Abstract
A new type of hydrogen, produced in situ in petroleum reservoirs, is proposed. This technology is based on ex situ catalytic gasification of biomass, combining two thermal enhanced oil recovery techniques currently used in industrial fields: cyclic steam stimulation and in situ combustion. This hydrogen, named “light-blue hydrogen”, is produced in reservoirs, like naturally occurring white hydrogen, and from fossil fuels, like blue hydrogen. The color light blue results from the blending of white and blue. This approach is particularly suitable for mature petroleum reservoirs, which are in the final stages of production or no longer producing oil. This manuscript describes the method for producing light-blue hydrogen in situ, its commercial application prospects, and the challenges for developing and scaling up this technology.
1. Introduction
Hydrogen is widely recognized as the sustainable fuel of the future, with diverse production sources, high energy density, and no emissions during combustion. It plays a key role in the energy transition toward a low-carbon future and is an alternative to fossil fuels, offering a route to reducing greenhouse gas emissions and combating climate change [1].
Hydrogen has several advantages: it does not produce CO2, it helps reduce NOx emissions, and its generation cost is expected to decrease as industrial acceptance grows. Additionally, it can be used as a sustainable aviation fuel (SAF) [2]. According to the International Energy Agency (IEA), from 2022 to 2035, more than half of the total funding (>27 billion USD) for clean energy projects has focused on hydrogen or hydrogen-based fuels, highlighting its importance as a future fuel [3].
Currently, most hydrogen production (approximately 70 million metric tons) comes from fossil fuels: 76% from natural gas via steam methane reforming (grey hydrogen), 22% from coal gasification (with China being the main producer), and only 2% from electrolysis (green hydrogen). Other hydrogen production technologies are still in development and are considered immature due to high production costs or low efficiency. The most significant new approaches to producing hydrogen are (1) green hydrogen (with renewable electricity) and (2) blue hydrogen (from fossil fuels with CO2 emissions reduced by carbon capture, utilization, and storage). However, both methods currently have higher costs compared to fossil-based hydrogen. Therefore, it is essential to develop new hydrogen production methods with acceptable efficiency, low carbon emissions, and low cost [4].
In this context, a new technology that uses ex situ conversion concepts to produce hydrogen from crude oils in mature reservoirs is an attractive alternative [5]. This hydrogen, produced by a combination of in situ combustion (ISC) and cyclic steam stimulation (CSS), is called aqua hydrogen [1]. Gasification, traditionally a thermal carbon rejection process used in petroleum refineries to convert vacuum residues into synthesis gas (syngas), has been extended to biomass conversion, enhanced by catalysts [6]. Catalytic gasification operates at lower temperatures than thermal gasification and can be used for in situ hydrogen production in mature crude oil reservoirs [7]. This method is positioned between white hydrogen (naturally formed and available in reservoirs) and blue hydrogen (produced from fossil fuels) [8]. The hydrogen produced from catalytic gasification deserves recognition as a new type of hydrogen. Therefore, this work introduces a method to produce hydrogen from petroleum reservoirs based on in situ catalytic gasification, named light-blue hydrogen, resulting from the mixing of white and blue colors.
2. Method to Produce Light-Blue Hydrogen
Two well-established and commercially proven thermal enhanced oil recovery (EOR) methods can be used together: [9]
- −
- Cyclic steam stimulation (CSS): The most widely used EOR method, CSS aims to reduce oil viscosity, increase reservoir pressure, and help release oils from the reservoir rock.
- −
- In situ combustion (ISC): ISC involves burning off the heavier components of petroleum with air to produce heat, thereby reducing the oil’s viscosity.
The combination of these two EOR methods—injecting water steam with air to improve heat transfer—is called wet ISC in the upstream sector. This is similar to the thermal gasification typically used in petroleum refineries (downstream sector) to convert bottom-of-barrel materials, like vacuum residue, into syngas. An example of this concept has been proposed by the University of Calgary and Proton Technologies Canada Inc. to produce hydrogen from oil sands (natural bitumen) without carbon emissions (Figure 1). The produced hydrogen, called aqua hydrogen, is extracted to the surface using membranes in production wells, while the carbon oxides are kept underground. The term “Aqua” is used to represent this type of hydrogen generation, as it is a color between green and blue, since it does not emit CO2 like green hydrogen and is produced from fossil fuels like blue hydrogen [1].
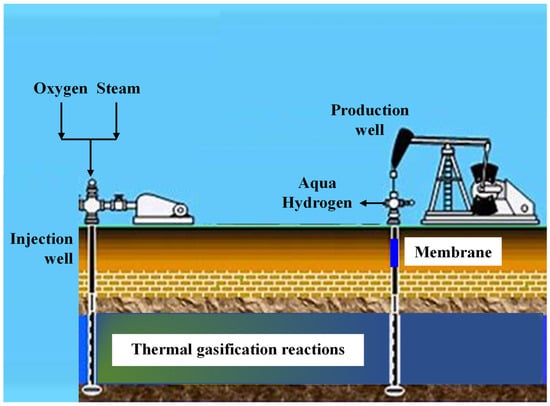
Figure 1.
Production of hydrogen by in situ thermal gasification.
This approach to producing hydrogen in petroleum reservoirs can be enhanced by using a catalyst, similar to how thermal gasification is improved by catalytic gasification in biomass conversion. In the case of catalytic biomass gasification, proper selection of a gasifier is needed depending on the feed properties. However, for in situ applications, the gasifier is the reservoir, and there is no way to change it.
The in situ catalytic hydrogen production shares key reaction mechanisms with in situ oil upgrading, with the primary distinction being the final product of each process. Some similarities between in situ hydrogen production methods and processes used in petroleum refineries, e.g., gasification, have been identified, in which hydrogen in situ production requires significantly less surface equipment than other technologies.
Catalytic biomass gasification has several advantages over thermal gasification, including higher conversion rates and greater syngas yield. For in situ applications, developing suitable catalysts with high stability and resistance to deactivation is strongly recommended. Ni-based catalysts, the most studied for biomass gasification, are anticipated to be the best candidates for in situ hydrogen production.
In addition to catalyst development, another challenge is the separation of hydrogen from other gases (primarily CO, CO2, and CH4). To produce aqua hydrogen, it has been proposed to use a membrane installed in a specially designed production well. The membrane, made of a palladium alloy, rejects COx gases underground while allowing hydrogen to dissolve in the palladium. This method separates hydrogen from other gases, sending it out of the reservoir for collection at surface facilities, while the other gases remain stored in the reservoir.
The approach proposed in this work for producing hydrogen in the reservoir can be considered an improvement of the aqua hydrogen technology, differentiated by the use of a catalyst [10]. Regarding the color of hydrogen, since it is produced in a reservoir, it can be seen as a type of white hydrogen, though it is not naturally formed, and it can also be considered a type of blue hydrogen since it is produced from fossil fuels [1]. Based on this analogy, this new type of hydrogen can be called “light-blue hydrogen”, resulting from the blending of white and blue colors (Figure 2).

Figure 2.
Definition of light-blue hydrogen.
Apart from the proposed method for separating hydrogen from other gases using a membrane in production wells, the separation can be performed at the surface. Once syngas has been produced inside the reservoir, it can be extracted, and the separation of hydrogen from other gases can be carried out using typical separation technologies. The other gases can be further used to produce high-value chemicals such as dimethyl ether and methanol (Figure 3).
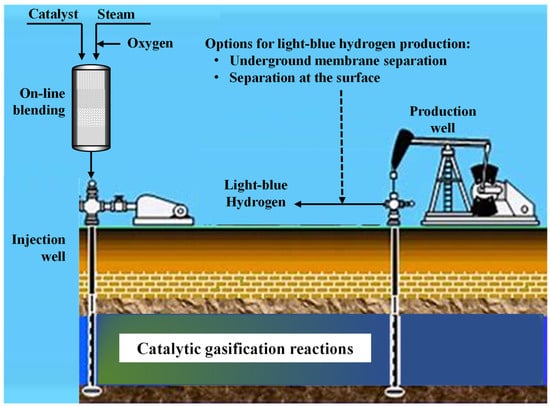
Figure 3.
Production of hydrogen by in situ catalytic gasification.
The in situ hydrogen production can be considered underground catalytic gasification, which involves drilling two wells, an injection well for introducing oxidants and steam to initiate oil ignition and combustion, and a production well for extracting the resulting syngas. Through a series of catalytic gasification reactions, syngas is generated. The composition of syngas varies depending on oil properties, catalysts, hydrogeological conditions, and operating conditions, affecting the concentrations of methane, hydrogen, carbon monoxide, carbon dioxide, nitrogen, and hydrogen sulfide.
Since the use of catalysts to enhance in situ gasification has not been extensively studied, this opens a new area for research to properly design catalysts and establish operating conditions for producing light-blue hydrogen tailored to the specific characteristics of petroleum reservoirs.
3. Perspectives of Light-Blue Hydrogen
Firstly, it should be noted that production costs of hydrogen indicate that aqua hydrogen (0.23 USD/Kg) has lower costs than green hydrogen (2.28–7.39 USD/Kg) and blue hydrogen (0.99–1.83 USD/Kg) [1]. Both green hydrogen and blue hydrogen still have higher production costs than grey hydrogen, commonly produced from natural gas (0.67–1.31 USD/Kg), which is the main reason they are not fully adopted for commercial applications. The use of a catalyst to produce the proposed light-blue hydrogen would increase production costs slightly, but it would also increase the hydrogen yield, making it economically attractive and cheaper than other hydrogen production technologies.
As for its commercial application, light-blue hydrogen technology would be more effective for heterogeneous heavy oil reservoirs, i.e., those that are mature with low oil saturation and are more challenging to develop. Mature reservoirs are the primary target for this technology since they are in declining production or reaching the end of their productive lives. For example, in Mexico, more than 65% of the total fields are mature, and the average recovery factor (around 15%) is notably lower than the international average of about 35% [11]. This presents a significant opportunity to apply this technology in such mature petroleum fields.
It is widely recognized that the production of green and blue hydrogen (and other types of hydrogen) would not be sufficient to meet global requirements. Therefore, the production of hydrogen from other sources in a more economical way, such as the light-blue hydrogen proposed here, is crucial to achieving net-zero CO2 emissions.
The current situation is opportune, as the percentage of mature petroleum reservoirs worldwide is high, and there is a tendency to avoid new field exploration and development. Additionally, there is a need for hydrogen production that can compete with grey hydrogen produced from natural gas. Thus, technology based on light-blue hydrogen production can be an attractive alternative for further research and development.
It is anticipated that light-blue hydrogen would represent a more challenging and cost-efficient technology than other types of hydrogen, especially compared to green and blue hydrogen. It is also important to mention that applying this technology to mature petroleum reservoirs would need to address the following challenges:
- −
- Optimization of oxygen injection;
- −
- Design of proper gasification catalysts;
- −
- Control of in situ combustion;
- −
- Evaluation of the potential risks of CO2 (and other gases) geological storage;
- −
- Suitable design of the membrane for the production well or surface facilities for the selective extraction of hydrogen from combustion gases;
- −
- Storage and transportation of the produced hydrogen;
- −
- Confirmation of the economics of producing light-blue hydrogen through feasibility studies;
- −
- Scaling-up of technology;
- −
- Testing the technology in petroleum fields.
4. Challenges for the Production of Light-Blue Hydrogen
The following main challenges for the production of light-blue hydrogen have been identified [2,12,13].
4.1. Efficient Catalyst Delivery into the Subsurface
Current supported porous metal-based catalysts used in reactors are not suitable for direct subsurface injection. While some nano-scale solid catalysts can be introduced into these environments, they face significant logistical and technical hurdles. Successful catalyst delivery requires stable suspension in water or hydrocarbon solvents, often achieved through emulsions. However, this approach increases costs and complicates injection strategies. Additionally, pore throat plugging during migration through porous media remains a risk, potentially impeding catalyst mobility and effectiveness.
To address these subsurface challenges, novel catalyst development strategies distinct from those used in reactors are being explored. One such approach is the in situ formation of catalysts, utilizing water-soluble or oil-dispersed precursors. However, the effectiveness of in situ catalysts is highly dependent on the composition of fossil fuels and rock minerals in the reservoir, necessitating extensive small-scale experimental studies. These studies aim to determine optimal active phase formation for hydrogen production and investigate preparation methods that ensure stable catalytic performance in subsurface conditions.
4.2. Catalyst Stability
Overall, developing active and stable catalysts for in situ hydrogen production in fossil fuel reservoirs remains a significant technological challenge. However, it also represents an exciting research frontier with substantial potential for advancing sustainable hydrogen production and carbon storage in subsurface environments.
Fossil fuels and rock minerals often contain impurities such as sulfur and heavy metals, which pose significant challenges for catalyst stability, i.e., catalyst deactivation. This problem becomes even more critical in complex subsurface conditions.
4.3. Use of Membranes Underground
Pd-based membranes, widely used in surface steam reforming for hydrogen separation, are fragile and unsuitable for downhole applications due to their limited durability and the difficulty of replacement. Alternatives such as Ti, V, and ceramic membranes offer greater mechanical robustness but suffer from low permeability, gas accumulation leading to pressure buildup, and potential blockage by multiphase fluids in wellbores. Critical aspects, including practical flow rates and delta-P requirements, remain unresolved, making hydrogen separation in subsurface conditions both technically and economically challenging.
Despite the potential of membrane technologies, applying them in subsurface reservoirs presents unique challenges, including extreme operating conditions, membrane durability, and incomplete reactant mixing, factors that limit hydrogen production efficiency.
5. Conclusions
A new approach to produce hydrogen in situ in petroleum reservoirs, based on concepts from catalytic gasification of biomass, is proposed. The hydrogen produced by this method is called light-blue hydrogen.
Light-blue hydrogen production is technically and economically more feasible than other types of hydrogen, such as green, blue, and grey, as it does not require high investment costs. Mature petroleum reservoirs, which are in the final stages of production, are the main targets for the application of light-blue hydrogen production. Currently, most of these petroleum fields are not economically viable, making them the best candidates for applying this technology.
It is recognized that the in situ production of light-blue hydrogen should consider the recent developments achieved in catalytic gasification of biomass, particularly the development of suitable catalysts. These catalysts should provide the highest hydrogen yield, be resistant to deactivation, and be cost-effective and properly designed for injection into the reservoir.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Yu, M.; Wang, K.; Vredenburg, H. Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen. Int. J. Hydrogen Energy 2021, 46, 21261–21273. [Google Scholar] [CrossRef]
- Okere, C.J.; Sheng, J.J. Review on clean hydrogen generation from petroleum reservoirs: Fundamentals, mechanisms, and field applications. Int. J. Hydrogen Energy 2023, 48, 38188–38222. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2024, Paris. 2024. Available online: https://www.iea.org/reports/world-energy-outlook-2024 (accessed on 28 February 2025).
- Chen, W.H.; Chen, C.Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Shi, H.; Ran, L.; Ancheyta, J. In-Situ upgrading of heavy crude oils inspired by ex-situ petroleum refining processes. Fuel 2024, 365, 131113. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Abbasi, G.R.; Lashari, N.; Patil, S.; Abdurrahman, M. A mini-review on underground hydrogen storage: Production to field studies. Energy Fuels 2023, 37, 8128–8141. [Google Scholar] [CrossRef]
- Ancheyta, J. Modeling of Processes and Reactors for Upgrading of Heavy Petroleum; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Tezer, O.; Karaba, N.; Ongen, A.; Colpan, C.O.; Ayo, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Varfolomeev, M.A.; Yuan, C.; Ancheyta, J. Catalytic In-Situ Upgrading of Heavy and Extra-Heavy Crude Oils; John Wiley & Sons, Inc.: New York, NY, USA, 2023. [Google Scholar]
- Wang, J.; Kang, D.; Shen, B.; Sun, H.; Wu, C. Enhanced hydrogen production from catalytic biomass gasification, with in-situ CO2 capture. Environ. Pollut. 2020, 267, 115487. [Google Scholar] [CrossRef] [PubMed]
- National Hydrocarbons Commission. Database of Crude Oil Mexican Reserves. 2018. Available online: https://www.gob.mx/cnh/es/articulos/centro-nacional-de-informacion-de-hidrocarburos-cnih-64831 (accessed on 28 February 2025).
- Hanfi, M.A.; Alade, O.S.; Tanimu, A.; Mahmoud, M.; Alarifi, S.A. Catalytic and noncatalytic in situ hydrogen production from heavy oil: A review of experimental studies. ACS Omega 2024, 9, 50118–50133. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Afanasev, P.; Askarova, A.; Popov, E.; Cheremisin, A. In situ hydrogen generation in subsurface reservoirs of fossil fuels by thermal methods: Reaction mechanisms, kinetics, and catalysis. Appl. Energy 2025, 381, 125219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).