Development of Volumetric Adsorption Isotherms for Volcanic Fly Ash from Egypt for Carbon Dioxide Capture Under Elevated Pressure and Temperature
Abstract
1. Introduction
2. Experimental Description
2.1. Experimental Material
- ▪
- Fly Ash: The fly ash used to conduct all the experiments in this research was provided as a greyish powder with a high aluminosilicate concentration. The volcanic fly ash provided was purified by the supplier to remove any traces of heavy metals and sulfur. The fly ash had 30% aluminum oxide, 10% iron oxide, 10% calcium oxide, 10% silicon oxide, and 25% sodium oxide, with the remaining concentration composed of sulfur oxide, potassium, and magnesium oxide, and loss on ignition. The surface area of the fly ash ranged between 3 and 7 m2/g;
- ▪
- Water Bath: A water bath with distilled water was used to provide a constant temperature to the experimental setup across the experiments. The water bath had a slotted lid on it to ensure minimal water evaporation, with no pressure buildup;
- ▪
- Precision Balance: A precision scale was used to weigh the fly ash packed into the adsorption vessel to ensure that all the experiments used the same weight of fly ash;
- ▪
- CO2 Cylinder: The CO2 cylinder was used to provide a source of CO2 for the experiment. It was provided as a CO2 cylinder with a purity of 99.99%;
- ▪
- Helium Cylinder: Helium was used to measure the void space volume between the fly ash particles prior to conducting the adsorption experiment;
- ▪
- Pressure Transducers: Pressure transducers were connected to the sample cell and the reference cell to measure the pressure differential and the pressure equilibrium values for adsorption calculation.
2.2. Experimental Setup
2.3. Experimental Procedure
- ▪
- The sample cell was fully packed with the volcanic fly ash sample. The weight of the fly ash sample was determined to be the same for all experiments. The sample cell was then sealed and vacuumed. The fly ash was used in the same manner in which it was provided, without the utilization of any additional alkaline activators. This was due to the high initial concentration of alkaline components in the fly ash;
- ▪
- The experimental setup is placed in the water bath and is left for 12 h until the temperature homogenizes;
- ▪
- The sample cell was filled with helium at the design pressure of the experiment. Following this, the void space volume was measured using helium expansion. This was used in the adsorption calculations to remove errors in the calculation due to the void space present between the fly ash particles. When the shale particles are placed in the reference cell, some small spaces will exist between the particles. This volume is referred to as the void volume and must be accounted for during each experiment, since this is considered excess volume that will be occupied by the CO2 and will not contribute to the adsorption. The void space therefore signifies the pores present between the grains and is thus similar to the total porosity of the system. The void volume is also an essential factor in the adsorption calculations, as was shown previously. The void volume is usually measured using a gas with extremely low adsorption; the gas used in this study to measure void volume is helium, which is the most widely used gas to measure adsorption. Helium has many advantages that make it extremely suitable to use when measuring the void space;
- ▪
- Once the void space is measured, the CO2 adsorption experiment commences. The pressure transducers begin recording the pressure in both cells. Initially, the sample cell reads a negative gauge pressure due to vacuum, while the reference cell reads the reference pressure of the experiment;
- ▪
- The valve connecting the sample cell and the reference cell is opened, and the CO2 is allowed to expand in the vacuumed sample cell. The pressure is then left to equilibrate between both cells. This can take between 8 h to 3 days, depending on the pressure and temperature conditions;
- ▪
- Once equilibrium pressure is reached, the experiment is concluded, and the pressure is used to calculate the adsorption capacity for the design pressure. The experiment is then repeated at the same temperature for different pressure values. By measuring the CO2 adsorption at the same temperature at different pressure values, the adsorption isotherm for the fly ash can be determined for this specific temperature;
- ▪
- Once the isotherm is developed at a specific temperature, the temperature is then changed, and the process is repeated to construct the isotherm for the elevated temperature.
3. Volumetric Adsorption
4. Results and Analysis
4.1. Adsorption Isotherm
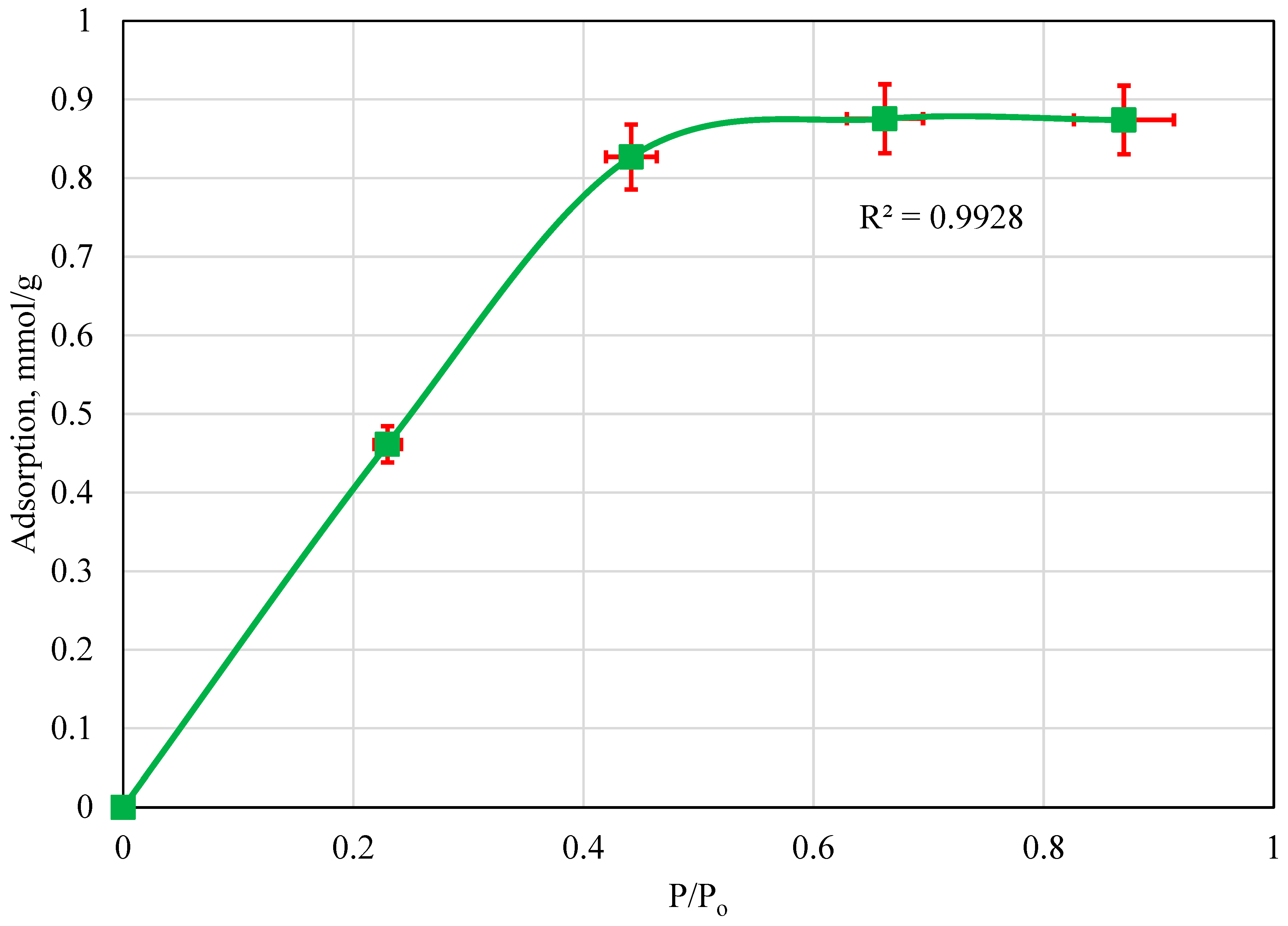
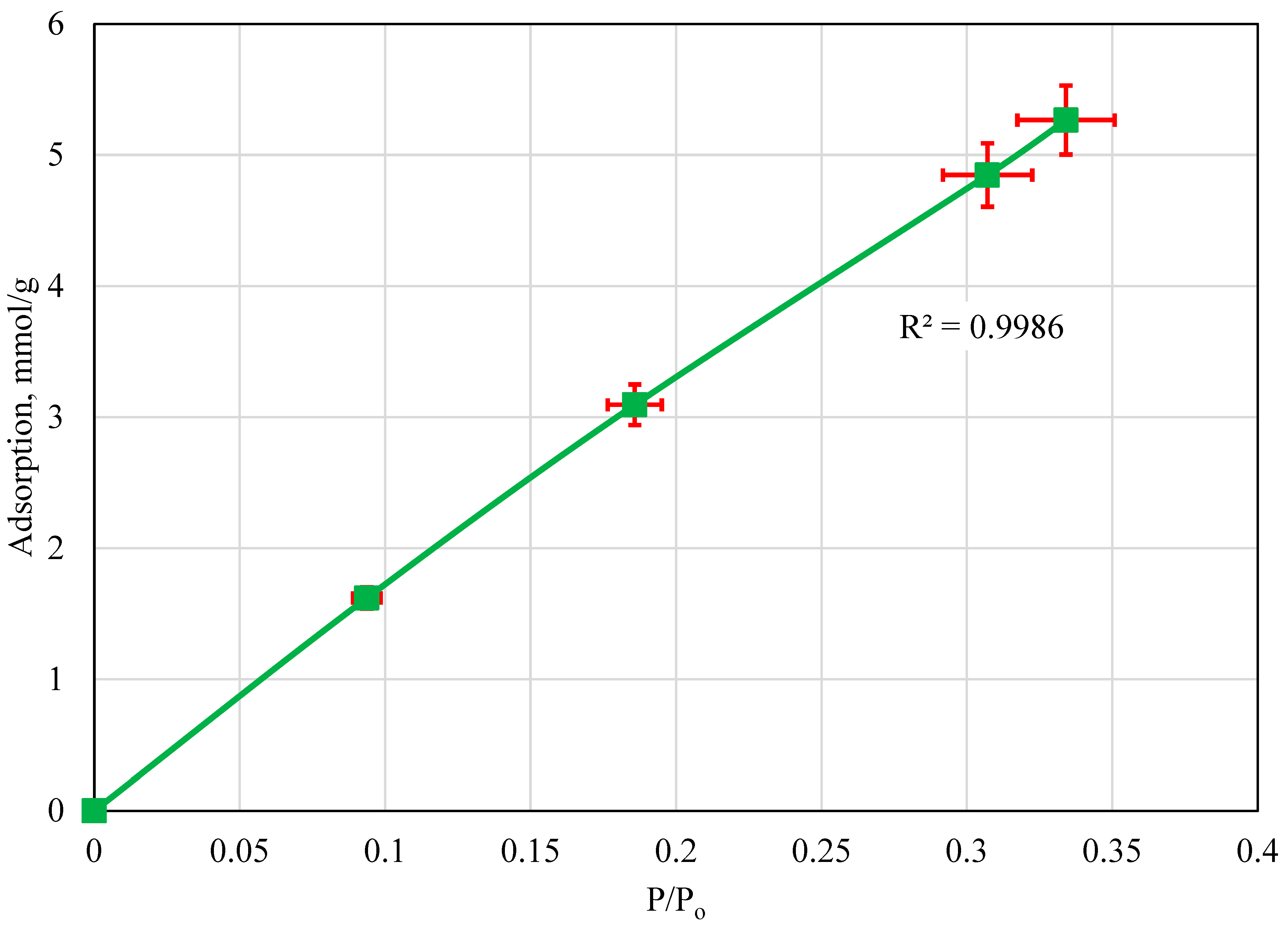
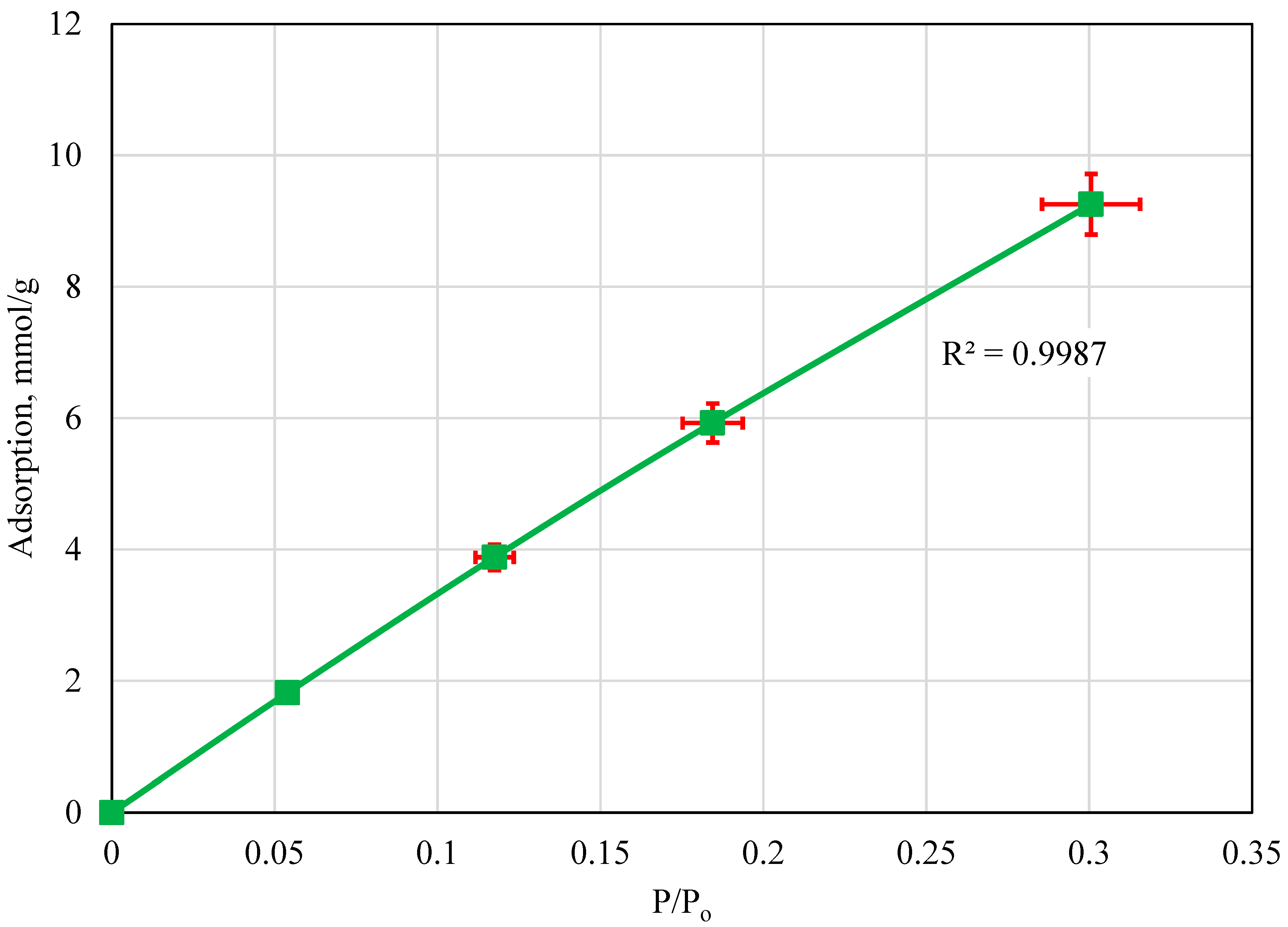
4.2. Adsorption Isotherm: 23 °C
4.3. Adsorption Isotherm: 40 °C
4.4. Adsorption Isotherm: 60 °C
4.5. Adsorption Isotherm: 80 °C
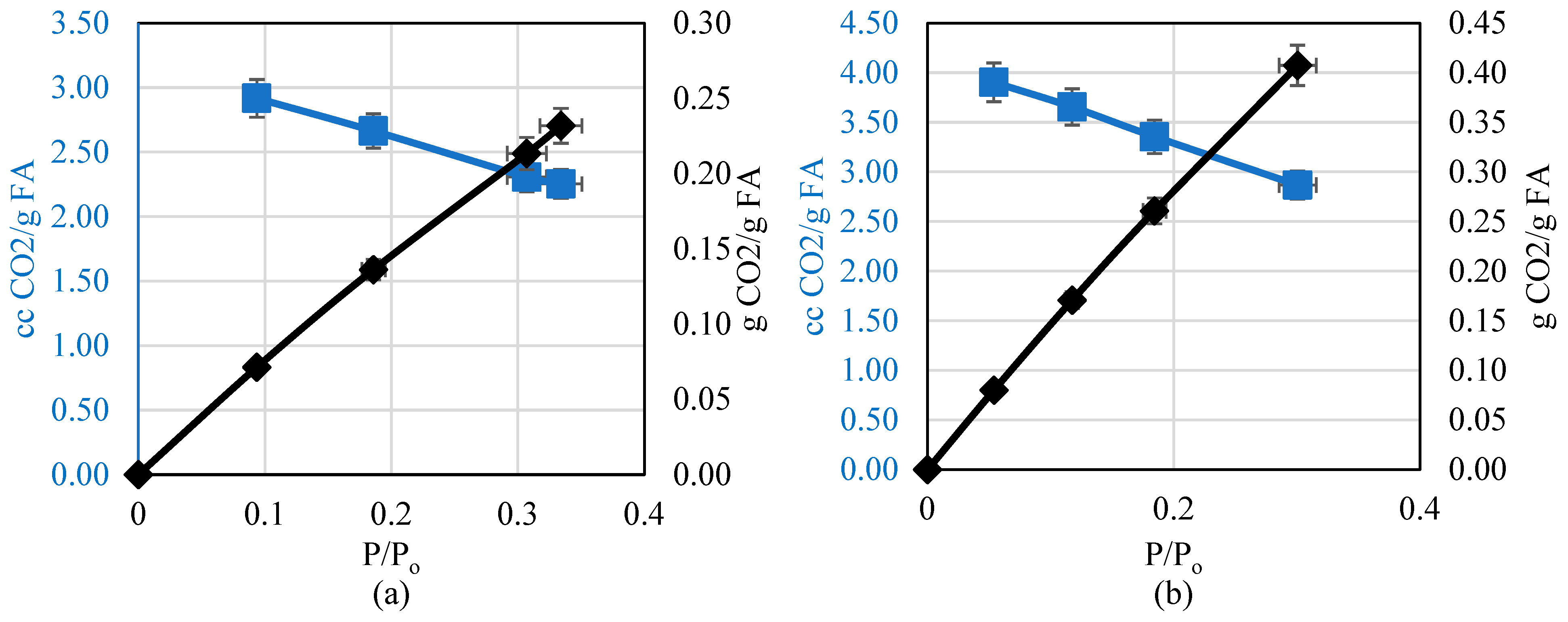
5. Discussion
6. Conclusions
- ▪
- The volcanic fly ash used in this research has a good potential for CO2 storage applications based on its ability to adsorb a large volume of CO2 on its surface;
- ▪
- Increasing the temperature of the fly ash resulted in expansion of the particles, which in turn increased the available surface area for adsorption, thus increasing CO2 capture;
- ▪
- Increasing the pressure of the injected CO2 resulted in an increase in the adsorption capacity. This trend was observed until the maximum available adsorption sites were occupied by CO2;
- ▪
- Increasing pressure allows for the compression of a larger volume of CO2 in the available adsorption sites. This was observed by comparing the change in volume and mass of CO2 with pressure at each temperature.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karimi, M.; Shirzad, M.; Silva, J.A.C.; Rodrigues, A.E. Carbon dioxide separation and capture by adsorption: A review. Environ. Chem. Lett. 2023, 21, 2041–2084. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon dioxide adsorption based on porous materials. RSC Adv. 2021, 11, 12658–12681. [Google Scholar] [CrossRef] [PubMed]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- Mehrmohammadi, P.; Ghaemi, A. Investigating the effect of textural properties on CO2 adsorption in porous carbons via deep neural networks using various training algorithms. Sci. Rep. 2023, 13, 21264. [Google Scholar] [CrossRef]
- Giraldo, L.; Vargas, D.P.; Moreno-Piraján, J.C. Study of CO2 Adsorption on Chemically Modified Activated Carbon with Nitric Acid and Ammonium Aqueous. Front. Chem. 2020, 8, 543452. [Google Scholar] [CrossRef]
- Zeng, H.; Qu, X.; Xu, D.; Luo, Y. Porous Adsorption Materials for Carbon Dioxide Capture in Industrial Flue Gas. Front. Chem. 2022, 10, 939701. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere 2022, 13, 397. [Google Scholar] [CrossRef]
- Maia, R.A.; Louis, B.; Gao, W.; Wang, Q. CO2 adsorption mechanisms on MOFs: A case study of open metal sites, ultra-microporosity and flexible framework. React. Chem. Eng. 2021, 6, 1118–1133. [Google Scholar] [CrossRef]
- Ren, F.; Liu, W. Review of CO2 Adsorption Materials and Utilization Technology. Catalysts 2023, 13, 1176. [Google Scholar] [CrossRef]
- Elsayed, A.; Fakher, S. Investigating Asphaltene Damage Reduction in Wellbores, and Pipelines Using Alkaline and Surfactant Chemical Agents. In Proceedings of the Mediterranean Offshore Conference, Alexandria, Egypt, 20–22 October 2024. [Google Scholar] [CrossRef]
- Fakher, S.; Tamer, S.; Eltohamy, M.; Ali, M.; Alsakkaf, A.; Gehad, M.; Haydar, R.; Mourad, K. Development and Optimization of Epoxy-Resin Based Cement Reinforced with Low-Cost Fly Ash for High Durability Environmentally Friendly Cement. In Proceedings of the 57th U.S. Rock Mechanics/Geomechanics Symposium, Atlanta, GA, USA, 25–28 June 2023. [Google Scholar] [CrossRef]
- Haydar, R.; Fakher, S. Harsh Environmental Effects on Low Density Fly Ash Proppants. In Proceedings of the Mediterranean Offshore Conference, Alexandria, Egypt, 20–22 October 2024. [Google Scholar] [CrossRef]
- Youssef, H.; Fakher, S. Evaluating the Performance of Class F Fly Ash Compared to Class G Cement for Hydrocarbon Wells Cementing: An Experimental Investigation. Materials 2024, 17, 2710. [Google Scholar] [CrossRef]
- Ebied, A.; Fakher, S.; Kayed, H. Experimental Investigation of Inclusion of Various Nanocarbon Black Concentrations on Mechanical Characteristics of Oil-Well Cement Slurries in High-Pressure High-Temperature Conditions. Int. J. Concr. Struct. Mater. 2025, 19, 10. [Google Scholar] [CrossRef]
- Raz, H.; Fakher, S. Investigating and Evaluating Novel Fly Ash-Based Proppant Compressive Strength Under Various Environmental Conditions. Materials 2025, 18, 399. [Google Scholar] [CrossRef] [PubMed]
- Sherif, F.; Khlaifat, A.; Mokhtar, K.; Abdelsamei, M. Assessment of Two Crosslinked Polymer Systems Including Hydrolyzed Polyacrylamide and Acrylic Acid–Hydrolyzed Polyacrylamide Co-Polymer for Carbon Dioxide and Formation Water Diversion Through Relative Permeability Reduction in Unconsolidated Sandstone Formation. Polymers 2024, 16, 3503. [Google Scholar] [CrossRef]
- Sherif, F.; Khlaifat, A.; Hassanien, A. Carbon Dioxide Capture under Low-Pressure Low-Temperature Conditions Using Shaped Recycled Fly Ash Particles. Gases 2024, 4, 117–132. [Google Scholar] [CrossRef]
- Sherif, F.; El-Sayed, A.; Sameh, L.; Abdeltawab, B. Evaluating a Novel Fly Ash Resin-Reinforced Cement’s Interactions under Acidic, Basic, High-Salinity, and High-Temperature Conditions. Polymers 2023, 15, 3404. [Google Scholar] [CrossRef]
- Sherif, F.; Khlaifat, A.L. Experimental Investigation of Polymer Injection in High Permeability Conduits for Material Sustainability and Behavior in Oil Reservoirs. Polymers 2023, 15, 2950. [Google Scholar] [CrossRef] [PubMed]
- Sherif, F.; Yousef, A.; Al-Sakkaf, A.; Eldakar, S. Asphaltene onset pressure measurement and calculation techniques: A review. Petroleum 2024, 10, 191–201. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Guo, T.; Hao, J.; Si, C.; Guo, Q. Efficient CO2 adsorption and mechanism on nitrogen-doped porous carbons. Front. Chem. Sci. Eng. 2021, 15, 493–504. [Google Scholar] [CrossRef]
- Sukkathanyawat, H.; Bampenrat, A.; Jarunglumlert, T.; Prommuak, C. Modification of waste sugarcane bagasse fly ash for CO2 capture application. Mater. Renew. Sustain. Energy 2022, 11, 267–276. [Google Scholar] [CrossRef]
- Majchrzak-Kucęba, I.; Nowak, W. Development of Fly Ash-Based Sorbent to Capture CO2 from Flue Gas. In Proceedings of the 20th International Conference on Fluidized Bed Combustion, Xi’an, China, 18–21 May 2009; Yue, G., Zhang, H., Zhao, C., Luo, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Islam, R.; Biswas, B.; Naidu, R. CO2 Capture Using Zeolite Synthesized from Coal Fly Ash and Its Subsequent Utilization for Fire Retardation and Dye Removal. ACS Sustain. Resour. Manag. 2024, 1, 799–809. [Google Scholar] [CrossRef]
- Shen, X.; Yan, F.; Li, C.; Zhang, Z.; Zhang, Z. A green synthesis of PEI-nano-SiO2 adsorbent from coal fly ash: Selective and efficient CO2 adsorption from biogas. Sustain. Energy Fuels 2021, 5, 1014–1025. [Google Scholar] [CrossRef]
- Guo, F.; He, J.; Johnson, P.A.; Aryana, S.A. Stabilization of CO2 foam using by-product fly ash and recyclable iron oxide nanoparticles to improve carbon utilization in EOR processes. Sustain. Energy Fuels 2017, 1, 814–822. [Google Scholar] [CrossRef]
- Boycheva, S.; Marinov, I.; Zgureva-Filipova, D. Studies on the CO2 Capture by Coal Fly Ash Zeolites: Process Design and Simulation. Energies 2021, 14, 8279. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, K.; Luo, J.; Wu, M.; Wang, X.; Wang, K.; Liu, S. A Study on the Mechanisms of Coal Fly Ash to Improve the CO2 Capture Efficiency of Calcium-Based Adsorbents. Sustainability 2024, 16, 8139. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, M.; Wang, C.; Tao, L.; Bu, J.; Zhu, Q. Harnessing Ash for Sustainable CO2 Absorption: Current Strategies and Future Prospects. Chem.—Asian J. 2024, 19, e202400180. [Google Scholar] [CrossRef]
- Czuma, N.; Casanova, I.; Baran, P.; Szczurowski, J.; Zarębska, K. CO2 sorption and regeneration properties of fly ash zeolites synthesized with the use of differentiated methods. Sci. Rep. 2020, 10, 1825. [Google Scholar] [CrossRef]
- Chen, L.; Lu, S.; Zhang, L.; Zhang, C.; Liu, L.; Kang, G.; Tang, H.; Chen, S. Solid Waste of Fly Ash toward Energy-Efficient CO2 Capture. ACS Sustain. Chem. Eng. 2023, 11, 8281–8293. [Google Scholar] [CrossRef]
- Liu, R.; Shi, X.; Wang, C.; Gao, Y.; Xu, S.; Hao, G.; Chen, S.; Lu, A. Advances in Post-Combustion CO2 Capture by Physical Adsorption: From Materials Innovation to Separation Practice. ChemSusChem 2021, 14, 1428–1471. [Google Scholar] [CrossRef]
- Lai, J.Y.; Ngu, L.H.; Hashim, S.S. A review of CO2 adsorbents performance for different carbon capture technology processes conditions. Greenh. Gases: Sci. Technol. 2021, 11, 1076–1117. [Google Scholar] [CrossRef]
- Madden, D.; Curtin, T. Carbon dioxide capture with amino-functionalised zeolite-β: A temperature programmed desorption study under dry and humid conditions. Microporous Mesoporous Mater. 2016, 228, 310–317. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Mouhtaris, T.; Charistos, D.; Kantiranis, N.; Filippidis, A.; Kassoli-Fournaraki, A.; Tsirambidis, A. GIS-type zeolite synthesis from Greek lignite sulphocalcic fly ashes promoted by NaOH solutions. Microporous Mesoporous Mater. 2003, 61, 57–67. [Google Scholar] [CrossRef]
- Medek, J. Possibility of micropore analysis of coal and coke from the carbon dioxide isotherm. Fuel 1977, 56, 131–133. [Google Scholar] [CrossRef]
- Derkowski, A.; Franus, W.; Waniak-Nowicka, H.; Czímerová, A. Textural properties vs. CEC and EGME retention of Na-X zeolite prepared from fly ash at room temperature. Int. J. Miner. Process. 2007, 82, 57–68. [Google Scholar] [CrossRef]
- Chen, X.B.; Tang, Y.T.; Ke, C.C.; Zhang, C.Y.; Ding, S.C.; Ma, X.Q. CO2 capture by double metal modified CaO-based sorbents from pyrolysis gases. Chin. J. Chem. Eng. 2022, 43, 40–49. [Google Scholar] [CrossRef]
- Modekwe, H.U.; Moothi, K.; Daramola, M.O.; Mamo, M.A. Corn cob char as catalyst support for developing carbon nanotubes from waste polypropylene plastics: Comparison of activation techniques. Polymers 2022, 14, 2898. [Google Scholar] [CrossRef]
- Lafmejani, M.K.A.; Parsa, A.; Mirmohammadi, M.; Ahmadi, T.; Mirmohammadi, H. A novel and facile synthesis of calcium silicate nanoparticles as a base for root canal cement/sealer under constant potential: Compared to chemical synthesis. Mater. Chem. Phys. 2024, 315, 128924. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Castañeda, J. Estudio de las Propiedades de Trasporte en Materiales Porosos Mediante Espectroscopía Infrarrojo con Trasformada de Fourier (FTIR). Master’s Thesis, Universidad Nacional de Colombia, Medellin, Colombia, 2012. [Google Scholar]
- Da Gama, C.; Navarro-Torres, V.; Falcao-Neves, A. Techological innovations on underground coal gasification and CO2 sequestration. Dyna 2010, 77, 101–108. [Google Scholar]
- Lira-Zúñiga, S.; Sáez-Navarrete, C.; Rodríguez-Córdova, L.; Herrera-Zeppelin, L.; Herrera-Urbina, R. CO2 adsorption on agricultural biomass combustion ashes. Maderas-Cienc. Tecnol. 2016, 18, 607–616. [Google Scholar] [CrossRef]
- Jiang, L.; Cheng, L.; Zhang, Y.; Liu, G.; Sun, J. A Review on CO2 Sequestration via Mineralization of Coal Fly Ash. Energies 2023, 16, 6241. [Google Scholar] [CrossRef]
- Verrecchia, G.; Cafiero, L.; de Caprariis, B.; Dell’Era, A.; Pettiti, I.; Tuffi, R.; Scarsella, M. Study of the parameters of zeolites synthesis from coal fly ash in order to optimize their CO2 adsorption. Fuel 2020, 276, 118041. [Google Scholar] [CrossRef]
- Ragipani, R.; Sreenivasan, K.; Anex, R.P.; Zhai, H.; Wang, B. Direct Air Capture and Sequestration of CO2 by Accelerated Indirect Aqueous Mineral Carbonation under Ambient Conditions. ACS Sustain. Chem. Eng. 2022, 10, 7852–7861. [Google Scholar] [CrossRef]
- Zgureva, D.; Boycheva, S. Experimental and model investigations of CO2 adsorption onto fly ash zeolite surface in dynamic conditions. Sustain. Chem. Pharm. 2020, 15, 100222. [Google Scholar] [CrossRef]
- International Zeolite Association (IZA). Available online: https://www.iza-online.org (accessed on 1 April 2025).
- Uppili, S.; Thomas, K.J.; Crompton, E.M.; Ramamurthy, V. Probing zeolites with organic molecules: Supercages of X and Y zeolites are superpolar. Langmuir 2000, 16, 265–274. [Google Scholar] [CrossRef]
- Osatiashtiani, A.; Puertolas, B.; Oliveira, C.C.S.; Manayil, J.C.; Barbero, B.; Isaacs, M.; Michailof, C.; Heracleous, E.; Pérez-Ramírez, J.; Lee, A.F.; et al. On the influence of Si:Al ratio and hierarchical porosity of FAU zeolites in solid acid catalysed esterification pretreatment of bio-oil. Biomass Conv. Bioref. 2017, 7, 331–342. [Google Scholar] [CrossRef]
- Nikolakis, V.; Xomeritakis, G.; Abibi, A.; Dickson, M.; Tsapatsis, M.; Vlachos, D.G. Growth of a faujasite-type zeolite membrane and its application in the separation of saturated/unsaturated hydrocarbon mixtures. J. Membr. Sci. 2001, 184, 209–219. [Google Scholar] [CrossRef]
- Julbe, A.; Drobek, M. Zeolite X: Type. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Su, F.; Lu, C. CO2 capture from gas stream by zeolite 13X using a dual-column temperature/vacuum swing adsorption. Energy Environ. Sci. 2012, 5, 9021–9027. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore sizedistribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Bolis, V. Fundamentals in adsorption at the solid-gas interface. Concepts and thermodynamic. In Calorimetry and Thermal Methods in Catalysis; Springer Series in Materials Science; Auroux, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 154, pp. 3–50. [Google Scholar]
- Ruthven, D.M. Adsorption (Chemical Engineering). In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 251–271. [Google Scholar]
- Chou, C.-T.; Wu, B.-C.; Wu, T.-L.; Yang, H.-S.; Shen, C.-H. Concentrating high purity CO2 from syngas after oxy-fuel combustion by pressure swing adsorption process. Comp. Aided Chem. Eng. 2018, 43, 1425–1431. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers Handbook, 7th ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Herraiz, L.; Palfi, E.; Sánchez, E.F.; Mathieu, L. Rotary Adsorption: Selective recycling of CO2 in combined cycle gas turbine power plants. Front. Energy. Res. 2020, 8, 308. [Google Scholar] [CrossRef]
- Kalvachev, Y.; Zgureva, D.; Boycheva, S.; Barbov, B.; Petrova, N. Synthesis of carbon dioxide adsorbents by zeolitization of fly ash. J. Therm. Anal. Calorim. 2016, 124, 101–106. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D. Studies on the CO2 adsorption onto coal fly ash zeolites at elevated Pressures. In Proceedings of the 17th International Conference on Environmental Science and Technology (CEST 2021), Athens, Greece, 1–4 September 2021. [Google Scholar]
- Kareem, F.A.A.; Shariff, A.M.; Ullah, S.; Dreisbach, F.; Keong, L.K.; Mellon, N.; Garg, S. Experimental measurements and modeling of supercritical CO2 adsorption on 13X and 5A zeolites. J. Nat. Gas Sci. Eng. 2018, 50, 115–127. [Google Scholar] [CrossRef]
- Siemons, N.; Busch, A. Measurement and interpretation of supercritical CO2 sorption on various coals. Int. J. Coal Geol. 2007, 69, 229–242. [Google Scholar] [CrossRef]
- Sircar, S. Measurement of Gibbsian surface excess. AIChE J. 2001, 47, 1169–1176. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakher, S.; Khlaifat, A.; Salib, A.M.; Elsayed, A. Development of Volumetric Adsorption Isotherms for Volcanic Fly Ash from Egypt for Carbon Dioxide Capture Under Elevated Pressure and Temperature. Processes 2025, 13, 1570. https://doi.org/10.3390/pr13051570
Fakher S, Khlaifat A, Salib AM, Elsayed A. Development of Volumetric Adsorption Isotherms for Volcanic Fly Ash from Egypt for Carbon Dioxide Capture Under Elevated Pressure and Temperature. Processes. 2025; 13(5):1570. https://doi.org/10.3390/pr13051570
Chicago/Turabian StyleFakher, Sherif, Abdelaziz Khlaifat, Ann Maria Salib, and Ali Elsayed. 2025. "Development of Volumetric Adsorption Isotherms for Volcanic Fly Ash from Egypt for Carbon Dioxide Capture Under Elevated Pressure and Temperature" Processes 13, no. 5: 1570. https://doi.org/10.3390/pr13051570
APA StyleFakher, S., Khlaifat, A., Salib, A. M., & Elsayed, A. (2025). Development of Volumetric Adsorption Isotherms for Volcanic Fly Ash from Egypt for Carbon Dioxide Capture Under Elevated Pressure and Temperature. Processes, 13(5), 1570. https://doi.org/10.3390/pr13051570








