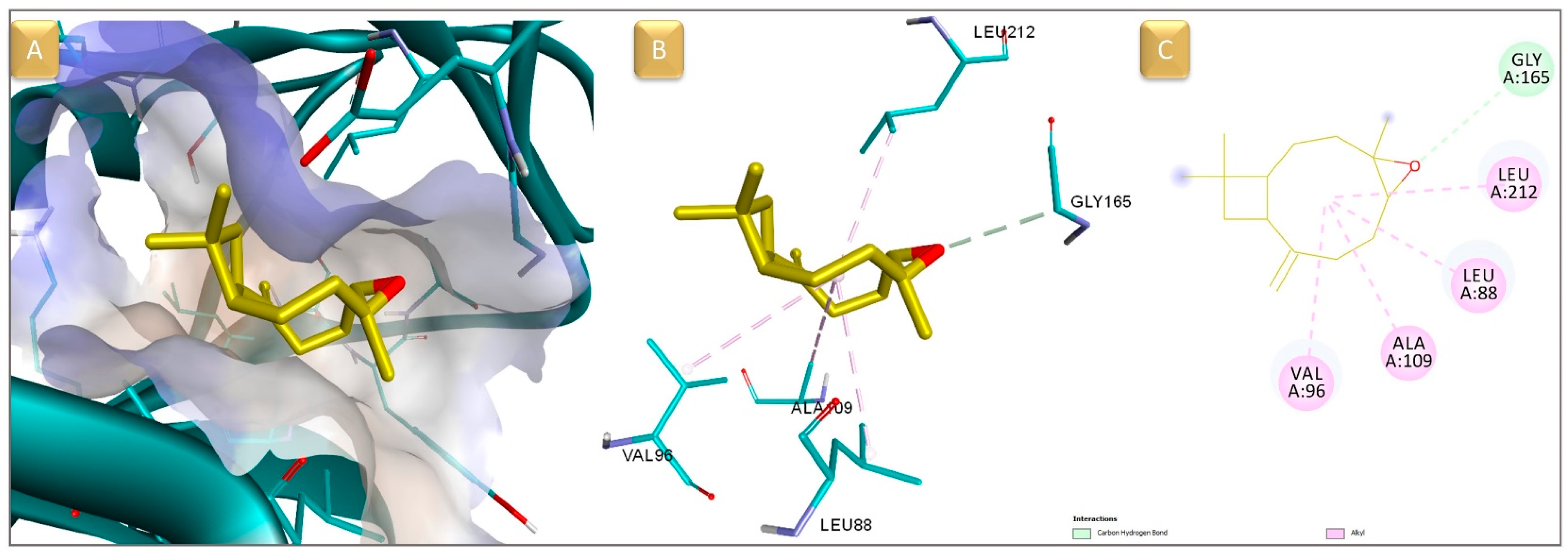
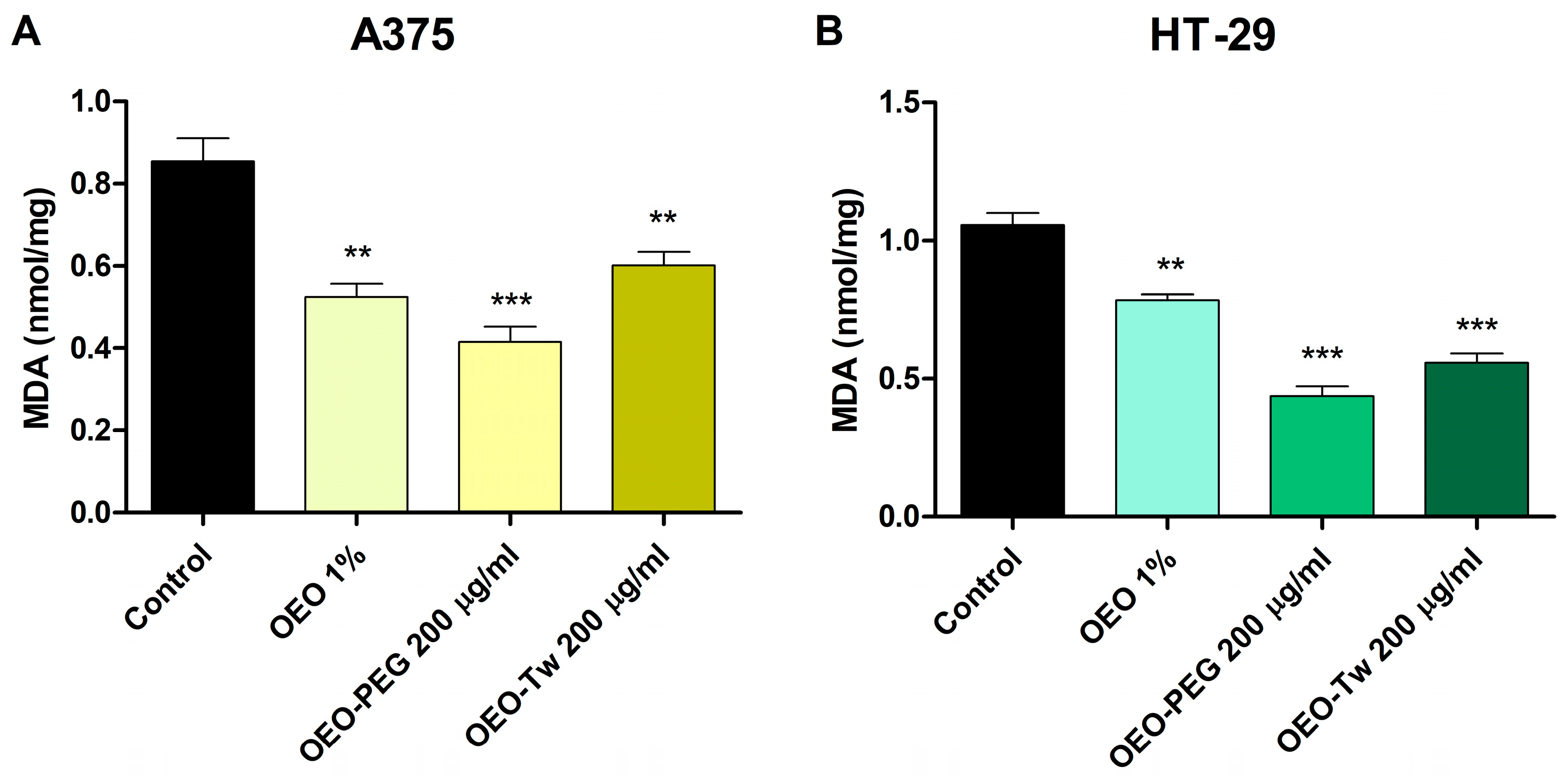
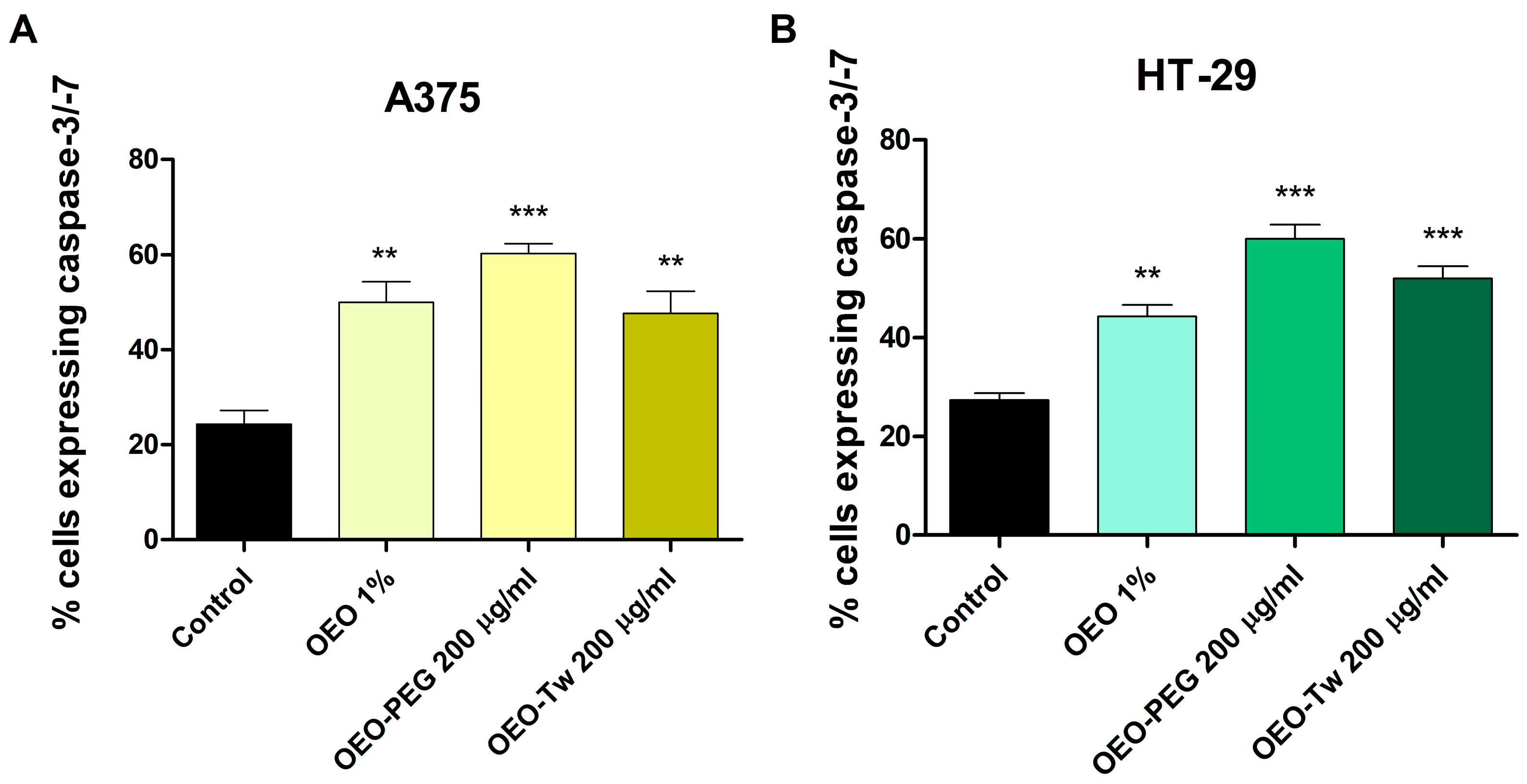
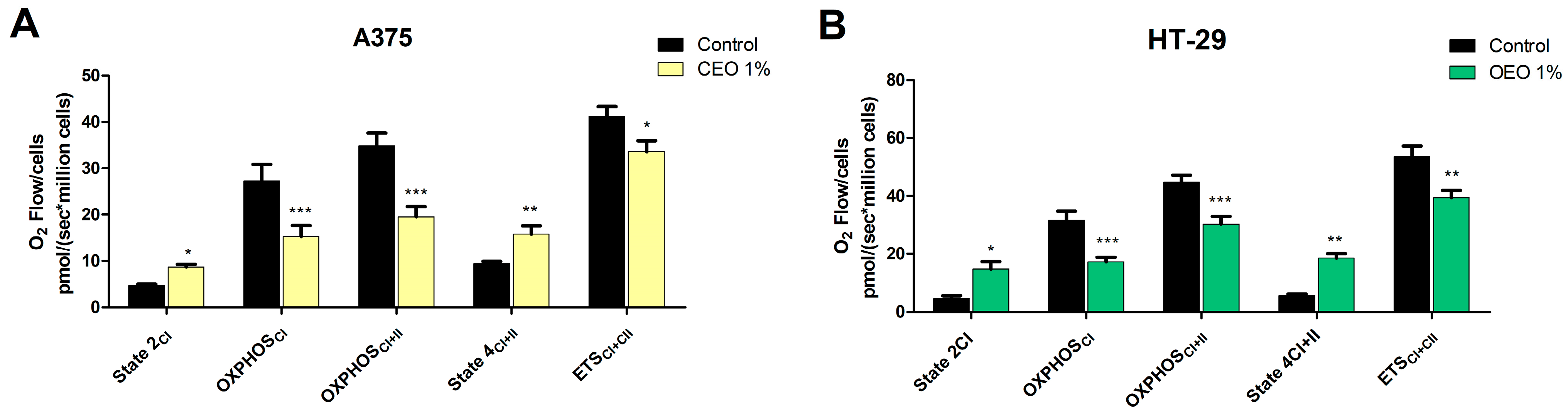
| Id | Compound Name | RT | RIcalc | RIlit | Area % Calc |
|---|---|---|---|---|---|
| 1 | β-Pinene | 5.31 | 959 | 974 | 4.84 |
| 2 | δ-Carene | 5.63 | 976 | — | 2.92 |
| 3 | Limonene | 5.96 | 993 | 1024 | 1.63 |
| 4 | γ-Terpinene | 6.84 | 1040 | 1008 | 22.16 |
| 5 | p-Cymene | 7.31 | 1065 | — | 43.98 |
| 6 | Linalool | 13.58 | 1395 | 1095 | 3.11 |
| 7 | β-Caryophyllene | 13.84 | 1409 | 1418 | 3.40 |
| 8 | 2-Isopropyl-4-methylanisole | 13.92 | 1413 | 1232 | 2.08 |
| 9 | Anethole | 15.19 | 1480 | 913 | 2.23 |
| 10 | β-Bisabolene | 16.25 | 1536 | 1505 | 1.10 |
| 11 | β-Caryophyllene oxide | 20.86 | 1780 | 1582 | 1.09 |
| 12 | Thymol | 24.34 | 1963 | 1289 | 11.46 |
| Sample | DPPH IC50 (µg/mL) | ABTS (Inh %) | TPC (mg GAE/g Extract) |
|---|---|---|---|
| OEO | 134.67 ± 1.32 | 88.15 ± 0.045 | 159.63 |
| AA | 10.53 ± 0.33 | 88.84 ± 0.002 * | - |
| BHA | 8.21 ± 0.46 | 88.79 ± 0.002 * | - |
| Compound | Protein Targets | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PDPK1 | mTOR | Mek1 | Akt | PI3Kα | PI3Kγ | Bcl-XL | Bcl-2 | Vegfr2 | Egfr1 | |
| Binding Affinity (kcal/mol) | ||||||||||
| Native Ligand | −8.8 | −11.2 | −9.5 | −9.4 | −8.8 | −9.3 | −10.9 | −11.5 | −12 | −11 |
| 1 | −5.6 | −5.1 | −5.3 | −5.1 | −5.4 | −5.3 | −6.2 | −5.7 | −6.4 | −5.3 |
| 2 | −6.1 | −6.1 | −6.3 | −6.2 | −5.7 | −6 | −6.2 | −6 | −6.6 | −5.9 |
| 3 | −6.2 | −6 | −6.5 | −6.1 | −5.6 | −5.9 | −6.5 | −5.9 | −6.8 | −6 |
| 4 | −6.2 | −6 | −6.6 | −6 | −5.7 | −5.9 | −6 | −5.7 | −6.7 | −5.8 |
| 5 | −6.3 | −6 | −6.7 | −6 | −5.7 | −6 | −6.1 | −5.8 | −6.7 | −5.8 |
| 6 | −5.4 | −5.6 | −5.4 | −5.1 | −5.5 | −5.6 | −5.4 | −5.5 | −6.2 | −5.3 |
| 7 | −7.2 | −6.6 | −6.6 | −6 | −6.3 | −6.2 | −7.1 | −6.9 | −7 | −7.1 |
| 8 | −6.1 | −5.8 | −5.5 | −6.2 | −6 | −6 | −6 | −5.5 | −6 | −5.9 |
| 9 | −6.1 | −6.3 | −6 | −5.9 | −5.9 | −6.1 | −5.9 | −5.9 | −6.7 | −5.9 |
| 10 | −7.1 | −7.3 | −7 | −6.7 | −7.2 | −7.4 | −6.6 | −7 | −8.5 | −7.5 |
| 11 | −7.9 | −6.2 | −7.2 | −6.2 | −6.4 | −6.3 | −7.5 | −6.9 | −7 | −7.1 |
| 12 | −6.4 | −6 | −6.5 | −6.2 | −6 | −5.8 | −6 | −5.9 | −7.2 | −6 |
| Compound | BBB | Caco2 | CYP_2C19_Inhibition | CYP_2C9_Inhibition | CYP_2D6_Inhibition | CYP_3A4_Inhibition | HIA | Plasma_Protein_Binding | SKlogP_Value |
|---|---|---|---|---|---|---|---|---|---|
| β-Pinene | 5.756 | 23.492 | Non | Inhib | Non | Inhib | 100 | 100 | 2.952 |
| δ-Carene | 5.533 | 23.631 | Non | Inhib | Non | Non | 100 | 100 | 2.918 |
| Limonene | 8.278 | 23.631 | Inhib | Inhib | Non | Non | 100 | 100 | 3.669 |
| γ-Terpinene | 8.037 | 23.640 | Inhib | Inhib | Non | Non | 100 | 100 | 3.634 |
| p-Cymene | 4.969 | 23.433 | Inhib | Inhib | Non | Inhib | 100 | 100 | 3.559 |
| Linalool | 6.125 | 29.355 | Inhib | Inhib | Non | Non | 100 | 100 | 2.749 |
| β-Caryophyllene | 13.319 | 23.631 | Inhib | Inhib | Non | Non | 100 | 100 | 4.896 |
| 2-Isopropyl-4-methylanisole | 2.393 | 57.964 | Inhib | Inhib | Non | Inhib | 100 | 100 | 3.544 |
| Anethole | 1.470 | 58.089 | Inhibi | Inhib | Non | Non | 100 | 89.24 | 2.938 |
| β-Bisabolene | 15.064 | 23.405 | Inhib | Inhib | Non | Non | 100 | 100 | 5.613 |
| β-Caryophyllene oxide | 3.752 | 56.347 | Non | Inhib | Non | Inhib | 100 | 90.84 | 3.700 |
| Thymol | 6.388 | 38.012 | Inhib | Inhib | Non | Inhib | 100 | 100 | 3.405 |
| Compound | Ames_Test | Carcino_Mouse | Carcino_Rat | hERG_Inhibition |
|---|---|---|---|---|
| β-Pinene | mutagen | negative | positive | medium_risk |
| δ-Carene | mutagen | negative | positive | medium_risk |
| Limonene | mutagen | negative | positive | medium_risk |
| γ-Terpinene | mutagen | positive | positive | medium_risk |
| p-Cymene | mutagen | positive | negative | medium_risk |
| Linalool | mutagen | negative | negative | low_risk |
| β-Caryophyllene | mutagen | negative | positive | medium_risk |
| 2-Isopropyl-4-methylanisole | mutagen | positive | negative | medium_risk |
| Anethole | mutagen | positive | negative | medium_risk |
| β-Bisabolene | mutagen | negative | positive | medium_risk |
| β-Caryophyllene oxide | mutagen | positive | positive | medium_risk |
| Thymol | mutagen | negative | negative | low_risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardale, G.; Caruntu, F.; Mioc, A.; Mioc, M.; Lukinich-Gruia, A.T.; Pricop, M.-A.; Jianu, C.; Gogulescu, A.; Maksimovic, T.; Șoica, C. Integrated In Silico and In Vitro Assessment of the Anticancer Potential of Origanum vulgare L. Essential Oil. Processes 2025, 13, 1695. https://doi.org/10.3390/pr13061695
Mardale G, Caruntu F, Mioc A, Mioc M, Lukinich-Gruia AT, Pricop M-A, Jianu C, Gogulescu A, Maksimovic T, Șoica C. Integrated In Silico and In Vitro Assessment of the Anticancer Potential of Origanum vulgare L. Essential Oil. Processes. 2025; 13(6):1695. https://doi.org/10.3390/pr13061695
Chicago/Turabian StyleMardale, Gabriel, Florina Caruntu, Alexandra Mioc, Marius Mioc, Alexandra Teodora Lukinich-Gruia, Maria-Alexandra Pricop, Calin Jianu, Armand Gogulescu, Tamara Maksimovic, and Codruța Șoica. 2025. "Integrated In Silico and In Vitro Assessment of the Anticancer Potential of Origanum vulgare L. Essential Oil" Processes 13, no. 6: 1695. https://doi.org/10.3390/pr13061695
APA StyleMardale, G., Caruntu, F., Mioc, A., Mioc, M., Lukinich-Gruia, A. T., Pricop, M.-A., Jianu, C., Gogulescu, A., Maksimovic, T., & Șoica, C. (2025). Integrated In Silico and In Vitro Assessment of the Anticancer Potential of Origanum vulgare L. Essential Oil. Processes, 13(6), 1695. https://doi.org/10.3390/pr13061695








