1. Introduction
Magnetic iron oxide nanoparticles, particularly maghemite (γ-Fe
2O
3) nanoparticles, have attracted considerable attention owing to their unique combination of superparamagnetic behavior, chemical stability, and biocompatibility [
1,
2,
3]. These attributes render γ-Fe
2O
3 nanoparticles suitable for various applications, including targeted drug delivery, magnetic resonance imaging, biosensing, catalysis, and environmental remediation [
4,
5,
6]. In such applications, surface chemistry plays a critical role in governing colloidal stability, dispersion behavior in various solvents, and interactions with functional molecules or biological systems. Although native iron oxide surfaces are typically rich in hydroxyl (–OH) groups, the density and chemical reactivity of these groups can vary significantly depending on the synthesis route and subsequent post-synthetic treatments. Therefore, surface functionalization strategies that enable the controlled and stable introduction of reactive moieties are essential for tailoring the physicochemical properties of γ-Fe
2O
3 nanoparticles to meet application-specific demands.
A widely employed strategy for modifying metal oxide nanoparticle surfaces involves the use of organosilanes, which form covalent Si–O–M bonds through condensation with surface –OH groups [
7]. Among these organosilanes, 3-aminopropyltriethoxysilane (APTES) is of particular interest as it introduces primary amine functionalities that serve as versatile anchoring sites for biomolecule immobilization, crosslinking reactions, or further chemical derivatization [
8,
9]. However, the efficiency of APTES grafting depends significantly on the surface density and accessibility of –OH groups. The insufficient availability of the –OH group can result in low grafting efficiency, non-uniform surface coverage, and the undesirable polymerization of APTES, which can lead to the formation of multilayer or aggregated structures rather than well-defined monolayers. Consequently, pre-functionalization strategies aimed at optimizing surface hydroxylation are vital for achieving consistent and effective silanization. Despite the widespread use of APTES in nanomaterials chemistry, comprehensive studies examining the relationship between surface –OH group density and silanization efficiency remain limited, particularly in the context of magnetic iron oxide systems.
Surface activation using acid or base treatments is commonly employed to enhance hydroxylation on oxide surfaces. Acidic conditions are generally believed to remove surface contaminants and increase terminal –OH group density [
10,
11], while basic conditions may promote the formation of bridging –OH species and alter surface roughness or porosity [
12]. However, the effects of these treatments are system-dependent and influenced by parameters such as pH, treatment duration, and thermal conditions. In addition, introducing an intermediate silica layer via the hydrolysis and condensation of tetraethoxysilane (TEOS) has been proposed to improve silanization by generating a more uniform and hydroxyl-rich interface. TEOS-derived silica coatings can serve as scaffolds that facilitate the homogeneous distribution of APTES molecules and promote the formation of densely packed, stable aminosilane layers [
13,
14,
15]. Moreover, SiO
2 layers derived from TEOS and APTES have been widely reported to exhibit low toxicity and excellent biocompatibility, providing a safe platform for the application of functionalized nanoparticles in biomedical and environmental fields [
16,
17]. However, the combined influence of acid/base pretreatments, TEOS interlayers, and subsequent APTES grafting in γ-Fe
2O
3 nanoparticle systems has not been thoroughly investigated, which has hindered the rational development of robust surface functionalization protocols.
This study investigates the effects of acid and base pretreatments on the surface –OH group density of γ-Fe2O3 nanoparticles and their subsequent impact on APTES-mediated amine functionalization. Furthermore, the incorporation of an intermediate TEOS-derived silica layer is explored as a strategy to enhance silanization by improving surface uniformity and –OH availability. The surface composition, grafting density, and amine incorporation are analyzed to examine the influence of each surface modification step. The novelty of this work lies in its comprehensive evaluation of sequential surface treatments, i.e., acid/base activation followed by TEOS and APTES functionalization, specifically tailored for γ-Fe2O3 nanoparticles. The findings provide valuable insights for the rational design of surface engineering strategies aimed at producing high-density, chemically robust amine-functionalized magnetic nanoparticles for use in advanced functional materials.
3. Results and Discussion
Figure 1 presents the FT-IR spectra used to analyze the changes in functional groups resulting from the surface modification of γ-Fe
2O
3 nanoparticles. The measurements were performed using the KBr pellet technique, in which the sample was homogenized with potassium bromide and compressed into a transparent pellet. In this analysis, the chemical modifications of as-received γ-Fe
2O
3 and γ-Fe
2O
3 nanoparticles surface-treated in acidic and basic environments using HCl and NH
4OH, respectively, were investigated. A broad absorption band was observed around 570 cm
−1 in all the samples, which is attributed to Fe–O stretching vibrations [
18].
In the FT-IR spectra of the Acid-γ-Fe
2O
3 (A-γ-Fe
2O
3) and Base-γ-Fe
2O
3 (B-γ-Fe
2O
3) samples, a broad O–H stretching vibration peak was observed in the region of 3000–3700 cm
−1 [
19], along with an H–O–H bending vibration peak around 1600 cm
−1 [
20]. This indicates an increase in the density of –OH groups and adsorbed water molecules on the surface of γ-Fe
2O
3 nanoparticles due to surface modification. The HCl and NH
4OH treatments effectively altered the surface of γ-Fe
2O
3 nanoparticles, promoting the formation of –OH groups and the adsorption of water molecules, which increased the intensities of the O–H and H–O–H vibration peaks.
Notably, the B-γ-Fe
2O
3 sample treated with NH
4OH exhibited stronger O–H and H–O–H peaks than the A-γ-Fe
2O
3 sample treated with HCl. This is because NH
4OH, a strong base, provides OH⁻ ions, facilitating hydroxylation reactions on the γ-Fe
2O
3 surface and altering the surface charge to a more negative state, which induces the additional adsorption of water molecules [
21]. In contrast, HCl provides an acidic environment, reacting with some of the Fe–OH groups on the surface, which may partially suppress –OH group formation through dehydration-condensation or Cl⁻ substitution [
22]. Because of these mechanistic differences, NH
4OH treatment is found to induce the greater formation of –OH groups on the surface of γ-Fe
2O
3 nanoparticles than HCl treatment.
Overall, the surface modification process was demonstrated to facilitate the adsorption of –OH groups and water molecules.
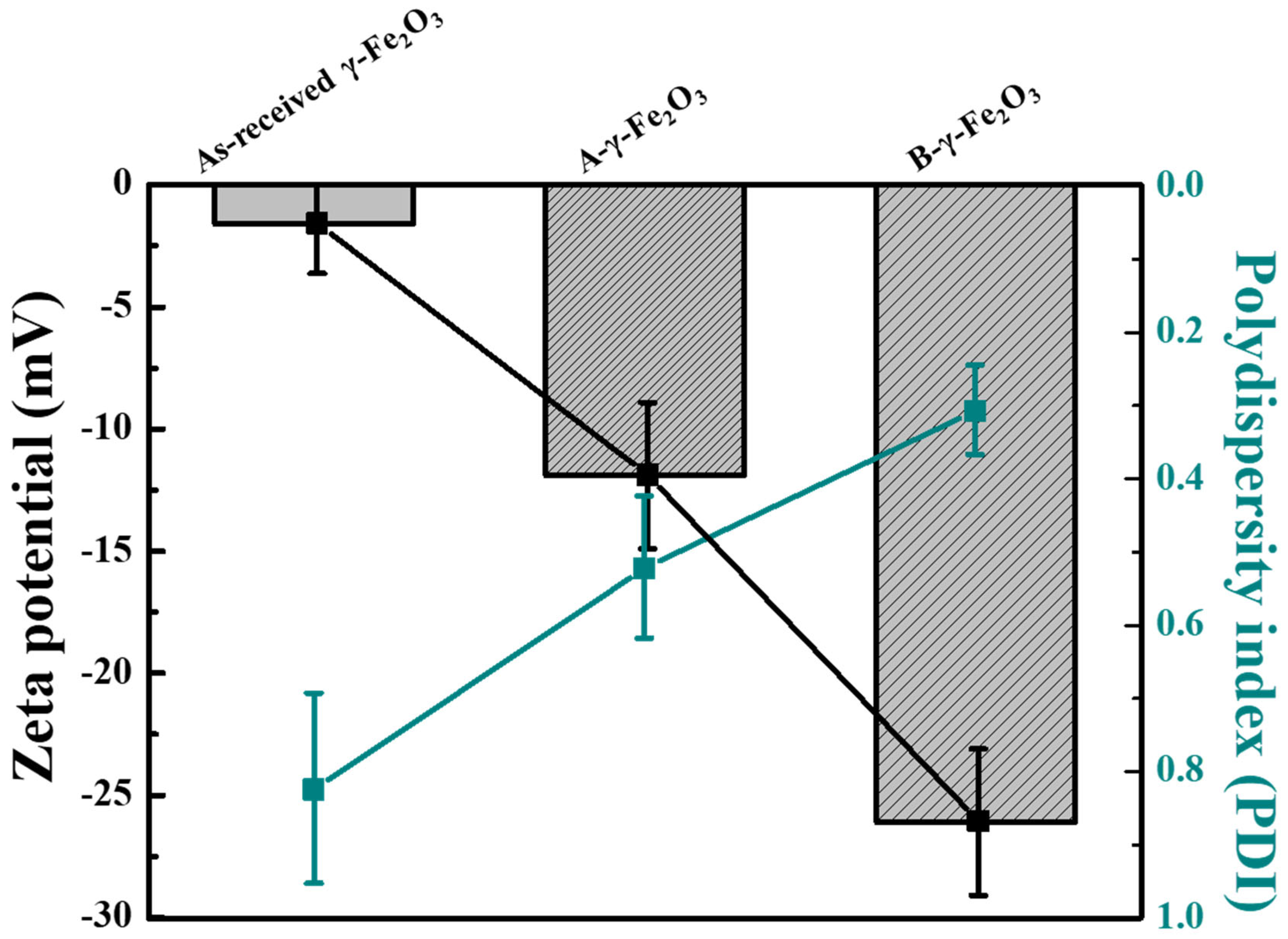
Figure 2 presents the zeta potential and polydispersity index (PDI) values measured to evaluate the dispersion stability of the as-received γ-Fe
2O
3 nanoparticles, as well as the surface-modified A-γ-Fe
2O
3 and B-γ-Fe
2O
3 nanoparticles treated with HCl and NH
4OH, respectively. All samples were dispersed in deionized water (DIW) prior to measurement. The measurement results indicated that the as-received γ-Fe
2O
3 nanoparticles had a zeta potential of −1.6 ± 2 mV and a PDI of 0.824 ± 0.13. This suggested that the weak electrostatic repulsion between γ-Fe
2O
3 nanoparticles led to aggregation, resulting in a broad particle-size distribution (PSD) and a high PDI value. The measured pH of the dispersion was 6.1.
In contrast, the surface-modified nanoparticles treated with HCl and NH4OH exhibited zeta-potential values of −11.9 ± 3 mV and −26.1 ± 3 mV, respectively, with corresponding PDI values of 0.522 ± 0.097 and 0.367 ± 0.061. The pH values of the dispersions were measured to be 4.3 for the acid-treated sample and 9.4 for the base-treated sample. Compared with as-received γ-Fe2O3, the surface-modified nanoparticles exhibited lower PDI values. This is attributed to the increased electrostatic repulsion between particles due to surface modification, which enhances the dispersion stability.
Notably, the B-γ-Fe
2O
3 nanoparticles treated with NH
4OH exhibited a higher absolute zeta-potential value and a lower PDI value than the A-γ-Fe
2O
3 nanoparticles treated with HCl. Consistent with the FT-IR spectral analysis in
Figure 1, this suggests that NH
4OH treatment led to the formation of a larger number of –OH groups on the surface, which increased the surface charge and electrostatic repulsion between particles and ultimately improved the dispersion stability. Therefore, base treatment is considered more effective for enhancing the dispersion stability of nanoparticles than acid treatment.
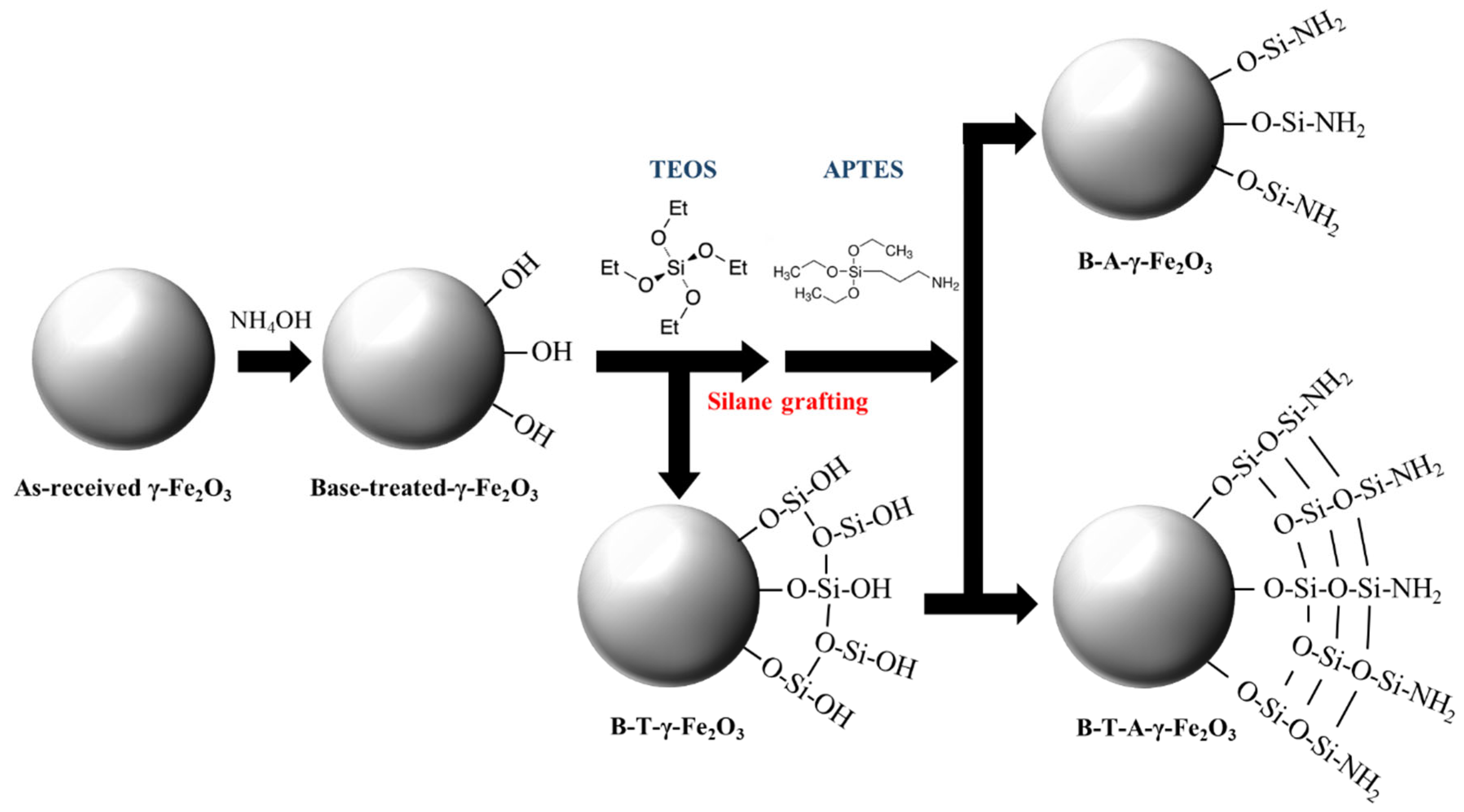
Figure 3 visually represents the surface modification of B-γ-Fe
2O
3 nanoparticles through alkaline treatment followed by functionalization using TEOS and APTES. Upon treatment with NH
4OH, –OH groups are introduced onto the surface of the B-γ-Fe
2O
3 nanoparticles. Subsequently, the nanoparticles undergo amine functionalization through two different approaches: (1) direct functionalization with APTES and (2) sequential treatment with TEOS followed by APTES functionalization.
In the first approach, APTES is directly introduced onto the surface of B-γ-Fe
2O
3 nanoparticles. The ethoxy (–OCH
2CH
3) groups of APTES undergo hydrolysis in the presence of water, leading to the formation of silanol (Si–OH) groups. These Si–OH groups react with the –OH groups present on the nanoparticle surface via condensation reactions, forming Si-O-Si bonds through silane grafting [
23]. This results in the introduction of NH
2 functional groups in a monolayer configuration. However, owing to the limited availability of active sites on the nanoparticle surface, the density of amine functional groups incorporated through this method is relatively low.
Conversely, in the second approach, TEOS is introduced before APTES functionalization. During the hydrolysis of TEOS, ethanol (C
2H
5OH) is eliminated, forming Si–OH groups. The generated Si(OH)
4 molecules react with surface –OH groups on B-γ-Fe
2O
3, forming Fe–O–Si(OH)
3 bonds. This is followed by condensation reactions, facilitating the development of a three-dimensional SiO
2 network on the nanoparticle surface [
24]. The increased density of Si–OH groups resulting from this process provides a larger number of active sites for subsequent APTES attachment. When APTES is introduced, its hydrolysis and condensation reactions with terminal Si–OH groups of the preformed SiO
2 network enable the formation of robust chemical bonds, increasing the density of amine (-NH
2) functional groups on the nanoparticle surface.
According to these findings, the pretreatment of B-γ-Fe2O3 nanoparticles with TEOS prior to APTES functionalization is a more effective strategy for introducing amine groups than direct APTES grafting. The formation of a three-dimensional SiO2 network enhances the available binding sites, increasing the attachment density of APTES and optimizing the surface modification efficiency.
Figure 4 presents the FT-IR spectral analysis of γ-Fe
2O
3 nanoparticles before and after TEOS and APTES treatments, highlighting the chemical modifications at the nanoparticle surface. The functional group variations in base-treated γ-Fe
2O
3 (B-T-γ-Fe
2O
3) nanoparticles following TEOS treatment were examined, revealing that the Fe-O bond remained intact. This observation suggests that TEOS treatment does not alter the fundamental structural characteristics of γ-Fe
2O
3 nanoparticles. Furthermore, new peaks corresponding to Si-O-Si bonds appeared at 1220 and 1070 cm
−1 [
25], confirming the successful formation of an SiO
2 shell on the nanoparticle surface after NH
4OH treatment. The FT-IR spectra also exhibited an increased intensity of –OH and H–O–H vibration peaks following TEOS treatment. This is attributed to the hydrolysis and condensation reactions of TEOS, which led to the formation of additional Si-OH groups on the surface [
26]. These Si-OH groups are likely to form hydrogen bonds with pre-existing –OH groups on the γ-Fe
2O
3 surface or facilitate water-molecule adsorption, increasing the O–H stretching and H-O-H bending vibration intensities.
Additionally, FT-IR analysis of base-APTES γ-Fe
2O
3 (B-A-γ-Fe
2O
3) and base-TEOS-APTES γ-Fe
2O
3 (B-T-A-γ-Fe
2O
3) nanoparticles confirmed the introduction of amine functionalities. Distinct absorption peaks were observed at 3000–3700 cm
−1 (N–H stretching vibration), approximately 2900 cm⁻¹ (C–H stretching vibration), and 1635 cm
−1 (N–H bending vibration), indicating the successful functionalization of the nanoparticle surface [
27,
28,
29]. These results confirm that APTES, an organosilane compound with a terminal amine (-NH
2) group, chemically bonded to the nanoparticle surface via covalent interactions.
To provide a comparative assessment of amine group incorporation, the FTIR absorption bands corresponding to N–H bending (~1635 cm−1) and C–H stretching (~2900 cm−1) were qualitatively evaluated relative to the Fe–O stretching band at 570 cm−1. The B-T-A-γ-Fe2O3 sample exhibited noticeably stronger N–H and C–H signals than the B-A-γ-Fe2O3 sample, indicating a higher level of surface amine functionalization. Although not quantitatively determined, this relative enhancement supports the conclusion that the TEOS-mediated silica interlayer promotes more effective and homogeneous APTES grafting.
Notably, in the B-T-A-γ-Fe2O3 sample, the SiO2 shell formed during TEOS treatment facilitated siloxane bond formation with APTES, leading to the retention of the Si–O–Si peaks at 1220 and 1070 cm−1. Simultaneously, the intensities of FT-IR peaks associated with amine functional groups were significantly increased. This suggests that TEOS treatment not only provides an abundance of Si-OH groups on the surface but also promotes strong APTES binding, facilitating a higher degree of amine functionalization on the nanoparticles.
In conclusion, TEOS treatment preserves the intrinsic properties of γ-Fe2O3 while introducing a thin SiO2 layer that enhances surface –OH and H–O–H bonding characteristics. Furthermore, the pre-deposited SiO2 layer improves the stability and uniformity of APTES functionalization, leading to an increase in surface hydrophilicity and a more homogeneous amine group distribution. These findings suggest that the combined TEOS and APTES treatment serves as an effective strategy for surface modification, significantly improving the functionality of γ-Fe2O3 nanoparticles for potential applications.
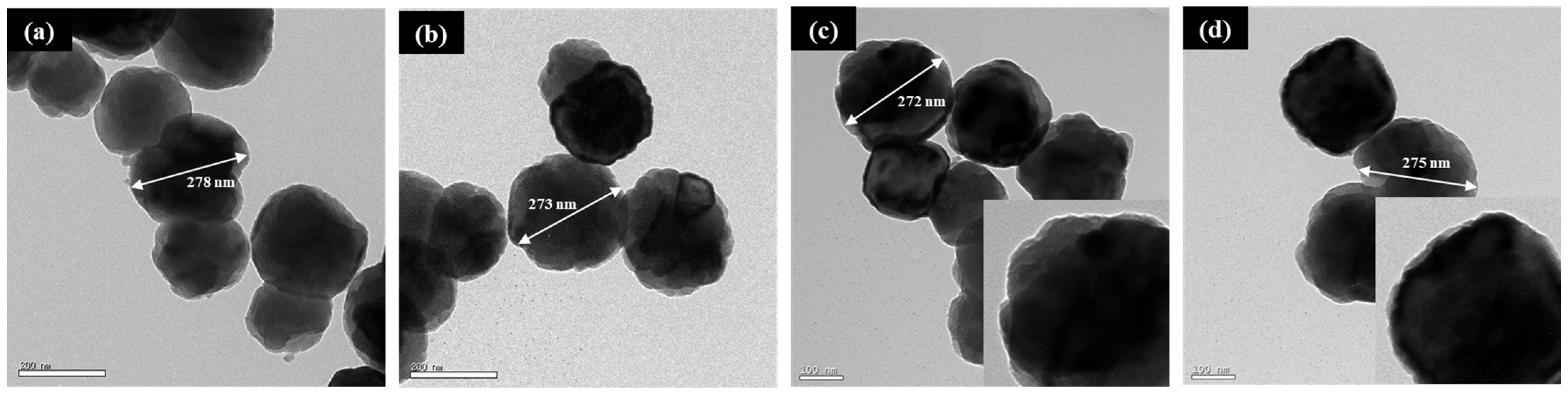
Figure 5 presents TEM images used to analyze the morphological characteristics of γ-Fe
2O
3 nanoparticles, including the particle size and surface structure. The images correspond to (a) as-received γ-Fe
2O
3, (b) base-treated B-A-γ-Fe
2O
3 nanoparticles modified with NH
4OH, (c) B-T-A-γ-Fe
2O
3 nanoparticles further coated with TEOS, and (d) B-T-A-γ-Fe
2O
3 nanoparticles subjected to additional amine functionalization using APTES. The average particle size for all the samples was measured to be in the range of approximately 270–290 nm, indicating that the NH
4OH-based base treatment, TEOS coating, and APTES functionalization processes were successfully conducted without causing structural degradation of the γ-Fe
2O
3 core.
Notably, the TEM images in
Figure 5c,d show no distinct shell structure or additional phase-separated materials, despite the application of TEOS and APTES treatments. This suggests that TEOS and APTES were adsorbed onto the nanoparticle surface in an ultrathin and uniform manner, forming SiO
2 and organic layers that were not sufficiently thick to be distinctly identified via TEM analysis. Additionally, the nanoparticles retained their spherical or quasi-spherical morphology even after surface modifications, indicating that the modification processes had minimal impact on the morphological stability of γ-Fe
2O
3 nanoparticles.
Consequently, the results confirm that the base treatment, TEOS coating, and amine functionalization processes applied in this study effectively modified the nanoparticle surface without inducing structural changes in the γ-Fe2O3 core.
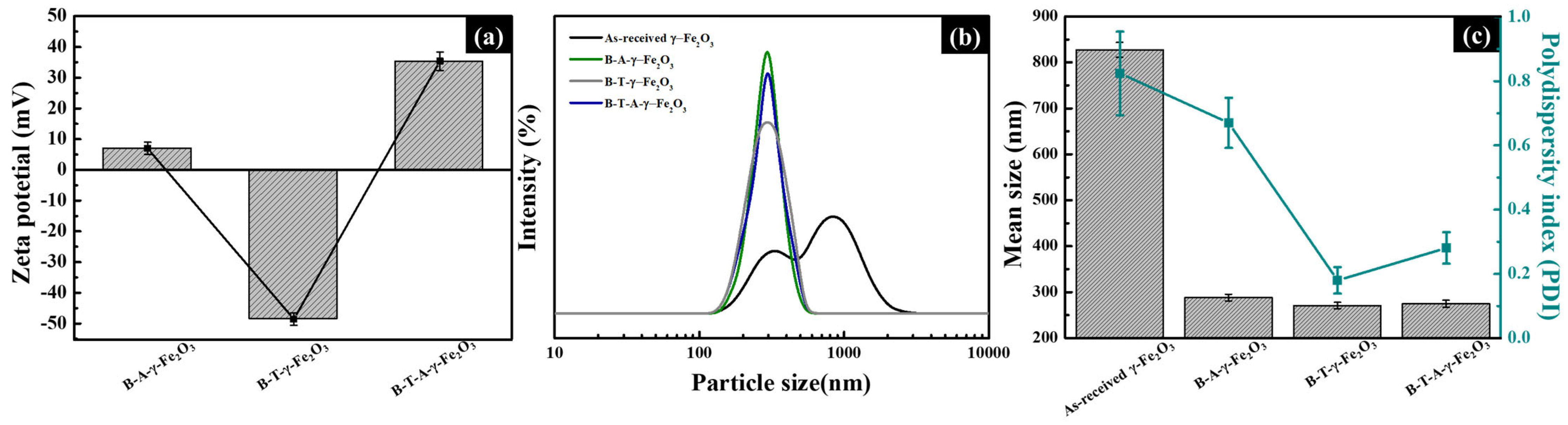
Figure 6a presents the zeta-potential measurements of B-A-γ-Fe
2O
3, B-T-γ-Fe
2O
3, and B-T-A-γ-Fe
2O
3 nanoparticles after amine functionalization via APTES treatment, indicating their dispersion stability. All samples were dispersed in deionized water (DIW), and the pH values of the respective dispersions were measured to be 7.3 for B-A-γ-Fe
2O
3, 10.0 for B-T-γ-Fe
2O
3 and 4.9 for B-T-A-γ-Fe
2O
3. The measured zeta-potential values were 7.1 ± 2 mV for B-A-γ-Fe
2O
3, −48.3 ± 2 mV for B-T-γ-Fe
2O
3, and 35.4 ± 3 mV for B-T-A-γ-Fe
2O
3. These variations are primarily attributed to TEOS treatment, which facilitates the effective adsorption of APTES amine groups onto the γ-Fe
2O
3 surface. Notably, the absolute zeta-potential value of B-T-A-γ-Fe
2O
3 increased more than threefold compared with that of B-A-γ-Fe
2O
3, indicating a significant enhancement in dispersion stability. Furthermore, the increase in the absolute zeta-potential value was directly correlated with the higher concentration of positively charged amine groups. The positive shift in zeta potential for B-T-A-γ-Fe
2O
3 confirms the successful incorporation of a larger number of amine groups, which aligns with the higher N–H bending peak intensity observed in the FT-IR spectrum of
Figure 4. These results collectively suggest that TEOS serves as a precursor for forming a thin SiO
2 layer on the γ-Fe
2O
3 surface, which subsequently facilitates siloxane bonding with APTES, leading to more efficient amine functionalization. The findings indicate that TEOS treatment significantly improves the dispersion stability of amine-functionalized γ-Fe
2O
3 nanoparticles.
Figure 6b,c illustrate the PSD, mean particle size, and PDI of as-received γ-Fe
2O
3 and surface-modified nanoparticles. The as-received γ-Fe
2O
3 nanoparticles exhibited a bimodal distribution with peaks at approximately 370 and 990 nm, with an average particle size of 827.4 ± 16.4 nm and a PDI of 0.824 ± 0.13. This significantly larger size compared with the individual particles observed in the TEM images (
Figure 5) suggests aggregation due to poor dispersion stability. In contrast, the average particle sizes and PDIs of the surface-modified nanoparticles, including B-A-γ-Fe
2O
3, B-T-γ-Fe
2O
3, and B-T-A-γ-Fe
2O
3, were measured as 289.1 ± 7.3 nm and 0.67 ± 0.078, 270.9 ± 6.9 nm and 0.18 ± 0.031, and 274.6 ± 7.6 nm and 0.281 ± 0.039, respectively. These values closely match those observed in the TEM analysis, confirming that surface modification effectively prevents aggregation.
Additionally, the changes in the PSD patterns indicate that surface modification enhances electrostatic repulsion between particles, suppressing aggregation and leading to a transition from a bimodal to a monomodal distribution. In
Figure 6a, the significantly higher absolute zeta-potential values of B-T-γ-Fe
2O
3 and B-T-A-γ-Fe
2O
3 compared with B-A-γ-Fe
2O
3 corroborate their superior dispersion stability. This is also reflected in the PSD graphs, where these samples exhibit narrower and more defined peaks.
In conclusion, the combined treatments of NH4OH, TEOS, and APTES effectively improve the surface characteristics and dispersion stability of γ-Fe2O3 nanoparticles while maintaining the core particle size without significant alteration.
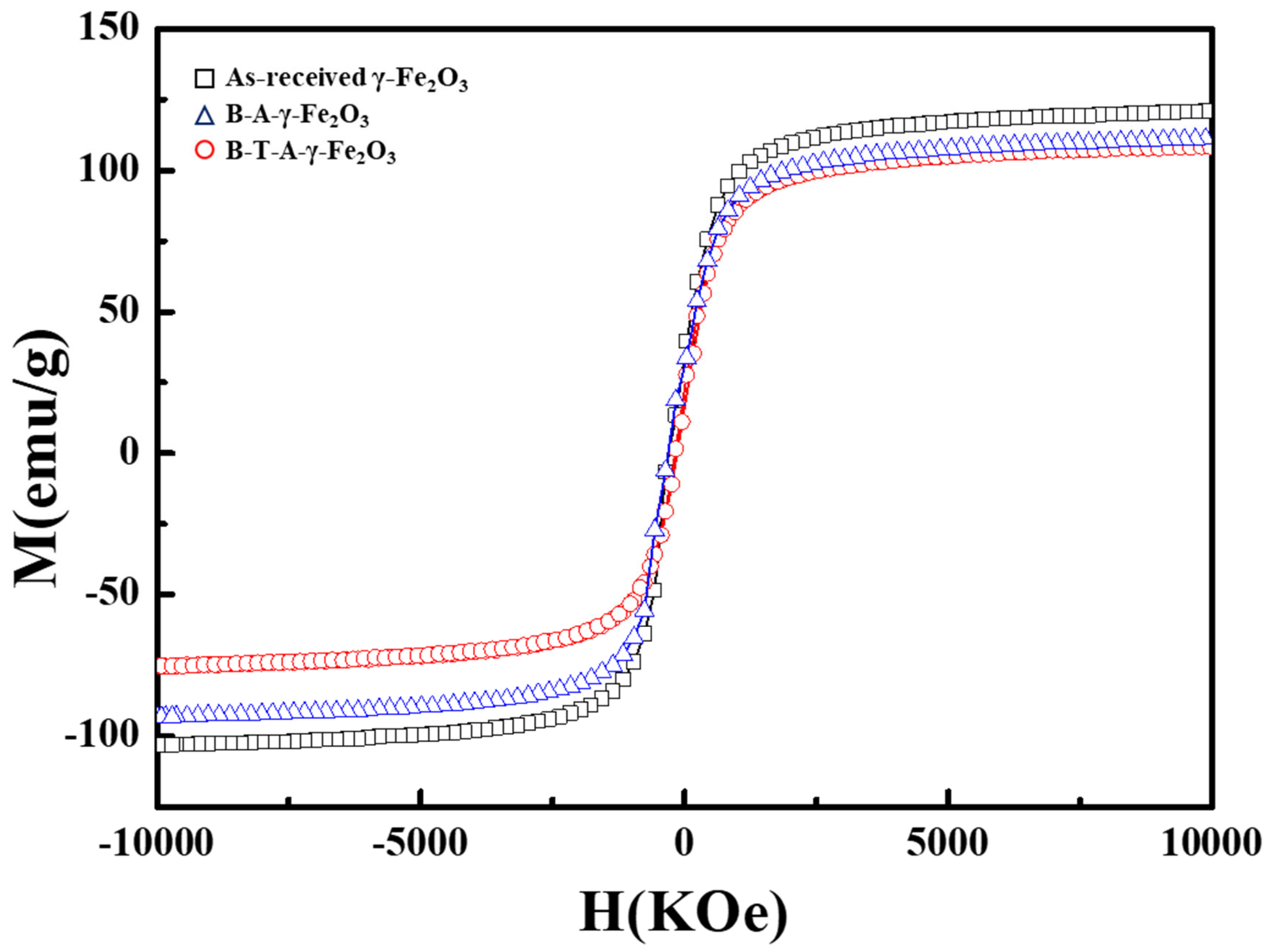
Figure 7 presents the VSM analysis results conducted to evaluate the effect of APTES-based amine functionalization on the magnetic properties of γ-Fe
2O
3 nanoparticles. The magnetic hysteresis curves for all the samples exhibited a linear form with no residual magnetization or coercivity, indicating that the synthesized nanoparticles retained their superparamagnetic properties.
The as-received γ-Fe2O3 nanoparticles exhibited a high saturation magnetization (Ms) value of 121.48 emu/g. After surface modification with APTES, the Ms values of B-A-γ-Fe2O3 and B-T-A-γ-Fe2O3 samples were slightly reduced to 111.24 and 109.81 emu/g, respectively.
This reduction in Ms is not primarily attributed to the surface-bound functional groups, which are present only in trace amounts and thus exert negligible influence on the overall magnetic response. Rather, it may be attributed to two main factors: (1) improved dispersion stability of the surface-modified particles, which minimizes magnetic interactions among aggregated particles during VSM measurement, and (2) minor structural changes such as surface etching or partial reduction in magnetic core volume caused by acid/base and silanization treatments.
However, all the samples maintained their superparamagnetic properties, suggesting that the surface modification did not significantly impair the magnetic characteristics of γ-Fe2O3 nanoparticles. Consequently, APTES amine functionalization is considered to have only a minor impact on the magnetic properties of γ-Fe2O3 nanoparticles.