Potential of Sugarcane Biomass-Derived Biochars for the Controlled Release of Sulfentrazone in Soil Solutions
Abstract
1. Introduction
2. Material and Methods
2.1. Biochar Production
2.2. Characterization of the Biochars and the Soil
2.3. Chromatographic Analyses
2.4. Sorption Kinetics and Batch Sorption–Desorption Experiments in Authentic or Biochar-Amended Soil Samples
2.4.1. Kinetic Study
2.4.2. Sorption and Desorption Isotherms
2.5. Sequential Desorption Assay of SFZ Incorporated into Biochars and Soil
2.5.1. Incorporation of SFZ into the Biochars and Soil
2.5.2. Consecutive Desorptions
2.6. Bioassay
2.7. Statistical Analyses
3. Results and Discussion
3.1. Characterization of the Biochars and Soil
3.2. Kinetic Study
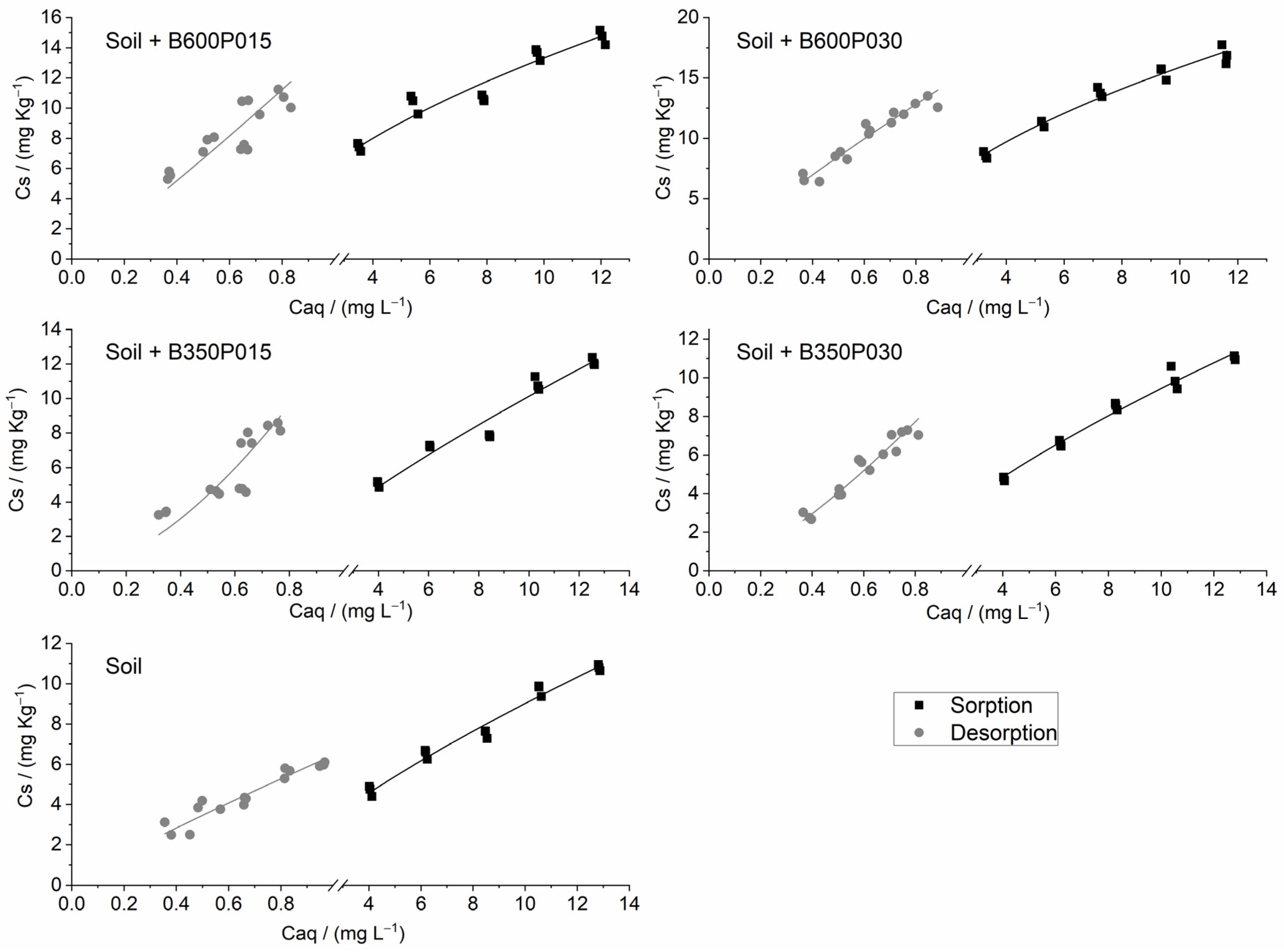
3.3. Sorption/Desorption of SFZ
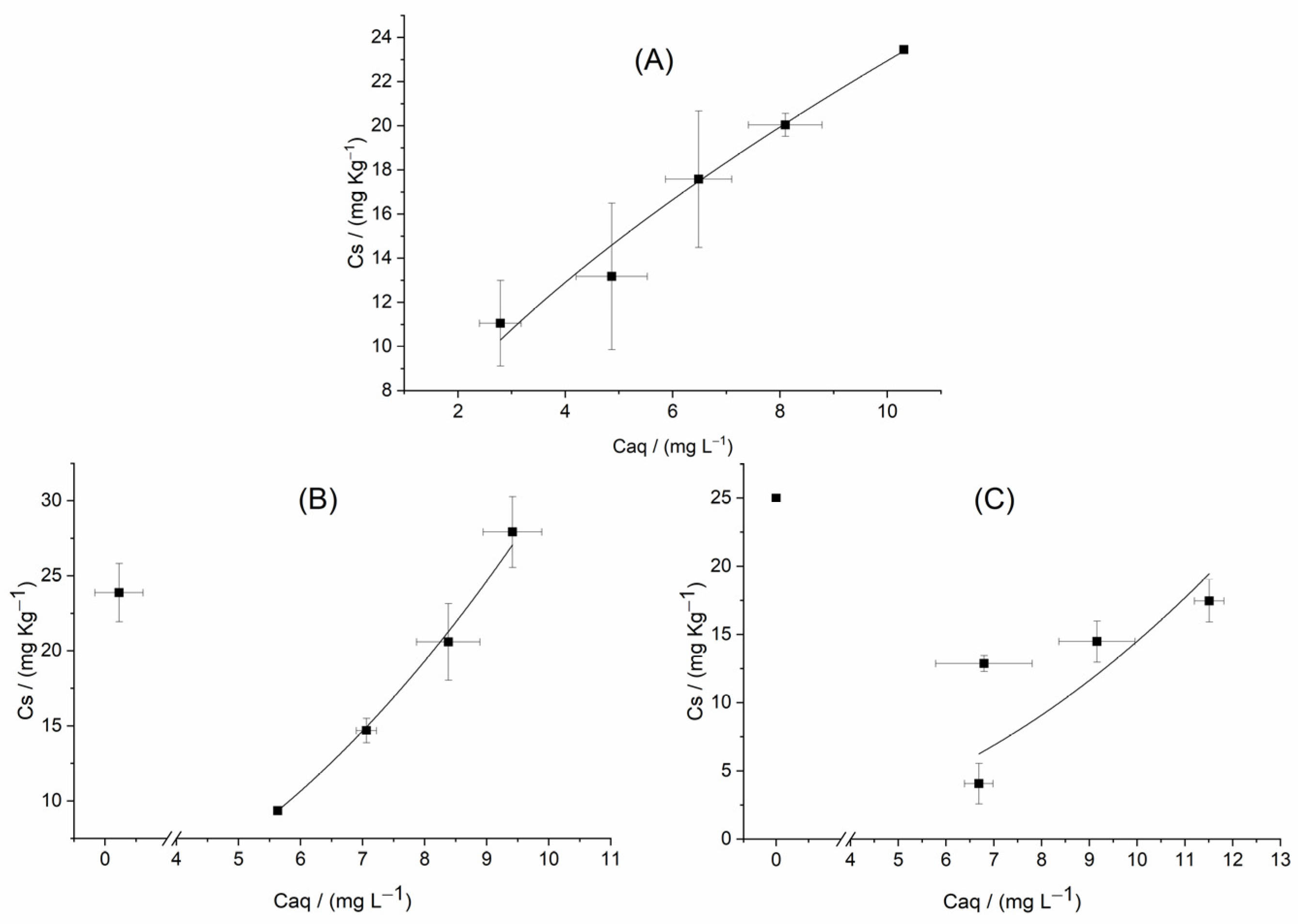
3.4. Sequential Release of SFZ in Soil and Biochars
3.5. Assessment of the Agronomic Efficiency of SFZ Incorporated into Biochars Through Bioassays
3.6. Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Yu, B.; Luo, K.; Liu, X.; Bai, L. Reduced Sulfentrazone Phytotoxicity through Increased Adsorption and Anionic Species in Biochar-Amended Soils. Environ. Sci. Pollut. Res. 2016, 23, 9956–9963. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; He, H.; Inthapanya, X.; Yang, C.; Lu, L.; Zeng, G.; Han, Z. Role of Biochar on Composting of Organic Wastes and Remediation of Contaminated Soils—A Review. Environ. Sci. Pollut. Res. 2017, 24, 16560–16577. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, W.; Sheng, G.D. Burned Rice Straw Reduces the Availability of Clomazone to Barnyardgrass. Sci. Total Environ. 2008, 392, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Gámiz, B.; Velarde, P.; Spokas, K.A.; Hermosín, M.C.; Cox, L. Biochar Soil Additions Affect Herbicide Fate: Importance of Application Timing and Feedstock Species. J. Agric. Food Chem. 2017, 65, 3109–3117. [Google Scholar] [CrossRef]
- Freitas, M.A.M.; Passos, A.; Torres, L.; Moraes, H.M.F.; Faustino, L.A.; Rocha, P.R.R.; Silva, A.A. Sulfentrazone Sorption in Different Types of Soil by Bioassays. Planta Daninha 2014, 32, 385–392. [Google Scholar] [CrossRef]
- Maski, D.; Durairaj, D. Effects of Charging Voltage, Application Speed, Target Height, and Orientation upon Charged Spray Deposition on Leaf Abaxial and Adaxial Surfaces. Crop. Prot. 2010, 29, 134–141. [Google Scholar] [CrossRef]
- Wen, P.; Wu, Z.; Han, Y.; Cravotto, G.; Wang, J.; Ye, B.C. Microwave-Assisted Synthesis of a Novel Biochar-Based Slow-Release Nitrogen Fertilizer with Enhanced Water-Retention Capacity. ACS Sustain. Chem. Eng. 2017, 5, 7374–7382. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Pandit, B.H.; Cornelissen, G.; Kammann, C.I. Biochar-Based Fertilization with Liquid Nutrient Enrichment: 21 Field Trials Covering 13 Crop Species in Nepal. Land. Degrad. Dev. 2017, 28, 2324–2342. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Medina, J.; González, A.; Diez, M.C.; Cartes, P.; Monreal, C.; Navia, R. Evaluation of Biodegradable Polymers as Encapsulating Agents for the Development of a Urea Controlled-Release Fertilizer Using Biochar as Support Material. Sci. Total Environ. 2015, 505, 446–453. [Google Scholar] [CrossRef]
- MAPA Agrofit. Available online: http://extranet.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 8 May 2025).
- ANVISA Monografia: Sulfentrazona. Available online: https://www.gov.br/anvisa/pt-br/setorregulado/regularizacao/agrotoxicos/monografias/monografias-autorizadas/q-r-s/4513json-file-1 (accessed on 8 May 2025).
- IUPAC Pesticide Properties Database. Available online: http://sitem.herts.ac.uk/aeru/iupac/Reports/601.htm (accessed on 30 September 2019).
- USEPA Fact Sheet for Sulfentrazone. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-129081_27-Feb-97.pdf (accessed on 28 May 2025).
- Wang, Z.; Han, L.; Sun, K.; Jin, J.; Ro, K.S.; Libra, J.A.; Liu, X.; Xing, B. Sorption of Four Hydrophobic Organic Contaminants by Biochars Derived from Maize Straw, Wood Dust and Swine Manure at Different Pyrolytic Temperatures. Chemosphere 2016, 144, 285–291. [Google Scholar] [CrossRef]
- Jiang, S.; Nguyen, T.A.H.; Rudolph, V.; Yang, H.; Zhang, D.; Ok, Y.S.; Huang, L. Characterization of Hard- and Softwood Biochars Pyrolyzed at High Temperature. Environ. Geochem. Health 2017, 39, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R.; Zimmerman, A.R. Effects of Biochar and Other Amendments on the Physical Properties and Greenhouse Gas Emissions of an Artificially Degraded Soil. Sci. Total Environ. 2014, 487, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Ahmedna, M.; Johns, M.M.; Clarke, S.J.; Marshall, W.E.; Rao, R.M. Potential of Agricultural By-Product-Based Activated Carbons for Use in Raw Sugar Decolourisation. J. Sci. Food Agric. 1997, 75, 117–124. [Google Scholar] [CrossRef]
- Gonçalves, G.d.C.; Pereira, N.C.; Veit, M.T. Production of Bio-Oil and Activated Carbon from Sugarcane Bagasse and Molasses. Biomass Bioenergy 2016, 85, 178–186. [Google Scholar] [CrossRef]
- Yang, Y.; Chun, Y.; Shang, G.; Huang, M. PH-Dependence of Pesticide Adsorption by Wheat-Residue-Derived Black Carbon. Langmuir 2004, 20, 6736–6741. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of Pyrolysis Temperature on Biochar Property and Function as a Heavy Metal Sorbent in Soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.I.; Wartelle, L.H.; Lima, I.M.; Klasson, K.T. Lead Retention by Broiler Litter Biochars in Small Arms Range Soil: Impact of Pyrolysis Temperature. J. Agric. Food Chem. 2012, 60, 5035–5044. [Google Scholar] [CrossRef]
- EMBRAPA. Manual de Métodos de Análise de Solo; Empresa Brasileira de Pesquisa Agropecuária: Rio de Janeiro, RJ, Brazil, 1997; ISBN 8585864036. [Google Scholar]
- ANVISA. Resolução Da Diretoria Colegiada No 166, de 24 de Julho de 2017. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2017/rdc0166_24_07_2017.pdf (accessed on 28 May 2025).
- SANTE. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed; SANTE/11813/2017. Available online: https://www.eurl-pesticides.eu/userfiles/file/eurlall/sante_11813_2017-fin.pdf (accessed on 28 May 2025).
- OECD. Test No. 106: Adsorption—Desorption Using a Batch Equilibrium Method; OECD Guidelines for the Testing of Chemicals, Section 1; OECD: Paris, France, 2000; ISBN 9789264069602. [Google Scholar]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q. Critical Review in Adsorption Kinetic Models. J. Zhejiang Univ.-Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A Review of the Kinetics Adsorption Models and Their Application to the Adsorption of Lead by an Activated Carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical Models of Sorption Kinetics Including a Surface Reaction Mechanism: A Review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. New Insights into Pseudo-Second-Order Kinetic Equation for Adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 275–278. [Google Scholar] [CrossRef]
- Sun, L.; Chen, D.; Wan, S.; Yu, Z. Performance, Kinetics, and Equilibrium of Methylene Blue Adsorption on Biochar Derived from Eucalyptus Saw Dust Modified with Citric, Tartaric, and Acetic Acids. Bioresour. Technol. 2015, 198, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, S.; Nie, G.; Zhang, Z.; Wang, J. Adsorption and Desorption of Herbicide Monosulfuron-Ester in Chinese Soils. J. Environ. Sci. 2011, 23, 1524–1532. [Google Scholar] [CrossRef]
- Hlaváčiková, H.; Novák, V.; Kameyama, K.; Brezianska, K.; Rodný, M.; Vitková, J. Two Types of Biochars: One Made from Sugarcane Bagasse, Other One Produced from Paper Fiber Sludge and Grain Husks and Their Effects on Water Retention of a Clay, a Loamy Soil and a Silica Sand. Soil Water Res. 2019, 14, 67–75. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-Logistic Analysis of Herbicide Dose-Response Relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Sun, X.; Shan, R.; Li, X.; Pan, J.; Liu, X.; Deng, R.; Song, J. Characterization of 60 Types of Chinese Biomass Waste and Resultant Biochars in Terms of Their Candidacy for Soil Application. GCB Bioenergy 2017, 9, 1423–1435. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Solar, J.; De Marco, I.; Caballero, B.M.; Rodriguez, N.; Agirre, I.; Adrados, A. Influence of Temperature and Residence Time in the Pyrolysis of Woody Biomass Waste in a Continuous Screw Reactor. Biomass Bioenergy 2016, 95, 416–423. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; de Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of Biochar Derived from Wood and High-Nutrient Biomasses with the Aim of Agronomic and Environmental Benefits. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liu, R. Effects of Pyrolysis Temperature and Heating Time on Biochar Obtained from the Pyrolysis of Straw and Lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Che, X.; Ding, Z.; Hu, X.; Creamer, A.E.; Chen, H.; Gao, B. Release of Soluble Elements from Biochars Derived from Various Biomass Feedstocks. Environ. Sci. Pollut. Res. 2016, 23, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Angin, D. Effect of Pyrolysis Temperature and Heating Rate on Biochar Obtained from Pyrolysis of Safflower Seed Press Cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, D. Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Ren, X.; Sun, H.; Wang, F.; Cao, F. The Changes in Biochar Properties and Sorption Capacities after Being Cultured with Wheat for 3 Months. Chemosphere 2016, 144, 2257–2263. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and Chemical Characterization of Biochars Derived from Different Agricultural Residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Zhang, X.; Silva, L.C.R.; Six, J.; Parikh, S.J. Use of Chemical and Physical Characteristics To Investigate Trends in Biochar Feedstocks. J. Agric. Food Chem. 2013, 61, 2196–2204. [Google Scholar] [CrossRef]
- Peruchi, L.M.; Fostier, A.H.; Rath, S. Sorption of Norfloxacin in Soils: Analytical Method, Kinetics and Freundlich Isotherms. Chemosphere 2015, 119, 310–317. [Google Scholar] [CrossRef]
- MacKay, A.A.; Vasudevan, D. Polyfunctional Ionogenic Compound Sorption: Challenges and New Approaches to Advance Predictive Models. Environ. Sci. Technol. 2012, 46, 9209–9223. [Google Scholar] [CrossRef]
- Keiluweit, M.; Kleber, M. Molecular-Level Interactions in Soils and Sediments: The Role of Aromatic π-Systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Mu, C.L.; Gu, C.; Liu, C.; Liu, X.J. Impact of Woodchip Biochar Amendment on the Sorption and Dissipation of Pesticide Acetamiprid in Agricultural Soils. Chemosphere 2011, 85, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Zhelezova, A.; Cederlund, H.; Stenström, J. Effect of Biochar Amendment and Ageing on Adsorption and Degradation of Two Herbicides. Water Air Soil Pollut. 2017, 228, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, C.; Guo, Y.; Wang, C. Experimental and Theoretical Studies on Methylene Blue and Methyl Orange Sorption by Wheat Straw-Derived Biochar with a Large Surface Area. Phys. Chem. Chem. Phys. 2016, 18, 30196–30203. [Google Scholar] [CrossRef]
- Poch, J.; Villaescusa, I. Orthogonal Distance Regression: A Good Alternative to Least Squares for Modeling Sorption Data. J. Chem. Eng. Data 2012, 57, 490–499. [Google Scholar] [CrossRef]
- Passos, A.B.R.J.; Freitas, M.A.M.; Torres, L.G.; Silva, A.A.; Queiroz, M.E.L.R.; Lima, C.F. Sorption and Desorption of Sulfentrazone in Brazilian Soils. J. Environ. Sci. Health B 2013, 48, 646–650. [Google Scholar] [CrossRef]
- IBAMA. Manual de Testes para Avaliação da Ecotoxicidade de Agentes Químicos, 2nd ed.; Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA): Brasília, DF, Brasil, 1990; p. 351.
- Cumming, G. Inference by Eye: Reading the Overlap of Independent Confidence Intervals. Stat. Med. 2009, 28, 205–220. [Google Scholar] [CrossRef]
- Cumming, G.; Finch, S. Inference by Eye Confidence Intervals and How to Read Pictures of Data. Am. Psychol. 2005, 60, 170–180. [Google Scholar] [CrossRef]
- Yu, X.; Pan, L.; Ying, G.; Kookana, R.S. Enhanced and Irreversible Sorption of Pesticide Pyrimethanil by Soil Amended with Biochars. J. Environ. Sci. 2010, 22, 615–620. [Google Scholar] [CrossRef]
- McBride, M.B. Organic Polluants in Soils. In Enviromental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994; p. 406. ISBN 0-19-507011-9. [Google Scholar]
- da Silva, M.R.F.; de Queiroz, M.E.L.R.; Neves, A.A.; da Silva, A.A.; de Oliveira, A.F.; Azevedo, M.M.; de Oliveira, R.L. Impact of Percentage and Particle Size of Sugarcane Biochar on the Sorption Behavior of Clomazone in Red Latosol. Ann. Braz. Acad. Sci. 2018, 90, 3745–3759. [Google Scholar] [CrossRef]
- Dionisio, A.C.; Rath, S. Abamectin in Soils: Analytical Methods, Kinetics, Sorption and Dissipation. Chemosphere 2016, 151, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Mamy, L.; Barriuso, E. Desorption and Time-Dependent Sorption of Herbicides in Soils. Eur. J. Soil Sci. 2007, 58, 174–187. [Google Scholar] [CrossRef]
- Borisover, M.; Gerstl, Z.; Burshtein, F.; Yariv, S.; Mingelgrin, U. Organic Sorbate-Organoclay Interactions in Aqueous and Hydrophobic Environments: Sorbate-Water Competition. Environ. Sci. Technol. 2008, 42, 7201–7206. [Google Scholar] [CrossRef] [PubMed]
- Leng, M.L.; Leovey, E.M.K.; Zubkoff, P.L. Agrochemical Environmental Fate: State of the Art; CRC Press: Boca Raton, FL, USA, 1995; ISBN 9781566700344. [Google Scholar]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in Adsorption. Part XI.* A System of Classification of Solution Adsorption Isotherms, and Its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids. J. Chem. Soc. 1960, 846, 3973–3993. [Google Scholar] [CrossRef]
- Lü, J.; Li, J.; Li, Y.; Chen, B.; Bao, Z. Use of Rice Straw Biochar Simultaneously as the Sustained Release Carrier of Herbicides and Soil Amendment for Their Reduced Leaching. J. Agric. Food Chem. 2012, 60, 6463–6470. [Google Scholar] [CrossRef]
- da Silva, M.; de Queiroz, M.; Neves, A.; da Silva, A.; de Oliveira, A.; de Oliveira, R.; Azevedo, M.; Pereira, G. Effect of the Incorporation of Sugarcane Bagasse Biochar in Leaching and Bioavailability of Clomazone in Soil. J. Braz. Chem. Soc. 2019, 30, 2386–2394. [Google Scholar] [CrossRef]
- Nag, S.K.; Kookana, R.; Smith, L.; Krull, E.; Macdonald, L.M.; Gill, G. Poor Efficacy of Herbicides in Biochar-Amended Soils as Affected by Their Chemistry and Mode of Action. Chemosphere 2011, 84, 1572–1577. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Saleem, M.; Yang, Y.; Zhang, Q. Effects of Corn Stalk Biochar and Pyrolysis Temperature on Wheat Seedlings Growth and Soil Properties Stressed by Herbicide Sulfentrazone. Environ. Technol. Innov. 2022, 25, 102208. [Google Scholar] [CrossRef]



| Sulfentrazone (SFZ) | Properties and Characteristics |
|---|---|
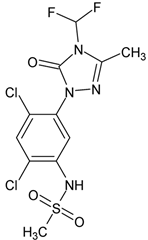 | Molecular formula: C11H10Cl2F2N4O3S |
| Molar mass: 387.19 g mol−1 | |
| Vapor pressure at 20 °C: 1.30 × 10−4 mPa | |
| log Kow at 20 °C and pH 7: 0.991 | |
| Water solubility at 20 °C: 780 mg L−1 | |
| pKa: 6.56 (weak acid) | |
| Aerobic half-life: 1.5 years | |
| Koc: 43 (cm3 g−1) |
| Kinetic Models | Kinetic Parameters | Treatments | ||
|---|---|---|---|---|
| Soil | Soil + B350 | Soil + B600 | ||
| Pseudo-first-order | (h−1) | 0.82 ± 0.22 | 0.70 ± 0.21 | 0.46 ± 0.11 |
| (mg g−1) | 4.93 ± 0.21 | 6.21 ± 0.28 | 11.48 ± 0.41 | |
| 0.997 | 0.993 | 0.988 | ||
| (mg g−1) | 0.68 | 0.91 | 1.21 | |
| Pseudo-second-order | (mg g−1 h−1) | 0.22 ± 0.07 | 0.15 ± 0.06 | 0.055 ± 0.016 |
| (mg g−1) | 5.32 ± 0.23 | 6.73 ± 0.34 | 12.58 ± 0.49 | |
| 0.998 | 0.995 | 0.992 | ||
| (mg g−1) | 0.56 | 0.79 | 0.95 | |
| Elovich | (mg g−1 h−1) | 68.0 ± 51.7 | 76.2 ± 79.9 | 57.4 ± 33.3 |
| (g mg−1) | 1.45 ± 0.19 | 1.15± 0.21 | 0.54 ± 0.07 | |
| 0.999 | 0.996 | 0.995 | ||
| (mg g−1) | 0.79 | 0.70 | 0.43 | |
| Weber-Morris | (mg g−1 h−1/2) | 0.56± 0.06 | 0.69 ± 0.16 | 1.37 ± 0.13 |
| 2.72 ± 0.19 | 3.40 ± 0.56 | 5.64 ± 0.46 | ||
| 0.999 | 0.992 | 0.994 | ||
| (mg g−1) | 0.38 | 0.84 | 1.01 | |
| Experiment | Evaluated Phenomenon | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| Kf (µg1−1/n(cm−3)1/ng−1) | CI of Kf a | 1/n | CI of 1/n | R2 | sres | H b | ||
| Soil | Sor c | 1.631 ± 0.138 | 1.333–1.929 | 0.742 ± 0.037 | 0.663–0.822 | 0.995 | 0.152 | 1.212 |
| Des d | 6.449 ± 0.216 | 5.982–6.916 | 0.900 ± 0.089 | 0.708–1.092 | 0.999 | 0.071 | ||
| Soil + B350P015 | Sor | 1.615 ± 0.210 | 1.161–2.068 | 0.797 ± 0.057 | 0.675–0.920 | 0.988 | 0.191 | 2.088 |
| Des | 13.977± 2.048 | 9.552–18.402 | 1.665 ± 0.279 | 1.061–2.269 | 0.999 | 0.075 | ||
| Soil + B350P030 | Sor | 1.780 ± 0.142 | 1.473–2.087 | 0.725 ± 0.035 | 0.650–0.800 | 0.995 | 0.151 | 1.902 |
| Des | 10.516 ± 0.554 | 9.318–11.714 | 1.379 ± 0.108 | 1.147–1.611 | 0.999 | 0.053 | ||
| Soil + B600P015 | Sor | 3.694 ± 0.407 | 2.815–4.573 | 0.557 ± 0.050 | 0.450–0.664 | 0.987 | 0.213 | 1.995 |
| Des | 14.388 ± 1.318 | 11.540–17.236 | 1.112 ± 0.192 | 0.697–1.526 | 0.999 | 0.079 | ||
| Soil + B600P030 | Sor | 4.605 ± 0.322 | 3.909–5.301 | 0.538 ± 0.032 | 0.468–0.607 | 0.996 | 0.178 | 1.635 |
| Des | 15.586 ± 0.531 | 14.440–16.732 | 0.879 ± 0.075 | 0.713–1.041 | 0.999 | 0.059 |
| Experiment | Freundlich | |||||
|---|---|---|---|---|---|---|
| Kf’ (µg1−1/n(cm−3)1/ng−1) | CI of Kf’ a | 1/n | CI of 1/n b | R2 | sres | |
| Soil-SFZ | 5.403 ± 0.556 | 3.634–7.172 | 0.628 ± 0.047 | 0.479–0.777 | 0.999 | 0.262 |
| Soil + B350-SFZ | 0.262 ± 0.023 | 0.162–0.362 | 2.069 ± 0.050 | 1.852–2.286 | 0.998 | 0.383 |
| Soil + B600-SFZ | 0.117 ± 0.312 | −1.224–1.458 | 2.091 ± 1.135 | −2.787–6.981 | 0.953 | 2.279 |
| Treatments | Equation | C50 (kg ha−1) | CI of C50 | R2 |
|---|---|---|---|---|
| Soil-SFZ a | Y = 98.011 + (−0.009 − 98.011)/(1 + (x/0.391)3.414) | 0.392 ± 0.016 | 0.347–0.435 | 0.999 |
| Soil+B350P015-SFZ b | Y = 100.82 + (3.486 × 10−6 − 100.82)/(1 + (x/0.482)2.217) | 0.482 ± 0.148 | 0.072–0.893 | 0.999 |
| Soil+B350P030-SFZ c | Y = 99.950 + (9.804 × 10−6 − 99.950)/(1 + (x/0.713)3.691) | 0.713 ± 0.065 | 0.532–0.894 | 0.999 |
| Soil+B600P015-SFZ d | Y = 100.50 + (0.019 − 100.50)/(1 + (x/1.395)4.802) | 1.395 ± 0.181 | 0.893–1.897 | 0.999 |
| Soil+B600P030-SFZ e | Y = 93.889 + (−0.028 − 93.889)/(1 + (x/2.547)8.400) | 2.547 ± 0.171 | 2.072–3.022 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.R.F.; Queiroz, M.E.L.R.; Neves, A.A.; da Silva, A.A.; de Oliveira, A.F.; Miranda, L.D.L.; Souza, R.A.R.; Rodrigues, A.A.Z.; da Rocha, J.G. Potential of Sugarcane Biomass-Derived Biochars for the Controlled Release of Sulfentrazone in Soil Solutions. Processes 2025, 13, 1965. https://doi.org/10.3390/pr13071965
da Silva MRF, Queiroz MELR, Neves AA, da Silva AA, de Oliveira AF, Miranda LDL, Souza RAR, Rodrigues AAZ, da Rocha JG. Potential of Sugarcane Biomass-Derived Biochars for the Controlled Release of Sulfentrazone in Soil Solutions. Processes. 2025; 13(7):1965. https://doi.org/10.3390/pr13071965
Chicago/Turabian Styleda Silva, Marcos R. F., Maria Eliana L. R. Queiroz, Antônio A. Neves, Antônio A. da Silva, André F. de Oliveira, Liany D. L. Miranda, Ricardo A. R. Souza, Alessandra A. Z. Rodrigues, and Janilson G. da Rocha. 2025. "Potential of Sugarcane Biomass-Derived Biochars for the Controlled Release of Sulfentrazone in Soil Solutions" Processes 13, no. 7: 1965. https://doi.org/10.3390/pr13071965
APA Styleda Silva, M. R. F., Queiroz, M. E. L. R., Neves, A. A., da Silva, A. A., de Oliveira, A. F., Miranda, L. D. L., Souza, R. A. R., Rodrigues, A. A. Z., & da Rocha, J. G. (2025). Potential of Sugarcane Biomass-Derived Biochars for the Controlled Release of Sulfentrazone in Soil Solutions. Processes, 13(7), 1965. https://doi.org/10.3390/pr13071965







