Fe2+-Coupled Organic-Substrate-Enhanced Nitrogen Removal in Two-Stage Anammox Biofilm Reactors
Abstract
1. Introduction
2. Materials and Methods
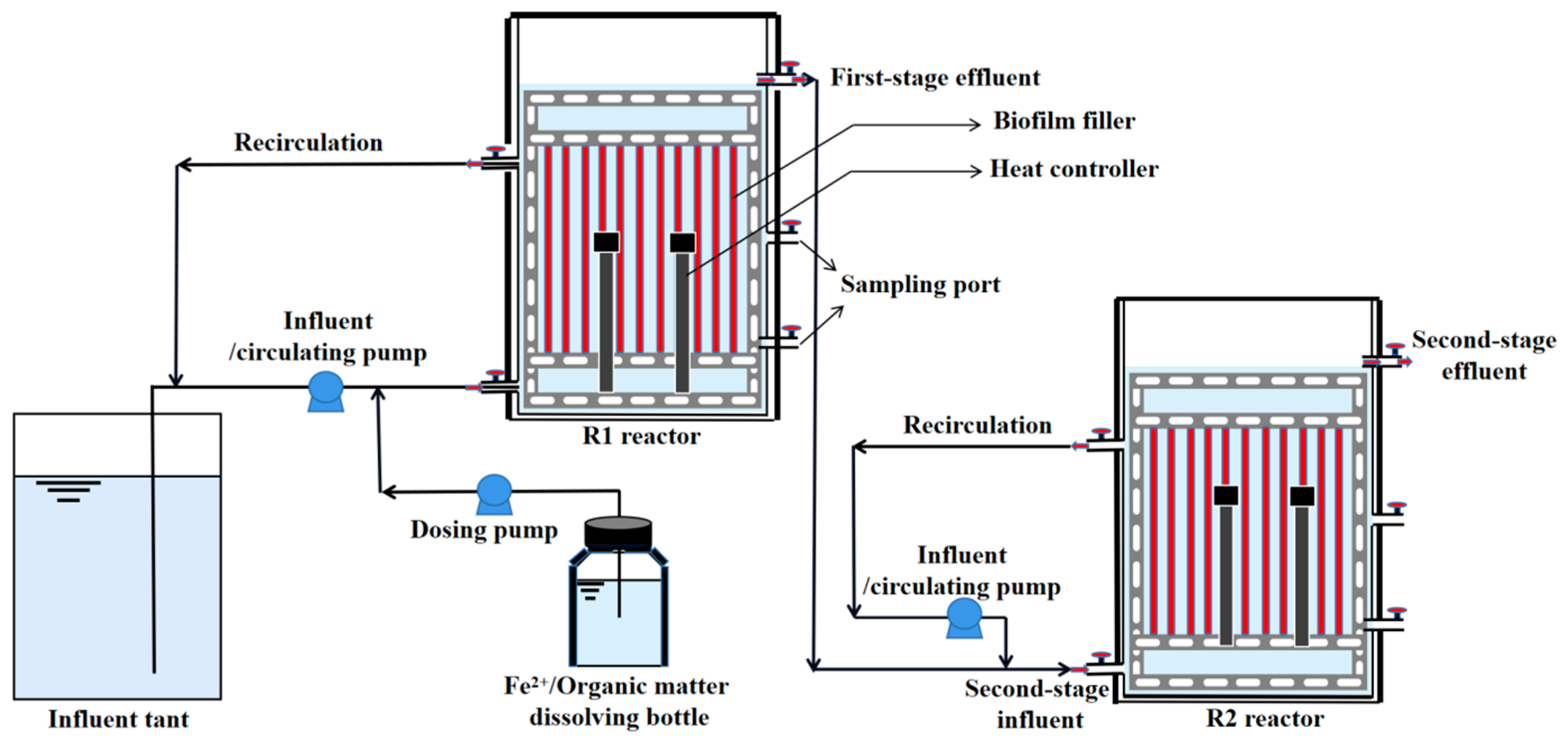
2.1. Reactor Configuration and Operational Strategy
2.2. Sludge Inoculation and Synthetic Wastewater Feeding
2.3. Water Quality Analysis and Calculations
2.4. Extraction and Analysis of Extracellular Polymeric Substance (EPS)
2.5. Extraction and Determination of Heme C
2.6. Scanning Electron Microscopy (SEM)
2.7. Data Analysis and Visualization
3. Results and Discussion
3.1. Effect of Organic Substrate on the Performance of the Two-Stage Anammox System
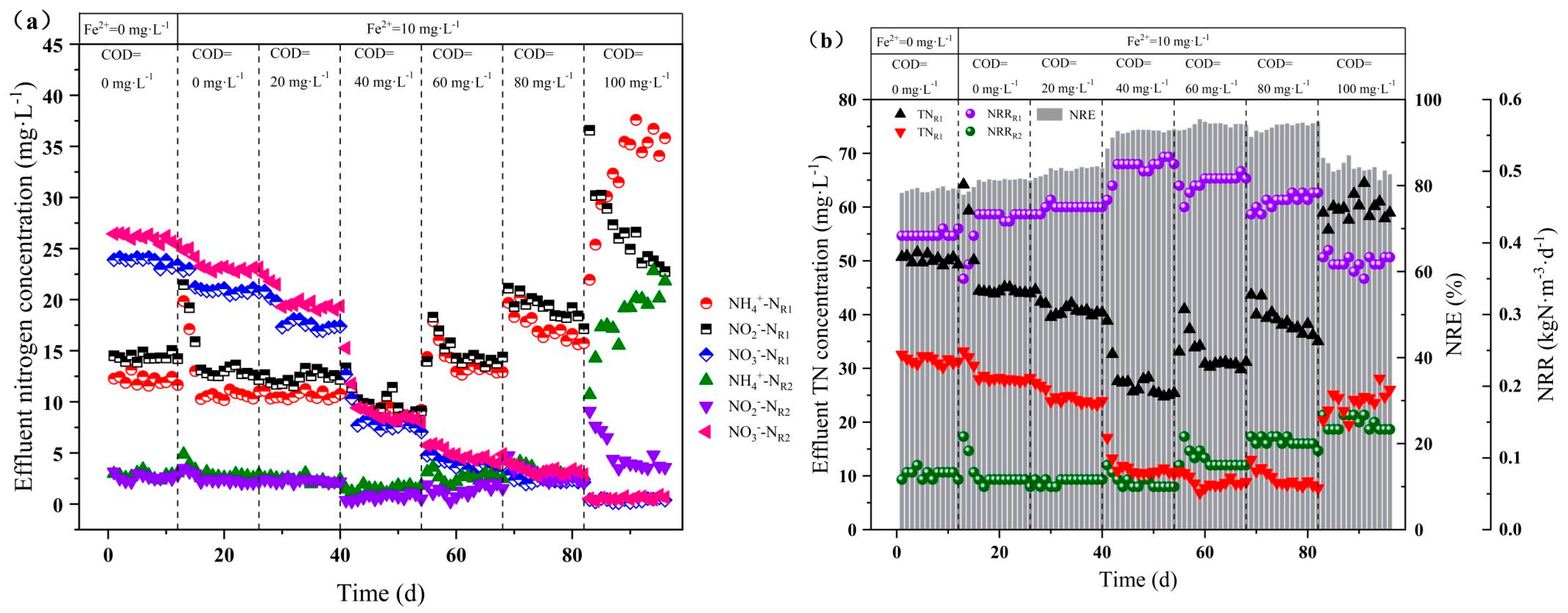
3.1.1. Effect of Organic Substrate Concentration on Nitrogen Removal
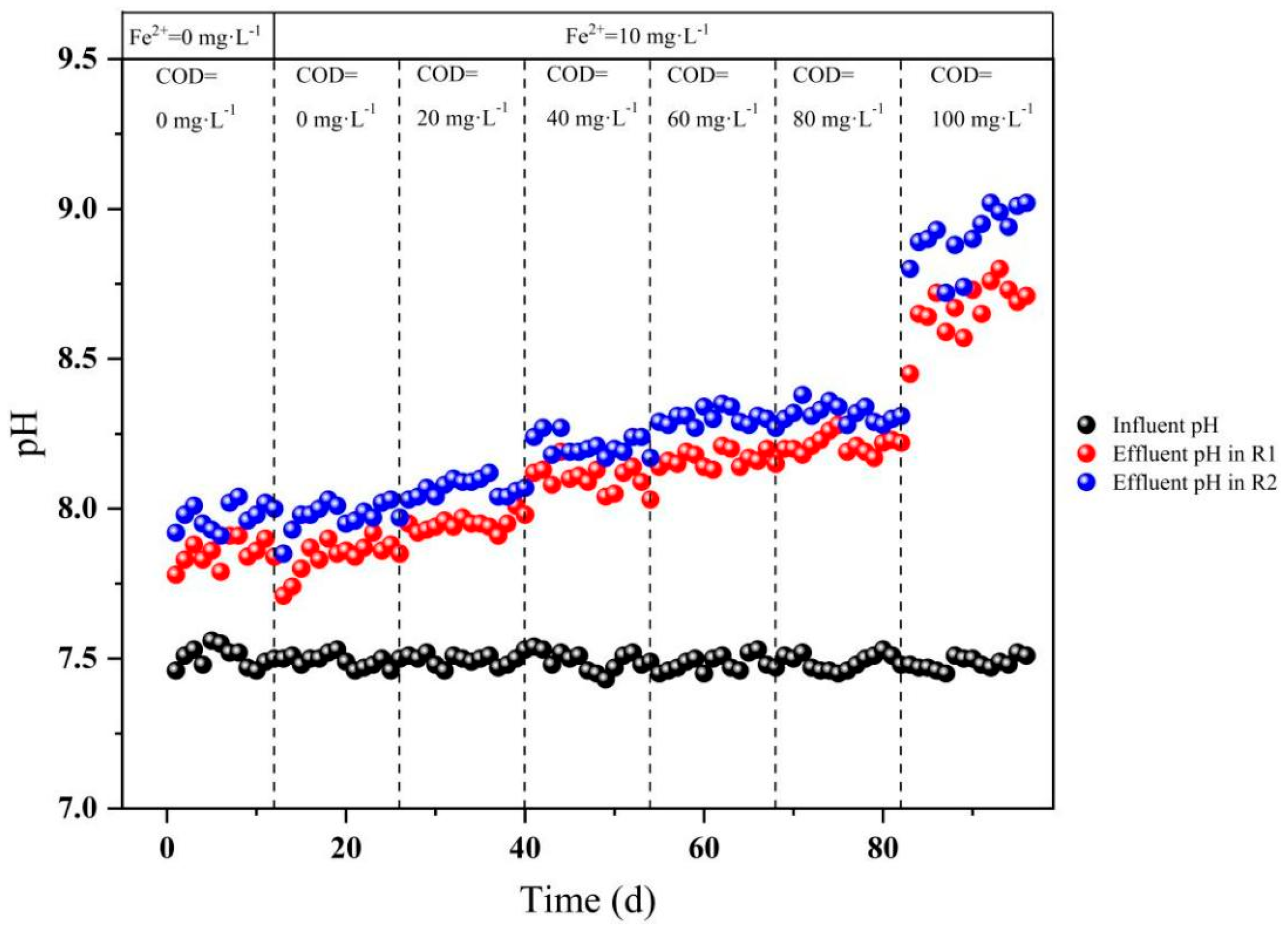
3.1.2. Analysis of pH Variations and Nitrogen Stoichiometric Ratios
3.2. Effects of Fe2+ Coupled with Organic Substrates on the Two-Stage Anammox System
3.2.1. Effect of Fe2+ on Nitrogen Removal
3.2.2. Effect of Fe2+ Coupled with Organic Substrates on Nitrogen Removal
3.2.3. Analysis of pH Dynamics and Nitrogen Stoichiometry
3.3. Effects of Fe2+ Coupled with Organic Substrates on Anammox Biofilm Morphology
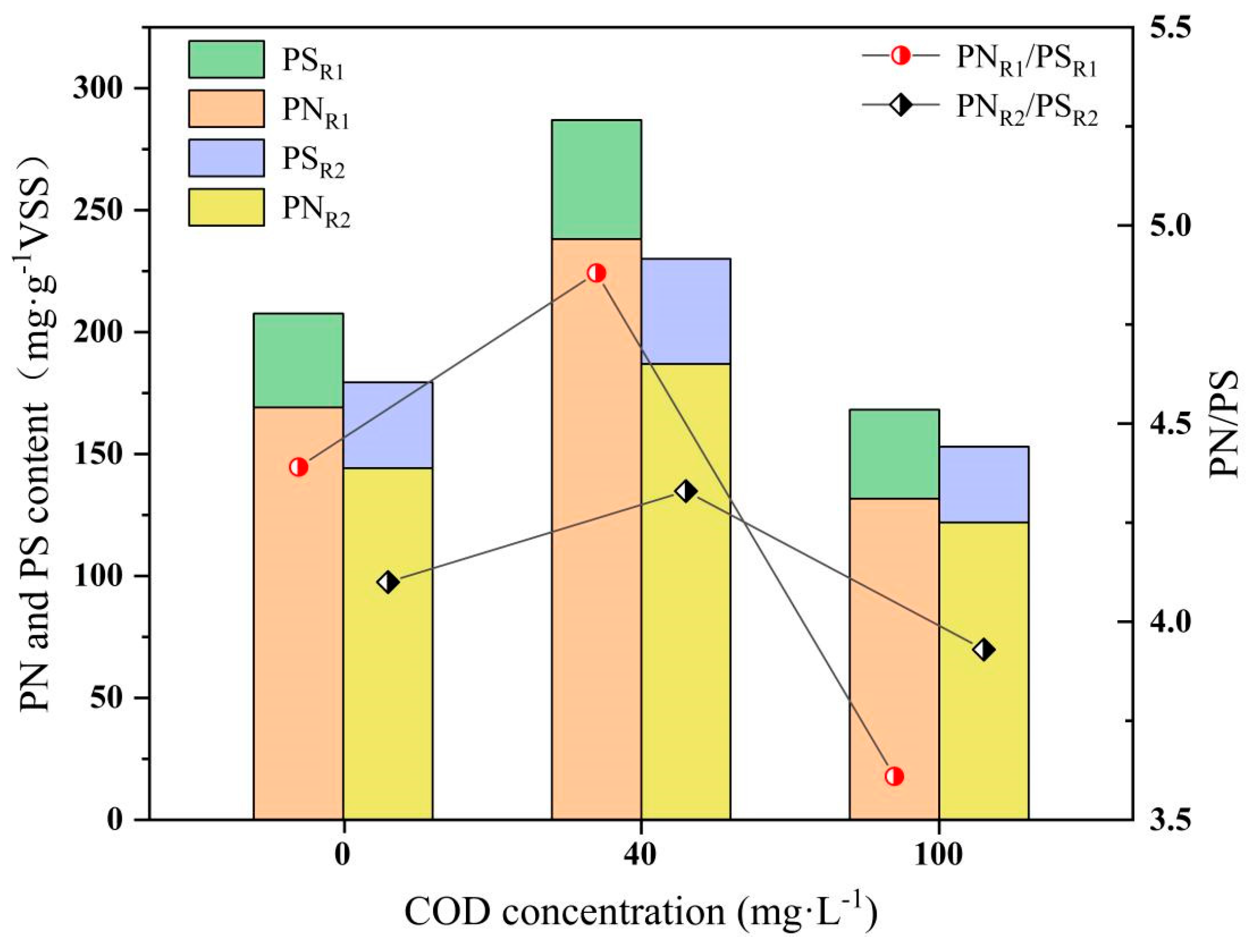
3.4. EPS Variation
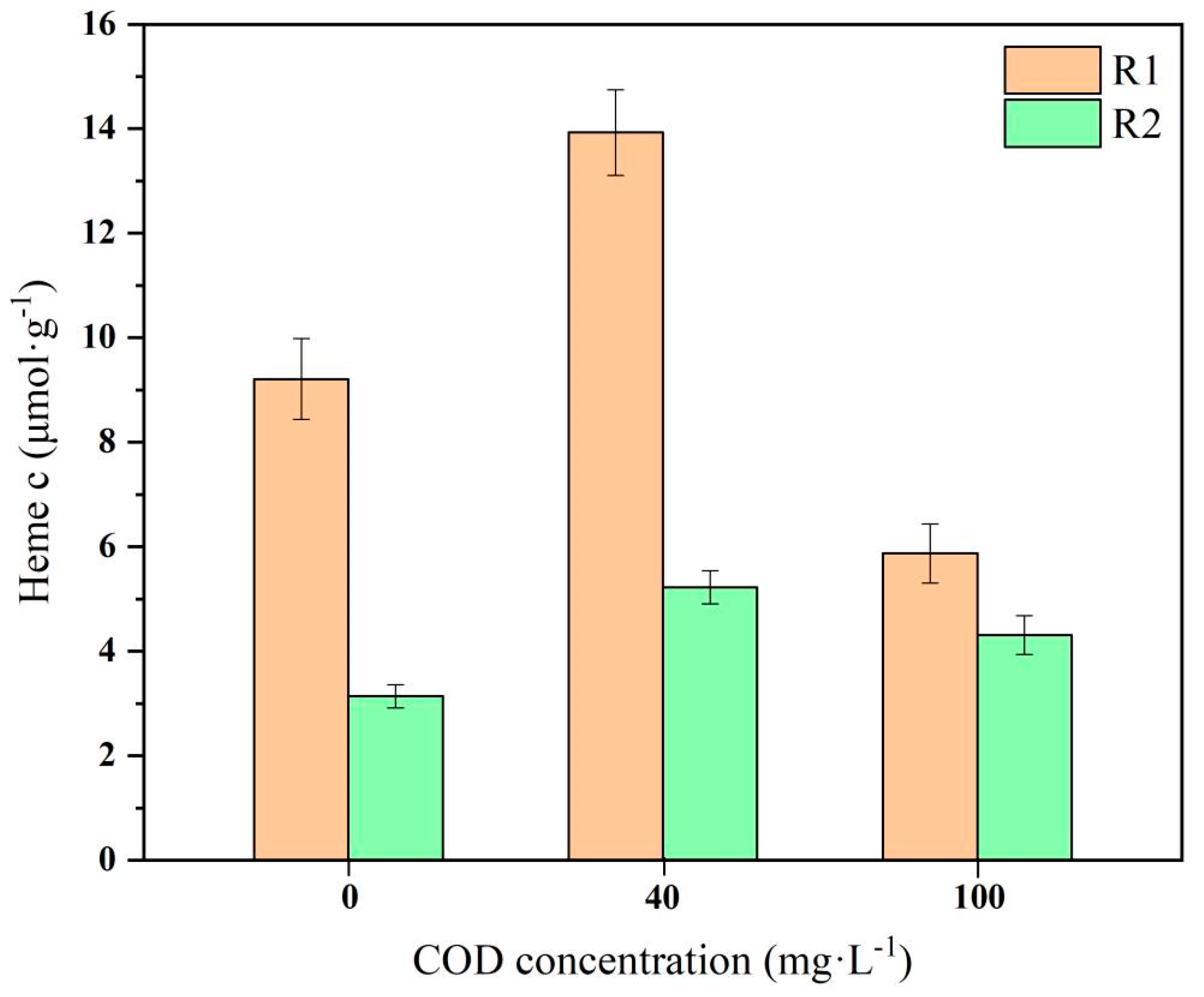
3.5. Variation in Cytochrome C Content
4. Conclusions
- (1)
- Low concentrations of sodium acetate (10–20 mg/L COD) had no inhibitory effect on nitrogen removal, while 40 mg/L COD significantly enhanced anammox performance, achieving an average NRE of 90.02%. In contrast, 60 mg/L COD led to the significant inhibition of the anammox process.
- (2)
- The optimal influent Fe2+ concentration was determined to be 10 mg/L for the enhancement of the nitrogen removal performance in a two-stage anammox system. Under this Fe2+ addition, the system showed enhanced NRE with increasing COD up to 40 mg/L. Under the condition of 10 mg/L Fe2+ coupled with 60 mg/L COD, the two-stage anammox system achieved the highest NRE, with an average NRE of 94.11%. When COD was further increased to 100 mg/L, denitrification was intensified while anammox activity in R1 was severely inhibited, resulting in a decline in the overall nitrogen removal performance.
- (3)
- At a COD concentration of 40 mg/L, both EPS and cytochrome c contents in anammox biofilm were significantly elevated compared to the condition with 10 mg/L Fe2+ alone. However, higher COD concentrations resulted in a decline in these indicators. SEM analysis further revealed that under the influence of Fe2+ coupled with organic substrates, the sludge surface exhibited abundant granular protrusions and porous structures, which contributed to the morphological stability of the biofilm. These findings provide insight into the regulatory role of Fe2+ and organic substrates on anammox technology and offer a theoretical basis for its application in low-strength nitrogen wastewater treatment.
- (4)
- This study demonstrated that an Fe2+-coupled organic substrate strategy could significantly enhance anammox activity and nitrogen removal efficiency under low-strength nitrogen conditions, with optimal performance observed at moderate COD levels. However, the observed inhibitory effects at higher COD concentrations underscored a key limitation of this approach when applied to real wastewater, which often exhibits fluctuating and complex compositions. In real-world treatment settings, the variability in COD levels and the presence of diverse organic compounds may reduce system stability and efficiency. Therefore, while the proposed strategy shows promise for enhancing anammox-based nitrogen removal, it is essential to further investigate its robustness across a broader range of wastewater characteristics, including varying COD/N ratios, organic compositions, and potential inhibitory substances. Future research should focus on optimizing operational parameters and pretreatment strategies to mitigate the effects of excessive or inhibitory organics, thereby ensuring reliable application in full-scale wastewater treatment systems.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AnAOB | Anaerobic ammonium-oxidizing bacteria |
| TCA | Tricarboxylic acid |
| EPS | Extracellular polymeric substances |
| NRE | Total nitrogen removal efficiency |
| SI | Sponge iron |
| NLR | Nitrogen loading rate |
| TN | Total nitrogen |
| NH4+-N | Ammonium–nitrogen |
| NO2−-N | Nitrite–nitrogen |
| NRR | Nitrogen removal rate |
| COD | Chemical oxygen demand |
| MLVSS | Mixed liquor volatile suspended solids |
| MLSS | Mixed liquor suspended solids |
| DO | Dissolved oxygen |
| HRT | Hydraulic retention time |
| PBS | Phosphate-buffered saline |
| PN | Protein |
| PS | Polysaccharide |
| SEM | Scanning electron microscopy |
| FA | Free ammonia |
| FNA | Free nitrous acid |
| NDFO | Nitrogen-dependent ferrous oxidation |
References
- Xu, Y.; Xu, Y.; Li, T.; Wang, G.; Dai, X. Two-step partial nitrification-anammox process for treating thermal-hydrolysis anaerobic digester effluent: Start-up and microbial characterisation. J. Clean. Prod. 2020, 252, 119784. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.; Sun, C.; Guo, H.; Xi, Z.; Zhang, T.; Hu, Y.; Li, Y.-Y.; Kong, Z. Characterization of start-up, long-term performance and resistance to ammonia inhibition during anaerobic treatment of high-strength pharmaceutical wastewater by up-flow anaerobic blanket reactor integrated with partial nitritation-anammox. J. Water Process Eng. 2024, 66, 105944. [Google Scholar] [CrossRef]
- Chen, X.Z.; Wang, X.J.; Zhong, Z.; Deng, C.; Chen, Z.; Chen, X. Biological nitrogen removal via combined processes of denitrification, highly efficient partial nitritation and Anammox from mature landfill leachate. Environ. Sci. Pollut. Res. 2020, 27, 29408–29421. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Z.; Liao, J.; Dai, R.; Lin, J.G.; Ling, J.; Xu, Y. Micro-aeration and low influent C/N are key environmental factors for achieving ANAMMOX in livestock farming wastewater treatment plants. Water Res. 2024, 253, 120141. [Google Scholar] [CrossRef]
- Yin, X.; Qiao, S.; Zhou, J.T. Using electric field to enhance the activity of anammox bacteria. Appl. Microbiol. Biotechnol. 2015, 99, 6921–6930. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Zhang, H.; Zhao, H.; Sun, B.; Wang, T.; Guo, K.; Dong, K.; Gu, S.; Wang, L. Novel insights into intrinsic mechanisms of magnetic field on long-term performance of anaerobic ammonium oxidation process. Bioresour. Technol. 2024, 402, 130839. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Sun, Y.; Zhou, S.; Li, L.; Shao, J. Using low frequency and intensity ultrasound to enhance start-up and operation performance of Anammox process inoculated with the conventional sludge. Ultrason. Sonochem. 2018, 42, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Chen, Z.; Zhou, Y.; Ma, Y.; Ma, C.; Li, Y.; Liang, Y.; Jia, J. Impacts of the heavy metals Cu (II), Zn (II) and Fe (II) on an Anammox system treating synthetic wastewater in low ammonia nitrogen and low temperature: Fe (II) makes a difference. Sci. Total Environ. 2019, 648, 798–804. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Huang, W.; Wang, D.; Yang, C.; Han, H. Unveiling environmental adaptability of magnetite-mediated Feammox system: Multiple pathways identification, metabolic responses and engineering potential analysis. Chem. Eng. J. 2025, 511, 161921. [Google Scholar] [CrossRef]
- Ferousi, C.; Lindhoud, S.; Baymann, F.; Kartal, B.; Jetten, M.S.; Reimann, J. Iron assimilation and utilization in anaerobic ammonium oxidizing bacteria. Curr. Opin. Chem. Biol. 2017, 37, 129–136. [Google Scholar] [CrossRef]
- Zhang, J.X.; Zhang, Y.B.; Yang, L.; Zhang, L.; Qiao, S.; Yang, F.; Quan, X. Enhancement of nitrogen removal in a novel anammox reactor packed with Fe electrode. Bioresour. Technol. 2012, 114, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Oikonomidis, I.; Burrows, L.J.; Carliell-Marquet, C.M. Mode of action of ferric and ferrous iron salts in activated sludge. J. Chem. Technol. Biotechnol. 2010, 85, 1067–1076. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhou, Y.; Zhao, S.Y.; Zhang, R.; Peng, Z.; Zhai, H.; Zhang, H. Effect of Fe (II) in low-nitrogen sewage on the reactor performance and microbial community of an ANAMMOX biofilter. Chemosphere 2018, 200, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guan, Z.; Li, B.; You, Y.; Cao, G.; Fan, Y.; Wu, J.; Ji, J. Denitrification enhancement, kinetic simulation and microbial assessment in the sponge iron-mediated anammox process: A method for low-strength nitrogen wastewater treatment. J. Water Process Eng. 2025, 72, 107550. [Google Scholar] [CrossRef]
- Xing, F.; Ma, X.; Sun, B.; Wang, T.; Lian, F.; Wang, L.; Fu, Z. Enhancing anammox granular sludge for mainstream anammox process by adding iron-loaded diatomite: Performance and intrinsic mechanism. Environ. Res. 2025, 268, 120806. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Jiang, Y.; Guo, M.; Cui, M.; Zhang, T.C. Effects of organic-matter-induced short-term stresses on performance and population dynamics of anammox systems. J. Environ. Eng. 2020, 146, 04020120. [Google Scholar] [CrossRef]
- Yin, X.; Rahaman, H.; Liu, W.; Mąkinia, J.; Zhai, J. Comparison of nitrogen and VFA removal pathways in autotrophic and organotrophic anammox reactors. Environ. Res. 2021, 197, 111065. [Google Scholar] [CrossRef]
- Hou, J.; You, G.X.; Yi, X.; Wang, C.; Wang, P.; Miao, L.; Li, Y.; Ao, Y.; Lv, B.; Yang, Y. Long-term effects of CuO nanoparticles on the surface physicochemical properties of biofilms in a sequencing batch biofilm reactor. Appl. Microbiol. Biotechnol. 2016, 100, 9629–9639. [Google Scholar] [CrossRef]
- Ma, H.Y.; Zhang, Y.L.; Xue, Y.; Zhang, Y.; Li, Y.Y. Relationship of heme c, nitrogen loading capacity and temperature in anammox reactor. Sci. Total Environ. 2019, 659, 568–577. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, Y.; Guo, Y.; Liu, S. Microbial transcript and metabolome analysis uncover discrepant metabolic pathways in autotrophic and mixotrophic anammox consortia. Water Res. 2018, 128, 402–411. [Google Scholar] [CrossRef]
- Jenni, S.; Vlaeminck, S.E.; Morgenroth, E.; Udert, K.M. Successful application of nitritation/anammox to wastewater with elevated organic carbon to ammonia ratios. Water Res. 2014, 49, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Thi, S.S.; Wuertz, S. Inhibition factors and kinetic model for anaerobic ammonia oxidation in a granular sludge bioreactor with Candidatus Brocadia. Chem. Eng. J. 2020, 389, 123618. [Google Scholar] [CrossRef]
- Zhang, L.; Okabe, S. Ecological niche differentiation among anammox bacteria. Water Res. 2020, 171, 115468. [Google Scholar] [CrossRef]
- Oshiki, M.; Satoh, H.; Okabe, S. Ecology and physiology of anaerobic ammonium oxidizing bacteria. Environ. Microbiol. 2016, 18, 2784–2796. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Ruan, W.; Ren, H.; Zhao, M. ANAMMOX performance, granulation, and microbial response under COD disturbance. J. Chem. Technol. Biotechnol. 2015, 90, 139–148. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Y.; Liu, J.; Adams, M.; Chang, Y.; Guo, M.; Xie, J.; Xie, J. The structure of anammox granular sludge under varying long-term organic matter stress: Performance, physiochemical and microbial community. J. Clean. Prod. 2021, 323, 129117. [Google Scholar] [CrossRef]
- Liu, Y.; An, T.; Xie, J.; Tang, K.; Wu, P.; Liu, W.; Sun, F.; Gayflor, S.F.; Chen, C. The influencing mechanisms and optimization strategies of organics on anammox process: A critical review. Chem. Eng. J. 2024, 493, 152743. [Google Scholar] [CrossRef]






| Content | Concentration (mg/L) |
|---|---|
| KH2PO4 | 68 |
| MgSO4·7H2O | 150 |
| CaCl2 | 68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Ge, Q.; Li, S.; Wang, X.; Zheng, X.; Chen, Z. Fe2+-Coupled Organic-Substrate-Enhanced Nitrogen Removal in Two-Stage Anammox Biofilm Reactors. Processes 2025, 13, 1603. https://doi.org/10.3390/pr13051603
Bao Y, Ge Q, Li S, Wang X, Zheng X, Chen Z. Fe2+-Coupled Organic-Substrate-Enhanced Nitrogen Removal in Two-Stage Anammox Biofilm Reactors. Processes. 2025; 13(5):1603. https://doi.org/10.3390/pr13051603
Chicago/Turabian StyleBao, Yingchun, Qilong Ge, Siyuan Li, Xiaowei Wang, Xuwen Zheng, and Zhenguo Chen. 2025. "Fe2+-Coupled Organic-Substrate-Enhanced Nitrogen Removal in Two-Stage Anammox Biofilm Reactors" Processes 13, no. 5: 1603. https://doi.org/10.3390/pr13051603
APA StyleBao, Y., Ge, Q., Li, S., Wang, X., Zheng, X., & Chen, Z. (2025). Fe2+-Coupled Organic-Substrate-Enhanced Nitrogen Removal in Two-Stage Anammox Biofilm Reactors. Processes, 13(5), 1603. https://doi.org/10.3390/pr13051603




