Technical Considerations for Designing an Electrocoagulation Reactor for Wastewater Treatment: A Brief Review
Abstract
1. Introduction
2. Electrochemical Engineering
3. Electrocoagulation (EC)
4. Design Criteria for an Electrocoagulation Reactor
5. Control Variables and Design Parameters of an Electrocoagulation Reactor
6. Operative Cost
7. Summary of Electrocoagulation Results from the Last Ten Years
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tegladza, I.D.; Xu, Q.; Xu, K.; Lv, G.; Lu, J. Electrocoagulation processes: A general review about role of electro-generated flocs in pollutant removal. Process Saf. Environ. Prot. 2021, 146, 169–189. [Google Scholar] [CrossRef]
- Díaz-Ponce, M.; Peñarrieta-Macias, F.F. Definiciones y Conceptos Básicos, En Actividades Antropogénicas en la Parroquia san Antonio y su Incidencia en la Calidad del agua del Humedal la Segua, 1st ed.; Grupo Compás: Guayaquil, Ecuador, 2020; p. 6. [Google Scholar]
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandagopal, S. Water resources: Agricultural and environmental issues. Bio. Sci. 2004, 54, 909–918. [Google Scholar] [CrossRef]
- Raffo-Lecca, E.; Ruiz-Lizama, E. Caracterización de las aguas residuales y la demanda bioquímica de oxígeno. Rev. Fac. Ing. Ind. 2014, 17, 71–80. [Google Scholar] [CrossRef]
- AWWA; ASCE. The Challenge of Water Treatment Plant Design. In Water Treatment Plant Design, 4th ed.; Lane, T.J., Ed.; McGrawHill: New York, NY, USA, 2004; pp. 1.1–1.6. [Google Scholar]
- Hammer, M.J.; Hammer, M.J. Wastewater Flows and Characteristics. In Water and Wastewater Technology, 7th ed.; Hammer, M.J., Ed.; Pearson Prentice Hall: Harlow, UK, 2014; pp. 259–275. [Google Scholar]
- UNESCO. Informe Mundial de las Naciones Unidas Sobre el Desarrollo de los Recursos Hídricos 2023: Alianzas y Cooperación por el agua, Datos, Cifras y Ejemplos de Acción; Koncagi, E., Connor, R., Eds.; UNESCO: Perugia, Italy, 2023; pp. 2–11. [Google Scholar]
- Metcalf & Eddy Inc. Introduction to Wasterwater Treatment Process Analysis Wasterwater Engeneering—Treatment Reuse, 5th ed.; Tchobanoglous, G., Stensel, H.D., Burton, F.L., Eds.; McGrawHill: Ney York, NY, USA, 2014; pp. 1–1.11. [Google Scholar]
- Salgot, M.; Folch, M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- CAEM. Sistemas de Tratamiento en el Estado de Mexico; Avances Semestrales: Toluca, México, 2023. [Google Scholar]
- Mercado, A.; de Blanco, M.L. Las normas oficiales mexicanas ecológicas para la industria mexicana: Alcances, exigencia y requerimientos de reforma. Gestión Y Política Pública 2003, 12, 93–128. [Google Scholar]
- Norma Oficial Mexicana NOM-001-SEMARNAT-2021; Que Establece los Límites Máximos Permisibles de Contaminantes en las Descargas de Aguas Residuales en Cuerpos Receptores Propiedad de la Nación. Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT): México City, México, 2021.
- Rodríguez-Peña, M.; Natividad, R.; Barrera-Díaz, C.E.; Hernández, P.B.; Ramírez, C.I.A.; Roa-Morales, G. Current perspective of advanced electrochemical oxidation processes in wastewater treatment and life cycle analysis. Int. J. Electrochem. Sci. 2024, 19, 100589. [Google Scholar] [CrossRef]
- Coeuret, F. Introducción a la Ingeniería Electroquímica, 1st ed.; Editorial REVERTÉ S.A: Barcelona, Spain, 1992; pp. 215–225. [Google Scholar]
- Barrera-Díaz, C.E. Electrocoagulación. Aplicaciones Electroquímicas al Tratamiento de Aguas Residuales, 1st ed.; Sociedad Mexicana de electroquímica; Reverté Ediciones S.A de C.V: Barcelona, Spain, 2014; pp. 165–181. [Google Scholar]
- Rodrigo, M.A.; Cañizares, P.; Lobato, J.; Sáez, C. Modeling of Electrochemical Process for the Treatment of Wastewater Containg Organic Pollutants; Electrochemistry for the Environment; Comninellis, C., Chen, G., Eds.; Springer: New York, NY, USA, 2010; pp. 99–125. [Google Scholar]
- Wang, J. Concepts Fundamental. Analytical Electrochemistry, 3rd ed.; Wiley-VCH: Ney York, NY, USA, 2006; pp. 1–28. [Google Scholar]
- Bratby, J. Treatmente with Metal Coagulants. Coagulation and Floculation in Water and Wastewater Treatment, 3rd ed.; IWA Publishing: London, UK, 2016; Volume 15, pp. 81–230. [Google Scholar]
- Yasri, N.; Hu, J.; Kibria, M.G.; Roberts, E.P.L. Electrocoagulation Separation Processes. In Multidisciplinary Advances in Efficient Separation Processes; American Chemical Society: Washington, DC, USA, 2020; Volume 1348, pp. 167–203. [Google Scholar]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Mechanistic models of electrocoagulation kinetics of pollutant removal in vinasse waste: Effect of voltage. J. Water Process Eng. 2020, 36, 101312. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent—A review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef]
- Vepsäläinen, M.; Sillanpää, M. Electrocoagulation in the Treatment of Industrial Waters and Wastewaters. In Advanced Water Treatment: Electrochemical Methods; Elsevier: Amsterdam, The Netherlands, 2020; Volume 801, pp. 267–299. [Google Scholar]
- Manikandan, S.; Deena, S.R.; Subbaiya, R.; Vijayan, D.S.; Vickram, S.; Preethi, B.; Karmegam, N. Waves of change: Electrochemical innovations for environmental management and resource recovery from water—A review. J. Environ. Manag. 2024, 366, 121879. [Google Scholar] [CrossRef]
- Howe, K.; Hand, D.; Crittenden, J.; Trussell, R.; Tchobbanoglous, G. Seleccion de Proceso. Principios de Tratamiento del agua, 1st ed.; Reyes-Martínez, J., Ed.; CENGAGE Learning: México, México, 2014; pp. 25–46. [Google Scholar]
- Barrera-Díaz, C.E.; Balderas-Hernández, P.; Bilyeu, B. Electrocoagulation: Fundamentals and Prospectives. In Electrochemical Water and Wastewater Treatment; Butterworth-Heinemann: Waltham, MA, USA, 2018; pp. 61–76. [Google Scholar]
- Mousazadeh, M.; Naghdali, Z.; Al-Qodah, Z.; Alizadeh, S.M.; Karamati Niaragh, E.; Malekmohammadi, S.; Nidheesh, P.V.; Roberts, E.P.L.; Sillanpää, M.; Emamjomeh, M.M. A systematic diagnosis of state of the art in the use of electrocoagulation as a sustainable technology for pollutant treatment: An updated review. Sustain. Energy Technol. Assess. 2021, 47, 101153. [Google Scholar] [CrossRef]
- Hansen, H.K.; Guti, C.; Gonzalez, J.L.; Lazo, A.; Hansen, M.E.; Lazo, P.; Ottosen, L.M.; Ortiz, R. Combined Electrodialysis and Electrocoagulation as Treatment for Industrial Wastewater Containing Arsenic and Copper. Membranes 2023, 13, 264. [Google Scholar] [CrossRef]
- Afsharnia, M.; Biglari, H.; Rasouli, S.S.; Karimi, A.; Kianmehr, M. Sono-Electrocoagulation of Fresh Leachate from Municipal Solid Waste; Simultaneous Applying of Iron and Copper Electrodes. Int. J. Electrochem. Sci. 2018, 13, 472–484. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Abfertiawan, M.S.; Syafila, M.; Handajani, M.; Hasan, F.; Oktaviani, H.; Gunawan, F.; Djali, D.F. Batch Electrocoagulation Process for the Removal of High Colloidal Clay from Open-Cast Coal Mine Water Using Al and Fe Electrodes. Mine Water Environ. 2024, 43, 516–528. [Google Scholar] [CrossRef]
- Garcia-Orozco, V.M.; Linares-Hernández, I.; Natividad, R.; Balderas-Hernandez, P.; Alanis-Ramírez, C.; Barrera-Díaz, C.E.; Roa-Morales, G. Solar-photovoltaic electrocoagulation of wastewater from a chocolate manufacturing industry: Anodic material effect (aluminium, copper and zinc) and life cycle assessment. J. Environ. Chem. Eng. 2022, 10, 107969. [Google Scholar] [CrossRef]
- García-García, A.; Martínez-Miranda, V.; Martínez-Cienfuegos, I.G.; Almazán-Sánchez, P.T.; Castañeda-Juárez, M.; Linares-Hernández, I. Industrial wastewater treatment by electrocoagulation—Electrooxidation processes powered by solar cells. Fuel 2015, 149, 46–54. [Google Scholar] [CrossRef]
- 1. García-Colindres, M.A.; Requena-Alvarez, B.L.; Castillo-Suárez, L.A.; Linares-Hernández, I. Removal of Nitrates in Drinking Water Polluted with Landfill Leachate by an Electrocoagulation System with Mg-Zn. Water Air Soil. Pollut. 2024, 235, 269. [Google Scholar] [CrossRef]
- Linares-Hernández, I.; Barrera-Díaz, C.E.; Valdés-Cerecero, M.; Almazán-Sánchez, P.T.; Castañeda-Juárez, M.; Lugo-Lugo, V. Soft drink wastewater treatment by electrocoagulation–electrooxidation processes. Environ. Technol. 2016, 38, 433–442. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Kong, W.; Fan, H. Recent advances in solar cells and photo-electrochemical water splitting by scanning electrochemical microscopy. Front. Optoelectron. 2018, 11, 333–347. [Google Scholar] [CrossRef]
- Lakshmi, J.; Sozhan, G.; Vasudevan, S. Recovery of hydrogen and removal of nitrate from water by electrocoagulation process. Environ. Sci. Pollut. Res. 2013, 20, 2184–2192. [Google Scholar] [CrossRef]
- CONACYT; CIDETEQ. Perspectivas de la Electrocoagulación, Seminario de Conocimientos; CONACYT, CIDETEQ: Querétaro, México, 2021. [Google Scholar]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Demirci, Y.; Pekel, L.C.; Alpbaz, M. Investigation of different electrode connections in electrocoagulation of textile wastewater treatment. Int. J. Electrochem. Sci. 2015, 10, 2685–2693. [Google Scholar] [CrossRef]
- Asaithambi, P.; Yesuf, M.B.; Govindarajan, R.; Selvakumar, P.; Niju, S.; Pandiyarajan, T.; Kadier, A.; Nguyen, D.D.; Alemayehu, E. Industrial wastewater treatment using batch recirculation electrocoagulation (BRE) process: Studies on operating parameters. Sustain. Chem. Environ. 2023, 2, 100014. [Google Scholar] [CrossRef]
- Bhuvanendran, R.K.; Bhuvaneshwari, S.; Prasannakumari, A.S.N.; Palani, M. Dairy wastewater treatment by peroxi-electrocoagulation method in hybrid electrocoagulation reactor and development of pigment from waste sludge. J. Mater. Cycles Waste Manag. 2024, 26, 1102–1118. [Google Scholar] [CrossRef]
- Khaled, B.; Wided, B.; Béchir, H.; Elimame, E.; Mouna, L.; Zied, T. Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem. 2019, 12, 1848–1859. [Google Scholar] [CrossRef]
- Arango-Ruiz, A.; Garcés-Giraldo, L.F. Diseño de una celda de electrocoagulación para el tratamiento de aguas residuales de la industria láctea. Rev. Univ. EAFIT 2007, 43, 56–67. [Google Scholar]
- Toussaint, N.K.; Raowia, L.; Zakia, Z.; Bouchra, B.; Anas, A.; Hassan, C.; Khalid, D.; Adil, D. Hydrodynamic modelling and performance assessment of continuous-flow single-channel reactor for river water turbidity removal using electrocoagulation process. Chem. Eng. Res. Des. 2023, 197, 496–508. [Google Scholar]
- Linares-Hernández, I.; Matínez-Miranda, V.; Barrera-Díaz, C.E.; Pavón-Romero, S.; Bernal-Martínez, L.; Lugo-Lugo, V. Oxidación de materia orgánica presistente en aguas residuales industriales mediante tratamientos electroquímicos. Av. En. Cienc. E Ing. 2011, 2, 21–36. [Google Scholar]
- Fuladpanjeh-Hojaghan, B.; Elsutohy, M.M.; Kabanov, V.; Heyne, B.; Trifkovic, M.; Roberts, E.P.L. In-Operando Mapping of pH Distribution in Electrochemical Processes. Angew. Chem. Int. Ed. 2019, 58, 16815–16819. [Google Scholar] [CrossRef]
- Tejocote-Pérez, M.; Balderas Hernández, P.; Barrera-Díaz, C.E.; Natividad, R. Treatment of industrial effluents by a continuous system: Electrocoagulation—Activated sludge. Bioresour. Technol. J. 2010, 101, 7761–7766. [Google Scholar]
- NMX-AA-093-SCFI-2000; Cancela a la NMX-AA-093-1984; Análisis de Agua- Determinación de la Conductividad Electrolítica- Método de Prueba. Secretaría de Comercio y Fomento Industrial (SCFI): México City, México, 2000.
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J. Removal of lead and zinc from battery industry wastewater using electrocoagulation process: Influence of direct and alternating current by using iron and stainless-steel rod electrodes. Sep. Purif. Technol. 2014, 135, 165–175. [Google Scholar] [CrossRef]
- Somasundaram, G.; Thavamani, T.; Thangavelu, S. Integration of sequential electrocoagulation and adsorption for effective removal of colour and total organic carbon in textile effluents and its utilization for seed germination and irrigation. Environ. Sci. Pollut. Res. 2024, 31, 30716–30734. [Google Scholar] [CrossRef]
- Ruotolo, L.A.M.; Gubulin, J.C. A mathematical model to predict the electrode potential profile inside a polyaniline-modified reticulate vitreous carbon electrode operating in the potentiostatic reduction of Cr(VI). Chem. Eng. J. 2011, 171, 1170–1177. [Google Scholar] [CrossRef]
- Zeboudji, B.; Drouiche, N.; Lounici, H.; Mameri, N.; Ghaffour, N. The Influence of Parameters Affecting Boron Removal by Electrocoagulation Process. Sep. Sci. Technol. 2013, 48, 1280–1288. [Google Scholar] [CrossRef]
- Escobar, C.; Soto, S.C.; Toral, M.I. Optimization of the Electrocoagulation Process for the Removal of Copper, Lead and Cadmium in Natural Waters and Simulated Wastewater Optimization of the electrocoagulation process for the removal of copper, lead and cadmium in natural waters and simula. J. Environ. Manag. 2006, 81, 384–391. [Google Scholar] [CrossRef]
- Verma, A.K. Treatment of textile wastewaters by electrocoagulation employing Fe-Al composite electrode. J. Water Process Eng. 2017, 20, 168–172. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef]
- Escaler, M.I.; Mujeriego, R. Eliminación biológica de nutrientes (nitrógeno y fósforo) mediante un proceso discontinuo de fangos activados. Ing. Del. Agua 2001, 8, 67–77. [Google Scholar] [CrossRef][Green Version]
- Montaño, E.; Vázquez, V.; Valenzuela, J.L. Estudio termodinámico y cinético para la recuperación de plata por proceso de electrocoagulación. Minería Metal. Y Explor. 2022, 39, 153–159. [Google Scholar]
- Ahmed, I.; Nosier, S.; Malash, G.; Huseein, M.; Abdel-Aziz, M.; Sedahmed, M.; Fathalla, A. Removal of Brilliant Yellow Azo Dye from Wastewater by Electrocoagulation in a Cell of Improved Design. Water Air Soil. Pollut. 2023, 234, 237. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M. Heavy metal ions removal from wastewater using electrocoagulation processes: A comprehensive review. Sep. Sci. Technol. 2017, 52, 2649–2676. [Google Scholar] [CrossRef]
- Bayar, S.; Yildiz, Y.S.; Yilmaz, A.E.; Irdemez, S. The effect of stirring speed and current density on removal efficiency of poultry slaughterhouse wastewater by electrocoagulation method. Desalination 2011, 280, 103–107. [Google Scholar] [CrossRef]
- Spellman, F.R. Handbook of Water and Wastewater Treatment Plant Operations, 1st ed.; CRC Press: New York, NY, USA, 2003; p. 696. [Google Scholar]
- Jiang, S.P.; Love, J.G.; Apateanu, L. Effect of contact between electrode and current collector on the performance of solid oxide fuel cells. Solid. State Ion. 2003, 160, 15–26. [Google Scholar] [CrossRef]
- Prihartini-Aryanti, P.T.; Nugroho, F.A.; Phalakornkule, C.; Kadier, A. Energy efficiency in electrocoagulation processes for sustainable water and wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 114124. [Google Scholar] [CrossRef]
- Diamantis, T.; Kontos, M.; Arvelakis, A.; Syroukis, S.; Koronarchis, D.; Papalois, A.; Agapitos, E.; Bastounis, E.; Lazaris, A.C. Comparison of monopolar electrocoagulation, bipolar electrocoagulation, ultracision, and ligasure. Surg. Today 2006, 36, 908–913. [Google Scholar] [CrossRef]
- Adhoum, N.; Monser, L. Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem. Eng. Process. 2004, 43, 1281–1287. [Google Scholar] [CrossRef]
- Arboleda-Camacho, J.M.; Herrera-López, P.J. Evaluación de un Proceso de Electrocoagulación en un Reactor tipo Batch Para la Remoción de Cromo Hexavalente (cr6+) con Electrodos de Aluminio—Aluminio y de Hierro—Aluminio en Condiciones de Laboratorio. Bachelor’s Thesis, Universidad Santo Tomás, Bogotá, Colombia, 2015. [Google Scholar]
- Kuroda, Y.; Kawada, Y.; Takahashi, T.; Ehara, Y.; Ito, T.; Zukeran, A.; Kono, Y.; Yasumoto, K. Effect of electrode shape on discharge current and performance with barrier discharge type electrostatic precipitator. J. Electrost. 2003, 57, 407–415. [Google Scholar] [CrossRef]
- Arias-A, V.; Lovera-D, D.; García-G, I. Diseño y construcción de un reactor electrolítico multielectródico para laboratorio. Rev. Inst. Investig. Fac. Minas Metal. Cienc. Geográficas 2011, 14, 53–57. [Google Scholar]
- Cazco-Sánchez, A.J.; Jarrín-Flores, S.A. Diseño, Construcción y Análisis de los Parámetros de una Operación de un Sistema de Coagulación. Bachelor’s Thesis, Escuela Politécnica Nacional, Quito, Ecuador, 2010. [Google Scholar]
- Eyvaz, M.; Kirlaroglu, M.; Aktas, T.S.; Yuksel, E. The effects of alternating current electrocoagulation on dye removal from aqueous solutions. Chem. Eng. J. 2009, 153, 16–22. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J. Effect of alternating and direct current in an electrocoagulation process on the removal of cadmium from water. Water Sci. Technol. 2012, 65, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.; Nava, J.L.; Cruz, R.; Lázaro, I.; Rodríguez, I. The importance of current distribution cell hydrodynamic analysis for the design of electrocoagulation reactors. J. Chem. Technol. Biotechnol. 2014, 89, 220–229. [Google Scholar] [CrossRef]
- Sandoval, M.A.; Fuentes, R.; Pérez, T.; Walsh, F.C.; Nava, J.L. Modelling and simulation of H2-H2O bubbly flow through a stack of three cells in a pre-pilot filter press electrocoagulation reactor. Sep. Purif. Technol. 2021, 261, 118235. [Google Scholar] [CrossRef]
- Cornejo, O.M.; Valentín-Reyes, J.; Rosales, M.; Nava, J.L. Simulations of aluminum dosage and H2O-H2 flow in a pre-pilot twelve-cell electrocoagulation stack. Chem. Eng. J. 2022, 450, 138222. [Google Scholar] [CrossRef]
- Castañeda, L.F.; Nava, J.L. Nava. Simulations of single-phase flow in an up-flow electrochemical reactor with parallel plate electrodes in a serpentine array. J. Electroanal. Chem. 2019, 832, 31–39. [Google Scholar] [CrossRef]
- Cengel, Y.A.; Cimbala, J.M. Mecánica de Fluidos Fundamentos y Aplicaciones, 1st ed.; McGrawHill: México, México, 2006; pp. 2–36. [Google Scholar]
- Fall, C. Comunicación Personal; Universidad Autónoma del Estado de México: Toluca, Estado de México, México, 2024. [Google Scholar]
- Justi, R. Teaching and Learning Chemical Kinetics. Chemical Education: Towards Research-Based Practice; Gilbert, J.K., De Jong, O., Justi, R., Treagust, D.F., Van Driel, J.H., Eds.; Science & Technology Education Library: New York, NY, USA, 2002; Volume 17, p. 293. [Google Scholar]
- lIhan, F.; Ulucan-Altuntas, K.; Avsar, Y.; Kurt, U.; Saral, A. Electrocoagulation process for the treatment of metal-plating wastewater: Kinetic modeling and energy consumption. Front. Environ. Sci. Eng. 2019, 13, 1–8. [Google Scholar]
- Boinpally, S.; Kolla, A.; Kainthola, J.; Kodal, R.; Vemuri, J. A state-of-the-art review of the electrocoagulation technology for wastewater treatment. Water Cycle 2023, 4, 26–36. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH Water Treatment Principles and Design, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 17–73. [Google Scholar]
- Hayden, J.; Abbassi, B. Continuous Flow Electrocoagulation System for Enhanced Phosphorous Removal in Decentralized Wastewater Treatment Systems. Water 2025, 17, 202. [Google Scholar] [CrossRef]
- Arnold, M.; Kangas, P.; Mäkinen, A.; Lakay, E.; Isomäki, N.; Lavén, G.; Gericke, M.; Pajuniemi, P.; Kaartinen, T.; Wendling, L. Mine Water as a Resource: Selective Removal and Recovery of Trace Antimony from Mine-Impacted Water. Mine Water Environ. 2019, 38, 431–446. [Google Scholar] [CrossRef]
- Brahmi, K.; Bouguerra, W.; Belhsan, H.; Elaloui, E.; Loungou, M.; Tlili, Z.; Hamrouni, B. Nutzung von Elektro-Koagulation mit Aluminiumelektroden für die Enthärtung von Prozesswasser im Phosphatbergbau in Tunesien. Mine Water Environ. 2016, 35, 310–317. [Google Scholar] [CrossRef]
- Victoria, D.D.; Toro, C.C.O.; Villegas, M.V.Z.; Marriaga-Cabrales, N.; Martínez, F.M.; Hernández, J.M.P.; Huitler, C.A.M. Electrocoagulation of indigo, carmine dye solution with magnesium and AZ31 alloy anodes. DYNA 2018, 85, 258–267. [Google Scholar]
- Montaño-Saavedra, M.D.; Paschino-Bissoto, F.; De Souza, R.A.; Cárdenas-Concha, V.O.; Gaspar-Bastos, R. Growth of Desmodesmus subspicatus green microalgae and nutrient removal from sugarcane vinasse clarified by electrocoagulation using aluminum or iron electrodes. Dyna 2019, 86, 225–232. [Google Scholar] [CrossRef]
- Apaza, H.; Daniel, E.; Monteagudo, C.; Katherine, D.; Colpaert, C.; Rodolfo, F.; Chambi, H. Model of a Treatment System for Effluents from the Tannery Industry. Rev. Investig. Esc. Posgrado 2020, 051, 1647–1658. [Google Scholar]
- López, P.; Harnisth, A. Electrocoagulación de aguas residuales de la industria láctea (Dairy industry wastewater electrocoagulation). Enfoque UTE 2016, 7, 13–21. [Google Scholar] [CrossRef][Green Version]
- Fekete, É.; Lengyel, B.; Cserfalvi, T.; Pajkossy, T. Electrocoagulation: An electrochemical process for water clarification. J. Electrochem. Sci. Eng. 2016, 6, 57–65. [Google Scholar] [CrossRef][Green Version]
- Cruz, K.D.; Francisco, J.T.J.; Mellendrez, K.J.M.; Pineda, J.M.F. Electrocoagulation Treatment of Swine Slaughterhouse Wastewater: Effect of Electrode Material. In E3S Web of Conferences 2019; EDP Sciences: Les Ulis, France, 2019; Volume 117, pp. 1–5. [Google Scholar]
- de la Fuente, A.; Muro-Pastor, A.M.; Merchán, F.; Madrid, F.; Pérez-Martínez, J.I.; Undabeytia, T. Electrocoagulation/flocculation of cyanobacteria from surface waters. J. Clean. Prod. 2019, 238, 117964. [Google Scholar] [CrossRef]
- Vaca, M.C.G.; Ubaque, C.A.G.; de Plaza Solórzano, J.S. Estudio exploratorio del tratamiento de agua de lavado de tintas por método de electrocoagulación/electroflotación. Rev. Tecnura 2016, 20, 107–117. [Google Scholar] [CrossRef]
- Watawati, C.S.; Shivayogimath, C.B. Treatment of Dairy Wastewater by Electrocoagulation Process Using Iron Electrodes. Recent. Adv. Sustain. Waste Manag. Pract. 2024, 430, 263–275. [Google Scholar]
- Carbaja-Varea, A.N.; Torres-Ccoyllar, N.I.; Huamani-Andrade, Y.J.; Cornjeo-Tueros, J.V. The Removal of Polypropylene Microplastics in Simulated Water Enriched with Organic Substances Through the Electrocoagulation Process. In The 8th International Conference on Energy and Environmental Science (ICEES 2024): ICEES 2024; Springer Nature: Cham, Switzerland, 2024; pp. 43–59. [Google Scholar]
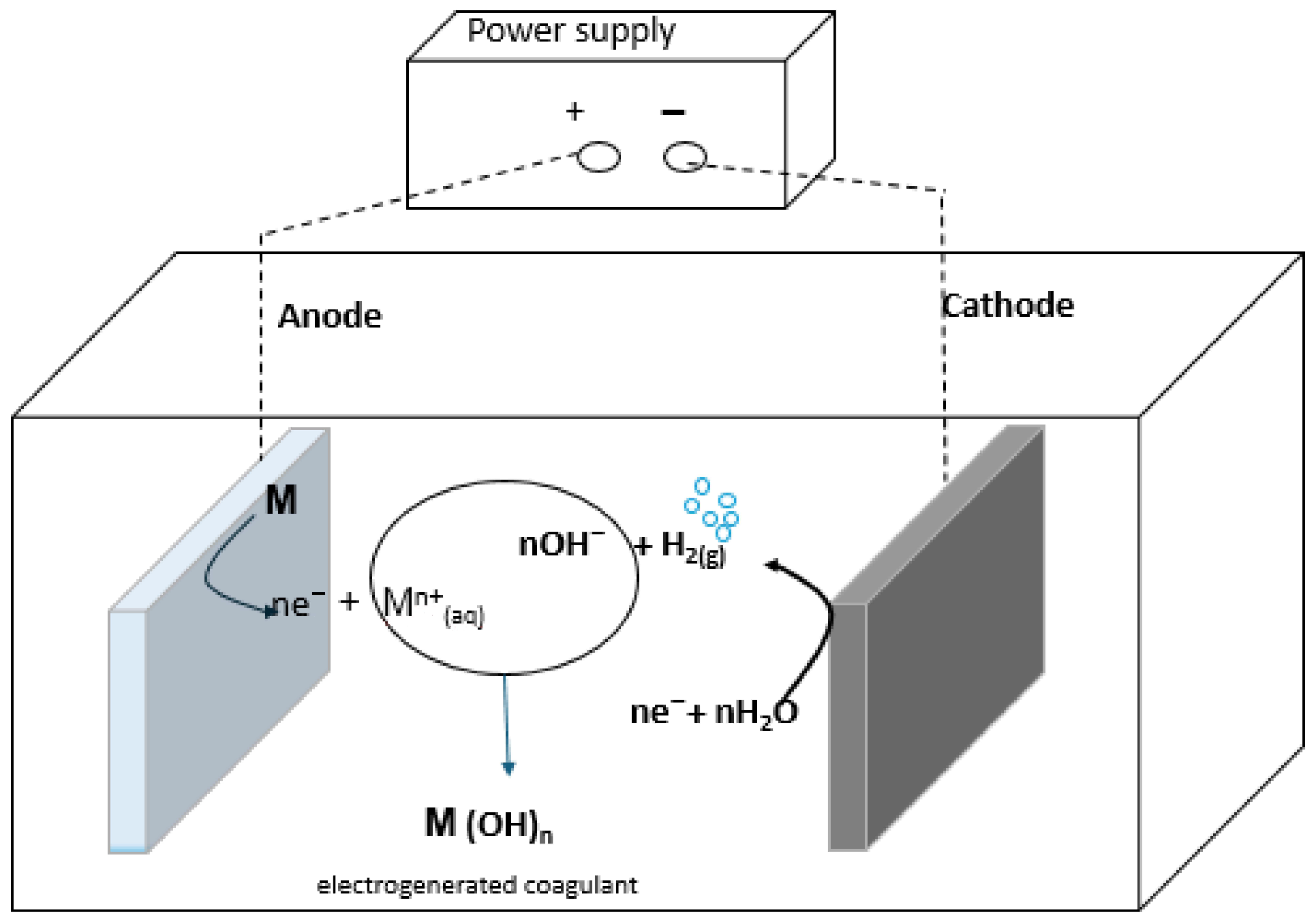
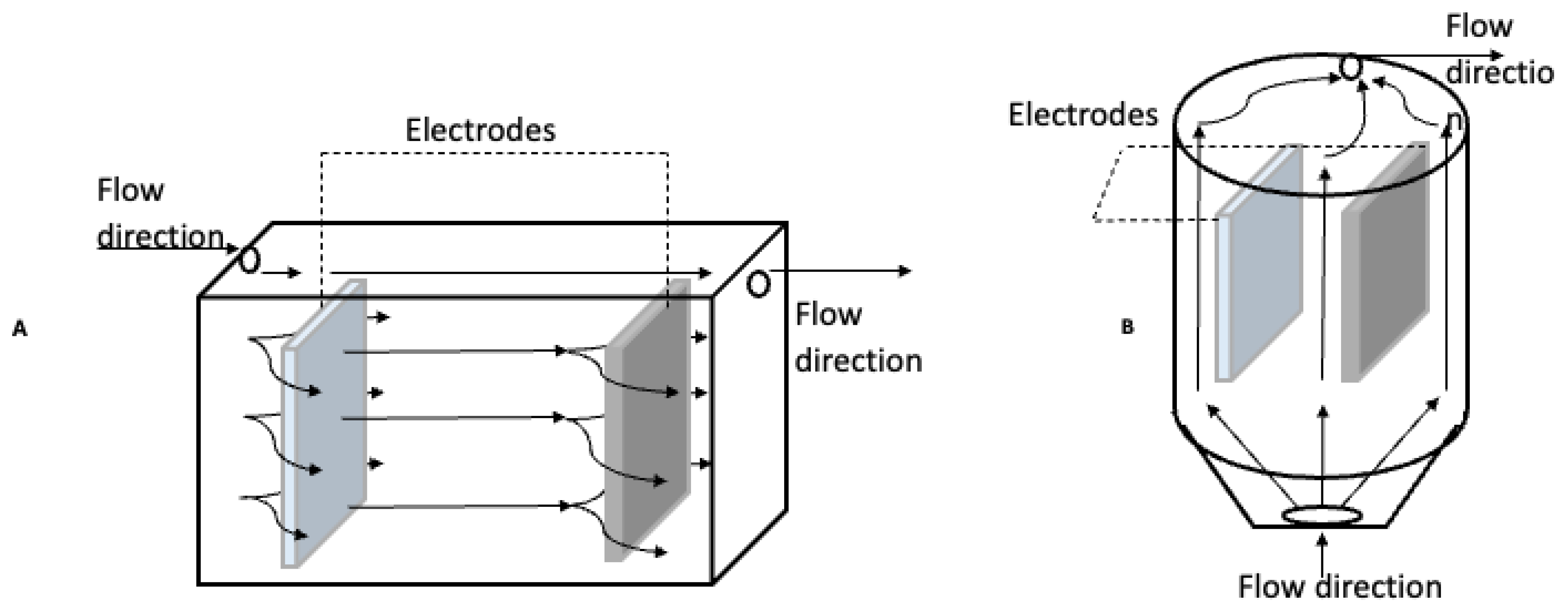

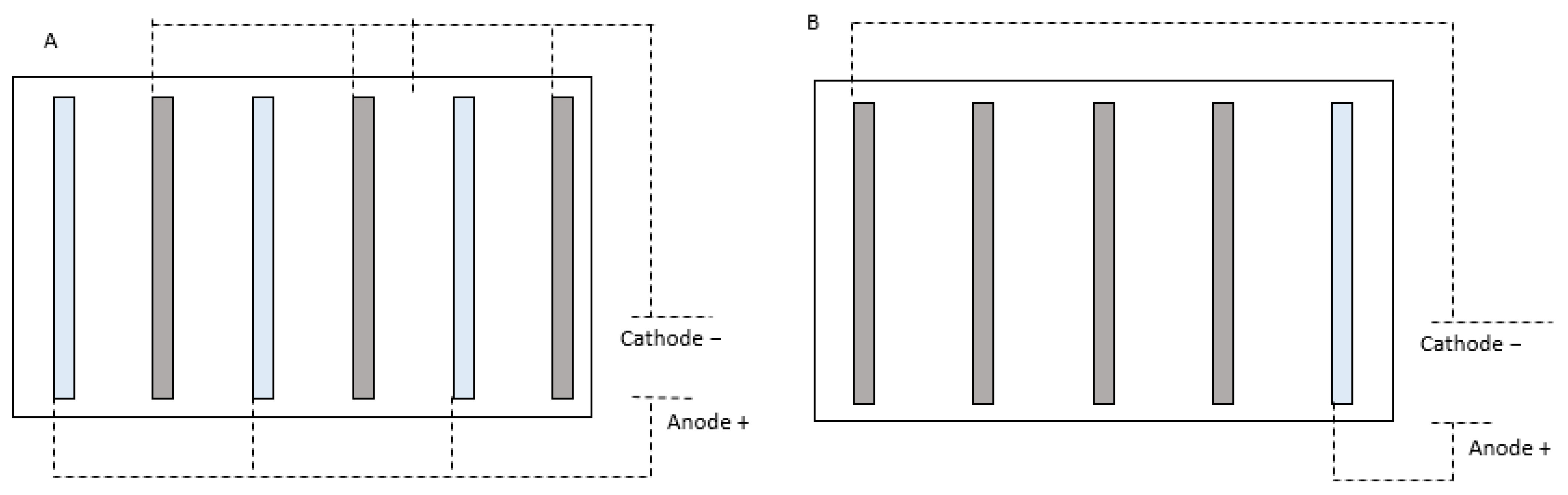



| Variable Process | Units | Variable Analysis | Reference |
|---|---|---|---|
| pH | pH |
| [46,48] |
| Electrical conductivity | µS/cm |
| [19,49] |
| Current density | A/m2 |
| [50,51,52] |
| Limiting current density | A/m2 |
| [52] |
| Electric current | A |
| [20,47,53] |
| Resistivity | Ω |
| [20,47,53,54] |
| Voltage | V |
| [20,47,53,54] |
| Zeta potential | mV |
| [42] |
| Hydraulic retention time 1 | min |
| [55,56] |
| Flow 1 | L/min |
| [56,57] |
| Temperature | °C |
| [58,59,60] |
| Stirring speed | rpm (Batch) |
| [60,61] |
| Initial concentration of the contaminant 1 | mg/L |
| [5,62] |
| Electrode contact area 1 | cm2 |
| [63] |
| Design Criteria | Units | Variable Analysis | Reference |
|---|---|---|---|
| Kind of cell | ---- |
| [15,39] |
| Operation mode | ---- |
| [8,27] |
| Electrode distance | cm |
| [50,54] |
| Electrode arrangement | ---- |
| [64,65] |
| Electrode material | ---- |
| [66,67] |
| Electrode shape | --- |
| [59,68] |
| Cell material | --- |
| [69] |
| Cell size | cm3 |
| [44,70] |
| Kind of current supplied: Direct current (dc) Alternating current (AC) | V |
| [71,72] |
| Author | Process’ Output | Treated Substrate | Electrode Material (Sacrificial Anode and Cathode), and/or Counter Electrode | pH | Treatment Time (min) | Current Density Applied (mA/cm2) | Efficiency Percentage (%) |
|---|---|---|---|---|---|---|---|
| [84] | Continuous | Sb contained in mine wastewater | Fe-Fe | 8 | 3.5 | 0.06–2.12 | 75 for Sb |
| [85] | Batch | Hardness in mining waters containing phosphates | Al-Al | 7 | 30 | 22.2 | 83.8 for hardness (CaCO3) |
| [86] | Batch | Indigo carmine in synthetic water | Mg pure-304 Stainless Steel AZ31 Magnesium alloy-304 Stainless Steel | 7.83 | 150 | 2–5 | 99 for color 84 for TOC |
| [87] | Batch | Vinasse (wastewater from ethanol production using sugarcane) | Al-Fe/cultivation of D. subspicatus | 6.4 | 240 (Time only for electrocoagulation process) | 20 | 66 for TOC 75 for nitrogen removal 98 for turbidity |
| [88] | Semi-continuous | Wastewater from the tanning industry | Processes including four steps: Sedimentation/porous filter/electrocoagulation/biomass filter | 7 | 15 (Time only for electrocoagulation process) | 20 A (current density nonreported) | 83.33 for COD 66.43 for BDO5 84 for chromium |
| [89] | Batch | Wastewater from the dairy industry | Al-Al | Non-reported | 60 | 12 V (current density non-reported) | 93.33 for BDO5 82.42 for COD 76.81 for SS |
| [90] | Continuous | Oil synthetic solution | Al-Fe | Non reported | 60 | 10 | 85 for CDO |
| [91] | Batch | Wastewater from pig slaughterhouse | Al-Fe | 2.43 | 100 | 25 | 97 for COD |
| [92] | Continuous | Cyanobacteria | Al-Al/anionic flocculants | 7–8 | 2.2 | 37 | 34.8 for bacterial inactivation |
| [93] | Batch | Wastewater from the printing ink industry | Al-Al | Non reported | 30 | 156 | 51 for COD |
| [94] | Batch | Dairy Wastewater | Fe-Fe | 9 | 60 | 30 V (current density non-reported) | 98 for COD |
| [95] | Continuous | Synthetic wastewater with microplastics | Ni-Ni | 8 | 50 | 5 A (current density non-reported) | 81 for microplastic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solano Huerta, I.S.; Roa Morales, G.; Balderas Hernández, P.; Barrera Díaz, C.E.; Pavón Silva, T.B.; Ávila Pérez, P.; Rodríguez Torres, I. Technical Considerations for Designing an Electrocoagulation Reactor for Wastewater Treatment: A Brief Review. Processes 2025, 13, 1679. https://doi.org/10.3390/pr13061679
Solano Huerta IS, Roa Morales G, Balderas Hernández P, Barrera Díaz CE, Pavón Silva TB, Ávila Pérez P, Rodríguez Torres I. Technical Considerations for Designing an Electrocoagulation Reactor for Wastewater Treatment: A Brief Review. Processes. 2025; 13(6):1679. https://doi.org/10.3390/pr13061679
Chicago/Turabian StyleSolano Huerta, Ismael Salvador, Gabriela Roa Morales, Patricia Balderas Hernández, Carlos Eduardo Barrera Díaz, Thelma Beatriz Pavón Silva, Pedro Ávila Pérez, and Israel Rodríguez Torres. 2025. "Technical Considerations for Designing an Electrocoagulation Reactor for Wastewater Treatment: A Brief Review" Processes 13, no. 6: 1679. https://doi.org/10.3390/pr13061679
APA StyleSolano Huerta, I. S., Roa Morales, G., Balderas Hernández, P., Barrera Díaz, C. E., Pavón Silva, T. B., Ávila Pérez, P., & Rodríguez Torres, I. (2025). Technical Considerations for Designing an Electrocoagulation Reactor for Wastewater Treatment: A Brief Review. Processes, 13(6), 1679. https://doi.org/10.3390/pr13061679







