Comparative Analysis of Sulfuric Acid Alkylation Technologies Based on a Reaction Kinetic Model
Abstract
1. Introduction
2. Industrial Alkylation Technology
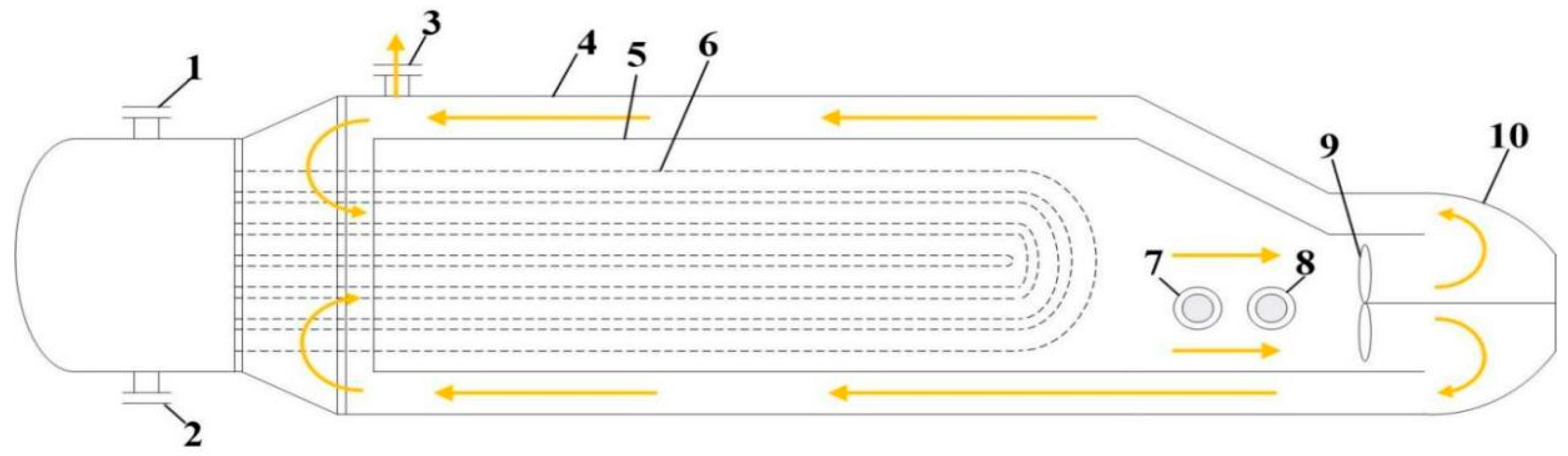
2.1. STRATCO Alkylation Technology
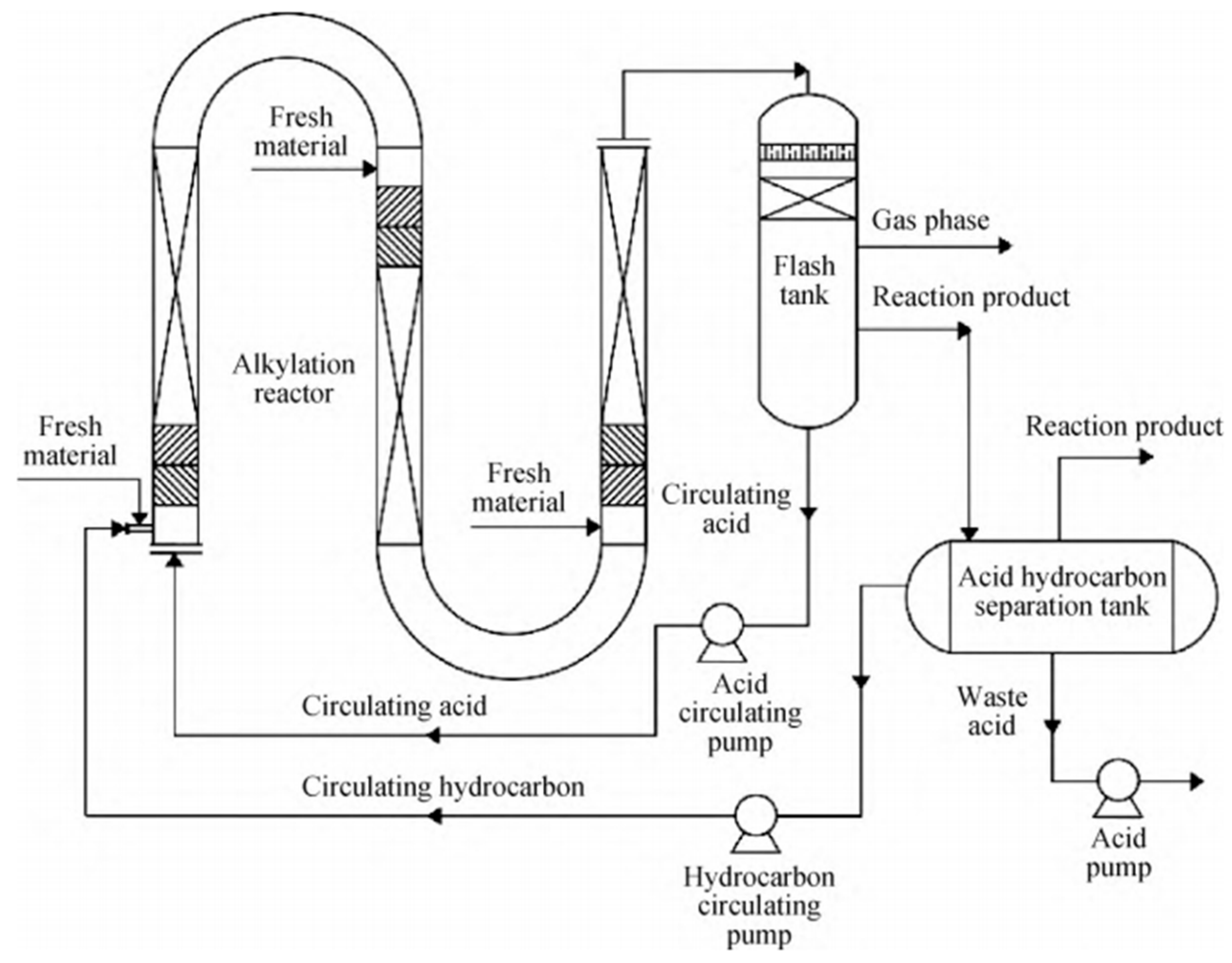
2.2. SINOALKY Alkylation Technology
3. Modelling Methodology
3.1. Reactor Model
3.1.1. STRATCO Reactor Model
- (a)
- Hydrocarbon-phase and acid-phase flows are modelled using a plug flow reactor (PFR) approach, with undetectable olefin concentrations at the outlet—indicating that the alkylation reactions are completed.
- (b)
- Given the typical industrial practice of maintaining constant impeller speeds, the circulating flow rate in the draft tube can be assumed as a fixed value.
- (c)
- The STRATCO reactor maintains near-isothermal conditions by extracting heat generated from alkylation reactions in the shell side through the reaction effluent in U-tubes, while the impeller-driven high fluid velocity ensures efficient heat removal, thereby stabilizing the shell-side temperatures. Distributed control system (DCS) data reveal that the temperature does not change significantly across the reactor, justifying the isothermal reactor assumption.
3.1.2. SINOALKY Reactor Model
- (a)
- The reactor is a plug flow reactor with three-stage feeding. The radial concentration distribution inside is uniform, and only the changes in concentration and temperature along the axial flow direction are considered.
- (b)
- In a typical alkylation process, the reactor internals are characterized by a sulfuric acid continuous phase and a hydrocarbon dispersed phase. Therefore, the acid-to-hydrocarbon ratio is operationally controlled within the range of 1.1–1.2. At the inlet of the SINOALKY reactor, the acid and hydrocarbon phases are rapidly homogenized into an emulsion phase via a static mixer, creating a pseudo-homogeneous fluid. The liquid-phase reaction system of sulfuric-acid-based alkylation is treated as a pseudo-homogeneous phase. The sulfuric acid concentration has a significant impact on the catalytic reaction rate.
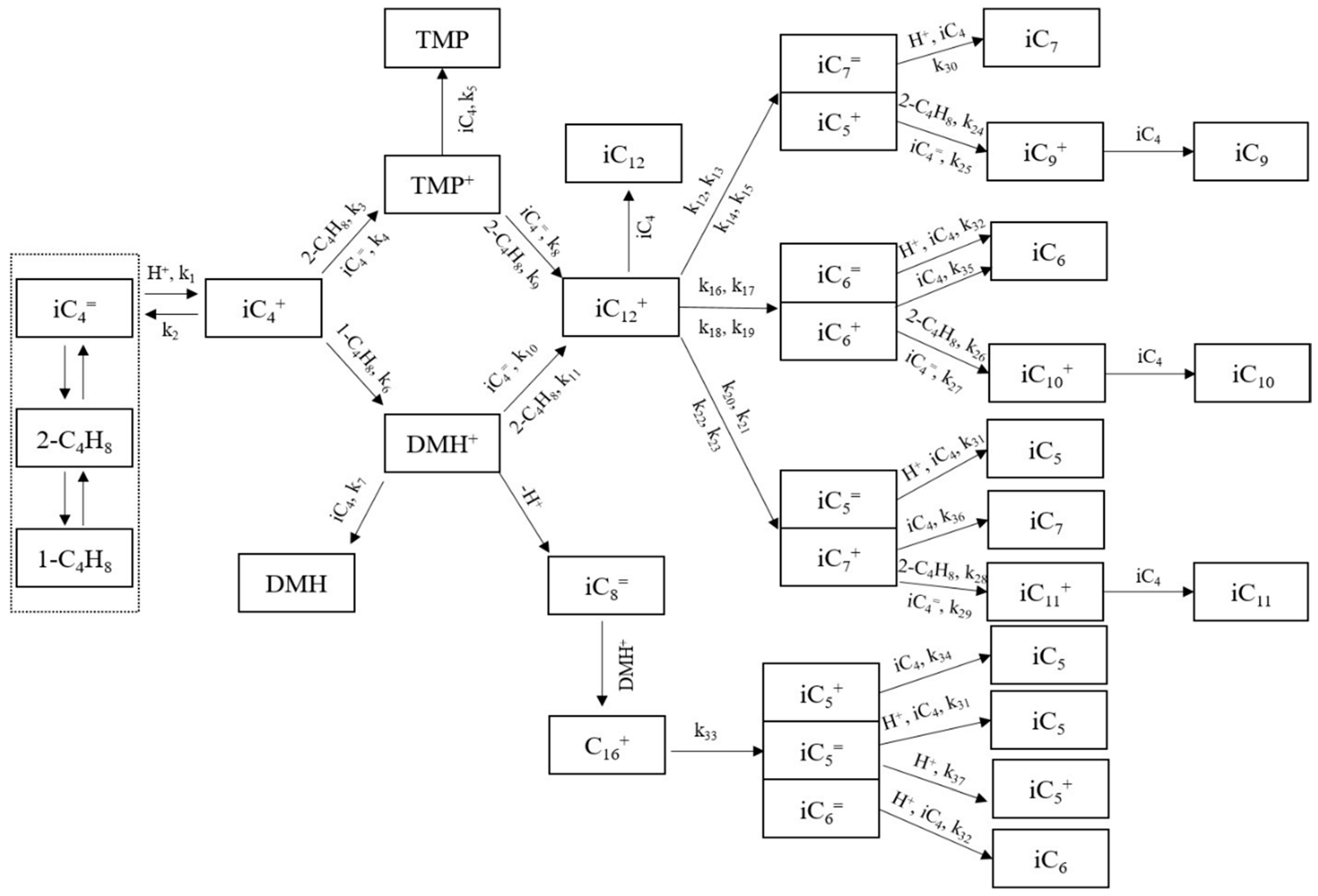
3.2. Kinetic Model
- (a)
- According to the daily analysis of the industrial data, the components in the reaction system are lumped based on the principle of similar kinetic characteristics in this study. Specifically, components with C5 and above are classified according to the number of carbon atoms. Among them, as the main product, C8 is mainly composed of trimethylpentane (TMP) and dimethylhexane (DMH), so it is divided into two lumped components, namely TMP and DMH. Components with less than 5 carbon atoms are lumped and classified according to specific components, with butene as an independent lumped component. Given that the alkylation reaction follows the carbocation reaction mechanism, a series of carbocations and olefin intermediates are generated during the reaction process, and these intermediates are also lumped separately. A total of 20 lumped components are finally classified, which are butene, TMP+, DMH+, isobutane, C5, C6, C7, TMP, DMH, C9, C10, C11, C12, iC4+, iC5+, iC6+, iC7+, iC5=, iC6=, and iC7=.
- (b)
- The isomerization reaction rates among the four isomers of butene are fast enough, so the four butene are considered to be in chemical equilibrium. The isomers of butene are grouped as one lumped component, and the contents of 1-butene, cis-2-butene, trans-2-butene, and isobutene can be calculated using the equilibrium constants.
- (c)
- The cracking reactions of C12+ and C16+ follow the β-cracking rule. In the initial stage of the alkylation reaction, the tert-butyl carbocation (formed via olefin protonation) reacts with olefins to generate C8 carbocations (C8+). These C8+ species further react with olefins to form larger carbocations (e.g., C12+, C16+). However, such high-carbon carbocations are unstable and undergo β-scission, yielding smaller olefins and intermediate carbocations. These intermediates subsequently participate in hydride transfer reactions with isobutane, ultimately forming alkanes such as C5, C6, and C7 hydrocarbons.
- (d)
- The reaction of TMP+ (DMH+) to generate C12+ is an instantaneous reaction.
- (e)
- As inert components, the propane and n-butane in the raw materials do not participate in the alkylation reactions.
- (f)
- The concentration effects of the hydrocarbon monomers and carbocation intermediates participating in the reactions are all first-order.
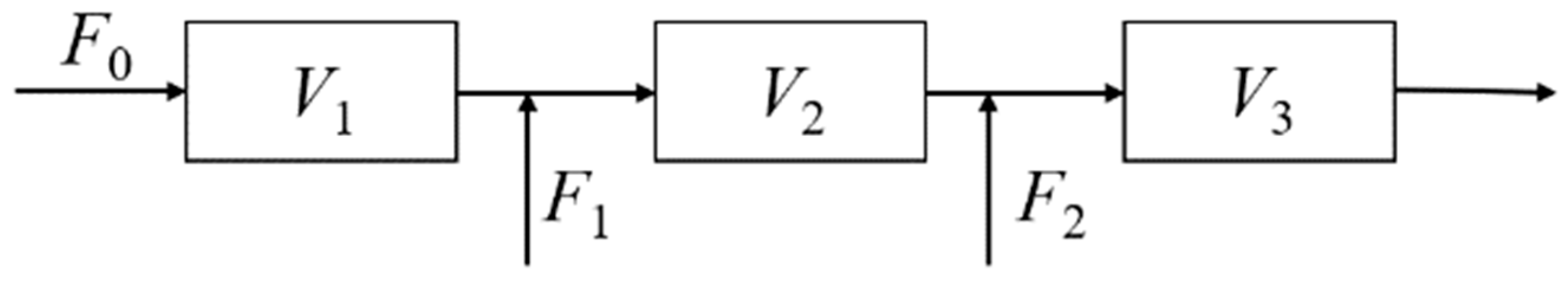
3.3. Model Transformation
4. Results and Discussion
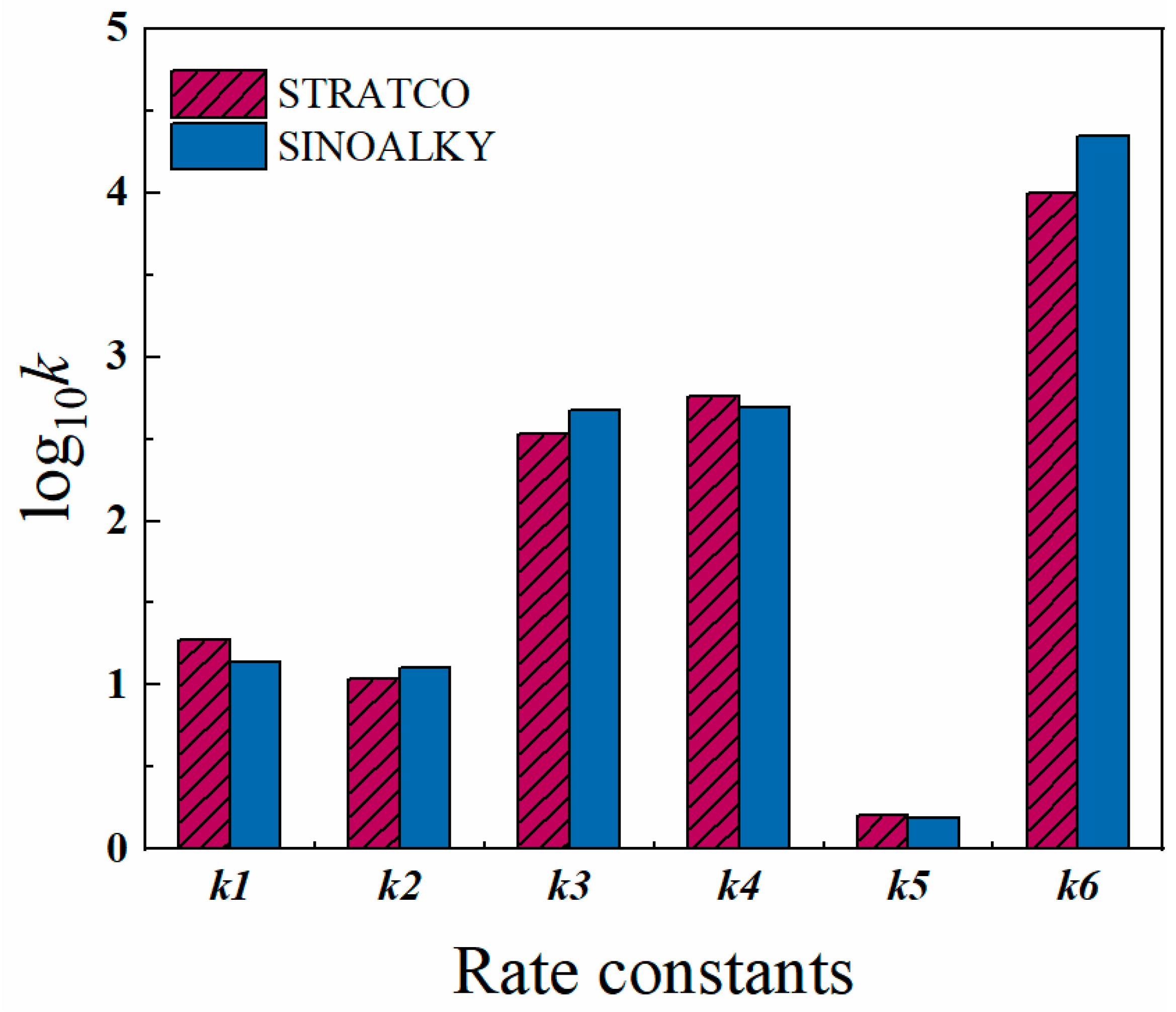
4.1. Parameter Estimation
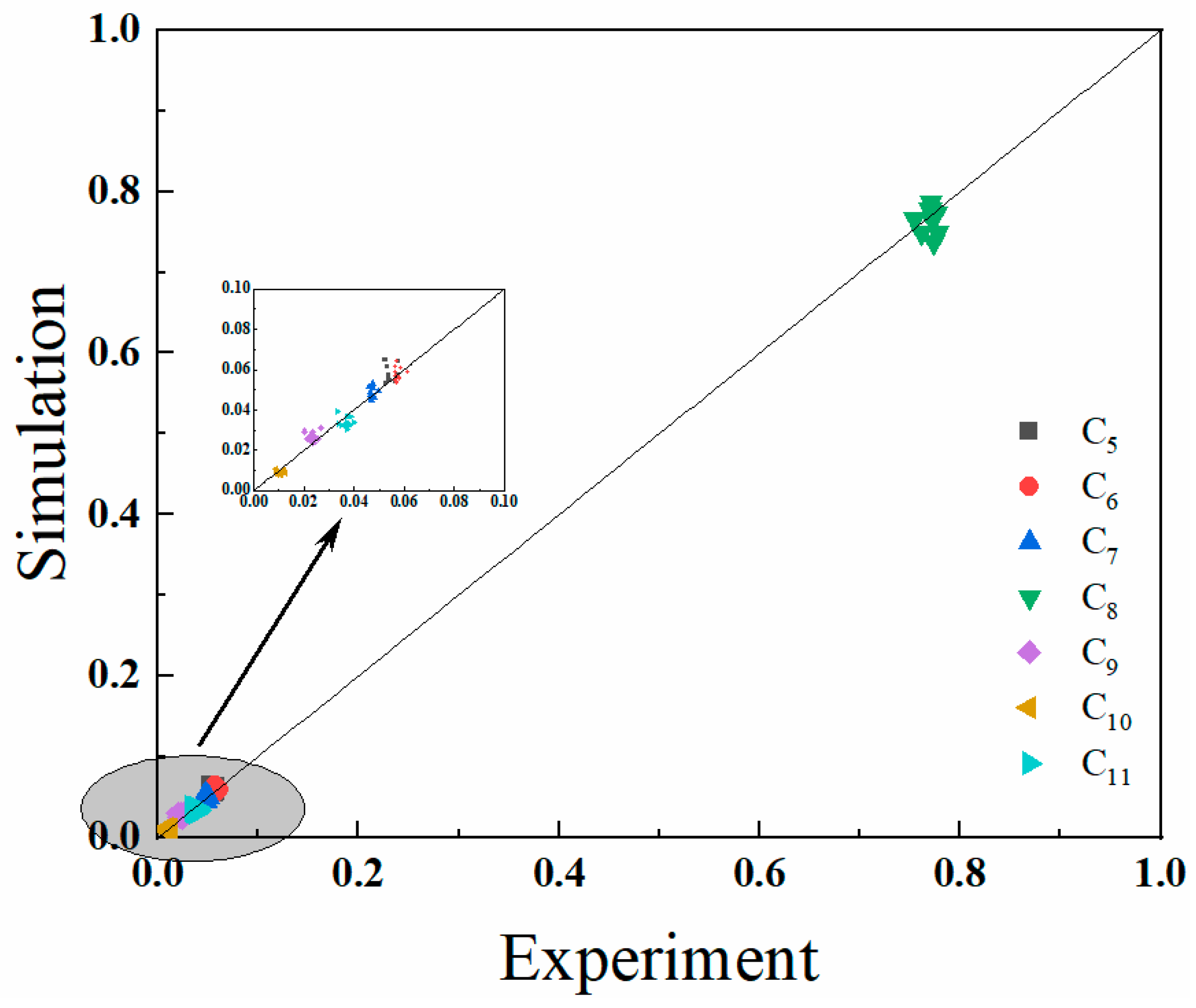
4.2. Model Validation
4.3. Model Prediction
4.3.1. Concentration Profile of the Reactor
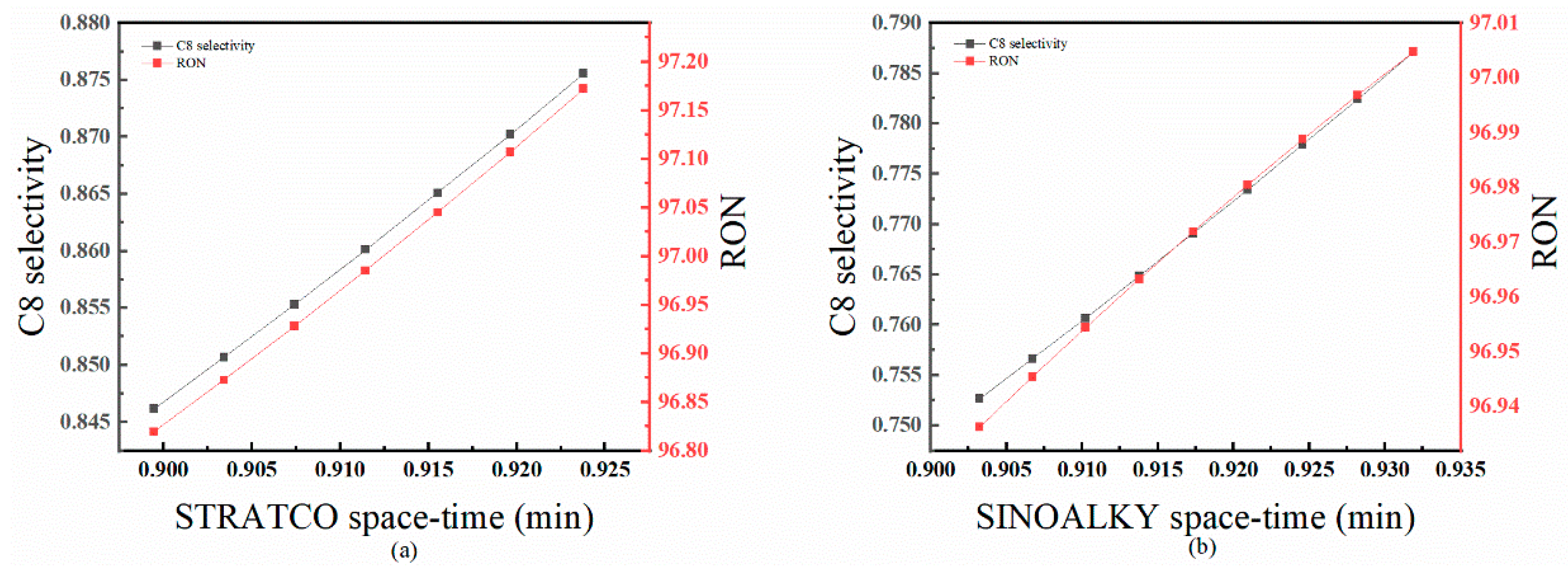
4.3.2. Effect of Space–Time
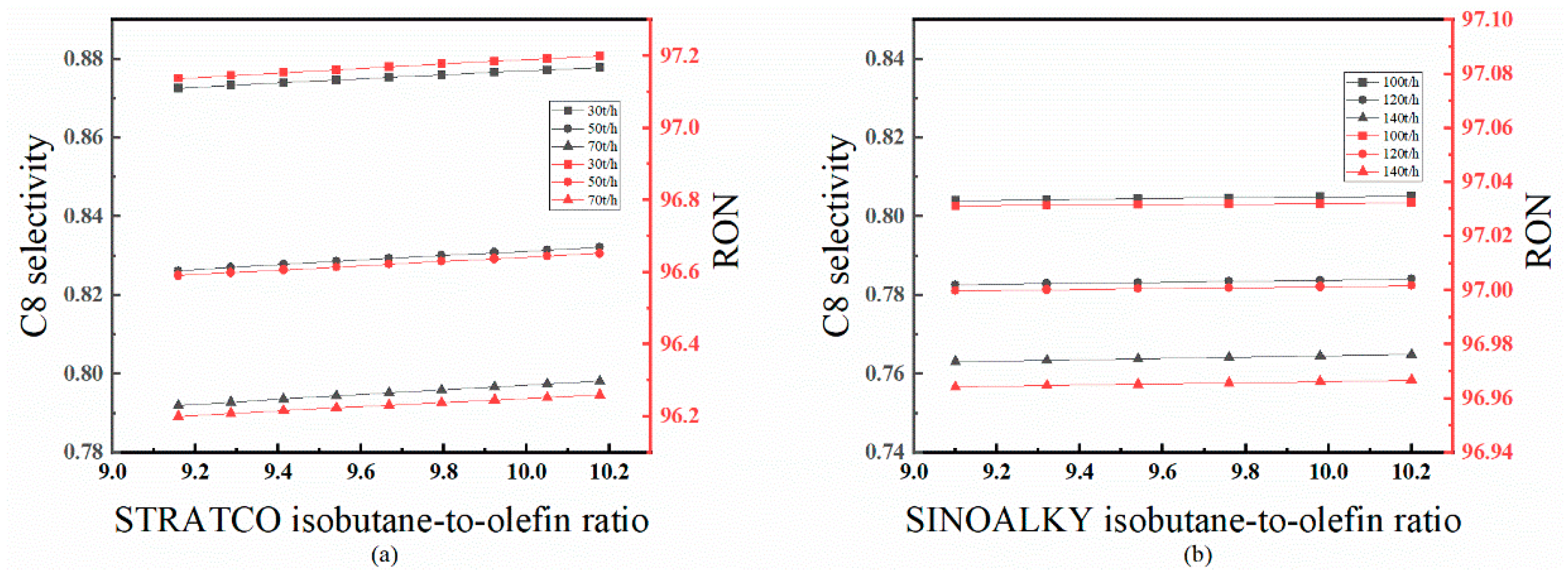
4.3.3. Effect of Isobutane-to-Olefin Ratio
4.3.4. Effect of Acid-to-Hydrocarbon Ratio
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RON | Research octane number |
| TMP | Trimethylpentane |
| DMH | Dimethylhexane |
| SM | Sequential modular method |
| EO | Equation-oriented method |
References
- Zhu, Q.Y.; Zheng, L.J.; Ren, W.P. New progress in alkylate oil production technologies. Petrochem. Technol. Appl. 2016, 34, 511–515. [Google Scholar]
- Zhu, Q.Y.; Qiao, M.; Ren, J. Trend in Technology of Gasoline Alkylated with Liquid Acid. Petrochem. Ind. Technol. 2010, 17, 49–53. [Google Scholar]
- Sun, H.; Song, Y.; Zhou, X.; He, M.; Ali, M.F.; Rizwan, M. Study on Isobutane/1-Butene Alkylation over Phosphorus-Modified HY Zeolite. Catal. Lett. 2024, 154, 651–663. [Google Scholar] [CrossRef]
- Zhang, H.K. Current status and progress of C4 alkylation technology. Qilu Petrochem. Technol. 2022, 50, 242–248. [Google Scholar]
- Li, Q.; Chi, Z.M.; Zhu, D.L. Research progress in the alkylation technology. Contemp. Chem. Ind. 2016, 45, 2440–2442. [Google Scholar]
- Li, W. Study on the C4 Alkylation Reaction Catalyzed by Ionic Liquid/Solid Acid Catalysts. Master’s Thesis, Inner Mongolia University, Hohhot, China, 2018. [Google Scholar]
- Ma, L.L.; Xu, H.G.; Gao, C. Summary for industry application of alkylation technology. Technol. Dev. Chem. Ind. 2013, 42, 24–27. [Google Scholar]
- Dang, X.F.; Shi, F.Y.; Wang, Y.B. The comparison of sulfuric acid and hydrofluoric acid alkylation technology. Pet. Plan. Eng. 2018, 29, 14–17. [Google Scholar]
- Ma, Z.; Zheng, W.; Dong, M.; Sun, W.; Zhao, L. Assessing industrial-scale H2SO4-catalyzed C4 alkylation enhanced by [N1,1,1,1][C10SO4] additives based on a complex kinetic model. Ind. Eng. Chem. Res. 2024, 63, 2177–2186. [Google Scholar] [CrossRef]
- Albright, L.F.; Li, K.W. Alkylation of isobutane with light olefins using sulfuric acid reaction mechanism and comparison with HF alkylation. Ind. Eng. Chem. Process Des. Dev. 1970, 9, 447–454. [Google Scholar] [CrossRef]
- Albright, L.F.; Doshi, B.M.; Ferman, M.A.; Ewo, A. Alkylation of Isobutane with C4 Olefins; American Chemical Society: Washington, DC, USA, 1977. [Google Scholar]
- Mi, H.G. Analysis of factors of sulfuric acid alkylation reaction. Chem. Enterp. Manag. 2020, 13, 94–95. [Google Scholar]
- Fu, K.; Chen, X.; Chen, Z.; Wu, C.; Wei, X.; Liang, J.; Nong, W.; Wang, L. Response surface methodology optimization and kinetic study of isobutene/2-butene alkylation reaction. React. Kinet. Mech. Catal. 2023, 136, 1891–1913. [Google Scholar] [CrossRef]
- Ivashkina, E.; Dolganova, I.; Dolganov, I.; Ivanchina, E.; Nurmakanova, A.; Bekker, A. Modeling the H2SO4-catalyzed isobutane alkylation with alkenes considering the process unsteadiness. Catal. Today 2019, 329, 206–213. [Google Scholar] [CrossRef]
- Geng, Y.J. Alkylation Process and Technology; China Petrochemical Press: Beijing, China, 1993. [Google Scholar]
- Schmerling, L. The mechanism of the alkylation of paraffins. J. Am. Chem. Soc. 1945, 67, 1778–1783. [Google Scholar] [CrossRef]
- Langley, J.R.; Pike, R.W. The kinetics of alkylation of isobutane with propylene. AIChE J. 1972, 18, 698–705. [Google Scholar] [CrossRef]
- Lee, L.M.; Harriott, P. The kinetics of isobutane alkylation in sulfuric acid. Ind. Eng. Chem. Process Des. Dev. 1977, 16, 282–287. [Google Scholar] [CrossRef]
- Sun, W.; Shi, Y.; Chen, J.; Xi, Z.; Zhao, L. Alkylation kinetics of isobutane by C4 olefins using sulfuric acid as catalyst. Ind. Eng. Chem. Res. 2013, 52, 15262–15269. [Google Scholar] [CrossRef]
- Xin, Z.C.; Jiang, H.B.; Zhang, Z.; Chen, Y.; Wang, J. Kinetic model of olefins/isobutane alkylation using sulfuric acid as catalyst. ACS Omega 2022, 7, 9513–9526. [Google Scholar] [CrossRef] [PubMed]
- Kranz, K. Cold Temperature Alkylation Process. U.S. Patent 5,095,168, 8 November 1989. [Google Scholar]
- Meyers, R.A. Handbook of Petroleum Refining Processes; McGrawHill: New York, NY, USA, 2003. [Google Scholar]
- Zhang, Q.X. Introduction of STRATCO sulfuric acid alkylation reactor. Petrochem. Equip. Technol. 1991, 1, 21. [Google Scholar]
- Dong, M.H.; Zong, B.N. Sinoalky technology for sulfuric acid alkylation and its application. Pet. Process. Petrochem. 2019, 50, 29–32. [Google Scholar]
- Cai, H.B.; Dai, G.C. CFD Simulation of Fluid Flow in Sulfuric Acid Alkylation Reactor—Effect of Structure Parameters. Chem. React. Eng. Technol. 2012, 28, 391–397. [Google Scholar]
- Zhang, L.; Xu, Z.M.; Yuan, X.Q. Principles of Chemical Reaction Engineering; East China University of Science and Technology Press: Shanghai, China, 2007. [Google Scholar]
- Nurmakanova, A.E.; Ivashkina, E.N.; Ivanchina, E.; Dolganov, I.; Boychenko, S. Predicting alkylate yield and its hydrocarbon composition for sulfuric acid catalyzed isobutane alkylation with olefins using the method of mathematical modeling. Procedia Chem. 2015, 15, 54–64. [Google Scholar] [CrossRef]
- Ma, P.S. Data Manual for Experimental Physical Properties of Organic Compounds; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Editorial Committee of the Sulfuric Acid Association. Sulfuric Acid Manual; Chemical Industry Press: Beijing, China, 1982. [Google Scholar]
- Cao, P.; Zheng, L.; Sun, W.Z.; Zhao, L. Multiscale Modeling of Isobutane Alkylation with Mixed C4 Olefins Using Sulfuric Acid as Catalyst. Ind. Eng. Chem. Res. 2019, 58, 6340–6349. [Google Scholar] [CrossRef]
- Wang, P.; Wang, D.; Xu, C.; Gao, J. DFT calculations of the alkylation reaction mechanisms of isobutane and 2-butene catalyzed by Bronsted acid. Appl. Catal. A Gen. 2007, 332, 22–26. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; Ancheyta, J. Detailed description of kinetic and reactor modeling for naphtha catalytic reforming. Fuel 2011, 90, 3492–3508. [Google Scholar] [CrossRef]
- Lanczos, C. Trigonometric Interpolation of Empirical, Analytical Functions. Stud. Appl. Math. 1938, 17, 85–88. [Google Scholar] [CrossRef]
- Villadsen, J.V.; Stewart, W.E. Solution of Boundary-value Problems by Orthogonal Collocation. Chem. Eng. Sci. 1995, 22, 1483–1501. [Google Scholar] [CrossRef]
- Jiang, H.B.; Sun, Y.; Jiang, S.B.; Li, Z.M.; Tian, J.H. Reactor model of counter-current continuous catalyst-regenerative reforming process toward real time optimization. Energy Fuels 2021, 35, 10770–10785. [Google Scholar] [CrossRef]
- Li, D.; Sun, W.; Xi, Z.; Zhang, M.; Zhao, L. Alkylation kinetics of mixed butenes/isobutane by sulfuric acid. CIESC J. 2015, 66, 584–590. [Google Scholar]
- Ma, Q.F. Analysis and optimization of influencing factors on product quality of sulfuric acid alkylation. Chem. Enterp. Manag. 2019, 15, 38–39. [Google Scholar]
- Yu, J.; Gou, X.L.; Yu, J.J. Comprehensive Chemical Kinetic Model of 2,6,10-Trimethyl Dodecane. Energy Fuels 2020, 34, 2366–2375. [Google Scholar] [CrossRef]




| Equation Number | ∆Hr/KJ·mol−1 | Equation Number | ∆Hr/KJ·mol−1 | Equation Number | ∆Hr/KJ·mol−1 |
|---|---|---|---|---|---|
| 1 | −97.44 | 14 | −10.14 | 27 | −69.45 |
| 2 | 97.44 | 15 | −16.31 | 28 | −67.24 |
| 3 | −92.58 | 16 | 1.82 | 29 | −61.07 |
| 4 | −86.41 | 17 | 7.99 | 30 | −89.52 |
| 5 | 6.44 | 18 | −11.63 | 31 | −92.14 |
| 6 | −89.58 | 19 | −5.47 | 32 | −93.54 |
| 7 | 8.78 | 20 | 1.87 | 33 | 0.06 |
| 8 | −51.67 | 21 | 8.04 | 34 | 2.86 |
| 9 | −57.83 | 22 | −11.58 | 35 | 5.47 |
| 10 | −65.13 | 23 | −5.42 | 36 | 0.75 |
| 11 | −71.29 | 24 | −94.67 | 37 | −95.00 |
| 12 | 3.31 | 25 | −88.51 | ||
| 13 | −2.85 | 26 | −75.61 |
| Component | A | B × 102 | C × 104 | D × 106 |
|---|---|---|---|---|
| Propane | 59.6420 | 32.8310 | −15.3770 | 3.6539 |
| n-Butane | 62.8730 | 58.9130 | −23.5880 | 4.2257 |
| Isobutane | 71.7910 | 48.4720 | −20.5190 | 4.0634 |
| Isobutene | 57.6110 | 56.2510 | −22.9850 | 4.1773 |
| 1-Butene | 74.5970 | 33.4340 | −13.9140 | 3.0241 |
| trans-2-Butene | 36.1620 | 79.7390 | −30.6740 | 4.8919 |
| Isopentane | 109.5040 | 11.7106 | −0.8803 | 1.0706 |
| Isoprene | 90.0050 | 39.7770 | −15.4620 | 2.9909 |
| 2,3-Dimethylbutane | 115.6688 | 21.7756 | −3.2285 | 1.3881 |
| 2,4-Dimethylpentane | 155.9063 | −17.8757 | 19.5046 | −1.9530 |
| 2,2,4-Trimethylpentane | 145.4385 | 13.3233 | 6.0554 | 0.0000 |
| 2,3-Dimethylhexane | 109.6920 | 109.1100 | −32.5790 | 4.1505 |
| 2,2,5-Trimethylhexane | 139.6900 | 86.3420 | −26.0670 | 3.6252 |
| Decane | 325.6033 | −29.6775 | 0.5202 | 2.7394 |
| 2-Methyldecane | 464.6960 | −196.4158 | 71.1196 | −6.4211 |
| Dodecane | 84.4850 | 203.5800 | −50.9810 | 5.2186 |
| Sulfuric Acid Concentration/wt.% | A | B × 104 | Sulfuric Acid Concentration/wt.% | A | B × 104 |
|---|---|---|---|---|---|
| 80 | 0.437 | 4.4 | 90 | 0.386 | 4.3 |
| 82 | 0.440 | 3.5 | 92 | 0.366 | 4.7 |
| 84 | 0.437 | 2.4 | 94 | 0.347 | 4.9 |
| 86 | 0.419 | 3.4 | 96 | 0.334 | 5.0 |
| 88 | 0.403 | 3.9 | 98 | 0.325 | 5.0 |
| Rate Constants | STRATCO | SINOALKY | Rate Constants | STRATCO | SINOALKY |
|---|---|---|---|---|---|
| k1/min−1 | 18.98 | 13.84 | k20/kg·mol−1·min−1 | 192.47 | 97.63 |
| k2/min−1 | 10.99 | 12.65 | k21/kg·mol−1·min−1 | 132.26 | 95.05 |
| k3/kg·mol−1·min−1 | 336.53 | 469.22 | k22/kg·mol−1·min−1 | 84.24 | 98.01 |
| k4/kg·mol−1·min−1 | 578.66 | 493.56 | k23/kg·mol−1·min−1 | 96.30 | 98.02 |
| k5/kg·mol−1·min−1 | 1.60 | 1.54 | k24/kg2·mol−2·min−1 | 26.04 | 139.32 |
| k6/kg·mol−1·min−1 | 10,028.01 | 22178.64 | k25/kg2·mol−2·min−1 | 73.73 | 132.16 |
| k7/kg2·mol−2·min−1 | 0.21 | 5.00 | k26/kg2·mol−2·min−1 | 31.93 | 35.47 |
| k8/kg2·mol−2·min−1 | 2.07 | 0.52 | k27/kg2·mol−2·min−1 | 27.32 | 37.94 |
| k9/kg2·mol−2·min−1 | 1.37 | 0.45 | k28/kg2·mol−2·min−1 | 27.03 | 145.67 |
| k10/kg2·mol−2·min−1 | 1.37 | 1.86 | k29/kg2·mol−2·min−1 | 81.08 | 149.72 |
| k11/kg2·mol−2·min−1 | 1.37 | 1.99 | k30/kg2·mol−2·min−1 | 11.48 | 11.49 |
| k12/kg·mol−1·min−1 | 71.30 | 84.39 | k31/kg2·mol−2·min−1 | 4.09 | 6.71 |
| k13/kg·mol−1·min−1 | 71.35 | 111.95 | k32/kg2·mol−2·min−1 | 51.35 | 51.72 |
| k14/kg·mol−1·min−1 | 115.91 | 119.98 | k33/kg·mol−1·min−1 | 3486.21 | 3129.43 |
| k15/kg·mol−1·min−1 | 107.04 | 119.01 | k34/kg·mol−1·min−1 | 1.30 | 1.19 |
| k16/kg·mol−1·min−1 | 135.72 | 91.45 | k35/kg·mol−1·min−1 | 2.10 | 1.25 |
| k17/kg·mol−1·min−1 | 90.43 | 96.23 | k36/kg·mol−1·min−1 | 3.37 | 0.30 |
| k18/kg·mol−1·min−1 | 90.45 | 90.96 | k37/min−1 | 29.98 | 29.12 |
| k19/kg·mol−1·min−1 | 90.46 | 90.94 |
| Sensitivity Coefficients | STRATCO | SINOALKY | Sensitivity Coefficients | STRATCO | SINOALKY |
|---|---|---|---|---|---|
| S1 | 6.36 × 10−2 | 7.27 × 10−3 | S20 | −1.37 × 10−2 | −8.54 × 10−4 |
| S2 | −5.56 × 10−2 | −6.93 × 10−3 | S21 | −6.07 × 10−2 | −5.63 × 10−3 |
| S3 | 1.01 × 10−2 | 4.55 × 10−4 | S22 | −2.93 × 10−4 | −6.31 × 10−5 |
| S4 | 6.70 × 10−2 | 2.51 × 10−3 | S23 | −2.22 × 10−3 | −4.33 × 10−4 |
| S5 | 7.11 × 10−2 | 8.72 × 10−3 | S24 | −4.23 × 10−4 | −8.66 × 10−5 |
| S6 | −9.62 × 10−2 | 1.53 × 10−3 | S25 | −6.35 × 10−3 | −4.13 × 10−4 |
| S7 | 3.20 × 10−2 | 9.68 × 10−4 | S26 | −3.24 × 10−4 | −4.63 × 10−5 |
| S8 | −9.23 × 10−3 | −2.66 × 10−4 | S27 | −1.68 × 10−3 | −2.69 × 10−4 |
| S9 | −9.11 × 10−4 | −3.59 × 10−5 | S28 | −2.09 × 10−4 | −3.04 × 10−5 |
| S10 | −1.86 × 10−4 | −7.63 × 10−5 | S29 | −3.36 × 10−3 | −1.26 × 10−4 |
| S11 | −2.78 × 10−5 | −1.17 × 10−5 | S30 | −9.86 × 10−5 | −1.72 × 10−5 |
| S12 | −3.35 × 10−2 | −6.10 × 10−3 | S31 | 1.30 × 10−3 | 1.40 × 10−4 |
| S13 | −5.11 × 10−3 | −9.23 × 10−4 | S32 | −6.37 × 10−5 | −5.36 × 10−6 |
| S14 | −2.67 × 10−3 | −4.76 × 10−4 | S33 | −1.86 × 10−2 | −3.22 × 10−4 |
| S15 | −3.74 × 10−4 | −6.91 × 10−5 | S34 | 3.84 × 10−3 | 4.42 × 10−4 |
| S16 | −9.12 × 10−3 | −6.23 × 10−4 | S35 | 7.50 × 10−4 | 1.52 × 10−4 |
| S17 | −3.98 × 10−2 | −4.18 × 10−3 | S36 | 1.86 × 10−3 | 2.09 × 10−4 |
| S18 | −2.60 × 10−4 | −4.73 × 10−5 | S37 | −1.93 × 10−3 | −2.10 × 10−4 |
| S19 | −1.73 × 10−3 | −3.25 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Jiang, H. Comparative Analysis of Sulfuric Acid Alkylation Technologies Based on a Reaction Kinetic Model. Processes 2025, 13, 1604. https://doi.org/10.3390/pr13051604
Zhang W, Jiang H. Comparative Analysis of Sulfuric Acid Alkylation Technologies Based on a Reaction Kinetic Model. Processes. 2025; 13(5):1604. https://doi.org/10.3390/pr13051604
Chicago/Turabian StyleZhang, Wenbin, and Hongbo Jiang. 2025. "Comparative Analysis of Sulfuric Acid Alkylation Technologies Based on a Reaction Kinetic Model" Processes 13, no. 5: 1604. https://doi.org/10.3390/pr13051604
APA StyleZhang, W., & Jiang, H. (2025). Comparative Analysis of Sulfuric Acid Alkylation Technologies Based on a Reaction Kinetic Model. Processes, 13(5), 1604. https://doi.org/10.3390/pr13051604






