Tertiary Treatment of Pulp Industry Effluents Using Activated Biochar Derived from Biological Sludge Within a Circular Economy Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Biomass and Industrial Effluent Acquisition
2.3. Synthesis of Biochars
2.4. Characterization of the Materials
2.5. Adsorption Assays
2.6. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Biochar Synthesis
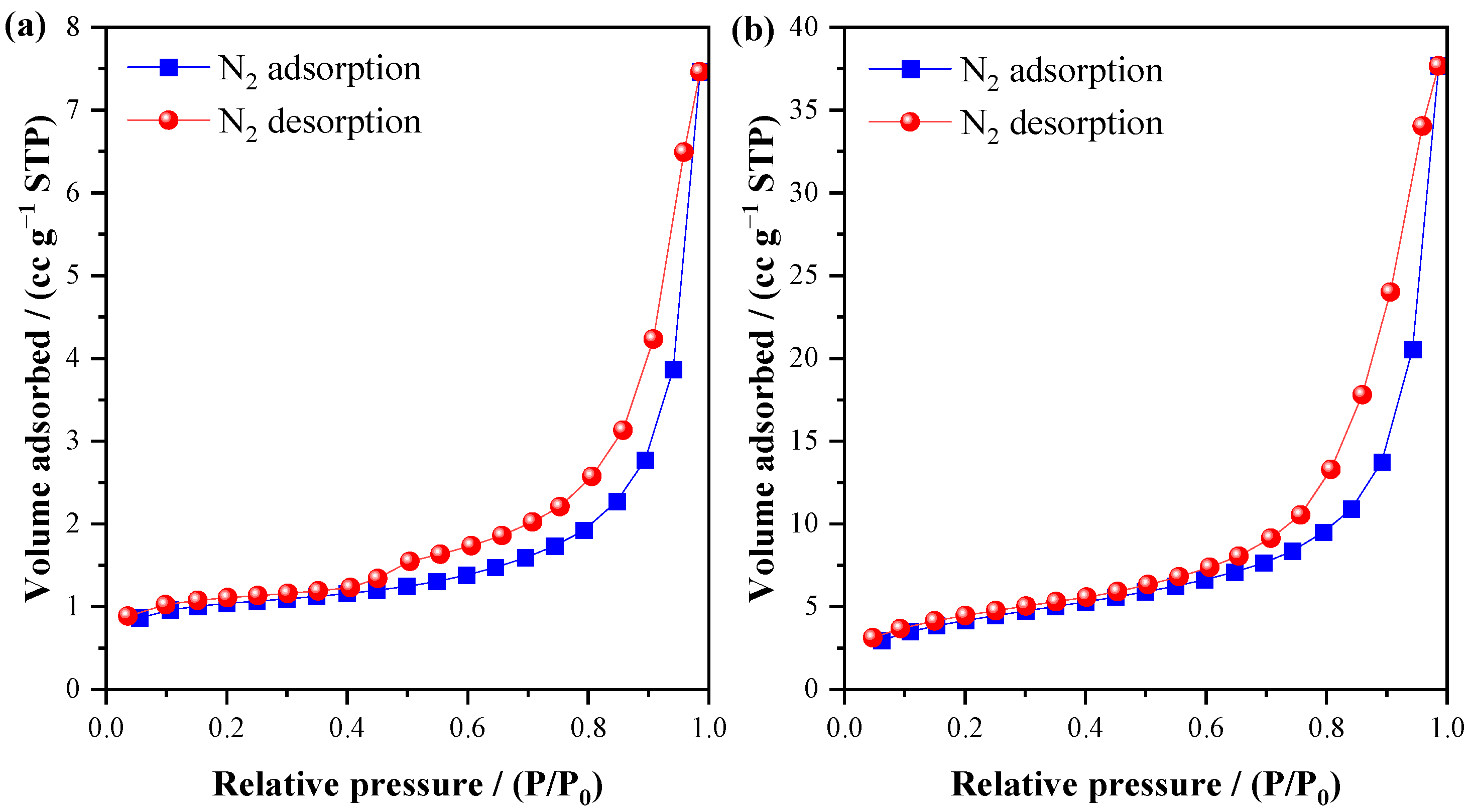
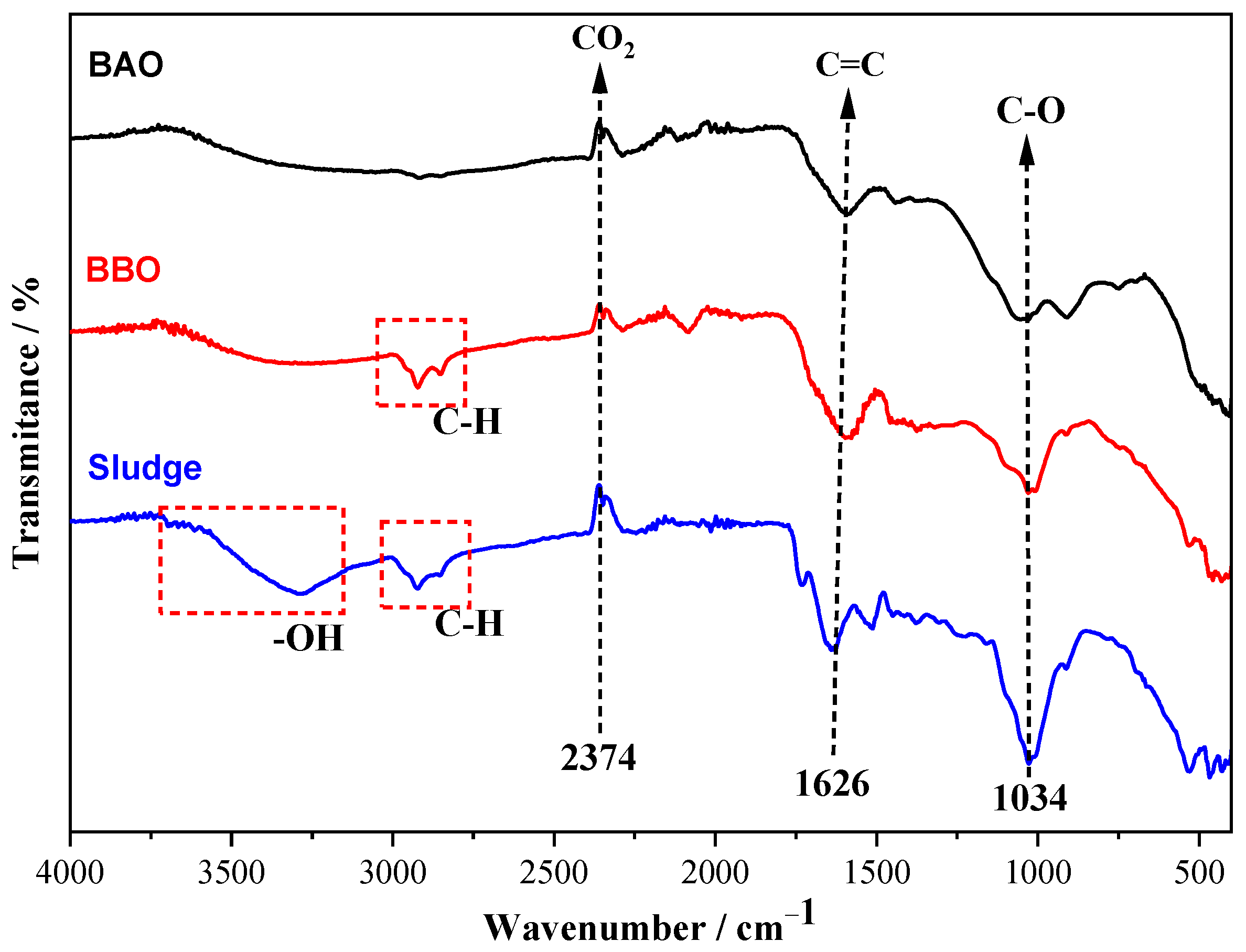
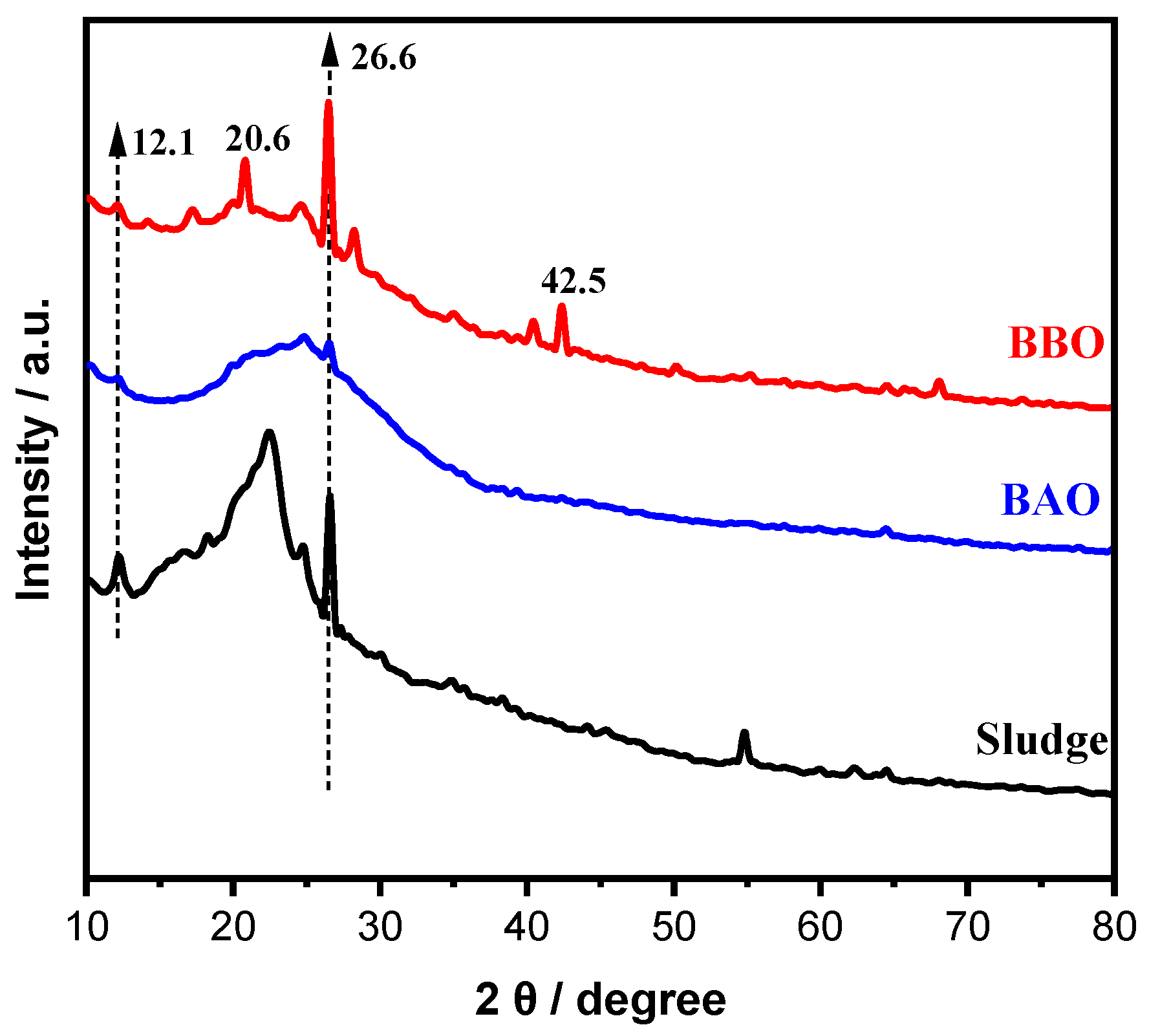
3.2. Characterization of Materials
| Metals | Concentration (mg L−1) | Maximum Permissible Limit (mg L−1) * |
|---|---|---|
| Cadmium | <LQ ** | 0.20 |
| Lead | <LQ ** | 0.50 |
| Chrome | <LQ ** | 1.0 |
| Magnesium | 2.53 ± 0.05 | - |
| Manganese | 0.240 ± 0.0003 | 1.0 |
| Potassium | 46.5 ± 0.05 | - |
| Zinc | 0.100 ± 0.0003 | 5.0 |
| Aluminum (Dissolved) | 0.903 ± 0.0003 | - |
| Copper (Dissolved) | <LQ ** | 1.0 |
| Iron (Dissolved) | 1.88 ± 0.05 | 15.0 |
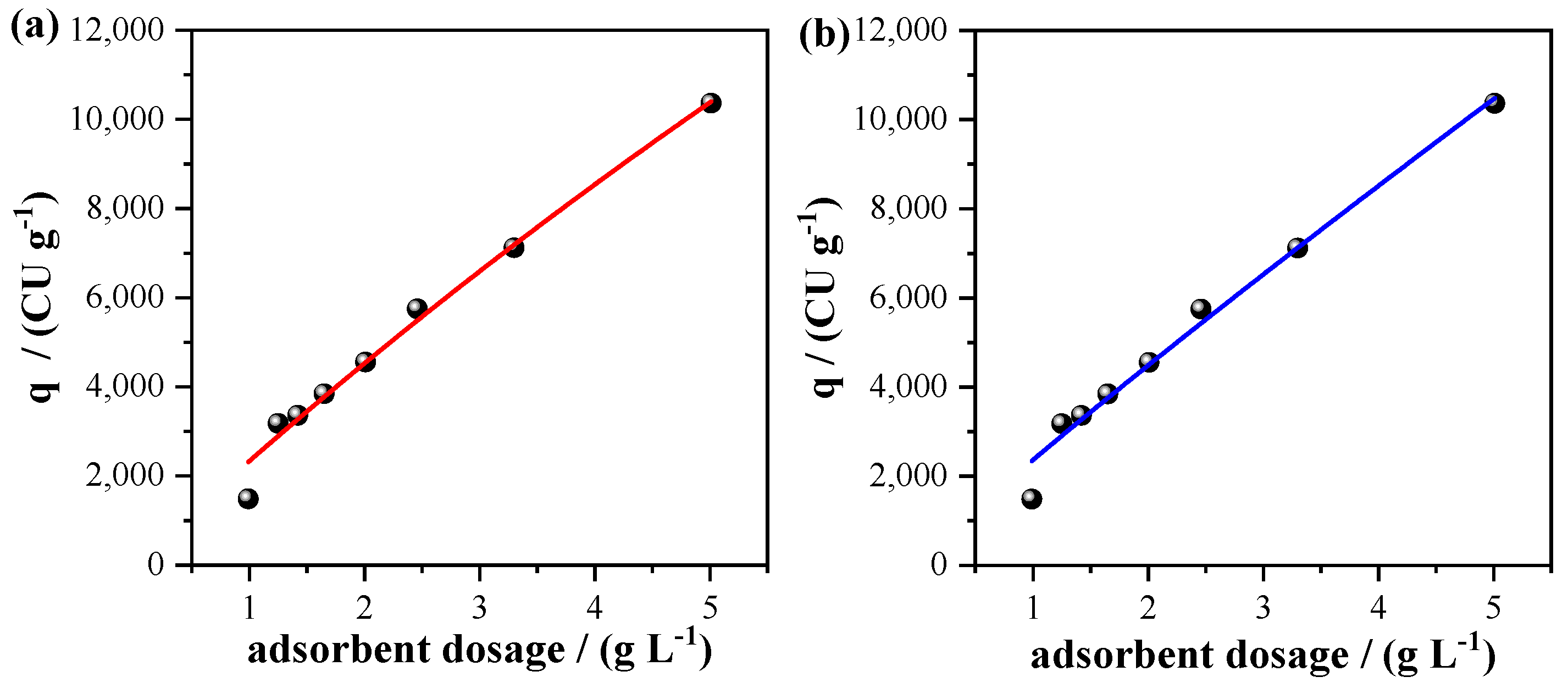
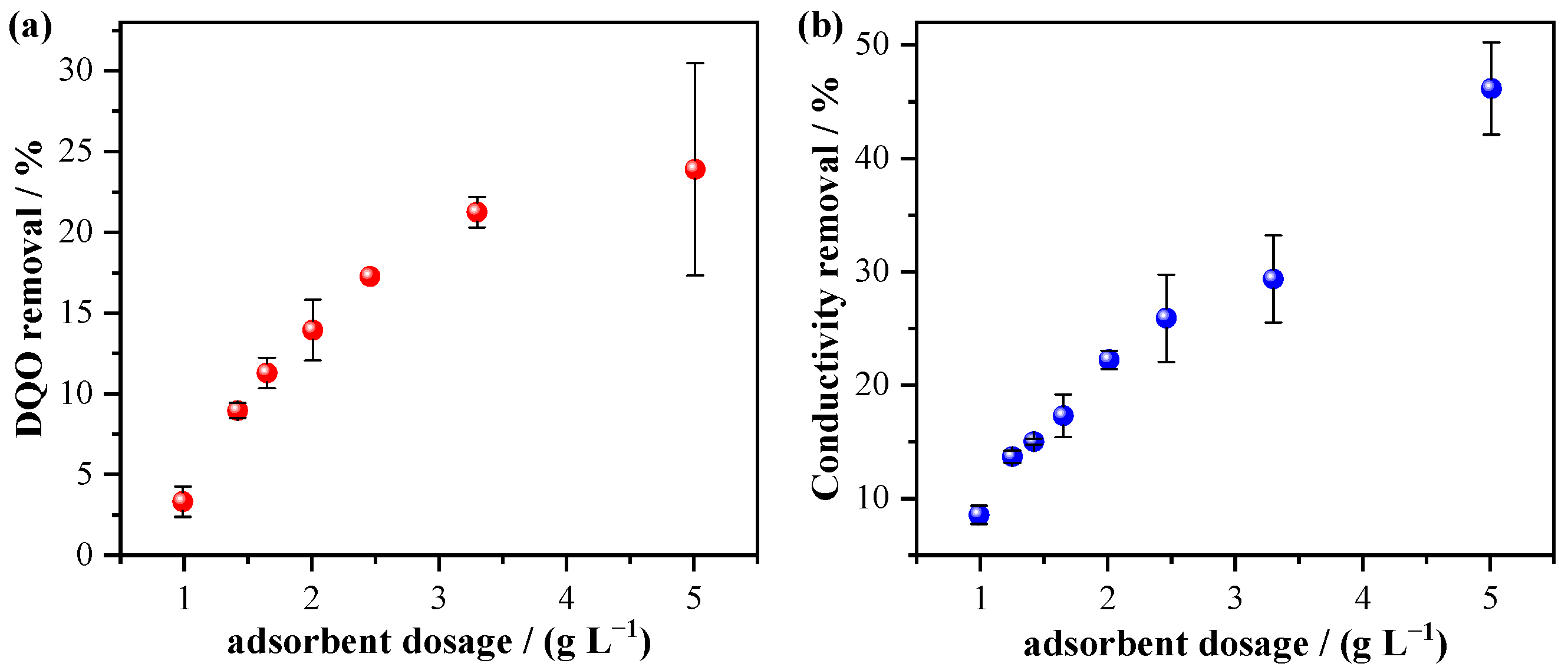
3.3. Adsorption of the Effluent as a Tertiary Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Biochar |
| COD | Chemical Oxygen Demand |
| CCRD | Central Composite Rotational Design |
| FTIR | Fourier Transform Infrared Spectroscopy |
| BET | Brunauer–Emmett–Teller (method for surface area analysis) |
| HHV | Higher Heating Value |
| SEM | Scanning Electron Microscopy |
| BS | Biological Sludge |
| BO | Bio-oil |
| BG | Biogas |
| BA | H3PO4-activated Biochar |
| BB | KOH-activated Biochar |
| BAO | Optimized H3PO4-activated Biochar |
| BBO | Optimized KOH-activated Biochar |
| TOC | Total Organic Carbon |
| SBET | BET Surface Area |
| EDS | Energy Dispersive Spectroscopy |
| PZC | Point of Zero Charge |
| CU | Color Unit |
| RSM | Response Surface Methodology |
| ANOVA | Analysis of Variance |
References
- Kataki, R.; Borkotoki, B.; Bora, N.; Maheshwari, S. Valorization of Pulp and Paper Mill Bio-Residues to Biochar for Environmental and Business Sustainability in Totality. IPPTA Q. J. Indian Pulp Pap. Tech. Assoc. 2024, 36, 179–181. [Google Scholar]
- Vilas-Boas, A.C.M.; Tarelho, L.A.C.; Kamali, M.; Hauschild, T.; Pio, D.T.; Jahanianfard, D.; Gomes, A.P.D.; Matos, M.A.A. Biochar from Slow Pyrolysis of Biological Sludge from Wastewater Treatment: Characteristics and Effect as Soil Amendment. Biofuels Bioprod. Biorefin. 2021, 15, 1054–1072. [Google Scholar] [CrossRef]
- Mehmood, K.; Rehman, S.K.U.; Wang, J.; Farooq, F.; Mahmood, Q.; Jadoon, A.M.; Javed, M.F.; Ahmad, I. Treatment of Pulp and Paper Industrial Effluent Using Physicochemical Process for Recycling. Water 2019, 11, 2393. [Google Scholar] [CrossRef]
- Mänttäri, M.; Nyström, M.; Nuortila-Jokinen, J.; Kallioinen, M. Nanofiltration in the Pulp and Paper Industry. In Nanofiltration; Schäefer, A.I., Fane, A.G., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 599–620. ISBN 978-3-527-34690-5. [Google Scholar]
- Shafeeyan, M.S. Application of Photocatalytic and Fenton Processes for the Degradation of Toxic Pollutants from Pulp and Paper Industry Effluents. Water Resour. Ind. 2024, 32, 100260. [Google Scholar] [CrossRef]
- Kamali, M.; Khodaparast, Z. Review on Recent Developments on Pulp and Paper Mill Wastewater Treatment. Ecotoxicol. Environ. Saf. 2015, 114, 326–342. [Google Scholar] [CrossRef]
- Pio, D.T.; Tarelho, L.A.C.; Tavares, A.M.A.; Matos, M.A.A.; Silva, V. Co-Gasification of Refused Derived Fuel and Biomass in a Pilot-Scale Bubbling Fluidized Bed Reactor. Energy Convers. Manag. 2020, 206, 112476. [Google Scholar] [CrossRef]
- Pio, D.T.; Tarelho, L.A.C.; Nunes, T.F.V.; Baptista, M.F.; Matos, M.A.A. Co-Combustion of Residual Forest Biomass and Sludge in a Pilot-Scale Bubbling Fluidized Bed. J. Clean. Prod. 2020, 249, 119309. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Hui, S. Ash-Related Issues during Biomass Combustion: Alkali-Induced Slagging, Silicate Melt-Induced Slagging (Ash Fusion), Agglomeration, Corrosion, Ash Utilization, and Related Countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Monlau, F.; Abdelouahdi, K.; Oukarroum, A.; Barakat, A. Pretreatment and Co-Digestion of Wastewater Sludge for Biogas Production: Recent Research Advances and Trends. Renew. Sustain. Energy Rev. 2019, 114, 109287. [Google Scholar] [CrossRef]
- Ribeiro, M.R.; De Moraes Guimarães, Y.; Silva, I.F.; Almeida, C.A.; Silva, M.S.V.; Nascimento, M.A.; Da Silva, U.P.; Varejão, E.V.; Dos Santos Renato, N.; Teixeira, A.P.D.C.; et al. Synthesis of Value-Added Materials from the Sewage Sludge of Cosmetics Industry Effluent Treatment Plant. J. Environ. Chem. Eng. 2021, 9, 105367. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of Biochar for the Adsorption of Organic Pollutants from Wastewater: Modification Strategies, Mechanisms and Challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Kapatel, D.V.; Rotliwala, Y.C.; Patel, H.J. Co-Pyrolysis Based Activated Bio-Char: Characterization and Its Utilization for Secondary Treated Pulp and Paper Industry Wastewater. Mater. Today Proc. 2022, 57, 1724–1729. [Google Scholar] [CrossRef]
- Li, Z.; Yu, D.; Wang, X.; Liu, X.; Xu, Z.; Wang, Y. A Novel Strategy of Tannery Sludge Disposal—Converting into Biochar and Reusing for Cr(VI) Removal from Tannery Wastewater. J. Environ. Sci. 2024, 138, 637–649. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the Preparation and Application of Modified Biochar for Improved Contaminant Removal from Water and Wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef]
- Reddy, B.S.; Reddy, N.S.; Nam, S.-Y.; Ahn, H.-J.; Ahn, J.-H. Potassium Hydroxide Activated Carbon Derived from Albumen as an Efficient Sulfur Host for Room Temperature Sodium-Sulfur Batteries. J. Energy Storage 2022, 45, 103666. [Google Scholar] [CrossRef]
- ABNT NBR 12073; Carvão Ativado Pulverizado—Determinação Do Número de Iodo—Método de Ensaio. Associação Brasileira de Normas Técnicas: São Paulo, Brasil, 1991.
- ASTM D1762−84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D5373-21; Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D2015-00; Standard Test Method for Gross Calorific Value of Coal and Coke by the Adiabatic Bomb Calorimeter. ASTM International: West Conshohocken, PA, USA, 2000.
- De Souza, N.C.S.; Do Carmo Dias, G.; Puiatti, G.A.; De Oliveira, K.L.A.; Vitorino, T.B.; Silva, T.A.; Moreira, R.P.L. Eco-Friendly Photodegradation of Direct Red 80 Dye Mediated by Biochar Decorated with Cobalt Ferrite. Int. J. Environ. Sci. Technol. 2025, 22, 4263–4280. [Google Scholar] [CrossRef]
- Standart Methods 6010 D; Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). U.S. EPA: Washington, DC, USA, 2023.
- Standart Methods 2120 C; Spectrophotometric—Single—Wavelength Method. U.S. EPA: Washington, DC, USA, 2023.
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Geloster Stoffe. K. Sven. Vetenskapsakademiens Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. A Kinetic Study of Dye Sorption by Biosorbent Waste Product Pith. Resour. Conserv. Recycl. 1999, 25, 171–193. [Google Scholar] [CrossRef]
- Langmuir, I. The Dissociation of Hyfrogen into Atoms. J. Am. Chem. Soc. 1912, 34, 860–877. [Google Scholar] [CrossRef]
- Freundlich, H. On Adsorption in Solutions. Z. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Standart Methods 5220 D; Closed Reflux, Colorimetric Method. U.S. EPA: Washington, DC, USA, 2023.
- Standart Methods 2510 B; Conductivity. U.S. EPA: Washington, DC, USA, 2023.
- Thivaly, D.A.; Setyawan, H.Y.; Yusoff, M.Z.M.; Mohamed, M.S.; Farid, M.A.A. Activated Biochar Production from Young Coconut Waste (Cocos Nucifera) as Bioadsorbent: A Pathway through Artificial Neural Network (ANN) Optimization. Environ. Monit. Assess. 2024, 196, 962. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, C.; Xing, X.; Chen, L.; Yao, B.; Chao, L.; Zhang, Y.; Wang, J.; Dong, J.; Liu, C.; et al. Interconnected Pyrolysis and Activation with In-Situ H3PO4 Activation of Biochar from Pear Wood Chips in a Pilot Scale Dual Fluidized Bed. Chem. Eng. J. 2024, 495, 153579. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into Activated Carbon from Different Kinds of Chemical Activating Agents: A Review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef]
- Beik, F.; Williams, L.; Brown, T.; Wagland, S.T. Development and Prototype Testing of a Novel Small-Scale Pyrolysis System for the Treatment of Sanitary Sludge. Energy Convers. Manag. 2023, 277, 116627. [Google Scholar] [CrossRef]
- Tarelho, L.A.C.; Hauschild, T.; Vilas-Boas, A.C.M.; Silva, D.F.R.; Matos, M.A.A. Biochar from Pyrolysis of Biological Sludge from Wastewater Treatment. Energy Rep. 2020, 6, 757–763. [Google Scholar] [CrossRef]
- da Luz Corrêa, A.P.; Da Silva, P.M.M.; Gonçalves, M.A.; Bastos, R.R.C.; Da Rocha Filho, G.N.; Da Conceição, L.R.V. Study of the Activity and Stability of Sulfonated Carbon Catalyst from Agroindustrial Waste in Biodiesel Production: Influence of Pyrolysis Temperature on Functionalization. Arab. J. Chem. 2023, 16, 104964. [Google Scholar] [CrossRef]
- Oasmaa, A.; Solantausta, Y.; Arpiainen, V.; Kuoppala, E.; Sipilä, K. Fast Pyrolysis Bio-Oils from Wood and Agricultural Residues. Energy Fuels 2010, 24, 1380–1388. [Google Scholar] [CrossRef]
- Rabichi, I.; Sekkouri, C.; Yaacoubi, F.E.; Ennaciri, K.; Izghri, Z.; Bouzid, T.; El Fels, L.; Baçaoui, A.; Yaacoubi, A. Experimental and Theoretical Investigation of Olive Mill Solid Waste Biochar for Vanillic Acid Adsorption Using DFT/B3LYP Analysis. Water Air Soil Pollut. 2024, 235, 369. [Google Scholar] [CrossRef]
- Almahbashi, N.M.Y.; Kutty, S.R.M.; Ayoub, M.; Noor, A.; Salihi, I.U.; Al-Nini, A.; Jagaba, A.H.; Aldhawi, B.N.S.; Ghaleb, A.A.S. Optimization of Preparation Conditions of Sewage Sludge Based Activated Carbon. Ain Shams Eng. J. 2021, 12, 1175–1182. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, C.; Siyal, A.A.; Song, Y.; Jiang, Z.; Dai, J.; Chtaeva, P.; Fu, J.; Ao, W.; Deng, Z.; et al. Comparative Study for Fluidized Bed Pyrolysis of Textile Dyeing Sludge and Municipal Sewage Sludge. J. Hazard. Mater. 2020, 396, 122619. [Google Scholar] [CrossRef] [PubMed]
- Eloy, E.; Pedrazz, C.; Coldebella, R.; Mangin, T.D.S.; Trevisan, R.; Caron, B.O.; Santos, A.D. Effect of Chemical Constituents on the Energetic and Physical Properties of Wood from Forestry Species. Ciência Rural. 2024, 54, e20230290. [Google Scholar]
- Meina, L.; Qiao, M.; Zhang, Q.; Xu, S.; Wang, D. Study on the Dynamic Adsorption and Recycling of Phosphorus by Fe–Mn Oxide/Mulberry Branch Biochar Composite Adsorbent. Sci. Rep. 2024, 14, 1235. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Koetlisi, K.A.; Muchaonyerwa, P. Biochar Types from Latrine Waste and Sewage Sludge Differ in Physico-Chemical Properties and Cadmium Adsorption. Am. J. Appl. Sci. 2017, 14, 1039–1048. [Google Scholar] [CrossRef]
- Yu, Q.; Ye, J.; Liu, G.; Liu, M.; Tang, M.; Li, L. Exploring Electron-Transfer Pathways in Co-Pyrolyzed Waste-Derived Carbocatalyst for Enhanced Peroxydisulfate Activation. Sep. Purif. Technol. 2025, 353, 128313. [Google Scholar] [CrossRef]
- Minaei, S.; Zoroufchi Benis, K.; McPhedran, K.N.; Soltan, J. Adsorption of Sulfamethoxazole and Lincomycin from Single and Binary Aqueous Systems Using Acid-Modified Biochar from Activated Sludge Biomass. J. Environ. Manag. 2024, 358, 120742. [Google Scholar] [CrossRef]
- Li, W.-H.; Yue, Q.-Y.; Gao, B.-Y.; Wang, X.-J.; Qi, Y.-F.; Zhao, Y.-Q.; Li, Y.-J. Preparation of Sludge-Based Activated Carbon Made from Paper Mill Sewage Sludge by Steam Activation for Dye Wastewater Treatment. Desalination 2011, 278, 179–185. [Google Scholar] [CrossRef]
- Xiang, J.; Luo, B.X.; Li, J.M.; Mi, Y.; Tian, B.; Gong, S.J.; Zhou, Y.R.; Ma, T.W. Development of KOH and H3PO4-Modified Composite Biochar from Corn Straw and Activated Sludge for Removing Methylene Blue. Int. J. Environ. Sci. Technol. 2023, 20, 1673–1688. [Google Scholar] [CrossRef]
- Fagbayigbo, B.O.; Opeolu, B.O.; Fatoki, O.S.; Akenga, T.A.; Olatunji, O.S. Removal of PFOA and PFOS from Aqueous Solutions Using Activated Carbon Produced from Vitis Vinifera Leaf Litter. Environ. Sci. Pollut. Res. 2017, 24, 13107–13120. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu(II) and Cd(II) from Aqueous Solutions by Ferromanganese Binary Oxide–Biochar Composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.J.; Zandvakili, O.R.; Xing, B.; Hashemi, M.; Herbert, S.; Mashayekhi, H.H. Dataset on the Effect of Hardwood Biochar on Soil Gravimetric Moisture Content and Nitrate Dynamics at Different Soil Depths with FTIR Analysis of Fresh and Aged Biochar. Data Brief 2019, 25, 104073. [Google Scholar] [CrossRef] [PubMed]
- Thue, P.S.; Lima, D.R.; Lima, E.C.; Teixeira, R.A.; Dos Reis, G.S.; Dias, S.L.P.; Machado, F.M. Comparative Studies of Physicochemical and Adsorptive Properties of Biochar Materials from Biomass Using Different Zinc Salts as Activating Agents. J. Environ. Chem. Eng. 2022, 10, 107632. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The Forms of Alkalis in the Biochar Produced from Crop Residues at Different Temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.-Y.; Cho, T.-S.; Choi, J.W. Influence of Pyrolysis Temperature on Physicochemical Properties of Biochar Obtained from the Fast Pyrolysis of Pitch Pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar] [CrossRef]
- Saucier, C.; Adebayo, M.A.; Lima, E.C.; Cataluña, R.; Thue, P.S.; Prola, L.D.T.; Puchana-Rosero, M.J.; Machado, F.M.; Pavan, F.A.; Dotto, G.L. Microwave-Assisted Activated Carbon from Cocoa Shell as Adsorbent for Removal of Sodium Diclofenac and Nimesulide from Aqueous Effluents. J. Hazard. Mater. 2015, 289, 18–27. [Google Scholar] [CrossRef]
- Streit, A.F.M.; Collazzo, G.C.; Druzian, S.P.; Verdi, R.S.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. Adsorption of Ibuprofen, Ketoprofen, and Paracetamol onto Activated Carbon Prepared from Effluent Treatment Plant Sludge of the Beverage Industry. Chemosphere 2021, 262, 128322. [Google Scholar] [CrossRef]
- Zhuang, Z.; Liu, Y.; Wei, W.; Shi, J.; Jin, H. Preparation of Biochar Adsorption Material from Walnut Shell by Supercritical CO2 Pretreatment. Biochar 2024, 6, 11. [Google Scholar] [CrossRef]
- Guo, K.; Gao, B.; Yue, Q.; Xu, X.; Li, R.; Shen, X. Characterization and Performance of a Novel Lignin-Based Flocculant for the Treatment of Dye Wastewater. Int. Biodeterior. Biodegrad. 2018, 133, 99–107. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Ramos, C.G.; Piccilli, D.G.A.; Lima, E.C.; Sher, F. A Review of the Antibiotic Ofloxacin: Current Status of Ecotoxicology and Scientific Advances in Its Removal from Aqueous Systems by Adsorption Technology. Chem. Eng. Res. Des. 2023, 193, 99–120. [Google Scholar] [CrossRef]
- Haq, I.; Roy, S.; Kalamdhad, A.S. Characterization of Pulp and Paper Mill Wastewater and Its Toxicity Analysis Using Vigna Radiata. In Environmental Degradation: Monitoring, Assessment and Treatment Technologies; Haq, I., Kalamdhad, A.S., Dash, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 261–269. ISBN 978-3-030-94147-5. [Google Scholar]
- BRASIL. Conselho Nacional do Meio Ambiente (CONAMA). Resolução CONAMA nº 430, de 13 de maio de 2011. Dispõe sobre as condições e padrões de lançamento de efluentes, complementa e altera a Resolução nº 357, de 17 de março de 2005. Diário Oficial da União, 16 May 2011. [Google Scholar]
- Sharma, P.; Iqbal, H.M.N.; Chandra, R. Evaluation of Pollution Parameters and Toxic Elements in Wastewater of Pulp and Paper Industries in India: A Case Study. Case Stud. Chem. Environ. Eng. 2022, 5, 100163. [Google Scholar] [CrossRef]
- Agarwal, S.; Singh, A.P.; Mathur, S. Removal of COD and Color from Textile Industrial Wastewater Using Wheat Straw Activated Carbon: An Application of Response Surface and Artificial Neural Network Modeling. Environ. Sci. Pollut. Res. 2023, 30, 41073–41094. [Google Scholar] [CrossRef] [PubMed]
- Magesh, N.; Renita, A.A.; Siva, R.; Harirajan, N.; Santhosh, A. Adsorption Behavior of Fluoroquinolone(Ciprofloxacin) Using Zinc Oxide Impregnated Activated Carbon Prepared from Jack Fruit Peel: Kinetics and Isotherm Studies. Chemosphere 2022, 290, 133227. [Google Scholar] [CrossRef]
- Feiyan, W.; Yali, Z.; Siling, L.; Zhiqin, C.; Shanshan, L.; Wenkui, L. Biochar from De-Oiled Chlorella vulgaris and Its Adsorption on Antibiotics. Open Chem. 2024, 22, 20230178. [Google Scholar] [CrossRef]
- Zou, M.; Tian, W.; Chu, M.; Gao, H.; Zhang, D. Biochar Composite Derived from Cellulase Hydrolysis Apple Branch for Quinolone Antibiotics Enhanced Removal: Precursor Pyrolysis Performance, Functional Group Introduction and Adsorption Mechanisms. Environ. Pollut. 2022, 313, 120104. [Google Scholar] [CrossRef]
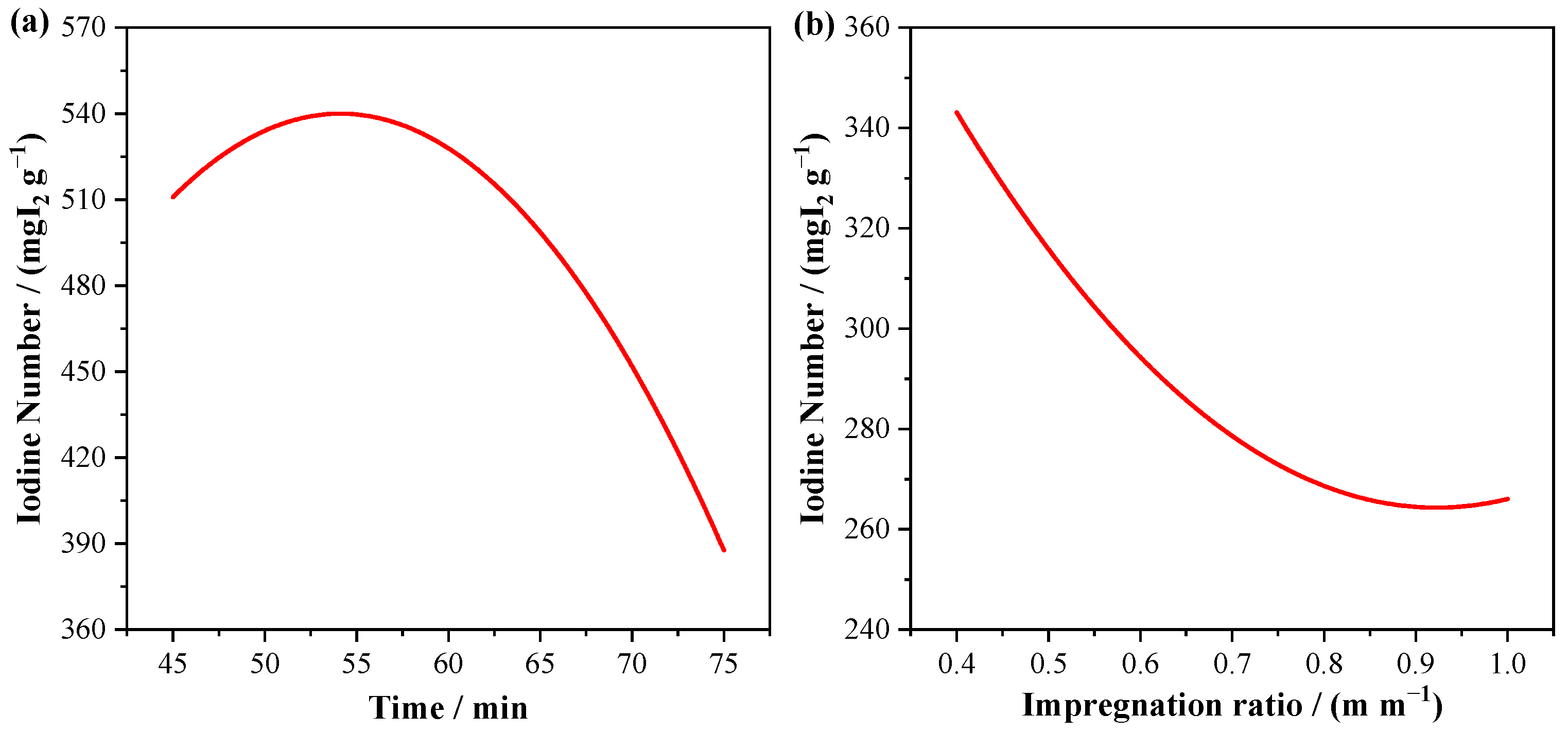

| Code | Final Pyrolysis Composition (%) | Final Yield * | Iodine Number | ||
|---|---|---|---|---|---|
| BC | BO | BG | (%) | (mg I2 g−1) | |
| BA1 | 67.76 | 0.64 | 31.6 | 27.53 | 518.4 |
| BA2 | 59.58 | 3.13 | 37.29 | 40.96 | 475.43 |
| BA3 | 65.14 | 2.04 | 32.82 | 42.87 | 537.82 |
| BA4 | 70.94 | 0.5 | 28.56 | 35.62 | 557.84 |
| BA5 | 56.06 | 4.95 | 38.99 | 44.7 | 441.28 |
| BA6 | 63.45 | 3.75 | 32.8 | 33.32 | 475.18 |
| BA7 | 60.12 | 1.29 | 38.59 | 41.89 | 536.75 |
| BA8 | 60.92 | 1.83 | 37.25 | 37.99 | 534.8 |
| BA9 | 58.07 | 1.78 | 40.15 | 40.98 | 375.09 |
| BA10 | 61.32 | 5.02 | 33.66 | 36.82 | 528.43 |
| BA11 | 61.6 | 1.86 | 36.53 | 37.24 | 495.33 |
| BA12 | 65.27 | 2.89 | 31.84 | 40.67 | 553.13 |
| BA13 | 61.67 | 3.27 | 35.07 | 40.13 | 519.31 |
| BAO | 59.94 | 1.95 | 38.11 | 56.44 | 510.66 |
| Average BAs | 62.27 | 2.49 | 35.23 | 39.8 | |
| BB1 | 81.18 | 0.24 | 18.58 | 15.95 | 274.86 |
| BB2 | 63.8 | 0.14 | 36.05 | 29.47 | 351.74 |
| BB3 | 69.06 | 1.33 | 29.61 | 23.13 | 296.83 |
| BB4 | 79.48 | 0.37 | 20.15 | 19.11 | 266.14 |
| BB5 | 61.32 | 0.15 | 38.53 | 25.11 | 295.76 |
| BB6 | 68.79 | 1.08 | 30.13 | 18.94 | 245 |
| BB7 | 64.72 | 0.34 | 34.94 | 26.04 | 324.51 |
| BB8 | 69.02 | 1.48 | 29.51 | 23.87 | 282.64 |
| BB9 | 67.15 | 1.08 | 31.77 | 24.76 | 262.87 |
| BB10 | 67.75 | 0.84 | 31.41 | 24.32 | 270.47 |
| BB11 | 68.87 | 2.03 | 29.11 | 21.79 | 287.68 |
| BB12 | 69.78 | 1.29 | 28.94 | 24.65 | 270.88 |
| BB13 | 67.71 | 2.28 | 30.01 | 22.07 | 296.46 |
| BBO | 61.58 | 1.43 | 36.99 | 29.01 | 328.44 |
| Average BBs | 68.59 | 1.01 | 30.41 | 23.44 | |
| Code | Predicted | Observed | Relative Error (%) |
|---|---|---|---|
| BAO * | 508.69 | 510.66 | 0.39% |
| BBO ** | 351.02 | 328.44 | 6.43% |
| Analysis | Characteristics | Sludge | BAO | BBO |
|---|---|---|---|---|
| Immediate Analysis (m m−1) | Moisture (%) | NP * | 6.04 | 3.73 |
| Volatile Compounds (%) | 71.40 | 27.92 | 35.16 | |
| Ashes (%) | 12.40 | 30.96 | 31.03 | |
| Fixed Carbon (%) | 16.20 | 35.08 | 30.08 | |
| Elemental Analysis (m m−1) | C (%) | 43.01 | 47.00 | 49.50 |
| H (%) | 6.43 | 3.09 | 4.19 | |
| N (%) | 4.65 | 5.03 | 3.52 | |
| S (%) | 3.09 | 0.45 | 0.64 | |
| O (%) | 42.82 | 44.43 | 42.15 | |
| H/C | 1.79 | 0.79 | 1.02 | |
| O/C | 0.75 | 0.71 | 0.64 | |
| Total Organic Carbon (m m−1) | TOC (%) | 41.53 | 35.82 | 42.45 |
| Higher Heating Value | HHV (Kcal kg−1) | 4565.9 | 4257.1 | 4469.2 |
| Energy Dispersive Spectroscopy (EDS) of Inorganics (m m−1) | Na (%) | NP * | 7.61 | 1.36 |
| Mg (%) | NP * | 2.58 | 1.56 | |
| Al (%) | NP * | 9.33 | 20.86 | |
| Si (%) | NP * | 10.98 | 22.60 | |
| P (%) | NP * | 63.93 | 26.20 | |
| K (%) | NP * | 1.30 | 18.77 | |
| Ca (%) | NP * | 4.26 | 8.65 | |
| SBET | m2 g−1 | NP * | 3.34 | 15.04 |
| Average Pore Diameter | Nm | NP * | 5.29 | 13.94 |
| Model | qe (CU g−1) | k1 (min−1) | k2 (g CU−1 min−1) | R2 | R2adj | p-Value |
|---|---|---|---|---|---|---|
| Pseudo-First-Order | 916.124 | 0.050 | - | 0.884 | 0.855 | 1.82 × 10−5 |
| Pseudo-Second-Order | 1037.448 | - | 6.516 | 0.896 | 0.870 | 1.46 × 10−5 |
| Model | qmax (CU g−1) | kL (L g−1) | kF (CU1-(1/n) g−2 L1/n) | n | R2 | R2adj | p-Value |
|---|---|---|---|---|---|---|---|
| Langmuir | 75,298.7 | 0.032 | - | - | 0.984 | 0.982 | 3.88 × 10−8 |
| Freundlich | - | - | 2363.32 | 1.082 | 0.982 | 0.979 | 5.33 × 10−8 |
| Materials | Initial COD (mgO2 L−1) | Final COD (mgO2 L−1) | Removed COD (%) |
|---|---|---|---|
| BAO | 80.00 | 34.98 ± 2.80 | 56.27 |
| Commercial activated carbon | 80.00 | 39.06 ± 2.21 | 51.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netto, A.M.; Nascimento, M.C.G.M.; de Caux, L.S.; Cortez, M.d.O.B.; Ferreira, J.P.R.; Monteiro, K.A.; Moreira, R.P.L. Tertiary Treatment of Pulp Industry Effluents Using Activated Biochar Derived from Biological Sludge Within a Circular Economy Framework. Processes 2025, 13, 1647. https://doi.org/10.3390/pr13061647
Netto AM, Nascimento MCGM, de Caux LS, Cortez MdOB, Ferreira JPR, Monteiro KA, Moreira RPL. Tertiary Treatment of Pulp Industry Effluents Using Activated Biochar Derived from Biological Sludge Within a Circular Economy Framework. Processes. 2025; 13(6):1647. https://doi.org/10.3390/pr13061647
Chicago/Turabian StyleNetto, Antonio Machado, Marília Christian Gomes Morais Nascimento, Leonardo Souza de Caux, Marcela de Oliveira Brahim Cortez, José Pedro Rodrigues Ferreira, Keivison Almeida Monteiro, and Renata Pereira Lopes Moreira. 2025. "Tertiary Treatment of Pulp Industry Effluents Using Activated Biochar Derived from Biological Sludge Within a Circular Economy Framework" Processes 13, no. 6: 1647. https://doi.org/10.3390/pr13061647
APA StyleNetto, A. M., Nascimento, M. C. G. M., de Caux, L. S., Cortez, M. d. O. B., Ferreira, J. P. R., Monteiro, K. A., & Moreira, R. P. L. (2025). Tertiary Treatment of Pulp Industry Effluents Using Activated Biochar Derived from Biological Sludge Within a Circular Economy Framework. Processes, 13(6), 1647. https://doi.org/10.3390/pr13061647








