Abstract
This study focuses on the synthesis and characterization of a cationic ion-exchange sorbent derived from vermiculite and epoxy acrylate copolymers, designed to address freshwater scarcity by removing toxic metal ions from aqueous environments. The sorbent was engineered to preserve the chemical integrity of freshwater while adhering to environmental safety standards. Vermiculite served as the base material, modified with glycidyl methacrylate (GMA), acrylonitrile (ACN), and orthophosphoric acid (H3PO4) in a mass ratio of 1:0.35:0.15:3. Optimization experiments explored varying H3PO4 proportions (two- and threefold increases) to refine the synthesis conditions. The materials underwent microwave irradiation at 300 W for 10 min. Infrared (IR) spectroscopy confirmed the presence of functional groups (P=O, P−O−C), enhancing sorption capacity, while scanning electron microscopy (SEM) revealed a porous structure crucial for adsorption. Sorption properties, assessed via atomic emission spectroscopy, demonstrated capacities of 39.80 mg/g for MoO42− and 39.06 mg/g for ReO4−, with extraction efficiencies of 79% and 78%, respectively. Chemical stability tests indicated the sorbent retained up to 90% of its functionality in aggressive environments, highlighting its robustness. The developed sorbent offers a high-performance, cost-effective solution for heavy metal removal from wastewater, advancing sustainable water purification technologies.
1. Introduction
The presence of heavy metals, such as molybdenum and rhenium, in wastewater from mining and chemical industries represents a serious environmental issue. Their high toxicity and potential for bioaccumulation necessitate the development of advanced technologies for removing these ions from water resources. One promising approach is the use of modified mineral sorbents, which combine environmental safety with high sorption capacity.
Pollution of water resources by heavy metals and their compounds is a global environmental problem. Among these, particular attention is given to molybdenum (MoO42−) and rhenium (ReO4−) ions, which are present in the wastewater of mining, metallurgical, and petrochemical enterprises. These substances are not only toxic but also capable of accumulating in organisms, leading to severe consequences for human health and ecosystems. As noted by authors [1,2,3,4], these elements exhibit a tendency for bioaccumulation, causing molecular-level damage to reproductive organs in humans.
The non-biodegradable nature of heavy metals and metalloids demands innovative strategies for the reclamation of contaminated soils and water sources. The growing contamination of soil and water resources by heavy metals has become a pressing environmental challenge [5,6]. Consequently, the development of effective reclamation strategies for removing heavy metal ions from these matrices has become a research priority. Among various approaches, the use of sorbing materials, both natural and synthetic, has emerged as a promising solution for water purification.
This study [7,8] investigates the recovery and separation of rhenium and molybdenum ions from acidic aqueous solutions using mechanoactivated graphite, which is effective in adsorbing these ions. Additionally, amino-functionalized mesoporous silica materials, specifically SS5-1h-5.5@95-APTES, were developed for Mo(VI) recovery from Re(VII)-containing solutions, showing high adsorption capacity (194.32 mg/g at pH 1) and excellent selectivity, confirmed through various characterization methods.
The application of vermiculite in water purification has shown particular promise in recent research. Additional modification of natural sorbents, such as vermiculite, significantly enhances their characteristics and functional properties. For example, ref. [9] activation through acid or alkaline treatment or the application of active modifying layers can improve sorption capacity by altering the material’s composition and structure. In this context, exfoliated vermiculite is an excellent candidate for such modifications. It possesses high ion-exchange properties, making it highly sought after in agriculture and industry. Acid treatment (modification) of vermiculite improves its sorption characteristics by altering the ionic composition of interlayer spaces [10,11]. Due to its crystalline structure, vermiculite is easily modified with various acids, bases, metals, and organic molecules, such as long-chain quaternary ammonium salts, phosphoric salts, silane agents, amino acids, and other organic additives. For example, vermiculite calcined at 250 °C for two hours demonstrated the ability to extract up to 99.9% of strontium from a solution with an initial concentration of 1.14 × 10−3 mol/L in a neutral medium [12].
The immobilization of azo dyes on poly (glycidyl methacrylate-co-methyl methacrylate) polymers represents a cutting-edge approach to creating advanced adsorbents specifically designed for water purification. These polymer-based materials offer flexibility and efficiency in addressing a broad range of contaminants, particularly heavy metals and dyes, commonly found in industrial wastewater. Their unique properties enable enhanced adsorption performance, making them a valuable tool in improving water quality and advancing sustainable wastewater management [13,14,15]. Similarly [16], impregnation and reaction of glycerol (Gly) on the surface of expanded vermiculite (EV) were used to produce a highly efficient absorbent for removing water-spilled oils.
However, the immobilization of epoxy compounds onto natural adsorbents like vermiculite offers an alternative strategy, combining the inherent porosity and high surface area of vermiculite with the chemical reactivity of epoxy groups. This hybrid approach enhances the adsorbent’s capacity to remove heavy metals and organic pollutants effectively. While polymer-based adsorbents focus on the versatility of synthetic composites, epoxy-modified vermiculite provides a more natural, resource-efficient option for addressing industrial wastewater challenges, demonstrating complementary pathways toward improved environmental remediation technologies [17,18,19,20].
Thus, the modification of natural vermiculite by various physicochemical methods enables the creation of affordable and cost-effective sorbents capable of adsorbing heavy metal ions. Developing efficient and economically feasible sorbents based on natural materials is a priority research direction. Vermiculite, in particular, is of interest due to its high ion-exchange capacity and porous structure.
In addition, the modification of natural vermiculite using various physicochemical methods leads to the creation of affordable and cost-effective sorbents that can adsorb heavy metal ions. Vermiculite, with its high ion-exchange capacity and porous structure, is particularly suitable for this purpose. Modification with epoxy compounds, such as copolymers of GMA, ACN, and orthophosphoric acid, significantly enhances the sorbent’s properties, allowing for the effective removal of toxic ions like MoO42− and ReO4−. While various methods of modifying natural sorbents have been explored, this approach offers significant advantages in terms of both efficiency and cost-effectiveness. The main goal of this work is to study the impact of these modifications on the sorption capacity of vermiculite, which is of both practical and theoretical interest for water purification and environmental protection.
2. Materials and Methods
2.1. Materials and Reagents
Natural vermiculite, dimethylformamide (DMF), glycidyl methacrylate (GMA), acrylonitrile (ACN), benzoyl peroxide, ethyl alcohol, sodium hydroxide (NaOH), orthophosphoric acid (H3PO4), hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3), hydrogen peroxide (H2O2), potassium nitrate (KNO3), and sulfate salts were purchased from Sigma-Aldrich (Germany) and used without further purification.
2.2. Procedure for Preparing the Modified Mineral Sorbent
The modification of the sorbent involved using natural vermiculite, which was pre-treated by thermal activation at 300 W for 10 min. The procedure for modifying the vermiculite-based sorbent with glycidyl methacrylate (GMA), acrylonitrile (ACN), and orthophosphoric acid (H3PO4) consisted of several steps aimed at enhancing the material’s sorption properties:
Thermally activated vermiculite was mixed with GMA and ACN at a weight ratio of 1:0.35:0.15. Specifically, 3.3 g of vermiculite, 1.17 g of GMA, and 0.50 g of ACN were used.
Then, 10.0 g of orthophosphoric acid (85% concentration) was added to the mixture, resulting in an overall component ratio of 1:0.35:0.15:3 (vermiculite:GMA:ACN:H3PO4).
The mixture was thoroughly stirred to ensure uniform distribution of all the components.
The resulting mixture (total initial mass ~15 g) was subjected to microwave treatment at 300 W and 110 °C for 10 min, with two 5 min intervals for cooling.
After microwave processing, the sample was washed with a 5% HCl solution to remove residual acidic impurities, rinsed with distilled water until neutral pH was achieved, and subsequently treated with 5% NaOH solution.
The modified sorbent was dried in two stages: initially at 60 °C for 6 h in a drying oven, followed by 6 h at room temperature to achieve constant weight and ensure the complete removal of residual moisture and solvents.
The practical yield of the modified sorbent was approximately 12 g, corresponding to 80% of the initial total mass. The static exchange capacity (SEC, mg-eq/g) of the product was determined using a 0.1 N HCl solution under static conditions [21].
2.3. Sorption Procedure for Molybdenum and Rhenium Ions
The sorption properties of the sorbents were investigated concerning ions from solutions of corresponding high-purity salts (produced by ALDRICH, St. Louis, MO, USA). The concentration of metal cations was determined using atomic emission spectroscopy.
For each experiment:
- A polymer sample weighing 0.05 g (accurate to 0.0001 g) was placed in an Erlenmeyer flask.
- 20 mL of the metal salt solution was added.
The sorption capacity (SC, mg/g) was calculated using the formula:
where
- V—is the volume of the solution (0.02 L),
- C1—is the initial ion concentration (mg/L),
- C2—is the final ion concentration after sorption (mg/L),
- m–is the mass of the sorbent (0.05 g).
The extraction rate (R) of ion sorption was calculated using the formula:
where
- R—is the degree of extraction,
- C1—is the concentration of ions before sorption,
- C2—is the concentration of ions after sorption.
2.4. Chemical Stability Testing
The chemical resistance of the sorbents was assessed using aggressive media such as acidic and alkaline solutions:
- Two 0.1 g samples were placed in 250 mL round-bottom flasks.
- One sample was treated with 100 mL of a 5 N H2SO4 solution, and the other with a 5 N NaOH solution.
- The mixtures were heated in a boiling water bath for 30 min, cooled to room temperature, and filtered.
- The ion-exchange sorbent was converted to its hydroxyl form, and the SEC was determined.
Chemical resistance (U) was calculated as follows:
where E0 and E1 are SEC (mg-eq/g) before and after contact with acid or alkali, respectively.
2.5. Determining Stability in Oxidative Solutions
A 0.1 g sample of the sorbent was treated with 100 mL of a 1 N HNO3 solution and left at room temperature for 48 h under periodic stirring. Chemical resistance was evaluated by comparing the SEC before and after treatment.
2.6. Physical and Chemical Characterization Methods
FT-IR Spectroscopy: Infrared spectra were recorded in the range of 400–4000 cm−1 using a Cary 600 FT-IR spectrophotometer (Agilent, Waldbronn, Germany) with a resolution of 4 cm−1.
Scanning Electron Microscopy (SEM): The pore structure and morphology were analyzed using a ZEISS Crossbeam 540 instrument to obtain high-resolution surface images. This allowed for the acquisition of high-resolution surface images of morphology and microstructure. The SEM method provided detailed information on the distribution, shape, and size of pores within the material, which is critical for understanding the functionality of the ion-exchanger and its potential application capabilities.
Sorption Analysis: The concentrations of molybdenum and rhenium ions were determined using atomic absorption spectroscopy (AAS) with a Shimadzu AA-6800 spectrophotometer equipped with an autosampler. Prior to analysis, the solutions were filtered and appropriately diluted. Calibration was performed using standard solutions of MoO42− and ReO4−. This method ensures high accuracy and reproducibility in the quantitative determination of metal ions in solution.
3. Results and Discussion
3.1. Optimization of Sorbent Synthesis Conditions. Study of the Influence of Various Factors (Mass Ratio, Temperature, and Duration) on the Modification of Organomineral Sorbents
To determine the optimal conditions for synthesizing modified natural mineral material (vermiculite), glycidyl methacrylate copolymers, and orthophosphoric acid, the effects of the ratio of the initial reacting components, temperature (microwave power), and process duration were studied. These factors were evaluated in terms of their impact on the static ion-exchange capacity using a 0.1 N HCl solution. The study explored the influence of various factors, including the mass ratio of components, temperature (microwave power), and duration of the process, on the static exchange capacity (SEC) of the sorbent.
3.1.1. Influence of Orthophosphoric Acid Concentration
It was observed that the static ion-exchange capacity (SEC) of the vermiculite–GMA–ACN–H3PO4 adsorbent increased with the concentration of orthophosphoric acid, reaching a peak value of 5.91 mg-eq/g at 3.0 mass parts (Figure 1). Beyond this point, further increases in acid concentration did not result in a corresponding enhancement of the SEC. This saturation effect can be attributed to the exhaustion of available active sites on the sorbent surface, the structural densification of the material, the formation of polymeric phosphate layers, and potential side reactions that negatively affected the sorbent’s performance. Therefore, the optimized acid concentration was selected, which maximized the static ion-exchange capacity while preserving the sorbent’s chemical and mechanical stability.
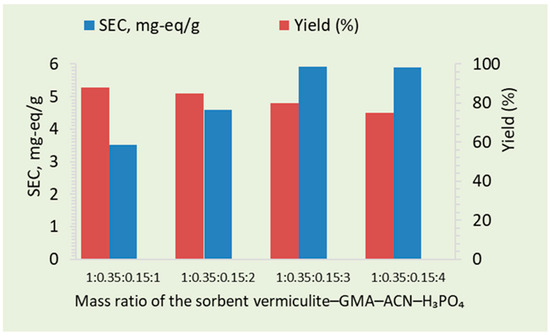
Figure 1.
Change in the SEC of the vermiculite–GMA–ACN–H3PO4 sorbent as a function of orthophosphoric acid concentration.
Similarly, the yield of the adsorbent exhibited a decreasing trend as the concentration of orthophosphoric acid increased. At concentrations of 1.0, 2.0, 3.0, and 4.0 mass parts, the yield was 88%, 85%, 80%, and 75%, respectively. The highest yield was observed at the lowest concentration (1.0 mass part), where the sorbent underwent less structural modification and retained more of its original form. As the concentration of orthophosphoric acid increased, the formation of denser polymeric layers reduced the amount of the adsorbent formed, leading to a decrease in yield. Therefore, an optimized acid concentration was selected that balanced the static ion-exchange capacity and yield while maintaining the chemical and mechanical stability of the sorbent.
3.1.2. Influence of Curing Duration and Microwave Power
Figure 2 shows that the optimal SEC of 5.91 mg-eq/g was achieved with 10 min of microwave treatment at 300 W and 110 °C (split into two 5 min intervals with cooling breaks). Prolonged exposure or increased microwave power beyond 300 W led to a decrease in SEC.
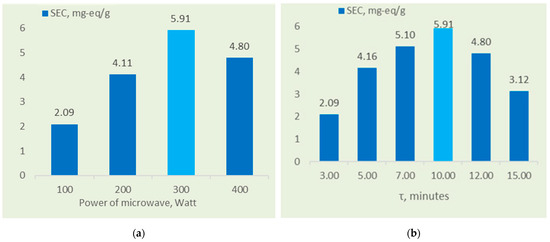
Figure 2.
(a) Effects of microwave power on the SEC of the sorbent. (b) Effects of curing duration on the sorbent’s SEC.
Figure 2a illustrates the effect of modification duration of the vermiculite–GMA–ACN–H3PO4 sorbent in a ratio of 1:0.35:0.15:3, on the static ion-exchange capacity (SEC) under microwave heating at 300 W (at 110 °C). The maximum SEC of the sorbent was achieved within a 10 min interval (with two 5 min pauses), reaching 5.91 mg-eq/g. The blue bars represent experimental conditions, and the light blue bars indicate the optimal condition with the highest sorption efficiency.
As shown in Figure 2b, increasing the microwave power from 300 to 400 W and extending the curing time from 10 to 15 min resulted in a slight decrease in the sorbent’s exchange capacity. This reduction in SEC at longer time intervals and higher microwave power can be attributed to the thermal degradation of the material. Prolonged microwave exposure may lead to partial destruction of chemical bonds and the sorbent structure due to localized overheating. This reduces the number of active functional groups, thereby decreasing sorption capacity. Additionally, prolonged heating and exposure to high microwave power may cause densification of the sorbent matrix. This densification could be associated with the formation of additional organomineral structures, reducing the accessibility of ionogenic groups to low-molecular-weight electrolyte molecules during ion exchange [22,23,24].
Thus, the optimal conditions for obtaining the modified natural mineral sorbent using the microwave method involve a component ratio of vermiculite–GMA–ACN–H3PO4 at 1:0.35:0.15:3 by weight. The resulting mineral sorbent exhibited a static ion-exchange capacity of 5.91 mg-eq/g in a 0.1 N HCl solution with an 80% yield, as shown in Table 1.

Table 1.
Optimal conditions for the microwave synthesis of vermiculite–GMA–ACN–H3PO4 sorbent.
3.2. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis of the Synthesized Sorbent
The FT-IR spectra (Figure 3) of vermiculite and its modified forms confirm the structural transformation of the sorbent during modification with GMA–ACN–H3PO4 and subsequent sorption of Mo (VI) and Re (VII) ions. The spectrum of unmodified vermiculite shows characteristic bands near 3620 cm−1 (–OH stretching), 1620 cm−1 (H2O bending), 1040 cm−1 (Si–O stretching), and 780 cm−1 (Al–O bending), typical for natural layered silicates. Upon modification, new bands appear at 2335 cm−1 and 1724 cm−1, corresponding to C≡N (from acrylonitrile) and C=O (from glycidyl methacrylate), respectively. Phosphate groups are confirmed by peaks at 1298, 1223, 1173, and 1081 cm−1, indicating P=O, P–O–CH3, and P–O–C functionalities, which enable hydrogen bonding and electrostatic interactions with metal ions. After Mo (VI) sorption, the spectrum reveals intensified OH stretching (~3432 cm−1), enhanced H2O deformation (1631 cm−1), and distinct new bands at 915, 778, and 626 cm−1, associated with Mo–O bonds, confirming metal uptake. Similarly, the spectrum after Re (VII) sorption exhibits preserved OH and Si–O bands along with new absorptions at 1038 and 541 cm−1, characteristic of Re–O interactions. These spectral changes demonstrate the successful incorporation of functional groups during modification and effective complexation of Mo and Re ions through ligand exchange and hydrogen bonding.
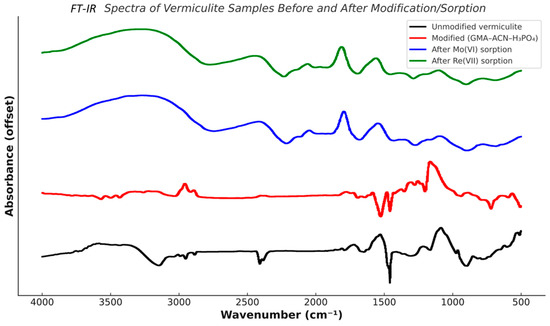
Figure 3.
FT-IR spectrum of the mineral sorbent vermiculite–GMA–ACN–
3.3. Microstructure Analysis of the Sorbent Based on Electron Microscopy
The surface morphology of the final modified sorbent based on vermiculite–GMA–ACN–H3PO4 was studied using scanning electron microscopy (SEM). The SEM results are shown in Figure 4A–D, which presents images of the same sorbent sample captured at different magnifications, providing a multiscale view of its microstructural features.
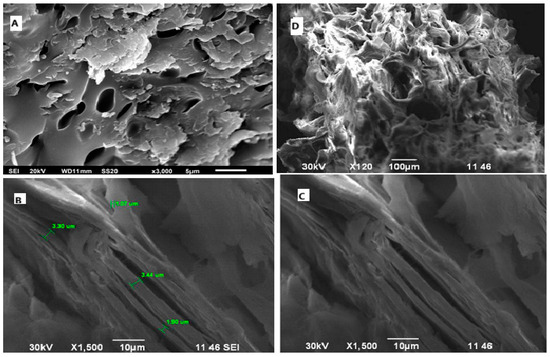
Figure 4.
Evaluation of the structure of the sorbent vermiculite–GMA–ACN– based on SEM images. (A) Surface morphology at ×3000 magnification; (B) Fibrous structure and pore sizes at ×1500; (C) Porous network and internal structure at ×1500; (D) Macroscopic texture and agglomerates at ×120.
Figure 4A (×3000) reveals the general surface topology of the sorbent, characterized by a rough and uneven structure. Fine granular inclusions and protrusions are visible, likely corresponding to residual polymeric or phosphate-containing fragments resulting from surface modification. The surface appears heterogeneous, with irregularities that may serve as active sites for ion sorption.
Figure 4B (×1500) focuses on the fibrous components embedded within the composite structure. These fibers, measuring 1.07–3.44 µm in diameter (as indicated in the image), are randomly distributed and entangled. Their presence suggests the formation of a partially polymerized, three-dimensional matrix on the vermiculite surface, which contributes to structural stability and an increase in the sorbent’s active surface area [25,26].
Figure 4C (×1500, closer view) offers a detailed examination of the internal porous network. The image shows a branched and partially interconnected system of channels and voids formed between the fibers and granules. This morphology indicates that the modification process not only grafted functional groups but also altered the physical architecture of the sorbent, promoting efficient access to internal active zones.
Figure 4D (×120) provides a low-magnification overview of the sample, where the general macroscopic texture and particle distribution are visible. The sorbent appears to form dense agglomerates with clearly distinguishable surface boundaries and hierarchical layering.
In summary, the SEM data confirm that the modified vermiculite-based sorbent exhibits a complex and hierarchical surface morphology, including fiber-like structures, granular domains, and interconnected voids. These features contribute to increased surface area and the availability of active functional groups, both of which are essential for effective adsorption of molybdenum and rhenium ions.
3.4. Sorption of Molybdenum Ions (MoO42−)
The problem of developing new highly efficient sorbents for wastewater treatment and the extraction of ions of heavy, multivalent, and transition metals in hydrometallurgy, medicine, the food industry, water treatment, isotope sorption and concentration, as well as for addressing oil spill issues on water surfaces, the comprehensive utilization of natural and energy-saving resources, and environmental protection remains relevant.
In laboratory conditions, the sorption properties of vermiculite–GMA–ACN–H3PO4 mineral sorbent with respect to MoO42− ions were studied in different solution concentrations (Table 2).

Table 2.
Sorption capacity for MoO42− ions.
As shown in Figure 5, with an increase in the concentration of molybdenum ions (MoO42−) in an aqueous solution of Na2MoO4∙2H2O, the sorption capacity of the vermiculite–GMA–ACN–H3PO4-based sorbent increases from 9.86 mg/g to 39.8 mg/g and stabilizes at a metal concentration above 125 mg/L. Further increases in the molybdenum ion concentration in the solution do not lead to an increase in sorption capacity, indicating that the maximum sorption capacity for MoO42− ions on 0.05 g of the vermiculite–GMA–ACN–H3PO4 sorbent is 39.8 mg/g, with a extraction rate (R) of 79%.
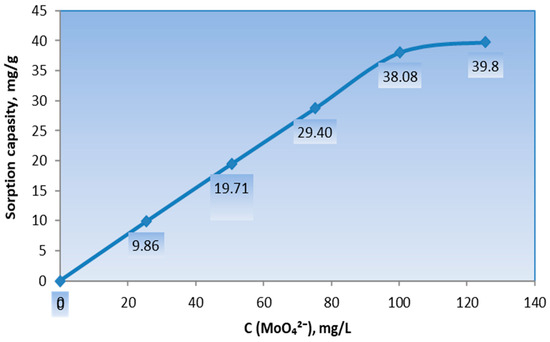
Figure 5.
The isotherm of the sorption of MoO42− ions from the salt by the ion-exchange sorbent vermiculite–GMA–ACN–
To describe the sorption process of molybdate ions (MoO42−), several key mechanisms and structural features can be highlighted. In the solution, molybdenum ions are shown in their free state (e.g., MoO42−). Active centers on the sorbent surface can be represented as distinct particles. Molybdenum ions come into contact with these active centers, which can be indicated by arrows illustrating the movement of ions toward the sorbent surface.
On the sorbent surface, ion exchange or electrostatic interactions may occur. The interaction mechanisms can include electrostatic forces or chemical bonding. After sorption, molybdenum ions stabilize on the sorbent surface and can be depicted in a bound state.
The process of sorption of molybdenum ions (MoO42−), moving from the solution to the sorbent surface, can be explained as follows: the sorbent is a material with active centers on its surface. These centers, represented by arrows symbolizing the sorption process, bind molybdenum ions through physicochemical interactions [27,28,29,30].
The molybdenum ion sorption process involves the binding of MoO42− ions to the active centers of the sorbent via mechanisms such as electrostatic forces or chemical bonding, resulting in their stable retention on the sorbent surface.
The sorption process of molybdenum ions (MoO42−) onto the sorbent involves the movement of the ions toward the sorbent’s active sites. These active sites, located on the material’s porous surface, facilitate ion-binding through mechanisms such as electrostatic interactions or chemical bonding. After the sorption process, molybdenum ions become immobilized on the sorbent surface, completing the adsorption cycle.
Despite the complexity of the process, the reaction can be generally described as follows:
where
Sorb-X + MoO42− → Sorb − MoO42− + X−
- −
- Sorb—active centers of the sorbent,
- −
- MoO42−—molybdate ion in solution.
3.5. Sorption of Rhenium Ions from Model Solutions
The study of the effect of NH4ReO4 solution concentration on the sorption properties of the organomineral sorbent vermiculite–GMA–ACN–H3PO4 demonstrated that the ReO4− ions were almost completely adsorbed from the solutions, achieving up to 98% removal (Table 3), in solutions containing 25.2–100.1 mg/mL of rhenium.

Table 3.
Sorption capacity and extraction rate of rhenium ions by the mineral sorbent vermiculite–GMA–ACN–H3PO4 using atomic emission spectrophotometry.
Figure 6 shows the sorption isotherms of the ReO4− ions from the NH4ReO4 salt solution using the vermiculite–GMA–ACN–H3PO4 sorbent. As the concentration of ReO4− ions in the aqueous NH4ReO4 solution increases, the sorption capacity of the sorbent rises from 10.04 mg/g to 39.06 mg/g and subsequently stabilizes. Additionally, the extraction rate (R) of the vermiculite–GMA–ACN–H3PO4 sorbent for ReO4− ions reached 78%.
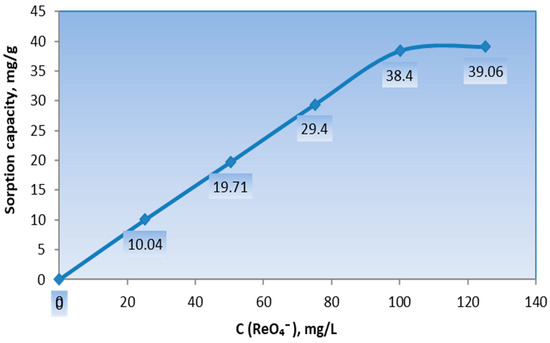
Figure 6.
Sorption isotherm of ReO4− ions from NH4ReO4 salt by the ion-exchange sorbent vermiculite–GMA–ACN–H3PO4.
Figure 6 illustrates the sorption of perrhenate ions (ReO4−) by the ion-exchange sorbent vermiculite–GMA–ACN–H3PO4. When NH4ReO4 salt dissolves in water, the ReO4− ions transition into the solution in free form. Upon contact between the solution containing the ReO4− ions and the sorbent, the phosphate groups of the sorbent, with the composition Sorbent-PO4H+, interact ionically with the ReO4− ions. During this interaction, NH4+ ions remain in the solution, maintaining overall electrical neutrality [31,32,33,34].
The primary active centers of the sorbent are hydroxyl groups (OH), capable of forming hydrogen bonds, and phosphate groups, which are ready for ion exchange with anions in the solution. The phosphate groups of the sorbent can form ionic complexes with ReO4− ions through electrostatic interactions.
As a result, the ReO4− ions displace weakly bound anions from the surface of the sorbent and occupy the active centers. After the ion exchange and electrostatic interaction processes are complete, the ReO4− ions are firmly bound to the sorbent surface. The hydroxyl and phosphate groups of the sorbent form hydrogen bonds with the rhenium ions, enhancing the ability of the phosphate groups to retain ReO4− ions through electrostatic interaction [35,36,37].
Figure 7 demonstrates the key stages of sorption and the physicochemical mechanisms that ensure the binding of ions on the sorbent. The process of ion exchange of rhenium ions (ReO4−) with an ion-exchange sorbent can be represented as follows:
where
Sorb-X + ReO4− → Sorb − ReO4− + X−
- −
- Sorb-X—an ion-exchange sorbent with exchange ions X− on the surface,
- −
- ReO4−—a perrhenate ion in solution,
- −
- X−—an exchange ion that is replaced by a rhenium ion.
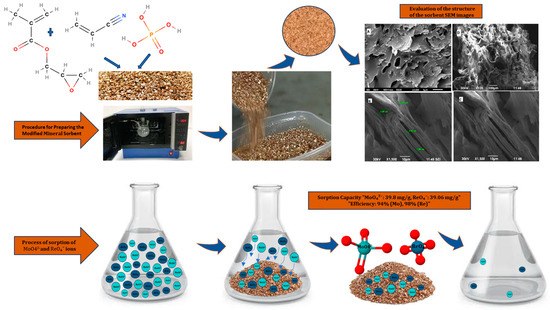
Figure 7.
Schematic representation of the sorbent synthesis process and the adsorption of molybdate (MoO42−) and perrhenate (ReO4−) ions onto the modified vermiculite-based material (vermiculite–GMA–ACN–H3PO4).
In addition to the classical ion-exchange mechanism, the sorption process may also involve the formation of coordination complexes between the sorbent and target anions. The modified vermiculite-based sorbent contains reactive functional groups such as phosphate, hydroxyl, and amine moieties, which are capable of acting as electron-donating ligands. These groups can interact directly with molybdate (MoO42−) and perrhenate (ReO4−) ions in solution, forming stable surface complexes.
Thus, the overall sorption mechanism is likely a combination of:
Ion exchange, where surface-bound anionic groups (X−) are replaced by MoO42− or ReO4− ions, and complexation, which involves coordination between the sorbent’s functional groups and the metal oxyanions.
This dual mechanism contributes to the high affinity and selectivity of the sorbent for molybdenum and rhenium ions.
3.6. Chemical Resistance of Sorbents in Aggressive Media
The aggressive nature of industrial effluents, in terms of their chemical composition, necessitates that any material exposed to such wastewater must withstand harsh chemical environments without significant degradation, at least until the intended purpose of its application is achieved. Therefore, it is essential to investigate the resistance of the ion exchanger in various chemical environments.
The results of chemical stability tests (Table 4) show that the synthesized ion exchangers exhibit sufficient resistance to chemical reagents. The degree of capacity loss for the vermiculite–GMA–ACN–H3PO4 ion exchanger when treated with oxidative, acidic, and alkaline solutions does not exceed 10%. It was also observed that the sorbent undergoes the most degradation, although not significantly, in the diprotic H2SO4 solution, with a capacity loss of about 5–10%.

Table 4.
Chemical stability of vermiculite–GMA–ACN–H3PO4 sorbent.
4. Conclusions
The study demonstrated that the synthesized vermiculite–GMA–ACN–H3PO4 sorbent exhibits improved sorption properties due to the incorporation of functional groups, such as phosphorus and epoxy groups, which enhance its ion-exchange capacity.
It was established that the optimal conditions for achieving the maximum static exchange capacity (SEC) of the sorbent are 5.91 mg-eq/g in a 0.1 N HCl solution under microwave heating for 10 min at 300 W.
The adsorption efficiency of the modified sorbent vermiculite–GMA–ACN–H3PO4 was investigated for rhenium and molybdenum ions from model solutions. The maximum sorption capacity reached 39.8 mg/g for the MoO42− ions and 39.06 mg/g for the ReO4− ions, with extraction rates of 79% and 78%, respectively.
In conclusion, the modified natural sorbent vermiculite–GMA–ACN–H3PO4 can be effectively used as a sorption material for the removal of rhenium and molybdenum ions from wastewater.
Author Contributions
Conceptualization, K.S. and N.B.; methodology, K.S., N.B., A.B. and E.K.; software, T.C., G.K. and K.S.; validation, A.B. and K.S.; formal analysis, K.S., A.B. and N.B.; investigation, G.K.; resources, E.K.; data curation, A.B., K.S. and N.B.; writing—original draft preparation, K.S., A.B. and N.C.; writing—review and editing, N.B. and A.B.; visualization, A.B., K.S. and N.B.; supervision, N.B. and T.C.; project administration, N.B., N.C., T.C. and A.B.; funding acquisition, N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was conducted at Abai Kazakh National Pedagogical University under the targeted funding program for scientific research on the topic: “Synthesis of new highly permeable ion-exchange materials based on minerals” in 2024. The research was supported by university grants for the implementation of integrative scientific projects in collaboration with research institutes of the Republic of Kazakhstan, according to Order No. 05-04/368, dated 24 May 2024.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oyaro, N.; Juddy, O.; Murago, E.N.M.; Gitonga, E. The contents of Pb, Cu, Zn and Cd in meat in Nairobi, Kenya. Int. J. Food Agric. Environ. 2007, 5, 119–121. [Google Scholar]
- Ahangarpour, A.; Oroojan, A.A.; Rezae, M.; Khodayar, M.J.; Alboghobeish, S.; Zeinvand, M. Effects of butyric acid and arsenic on isolated pancreatic islets and liver mitochondria of male mouse. Gastroenterol. Hepatol. Bed Bench 2017, 10, 44–53. [Google Scholar] [PubMed]
- Hou, Y.; Fu, Z.; Luo, J.; Liu, X.; Sun, H.; Li, G. Selective separation of rhenium from oxygen-pressure leach solution of molybdenite concentrate using modified D201 resin: Experiments and theoretical calculations. J. Mol. Liq. 2024, 408, 125371. [Google Scholar] [CrossRef]
- Fathi, M.B.; Rezai, B.; Alamdari, E.K. Competitive adsorption characteristics of rhenium in single and binary (Re-Mo) systems using Purolite A170. Int. J. Miner. Process. 2017, 169, 1–6. [Google Scholar] [CrossRef]
- Mao, J.; Hong, W.; Li, Q.; Gao, Y.; Jiang, Y.; Li, Y.; Li, B.; Gao, B.; Xu, X. The application strategies and progresses of silicon-based minerals in advanced oxidation processes for water decontamination. Coord. Chem. Rev. 2024, 511, 215871. [Google Scholar] [CrossRef]
- Arjmandi, R.; Hassan, A.; Mohamad Haafiz, M.K.; Zakaria, Z. Synthesis and characterization of chitosan-coated magnetic nanoparticles for removal of heavy metals from aqueous solutions. Int. J. Biol. Macromol. 2015, 81, 91–98. [Google Scholar] [CrossRef]
- Korobitsyna, A.D.; Pechishcheva, N.V.; Konysheva, E.Y.; Shunyaev, K.Y. Adsorption of Molybdenum(VI) and Rhenium(VII) on Mechanoactivated Graphite. Russ. J. Phys. Chem. A 2025, 99, 570–580. [Google Scholar] [CrossRef]
- Shan, W.; Shu, Y.; Chen, H.; Zhang, D.; Wang, W.; Ru, H.; Xiong, Y. The recovery of molybdenum(VI) from rhenium(VII) on amino-functionalized mesoporous materials. Hydrometallurgy 2016, 165, 251–260. [Google Scholar] [CrossRef]
- Melnikov, A.; Gordina, N.; Sinitsyn, A.; Gusev, G.; Gushchin, A. Rumyantsev Investigation of the influence of mechanochemical effects on the structure and properties of vermiculite sorbents. J. Solid State Chem. 2021, 306, 122795. [Google Scholar] [CrossRef]
- Neves, H.S.d.C.; da Silva, T.L.; da Silva, M.G.C.; Guirardello, R.; Vieira, M.G.A. Ion exchange and adsorption of cadmium from aqueous media in sodium-modified expanded vermiculite. Environ. Sci. Pollut. Res. 2022, 29, 79903–79919. [Google Scholar] [CrossRef] [PubMed]
- Shapkin, N.P.; Panasenko, A.E.; Khal’chenko, I.G.; Pechnikov, V.S.; Maiorov, V.Y.; Maslova, N.V.; Razov, V.I.; Papynov, E.K. Synthesis and characterization of new hybrid materials based on vermiculite and polyaniline. Russ. J. Inorg. Chem. 2020, 65, 1614–1622. [Google Scholar] [CrossRef]
- Sivaiah, M.V.; Kumar, S.S.; Venkatesan, K.A.; Sasidhar, P.; Krishna, R.M.; Murthy, G.S. Sorption of Strontium on Zirconia Modified Vermiculite. J. Nucl. Radiochem. Sci. 2004, 5, 33–36. [Google Scholar] [CrossRef][Green Version]
- Adjei, J.K.; Ofori, A.; Megbenu, H.K.; Ahenguah, T.; Boateng, A.K.; Adjei, G.A.; Bentum, J.K.; Essumang, D.K. Health risk and source assessment of semi-volatile phenols, p-chloroaniline and plasticizers in plastic packaged (sachet) drinking water. Sci. Total Environ. 2021, 797, 149008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Zhao, W.; Li, Y. Arsenic removal from aqueous solutions by diethylenetriamine-functionalized resin: Isotherm, kinetics, selectivity and mechanism. R. Soc. Open Sci. 2018, 5, 181013. [Google Scholar] [CrossRef]
- Garipov, I.; Khaydarov, R.; Gapurova, O.; Khaydarov, R.; Evgrafova, S. The Application of Fiber Ion Exchange Sorbents for Wastewater Treatment and Purification of Gas Mixtures. J. Energy Environ. Chem. Eng. 2020, 5, 10. [Google Scholar] [CrossRef]
- Medeiros, M.d.A.; Sansiviero, M.T.; Araújo, M.H.; Lago, R.M. Modification of vermiculite by polymerization and carbonization of glycerol to produce highly efficient materials for oil removal. Appl. Clay Sci. 2009, 45, 213–219. [Google Scholar] [CrossRef]
- Han, B.; He, B.; Geng, R.; Zhao, X.; Li, P.; Liang, J.; Fan, Q. Ni(II) sorption mechanism at the vermiculite-water interface: Effects of interlayer. J. Mol. Liq. 2019, 274, 362–369. [Google Scholar] [CrossRef]
- Moraes, D.S.; Rodrigues, E.M.; Lamarão, C.N.; Marques, G.T.; Rente, A.F. New sodium activated vermiculite process. Testing on Cu2+ removal from tailing dam waters. J. Hazard. Mater. 2019, 366, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Hashem, F.S.; Amin, M.S.; El-Gamal, S.M.A. Chemical activation of vermiculite to produce highly efficient material for Pb2+ and Cd2+ removal. Appl. Clay Sci. 2015, 115, 189–200. [Google Scholar] [CrossRef]
- Balgysheva, B.; Massalimov, I.; Urakaev, F.; Sassykova, L.; Zhakirova, N.; Boranbayeva, G.; Dalabayeva, N.; Azatkyzy, S. Modified vermiculite of the mugodzhary deposit and its sorption properties. J. Chem. Technol. Metall. 2022, 57, 533–544. [Google Scholar]
- GOST 20255.1-89; Ionites. Method for Determining Static Exchange Capacity. Standartinform: Moscow, Russia, 2002; 5p. Available online: https://allgosts.ru/71/100/gost_20255.1-89?utm_source (accessed on 9 May 2025).
- Murzakasymova, N.S.; Bektenov, N.A.; Serebriakov, K.V.; Yelkin, E.S.; Gavrilenko, M.A. Control for selective sorption of heavy metals cations with anion exchanger AB-17-8 by modifying the surface with citrate groups. Mendeleev Commun. 2024, 34, 755–757. [Google Scholar] [CrossRef]
- Ergozhin, E.E.; Bektenov, N.A.; Arup, K. Sorption of ions strontium with new complex-forming ionites on the basis of epoxyacrylates and complexones. News of the Academy of sciences of the Republic of Kazakhstan. Ser. Chem. Technol. 2018, 1, 6–11. [Google Scholar]
- Zhang, B.; Gao, B.; Ma, W.; Mo, Z.; Song, Y.; Xie, S.; Jiang, F.; Hu, X. Adsorption of uranium(VI) by natural vermiculite: Isotherms, kinetic, thermodynamic and mechanism studies. J. Environ. Radioact. 2023, 270, 107305. [Google Scholar] [CrossRef] [PubMed]
- Kambarova, E.A.; Murzakasymova, N.S.; Bektenov, N.A. Investigation of the Possibility of Purifying Wastewater from Metal Ions Using New Sorbents. Problems of Geology and Subsurface Development. In Proceedings of the XXIV International Symposium Named after Academician M. A. Usov for Students and Young Scientists, Dedicated to the 75th Anniversary of Victory in the Great Patriotic War, Tomsk, Russia, 6–10 April 2020; Volume 2, pp. 348–350. Available online: https://earchive.tpu.ru/bitstream/11683/62754/1/conference_tpu-2020-C11_V2_p348-350.pdf (accessed on 9 May 2025).
- Deivasigamani, P.; Kumar, P.S.; Sundaraman, S.; Soosai, M.R.; Renita, A.A.; Karthikeyan, M.; Bektenov, N.; Baigenzhenov, O.; Venkatesan, D.; Kumar, A. Deep insights into kinetics, optimization and thermodynamic estimates of methylene blue adsorption from aqueous solution onto coffee husk (Coffee arabica) activated carbon. Environ. Res. 2023, 236, 116735. [Google Scholar] [CrossRef] [PubMed]
- Bektenov, N.; Baidullayeva, A.; Chalov, T.; Jumadilov, T.; Kanat, S. Modified Adsorbents Based on Glycidyl Methacrylate Copolymers for the Removal of Copper and Lead Ions from Wastewater. Eng. Sci. 2024, 31, 1237. [Google Scholar] [CrossRef]
- Sarı, A.; Saleh, T.A.; Tuzen, M. Development and characterization of polymer-modified vermiculite composite as novel highly-efficient adsorbent for water treatment. Surf. Interfaces 2021, 27, 101504. [Google Scholar] [CrossRef]
- da Fonseca, M.G.; de Oliveira, M.M.; Arakaki, L.N.; Espinola, J.G.; Airoldi, C. Natural vermiculite as an exchanger support for heavy cations in aqueous solution. J. Colloid Interface Sci. 2005, 285, 50–55. [Google Scholar] [CrossRef]
- Prakash, N.; Soundarrajan, M.; Vendan, S.A.; Sudha, P.N.; Renganathan, N.G. Contemplating the feasibility of vermiculate blended chitosan for heavy metal removal from simulated industrial wastewater. Appl. Water Sci. 2017, 7, 4207–4218. [Google Scholar] [CrossRef]
- Suručić, L.; Janjić, G.; Marković, B.; Tadić, T.; Vuković, Z.; Nastasović, A.; Onjia, A. Speciation of Hexavalent Chromium in Aqueous Solutions Using a Magnetic Silica-Coated Amino-Modified Glycidyl Methacrylate Polymer Nanocomposite. Materials 2023, 16, 2233. [Google Scholar] [CrossRef]
- Wen, D.; Xie, C.; Zhang, M.; Dong, Z.; Zhai, M.; Zhao, L. DOPO-Modified Cellulose Microsphere: Preparation and Application for Selective Adsorption U(VI) under Acidic Solutions. Res. Sq. 2021. Preprint. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, J.; Jin, X.; Wan, L.; Wang, Y.; Chen, H.; Shan, W.; Xiong, Y. Brown algae based new sorption material for fractional recovery of molybdenum and rhenium from wastewater. Chem. Eng. J. 2015, 273, 231–239. [Google Scholar] [CrossRef]
- Marković, B.M.; Vuković, Z.M.; Spasojevic, V.; Kusigerski, V.; Pavlović, V.; Onjia, A.; Nastasović, A.B. Selective magnetic GMA based potential sorbents for molybdenum and rhenium sorption. J. Alloys Compd. 2017, 705, 38–50. [Google Scholar] [CrossRef]
- Batueva, T.; Scherban, M.; Kondrashova, N.; Chekanova, L. Sorption of rhenium (VII) and molybdenum (VI) by modified mesoporous silicas. Sep. Sci. Technol. 2021, 57, 532–541. [Google Scholar] [CrossRef]
- Joo, S.-H.; Kim, Y.-U.; Kang, J.-G.; Kumar, J.R.; Yoon, H.-S.; Parhi, P.K.; Shin, S.M. Recovery of Rhenium and Molybdenum from Molybdenite Roasting Dust Leaching Solution by Ion Exchange Resins. Mater. Trans. 2012, 53, 2034–2037. [Google Scholar] [CrossRef]
- Pechishcheva, N.; Korobitsyna, A.; Ordinartsev, D.; Zaitceva, P.; Melchakova, O.; Estemirova, S. Effective simultaneous separation of copper (II) and molybdenum (VI) from rhenium (VII) by adsorption on X-alumina. Sep. Sci. Technol. 2021, 57, 180–191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).