Energy-Saving Design of Urea Method for Hydrazine Hydrate Process
Abstract
1. Introduction
2. Production Process
2.1. Process Design and Simulation
2.2. Experimental Methodology
2.3. Sensitivity Analysis
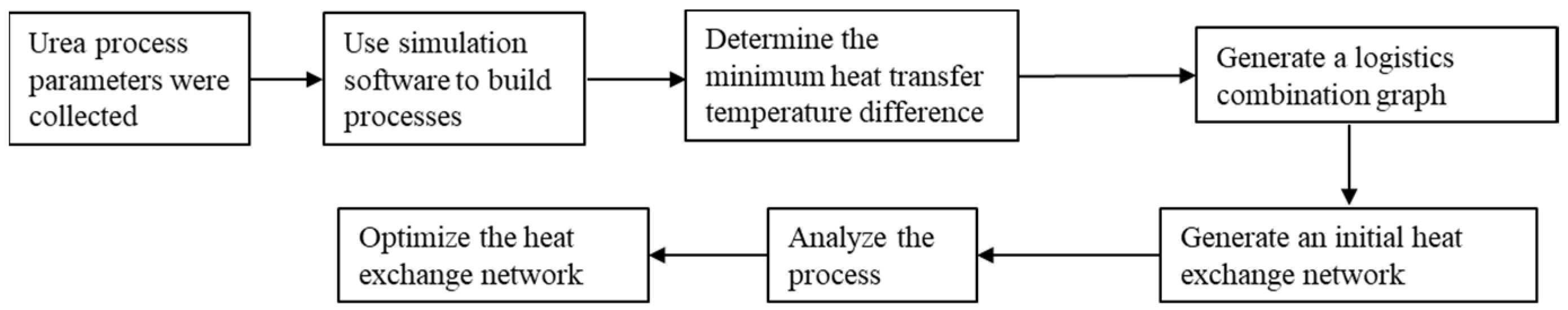
3. Energy-Saving Optimization
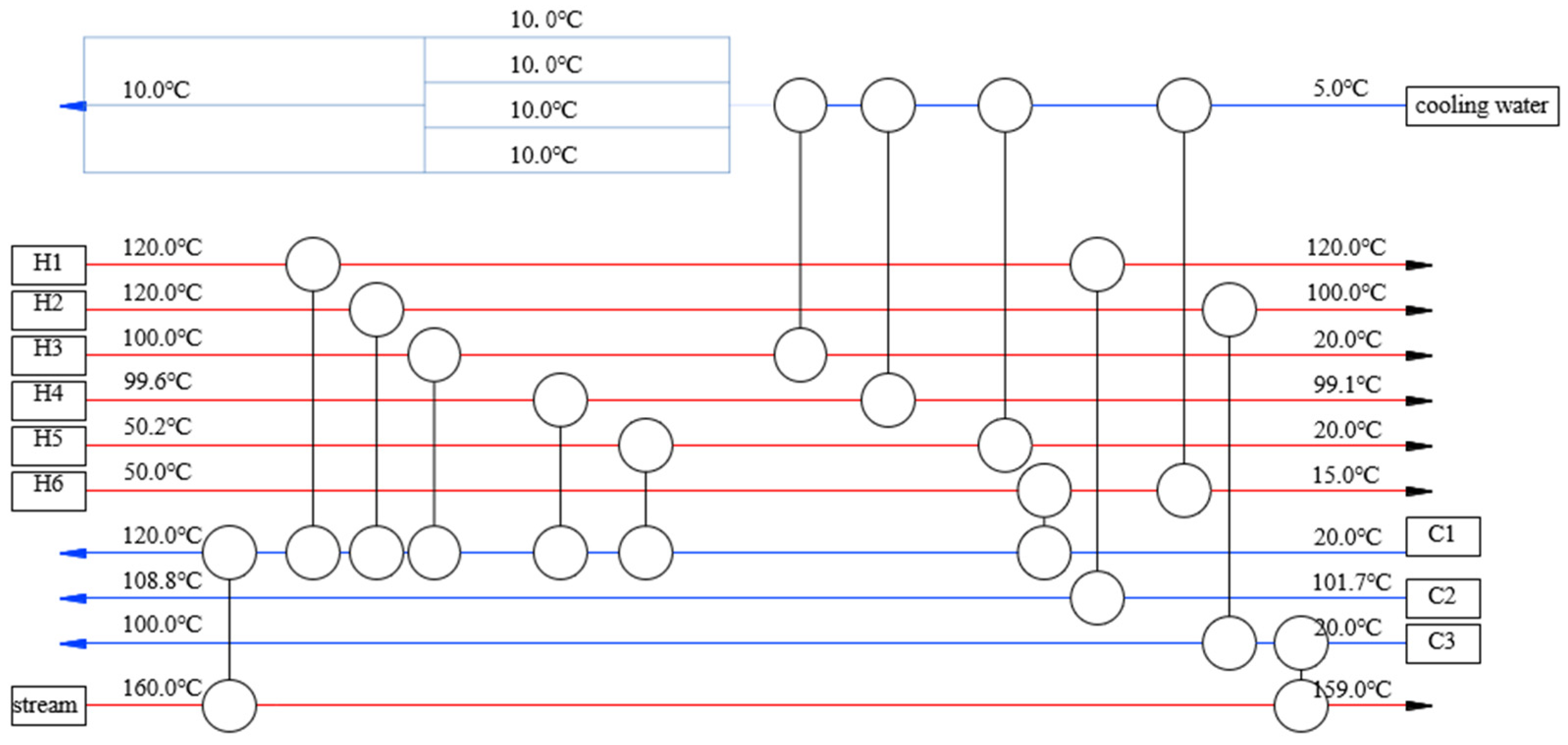
3.1. Initial HEN Synthesis
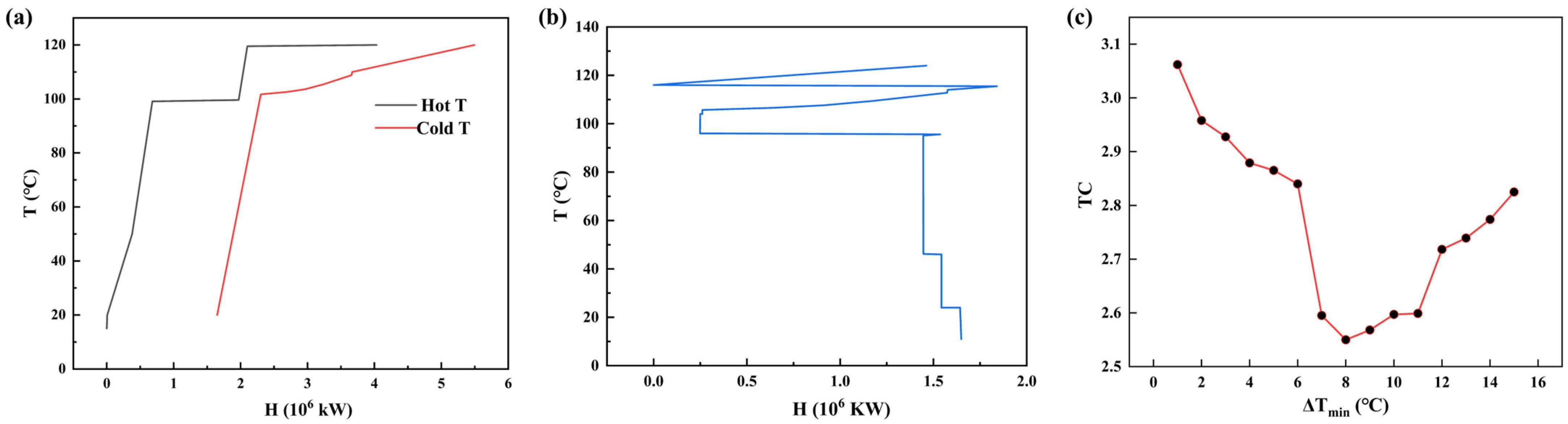
3.2. Optimization of Heat Exchanger Network

3.3. Analysis of Optimization Results
4. Conclusions
- Energy conservation: hot utility consumption decreased by 65.8% (from 3846 to 1317 MJ/h), and cold utility demand was reduced by 62.7% (from 4032 to 1503 MJ/h). Approximately 67% of waste heat from exothermic reactions was recovered through temperature-cascaded heat exchange.
- Cost efficiency: Total operational costs declined by 12%, driven by reduced utility expenditures, despite additional heat exchanger investments.
- Process optimization: Distillation parameters (nine theoretical stages, fifth-stage feed, 0.6 reflux ratio) minimized reboiler energy demand while ensuring stable product quality (20% hydrazine hydrate in column bottoms).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviation
| ADC | Azodicarbonamide |
| NRTL | Non-Random Two Liquid |
| ELECNRTL | Electrolyte Non-Random Two Liquid Model |
| HX | Heat Exchanger |
| HEN | Heat Exchanger Network |
| wt% | Weight Percentage |
| DSTWU | Distillation Shortcut (Winn–Underwood) Method |
| Radfrac | Rigorous Fractionation Model |
| CP | Heat Capacity Flow Rate |
| TC | Total Cost |
| A | The Annualization Factor |
| CC | Capital Cost of The Installed Heat Exchanger |
| OC | Operation Cost |
| ROR | Rate of Return |
| PL | Project Life |
| a | Base Installation Cost of The Heat Exchanger |
| b | Heat Transfer Area/Duty |
| c | Cost Coefficient |
| Area | Total Heat Transfer Area of The Heat Exchanger |
| Nshell | Number of Shells In The Heat Exchanger |
References
- Xie, X.; Luo, M.; Hu, S. Green assessment method for industrial technology: A case study of the saline lake industry. ACS Sustain. Chem. Eng. 2022, 10, 1544–1553. [Google Scholar] [CrossRef]
- Kong, C.; Hu, F.; Liu, Y.; Yue, H.; Hu, H. Planning of Chlor-Alkali Enterprises in Circular Chemical Industrial Parks: A Discussion. Mod. Salt Chem. Ind. 2020, 47, 113–114. [Google Scholar]
- Jeffreys, G.V.; Wharton, J.T. Raschig Synthesis of Hydrazine: Formation of Hydrazine from Chloramine. Ind. Eng. Chem. Process Des. Dev. 1965, 4, 71–76. [Google Scholar] [CrossRef]
- Rehse, K.; Shahrouri, T. Hydrazine Derivatives. Arch. Pharm. 1998, 331, 308–312. [Google Scholar] [CrossRef]
- Hayashi, H. Hydrazine Synthesis: Commercial Routes, Catalysis and Intermediates. Res. Chem. Intermed. 1998, 24, 183–196. [Google Scholar] [CrossRef]
- Sari, T.; Akgul, D. Hydrazine (Bio)Synthesis and Separation: Advances, State-of-the-Art Methods, and Patent Review. Biomass Convers. Biorefinery 2025, prepublish. [Google Scholar] [CrossRef]
- Zhou, Z.G.; Shi, S.L. Comparison of Hydrazine Hydrate Production Technologies: Ketazine Method vs. Urea Method. J. Salt Sci. Chem. Ind. 2019, 48, 5–8. [Google Scholar]
- Li, B.; Tian, Z.; Song, C.; Zhang, Y.; Xu, X. Study on the Preparation of Hydrazine Hydrate by Urea Method. Appl. Chem. Ind. 2006, 6, 422–424. [Google Scholar]
- Sitompul, J.P. Study on Hydrazine Production via Urea Process. Reaktor 2005, 9, 20–25. [Google Scholar] [CrossRef][Green Version]
- Francis, P.; Lim, K. Conversion of Urea to Hydrazine: Hofmann Reaction or Favorskii Analogue? Exploring Reaction Mechanism through Isotopic Labeling Studies. Chem. Educ. 2007, 12, 307–313. [Google Scholar]
- Wang, Y.X.; Xue, W.P. Process Optimization of Hydrazine Hydrate in ADC Production. Shandong Chem. Ind. 2013, 42, 184–185, 188. [Google Scholar]
- Sun, H.; Geng, G.; Lin, S.; Wu, D.; Yu, S.; Gao, C. Optimizing Organic Pollutant Removal from Hydrazine Hydrate Waste Brine through Thermal Activation of Sodium Persulfate Assisted by Response Surface Methodology. J. Water Process Eng. 2025, 69, 106687. [Google Scholar] [CrossRef]
- Feng, L.; Tian, B.; Zhang, L.; Yang, M. Pyrolysis of Hydrazine Hydrate Waste Salt: Thermal Behaviors and Transformation Characteristics of Organics under Aerobic/Anaerobic Conditions. J. Environ. Manag. 2022, 323, 116304. [Google Scholar] [CrossRef]
- Yu, X.F.; Wang, W.Z.; Qu, X.R.; Dong, C.J. Study on the Process of Removing Na2CO3 from Crude Hydrazine Hydrate Solution Using CaCl2. J. Salt Sci. Chem. Ind. 2018, 47, 24–26. [Google Scholar]
- Wang, X.J.; Tang, L.; Jiang, Z. Numerical Simulation of Venturi Ejector Reactor in Yellow Phosphorus Purification System. Nucl. Eng. Des. 2014, 268, 18–23. [Google Scholar] [CrossRef]
- Yan, S.; Lv, F.; Wang, B.; Dong, X.; Yang, X.; Ma, L.; Chen, S.; Han, B.; Bai, Z. Mixing Characteristics and Intensification Mechanism of an Improved Swirl Jet Mixer for High Phase Ratios. J. Ind. Eng. Chem. 2024, 135, 457–470. [Google Scholar] [CrossRef]
- Shen, D.; Ma, J.; Liu, Y.; Zhao, C.; Chen, S. Study on Nanofiltration Membrane Treatment of Hydrazine Hydrate Production Wastewater and Membrane Fouling Mechanism. Water Treat. Technol. 2018, 44, 54–57. [Google Scholar]
- Li, F.; Guo, Y.; Wang, S. Pilot-Scale Selective Electrodialysis for the Separation of Chloride and Sulphate from High-Salinity Wastewater. Membranes 2022, 12, 610. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Huang, Y.; Wang, J.; Bao, W.; Chang, L.; Shi, L.; Yi, Q. Pinch Analysis for Heat Integration of Pulverized Coke Chemical Looping Gasification Coupled with Coke-Oven Gas to Methanol and Ammonia. Processes 2022, 10, 1879. [Google Scholar] [CrossRef]
- Beijing Huateyuan Technology Co., Ltd. Method for Separating Sodium Carbonate and Sodium Chloride in Crude Hydrazine. Patent CN202311432907.0, 26 January 2024. [Google Scholar]
- Oliveira, M.; Borges, S.D.A. Enhancing Energy Efficiency in Integrated Electrolyser Stack and Methanation Reactor Systems through Pinch Analysis. Reactions 2024, 5, 984–998. [Google Scholar] [CrossRef]
- Anantpinijwatna, A.; Simasatitkul, L.; Yooyen, K.; Amornraksa, S.; Assabumrungrat, S.; Im-orb, K. Process Improvement and Economic and Environmental Evaluation of Bio-Hydrogenated Diesel Production from Re-fined Bleached Deodorized Palm Oil. Processes 2025, 13, 75. [Google Scholar] [CrossRef]
- Mohsenpour, M.; Pazuki, M.-M.; Salimi, M.; Amidpour, M. Optimized Heat Exchanger Network Design for a Phthalic Anhydride Plant Using Pinch Technology: A Maximum Energy Recovery Approach with Economic Analysis. Results Eng. 2024, 24, 103438. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Q.; Cui, G.; Bao, Z.; Zhang, G. Synthesis of Cost-Optimal Heat Exchanger Networks Using a Novel Stochastic Algorithm and a Modified Stage-Wised Superstructure. Processes 2021, 9, 2060. [Google Scholar] [CrossRef]


| Component | NaCl | Na2CO3 | Hydrazine Hydrate |
|---|---|---|---|
| Rejection rate | 99.60% | 99.90% | 5.60% |
| Stream Type | ID | Tag | T0/°C | T1/°C | CP/kW/°C | Q/kW |
|---|---|---|---|---|---|---|
| Hot | H1 | 0111 | 120 | 100 | 1.76 | 36.39 |
| H2 | 0109 | 120 | 119.5 | 1.8 | 535.56 | |
| H3 | 0113 | 100 | 20 | 1.77 | 136.03 | |
| H4 | 0205 | 99.63 | 99.13 | 0.4 | 357.78 | |
| H5 | 0103 | 50 | 20 | 1.4 | 42.03 | |
| H6 | 0105 | 50 | 15 | 0.34 | 12.42 | |
| Cold | C1 | 0107 | 20 | 120 | 6.72 | 671.82 |
| C2 | 0206 | 101.7 | 108.8 | 0.06 | 358.61 | |
| C3 | 0203 | 20 | 100 | 0.47 | 37.83 |
| Cost Items | Heat Utility Cost (USD/s) | Cold Utility Cost (USD/s) | Operating Cost (USD/s) | Equipment Cost (USD) | Total Cost (USD/s) |
|---|---|---|---|---|---|
| Before Optimization | 2.03 × 10−3 | 2.375 × 10−4 | 2.267 × 10−3 | 1.175 × 105 | 3.250 × 10−3 |
| After Optimization | 6.649 × 10−4 | 8.853 × 10−5 | 7.534 × 10−4 | 2.536 × 105 | 2.874 × 10−3 |
| Heat Exchange Network | Heat Utility Consumption (MJ/h) | Cold Utility Consumption (MJ/h) | Number of Heat Exchangers |
|---|---|---|---|
| Before Optimization | 3846 | 4032 | 9 |
| After Optimization | 1317 | 1503 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, X.; Wu, H.; Li, S.; Xu, Y. Energy-Saving Design of Urea Method for Hydrazine Hydrate Process. Processes 2025, 13, 1585. https://doi.org/10.3390/pr13051585
Wang Z, Wang X, Wu H, Li S, Xu Y. Energy-Saving Design of Urea Method for Hydrazine Hydrate Process. Processes. 2025; 13(5):1585. https://doi.org/10.3390/pr13051585
Chicago/Turabian StyleWang, Zhihao, Xiaojing Wang, Haibin Wu, Shengting Li, and Yongjie Xu. 2025. "Energy-Saving Design of Urea Method for Hydrazine Hydrate Process" Processes 13, no. 5: 1585. https://doi.org/10.3390/pr13051585
APA StyleWang, Z., Wang, X., Wu, H., Li, S., & Xu, Y. (2025). Energy-Saving Design of Urea Method for Hydrazine Hydrate Process. Processes, 13(5), 1585. https://doi.org/10.3390/pr13051585






